Pan-Atlantic Comparison of Deep-Sea Macro- and Megabenthos
Abstract
1. Introduction
2. Materials and Methods
| Project | Cruise Number | Date | Vessel | Sample Area | Reference |
|---|---|---|---|---|---|
| ANDEEP-SYSTCO II | ANT-XXVIII/3 | 7 January 2012– 11 March 2012 | Polarstern | South Atl | [26] |
| BIOPEARL I | JR 144 | 26 February 2006–17 April 2006 | RRS James Clark Ross | SO | [62] |
| DIVA 1 | M48/1 | 6 July 2000– 02 August2000 | RV Meteor | South Atl | [32] |
| DIVA 2 | M63/2 | 26 February 2005– 31 March 2005 | RV Meteor | South Atl | [41] |
| DIVA 3 | M79/1 | 10 June 2009– 26 August2009 | RV Meteor | South Atl | [33] |
| IceAGE | M85/3 | 27 August 2011–28 September 2011 | RV Meteor | North Atl | [34] |
| IceAGE 2 | POS456 | 20 July 2013– 4 August 2013 | RV Poseidon | North Atl | [40] |
| IceAGE RR | MSM75 | 29 June 2018– 8 September 2018 | RV Ms Merian | North Atl | [37] |
| IceAGE 3 | SO276 (MerMet17-06) | 22 June 2020– 26 July2020 | RV Sonne | North Atl | [35] |
| IceDIVA | SO280 | 8 January 2021– 7 February 2021 | RV Sonne | North Atl | [63] |
| IceDIVA 2 | S0286 | 5 November 2021– 8 December 2021 | RV Sonne | North Atl | [38] |
| JR275 | JR275 | 7 February 2012– 2 March 2012 | RRS James Clark Ross | SO, South Atl | [39] |
| Vema-TRANSIT | SO237 | 14 December 2014–26 January 2015 | RV Sonne | VFZ, PRT | [64] |
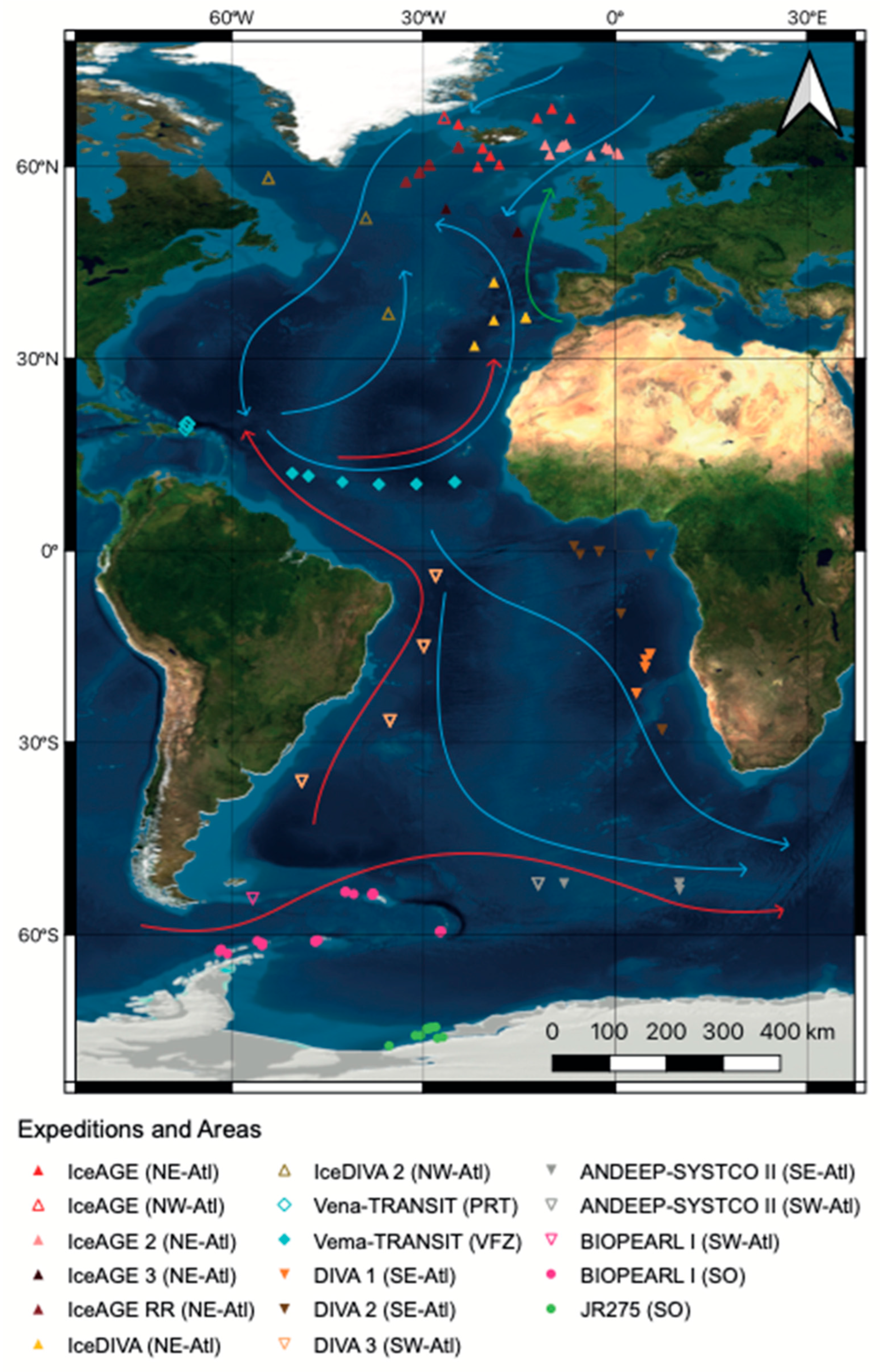
2.1. Sampling and Sample Processing
2.2. Environmental Data
2.3. Statistical Analyses
3. Results
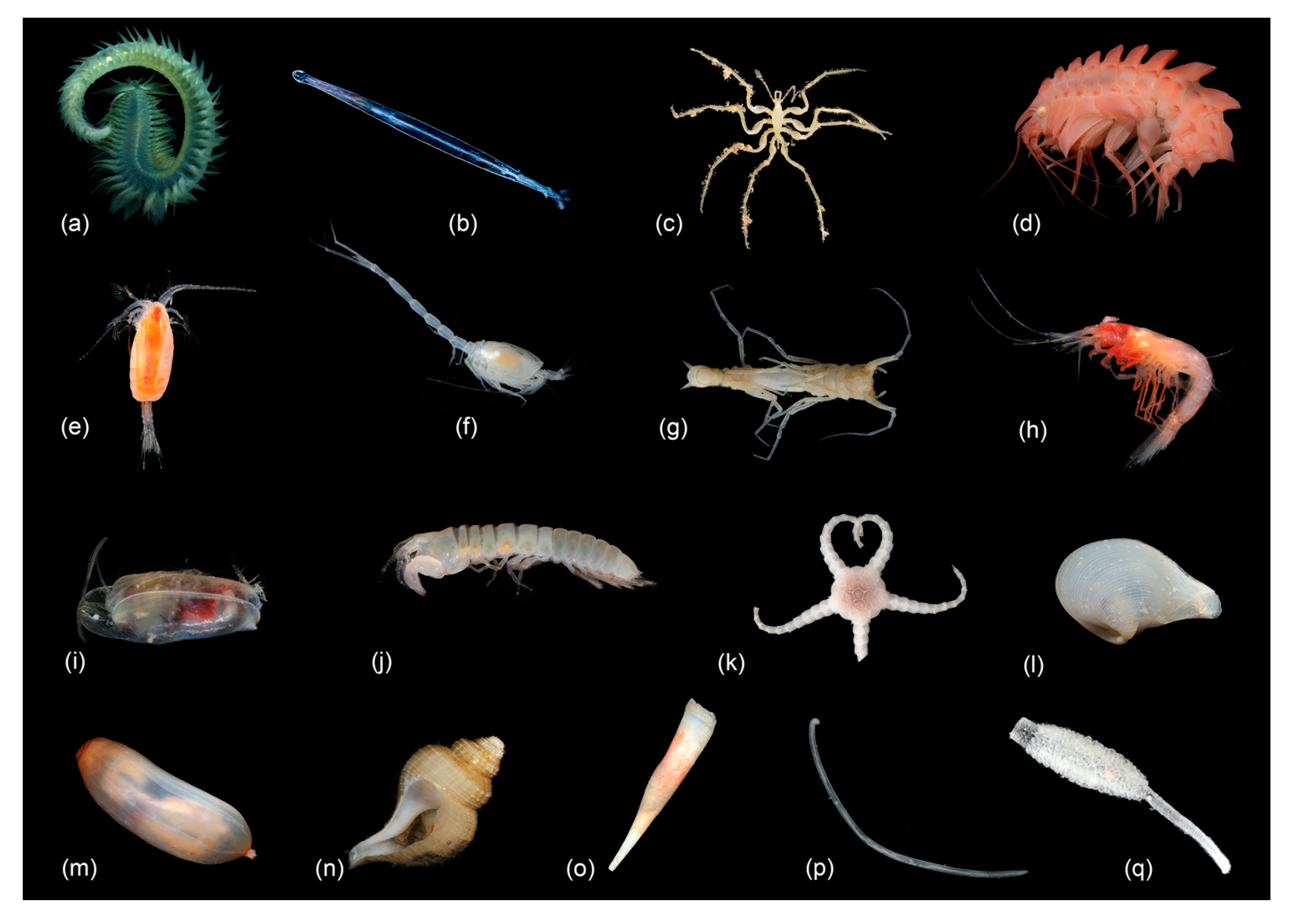
Macro- and Mega Fauna Composition
4. Discussion
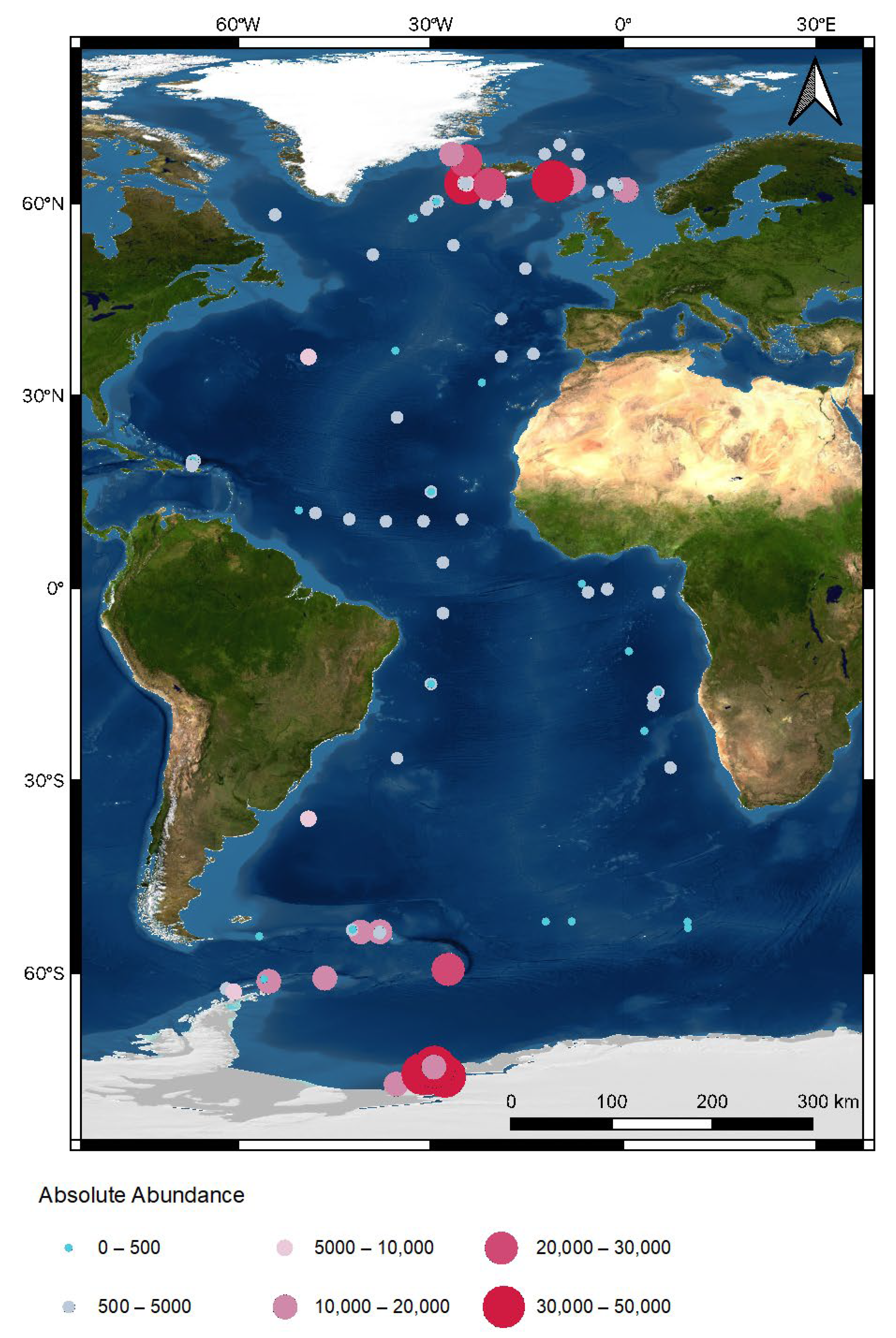
4.1. Patterns in Absolute Abundance
4.2. Patterns in Community Composition
4.3. Environmental Drivers in Community Composition
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Giblin, A.E.; Foreman, K.H.; Banta, G.T. Biogeochemical processes and marine benthic community structure: Which follows which? In Linking Species & Ecosystems; Springer: Berlin/Heidelberg, Germany, 1995; pp. 37–44. [Google Scholar] [CrossRef]
- Snelgrove, P.V.; Thrush, S.F.; Wall, D.H.; Norkko, A. Real world biodiversity–ecosystem functioning: A seafloor perspective. Trends Ecol. Evol. 2014, 29, 398–405. [Google Scholar] [CrossRef]
- Snelgrove, P.V. The importance of marine sediment biodiversity in ecosystem processes. Ambio 1997, 26, 578–583. [Google Scholar]
- Snelgrove, P.V. Getting to the bottom of marine biodiversity: Sedimentary habitats: Ocean bottoms are the most widespread habitat on earth and support high biodiversity and key ecosystem services. BioScience 1999, 49, 129–138. [Google Scholar] [CrossRef]
- Giller, P.; Hillebrand, H.; Berninger, U.G.; Gessner, M.O.; Hawkins, S.; Inchausti, P.; Inglis, C.; Leslie, H.; Malmqvist, B.; Monaghan, M.T. Biodiversity effects on ecosystem functioning: Emerging issues and their experimental test in aquatic environments. Oikos 2004, 104, 423–436. [Google Scholar] [CrossRef]
- Graf, G. Benthic-pelagic coupling: A benthic view. Oceanography and Marine Biology: An Annual Review. 1992, 30, 149–190. [Google Scholar]
- Mermillod-Blondin, F. The functional significance of bioturbation and biodeposition on biogeochemical processes at the water–sediment interface in freshwater and marine ecosystems. J. N. Am. Benthol. Soc. 2011, 30, 770–778. [Google Scholar] [CrossRef]
- Butler IV, M.J.; Hunt, J.H.; Herrnkind, W.F.; Childress, M.J.; Bertelsen, R.; Sharp, W.; Matthews, T.; Field, J.M.; Marshall, H.G. Cascading disturbances in Florida Bay, USA: Cyanobacteria blooms, sponge mortality, and implications for juvenile spiny lobsters Panulirus argus. Mar. Ecol. Prog. Ser. 1995, 129, 119–125. [Google Scholar] [CrossRef]
- Ebeling, A.W.; Hixon, M.A. Tropical and temperate reef fishes: Comparison of community structures. In The Ecology of Fishes on Coral Reefs; Sale, P.F., Ed.; Elsevier: Amsterdam, The Netherlands, 1991; pp. 509–563. [Google Scholar] [CrossRef]
- Miller, R.J.; Hocevar, J.; Stone, R.P.; Fedorov, D.V. Structure-forming corals and sponges and their use as fish habitat in Bering Sea submarine canyons. PLoS ONE 2012, 7, e33885. [Google Scholar] [CrossRef]
- Ehrnsten, E.; Savchuk, O.P.; Gustafsson, B.G. Modelling the effects of benthic fauna on carbon, nitrogen, and phosphorus dynamics in the Baltic Sea. Biogeosciences Discuss. 2022, 19, 3337–3367. [Google Scholar] [CrossRef]
- Thrush, S.; Hewitt, J.; Pilditch, C.; Norkko, A. Ecology of Coastal Marine Sediments: Form, Function, and Change in the Anthropocene; Oxford University Press: Oxford, UK, 2021. [Google Scholar]
- Strong, J.A.; Andonegi, E.; Bizsel, K.C.; Danovaro, R.; Elliott, M.; Franco, A.; Garces, E.; Little, S.; Mazik, K.; Moncheva, S. Marine biodiversity and ecosystem function relationships: The potential for practical monitoring applications. Estuar. Coast. Shelf Sci. 2015, 161, 46–64. [Google Scholar] [CrossRef]
- Chapin III, F.S.; Walker, B.H.; Hobbs, R.J.; Hooper, D.U.; Lawton, J.H.; Sala, O.E.; Tilman, D. Biotic control over the functioning of ecosystems. Science 1997, 277, 500–504. [Google Scholar] [CrossRef]
- Clark, M.R.; Consalvey, M.; Rowden, A.A. Biological Sampling in the Deep Sea; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Clark, M. Fisheries for orange roughy (Hoplostethus atlanticus) on seamounts in New Zealand. Oceanol. Acta 1999, 22, 593–602. [Google Scholar] [CrossRef]
- Almeida, M.; Frutos, I.; Company, J.B.; Martin, D.; Romano, C.; Cunha, M.R. Biodiversity of suprabenthic peracarid assemblages from the Blanes Canyon region (NW Mediterranean Sea) in relation to natural disturbance and trawling pressure. Deep Sea Res. Part II Top. Stud. Oceanogr. 2017, 137, 390–403. [Google Scholar] [CrossRef]
- Miller, K.A.; Thompson, K.F.; Johnston, P.; Santillo, D. An overview of seabed mining including the current state of development, environmental impacts, and knowledge gaps. Front. Mar. Sci. 2018, 4, 418. [Google Scholar] [CrossRef]
- Glover, A.G.; Smith, C.R. The deep-sea floor ecosystem: Current status and prospects of anthropogenic change by the year 2025. Environ. Conserv. 2003, 30, 219–241. [Google Scholar] [CrossRef]
- Frutos, I.; Sorbe, J.C. Suprabenthic assemblages from the Capbreton area (SE Bay of Biscay). Faunal recovery after a canyon turbidity disturbance. Deep Sea Res. Part I Oceanogr. Res. Pap. 2017, 130, 36–46. [Google Scholar] [CrossRef]
- Angulo-Preckler, C.; Pernet, P.; García-Hernández, C.; Kereszturi, G.; Álvarez-Valero, A.M.; Hopfenblatt, J.; Gómez-Ballesteros, M.; Otero, X.L.; Caza, J.; Ruiz-Fernández, J. Volcanism and rapid sedimentation affect the benthic communities of Deception Island, Antarctica. Cont. Shelf Res. 2021, 220, 104404. [Google Scholar] [CrossRef]
- Fanelli, E.; Di Giacomo, S.; Gambi, C.; Bianchelli, S.; Da Ros, Z.; Tangherlini, M.; Andaloro, F.; Romeo, T.; Corinaldesi, C.; Danovaro, R. Effects of Local Acidification on Benthic Communities at Shallow Hydrothermal Vents of the Aeolian Islands (Southern Tyrrhenian, Mediterranean Sea). Biology 2022, 11, 321. [Google Scholar] [CrossRef] [PubMed]
- Lucey, S.M.; Nye, J.A. Shifting species assemblages in the northeast US continental shelf large marine ecosystem. Mar. Ecol. Prog. Ser. 2010, 415, 23–33. [Google Scholar] [CrossRef]
- Walther, G.-R.; Post, E.; Convey, P.; Menzel, A.; Parmesan, C.; Beebee, T.J.; Fromentin, J.-M.; Hoegh-Guldberg, O.; Bairlein, F. Ecological responses to recent climate change. Nature 2002, 416, 389–395. [Google Scholar] [CrossRef]
- Brierley, A.S.; Kingsford, M.J. Impacts of climate change on marine organisms and ecosystems. Curr. Biol. 2009, 19, R602–R614. [Google Scholar] [CrossRef] [PubMed]
- Brandt, A.; Havermans, C.; Janussen, D.; Jörger, K.; Meyer-Löbbecke, A.; Schnurr, S.; Schüller, M.; Schwabe, E.; Brandao, S.; Würzberg, L. Composition and abundance of epibenthic-sledge catches in the South Polar Front of the Atlantic. Deep Sea Res. Part II Top. Stud. Oceanogr. 2014, 108, 69–75. [Google Scholar] [CrossRef]
- Linse, K.; Griffiths, H.J.; Barnes, D.K.; Clarke, A. Biodiversity and biogeography of Antarctic and sub-Antarctic mollusca. Deep Sea Res. Part II Top. Stud. Oceanogr. 2006, 53, 985–1008. [Google Scholar] [CrossRef]
- Kaiser, S.; Brandt, A.; Brix, S.; Brenke, N.; Kürzel, K.; Martinez Arbizu, P.; Pinkerton, M.H.; Saeedi, H. Community structure of abyssal macrobenthos of the South and equatorial Atlantic Ocean–identifying patterns and environmental controls. Deep. Sea Res. Part I Oceanogr. Res. Pap. 2023, 197, 104066. [Google Scholar] [CrossRef]
- Riehl, T.; Lins, L.; Brandt, A. The effects of depth, distance, and the Mid-Atlantic Ridge on genetic differentiation of abyssal and hadal isopods (Macrostylidae). Deep Sea Res. Part II Top. Stud. Oceanogr. 2018, 148, 74–90. [Google Scholar] [CrossRef]
- Devey, C.; Brandt, A.; Arndt, H.; Augustin, N.; Bober, S.; Borges, V.; Brenke, N.; Brix, S.; Elsner, N.; Frutos, I.; et al. RV SONNE Fahrtbericht/Cruise Report SO237 Vema-TRANSIT; GEOMAR Helmholtz-Zentrum für Ozeanforschung Kiel: Kiel, Germany, 2015. [Google Scholar]
- Lörz, A.N.; Oldeland, J.; Kaiser, S. Niche breadth and biodiversity change derived from marine Amphipoda species off Iceland. Ecol. Evol. 2022, 12, e8802. [Google Scholar] [CrossRef] [PubMed]
- Türkay, M. Research program. In South-East Atlantic 2000. Cruise No. 48, 6 July 2000–3 November 2000. METEOR-Berichte, Universität Hamburg, 06–05:1–3; Balzer, W., Alheit, J., Emeis, K.-C., Lass, H.U., Türkay, M., Eds.; Universität Hamburg: Hamburg, Germany, 2006. [Google Scholar]
- Martínez Arbizu, P.; Brix, S.; Kaiser, S.; Brandt, A.; George, K.H.; Arndt, H.; Hausmann, K.; Türkay, M.; Renz, J.; Hendrycks, E.; et al. Deep-Sea Biodiversity, Current Activity, and Seamounts in the Atlantic–Cruise No. M79/1–June 10–August 26, 2009–Montevideo (Uruguay)–Ponta Delgada (Portugal); DFG-Senatskommission für Ozeanographie: Bremen, Germany, 2015; pp. 1–92. [Google Scholar]
- Brix, S.; Meissner, K.; Stansky, B.; Halanych, K.M.; Jennings, R.M.; Kocot, K.M.; Svavarsson, J. The IceAGE project–A follow up of BIOICE. Pol. Polar Res. 2014, 35, 141–150. [Google Scholar] [CrossRef]
- Brix, S.; Taylor, J.; Le Saout, M.; Mercado-Salas, N.; Kaiser, S.; Lörz, A.-N.; Gatzemeier, N.; Jeskulke, K.; Kürzel, K.; Neuhaus, J. Depth Transects and Connectivity along Gradients in the North Atlantic and Nordic Seas in the Frame of the IceAGE Project (Icelandic Marine Animals: Genetics and Ecology), Cruise No. SO276 (MerMet17-06), 22.06. 2020-26.07. 2020, Emden (Germany)-Emden (Germany); Gutachterpanel Forschungsschiffe: Hamburg, Germany, 2020; p. 48. [Google Scholar]
- Brix, S.; Devey, C. Stationlist of the IceAGE project (Icelandic marine Animals: Genetics and Ecology) expeditions. Mar. Data Arch. 2019, 10, 349. [Google Scholar] [CrossRef]
- Devey, C. Short Cruise Report MERIAN MSM75, Reykjavik–Reykjavik 29.06.18–08.08.18; Leitstelle Deutsche Forschungsschiffe: Hamburg, Germany, 2018; p. 13. [Google Scholar]
- Brix, S.; Taylor, J. Master Tracks in Different Resolutions of SONNE Cruise SO286, Emden—Las Palmas, 2021-11-05–2021-12-08; Senckenberg am Meer: Wilhelmshaven, Germany, 2022. [Google Scholar] [CrossRef]
- Griffiths, H.; Downey, R.; Hamilton, D.S.; Heuzé, C.; Jackson, J.; Mackenzie, M.; Moreau, C.; Reed, A.; Sads, C.J. RRS James Clark Ross JR275 Cruise Report: Benthic Biology of the Weddell Sea; British Antarctic Survey: Camebridge, UK, 2012. [Google Scholar]
- Brix, S.; Martinez, P.; Svavarsson, J.; Kenning, M.; Jennings, R.; Holst, S.; Cannon, J.; Eilertsen, M.; Schnurr, S.; Jeskulke, K. IceAGE-Icelandic Marine Animals: Genetics and Ecology, Cruise No. POS456, IceAGE2, 20.07. 2013–04.08. 2013, Kiel (Germany)-Reykjavik (Iceland); Deutsches Zentrum für Marine Biodiversitätsforschung, Senkenbereg am Meer: Wilhelmshaven, Germany, 2013; p. 12. [Google Scholar] [CrossRef]
- Türkay, M.; Pätzold, J. METEOR-Berichte 09-3, Southwestern Indian Ocean Eastern Atlantic Ocean, Cruise No. 63, January 24 March 30, 2005, Cape Town (South Africa) Mindelo (Cabo Verde); Meteor-Berichte, Institut für Meereskunde der Universität Hamburg: Bremerhaven, Germany, 2009; p. 100. [Google Scholar]
- Brix, S.; Svavarsson, J. Distribution and diversity of desmosomatid and nannoniscid isopods (Crustacea) on the Greenland–Iceland–Faeroe Ridge. Polar Biol. 2010, 33, 515–530. [Google Scholar] [CrossRef]
- Kürzel, K.; Kaiser, S.; Lörz, A.-N.; Rossel, S.; Paulus, E.; Peters, J.; Schwentner, M.; Arbizu, P.M.; Coleman, C.O.; Svavarsson, J. Correct species identification and its implications for conservation using Haploniscidae (Crustacea, Isopoda) in Icelandic waters as a proxy. Recent Emerg. Innov. Deep-Sea Taxon. Enhanc. Biodivers. Assess. Conserv. 2022, 8, 795196. [Google Scholar] [CrossRef]
- Downey, R.V.; Griffiths, H.J.; Linse, K.; Janussen, D. Diversity and distribution patterns in high southern latitude sponges. PLoS ONE 2012, 7, e41672. [Google Scholar] [CrossRef]
- Kuhlbrodt, T.; Griesel, A.; Montoya, M.; Levermann, A.; Hofmann, M.; Rahmstorf, S. On the driving processes of the Atlantic meridional overturning circulation. Rev. Geophys. 2007, 45, RG2001. [Google Scholar] [CrossRef]
- Lörz, A.-N.; Kaiser, S.; Oldeland, J.; Stolter, C.; Kürzel, K.; Brix, S. Biogeography, diversity and environmental relationships of shelf and deep-sea benthic Amphipoda around Iceland. PeerJ 2021, 9, e11898. [Google Scholar] [CrossRef] [PubMed]
- Di Franco, D.; Linse, K.; Griffiths, H.J.; Brandt, A. Drivers of abundance and spatial distribution in Southern Ocean peracarid crustacea. Ecol. Indic. 2021, 128, 107832. [Google Scholar] [CrossRef]
- Saeedi, H.; Warren, D.; Brandt, A. The environmental drivers of benthic fauna diversity and community composition. Front. Mar. Sci. 2022, 9, 804019. [Google Scholar] [CrossRef]
- Fonseca, G.; Soltwedel, T. Regional patterns of nematode assemblages in the Arctic deep seas. Polar Biol. 2009, 32, 1345–1357. [Google Scholar] [CrossRef]
- Frutos, I.; Brandt, A.; Sorbe, J. Deep-sea suprabenthic communities: The forgotten biodiversity. In Marine Animal Forests: The Ecology of Benthic Biodiversity Hotspots; Rossi, S., Bramanti, L., Gori, A., Orejas, C., Eds.; Springer: Cham, Switzerland, 2017; pp. 475–503. [Google Scholar] [CrossRef]
- Stransky, B.; Brandt, A. Occurrence, diversity and community structures of peracarid crustaceans (Crustacea, Malacostraca) along the southern shelf of Greenland. Polar Biol. 2010, 33, 851–867. [Google Scholar] [CrossRef]
- Stransky, B.; Svavarsson, J. Diversity and species composition of peracarids (Crustacea: Malacostraca) on the South Greenland shelf: Spatial and temporal variation. Polar Biol. 2010, 33, 125–139. [Google Scholar] [CrossRef]
- Frutos, I.; Sorbe, J.C. Bathyal suprabenthic assemblages from the southern margin of the Capbreton Canyon (“Kostarrenkala” area), SE Bay of Biscay. Deep Sea Res. Part II Top. Stud. Oceanogr. 2014, 104, 291–309. [Google Scholar] [CrossRef]
- Brandt, A.; Brökeland, W.; Brix, S.; Malyutina, M. Diversity of Southern Ocean deep-sea Isopoda (Crustacea, Malacostraca)—A comparison with shelf data. Deep Sea Res. Part II Top. Stud. Oceanogr. 2004, 51, 1753–1768. [Google Scholar] [CrossRef]
- Brandt, A.; Brenke, N.; Andres, H.-G.; Brix, S.; Guerrero-Kommritz, J.; Mühlenhardt-Siegel, U.; Wägele, J.-W. Diversity of peracarid crustaceans (Malacostraca) from the abyssal plain of the Angola Basin. Org. Divers. Evol. 2005, 5, 105–112. [Google Scholar] [CrossRef]
- Brandt, A.; Frutos, I.; Bober, S.; Brix, S.; Brenke, N.; Guggolz, T.; Heitland, N.; Malyutina, M.; Minzlaff, U.; Riehl, T. Composition of abyssal macrofauna along the Vema Fracture Zone and the hadal Puerto Rico Trench, northern tropical Atlantic. Deep Sea Res. Part II Top. Stud. Oceanogr. 2018, 148, 35–44. [Google Scholar] [CrossRef]
- Brandt, A.; Bathmann, U.; Brix, S.; Cisewski, B.; Flores, H.; Göcke, C.; Janussen, D.; Krägefsky, S.; Kruse, S.; Leach, H. Maud Rise–a snapshot through the water column. Deep Sea Res. Part II Top. Stud. Oceanogr. 2011, 58, 1962–1982. [Google Scholar] [CrossRef]
- Brandt, A.; De Broyer, C.; De Mesel, I.; Ellingsen, K.E.; Gooday, A.J.; Hilbig, B.; Linse, K.; Thomson, M.R.A.; Tyler, P.A. The biodiversity of the deep Southern Ocean benthos. Philos. Trans. R. Soc. B Biol. Sci. 2007, 362, 39–66. [Google Scholar] [CrossRef] [PubMed]
- Brandt, A.; Brix, S.; Brökeland, W.; Choudhury, M.; Kaiser, S.; Malyutina, M. Deep-sea isopod biodiversity, abundance, and endemism in the Atlantic sector of the Southern Ocean—Results from the ANDEEP I–III expeditions. Deep Sea Res. Part II Top. Stud. Oceanogr. 2007, 54, 1760–1775. [Google Scholar] [CrossRef]
- Brandt, A.; Gooday, A.J.; Brandao, S.N.; Brix, S.; Brökeland, W.; Cedhagen, T.; Choudhury, M.; Cornelius, N.; Danis, B.; De Mesel, I. First insights into the biodiversity and biogeography of the Southern Ocean deep sea. Nature 2007, 447, 307–311. [Google Scholar] [CrossRef]
- Kuerzel, K.; Linse, K.; Brandt, A.; Brenke, N.; Enderlein, P.; Griffiths, H.; Kaiser, S.; Svavarsson, J.; Loerz, A.; Frutos, I.; et al. Pan-Atlantic Comparison of Deep-Water Macrobenthos Diversity Collected by Epibenthic Sledge Sampling and Analysis of Patterns and Environmental Drivers. 2023, Version 1. NER EDS UK Polar Data Centre. Available online: https://doi.org/10.5285/58080f33-884c-4e13-a419-c00cf1bab6a6 (accessed on 26 June 2023).
- Linse, K. Cruise Report: JR 144, 145, 146, 147 and 149 Stanley 26.02.2006–Montevideo 17.04.2006. 2006. Available online: https://www.bodc.ac.uk/resources/inventories/cruise_inventory/reports/jr144-149.pdf (accessed on 1 April 2023).
- Brix, S.; Taylor, J. Short Cruise Report R/V SONNE, cruise SO280 (GPF 20-3_087) Emden—Emden (Germany) 08.01.2021–07.02.2021; Universität Hamburg: Hamburg, Germany, 2021. [Google Scholar]
- Devey, C.W. and shipboard scientific party. RV SONNE Fahrtbericht/Cruise Report SO237 Vema-TRANSIT: Bathymetry of the Vema-Fracture-Zone and Puerto Rico TRench and Abyssal AtlaNtic BiodiverSITy Study, Las Palmas (Spain)-Santo Domingo (Dom. Rep.) 14.12. 14-26.01. 15; GEOMAR Helmholtz-Zentrum für Ozeanforschung Kiel: Kiel, Germany, 2015. [Google Scholar] [CrossRef]
- Stow, D.; Smillie, Z.; Esentia, I. Deep-Sea Bottom Currents: Their Nature and Distribution. In Encyclopedia of Ocean Sciences, 3rd ed.; Cochran, J.K., Bokuniewicz, H.J., Yager, P.L., Eds.; Academic Press: Oxford, UK, 2019; pp. 90–96. [Google Scholar]
- Clarke, A.; Johnston, N.M. Antarctic marine benthic diversity. In Oceanography and Marine Biology, An Annual Review, Volume 41; CRC Press: Boca Raton, FL, USA, 2003; pp. 55–57. [Google Scholar]
- Thistle, D. The deep-sea floor: An overview. In Ecosystems of the Deep Oceans; Elsevier: Amsterdam, The Netherlands, 2003; p. 5. [Google Scholar]
- Watling, L.; Guinotte, J.; Clark, M.R.; Smith, C.R. A proposed biogeography of the deep ocean floor. Prog. Oceanogr. 2013, 111, 91–112. [Google Scholar] [CrossRef]
- Wolff, T. The hadal community, an introduction. Deep Sea Research (1953) 1959, 6, 95–124. [Google Scholar] [CrossRef]
- Brandt, A.; Barthel, D. An improved supra-and epibenthic sledge for catching Peracarida (Crustacea, Malacostraca). Ophelia 1995, 43, 15–23. [Google Scholar] [CrossRef]
- Brenke, N. An epibenthic sledge for operations on marine soft bottom and bedrock. Mar. Technol. Soc. J. 2005, 39, 10–21. [Google Scholar] [CrossRef]
- Watling, L. Macrofauna. In Encyclopedia of Ocean Sciences, 3rd ed.; Cochran, J.K., Bokuniewicz, H.J., Yager, P.L., Eds.; Academic Press: Oxford, UK, 2019; pp. 728–734. [Google Scholar]
- Assis, J.; Tyberghein, L.; Bosch, S.; Verbruggen, H.; Serrão, E.A.; De Clerck, O. Bio-ORACLE v2. 0: Extending marine data layers for bioclimatic modelling. Glob. Ecol. Biogeogr. 2018, 27, 277–284. [Google Scholar] [CrossRef]
- Tyberghein, L.; Verbruggen, H.; Pauly, K.; Troupin, C.; Mineur, F.; De Clerck, O. Bio-ORACLE: A global environmental dataset for marine species distribution modelling. Glob. Ecol. Biogeogr. 2012, 21, 272–281. [Google Scholar] [CrossRef]
- Diesing, M. Deep-sea sediments of the global ocean. Earth Syst. Sci. Data 2020, 12, 3367–3381. [Google Scholar] [CrossRef]
- Bosch, S.; Tyberghein, L.; Deneudt, K.; Hernandez, F.; De Clerck, O. In search of relevant predictors for marine species distribution modelling using the MarineSPEED benchmark dataset. Divers. Distrib. 2018, 24, 144–157. [Google Scholar] [CrossRef]
- Gordillo, S.; Muñoz, D.F.; Bayer, M.S.; Malvé, M.E. How physical and biotic factors affect brachiopods from the Patagonian Continental Shelf. J. Mar. Syst. 2018, 187, 223–234. [Google Scholar] [CrossRef]
- Quintanar-Retama, O.; Vázquez-Bader, A.R.; Gracia, A. Macrofauna abundance and diversity patterns of deep sea southwestern Gulf of Mexico. Front. Mar. Sci. 2023, 9, 1033596. [Google Scholar] [CrossRef]
- Microsoft Corporation. Microsoft Excel 16.69.1; Microsoft: Redmond, WA, USA, 2022. [Google Scholar]
- Clarke, K.; Gorley, R. PRIMER: Getting Started with v6; PRIMER-E Ltd.: Plymouth, UK, 2005; Volume 931, p. 932. [Google Scholar]
- Pérez-Mendoza, A.Y.; Hernández-Alcántara, P.; Solís-Weiss, V. Bathymetric distribution and diversity of deep water polychaetous annelids in the Sigsbee Basin, northwestern Gulf of Mexico. Hydrobiologia 2003, 496, 361–370. [Google Scholar] [CrossRef]
- Thurston, M.; Bett, B.; Rice, A.; Jackson, P. Variations in the invertebrate abyssal megafauna in the North Atlantic Ocean. Deep Sea Res. Part I Oceanogr. Res. Pap. 1994, 41, 1321–1348. [Google Scholar] [CrossRef]
- Rex, M.A.; Etter, R.J. Deep-Sea Biodiversity: Pattern and Scale; Harvard University Press: Cambridge, MA, USA, 2010. [Google Scholar]
- Bridges, A.E.; Barnes, D.K.; Bell, J.B.; Ross, R.E.; Howell, K.L. Depth and latitudinal gradients of diversity in seamount benthic communities. J. Biogeogr. 2022, 49, 904–915. [Google Scholar] [CrossRef]
- Di Franco, D.; Linse, K.; Griffiths, H.J.; Haas, C.; Saeedi, H.; Brandt, A. Abundance and distributional patterns of Benthic Peracarid Crustaceans from the Atlantic sector of the Southern Ocean and Weddell Sea. Front. Mar. Sci. 2020, 7, 554663. [Google Scholar] [CrossRef]
- Rex, M.A.; Stuart, C.T.; Etter, R.J. Do deep-sea nematodes show a positive latitudinal gradient of species diversity? The potential role of depth. Mar. Ecol. Prog. Ser. 2001, 210, 297–298. [Google Scholar] [CrossRef]
- Rex, M.A.; Stuart, C.T.; Coyne, G. Latitudinal gradients of species richness in the deep-sea benthos of the North Atlantic. Proc. Natl. Acad. Sci. USA 2000, 97, 4082–4085. [Google Scholar] [CrossRef] [PubMed]
- Poore, G.C.; Wilson, G.D. Marine species richness. Nature 1993, 361, 597–598. [Google Scholar] [CrossRef]
- Rex, M.A.; McClain, C.R.; Johnson, N.A.; Etter, R.J.; Allen, J.A.; Bouchet, P.; Warén, A. A source-sink hypothesis for abyssal biodiversity. Am. Nat. 2005, 165, 163–178. [Google Scholar] [CrossRef] [PubMed]
- Rex, M.A.; Etter, R.J. Bathymetric patterns of body size: Implications for deep-sea biodiversity. Deep Sea Res. Part II Top. Stud. Oceanogr. 1998, 45, 103–127. [Google Scholar] [CrossRef]
- Svavarsson, J.; Stromberg, J.-O.; Brattegard, T. The deep-sea asellote (Isopoda, Crustacea) fauna of the Northern Seas: Species composition, distributional patterns and origin. J. Biogeogr. 1993, 20, 537–555. [Google Scholar] [CrossRef]
- Svavarsson, J. Diversity of isopods (Crustacea): New data from the Arctic and Atlantic Oceans. Biodivers. Conserv. 1997, 6, 1571–1579. [Google Scholar] [CrossRef]
- Neal, L.; Linse, K.; Brasier, M.J.; Sherlock, E.; Glover, A.G. Comparative marine biodiversity and depth zonation in the Southern Ocean: Evidence from a new large polychaete dataset from Scotia and Amundsen seas. Mar. Biodivers. 2018, 48, 581–601. [Google Scholar] [CrossRef]
- Gage, J.D. Diversity in deep-sea benthic macrofauna: The importance of local ecology, the larger scale, history and the Antarctic. Deep Sea Res. Part II Top. Stud. Oceanogr. 2004, 51, 1689–1708. [Google Scholar] [CrossRef]
- Connolly, S.R.; MacNeil, M.A.; Caley, M.J.; Knowlton, N.; Cripps, E.; Hisano, M.; Thibaut, L.M.; Bhattacharya, B.D.; Benedetti-Cecchi, L.; Brainard, R.E. Commonness and rarity in the marine biosphere. Proc. Natl. Acad. Sci. USA 2014, 111, 8524–8529. [Google Scholar] [CrossRef]
- Kröncke, I.; Türkay, M.; Fiege, D. Macrofauna communities in the Eastern Mediterranean deep sea. Mar. Ecol. 2003, 24, 193–216. [Google Scholar] [CrossRef]
- Dahl, E. The distribution of deep-sea Crustacea. Int. Union Biol. Sci. B 1954, 16, 43–46. [Google Scholar]
- Hessler, R.R.; Sanders, H.L. Faunal diversity in the deep-sea. In Deep Sea Research and Oceanographic Abstracts; Elsevier: Amsterdam, The Netherlands, 1967; pp. 65–78. [Google Scholar]
- Gage, J.D.; Tyler, P.A. Deep-Sea Biology: A Natural History of Organisms at the Deep-Sea Floor; Cambridge University Press: Cambridge, UK, 1991. [Google Scholar]
- Rex, M.A.; Etter, R.J.; Morris, J.S.; Crouse, J.; McClain, C.R.; Johnson, N.A.; Stuart, C.T.; Deming, J.W.; Thies, R.; Avery, R. Global bathymetric patterns of standing stock and body size in the deep-sea benthos. Mar. Ecol. Prog. Ser. 2006, 317, 1–8. [Google Scholar] [CrossRef]
- Levin, L.A.; Etter, R.J.; Rex, M.A.; Gooday, A.J.; Smith, C.R.; Pineda, J.; Stuart, C.T.; Hessler, R.R.; Pawson, D. Environmental influences on regional deep-sea species diversity. Annu. Rev. Ecol. Syst. 2001, 32, 51–93. [Google Scholar] [CrossRef]
- Bernardino, A.F.; Berenguer, V.; Ribeiro-Ferreira, V.P. Bathymetric and regional changes in benthic macrofaunal assemblages on the deep Eastern Brazilian margin, SW Atlantic. Deep Sea Res. Part I Oceanogr. Res. Pap. 2016, 111, 110–120. [Google Scholar] [CrossRef]
- Woolley, S.N.; Tittensor, D.P.; Dunstan, P.K.; Guillera-Arroita, G.; Lahoz-Monfort, J.J.; Wintle, B.A.; Worm, B.; O’Hara, T.D. Deep-sea diversity patterns are shaped by energy availability. Nature 2016, 533, 393–396. [Google Scholar] [CrossRef]
- McClain, C.R. Connecting species richness, abundance and body size in deep-sea gastropods. Glob. Ecol. Biogeogr. 2004, 13, 327–334. [Google Scholar] [CrossRef]
- Carney, R.S. Zonation of deep biota on continental margins. In Oceanography and Marine Biology; CRC Press: Boca Raton, FL, USA, 2005; pp. 221–288. [Google Scholar]
- Nelson, D.M.; DeMaster, D.J.; Dunbar, R.B.; Smith, W.O., Jr. Cycling of organic carbon and biogenic silica in the Southern Ocean: Estimates of water-column and sedimentary fluxes on the Ross Sea continental shelf. J. Geophys. Res. Ocean. 1996, 101, 18519–18532. [Google Scholar] [CrossRef]
- Barnes, D.K.A. A benthic richness hotspot in the Southern Ocean: Slope and shelf cryptic benthos of Shag Rocks. Antarct. Sci. 2008, 20, 263–270. [Google Scholar] [CrossRef]
- Kaiser, S.; Griffiths, H.; Barnes, D.; Brandão, S.; Brandt, A.; O’Brien, P. Is there a distinct continental slope fauna in the Antarctic? Deep Sea Res. Part II Top. Stud. Oceanogr. 2011, 58, 91–104. [Google Scholar] [CrossRef]
- Thatje, S.; Hillenbrand, C.-D.; Larter, R. On the origin of Antarctic marine benthic community structure. Trends Ecol. Evol. 2005, 20, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Bodil, B.A.; Ambrose, W.G.; Bergmann, M.; Clough, L.M.; Gebruk, A.V.; Hasemann, C.; Iken, K.; Klages, M.; Macdonald, I.R.; Renaud, P.E.; et al. Diversity of the arctic deep-sea benthos. Mar. Biodivers. 2011, 41, 87–107. [Google Scholar] [CrossRef]
- Degen, R.; Vedenin, A.; Gusky, M.; Boetius, A.; Brey, T. Patterns and trends of macrobenthic abundance, biomass and production in the deep Arctic Ocean. Polar Res. 2015, 34, 24008. [Google Scholar] [CrossRef]
- Griffiths, H.J.; Linse, K.; Barnes, D.K. Distribution of macrobenthic taxa across the Scotia Arc, Southern Ocean. Antarct. Sci. 2008, 20, 213–226. [Google Scholar] [CrossRef]
- Saeedi, H.; Costello, M.J.; Warren, D.; Brandt, A. Latitudinal and bathymetrical species richness patterns in the NW Pacific and adjacent Arctic Ocean. Sci. Rep. 2019, 9, 9303. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, C.; Saeedi, H.; Costello, M.J. Bimodality of latitudinal gradients in marine species richness. Trends Ecol. Evol. 2016, 31, 670–676. [Google Scholar] [CrossRef]
- Blake, J.A.; Grassle, J.F. Benthic community structure on the US South Atlantic slope off the Carolinas: Spatial heterogeneity in a current-dominated system. Deep Sea Res. Part II Top. Stud. Oceanogr. 1994, 41, 835–874. [Google Scholar] [CrossRef]
- Brey, T.; Dahm, C.; Gorny, M.; Klages, M.; Stiller, M.; Arntz, W. Do Antarctic benthic invertebrates show an extended level of eurybathy? Antarct. Sci. 1996, 8, 3–6. [Google Scholar] [CrossRef]
- Allen, J.; Sanders, H. The zoogeography, diversity and origin of the deep-sea protobranch bivalves of the Atlantic: The epilogue. Prog. Oceanogr. 1996, 38, 95–153. [Google Scholar] [CrossRef]
- Gunton, L.M.; Gooday, A.J.; Glover, A.G.; Bett, B.J. Macrofaunal abundance and community composition at lower bathyal depths in different branches of the Whittard Canyon and on the adjacent slope (3500 m; NE Atlantic). Deep Sea Res. Part I Oceanogr. Res. Pap. 2015, 97, 29–39. [Google Scholar] [CrossRef]
- De Broyer, C.; Jazdzewska, A. Biogeographic patterns of Southern Ocean benthic amphipods. In Biogeographic Atlas of the Southern Ocean; SCAR: Camebridge, UK, 2014; pp. 155–165. [Google Scholar] [CrossRef]
- Legeżyńska, J.; De Broyer, C.; Węsławski, J.M. Invasion of the poles. In Evolution and Biogeography: Volume 8; Oxford University Press: Oxford, UK, 2020; pp. 216–246. [Google Scholar] [CrossRef]
- De Broyer, C.; Koubbi, P.; Griffiths, H.J.; Raymond, B.; Udekem d’Acoz, C.; Van de Putte, A.P.; Danis, B.; David, B.; Grant, S.; Gutt, J.; et al. Biogeographic Atlas of the Southern Ocean; SCAR: Camebridge, UK, 2014. [Google Scholar]
- Fauchald, K.; Jumars, P.A. The diet of worms: A study of polychaete feeding guilds. Oceanogr. Mar. Biol. Annu. Rev. 1979, 17, 193–284. [Google Scholar]
- Grassle, J.F.; Maciolek, N.J. Deep-sea species richness: Regional and local diversity estimates from quantitative bottom samples. Am. Nat. 1992, 139, 313–341. [Google Scholar] [CrossRef]
- Piepenburg, D.; Ambrose, W.G., Jr.; Brandt, A.; Renaud, P.E.; Ahrens, M.J.; Jensen, P. Benthic community patterns reflect water column processes in the Northeast Water polynya (Greenland). J. Mar. Syst. 1997, 10, 467–482. [Google Scholar] [CrossRef]
- Brökeland, W.; Choudhury, M.; Brandt, A. Composition, abundance and distribution of Peracarida from the Southern Ocean deep sea. Deep Sea Res. Part II Top. Stud. Oceanogr. 2007, 54, 1752–1759. [Google Scholar] [CrossRef]
- Poore, G.C.B.; Bruce, N.L. Global Diversity of Marine Isopods (Except Asellota and Crustacean Symbionts). PLoS ONE 2012, 7, e43529. [Google Scholar] [CrossRef]
- Brusca, R.; Isopoda. Version 06 August 1997. The Tree of Life Web Project. 1997. Available online: http://tolweb.org/Isopoda/6320/1997.08 (accessed on 1 April 2023).
- Brandt, A. Abundance, diversity and community patterns of epibenthic-and benthic-boundary layer peracarid crustaceans at 75 N off East Greenland. Polar Biol. 1997, 17, 159–174. [Google Scholar] [CrossRef]
- Hessler, R.; Wilson, G. The origin and biogeography of malacostracan crustaceans in the deep sea. In Evolution, Time and Space: The Emergence of the Biosphere; Academic Press: London, UK; New York, NY, USA, 1983; pp. 227–254. [Google Scholar]
- Gaard, E.; Gislason, A.; Falkenhaug, T.; Søiland, H.; Musaeva, E.; Vereshchaka, A.; Vinogradov, G. Horizontal and vertical copepod distribution and abundance on the Mid-Atlantic Ridge in June 2004. Deep Sea Res. Part II Top. Stud. Oceanogr. 2008, 55, 59–71. [Google Scholar] [CrossRef]
- Yayanos, A.A. Reversible inactivation of deep-sea amphipods (Paralicella capresca) by a decompression from 601 bars to atmospheric pressure. Comp. Biochem. Physiol. Part A Physiol. 1981, 69, 563–565. [Google Scholar] [CrossRef]
- Weston, J.N.J.; Jensen, E.L.; Hasoon, M.S.R.; Kitson, J.J.N.; Stewart, H.A.; Jamieson, A.J. Barriers to gene flow in the deepest ocean ecosystems: Evidence from global population genomics of a cosmopolitan amphipod. Sci. Adv. 2022, 8, eabo6672. [Google Scholar] [CrossRef] [PubMed]
- Horton, T.; Thurston, M.H.; Vlierboom, R.; Gutteridge, Z.; Pebody, C.A.; Gates, A.R.; Bett, B.J. Are abyssal scavenging amphipod assemblages linked to climate cycles? Prog. Oceanogr. 2020, 184, 102318. [Google Scholar] [CrossRef]
- Hessler, R.R.; Ingram, C.L.; Yayanos, A.A.; Burnett, B.R. Scavenging amphipods from the floor of the Philippine Trench. Deep Sea Res. 1978, 25, 1029–1047. [Google Scholar] [CrossRef]
- Thurston, M.H.; Petrillo, M.; Della Croce, N. Population structure of the necrophagous amphipod Eurythenes gryllus (Amphipoda: Gammaridea) from the Atacama Trench (south-east Pacific Ocean). J. Mar. Biol. Assoc. UK 2002, 82, 205–211. [Google Scholar] [CrossRef]
- Blankenship, L.E.; Yayanos, A.A.; Cadien, D.B.; Levin, L.A. Vertical zonation patterns of scavenging amphipods from the Hadal zone of the Tonga and Kermadec Trenches. Deep Sea Res. Part I Oceanogr. Res. Pap. 2006, 53, 48–61. [Google Scholar] [CrossRef]
- Rex, M.A.; Etter, R.J.; Nimeskern, P.W., Jr. Density estimates for deep-sea gastropod assemblages. Deep Sea Res. Part A Oceanogr. Res. Pap. 1990, 37, 555–569. [Google Scholar] [CrossRef]
- Schrödl, M.; Bohn, J.; Brenke, N.; Rolán, E.; Schwabe, E. Abundance, diversity, and latitudinal gradients of southeastern Atlantic and Antarctic abyssal gastropods. Deep Sea Res. Part II Top. Stud. Oceanogr. 2011, 58, 49–57. [Google Scholar] [CrossRef]
- Frensel, R.; Barboza, C.; Moura, R. Southwest Atlantic deep-sea brittle stars (Echinodermata: Ophiuroidea) from Campos Basin, Brazil. In Proceedings of the 12th International Echinoderm Conference, Durham, NH, USA, 7–11 August 2006. [Google Scholar]
- Martynov, A.V.; Litvinova, N.M. Deep-water Ophiuroidea of the northern Atlantic with descriptions of three new species and taxonomic remarks on certain genera and species. Mar. Biol. Res. 2008, 4, 76–111. [Google Scholar] [CrossRef]
- Gutt, J.; Piepenburg, D.; Voß, J. Asteroids, ophiuroids and holothurians from the southeastern Weddell Sea (Southern Ocean). ZooKeys 2014, 434, 1–15. [Google Scholar] [CrossRef]
- Gutt, J.; Koltun, V.M. Sponges of the Lazarev and Weddell Sea, Antarctica: Explanations for their patchy occurrence. Antarct. Sci. 1995, 7, 227–234. [Google Scholar] [CrossRef]
- Corrêa, P.V.F.; Jovane, L.; Murton, B.J.; Sumida, P.Y.G. Benthic megafauna habitats, community structure and environmental drivers at Rio Grande Rise (SW Atlantic). Deep Sea Res. Part I Oceanogr. Res. Pap. 2022, 186, 103811. [Google Scholar] [CrossRef]
- Macpherson, E. Large–scale species–richness gradients in the Atlantic Ocean. Proc. R. Soc. London. Ser. B Biol. Sci. 2002, 269, 1715–1720. [Google Scholar] [CrossRef]
- Crame, J.A. Evolution of Taxonomic Diversity Gradients in the Marine Realm: Evidence From the Composition of Recent Bivalve Faunas. Paleobiology 2000, 26, 188–214. [Google Scholar] [CrossRef]
- Miranda, T.P.; Cantero, Á.L.P.; Marques, A.C. Southern Ocean areas of endemism: A reanalysis using benthic hydroids (Cnidaria, Hydrozoa). Lat. Am. J. Aquat. Res. 2013, 41, 1003–1009. [Google Scholar] [CrossRef]
- Kidawa, A.; Janecki, T. Antarctic benthic fauna in the global climate change. Pap. Glob. Chang. IGBP 2011, 18, 71–86. [Google Scholar] [CrossRef]
- Griffiths, H.J. Antarctic marine biodiversity–what do we know about the distribution of life in the Southern Ocean? PLoS ONE 2010, 5, e11683. [Google Scholar] [CrossRef] [PubMed]
- Rogers, A.D. Evolution and biodiversity of Antarctic organisms: A molecular perspective. Philos. Trans. R. Soc. B Biol. Sci. 2007, 362, 2191–2214. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.; Bindschadler, R.; Convey, P.; Di Prisco, G.; Fahrbach, E.; Gutt, J.; Hodgson, D.; Mayewski, P.; Summerhayes, C. Antarctic Climate Change and the Environment; SCAR: Camebridge, UK, 2009. [Google Scholar]
- Jacques, G.; Tréguer, P. Ecosystèmes Pélagiques Marins; Masson: Paris, France, 1986. [Google Scholar]
- Henley, S.F.; Cavan, E.L.; Fawcett, S.E.; Kerr, R.; Monteiro, T.; Sherrell, R.M.; Bowie, A.R.; Boyd, P.W.; Barnes, D.K.; Schloss, I.R. Changing biogeochemistry of the Southern Ocean and its ecosystem implications. Front. Mar. Sci. 2020, 7, 581. [Google Scholar] [CrossRef]
- Rivaro, P.; Ardini, F.; Grotti, M.; Aulicino, G.; Cotroneo, Y.; Fusco, G.; Mangoni, O.; Bolinesi, F.; Saggiomo, M.; Celussi, M. Mesoscale variability related to iron speciation in a coastal Ross Sea area (Antarctica) during summer 2014. Chem. Ecol. 2019, 35, 1–19. [Google Scholar] [CrossRef]
- Smetacek, V. The Supply of Food to the Benthos. In Flows of Energy and Materials in Marine Ecosystems: Theory and Practice; Fasham, M.J.R., Ed.; Springer: Boston, MA, USA, 1984; pp. 517–547. [Google Scholar]
- Rowe, G.; Staresinic, N. Sources of Organic Matter to the Deep-Sea Benthos. Ambio Spec. Rep. 1979, 6, 19. [Google Scholar] [CrossRef]
- de Baar, H.J.; Buma, A.G.; Nolting, R.F.; Cadée, G.C.; Jacques, G.; Tréguer, P.J. On iron limitation of the Southern Ocean: Experimental observations in the Weddell and Scotia Seas. Mar. Ecol. Prog. Ser. 1990, 50, 105–122. [Google Scholar] [CrossRef]
- Moore, C.; Mills, M.; Arrigo, K.; Berman-Frank, I.; Bopp, L.; Boyd, P.; Galbraith, E.; Geider, R.; Guieu, C.; Jaccard, S. Processes and patterns of oceanic nutrient limitation. Nat. Geosci. 2013, 6, 701–710. [Google Scholar] [CrossRef]
- Wefer, G.; Berger, W.H.; Siedler, G.; Webb, D.J. The South Atlantic: Present and Past Circulation; Springer: Berlin/Heidelberg, Germany, 1996. [Google Scholar]
- Street, J. Iron, Phytoplankton Growth, and the Carbon Cycle. Met. Ions Biol. Syst. 2005, 43, 153–193. [Google Scholar] [CrossRef] [PubMed]
- Korb, R.E.; Whitehouse, M.J.; Ward, P. SeaWiFS in the southern ocean: Spatial and temporal variability in phytoplankton biomass around South Georgia. Deep Sea Res. Part II Top. Stud. Oceanogr. 2004, 51, 99–116. [Google Scholar] [CrossRef]
- Fung, I.Y.; Meyn, S.K.; Tegen, I.; Doney, S.C.; John, J.G.; Bishop, J.K. Iron supply and demand in the upper ocean. Glob. Biogeochem. Cycles 2000, 14, 281–295. [Google Scholar] [CrossRef]
- Jickells, T.; An, Z.; Andersen, K.K.; Baker, A.; Bergametti, G.; Brooks, N.; Cao, J.; Boyd, P.; Duce, R.; Hunter, K. Global iron connections between desert dust, ocean biogeochemistry, and climate. Science 2005, 308, 67–71. [Google Scholar] [CrossRef]
- Raiswell, R.; Benning, L.G.; Davidson, L.; Tranter, M. Nanoparticulate bioavailable iron minerals in icebergs and glaciers. Mineral. Mag. 2008, 72, 345–348. [Google Scholar] [CrossRef]
- Brasier, M.J.; Barnes, D.; Bax, N.; Brandt, A.; Christianson, A.B.; Constable, A.J.; Downey, R.; Figuerola, B.; Griffiths, H.; Gutt, J. Responses of Southern Ocean seafloor habitats and communities to global and local drivers of change. Front. Mar. Sci. 2021, 8, 622721. [Google Scholar] [CrossRef]
- McClain, C.R.; Rex, M.A.; Etter, R.J. Paterns in deep -sea macroecology. In Marine Macroecology; University of Chicago Press: Chicago, IL, USA, 2009. [Google Scholar]
- Gebruk, A.V.; Budaeva, N.E.; King, N.J. Bathyal benthic fauna of the Mid-Atlantic Ridge between the Azores and the Reykjanes Ridge. J. Mar. Biol. Assoc. UK 2010, 90, 1–14. [Google Scholar] [CrossRef]
- Mironov, A.; Southward, A.J.; Gebruk, A.V. Biogeography of the North Atlantic Seamounts; KMK Scientific Press: Moscow, Russia, 2006. [Google Scholar]
- Priede, I.G.; Bergstad, O.A.; Miller, P.I.; Vecchione, M.; Gebruk, A.; Falkenhaug, T.; Billett, D.S.; Craig, J.; Dale, A.C.; Shields, M.A. Does presence of a mid-ocean ridge enhance biomass and biodiversity? PLoS ONE 2013, 8, e61550. [Google Scholar] [CrossRef]
- McClain, C.R.; Hardy, S.M. The dynamics of biogeographic ranges in the deep sea. Proc. R. Soc. B Biol. Sci. 2010, 277, 3533–3546. [Google Scholar] [CrossRef]
- Levin, L.A.; Gooday, A.J. The deep atlantic ocean. In Ecosystems of the World, Vol 28, Ecosystems of the Deep Oceans; Elsevier: Amsterdam, the Neatherlands, 2003; 569p. [Google Scholar]
- Shields, M.A.; Blanco-Perez, R. Polychaete abundance, biomass and diversity patterns at the Mid-Atlantic Ridge, North Atlantic Ocean. Deep Sea Res. Part II Top. Stud. Oceanogr. 2013, 98, 315–325. [Google Scholar] [CrossRef]
- Bergstad, O.A.; Falkenhaug, T.; Astthorsson, O.; Byrkjedal, I.; Gebruk, A.; Piatkowski, U.; Priede, I.; Santos, R.; Vecchione, M.; Lorance, P. Towards improved understanding of the diversity and abundance patterns of the mid-ocean ridge macro-and megafauna. Deep Sea Res. Part II Top. Stud. Oceanogr. 2008, 55, 1–5. [Google Scholar] [CrossRef]
- van der Heijden, K.; Petersen, J.M.; Dubilier, N.; Borowski, C. Genetic connectivity between north and south Mid-Atlantic Ridge chemosynthetic bivalves and their symbionts. PLoS ONE 2012, 7, e39994. [Google Scholar] [CrossRef] [PubMed]
- Pierrot-Bults, A. A short note on the biogeographic patterns of the Chaetognatha fauna in the North Atlantic. Deep Sea Res. Part II Top. Stud. Oceanogr. 2008, 55, 137–141. [Google Scholar] [CrossRef]
- Brix, S.; Bober, S.; Tschesche, C.; Kihara, T.-C.; Driskell, A.; Jennings, R.M. Molecular species delimitation and its implications for species descriptions using desmosomatid and nannoniscid isopods from the VEMA fracture zone as example taxa. Deep Sea Res. Part II Top. Stud. Oceanogr. 2018, 148, 180–207. [Google Scholar] [CrossRef]
- Menzel, L.; George, K.H.; Arbizu, P.M. Submarine ridges do not prevent large-scale dispersal of abyssal fauna: A case study of Mesocletodes (Crustacea, Copepoda, Harpacticoida). Deep Sea Res. Part I Oceanogr. Res. Pap. 2011, 58, 839–864. [Google Scholar] [CrossRef]
- Brandt, A.; Linse, K.; Ellingsen, K.E.; Somerfield, P.J. Depth-related gradients in community structure and relatedness of bivalves and isopods in the Southern Ocean. Prog. Oceanogr. 2016, 144, 25–38. [Google Scholar] [CrossRef]
- Linse, K.; Schwabe, E. Diversity of macrofaunal Mollusca of the abyssal Vema Fracture Zone and hadal Puerto Rico Trench, Tropical North Atlantic. Deep Sea Res. Part II Top. Stud. Oceanogr. 2018, 148, 45–53. [Google Scholar] [CrossRef]
- Malyutina, M.V.; Frutos, I.; Brandt, A. Diversity and distribution of the deep-sea Atlantic Acanthocope (Crustacea, Isopoda, Munnopsidae), with description of two new species. Deep Sea Res. Part II Top. Stud. Oceanogr. 2018, 148, 130–150. [Google Scholar] [CrossRef]
- Bober, S.; Brix, S.; Riehl, T.; Schwentner, M.; Brandt, A. Does the Mid-Atlantic Ridge affect the distribution of abyssal benthic crustaceans across the Atlantic Ocean? Deep Sea Res. Part II Top. Stud. Oceanogr. 2018, 148, 91–104. [Google Scholar] [CrossRef]
- Guggolz, T.; Lins, L.; Meißner, K.; Brandt, A. Biodiversity and distribution of polynoid and spionid polychaetes (Annelida) in the Vema Fracture Zone, tropical North Atlantic. Deep Sea Res. Part II Top. Stud. Oceanogr. 2017, 148, 54–63. [Google Scholar] [CrossRef]
- Kroeker, K.J.; Kordas, R.L.; Crim, R.; Hendriks, I.E.; Ramajo, L.; Singh, G.S.; Duarte, C.M.; Gattuso, J.P. Impacts of ocean acidification on marine organisms: Quantifying sensitivities and interaction with warming. Glob. Chang. Biol. 2013, 19, 1884–1896. [Google Scholar] [CrossRef] [PubMed]
- Bopp, L.; Aumont, O.; Cadule, P.; Alvain, S.; Gehlen, M. Response of diatoms distribution to global warming and potential implications: A global model study. Geophys. Res. Lett. 2005, 32, L19606. [Google Scholar] [CrossRef]
- Buesseler, K.O.; Lamborg, C.H.; Boyd, P.W.; Lam, P.J.; Trull, T.W.; Bidigare, R.R.; Bishop, J.K.; Casciotti, K.L.; Dehairs, F.; Elskens, M. Revisiting carbon flux through the ocean’s twilight zone. Science 2007, 316, 567–570. [Google Scholar] [CrossRef] [PubMed]
- Steinacher, M.; Joos, F.; Frölicher, T.; Bopp, L.; Cadule, P.; Cocco, V.; Doney, S.; Gehlen, M.; Lindsay, K.; Moore, J. Projected 21st century decrease in marine productivity: A multi-model analysis. Biogeosciences 2010, 7, 979–1005. [Google Scholar] [CrossRef]
- Comiso, J.C.; Cavalieri, D.J.; Markus, T. Sea ice concentration, ice temperature, and snow depth using AMSR-E data. IEEE Trans. Geosci. Remote Sens. 2003, 41, 243–252. [Google Scholar] [CrossRef]
- D’alba, L.; Monaghan, P.; Nager, R.G. Advances in laying date and increasing population size suggest positive responses to climate change in common eiders Somateria mollissima in Iceland. Ibis 2010, 152, 19–28. [Google Scholar] [CrossRef]
- Pecl, G.T.; Araújo, M.B.; Bell, J.D.; Blanchard, J.; Bonebrake, T.C.; Chen, I.-C.; Clark, T.D.; Colwell, R.K.; Danielsen, F.; Evengård, B. Biodiversity redistribution under climate change: Impacts on ecosystems and human well-being. Science 2017, 355, eaai9214. [Google Scholar] [CrossRef]





| Depth (Phylum) | Global R: 0.264 | |
|---|---|---|
| Groups | RStatistic | Significance Level (%) |
| Abyssal, Hadal | 0.035 | 37.8 |
| Abyssal, Shelf | 0.591 | 0.1 |
| Abyssal, Lower Slope | 0.143 | 0.1 |
| Abyssal, Upper Slope | 0.476 | 0.1 |
| Hadal, Shelf | 0.35 | 0.9 |
| Hadal, Lower Slope | 0.077 | 27.2 |
| Hadal, Upper Slope | 0.186 | 10.4 |
| Shelf, Lower Slope | 0.209 | 0.7 |
| Shelf, Upper Slope | –0.062 | 81.8 |
| Bathyal, Slope | 0.146 | 0.1 |
| Depth (Multitaxa) | Global R: 0.321 | |
|---|---|---|
| Groups | R Statistic | Significance Level (%) |
| Abyssal, Hadal | 0.545 | 0.2 |
| Abyssal, Shelf | 0.715 | 0.1 |
| Abyssal, Lower Slope | 0.196 | 0.1 |
| Abyssal, Upper Slope | 0.501 | 0.1 |
| Hadal, Shelf | 0.528 | 0.3 |
| Hadal, Lower Slope | 0.308 | 5.5 |
| Hadal, Upper Slope | 0.356 | 1.4 |
| Shelf, Lower Slope | 0.256 | 0.8 |
| Shelf, Upper Slope | –0.026 | 58.5 |
| Bathyal, Slope | 0.13 | 0.2 |
| Regions (Phylum) | Global R: 0.111 | |
|---|---|---|
| Groups | R Statistic | Significance Level (%) |
| VFZ, PRT | 0.244 | 3.8 |
| VFZ, SW-Atl | 0.054 | 18.1 |
| VFZ, SO | 0.066 | 22.7 |
| VFZ, SE-Atl | 0.169 | 2.5 |
| VFZ, NE-Atl | –0.059 | 70.7 |
| VFZ, NW-Atl | 0.059 | 24.6 |
| PRT, SW-Atl | –0.094 | 77.3 |
| PRT, SO | 0.013 | 39.2 |
| PRT, SE-Atl | 0.061 | 26.2 |
| PRT, NE-Atl | 0.02 | 42.3 |
| PRT, NW-Atl | 0.163 | 9.5 |
| SW-Atl, SO | 0.062 | 15.5 |
| SW-Atl, SE-Atl | 0.06 | 8.4 |
| SW-Atl, NE-Atl | 0.106 | 8.3 |
| SW-Atl, NW-Atl | 0.106 | 20 |
| SO, SE-Atl | 0.2 | 0.3 |
| SO, NE-Atl | 0.157 | 0.1 |
| SO, NW-Atl | 0.039 | 33.8 |
| SE-Atl, NE-Atl | 0.248 | 0.1 |
| SE-Atl, NW-Atl | 0.186 | 9.8 |
| NE-Atl, NW-Atl | –0.054 | 63.3 |
| Regions (Multitaxa) | Global R: 0.169 | |
|---|---|---|
| Groups | R Statistic | Significance Level (%) |
| VFZ, PRT | 0.548 | 0.3 |
| VFZ, SW-Atl | 0.066 | 14.1 |
| VFZ, SO | 0.034 | 33.3 |
| VFZ, SE-Atl | 0.345 | 0.1 |
| VFZ, NE-Atl | 0.039 | 32.7 |
| VFZ, NW-Atl | 0.374 | 0.9 |
| PRT, SW-Atl | 0.301 | 2.1 |
| PRT, SO | 0.058 | 27.7 |
| PRT, SE-Atl | 0.568 | 0.1 |
| PRT, NE-Atl | 0.314 | 2.2 |
| PRT, NW-Atl | 0.378 | 3 |
| SW-Atl, SO | 0.062 | 16.6 |
| SW-Atl, SE-Atl | 0.221 | 0.2 |
| SW-Atl, NE-Atl | 0.187 | 1.3 |
| SW-Atl, NW-Atl | 0.221 | 6.8 |
| SO, SE-Atl | 0.206 | 0.2 |
| SO, NE-Atl | 0.208 | 0.1 |
| SO, NW-Atl | –0.017 | 52.1 |
| SE-Atl, NE-Atl | 0.267 | 0.1 |
| SE-Atl, NW-Atl | 0.27 | 3.6 |
| NE-Atl, NW-Atl | –0.13 | 84.5 |
| Variables | PC1 | PC2 |
|---|---|---|
| Depth (m) | −0.094 | −0.414 |
| Latitude (Decimal) | −0.325 | 0.076 |
| Longitude (Decimal) | −0.084 | 0.041 |
| Temperature, benthic (°C) | −0.215 | 0.137 |
| Chlorophyll, surface (mg/m3) | 0.106 | 0.400 |
| Chlorophyll, benthic (mg/cm3) | 0.000 | 0.278 |
| Dissolved oxygen, benthic (mol/m3) | −0.100 | 0.196 |
| Iron, surface (mmol/m3) | −0.074 | −0.253 |
| Iron, benthic (mol/m3) | −0.176 | 0.210 |
| Nitrate, surface (mmol/m3) | 0.305 | 0.195 |
| Nitrate, benthic (mol/m3) | 0.317 | −0.160 |
| Phosphate, surface (mmol/m3) | 0.307 | 0.191 |
| Phosphate, benthic (mol/m3) | 0.324 | −0.136 |
| Salinity, surface (PSS) | −0.256 | −0.189 |
| Salinity, benthic (PSS) | −0.195 | 0.037 |
| Primary productivity, surface (g/m3/day) | −0.081 | 0.232 |
| Primary productivity, benthic (g/m3/day) | 0.094 | 0.080 |
| Silicate, benthic (mol/m3) | 0.319 | −0.137 |
| Silicate, surface (mol/m3) | 0.307 | 0.102 |
| Calcite, surface (mol/m3) | −0.031 | 0.409 |
| Current velocity, benthic (m/s) | −0.129 | 0.081 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kürzel, K.; Brix, S.; Brandt, A.; Brenke, N.; Enderlein, P.; Griffiths, H.J.; Kaiser, S.; Svavarsson, J.; Lörz, A.-N.; Frutos, I.; et al. Pan-Atlantic Comparison of Deep-Sea Macro- and Megabenthos. Diversity 2023, 15, 814. https://doi.org/10.3390/d15070814
Kürzel K, Brix S, Brandt A, Brenke N, Enderlein P, Griffiths HJ, Kaiser S, Svavarsson J, Lörz A-N, Frutos I, et al. Pan-Atlantic Comparison of Deep-Sea Macro- and Megabenthos. Diversity. 2023; 15(7):814. https://doi.org/10.3390/d15070814
Chicago/Turabian StyleKürzel, Karlotta, Saskia Brix, Angelika Brandt, Nils Brenke, Peter Enderlein, Huw J. Griffiths, Stefanie Kaiser, Jörundur Svavarsson, Anne-Nina Lörz, Inmaculada Frutos, and et al. 2023. "Pan-Atlantic Comparison of Deep-Sea Macro- and Megabenthos" Diversity 15, no. 7: 814. https://doi.org/10.3390/d15070814
APA StyleKürzel, K., Brix, S., Brandt, A., Brenke, N., Enderlein, P., Griffiths, H. J., Kaiser, S., Svavarsson, J., Lörz, A.-N., Frutos, I., Taylor, J., & Linse, K. (2023). Pan-Atlantic Comparison of Deep-Sea Macro- and Megabenthos. Diversity, 15(7), 814. https://doi.org/10.3390/d15070814









