Assessing the Relative Vulnerability of Chondrichthyan Species as Bycatch Using Spatially Reported Catch Data Series
Abstract
1. Introduction
2. Materials and Methods
2.1. The Southern and Eastern Scalefish and Shark Fishery
2.2. Species Occurrence Database
2.3. Fishery Interaction Index
2.4. Species Vulnerability
2.5. Assessing Vulnerability
3. Results
3.1. Catch Events
3.2. Fishery Interaction Index
3.3. Productivity Values
3.4. Vulnerability
3.5. Overall Priorities
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bonfil, R. Overview of World Elasmobranch Fisheries; Food & Agriculture Org.: Rome, Italy, 1994. [Google Scholar]
- IUCN. The IUCN Red List of Threatened Species, version 2022-2; IUCN: Gland, Switzerland, 2022. [Google Scholar]
- Hoenig, J.M. Life-history patterns in the elasmobranchs: Implications for fisheries management. Elasmobranchs as Living Source: Advances in the Biology, Ecology Systematics, and the Status of the Fisheries. NOAA Tech. Rep. 1990, 90, 1–16. [Google Scholar]
- Camhi, M. Sharks and Their Relatives: Ecology and Conservation; IUCN: Gland, Switzerland, 1998. [Google Scholar]
- Stevens, J.D.; Bonfil, R.; Dulvy, N.K.; Walker, P.A. The effects of fishing on sharks, rays, and chimaeras (chondrichthyans), and the implications for marine ecosystems. ICES J. Mar. Sci. 2000, 57, 476–494. [Google Scholar] [CrossRef]
- Smith, S.E.; Au, D.W.; Show, C. Intrinsic rebound potentials of 26 species of Pacific sharks. Mar. Freshw. Res. 1998, 49, 663–678. [Google Scholar] [CrossRef]
- Kennelly, S.J. The issue of bycatch in Australia’s demersal trawl fisheries. Rev. Fish Biol. Fish. 1995, 5, 213–234. [Google Scholar] [CrossRef]
- Lewison, R.L.; Crowder, L.B.; Wallace, B.P.; Moore, J.E.; Cox, T.; Zydelis, R.; McDonald, S.; DiMatteo, A.; Dunn, D.C.; Kot, C.Y.; et al. Global patterns of marine mammal, seabird, and sea turtle bycatch reveal taxa-specific and cumulative megafauna hotspots. Proc. Natl. Acad. Sci. USA 2014, 111, 5271–5276. [Google Scholar] [CrossRef] [PubMed]
- Commonwealth of Australia. A Guide to the Integrated Marine and Coastal Regionalisation of Australia Version 4.0; Department of the Environment and Heritage: Canberra, Australia, 2006.
- Musick, J.A.; Bonfil, R. Management Techniques for Elasmobranch Fisheries; Food & Agriculture Org.: Rome, Italy, 2005. [Google Scholar]
- Last, P.R.; Stevens, J.D. Sharks and Rays of Australia; CSIRO: Canberra, Australia, 2009. [Google Scholar]
- Fishery Status Reports 2022. In Australian Bureau of Agricultural and Resource Economics and Sciences; Australian Bureau of Agricultural and Resource Economics and Sciences: Canberra, Australia, 2022.
- AFMA. Guide to AFMA’s Ecological Risk Management; Australian Fisheries Managment Agency: Canberra, Australia, 2022. [Google Scholar]
- Cortés, E.; Brooks, E.N.; Shertzer, K.W. Risk assessment of cartilaginous fish populations. ICES J. Mar. Sci. 2014, 72, 1057–1068. [Google Scholar] [CrossRef]
- Hobday, A.; Smith, A.; Stobutzki, I.; Bulman, C.; Daley, R.; Dambacher, J.; Deng, R.; Dowdney, J.; Fuller, M.; Furlani, D.; et al. Ecological risk assessment for the effects of fishing. Fish. Res. 2011, 108, 372–384. [Google Scholar] [CrossRef]
- Zhou, S.; Smith, A.D.; Fuller, M. Quantitative ecological risk assessment for fishing effects on diverse data-poor non-target species in a multi-sector and multi-gear fishery. Fish. Res. 2011, 112, 168–178. [Google Scholar] [CrossRef]
- Zhou, S.; Griffiths, S.P. Sustainability Assessment for Fishing Effects (SAFE): A new quantitative ecological risk assessment method and its application to elasmobranch bycatch in an Australian trawl fishery. Fish. Res. 2008, 91, 56–68. [Google Scholar] [CrossRef]
- Sporcic, M.; Bulman, C.M.; Fuller, M. Ecological Risk Assessment for the Effects of Fishing. Report for the Southern and Eastern Scalefish and Shark Fishery (Gillnet Hook and Trap Sector): Shark Gillnet Sub-Fishery 2012–2016; Report for the Australian Fisheries Management Authority; Australian Fisheries Management Authority: Canberra, Australia, 2021; p. 218. [Google Scholar]
- Sporcic, M.; Bulman, C.M.; Fuller, M. Ecological Risk Assessment for the Effects of Fishing. Report for Southern and Eastern Scalefish and Shark Fishery (Commonwealth Trawl Sector): Danish Seine Subfishery 2012–2016; Report for the Australian Fisheries Management Authority; Australian Fisheries Management Authority: Canberra, Australia, 2021; p. 197. [Google Scholar]
- Sporcic, M.; Bulman, C.M.; Fuller, M. Ecological Risk Assessment for the Effects of Fishing. Report for Southern and Eastern Scalefish and Shark Fishery, Great Australian Bight Sector: Otter Trawl Sub-Fishery 2012–2016; Report for the Australian Fisheries Management Authority; Australian Fisheries Management Authority: Canberra, Australia, 2021; p. 174. [Google Scholar]
- Stobutzki, I.; Miller, M.; Brewer, D. Sustainability of fishery bycatch: A process for assessing highly diverse and numerous bycatch. Environ. Conserv. 2001, 28, 167–181. [Google Scholar] [CrossRef]
- Hordyk, A.R.; Carruthers, T.R. A quantitative evaluation of a qualitative risk assessment framework: Examining the assumptions and predictions of the Productivity Susceptibility Analysis (PSA). PLoS ONE 2018, 13, e0198298. [Google Scholar] [CrossRef] [PubMed]
- Patrick, W.S.; Spencer, P.; Ormseth, O.A.; Cope, J.M.; Field, J.C.; Kobayashi, D.R.; Gedamke, T.; Cortés, E.; Bigelow, K.; Overholtz, W.J.; et al. Use of productivity and susceptibility indices to determine stock vulnerability, with example applications to six US fisheries. In NOAA Technical Memorandum NMFS-F/SPO-101; U.S. Department of Commerce: Washington, DC, USA, 2009. [Google Scholar]
- Patrick, W.S.; Spencer, P.; Link, J.; Cope, J.; Field, J.; Kobayashi, D.; Lawson, P.; Gedamke, T.; Cortés, E.; Ormseth, O.; et al. Using productivity and susceptibility indices to assess the vulnerability of United States fish stocks to overfishing. Fish. Bull. 2010, 108, 305–323. [Google Scholar]
- Emery, T.; Woodhams, J.; Curtotti, R. Southern and Eastern Scalefish and Shark Fishery. In Fishery Status Reports 2022; Austrslian Government: Canberra, Australia, 2021; pp. 86–89. [Google Scholar]
- Alverson, D.L.; Freeber, M.H.; Murawsk, S.A.; Pope, J.G. A Global Assessment of Fisheries Bycatch and Discards; Food & Agriculture Org: Rome, Italy, 1994; Volume 339. [Google Scholar]
- Kaschner, K.; Kesner-Reyes, K.; Garilao, C.; Rius-Barile, J.; Rees, T.; Froese, R. AquaMaps: Predicted Range Maps for Aquatic Species. World Wide Web Electronic Publication, Version 08/2013. 2013. Available online: www.AquaMaps.org (accessed on 24 June 2015).
- Froese, R.; Pauly, D. FishBase: A Global Information System on Fishes. World Wide Web Electronic Publication. 2002. Available online: www.fishbase.org (accessed on 20 May 2023).
- Watson, D.F. A refinement of inverse distance weighted interpolation. Geo-Processing 1985, 2, 315–327. [Google Scholar]
- Esri, R. ArcGIS Desktop: Release 10; Environmental Systems Research Institute: Redlands, CA, USA, 2011. [Google Scholar]
- Musick, J.A. Criteria to Define Extinction Risk in Marine Fishes: The American Fisheries Society Initiative. Fisheries 1999, 24, 6–14. [Google Scholar] [CrossRef]
- Gallagher, A.J.; Kyne, P.; Hammerschlag, N. Ecological risk assessment and its application to elasmobranch conservation and management. J. Fish Biol. 2012, 80, 1727–1748. [Google Scholar] [CrossRef] [PubMed]
- Plagányi, É.E.; Punt, A.E.; Hillary, R.; Morello, E.B.; Thebaud, O.; Hutton, T.; Pillans, R.D.; Thorson, J.T.; Fulton, E.A.; Smith, A.D.M.; et al. Multispecies fisheries management and conservation: Tactical applications using models of intermediate complexity. Fish Fish. 2012, 15, 1–22. [Google Scholar] [CrossRef]
- Graham, K.J.; Andrew, N.L.; Hodgson, K.E. Changes in relative abundance of sharks and rays on Australian South East Fishery trawl grounds after twenty years of fishing. Mar. Freshw. Res. 2001, 52, 549–561. [Google Scholar] [CrossRef]

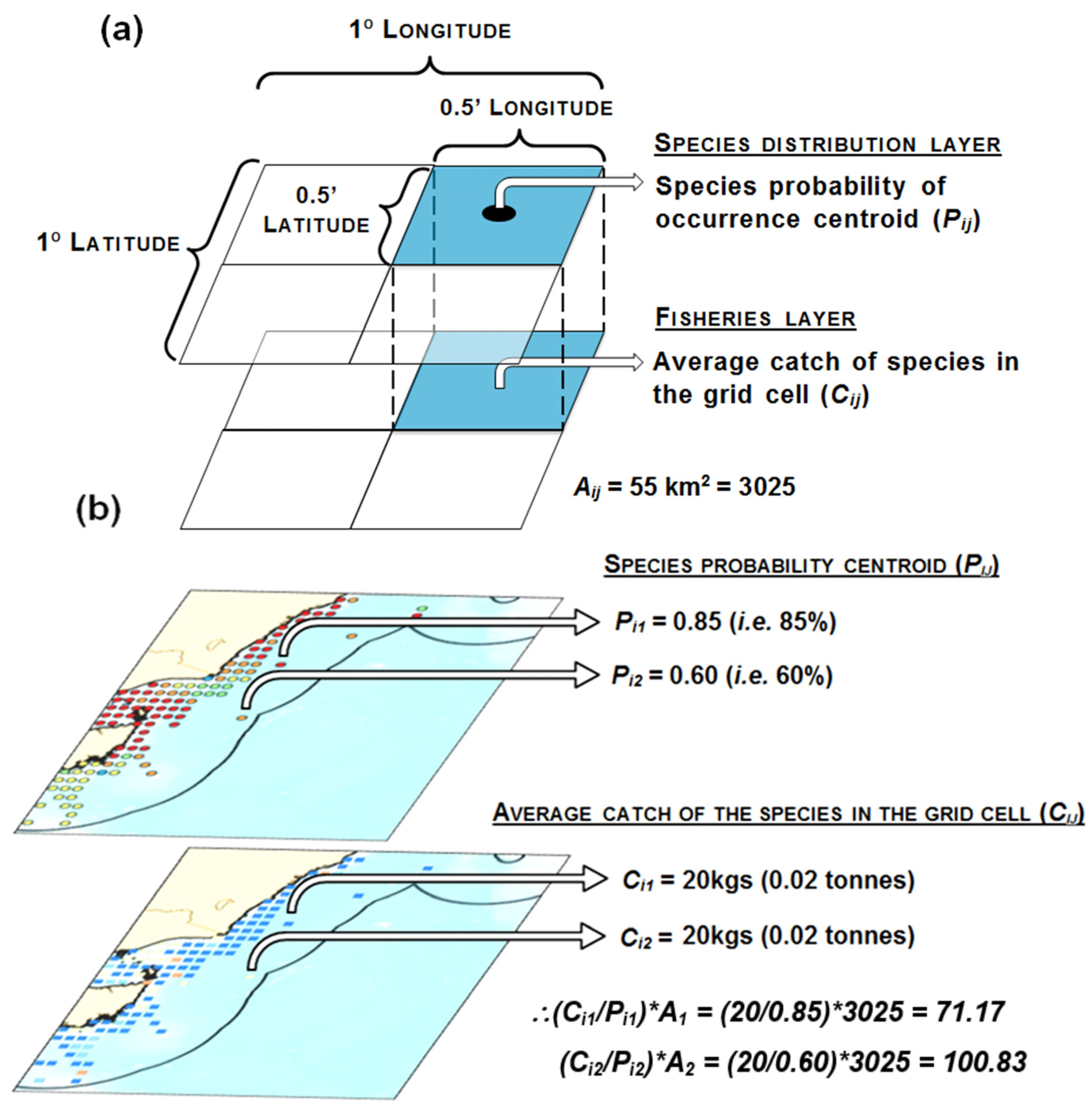
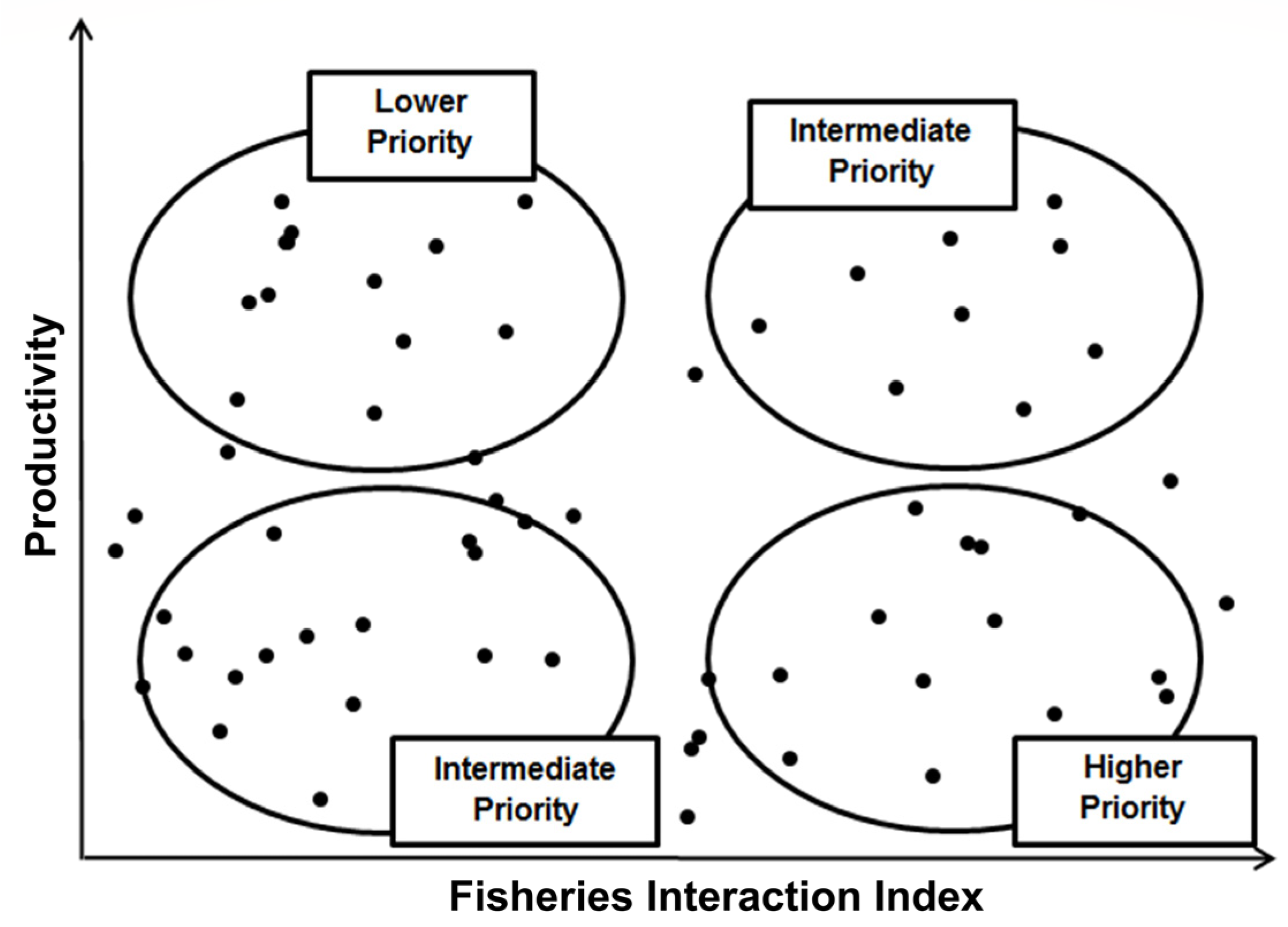
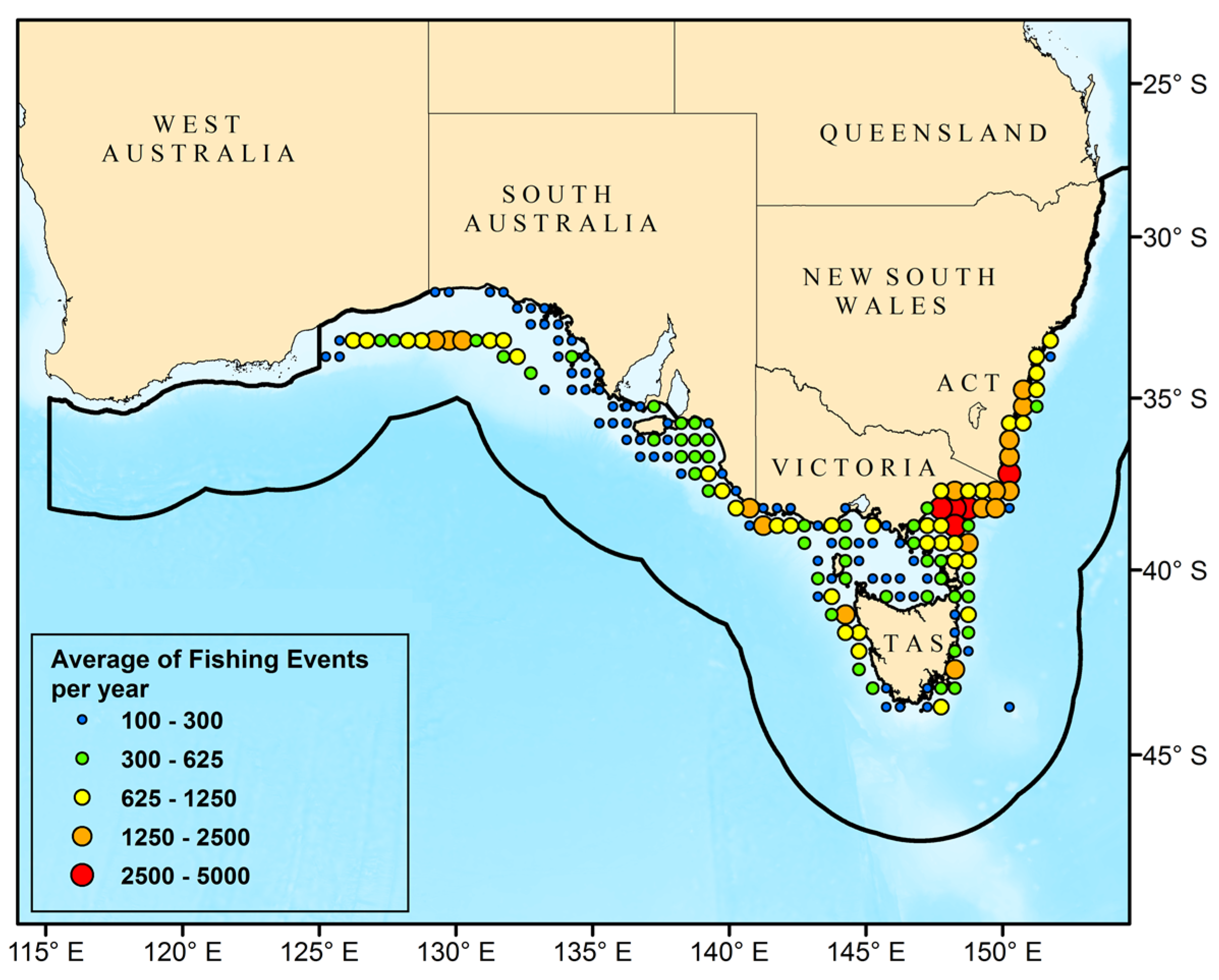
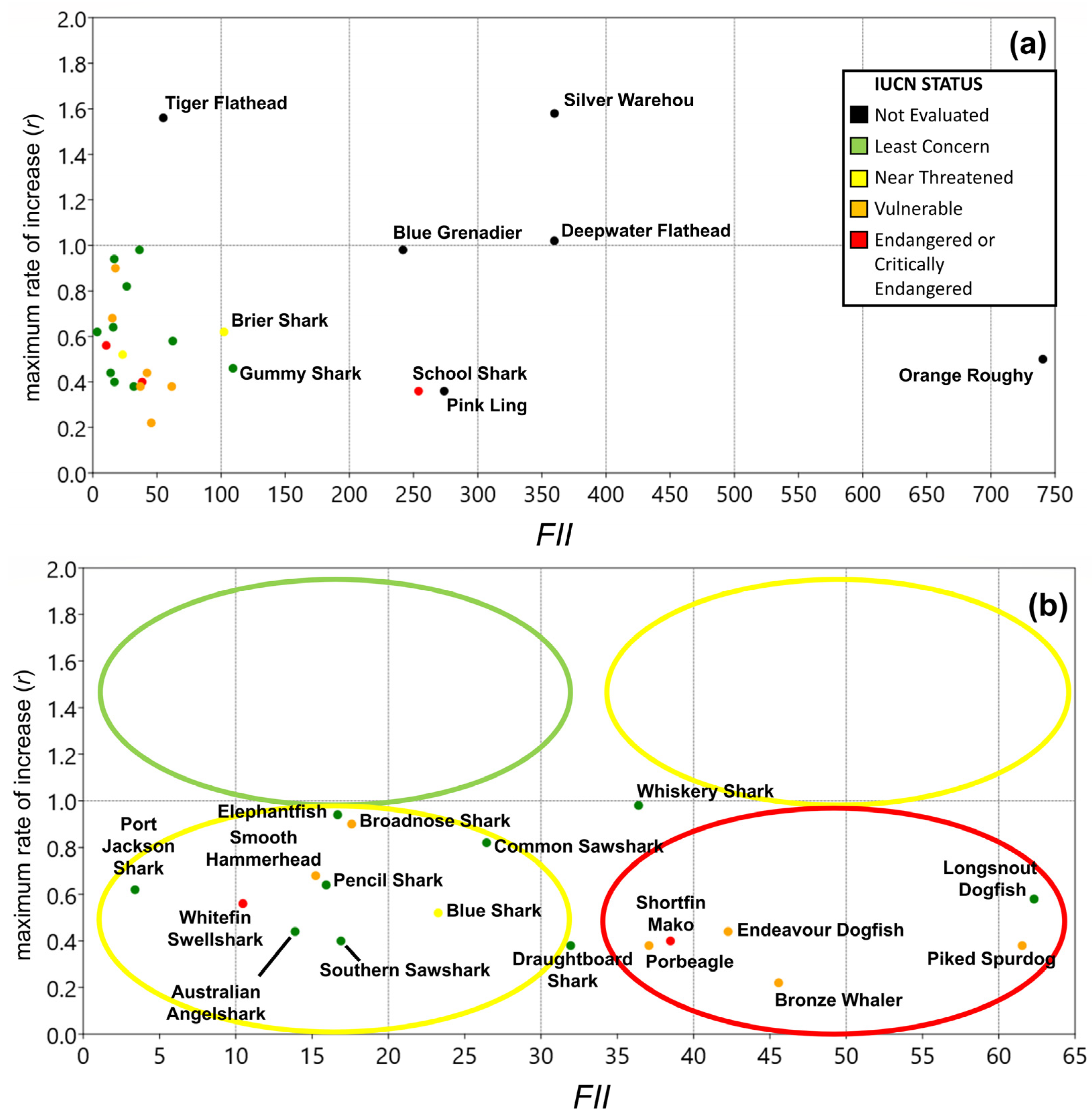
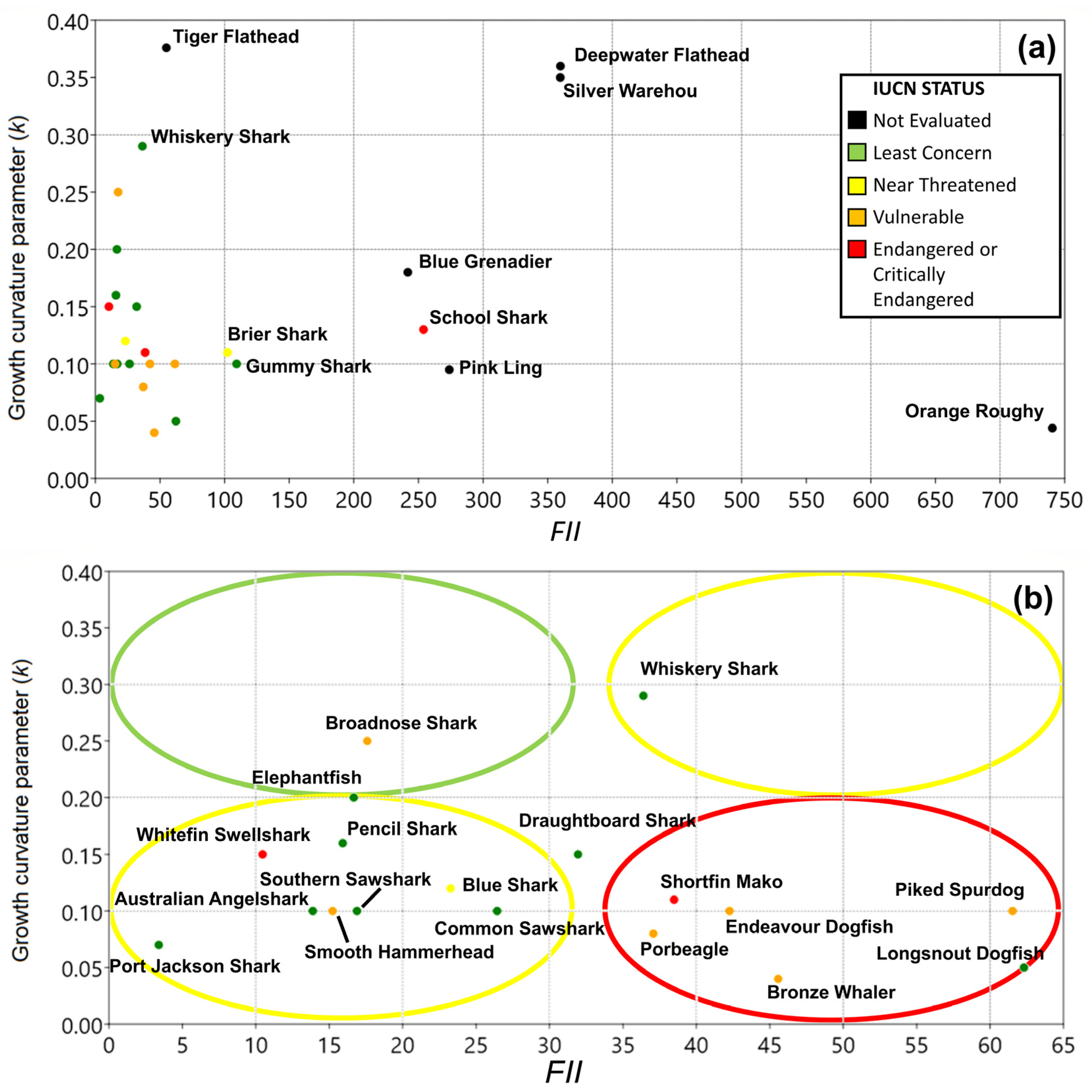
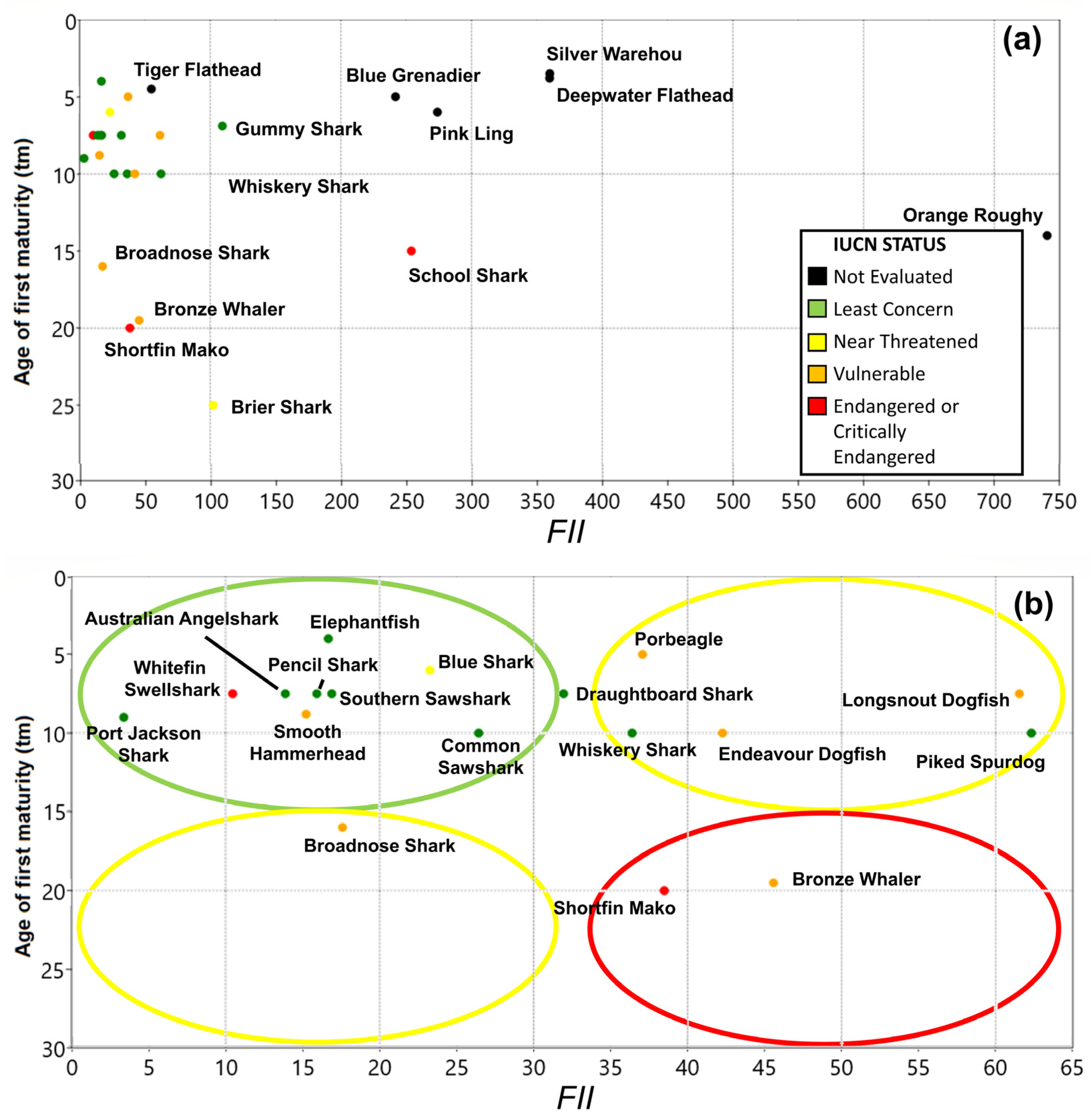
| Class | Family | Species | Common Name | Type | r | k | tm | Avg C Y−1 |
|---|---|---|---|---|---|---|---|---|
| Chondrichthyes | Callorhinchidae | Callorhinchus milii | Elephantfish | Bycatch | 0.94 | 0.20 | 4 | 58.27 |
| Heterodontidae | Heterodontus portusjacksoni | Port Jackson Shark | Bycatch | 0.62 | 0.10 | 9 | 0.38 | |
| Lamnidae | Isurus oxyrinchus | Shortfin Mako | Bycatch | 0.40 | 0.10 | 20 | 6.10 | |
| Lamna nasus | Porbeagle | Bycatch | 0.38 | 0.12 | 5 | 0.17 | ||
| Scyliorhinidae | Cephaloscyllium albipinnum | Whitefin Swellshark | Bycatch | 0.56 | 0.10 | 7.5 | 1.96 | |
| Cephaloscyllium laticeps | Draughtboard Shark | Bycatch | 0.38 | 0.10 | 7.5 | 10.86 | ||
| Triakidae | Furgaleus macki | Whiskery Shark | Bycatch | 0.98 | 0.30 | 10 | 27.57 | |
| Galeorhinus galeus | School Shark | Bycatch | 0.36 | 0.10 | 15 | 199.12 | ||
| Hypogaleus hyugaensis | Pencil Shark | Bycatch | 0.64 | 0.10 | 7.5 | 0.10 | ||
| Mustelus antarcticus | Gummy Shark | Target | 0.46 | 0.06 | 6.9 | 1503.81 | ||
| Carcharhinidae | Carcharhinus brachyurus | Bronze Whaler | Bycatch | 0.22 | 0.04 | 19.5 | 104.46 | |
| Prionace glauca | Blue Shark | Bycatch | 0.52 | 0.16 | 6 | 2.00 | ||
| Sphyrnidae | Sphyrna zygaena | Smooth Hammerhead | Bycatch | 0.68 | 0.10 | 8.8 | 6.60 | |
| Hexanchidae | Notorynchus cepedianus | Broadnose Shark | Bycatch | 0.90 | 0.25 | 16 | 36.08 | |
| Squalidae | Squalus megalops | Piked Spurdog | Bycatch | 0.58 | 0.05 | 10 | 19.62 | |
| Centrophoridae | Deania calcea | Brier Shark | Bycatch | 0.62 | 0.10 | 25 | 5.40 | |
| Deania quadrispinosa | Longsnout Dogfish | Bycatch | 0.38 | 0.10 | 7.5 | 24.06 | ||
| Centrophorus moluccensis | Endeavour Dogfish | Bycatch | 0.44 | 0.05 | 10 | 4.71 | ||
| Squatinidae | Squatina australis | Australian Angelshark | Bycatch | 0.44 | 0.10 | 7.5 | 54.43 | |
| Pristiophoridae | Pristiophorus cirratus | Common Sawshark | Bycatch | 0.82 | 0.05 | 10 | 86.11 | |
| Pristiophorus nudipinnis | Southern Sawshark | Bycatch | 0.40 | 0.10 | 7.5 | 46.27 | ||
| Actinopterygii | Merlucciidae | Macruronus novaezelandiae | Blue Grenadier | Target | 0.98 | 0.20 | 5 | 5011.30 |
| Ophidiidae | Genypterus blacodes | Pink Link | Target | 0.36 | 0.09 | 6 | 1091.42 | |
| Trachichthyidae | Hoplostethus atlanticus | Orange Roughy | Target | 0.50 | 0.06 | 14 | 1754.39 | |
| Platycephalidae | Platycephalus conatus | Deepwater Flathead | Target | 1.02 | 0.20 | 3.8 | 1054.14 | |
| Platycephalus richardsoni | Tiger Flathead | Target | 1.56 | 0.38 | 4.5 | 2574.76 | ||
| Centrolophidae | Seriolella punctata | Silver Warehou | Target | 1.58 | 0.36 | 3.5 | 1945.59 |
| Common Name | Scientific Name | FII | k | r | tm | IUCN Status | Priority |
|---|---|---|---|---|---|---|---|
| School Shark | Galeorhinus galeus | 253.90 | 0.08 | 0.36 | 15 | Crit. Endangered | VH |
| Gummy Shark | Mustelus antarcticus | 109.27 | 0.12 | 0.46 | 6.9 | Least Concern | H |
| Brier Shark | Deania calcea | 102.24 | 0.13 | 0.62 | 25 | Near Threatened | H |
| Piked Spurdog | Squalus megalops | 62.31 | 0.09 | 0.58 | 10 | Least Concern | M |
| Longsnout Dogfish | Deania quadrispinosa | 61.53 | 0.09 | 0.38 | 7.5 | Vulnerable | H |
| Bronze Whaler | Carcharhinus brachyurus | 45.57 | 0.04 | 0.22 | 20 | Vulnerable | H |
| Endeavour Dogfish | Centrophorus moluccensis | 42.26 | 0.10 | 0.44 | 10 | Vulnerable | H |
| Shortfin Mako | Isurus oxyrinchus | 38.48 | 0.09 | 0.40 | 20 | Endangered | VH |
| Porbeagle | Lamna nasus | 37.07 | 0.12 | 0.38 | 5 | Vulnerable | H |
| Whiskery Shark | Furgaleus macki | 36.39 | 0.29 | 0.98 | 10 | Least Concern | M |
| Draughtboard Shark | Cephaloscyllium laticeps | 31.94 | 0.09 | 0.38 | 7.5 | Least Concern | M |
| Common Sawshark | Pristiophorus cirratus | 26.43 | 0.19 | 0.82 | 10 | Least Concern | M |
| Blue Shark | Prionace glauca | 23.26 | 0.16 | 0.52 | 6 | Near Threatened | H |
| Broadnose Shark | Notorynchus cepedianus | 17.59 | 0.25 | 0.90 | 16 | Vulnerable | H |
| Southern Sawshark | Pristiophorus nudipinnis | 16.89 | 0.08 | 0.40 | 7.5 | Least Concern | L |
| Elephantfish | Callorhinchus milii | 16.67 | 0.23 | 0.94 | 4 | Least Concern | L |
| Pencil Shark | Hypogaleus hyugaensis | 15.92 | 0.18 | 0.64 | 7.5 | Least Concern | L |
| Smooth Hammerhead | Sphyrna zygaena | 15.23 | 0.14 | 0.68 | 8.8 | Vulnerable | M |
| Australian Angelshark | Squatina australis | 13.88 | 0.11 | 0.44 | 5 | Least Concern | L |
| Whitefin Swellshark | Cephaloscyllium albipinnum | 10.46 | 0.13 | 0.56 | 7.5 | Crit. Endangered | H |
| Port Jackson Shark | Heterodontus portusjacksoni | 3.39 | 0.11 | 0.62 | 9 | Least Concern | L |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reis, M.; Figueira, W.F. Assessing the Relative Vulnerability of Chondrichthyan Species as Bycatch Using Spatially Reported Catch Data Series. Diversity 2023, 15, 752. https://doi.org/10.3390/d15060752
Reis M, Figueira WF. Assessing the Relative Vulnerability of Chondrichthyan Species as Bycatch Using Spatially Reported Catch Data Series. Diversity. 2023; 15(6):752. https://doi.org/10.3390/d15060752
Chicago/Turabian StyleReis, Marcelo, and Will F. Figueira. 2023. "Assessing the Relative Vulnerability of Chondrichthyan Species as Bycatch Using Spatially Reported Catch Data Series" Diversity 15, no. 6: 752. https://doi.org/10.3390/d15060752
APA StyleReis, M., & Figueira, W. F. (2023). Assessing the Relative Vulnerability of Chondrichthyan Species as Bycatch Using Spatially Reported Catch Data Series. Diversity, 15(6), 752. https://doi.org/10.3390/d15060752






