Abstract
The Água Clara Cave System (ACCS) in Brazil is the richest hotspot of subterranean biodiversity in South America. In this study, we present an updated list of cave-restricted species in the ACCS and compare it with previously published hotspots in Brazil. Our list of cave-obligate fauna comprises 31 species, including 23 troglobionts and 8 stygobionts. The exceptional diversity of the ACCS can be attributed to factors related to the high dispersal potential of cave fauna within the system, high surface productivity, and the large size of the cave system size. Notably, we observed highly troglomorphic species in the ACCS, some of which are the most troglomorphic species in their respective groups in Brazil. The huge volume of galleries, high humidity, and trophic conditions prevailing in the ACCS may have played a role in shaping the strong troglomorphic traits observed in these species. However, all the obligate cave species in the ACCS require conservation attention and are at an elevated risk of extinction due to their limited ranges, few occurrences, and many potential threats. This study sheds light on the biodiversity and conservation status of cave-restricted fauna in the ACCS and highlights the importance of protecting these unique ecosystems.
1. Introduction
Subterranean environments are home to a distinct biodiversity that thrives under restricted food resources and stable environmental conditions, making them highly vulnerable to alterations in their pristine habitat characteristics [1]. However, our understanding of the diversity patterns of Neotropical cave fauna remains limited compared with other regions in the world [2], and the factors influencing their distribution are still largely unknown [3]. In South America, Brazil has the highest proportion of karst landscapes, and recent studies have identified many obligate cave species in these areas [4,5,6]. Karst landscapes in Brazil occur in different rock types, including granites, sandstone, iron ore, limestone, and dolomite, and are found in regions such as the Amazon Basin, the Atlantic Rain Forest, the Brazilian Savana, and the Caatinga under distinct climate conditions and geological ages [5,6,7,8].
Regrettably, industrial, economic, and human population growth in surrounding areas has had adverse effects on subterranean fauna and the karst landscape [1,9]. To safeguard these remarkable and delicate habitats and their fauna, some conservation strategies have been implemented globally to protect and preserve cave ecosystems [1]. The Red List of Threatened Species, established by the International Union for Conservation of Nature (IUCN), is one prominent initiative for indicating subterranean species that should be protected [2]. Furthermore, Culver and Sket [10] introduced the term “hotspots of subterranean biodiversity” to define subterranean habitats that have at least twenty or more obligate stygobitic and troglobitic species. However, they did not take into consideration the threats to biodiversity loss in those areas [4].
This approach of identifying hotspots based solely on species richness may not necessarily account for the ecological importance of these habitats or their vulnerability to anthropogenic impacts. Therefore, it is important to consider both the biodiversity value and the threats to the habitat in the process of identifying and prioritizing conservation areas [3,4]. Insufficient comprehension of subterranean systems has constrained the formulation of protective benchmarks, resulting in prioritization strategies that concentrate on identifying particular sites, such as caves, wells, or small aquifers, that display heightened levels of biodiversity. Nonetheless, these single-site methodologies are circumscribed by practical and financial limitations and neglect the interconnectivity among subterranean habitats as well as their mutual dependence with surface systems [11].
Despite the multifarious benefits that subterranean ecosystems and their biodiversity provide to humankind, they are infrequently incorporated into conservation plans on a large scale [12]. In order to safeguard cave biodiversity, it is imperative to not only preserve the intrinsic characteristics of subterranean habitats but also the pristine environmental conditions of the surrounding epigean environment [11]. Efforts focused on the development and implementation of conservation measures, as well as initiatives aimed at fostering collective action, are widely regarded as the most effective approach for identifying and safeguarding priority areas for conservation [13].
Despite persisting in a vision of conservation that remains constrained and fragmented in relation to subterranean habitats, identifying hotspots of subterranean biodiversity enables researchers and policymakers to optimize resource allocation and safeguard these exceptional and delicate ecosystems. Developing effective conservation strategies requires an understanding of the hazards that threaten these regions, including human development, pollution, and climate change. By investigating the taxonomic biodiversity, climate conditions, and organic resources present in these hotspots, researchers can improve their comprehension of the ecological mechanisms that govern these ecosystems and develop targeted conservation measures.
In Brazil, the speleological heritage is partially protected by a decree that requires that caves be classified according to their relevance degree prior to the installation of any activities that could potentially impact subterranean ecosystems. Currently, caves in Brazil are classified into four relevance categories (low, average, high, and maximum) through a multiparametric analysis. Only caves classified as having maximum relevance receive complete protection, while those in the remaining categories are susceptible to various degrees of impact, including complete cave suppression.
As part of the Diversity journal’s Special Issue titled “Hotspots of Subterranean Biodiversity”, we have compiled data on one of Brazil’s most remarkable cave systems, utilizing both existing literature and original findings. This contribution presents the extensive biological diversity within the system and offers hypotheses on why such a rich cave-restricted community evolved in this particular cave system. Therefore, it aims to serve as a reference for future research on evolution and conservation, as well as to inspire cave exploration in the numerous unexplored cave systems in Brazil.
2. Regional Geology
About 2.5% of Brazil’s territory is occupied by carbonate rocks, mostly in the central-eastern portion in an extensive strip of relatively continuous exposures between parallels 10° and 21° (Figure 1A). Some of the country’s most significant karst areas are located in this wide region that is part of the São Francisco Craton (CSF). Eleven “speleological regions” are discriminated, taking into account the largest clusters of caves [14], one of which is the region of Serra do Ramalho karst (Figure 1B).
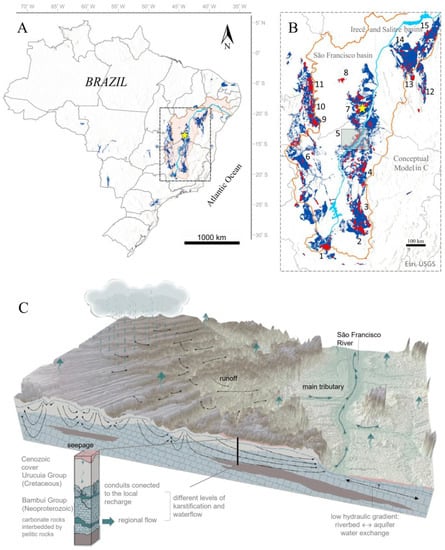
Figure 1.
(A) Distribution of predominantly carbonate lithostratigraphic units in Brazil as dark blue areas; São Francisco River Basin (BHSF) as red shaded with São Francisco River in light blue; the Água Clara Cave System pointed out by the yellow star. (B) Neoproterozoic carbonate units that cover the São Francisco Craton: São Francisco Sedimentary Basin, Bambuí Group (central and western region); Irecê and Salitre Basins, Una Group (northeastern region). Numbers representing 11 Speleological Regions related to the carbonate rocks of the Bambuí Group plus 4 Regions involving the Una Group (sensu [14]), placed around the major concentrations of caves (red spots). “Serra do Ramalho and surroundings” is the region number 7. (C) Illustrative scheme of water dynamics in the São Francisco River basin in the northern portion of Minas Gerais/southwest Bahia states. The model can be considered representative of the hydrogeological Subdomain involving the Serra do Ramalho Karst (slightly modified from [15]).
Karstification in these regions occurs in limestones and dolomites of siliciclastic-carbonate sedimentary successions belonging to the Bambuí Group, more specifically involving the Sete Lagoas and Lagoa do Jacaré formations (basal and intermediate units, respectively). The Bambuí mega-sequence is also composed by the Serra de Santa Helena Formation, a thick interval of pelitic rocks between the two carbonate units, and the Serra da Saudade and Três Marias formations, which gather siltstones, mudstones, and arkose sandstones at the top of the classic stratigraphic column [16,17].
The entire package can reach 3000 thick [18], but given the complex interplay of lithology, depositional environment, and tectonic activity, the sedimentary successions of the Bambuí Group may exhibit different stratigraphic patterns in different locations along the São Francisco Sedimentary Basin [17,19,20,21,22]. Still under discussion, the age of the basin filling by Bambuí sediments comprises the end of the Neoproterozoic and the beginning of the Paleozoic (Ediacaran-Cambrian), between 635?–520 Ma [23,24,25].
The Phanerozoic sedimentary cover of the CSF is composed of Cretaceous sediments, including siltstones, volcaniclastic rocks, and sandstones from the Areado, Mata da Corda, and Urucuia groups, which partially overlay the rocks of the Bambuí Group in erosive unconformity [26,27]. These sediments form the continuous plateaus of the Serra Geral de Goiás, which span from the north of Minas Gerais to the south of Piauí [28]. Additionally, Cenozoic covers consist of ferruginous detritus-lateritic sediments and alluvial deposits.
3. Hydrogeology
The regional geology plays a crucial role in the development of karst systems, particularly with respect to the aquifer system and the hydraulic conditions related to the regional sedimentary and tectonic settings [29]. Some factors inherited from regional geological processes include: (i) relationships between lithologies with different permeabilities; (ii) reduced primary porosity vs. increased secondary porosity; (iii) the existence of large geological structures such as faults and lineaments; and (iv) potentially fluidized mineralizations. Together with climatological parameters, these aspects must be addressed from integrated perspectives at different scales.
The carbonate units of the Bambuí Group are widely distributed within the São Francisco River basin, where extensive exposures of its carbonate formations are found (Figure 1C). These units are karst aquifers or fissured-karst when interbedded with siliciclastic rocks (i.e., interfaces with pelitic rocks of the Serra de Santa Helena formation). They are of high environmental and socioeconomic importance within the geopolitical context of the hydrogeographic basin, as they play a significant role in preserving ecological streamflows and are the sole source for urban, industrial, and rural supplies in many areas [30,31,32]. Karst springs and resurgences are heavily exploited in diffuse rural settlements, often through rudimentary catchment systems providing water for small-scale agriculture, animals, and even direct human consumption.
The management of these aquifers is carried out based on differentiated hydrogeological, geomorphological, and climatic scenarios within the river basin (BHSF) as a whole. These “scenarios” refer to hydrogeological domains and subdomains that exhibit distinct hydrodynamic signatures. These include relationships between carbonate and non-carbonate units, the deformational conditions, the nature of non-karst coverings, and the karstification patterns in terms of the general arrangement, size, and types of morphogenetic structures found in both exokarst and endokarst environments. From this overview, fifteen hydrogeological units are recognized in the BHSF [33]. The Serra do Ramalho karst, where the ACCS is located, is part of the “Subdomain IIIe”.
This subdomain features highly karstified areas in an exposure strip of undisturbed carbonate rocks located between the Urucuia plateaus and the middle reaches of the São Francisco River, spanning an elevation range of 450 to 800 m. The regional karstification in these areas is actively associated with the draining capabilities of the São Francisco River and large tributaries of its left bank, combined with high hydraulic gradient and permeability conditions, as illustrated in the general conceptual model applied to the Itacarambi-Montalvânia region [15], north of the state of Minas Gerais, middle São Francisco (Figure 1C).
4. The Local Karst
The Serra do Ramalho karst system is delimited to the south by the Carinhanha River and to the north by the Corrente River, two large perennial tributaries that are very important for the São Francisco River flow [34]. These rivers are formed on the Urucuia plateaus (Cerrado biome [35]) and cross in a W–E direction over 100 km of dissected karst areas of the so-called “Sanfranciscana depression” (Figure 2A).
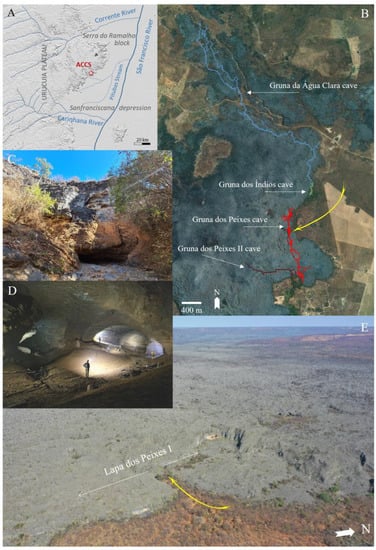
Figure 2.
Geomorphological-hydrological context of the Serra do Ramalho Karst and the Agua Clara Cave System (ACCS), highlighting the overall interrelationship with the São Francisco River: (A) Detached karst block in the interfluve of the Carinhanha (south) and Corrente (north) rivers; the ACCS placed on the eastern edge of the karst block; (B) Spatial distribution of the caves Gruna da Água Clara, Gruna dos Índios, Lapa dos Peixes I and Lapa dos Peixes II; the yellow arrow indicates some entrances of the Lapa dos Peixes cave; (C) One of the entrances of the Água Clara cave, sink of the seasonalstream that drains sections of the ACCS; (D) Inner conduit of the Água Clara cave; (E) Aerial view of the exposed massif at the edge of the Serra do Ramalho block, where the ACCS is located; the yellow arrow indicates some entrances of the Lapa dos Peixes cave. Bambuí Speleological group provides the cave maps (https://bambuiespeleo.wordpress.com/, accessed on 4 May 2023).
The interfluve of these rivers covers the broader region of Serra do Ramalho karst (approximately 12,000 km2), including the areas and potential areas of more direct karst recharge closer to the edge of Serra do Urucuia, where the sandstone cover is less thick. The wider karst also includes the discharge areas at local base levels and the discharge areas at the regional base level represented by the São Francisco River. The municipalities of Cocos, Feira da Mata, Coribe, Carinhanha, Serra do Ramalho, and São Félix do Coribe are all located entirely within this region.
A significant portion of the “broad karst system” of Serra do Ramalho corresponds to a large elevated block configuring an already quite incised extension of the Urucuia plateau, where the main karst structures that characterize the local karst are found. The essentially horizontal lithostratigraphic units of the Bambuí Group that support this block [36,37] gain geomorphological expressions in the terraces and erosive steps towards the plateau boundaries, as well as in the locally developed “erosional windows” inside the plateau. The karst features are notably associated with the two carbonate units depicted by the Sete Lagoas and Lagoa do Jacaré formations (calcilutites, calcarenites, calcirudites, and dolomites), although other lithostratigraphic units exert some kind of influence on the active karst processes [38].
In the descriptive model for the local karst system [39], the highest levels of the block are at the interface with the sandstone plateaus and present less evolved dissolution features. In intermediate elevations, the karst relief has strongly undulating surfaces with many dolines and limestone towers in conformation or exhumation. There is progressive dissection towards the margins of the block, gradually inverting the dominance between negative and positive forms. The endokarst is characterized by several underground river systems that, observed on a macro scale, constitute multiple cave conduits organized in a predominantly dendritic and regionally centrifugal pattern, which can extend for a few tens of kilometers. In general, these systems are linked to the multiple autochthonous recharge points associated with polygonal depressions and karst windows located in the central portions of the high and middle plateaus. They present convergent flow towards punctual discharges in several locations along the entire perimeter of the large rocky block. Recharge within the block also occurs diffusely in non-karstic coverings (e.g., residual sandstones from Urucuia; interbedded fissured pelitic rocks; soils). The contour of the karst block is marked by deep incisions that form large canyons and steep valleys. Although they are large, most of these marginal valleys are currently associated with intermittent or ephemeral drainage. Marginal groundwater outlets are perennial springs (phreatic level) or resurgences of highly seasonal flow associated with conduit systems.
Based on these hydrogeological, geomorphological, and speleogenetic aspects, one may distinguish, from the center to the edges of the plateau, karst domains with their own morphological patterns and hydrological and sedimentary dynamics [39].
Among them, the eastern margin of the block is exceptionally prominent in the local geomorphology, rising abruptly from the low levels of the São Francisco plains (460–480 m). This boundary extends for over 70 km in a very peculiar, strongly indented path, with contours delimited by cliffs tens of meters high, under which several springs and resurgences are located. On this face are the main discharges of the local karst system, and it is in this section that the ACCS is housed. This extensive discharge zone is also marked by the increased expression of the epikarst and, consequently, the movement of clastic materials that cover the karstified rocks. Under these conditions, soil volumes can be more easily injected into and later removed from the dissolution networks by water activity. Cyclical phases of aggradation and erosion are expressed in the underground and interstitial environments, while karren fields are progressively formed on the surface of the massifs.
All the outflows from this margin converge on the São Francisco plain, forming the Pitubas stream basin, a direct tributary of the São Francisco River that flows about 60 km along its alluvial plain. The tributaries in this “terminal basin of the karst system” have a strongly meandering pattern, with generations of wandering meanders characteristic of alluvial plains.
5. The Água Clara Cave System (ACCS)
The ACCS consists of a sequence of four large sets of underground conduits developed in the Sete Lagoas calcarenites. This “cave complex” is hydrologically connected by one of the multiple fluvial outlets located on the eastern periphery of the Serra do Ramalho karst block. From upstream to downstream, it involves the caves Gruna da Água Clara (13,880 m), Gruna dos Índios (510 m), Lapa dos Peixes I (9320 m), and Lapa dos Peixes II (2100 m). Linear development measurements and plan projections are indicated by Franco-Brazilian speleotographies carried out by Grupo Bambuí de Pesquisas Espeleológicas (GBPE) and Groupe Spéléo Bagnols Marcoule (GSBM) between 1998 and 2001 (Figure 2B) [14,40,41,42,43].
By direct observation of the relief and the surface drainage pattern, a maximum area of ca. 130 km2 is inferred for the possible catchment basin corresponding to the ACCS outflow, a tributary of the Riacho das Pitubas (Figure 2A). This calculation does not consider possible aquifer interactions of greater extension that may be associated with the regional stratigraphic and geomorphological framework.
The conduit system (Figure 2D) extends along a linear axis of about 8 km and a different level of ca. 80 m from the innermost end in the massif (“distal”, upstream) to the last resurgence (“proximal”, downstream), where the massif contour projects towards the alluvial plain of the São Francisco River. The stream that drains the basin at this discharge point has an intermittent flow and runs only 3.5 km underground, along two sections interspersed with the riverbed that surrounds the massif externally (Figure 2E). Some entrances also receive a seasonal water contribution (Figure 2C).
Considering the position, geometry, morphology, and sedimentary content, some hydrologically inactive subterranean segments may possibly depict ancient tracings of the main course (i.e., Gruna dos Índios) or secondary flow pathways active only under overflow conditions. Other segments correspond to smaller underground tributary channels, or palaeochannels. Floodwater mazes are especially close to the sinks and next to the final outlet of the system.
Overall, the system comprises the following subcompartments: (i) a sinuous main conduit with intermittent flow, with permanently inactive sections (fossil compartments); local anastomoses possibly related to overflow regimes, with eventual diversion of the main course to new parallel flow paths; channels with a markedly elliptical cross-section resulting from pressure flow, locally evolved to the “keyhole” type and tending towards quadrangular and irregular (polygonal) in the vicinity of entrances (sinks and resurgences) and gallery intersections; (ii) secondary conduits of ephemeral tributaries, or perennial drainages of low flow associated with runoffs of diffusely stored water; they form sinuous to straight sections of smaller diameter with diverse morphology; (iii) sections with lateral “accessory galleries” in a reticulated pattern or isolated fissures, associated with gravitational infiltrations from fissures connected to the exposed surface of the rocky mass (epikarst with higher karstification degree); they typically develop or reach the highest levels of the system; (iv) maze sectors lateral to the main conduit located near entrances (sink and resurgences), with galleries of diversified morphology in a reticular or braided arrangement, frequency of collapsed blocks and sedimentary cones (talus) injected from the surface, partially rearranged by backflows from the main channel, phreatic oscillations, and/or supervening floods; (v) lower proto-levels of punctual runoff to which fine sediments previously deposited are being selectively relocated.
The morphological pattern of the ACCS may represent a general speleogenetic model valid for other flow systems in similar transects, encompassing the upper portions of autochthonous recharges (diffuse and concentrated), the front steps of erosive retreat of non-karst coverings, the frank exposure of the carbonate rocks, and the peripheral gradient of karstification, ending with the arrangement of the discharges in the São Francisco plain that is linked to the São Francisco river flow dynamics [39].
Observing the currently prevailing hydrological conditions, the ACCS fits into a mixed condition of convergent surface flow towards a short underground transit channel, seasonally enhanced by a regime of rapid rainfall infiltration through an intensely fissured rocky substrate. Such conditions favor seasonal overflows in the system as well as water table oscillations, mainly in the lowest terminations of the system.
Considering the diameter of the fossil conduit that constitutes the distal (upstream) portion of the Gruna da Água Clara cave, it is plausible that the system extends into the inner massif after the collapses and sedimentary blockages. Sedimentary and morphogenetic studies may contribute to elucidating past flow regimes and related sources.
6. Compiling the Data (Overview of Invertebrate Sampling)
The database of the obligate cave fauna was compiled through a combination of published literature [44,45,46,47,48,49,50,51,52,53,54,55] and visits to the ACCS, with 10 visits conducted in total. To determine potentially troglobitic or stygobitic species, we identified ‘troglomorphisms’ in unknown sampled specimens and consulted with specialist researchers (specialists are acknowledged further on) or referred to previously identified and described species from the literature. All collected organisms were preserved in 70% ethanol, identified to an accessible taxonomic level, and deposited in the Subterranean Invertebrate Collection (ISLA) of the Center for Studies on Subterranean Biology at the Federal University of Lavras (CEBS-UFLA).
The cartographic sources for Figure 1A,B were obtained through ArcGIS Pro 3.1 geo-processing, specifically from the Geologic Maps of Brazil at a scale of 1:1,000,000 provided by the Serviço Geológico do Brasil (https://geoportal.cprm.gov.br/server/rest/services/geologia/litoestratigrafia_1000000/MapServer, accessed on 4 May 2023) and the Cadastro Nacional de Informações Espeleológicas (https://www.gov.br/icmbio/pt-br/assuntos/centros-de-pesquisa/cecav/cadastro-nacional-de-informacoes-espeleologicas/canie, 19 December 2022 updated data, accessed on 4 May 2023). The BHSF data in Figure 1C was sourced from open data provided by the Agência Nacional de Águas (ANA) (https://dadosabertos.ana.gov.br/, 29 March 2022 update, accessed on 4 May 2023). The shaded relief in Figure 2A was obtained from the Instituto de Pesquisas Espaciais—INPE (Brazilian Space Research Institute), specifically from the Topodata Project maps 14S45 and 13S45 (http://www.webmapit.com.br/inpe/topodata/, accessed on 4 May 2023). The cave maps in Figure 2B were provided by the Bambuí Speleological Group and can be accessed via their website at https://bambuiespeleo.wordpress.com/, accessed on 4 May 2023. The photographs of living specimens in Figure 3 were captured using a Canon EOS 80D digital camera (Canon, Tokyo, Japan). Lastly, the aerial photographs of the external landscape in Figure 2E were taken using a DJI Mavic 2 Pro drone (DJI, Shenzhen, China).

Figure 3.
Some of the cave-restricted species found in the Água Clara cave system (ACCS), Brazil. (A) Ochyroceratidae sp.1; (B) Charinus troglobius Baptista & Giupponi 2002; (C) Pseudochthonius koinopoliteia Prado & Ferreira 2023; (D) Giupponia chagasi Perez & Kury 2002; (E) Eukoenenia sp.1; (F) Pectenoniscus carinhanhensis Cardoso, Bastos-Pereira, Souza & Ferreira 2020; (G) Xangoniscus aganju Campos-Filho, Araujo & Taiti 2014; (H) Styloniscidae sp.1; (I) Styloniscidae sp.2; (J) Trichorhina sp.; (K) Troglobentosminthurus luridus Souza, Medeiros & Bellini 2022; (L) Blattodea sp.1; (M) Nylanderia sp.1; (N) Endecous infernalis Carvalho, Junta, Castro-Souza & Ferreira 2023; (O) Mesodiplatys falcifer Kamimura 2018; (P) Oniscodesmidae sp.1; (Q) Phaneromerium sp.1; (R) Chelodesmidae sp.1; (S) Pyrgodesmidae sp.1; (T) Geophilomorpha sp.1; (U) Girardia spelaea; (V) Spiripockia punctata; (W) Trichomycterus rubbioli.
7. The Checklist of Cave-Restricted Taxa in ACCS
The ACCS contains a total of 31 species that are restricted to caves, with distribution across Hexapoda (9 species), Arachnida (7 species), Crustacea (6 species), Myriapoda (5 species), Gastropoda (2 species), Turbellaria (1 species), and Siluriformes (1 species) (Figure 3). This makes the ACCS the South American cave system with the highest number of cave-restricted species. Terrestrial species were the most predominant (22 species), followed by amphibious (5 species) and aquatic species (3 species).
The low richness of aquatic species in the ACCS may be attributed to the intermittent nature of the drainage that traverses the system. The three aquatic species comprise a fish (Trichomycterus rubiolli Bichuette & Rizzato 2012), a snail (Spiripockia punctata Simone 2012), and a flatworm (Girardia spelaea Hellmann & Leal-Zanchet 2020), all associated with permanent water bodies (locally restricted within the system) that receive water input from epikarstic epigenic diffuse recharge. Consequently, populations of these species are only observed in specific areas along the ACCS, such as travertine pools or permanent ponds located in topographically lowered regions (as in the Peixes and Peixes II caves). However, during periods of rainfall, there can be dispersion events of organisms between different areas of the system as the main drainage becomes active. As an illustrative example, on a particular occasion, a solitary fish specimen (T. rubiolli) was observed in a minuscule travertine pool situated in an elevated area. It is plausible that this specimen was transported to that location during a flood event. It is worth noting that specific sampling methods targeting minute invertebrates, such as microcrustaceans, have not been employed. Therefore, the possibility of encountering additional stygobitic species in the ACCS in the future cannot be ruled out.
Among the caves in the ACCS, the Gruna da Água Clara cave was found to have the highest richness of troglobitic species, with a total of 23 species, including four species that were exclusively observed in this cave (Symphypleona sp.2, Rhagidiidae sp.1, Caponiidae sp.1, Trichorhina sp.1). The Lapa dos Peixes I cave had 19 species, while the Lapa dos Peixes II cave had 17 species, and the Gruna dos Índios cave had only five species (see Table 1). The Gruna da Água Clara and Lapa dos Peixes I caves had the largest number of shared species, even though their nearest entrances were approximately 3 km apart. It is noteworthy that the Gruna dos Índios cave, located in the intermediate portion of the ACCS, has a relatively dry main conduit due to the airflow that comes from the entrances on both sides of its main conduit. As a result, it is likely that the cave-restricted species shared by the Gruna da Água Clara and Lapa dos Peixes I caves are migrating through mesocaverns that connect these macrocaves. Only two species (Chelodesmidae sp.1 and Endecous infernalis) were found in all caves in the system (see Table 1). According to Souza-Silva et al. [51], the estimated troglobitic species richness suggests that the sampling effort reached adequate levels of completeness (obtained by Jack-Knife estimators), as the observed richness corresponded to over 78% of the estimated richness [56].

Table 1.
Variation in the number of troglobitic and stygobitic species across different taxa in the three South American hotspots of subterranean biodiversity.
It should be noted that, in addition to the troglobitic species, the ACCS also harbors 142 non-troglomorphic invertebrate species, distributed among Hexapoda (85 spp.), Arachnida (43 spp.), Myriapoda (5 spp.), Annelida (3 spp.), Mollusca (3 spp.), Turbellaria (2 spp.), and Nematoda (1 sp.). This makes the ACCS one of the most biologically diverse cave systems in South America, with at least 173 species. Importantly, most of these species were found in areas far from the cave entrances, indicating that future sampling efforts in the cave, especially in para-epigean communities, could further increase this number.
Notable Cave Species
Among the troglobitic species observed in the ACCS, some are highly troglomorphic, such as the springtail Troglobentosminthurus luridus (Figure 3K), the whip spider Charinus troglobius (Figure 3B), and the harvestmen Giupponia chagasi (Figure 3D), all of which represent the most troglomorphic species of their respective groups in Brazil. It is possible that the voluminous galleries associated with the oligotrophic conditions prevailing in the system have played a role in shaping the strong troglomorphic traits observed in these species. The elongated appendages found in these species confer advantages for both ambulation and detection of organic resources in such an ample subterranean environment. This hypothesis is in accordance with Trontelj et al. [57], who have discussed the significance of pore size on the evolution of cave organisms using amphipods from the genus Niphargus as models.
The high richness of isopods is also outstanding. From the six troglobitic species registered, five belong to the Styloniscidae family. It is noteworthy that the occurrence of phylogenetically related species within the same set of habitats is typically precluded by the competitive exclusion principle [58]. However, the coexistence of isopod species in the ACCS may be due to niche displacement, as they occupy distinct microhabitats within the system. Some species, such as Xangoniscus aganju (Figure 3G), are amphibious, whereas others, like Pectenoniscus carinhanensis (Figure 3F), are strictly terrestrial. Additionally, preferences for trophic resources can vary, with Trichorhina sp. (Figure 3J) typically found in plant organic debris (decaying trunks) and P. carinhanensis being more attracted to bat guano.
Among the 31 species that are restricted to caves within the ACCS, two species in particular warrant further discussion regarding their cave-restricted status: the earwig Mesodiplatys falcifer and the ant Nylanderia sp. The earwig M. falcifer has only been observed within the ACCS, despite extensive biological inventories conducted in numerous other caves within the Serra do Ramalho region. Although possessing relatively developed eyes, this species displays weak pigmentation (even in non-teneral adults) and elongated appendages compared with other species within its genus. In the original species description, Kamimura and Ferreira [49] suggested the possibility of it being troglobitic but did not definitively confirm this diagnosis, mainly because a single specimen was found. However, subsequent surveys within the ACCS revealed the presence of immature specimens consistently located in deep sections of the caves, with no observations of individuals in external habitats. Hence, we are herein considering this species as troglobitic. The ant species Nylanderia sp. exhibits all the typical troglomorphic traits. While all species in this genus are dark-pigmented and present well-developed eyes, the species from the ACCS displays weak pigmentation and considerably reduced eyes. Furthermore, an entire colony was observed rather than a single or a few specimens. Additionally, an expert (R. Feitosa, pers. com.) confirmed the troglomorphic traits and the undescribed status of this species.
Finally, it is important to mention that of the 31 cave-restricted species occurring in the ACCS, 22 are endemic to this cave system. The remaining eight species, including C. troglobius, G. chagasi, and X. aganju, which have relatively wide distributions across the Serra do Ramalho area, may comprise cryptic species complexes. Ongoing studies of C. troglobius and X. aganju have identified morphological and genetic differences between populations in distinct caves, suggesting that each species is actually a set of cryptic species, each endemic to a single cave or cave system. Therefore, it is likely that the number of endemic species in the ACCS will increase in the future, underscoring the system’s importance as a unique biodiversity hotspot.
8. Discussion
8.1. Importance of Continuous Surveys and Updated Checklists
Sampling subterranean environments can be a daunting task, especially in the case of invertebrates. This challenge is largely due to the difficulty of accessing crucial microhabitats such as fissures, mesocaves, and interstitial voids [1,57,59]. Thus, to document subterranean biodiversity effectively, it is crucial to conduct multiple collections over time. Unfortunately, Brazil has a history of insufficient long-term studies in caves, mainly due to two factors. Firstly, the continuous discovery of new caves and karst areas presents researchers with tempting opportunities to identify new species and ecological patterns, drawing them away from performing long-term studies in a same cave or cave system. Secondly, the legal framework for protecting Brazilian caves only requires two samplings of a given cave for environmental licensing purposes. While the allure of new karst areas attracts researchers, the minimal legal requirement for sampling also hinders long-term studies.
Therefore, it is highly probable that many undiscovered subterranean hotspots of biodiversity exist in tropical regions, and that the currently known hotspots represent only a small portion of the total. In Brazil, all identified hotspots consist of caves or cave systems that have undergone successive sampling, indicating that the scarcity of long-term studies may be a major obstacle to the discovery of new hotspots in tropical areas. As such, countries with cave conservation policies, like Brazil, should consider recommending a greater number of sampling events in caves to reveal their true diversity. Given that numerous troglobitic species are rare, it is improbable that the entire range of species present in a cave will be detected through just one or a few sampling efforts.
8.2. Taxonomic Impediment of Megadiverse Tropical Areas and the Challenge of Determining Cave-Restricted Species
The conservation of subterranean environments is often hindered by the fact that many of the species that inhabit these ecosystems remain undescribed and are therefore often overlooked in conservation efforts [60,61]. In tropical and subtropical regions, where most cave-restricted species are yet to be formally described [4,61], caves are typically considered to be relatively species-poor [60]. Therefore, describing the troglobitic species found in a given area is a crucial step towards their conservation.
As an example, among the 31 cave-restricted species observed in the ACCS, only 11 (35.5%) have been formally described (Table 1): Girardia spelaea (Platyhelminthes: Dugesiidae) [52], Spiripockia punctata (Mollusca: Caenogastropoda) [47], G. chagasi (Opiliones, Gonyleptidae) [45], C. troglobius (Amblypygi: Charinidae) [44], Pseudochthonius koinopoliteia (Pseudoscorpiones: Chthoniidae) [55], X. aganju (Isopoda, Styloniscidae) [48], P. carinhanhensis (Isopoda, Styloniscidae) [50], T. luridus (Collembola: Sminthuridae) [51], E. infernalis (Orthoptera: Phalangopsidae) [54], Mesodiplatys falcifer (Dermaptera: Diplatyidae) [49], and Trichomycterus rubbioli (Siluriformes: Trichomycteridae) [46]. Moreover, of the 11 species, six were only recently discovered during ecological surveys of the caves comprising the ACCS [53]. Prior to 2016, only six of the 24 known cave-restricted species in the region were formally described [62]. However, recent ecological studies conducted in 26 caves in the Serra do Ramalho region indicate the existence of at least 70 additional cave-restricted species [Ferreira et al. unpublished data], indicating the vast potential for discovering new species in this area.
The slow pace of species description of cave-dwelling organisms in Brazil highlights the lack of taxonomists who specialize in taxa commonly found in caves, as well as the difficulties encountered by foreign taxonomists in studying this fauna due to legal constraints. Despite some financial support being allocated towards the description of subterranean taxa, the number of species described in Brazil in recent years remains relatively low compared with the vast number of newly discovered species each year. Therefore, it is imperative not only to encourage species description but also to train new taxonomists, particularly for taxonomic groups with limited or no specialists capable of identifying and describing this unique, endemic, and threatened fauna.
Finally, identifying species that are exclusively restricted to caves is a difficult task in tropical areas. While the concept of troglobitic species is widely accepted, it can be challenging to determine this status with certainty. The only surefire way of confirming whether a species is exclusively restricted to subterranean habitats is to demonstrate its absence in surface habitats, which can be difficult, if not impossible, in mega-diverse tropical regions. To address this challenge, alternative approaches, such as the use of troglomorphic traits, have been employed to define such species. However, it should be noted that although typical troglomorphic traits, such as lack of pigmentation, eye reduction, and appendage elongation, are easily recognizable, there are several specific traits, especially for groups naturally devoid of pigment and eyes (e.g., Palpigradi), which can make identification challenging. Additionally, the use of troglomorphic traits can often lead to misdiagnosis. In some cases, a troglobitic species may not be recognized if it presents weak troglomorphisms, even though it is already restricted to caves. Conversely, an epigean troglomorphic species found in caves may be erroneously considered a troglobite.
8.3. Is 25 Cave-Restricted Species a “Magic” Number?
In their original proposal, Culver and Sket [10] introduced the term “hotspots of subterranean biodiversity” (HSB) to refer to subterranean habitats containing a minimum of twenty cave-restricted species. Although somewhat subjective, this threshold was most likely established based on the researchers’ extensive expertise in cave faunas globally and their specific interest in investigating the biogeographic patterns that underlie variations in the number of cave-restricted species across different areas. However, more recently, there has been a movement to raise this threshold to at least 25 cave-restricted species for a cave or cave system to qualify as a hotspot. This increase in the cutoff is still arbitrary, nonetheless. Consequently, an inevitable question arises: does this higher cutoff reflect the global scenario or is it primarily based on the already recognized regions that are known for their richness in troglobitic and stygobitic faunas?
Cave ecosystems in tropical regions are unlikely to exhibit the same ratio of troglobitic to non-troglobitic species as those found in temperate regions. This disparity arises due to the greater temperature fluctuations that occur during glacial maximums in higher latitude areas, which have profoundly affected the isolation and evolution of troglobitic species, leading to a high species richness in temperate caves. In contrast, tropical caves have not experienced such severe temperature changes over their geological history, although they are usually located within highly diverse external landscapes. As a result, while only a small proportion of epigean species may become isolated and evolve to become cave-restricted, this number can still be relatively high in tropical caves. Nonetheless, it is important to emphasize that tropical caves will never attain the same ratios of troglobitic species observed in temperate caves. This effect is evident when comparing the proportions of hotspots of subterranean biodiversity (HSB) between temperate and tropical regions. While 81.6% of these hotspots occur in temperate areas, only 18.4% are located in the tropics [1,4,63,64].
Therefore, it is crucial to consider various parameters while defining subterranean hotspots. As an initial step, it is crucial to consider different scales when identifying hotspots, distinguishing between regional and global levels. Furthermore, the presence of natural breakpoints in datasets can provide valuable insights and serve as indicators of potential hotspots. Another significant criterion to be taken into account is the latitudinal range where the cave is located. A cave located in a high-latitude region with few cave-restricted species may still be considered a hotspot, given the extreme external climate conditions that would typically preclude the existence of any species. In contrast, in tropical areas, where external conditions are less severe, a smaller number of cave-restricted species (compared with temperate regions) should be taken into account when defining a cave as a hotspot. Another important factor to consider when defining HSB is the lithology associated with the cave. It has been consistently observed that iron-ore caves in Brazil generally display a higher average richness of cave-restricted species compared with caves associated with other lithologies [65]. Conversely, granite caves tend to exhibit a lower abundance of troglobitic fauna. Consequently, a HSB located in granite caves may potentially have a lower number of cave-restricted species in comparison to HSBs in iron-ore caves. An interesting example illustrating this pattern is the Wynberg Cave System (WCS) in South Africa. This cave system is situated within quartzite rocks and harbors an impressive diversity of 19 cave-restricted species [66]. This substantial number of species within a lithology typically considered less favorable for supporting cave-restricted organisms further confirms the designation of WCS as a HSB.
Consequently, it is essential to rethink the HSB concept and propose adaptations, as many countries can use or consider it for public policies regarding cave conservation. Hence, there is a risk of overlooking significant caves or systems on these lists if we always consider a high number of cave-restricted species. Nonetheless, further studies are necessary to determine the appropriate width of the latitudinal range and the proportion of troglobitic richness relative to the average that should be considered when defining a hotspot.
It is worth noting that Culver and Sket [10] did not take into account the level of threat to which subterranean habitats are exposed, as proposed by the hotspot model of Myers et al. [67]. Well-preserved landscapes can quickly turn into pastures or be destroyed by mining activities, as has happened in many karst regions around the world in recent years [68,69]. Therefore, relying solely on the number of cave-restricted species might not provide an accurate indication of the “health” of a given subterranean system, as this depends on the type of impact it has experienced, especially in recent times [4]. The Água Clara Cave System (ACCS) is a prime example, as it faces unprecedented threats from external factors (see Section 8.7). Thus, it is crucial to incorporate the level of threat to which a cave is exposed in this concept, as proposed by Myers et al. [67], particularly given that conservation policies often prioritize investment in areas of high conservation value [4].
Finally, every HSB is undoubtedly important, not only because of the richness of cave-restricted species it presents but mainly due to the high degree of endemism displayed by a large proportion of its species. Thus, another attribute that should always be taken into account when assessing the significance of a hotspot is the number of cave-restricted species exclusively found within that cave (or system) in relation to the total number of troglobitic/stygobitic species it presents.
8.4. Why Is ACCS So Rich in Cave Restricted Species?
In temperate regions, cave-restricted species are significantly influenced by epigean primary productivity since the amount of organic resources in surface environments potentially affects the availability of resources in subterranean ecosystems [1]. However, in contrast to temperate regions, external primary productivity was not found to have a significant influence on the richness of troglobitic species in Brazil [70]. This is possibly due to the high productivity observed in tropical regions, where even areas with relatively low productivity can provide sufficient resources for cave-restricted species [70]. As a result, factors other than external productivity are likely to have shaped the ecology and evolution of these species in tropical regions.
The intermediate disturbance hypothesis (IDH) posits that local species diversity can be increased when ecological disturbances occur at intermediate frequencies, neither too rare nor too frequent. At low disturbance levels, competitive organisms outcompete less competitive species, leading to their extinction and the dominance of the ecosystem by the more competitive species [71]. Conversely, at high disturbance levels, all species are at risk of extinction. The IDH suggests that at intermediate levels of disturbance, species that thrive at both early and late successional stages can coexist, promoting diversity. This hypothesis is based on three premises: (i) ecological disturbances have significant impacts on species richness within the disturbance area; (ii) interspecific competition results in one species driving a competitor to extinction, thus becoming dominant in the ecosystem; and (iii) moderate ecological scale disturbances prevent interspecific competition [72,73,74]. Despite criticism of this hypothesis [75], many authors continue to rely on its theoretical and empirical foundations [76]. Although the IDH is mostly used to explain ecological scenarios, the role of disturbances in evolutionary processes is widely accepted [75]. Therefore, species diversity in disturbance-mediated coexistence could be enhanced by the presence of a disturbance regime that resembles historical processes since species generally adapt to the level of disturbance in their ecosystem during their evolution.
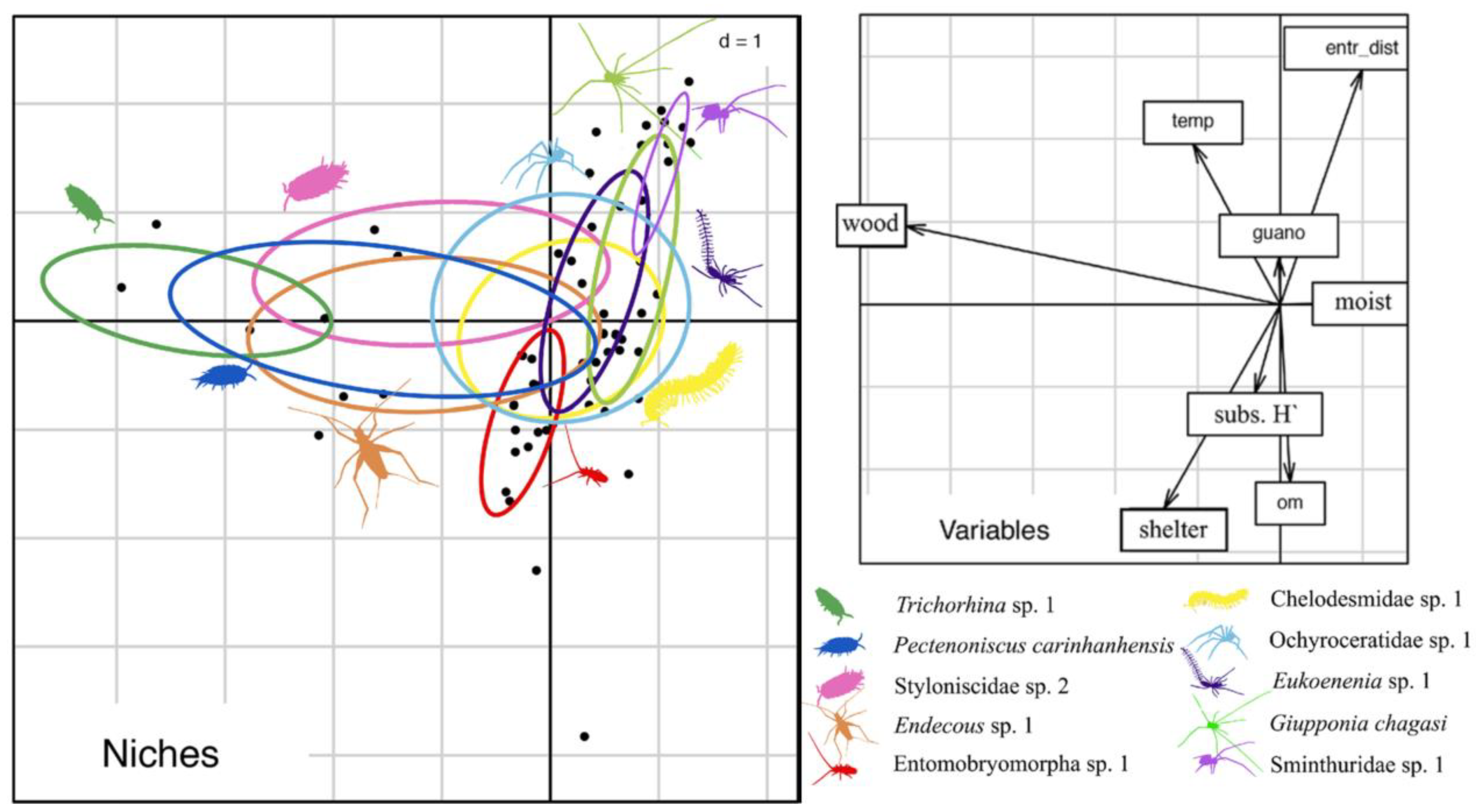
The ACCS presents an intermittent drainage that flows along the system during the rainy periods. The cave system receives a vast amount of water from the external micro basin, which is significant due to intense and localized rainfall events common in the region. The flashflood pulses associated with these events can transport external materials, including large tree trunks, which often become lodged within the caves (see Figure 2D). Moreover, these flood pulses seasonally alter many cave substrates, modifying the cave floor and affecting numerous microhabitats (as bat guano piles, that can be washed away). These disturbances can be classified as intermediate since they partially and temporarily modify the cave’s microhabitats. Therefore, flashflood pulses have not only shaped the invertebrate community structure of the ACCS but also likely influenced species evolution. By periodically changing the cave substrates, flashflood pulses prevent the establishment of dominant species, leading to the exploitation of various niches within the cave. Souza-Silva et al. [53], who analyzed the niches of ten troglobitic species from the ACCS, demonstrated that the most widespread troglobitic species could utilize microhabitats with distinct characteristics, thus avoiding niche overlap and promoting coexistence (Figure 4).
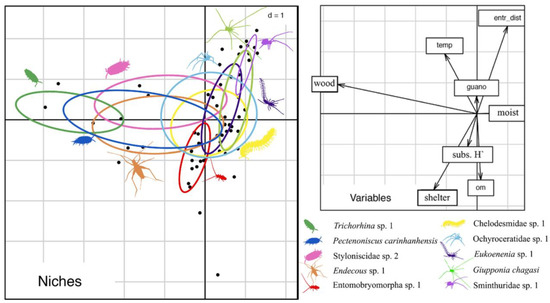
Figure 4.
Niches of some cave-restricted species from the ACCS (modified from [53]). Results of the Outlying Mean Index (omi) analysis for the ten most widespread troglobitic species in ACCS that occupy the environmental niche according to the physical, trophic and microclimate characteristics of each transect.
Thus, despite cave-dwelling species often being classified as generalists, the presence of flashflood disturbances within the ACCS has likely resulted in reduced niche overlap. This reduction, in turn, has played a significant role in facilitating the coexistence of multiple troglobitic species within the system.
It is imperative to acknowledge that caves or cave systems harboring a high number of cave-restricted species typically correspond to oversized caves found in regions of high external productivity or with isolated water bodies from the surface [1,53]. The extent of a cave plays a significant role in fostering a greater diversity of cave-restricted species. Larger caves, by virtue of their size, offer a wider range of microhabitats, thereby providing the potential for accommodating a larger number of species [53,66]. The ACCS, situated in the Brazilian Tropical Dry Forest (specifically the Caatinga domain), is no exception. However, the remarkable richness observed in this system may be attributed not only to its extension, but also to both the IDH and the historical transformations undergone by the biome where the cave is located [53]. In particular, many caves in the Caatinga, especially those with perennial water sources, exhibit a remarkable diversity and endemism of cave-restricted fauna. It is noteworthy that the Caatinga biome had tropical rainforests spread over several areas during the Last Glacial Maximum (LGM) [77], which may have served as a refugia for the ancestors of current troglobitic species that were subsequently “trapped” inside caves following the retraction of the humid regions [78,79]. Supporting this hypothesis, three of the four known hotspots of subterranean biodiversity in Brazil, one of which remains undisclosed, are located in the Caatinga domain [4,53].
8.5. Comparison with Other Subterranean Hotspots in Brazil
South America is home to three recognized hotspots of subterranean biodiversity, all of which are located in Brazil. These include the Água Clara cave System and Toca do Gonçalo cave, located in the Bahia state (Northeastern Brazil), as well as the Areias cave systems situated in the São Paulo state (Southeastern Brazil). All these subterranean systems are associated with carbonate rocks. The Água Clara and Toca do Gonçalo caves are located in the semi-arid Caatinga Biome and harbor 31 and 22 species of obligate cave dwellers, respectively. The Areias Cave System, which is a unique Brazilian hotspot located within a conservation unit (Park Estadual Turístico do Alto Ribeira—PETAR), accommodates 28 species and is located in the Brazilian Atlantic Forest. These findings were reported in studies conducted by Souza-Silva and Ferreira [4] and Souza-Silva et al. [53].
The presence of either permanent or temporary water bodies provides a suitable habitat for troglobites and stygobites species in all three Brazilian hotspots of subterranean biodiversity. Additionally, the occurrence of siluriform fish species (Trichomycterus rubbioli, Rhandiopsis sp.n., Pimelodella kronei (Ribeiro, 1907)) is common in all three areas. These extensive subterranean systems (spanning over 500 m) are dependent on allochthonous donors for the input of organic resources into food chains, including percolation water, permanent runoff, streams, and bats. Among these hotspots, the Areias cave system is the only one that presents cave-restricted species from the epikarst zone compartments [4,53]. The occurrence of roots does not appear to be an essential resource for the maintenance of the fauna, as they are scarce in all three hotspots [4,53].
The Água Clara Cave System exhibits a predominance of terrestrial species (23 spp.), followed by amphibious species (5 spp.), and exclusively aquatic species (3 spp.). Similarly, in Toca do Gonçalo, terrestrial species are predominant (17 spp.), followed by species living in lentic aquatic habitats (5 spp.). The Areias Cave System also presents a predominance of terrestrial species (22 spp.), with the remaining species found in the aquatic lotic habitat (4 spp.) and epikarst zone (2 spp.) [4,53]. It is noteworthy that certain taxa such as Crustacea, Hexapoda, Arachnida, and Myriapoda consistently dominate as the richest taxa across all three hotspots, suggesting a pattern of successful colonization and establishment in subterranean habitats (Table 2).

Table 2.
Taxonomic diversity and distribution of 31 obligate cave species within the Água Clara cave system, located in the western region of Bahia state, Brazil. The study includes observations from four specific caves within the cave system: Água Clara cave (AC), Índios cave (IN), Lapa dos Peixes I (LPI) and Lapa dos Peixes II (LPII). Additionally, the study distinguishes fauna between terrestrial (T) and aquatic (A) or both habitats.
It is crucial to emphasize the ecological significance of the three South American hotspots of subterranean biodiversity and to prioritize conservation efforts for their preservation. The presence of permanent or temporary water bodies seems to be a key factor in the occurrence of troglobitic and stygobitic species, particularly in those hotspots located in the semi-arid region of Brazil. Moreover, the dependence of these systems on allochthonous sources of organic resources underscores the importance of the surrounding landscapes in supporting subterranean life. The variation in species composition and diversity among the three Brazilian hotspots highlights their uniqueness and necessitates the development of tailored conservation strategies that account for the distinctive features of each ecosystem.
8.6. Global Relevance of the Brazilian Hotspots of Subterranean Biodiversity
Each year, more hotspots of subterranean biodiversity (HSB) are discovered, particularly in large cave systems, through long-term studies. However, tropical regions continue to be underrepresented in the discovery of new HSB. This lack of discovery in tropical areas is likely due to the relatively lower investment in research in these regions, particularly in Africa, the South and Central Americas, and Asia. Thus, the few known HSBs in tropical regions hold great contextual importance globally. It is crucial to note that although new HSB may be discovered in the future due to intensified research, it is improbable that they will be found as abundantly as those in some temperate regions. Therefore, existing HSB in tropical areas should be considered infrequent and protected, given their contextual rarity. Unfortunately, anthropogenic impacts in tropical regions have been rapidly increasing, particularly regarding deforestation in various biomes for agricultural expansion or logging purposes. This replacement of native vegetation with monocultures or pastures can severely disrupt the trophic webs of subterranean ecosystems that rely heavily on epigean organic sources. Therefore, the rarity of HSB in tropical regions, combined with the escalating human-induced impacts, raises concerns about the long-term continuity and viability of these ecosystems.
The HSB identified in Brazil carry a profound contextual significance, owing to the fact that three of the eight HSB that are currently known to occur in tropical regions are located in Brazil. Moreover, a recently discovered HSB in the country, although yet to be officially published (Ferreira et al. in prep.), further underscores the importance of the Brazilian Caatinga biome. The unearthing of this new HSB reveals the ongoing advancements in speleological research in Brazil, especially over the last two decades. It is thus anticipated that forthcoming research investments will certainly contribute to revealing additional HSB from other tropical regions in the future.
8.7. Challenges in Cave Conservation in Brazil
The conservation of caves in Brazil has been characterized by a turbulent history of policies that have oscillated between strengthening and weakening the protection of this invaluable natural heritage. This inconsistent and sometimes precarious state of protection has largely resulted from the ongoing conflicts between several productive sectors, notably the mining industry, and conservationists who are committed to preserving these unique ecosystems.
Prior to 1988, Brazilian caves received inadequate legal protection. However, since that year, they have been legally designated as “Assets of the Union” and have become increasingly recognized in conservation policies. In 1990, the enactment of Decree No. 99,556 granted complete legal protection to Brazilian caves, although, in practice, some caves have been subject to destruction even after the publication of this decree. In 2008, the publication of Decree No. 6640 (www.planalto.gov.br/ccivil_03/_Ato2007-2010/2008, accessed on 15 January 2010.) required the classification of Brazilian caves according to their degree of relevance for decision-making concerning the installation of commercial or industrial enterprises. Nonetheless, only caves deemed of utmost relevance received full legal protection, whereas others remained potentially vulnerable to destruction.
While Decree 6640 raised concerns by allowing the destruction of caves, it also spurred significant advancements in speleological research in Brazil. In comparison to the approximately 6000 registered caves prior to the decree’s enactment, there are now more than 23,000. The number of described troglobitic species in the country has also risen from 75 in 2008 to 285 today. Despite these advancements, the permission to destroy caves in Brazil remains a subject of scrutiny, particularly with regards to the criteria used for determining their relevance. However, the situation has been significantly exacerbated by the January 2022 publication of Decree 10,935 (www.in.gov.br/en/web/dou/-/decreto-n-10.935-de-12-de-janeiro-de-2022-373591582, accessed on 20 March 2023), which permits the destruction of even the most significant Brazilian caves [66]. Although the Brazilian Supreme Court has revoked parts of the decree, it is uncertain whether the country’s most important caves will be protected going forward. Thus, it is imperative to use arguments beyond just those concerning HSB to advocate for the conservation of unique caves and those that could provide essential ecosystem services at various spatial scales.
Regarding the ACCS specifically, concerns are also severe, particularly in light of recent trends towards deforestation in the region where these caves are situated. This trend has led to the removal of significant portions of the original vegetation for the opening of arable lands (Figure 5C) and charcoal production (Figure 5D). Moreover, given the semi-arid nature of the region and the underground water sources accessible through the caves, pumps, frequently diesel-powered, are often employed to extract water for human or animal consumption as well as for irrigation purposes (Figure 5A,B). This practice, besides inducing a progressive reduction of the water table, often leads to contamination, especially when diesel pumps are utilized—the most common type in the area. Therefore, a highly recommended course of action is to establish a conservation unit with strict limitations in the region. Ideally, this unit should encompass the ACCS as well as all its catchment micro-basins and the corresponding area of influence.

Figure 5.
Anthropic impacts in the caves and external surroundings of the ACCS: (A) Water capture in karstic resurgences, for diffuse rural supply; (B) Water capture in cave interior; (C) Deforestation near limestone outcrops; (D) Vegetal coal furnace.
8.8. ACCS Outreach and Public Awareness
The ACCS has recently received attention through various outreach and public awareness initiatives; however, these efforts remain in their early stages. The primary objective of these efforts is to increase public knowledge and appreciation of the unique subterranean biodiversity found in the cave system as well as promote the conservation of this valuable cave system.
Researchers, such as those from the Center of Studies on Subterranean Biology (CEBS/UFLA), extend an invitation to local community members to partake in most sampling activities and expeditions carried out within the ACCS, in addition to other caves located in the vicinity. The primary purpose of these opportunities is to elucidate the characteristics and significance of cave systems as well as engage local residents in the sampling activities. The overarching objective of this engagement is to mitigate any apprehension and misunderstandings concerning the cave ecosystem and its diverse fauna.
Educational lectures on the subject of cave systems were presented at schools within the region, and informative booklets detailing the topic of caves were freely distributed to students at the main school situated in the nearby small village of Agrovila 23. Additionally, informal conversations were held with local residents while conducting routine errands at markets, drugstores, and bakeries. The importance of engaging the local population and fostering their interest in preserving this unique natural heritage is recognized, as public support is pivotal for the conservation of cave fauna. Through technical visits, lectures, and informal talks, information regarding the subterranean environment can be subtly introduced, thereby expanding people’s awareness of the subject matter. This approach helps to establish a strong link between knowledge production and dissemination while also enhancing the quality of work provided by local professionals [80].
Undoubtedly, these outreach and public awareness initiatives have a pivotal role in promoting the conservation of the ACCS and other subterranean habitats throughout Brazil. Through augmenting public knowledge and appreciation of these unique environments and engaging local communities in conservation efforts, significant strides can be taken towards ensuring that these invaluable resources are safeguarded for future generations.
Author Contributions
Conceptualization, R.L.F.; methodology and analysis, R.L.F.; data acquisition, R.L.F., M.B.-B. and M.S.-S.; original draft preparation, R.L.F.; review and editing, R.L.F., M.B.-B. and M.S.-S. All authors have read and agreed to the published version of the manuscript.
Funding
The authors would like to thank the Centro Nacional de Pesquisa e Conservação de Cavernas—CECAV and Instituto Brasileiro de Desenvolvimento e Sustentabilidade—IABS for the financial support (TCCE ICMBio/Vale 01/2018). We are also thankful to the CNPq (National Council for Scientific and Technological Development, grant n. 302925/2022-8) for the productivity scholarship provided to R.L.F., and to the team from the Center of Studies in Subterranean Biology (CEBS/UFLA) for the support in the field trips.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this manuscript. Data sharing is not applicable for this manuscript.
Acknowledgments
The authors would like to thank the Centro Nacional de Pesquisa e Conservação de Cavernas—CECAV and Instituto Brasileiro de Desenvolvimento e Sustentabilidade—IABS for the financial support (TCCE ICMBio/Vale 01/2018). We are also thankful to the CNPq (National Council for Scientific and Technological Development, grant n. 302925/2022-8) for the productivity scholarship provided to R.L.F., and to the team from the Center of Studies in Subterranean Biology (CEBS/UFLA) for the support in the field trips. M. Berbert-Born thanks to Serviço Geológico do Brasil-CPRM for the funding and logistics supported by the GEOKARST project—Geodiversity of the Sanfranciscana Depression. Rafael Costa da Silva, Rafael Henrique Grudka Barroso, Dandara Evangelista Ferreira Bustamante, Taís Novaes Santoro, Victor Scardua, Guilherme Neiva Rodrigues Oliveira, Livia Medeiros Cordeiro, Leda Zogbi, Allan Silas Calux and Nogueira for cooperation in field surveys.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Culver, D.C.; Pipan, T. The Biology of Caves and Other Subterranean Habitats; Oxford University Press: Oxford, UK, 2019; ISBN 978-0-19-882076-5. [Google Scholar]
- Niemiller, M.L.; Taylor, S.J.; Bichuette, M.E. Conservation of cave fauna, with an emphasis on Europe and the Americas. In Cave Ecology; White, W.B., Culver, D.C., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 451–478. ISBN 978-0-12-813036-2. [Google Scholar]
- Mendes-Rabelo, L.; Souza-Silva, M.; Ferreira, R.L. Priority caves for biodiversity conservation in a key karst area of Brazil: Comparing the applicability of cave conservation indices. Biodivers. Conserv. 2018, 27, 2097–2129. [Google Scholar] [CrossRef]
- Souza-Silva, M.; Ferreira, R.L. The first two hotspots of subterranean biodiversity in South America. Subterr. Biol. 2016, 19, 1–21. [Google Scholar] [CrossRef]
- Ferreira, R.L.; de Oliveira, M.P.A.; Souza-Silva, M. Subterranean biodiversity in ferruginous landscapes. In Cave Ecology; White, W.B., Culver, D.C., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 435–447. ISBN 978-0-12-813036-2. [Google Scholar]
- Bento, M.D.; Souza-Silva, M.; Vasconcellos, A.C.; Bellini, B.C.; Prous, X.; Ferreira, R.L. Subterranean “oasis” in the Brazilian semiarid region: Neglected sources of biodiversity. Biodivers. Conserv. 2021, 30, 3837–3857. [Google Scholar] [CrossRef]
- Auler, A.; Farrant, A.R. A brief introduction to karst and caves in Brazil. Proc. Univ. Bristol Spelaeol. Soc. 1996, 20, 187–200. [Google Scholar]
- Souza-Silva, M.; Martins, R.P.; Ferreira, R.L. Cave conservation priority index to adopt a rapid protection strategy: A case study in Brazilian Atlantic rain forest. Environ. Manag. 2015, 55, 279–295. [Google Scholar] [CrossRef]
- Lee, N.M.; Meisinger, D.B.; Aubrecht, R.; Kovacik, L.; Saiz-Jimenez, C.; Baskar, S.; Engel, A.S. Caves and karst environments. In Life at Extremes: Environments, Organisms and Strategies for Survival; CABI: Wallingford, UK, 2012; pp. 320–344. [Google Scholar]
- Culver, D.C.; Sket, B. Hotspots of subterranean biodiversity in caves and wells. J. Cave Karst Stud. 2000, 62, 11–17. [Google Scholar]
- Iannella, M.; Fiasca, B.; Di Lorenzo, T.; Di Cicco, M.; Biondi, M.; Mammola, S.; Galassi, D.M. Getting the ‘most out of the hotspot’ for practical conservation of groundwater biodiversity. Glob. Ecol. Conserv. 2021, 31, e01844. [Google Scholar] [CrossRef]
- Canedoli, C.; Ficetola, G.F.; Corengia, D.; Tognini, P.; Ferrario, A.; Padoa-Schioppa, E. Integrating landscape ecology and the assessment of ecosystem services in the study of karst areas. Landsc. Ecol. 2022, 37, 347–365. [Google Scholar] [CrossRef]
- Kukkala, A.S.; Moilanen, A. Core concepts of spatial prioritisation in systematic conservation planning. Biol. Rev. 2013, 88, 443–464. [Google Scholar] [CrossRef]
- Rubbioli, E.; Auler, A.; Menin, D.; Brandi, R. Cavernas. In Atlas do Brasil Subterrâneo; ICMBio: Brasília, Brazil, 2019; 340p. [Google Scholar]
- ANA—Agência Nacional de Águas. Hidrogeologia dos Ambientes Cársticos da Bacia do Rio São Francisco Para a Gestão de Recursos Hídricos: Resumo Executivo; ANA/TPF-Techne: Brasília, Brazil, 2018; p. 71. [Google Scholar]
- Dardenne, M.A. Síntese sobre a estratigrafia do Grupo Bambuí no Brasil Central. In Proceedings of the Congresso Brasileiro de Geologia, Recife, Brazil, 30 November 1978; pp. 507–610. [Google Scholar]
- Alkmim, F.F.; Martins-Neto, M.A. Proterozoic first-order sedimentary sequences of the São Francisco craton, eastern Brazil. Mar. Pet. Geol. 2012, 33, 127–139. [Google Scholar] [CrossRef]
- Reis, H.L.S.; Suss, J.F. Mixed carbonate-siliciclastic sedimentation in forebulge grabens: An example from the Ediacaran Bambuí Group, São Francisco Basin, Brazil. Sediment. Geol. 2016, 339, 83–103. [Google Scholar] [CrossRef]
- Brito Neves, B.B.; Campos, D.A.; Fuck, R.A.; Cordani, U.G. From Rodinia to Western Gondwana: An approach to the Brasiliano-Pan African Cycle and orogenic collage. Epis. J. Int. Geosci. 1999, 22, 155–166. [Google Scholar]
- Reis, H.L.S.; Suss, J.F.; Fonseca, R.C.S.; Alkmim, F.F. Ediacaran forebulge grabens of the southern São Francisco basin, SE Brazil: Craton interior dynamics during West Gondwana assembly. Precambrian Res. 2017, 302, 150–170. [Google Scholar] [CrossRef]
- Caetano-Filho, S.; Paula-Santos, G.M.; Guacaneme, C.; Babinski, M.; Bedoya-Rueda, C.; Peloso, M.; Amorim, K.; Afonso, J.; Kuchenbecker, M.; Reis, H.L.S.; et al. Sequence stratigraphy and chemostratigraphy of an Ediacaran-Cambrian foreland-related carbonate ramp (Bambuí Group, Brazil). Precambrian Res. 2019, 331, 105365. [Google Scholar] [CrossRef]
- Uhlein, G.J.; Uhlein, A.; Pereira, E.; Caxito, F.A.; Okubo, J.; Warren, L.V.; Sial, A.N. Ediacaran paleoenvironmental changes recorded in the mixed carbonate-siliciclastic Bambuí Basin, Brazil. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2019, 517, 39–51. [Google Scholar] [CrossRef]
- Kuchenbecker, M.; Pedrosa-Soares, A.C.; Babinski, M.; Reis, H.L.S.; Atman, D.; Costa, R.D. Towards an integrated tectonic model for the interaction between the Bambuí basin and the adjoining orogenic belts: Evidences from the detrital zircon record of syn-orogenic units. J. S. Am. Earth Sci. 2020, 104, 102831. [Google Scholar] [CrossRef]
- Da Silva, L.; Pufahl, P.; James, N.; Guimaraes, E.; Reis, C. Sequence stratigraphy and paleoenvironmental significance of the Neoproterozoic Bambui Group, Central Brazil. Precambrian Res. 2022, 379, 106710. [Google Scholar] [CrossRef]
- Dantas, M.V.S.; Uhlein, A.; Uhlein, G.J.; Okubo, J.; Moura, S.A. Stratigraphy, isotope geochemistry, seismic stratigraphy and paleogeography of the Lagoa do Jacaré Formation, Bambuí Foreland Basin (Ediacaran-Cambrian), Southeast Brazil. J. S. Am. Earth Sci. 2023, 121, 104137. [Google Scholar] [CrossRef]
- Campos, J.E.G.; Dardenne, M.A. Estratigrafia e Sedimentação da Bacia Sanfranciscana: Uma Revisão. Rev. Bras. Geocienc. 1997, 27, 269–282. [Google Scholar] [CrossRef]
- Sgarbi, G.N.C.; Sgarbi, P.B.A.; Campos, J.E.G.; Dardenne, M.A.; Penha, U.C. Bacia Sanfranciscana: O registro Fanerozóico da Bacia do São Francisco. In Bacia do São Francisco: Geologia e Recursos Naturais; Pinto, C.P., Martins-Neto, M.A., Eds.; SBG: Belo Horizonte, Brazil, 2001; pp. 93–138. [Google Scholar]
- Zalán, P.V.; Romeiro-Silva, P.C. Bacia do São Francisco. Bol. Geociênc. Petrobrás. 2007, 15, 561–571. [Google Scholar]
- Ford, D.C.; Williams, P. Karst Hydrogeology and Geomorphology; John Wiley: Chichester, UK, 2007; p. 562. [Google Scholar]
- ANA–Agência Nacional de Águas. Diagnóstico dos Meios Físico e Socioeconômico—Resumo Executivo. In Hidrogeologia dos Ambientes Cársticos da Bacia do Rio São Francisco Para a Gestão de Recursos Hídricos: Relatório Final v.1; ANA/TPF-Techne: Brasília, Brazil, 2018; p. 264. [Google Scholar]
- Bhering, A.P.; Antunes, I.M.H.R.; Marques, A.G.; de Paula, R.S. Geological and hydrogeological review of a semi-arid region with conflicts to water availability (southeastern Brazil). Environ. Res. 2021, 202, 111756. [Google Scholar] [CrossRef] [PubMed]
- Assunção, P.A.; Galvão, P.; Lucon, T.; Doi, B.; Fleming, P.M.; Marques, T.; Costa, F. Hydrodynamic and hydrodispersive behavior of a highly karstified neoproterozoic hydrosystem indicated by tracer tests and modeling approach. J. Hydrol. 2023, 619, 129300. [Google Scholar] [CrossRef]
- ANA—Agência Nacional de Águas. Relatório técnico e temático dos Domínios e Subdomínios Hidrogeológicos. In Hidrogeologia dos Ambientes Cársticos da Bacia do Rio São Francisco Para a Gestão de Recursos Hídricos; ANA/TPF-Techne: Brasília, Brazil, 2018; p. 809. [Google Scholar]
- ANA—Agência Nacional de Águas. Hidrogeologia dos Ambientes Cársticos da Bacia do Rio São Francisco Para a Gestão de Recursos Hídricos: Relatório Final v.2; Hidrogeologia; ANA/TPF-Techne: Brasília, Brazil, 2018; 217p. [Google Scholar]
- IBGE—Instituto Brasileiro de Geografia e Estatística. Biomas e Sistema Costeiro—Marinho do Brasil—1:250,000. Available online: https://www.ibge.gov.br/geociencias/cartas-e-mapas/informacoes-ambientais/15842-biomas.html (accessed on 25 April 2023).
- Bittencourt, A.L.V.; Rodet, J. Evolução morfológica do canion do Morro Furado no contexto dos calcários carstificados do Grupo Bambuí (Serra do Ramalho, Bahia, Brasil). O Carste 2002, 14, 224–237. [Google Scholar]
- Santos, C.C.; Reis, C. Geologia e Recursos Minerais da Folha Santa Maria da Vitória—SD.23-X-C-II: Escala 1:100,000, Estado da Bahia; Nota Explicativa; Serviço Geológico do Brasil—CPRM: Salvador, Brazil, 2021; 75p. [Google Scholar]
- Silva, R.C.; Berbert-Born, M.; Bustamante, D.E.F.; Santoro, T.N.; Sedor, F.; Avilla, L.S. Diversity and preservation of Pleistocene tetrapods from caves of southwestern Bahia, Brazil. J. S. Am. Earth Sci. 2019, 90, 233–254. [Google Scholar] [CrossRef]
- Berbert-Born, M.; Silva, R.C. Projeto Geokarst—Geodiversidade da Depressão Sanfranciscana: Relatório de Campo; Serviço Geológico do Brasil—CPRM: Rio de Janeiro, Brazil, 2019; Unpublished. [Google Scholar]
- Brandi, R. A descoberta da Gruta do Peixe. O Carste 2001, 13, 38–41. [Google Scholar]
- Frágola, L. A Gruta dos Peixes II. O Carste 2001, 13, 56–62. [Google Scholar]
- Frágola, L. O retorno à Gruta dos Peixes. O Carste 2001, 13, 66–67. [Google Scholar]
- Jolivet, J. A Gruta de Água Clara—Ponto de encontro rue Mourfftard. O Carste 2001, 13, 28–31. [Google Scholar]
- Baptista, R.L.C.; Giupponi, A.D.L. A new troglomorphic Charinus from Brazil (Arachnida: Amblypygi: Charinidae). Rev. Ibér. Arac. 2002, 6, 105–110. [Google Scholar]
- Kury, A.B.; González, A.P. A new remarkable troglomorphic gonyleptid from Brazil (Arachnida, Opiliones, Laniatores). Rev. Ibér. Arachnol. 2002, 246, 43–50. [Google Scholar]
- Rizzato, P.P.; Bichuette, M.E. A new species of cave catfish from Brazil, Trichomycterus rubbioli sp. n., from Serra do Ramalho karstic area, São Francisco River basin, Bahia State (Silurifomes: Trichomycteridae). Zootaxa 2012, 3480, 48–66. [Google Scholar]
- Simone, L.R.L. A new genus and species of cavernicolous Pomatiopsidae (Mollusca, Caenogastropoda) in Bahia, Brazil. Pap. Avulsos Zool. 2012, 52, 515–524. [Google Scholar] [CrossRef]
- Campos-Filho, I.S.; Araujo, P.B.; Bichuette, M.E.; Trajano, E.; Taiti, S. Terrestrial isopods (Crustacea: Isopoda: Oniscidea) from Brazilian caves. Zool. J. Linn. Soc. 2014, 172, 360–425. [Google Scholar] [CrossRef]
- Kamimura, Y.; Ferreira, R.L. Description of a second South American species in the Malagasy earwig genus Mesodiplatys from a cave habitat, with notes on the definition of Haplodiplatyidae (Insecta, Dermaptera). ZooKeys 2018, 790, 87–100. [Google Scholar] [CrossRef]
- Cardoso, G.M.; Bastos-Pereira, R.; Souza, L.A.; Ferreira, R.L. New cave species of Pectenoniscus Andersson, 1960 (Isopoda: Oniscidea: Styloniscidae) and an identification key for the genus. Nauplius 2020, 28, 1–30. [Google Scholar] [CrossRef]
- Souza, P.G.C.; Medeiros, G.D.S.; Ferreira, R.L.; Souza-Silva, M.; Bellini, B.C. A highly troglomorphic new genus of Sminthuridae (Collembola, Symphypleona) from the Brazilian semiarid region. Insects 2022, 13, 650. [Google Scholar] [CrossRef]
- Hellmann, L.; Ferreira, R.L.; Rabelo, L.; Leal-Zanchet, A.M. Enhancing the still scattered knowledge on the taxonomic diversity of freshwater triclads (Platyhelminthes: Dugesiidae) in caves from two Brazilian Biomes. Stud. Neotrop. Fauna Envionr. 2022, 57, 148–163. [Google Scholar] [CrossRef]
- Souza-Silva, M.; Cerqueira, R.F.V.; Pellegrini, T.G.; Ferreira, R.L. Habitat selection of cave-restricted fauna in a new hotspot of subterranean biodiversity in Neotropics. Biodiv. Conserv. 2021, 30, 4223–4250. [Google Scholar] [CrossRef]
- Carvalho, P.H.M.; Junta, V.G.P.; Castro-Souza, R.A.; Ferreira, R.L. Three new cricket species and a new subgenus of Endecous Saussure, 1878 (Grylloidea: Phalangopsidae) from caves in northeastern Brazil. Zootaxa 2023, 5263, 001–039. [Google Scholar] [CrossRef] [PubMed]
- Prado, G.C.; Ferreira, R.L. Three new troglobitic species of Pseudochthonius Balzan, 1892 (Pseudoscorpiones, Chthoniidae) from northeastern Brazil. Zootaxa 2023, 5249, 92–110. [Google Scholar] [CrossRef] [PubMed]
- Ávila, A.C.; Pires, M.M.; Rodrigues, E.N.; Costi, J.A.; Stenert, C.; Maltchik, L. Drivers of the beta diversity of spider assemblages in southern Brazilian temporary wetlands. Ecol. Entomol. 2019, 45, 466–475. [Google Scholar] [CrossRef]
- Trontelj, P.; Blejec, A.; Fišer, C. Ecomorphological convergence of cave communities. Evolution 2012, 66, 3852–3865. [Google Scholar] [CrossRef]
- Den Boer, P.J. The present status of the competitive exclusion principle. Trends Ecol. Evol. 1986, 1, 25–28. [Google Scholar] [CrossRef] [PubMed]
- Ortuño, V.M.; Gilgado, J.D.; Jiménez-Valverde, A.; Sendra, A.; Pérez-Suárez, G.; Herrero-Borgoñón, J.J. The “aluvial mesovoid shallow substratum”, a new subterranean habitat. PLoS ONE 2013, 8, e76311. [Google Scholar] [CrossRef]
- Cardoso, P.; Erwin, T.L.; Borges, P.A.; New, T.R. The seven impediments in invertebrate conservation and how to overcome them. Biol. Conserv. 2011, 144, 2647–2655. [Google Scholar] [CrossRef]
- Pipan, T.; Deharveng, L.; Culver, D.C. Hotspots of subterranean biodiversity. Diversity 2020, 12, 209. [Google Scholar] [CrossRef]
- Trajano, E.; Gallão, J.E.; Bichuette, M.E. Spots of high diversity of troglobites in Brazil: The challenge of measuring subterranean diversity. Biodiv. Conserv. 2016, 25, 1805–1828. [Google Scholar] [CrossRef]
- Huang, S.; Wei, G.; Wang, H.; Liu, W.; Bedos, A.; Deharveng, L.; Tian, M. Ganxiao Dong: A hotspot of cave biodiversity in northern Guangxi, China. Diversity 2021, 13, 355. [Google Scholar] [CrossRef]
- Iliffe, T.M.; Calderón-Gutiérrez, F. Bermuda’s Walsingham Caves: A global hotspot for anchialine stygobionts. Diversity 2021, 13, 352. [Google Scholar] [CrossRef]
- Souza-Silva, M.S.; Martins, R.P.; Ferreira, R.L. Cave lithology determining the structure of the invertebrate communities in the Brazilian Atlantic Rain Forest. Biodivers. Conserv. 2011, 20, 1713–1729. [Google Scholar] [CrossRef]
- Ferreira, R.L.; Giribet, G.; Du Preez, G.; Ventouras, O.; Janion, C.; Souza-Silva, M. The Wynberg cave system, the most important site for cave fauna in South Africa at risk. Subterr. Biol. 2020, 36, 73–81. [Google Scholar] [CrossRef]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; Da Fonseca, G.A.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2020, 403, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.F.; Li, M.; Xu, K.W.; Zhang, L.; Zhang, L.B. Protect China’s karst cave habitats. Science 2021, 374, 699. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.L.; Bernard, E.; da Cruz Júnior, F.W.; Piló, L.B.; Calux, A.; Souza-Silva, M.; Frick, W.F. Brazilian cave heritage under siege. Science 2020, 375, 1238–1239. [Google Scholar] [CrossRef]
- Mendes-Rabelo, L.; Souza-Silva, M.; Ferreira, R.L. Epigean and hypogean drivers of Neotropical subterranean communities. J. Biogeogr. 2021, 48, 662–675. [Google Scholar] [CrossRef]
- Dial, R.; Roughgarden, J. Theory of marine communities: The intermediate disturbance hypothesis. Ecology 1988, 79, 1412–1424. [Google Scholar] [CrossRef]
- Wilkinson, D.M. The Disturbing History of Intermediate Disturbance. Oikos 1999, 84, 145–147. [Google Scholar] [CrossRef]
- Kricher, J.C. Tropical Ecology; Princeton University Press: Princeton, NJ, USA, 2011. [Google Scholar]
- Catford, J.A.; Daehler, C.C.; Murphy, H.T.; Sheppard, A.W.; Hardesty, B.D.; Westcott, D.A.; Rejmánek, M.; Bellingham, P.J. The intermediate disturbance hypothesis and plant invasions: Implications for species richness and management. Perspectives in Plant Ecology. Evol. Syst. 2012, 14, 231–241. [Google Scholar]
- Fox, J.W. The intermediate disturbance hypothesis should be abandoned. Trends Ecol. Evol. 2013, 28, 86–92. [Google Scholar] [CrossRef]
- Sheil, D.; Burslem, D.F.R.P. Defining and defending Connell’s intermediate disturbance hypothesis: A response to Fox. Trends Ecol. Evol. 2013, 28, 571–572. [Google Scholar] [CrossRef]
- Collevatti, R.G.; Terribile, L.C.; de Oliveira, G.; Lima-Ribeiro, M.S.; Nabout, J.C.; Rangel, T.F.; Diniz-Filho, J.A.F. Draw-backs to palaeodistribution modelling: The case of South American seasonally dry forests. J. Biogeogr. 2013, 40, 345–358. [Google Scholar] [CrossRef]
- Wang, X.; Auler, A.S.; Edwards, L.; Cheng, H.; Cristalli, P.S.; Smart, P.L.; Richards, D.A.; Shen, C.C. Wet periods in northeastern Brazil over the past 210 kyr linked to distant climate anomalies. Lett. Nat. 2004, 432, 740–743. [Google Scholar] [CrossRef] [PubMed]
- Polhemus, D.A.; Ferreira, R.L. Two unusual new genera of cavernicolous Hydrometridae (Insecta: Heteroptera) from Eastern Brazil. Tijdschr. Voor Entomol. 2018, 161, 25–38. [Google Scholar] [CrossRef]
- Souza-Silva, M.S.; Ferreira, R.L.; Gonçalves, L.V. Public support as a key element for fauna conservation in Maquiné and Rei do Mato show caves. Rev. Espeleo Tema 2019, 29, 65–76. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).