Microplastics Occurrence in Fish from Tocagua Lake, Low Basin Magdalena River, Colombia
Abstract
:1. Introduction
2. Materials and Methods
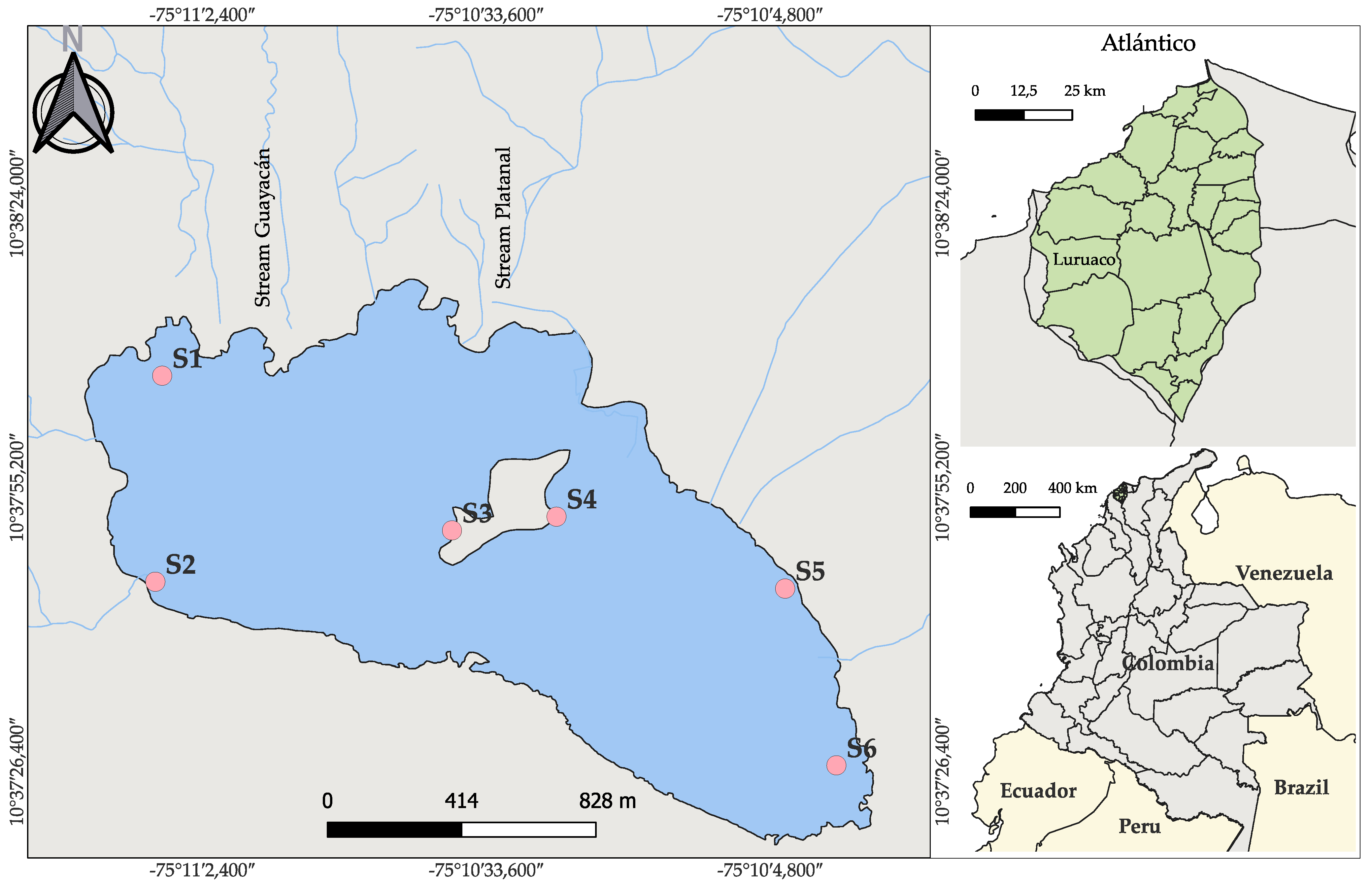
2.1. Study Area
2.2. Water Sampling
2.3. Sampling and Processing of Fish
2.4. Quality Assurance/Quality Control (QA/QC)
2.5. Physical and Chemical MPs Characterization
2.6. Statistical Analysis
3. Results
3.1. Habitat Physicochemical Characterization
3.2. Abundance and Characteristics of Microplastics in Water
3.3. Abundance and Characteristics of Microplastics in Fish Samples
3.4. Seasonal Variation of MPs in Fish
3.5. Transfer of MPs between Water and Dietary Trends of Fish
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Williams, A.T.; Rangel-Buitrago, N. The past, present, and future of plastic pollution. Mar. Pollut. Bull. 2022, 176, 113429. [Google Scholar] [CrossRef] [PubMed]
- Rangel-Buitrago, N.; Velez Mendoza, A.; Mantilla-Barbosa, E.; Arroyo-Olarte, H.; Arana, V.A.; Trilleras, I.; Gracia, A.; Neal, W.J.; Williams, A.T. Plastic pollution on the Colombian central Caribbean beaches. Mar. Pollut. Bull. 2021, 162, 111837. [Google Scholar] [CrossRef] [PubMed]
- Garcés-Ordóñez, O.; Espinosa, L.F.; Pereira Cardoso, R.; Barroso Issa Cardozo, B.; Meigikos dos Anjos, R. Plastic litter pollution along sandy beaches in the Caribbean and Pacific coast of Colombia. Environ. Pollut. 2020, 267, 115495. [Google Scholar] [CrossRef] [PubMed]
- Chawla, S.; Varghese, B.S.; Chithra, A.; Hussain, C.G.; Keçili, R.; Hussain, C.M. Environmental impacts of post-consumer plastic wastes: Treatment technologies towards eco-sustainability and circular economy. Chemosphere 2022, 308, 135867. [Google Scholar] [CrossRef]
- Chen, W.Q.; Ciacci, L.; Sun, N.N.; Yoshioka, T. Sustainable cycles and management of plastics: A brief review of RCR publications in 2019 and early 2020. Res. Conserv. Recycl. 2020, 159, 104822. [Google Scholar] [CrossRef]
- Huang, S.; Wang, H.; Ahmad, W.; Ahmad, A.; Ivanovich Vatin, N.; Mohamed, A.M.; Deifalla, A.F.; Mehmood, I. Plastic Waste Management Strategies and Their Environmental Aspects: A Scientometric Analysis and Comprehensive Review. Int. J. Environ. Res. Public Health 2022, 19, 4556. [Google Scholar] [CrossRef]
- Lorang, S.; Yang, Z.; Zhang, H.; Lü, F.; He, P. Achievements and policy trends of extended producer responsibility for plastic packaging waste in Europe. Waste Dispos. Sustain. Energy 2022, 4, 91–103. [Google Scholar] [CrossRef]
- Babaremu, K.O.; Okoya, S.A.; Hughes, E.; Tijani, B.; Teidi, D.; Akpan, A.; Igwe, J.; Karera, S.; Oyinlola, M.; Akinlabi, E.T. Sustainable plastic waste management in a circular economy. Heliyon 2022, 8, e09984. [Google Scholar] [CrossRef]
- Plásticos en Colombia 2022–2023—Acoplásticos. Available online: https://bit.ly/acoPeC22 (accessed on 28 January 2023).
- Jiang, J.; Shi, K.; Zhang, X.; Yu, K.; Zhang, H.; He, J.; Ju, Y.; Liu, J. From plastic waste to wealth using chemical recycling: A review. J. Environ. Chem. Eng. 2022, 10, 106867. [Google Scholar] [CrossRef]
- Plastics Europe, EPRO. Plastics—The Facts 2022: An Analysis of the Latest Data Related to Plastics Production, Demand, Conversion and Waste Management in Europe. 2022. Available online: https://plasticseurope.org/knowledge-hub/plastics-the-facts-2022/ (accessed on 16 April 2023).
- Manzoor, S.; Naqash, N.; Rashid, G.; Singh, R. Plastic Material Degradation and Formation of Microplastic in the Environment: A Review. Mater. Today Proc. 2022, 56, 3254–3260. [Google Scholar] [CrossRef]
- Huber, M.; Archodoulaki, V.-M.; Pomakhina, E.; Pukánszky, B.; Zinöcker, E.; Gahleitner, M. Environmental degradation and formation of secondary microplastics from packaging material: A polypropylene film case study. Polym. Degrad. Stab. 2022, 195, 109794. [Google Scholar] [CrossRef]
- Zhang, K.; Hamidian, A.H.; Tubić, A.; Zhang, Y.; Fang, J.K.; Wu, C.; Lam, P.K. Understanding plastic degradation and microplastic formation in the environment: A review. Environ. Pollut. 2021, 274, 116554. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Zhang, Z.; Chen, L.; Cui, Q.; Cui, Y.; Song, D.; Fang, L. Review on migration, transformation and ecological impacts of microplastics in soil. App. Soil Ecol. 2022, 176, 104486. [Google Scholar] [CrossRef]
- Koutnik, V.S.; Leonard, J.; Alkidim, S.; DePrima, F.J.; Ravi, S.; Hoek, E.M.V.; Mohanty, S.K. Distribution of microplastics in soil and freshwater environments: Global analysis and framework for transport modeling. Environ. Pollut. 2021, 274, 116552. [Google Scholar] [CrossRef]
- An, L.; Liu, Q.; Deng, Y.; Wu, W.; Gao, Y.; Ling, W. Sources of microplastic in the environment. In Microplastics in Terrestrial Environments. The Handbook of Environmental Chemistry; He, D., Luo, Y., Eds.; Springer: Cham, Switzerland, 2020; Volume 95, pp. 143–159. [Google Scholar] [CrossRef]
- Klavins, M.; Klavins, L.; Stabnikova, O.; Stabnikov, V.; Marynin, A.; Ansone-Bertina, L.; Mezulis, M.; Vaseashta, A. Interaction between Microplastics and Pharmaceuticals Depending on the Composition of Aquatic Environment. Microplastics 2022, 1, 520–535. [Google Scholar] [CrossRef]
- Stabnikova, O.; Stabnikov, V.; Marinin, A.; Klavins, M.; Vaseashta, A. The role of microplastics biofilm in accumulation of trace metals in aquatic environments. World J. Microbiol. Biotechnol. 2022, 38, 117. [Google Scholar] [CrossRef]
- Waldschläger, K.; Lechthaler, S.; Stauch, G.; Schüttrumpf, H. The way of microplastic through the environment–Application of the source-pathway-receptor model. Sci. Total Environ. 2020, 713, 136584. [Google Scholar] [CrossRef]
- Qu, H.; Wang, F.; Barrett, H.; Wang, B.; Han, J.; Wu, J.; Huang, X.; Hu, Y.; Yu, G. Synthetical effect of microplastics and chiral drug amphetamine on a primary food source algae Chlorella pyrenoids. Food Chem. Toxicol. 2022, 69, 113415. [Google Scholar] [CrossRef]
- Lee, H.-S.; Amarakoon, D.; Wei, C.; Choi, K.Y.; Smolensky, D.; Lee, S.-H. Adverse effect of polystyrene microplastics (PS-MPs) on tube formation and viability of human umbilical vein endothelial cells. Food Chem. Toxicol. 2021, 154, 112356. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Sun, X.; Shi, X.; Xu, S. Microplastics and di (2-ethylhexyl) phthalate synergistically induce apoptosis in mouse pancreas through the GRP78/CHOP/Bcl-2 pathway activated by oxidative stress. Food Chem. Toxicol. 2022, 167, 113315. [Google Scholar] [CrossRef]
- Chen, W.; Zhu, R.; Ye, X.; Sun, Y.; Tang, Q.; Liu, Y.; Yan, F.; Yu, T.; Zheng, X.; Tu, P. Food-derived cyanidin-3-O-glucoside reverses microplastic toxicity via promoting discharge and modulating the gut microbiota in mice. Food Funct. 2022, 13, 1447–1458. [Google Scholar] [CrossRef]
- Wang, A.; Han, Q.; Wei, Z.; Wang, Y.; Xie, J.; Chen, M. Polystyrene microplastics affect learning and memory in mice by inducing oxidative stress and decreasing the level of acetylcholine. Food Chem. Toxicol. 2022, 162, 112904. [Google Scholar] [CrossRef] [PubMed]
- Bertoli, M.; Pastorino, P.; Lesa, D.; Renzi, M.; Anselmi, S.; Prearo, M.; Pizzul, E. Microplastics accumulation in functional feeding guilds and functional habit groups of freshwater macrobenthic invertebrates: Novel insights in a riverine ecosystem. Sci. Total Environ. 2021, 804, 150207. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, R.; Khan, M.T.; Bilal, H.; Aslam, M.M.; Khan, I.A.; Arslan, M.; Nguyen, P.M. Microplastics as vectors of environmental contaminants: Interactions in the natural ecosystems. Hum. Ecol. Risk Assess. 2022, 28, 1022–1042. [Google Scholar] [CrossRef]
- Xiang, Y.; Jiang, L.; Zhou, Y.; Luo, Z.; Zhi, D.; Yang, J.; Lam, S.S. Microplastics and environmental pollutants: Key interaction and toxicology in aquatic and soil environments. J. Hazard. Mater. 2022, 422, 126843. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Chen, Y. Effects of microplastics on wastewater and sewage sludge treatment and their removal: A review. Chem. Eng. J. 2020, 382, 122955. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, J.; Xing, B. Environmental source, fate, and toxicity of microplastics. J. Hazard. Mater. 2021, 407, 124357. [Google Scholar] [CrossRef]
- O’Hara, P.D.; Avery-Gomm, S.; Wood, J.; Bowes, V.; Wilson, L.; Morgan, K.H.; Boyd, W.S.; Hipfner, J.M.; Desforges, J.-P.; Bertram, D.F.; et al. Seasonal variability in vulnerability for Cassin’s auklets (Ptychoramphus aleuticus) exposed to microplastic pollution in the Canadian Pacific region. Sci. Total Environ. 2019, 649, 50–60. [Google Scholar] [CrossRef]
- Talbot, R.; Chang, H. Microplastics in freshwater: A global review of factors affecting spatial and temporal variations. Environ. Pollut. 2022, 292, 118393. [Google Scholar] [CrossRef]
- Merga, L.B.; Redondo-Hasselerharm, P.E.; Van den Brink, P.J.; Koelmans, A.A. Distribution of microplastic and small macroplastic particles across four fish species and sediment in an African lake. Sci. Total Environ. 2020, 741, 140527. [Google Scholar] [CrossRef]
- Wu, C.; Xiong, X.; Hamidian, A.H.; Zhang, Y.; Xu, X. A review on source, occurrence, and impacts of microplastics in freshwater aquaculture systems in China. Water Biol. Secur. 2022, 1, 100040. [Google Scholar] [CrossRef]
- Koraltan, I.; Mavruk, S.; Güven, O. Effect of biological and environmental factors on microplastic ingestion of commercial fish species. Chemosphere 2022, 303, 135101. [Google Scholar] [CrossRef] [PubMed]
- Wootton, N.; Reis-Santos, P.; Gillanders, B.M. Microplastic in fish—A global synthesis. Rev. Fish. Biol. Fish. 2021, 31, 753–771. [Google Scholar] [CrossRef]
- D’Avignon, G.; Gregory-Eaves, I.; Ricciardi, A. Microplastics in lakes and rivers: An issue of emerging significance to limnology. Environ. Rev. 2022, 30, 228–244. [Google Scholar] [CrossRef]
- Wang, W.; Ge, J.; Yu, X. Bioavailability and toxicity of microplastics to fish species: A review. Ecotoxicol. Environ. Saf. 2020, 189, 109913. [Google Scholar] [CrossRef] [PubMed]
- Garcés–Ordóñez, O.; Saldarriaga–Vélez, J.F.; Espinosa–Díaz, L.F.; Patiño, A.D.; Cusba, J.; Canals, M.; Mejía–Esquivia, K.; Fragozo–Velásquez, L.; Sáenz–Arias, S.; Córdoba–Meza, T.; et al. Microplastic pollution in water, sediments and commercial fish species from Laguna Grande de Santa Marta lagoon complex, Colombian Caribbean. Sci. Total Environ. 2022, 829, 154643. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Cárdenas, V.; Luna-Acosta, A.; Gómez-Méndez, L.D. Differential Presence of Microplastics and Mesoplastics in Coral Reef and Mangrove Fishes in Isla Grande, Colombia. Microplastics 2022, 1, 477–493. [Google Scholar] [CrossRef]
- Rusinque-Quintero, L.L.; Montoya-Rojas, G.A.; Moyano-Molano, A.L. Environmental risks due to the presence of microplastics in coastal and marine environments of the Colombian Caribbean. Mar. Pollut. Bull. 2022, 185, 114357. [Google Scholar] [CrossRef]
- Rangel-Buitrago, N.; Arroyo-Olarte, H.; Trilleras, J.; Arana, V.A.; Mantilla-Barbosa, E.; Gracia, A.; Velez Mendoza, A.; Neal, W.J.; Williams, A.T.; Micalle, A. Microplastics pollution on Colombian Central Caribbean beaches. Mar. Pollut. Bull. 2021, 170, 112685. [Google Scholar] [CrossRef]
- Garcés-Ordóñez, O.; Espinosa, L.F.; Costa Muniz, M.; Salles Pereira, L.B.; Meigikos Dos Anjos, R. Abundance, distribution, and characteristics of microplastics in coastal surface waters of the Colombian Caribbean and Pacific. Environ Sci. Pollut. Res. 2021, 28, 43431–43442. [Google Scholar] [CrossRef]
- Vásquez-Molano, D.; Molina, A.; Duque, G. Spatial distribution and increase of microplastics over time in sediments of Buenaventura Bay, Colombian Pacific. Bol. Investig. Mar. Cost. 2021, 50, 27–42. [Google Scholar] [CrossRef]
- Acosta-Coley, I.; Olivero-Verbel, J. Microplastic resin pellets on an urban tropical beach in Colombia. Environ. Monit. Assess. 2015, 187, 435. [Google Scholar] [CrossRef] [PubMed]
- Corporación Autónoma Regional del Atlántico CRA (2021). Acuerdo Número 000008, por Medio del Cual se Adopta el Plan de Manejo Ambiental del Distrito Regional del Manejo Integrado (DRMI), Palmar del Tití Como Área Protegida y se Establecen Otras Disposiciones. Available online: https://www.crautonoma.gov.co/la-c-r-a/consejo-directivo/acuerdos-consejo (accessed on 27 May 2023).
- Arias-González, C.; González-Maya, J.F.; González Zamorano, P.; Ortega Rubiuo, A. Climate refugia for two Colombian endemic tamarin primates are critically under-protected. Mamm. Biol. 2021, 101, 531–543. [Google Scholar] [CrossRef]
- Martínez, L.; Dámato, G. Identity and environmental law protection of the Mokaná indigenous people in Malambo, Atlántico. Jurídicas CUC 2022, 18, 303–334. [Google Scholar] [CrossRef]
- Rojas–Luna, R.A.; Oquendo–Ruiz, L.; García–Alzate, C.A.; Arana, V.A.; García–Alzate, R.; Trilleras, J. Identification, Abundance, and Distribution of Microplastics in Surface Water Collected from Luruaco Lake, Low Basin Magdalena River, Colombia. Water 2023, 15, 344. [Google Scholar] [CrossRef]
- Parker, B.; Britton, J.R.; Green, I.D.; Amat-Trigo, F.; Andreou, D. Parasite infection but not chronic microplastic exposure reduces the feeding rate in a freshwater fish. Environ. Pollut. 2023, 320, 121120. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.; Rodrigues, S.M.; Silva, D.; Freitas, V.; Almeida, C.M.R.; Ramos, S. Microplastic contamination in large migratory fishes collected in the open Atlantic Ocean. Mar. Pollut. Bull. 2023, 186, 114454. [Google Scholar] [CrossRef] [PubMed]
- Foekema, E.M.; De Gruijter, C.; Mergia, M.T.; Van Franeker, J.A.; Murk, A.J.; Koelmans, A.A. Plastic in North Sea fish. Environ. Sci. Technol. 2013, 47, 8818–8824. [Google Scholar] [CrossRef]
- Dehaut, A.; Cassone, A.L.; Frère, L.; Hermabessiere, L.; Himber, C.; Rinnert, E.; Rivière, G.; Lambert, C.; Soudant, P.; Huvet, A.; et al. Microplastics in seafood: Benchmark protocol for their extraction and characterization. Environ. Pollut. 2016, 215, 223–233. [Google Scholar] [CrossRef] [Green Version]
- Enders, K.; Lenz, R.; do Sul, J.A.I.; Tagg, A.S.; Labrenz, M. When every particle matters: A QuEChERS approach to extract microplastics from environmental samples. MethodsX 2020, 7, 100784. [Google Scholar] [CrossRef]
- Koelmans, A.A.; Nor, N.H.M.; Hermsen, E.; Kooi, M.; Mintenig, S.M.; De France, J. Microplastics in freshwaters and drinking water: Critical review and assessment of data quality. Water Res. 2019, 155, 410–422. [Google Scholar] [CrossRef]
- Zhang, Z.; Wu, X.; Liu, H.; Huang, X.; Chen, Q.; Guo, X.; Zhang, J. A systematic review of microplastics in the environment: Sampling, separation, characterization and coexistence mechanisms with pollutants. Sci. Total Environ. 2023, 859, 160151. [Google Scholar] [CrossRef] [PubMed]
- Cabanilles, P.; Acle, S.; Arias, A.; Masiá, P.; Ardura, A.; Garcia-Vazquez, E. Microplastics Risk into a Three-Link Food Chain Inside European Hake. Diversity 2022, 14, 308. [Google Scholar] [CrossRef]
- Pastorino, P.; Prearo, M.; Di Blasio, A.; Barcelò, D.; Anselmi, S.; Colussi, S.; Alberti, S.; Tedde, G.; Dondo, A.; Ottino, M.; et al. Microplastics Occurrence in the European Common Frog (Rana temporaria) from Cottian Alps (Northwest Italy). Diversity 2022, 14, 66. [Google Scholar] [CrossRef]
- Chen, G.; Li, Y.; Wang, J. Occurrence and ecological impact of microplastics in aquaculture ecosystems. Chemosphere 2021, 274, 129989. [Google Scholar] [CrossRef]
- Andrade, J.M.; Ferreiro, B.; López–Mahía, P.; Muniategui–Lorenzo, S. Standardization of the minimum information for publication of infrared-related data when microplastics are characterized. Mar. Pollut. Bull. 2020, 154, 111035. [Google Scholar] [CrossRef] [PubMed]
- Primpke, S.; Wirth, M.; Lorenz, C.; Gerdts, G. Reference database design for the automated analysis of microplastic samples based on Fourier transform infrared (FTIR) spectroscopy. Analyt. Bioanalyt. Chem. 2018, 410, 5131–5141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venables, W.N.; Ripley, B.D. Random and Mixed Effects. In Modern Applied Statistics with S. Statistics and Computing; Springer: New York, NY, USA, 2002. [Google Scholar] [CrossRef]
- Gao, S.; Yan, K.; Liang, B.; Shu, R.; Wang, N.; Zhang, S. The different ways mmicroplastics from the water column and sediment accumulate in fish in Haizhou Bay. Sci. Total Environ. 2023, 854, 158575. [Google Scholar] [CrossRef]
- Wang, T.; Hu, M.; Xu, G.; Shi, H.; Leung, J.Y.S.; Wang, Y. Microplastic accumulation via trophic transfer: Can a predatory crab counter the adverse effects of microplastics by body defence? Sci. Total Environ. 2021, 754, 142099. [Google Scholar] [CrossRef]
- Wu, J.; Jiang, Z.; Liu, Y.; Zhao, X.; Liang, Y.; Lu, W.; Song, J. Microplastic contamination assessment in water and economic fishes in different trophic guilds from an urban water supply reservoir after flooding. J. Environ. Manage. 2021, 299, 113667. [Google Scholar] [CrossRef]
- Chen, Y.; Shen, Z.; Li, G.; Wang, K.; Cai, X.; Xiong, X.; Wu, C. Factors affecting microplastic accumulation by wild fish: A case study in the Nandu River, South China. Sci. Total Environ. 2022, 847, 157486. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.; Christie-Oleza, J.A.; Xu, E.G.; Li, J.; Zhang, H.; Wang, W.; Lin, L.; Zhang, W.; Yang, Y. Environmental fate of microplastics in the world’s third-largest river: Basin-wide investigation and microplastic community analysis. Water Res. 2022, 210, 118002. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. 2022. Available online: https://www.R-project.org/ (accessed on 27 March 2023).
- Dusaucy, J.; Gateuille, D.; Perrette, Y.; Naffrechoux, E. Microplastic pollution of worldwide lakes. Environ. Pollut. 2021, 284, 117075. [Google Scholar] [CrossRef] [PubMed]
- Garcés-Ordóñez, O.; Castillo-Olaya, V.A.; Granados-Briceño, A.F.; García, L.M.B.; Díaz, L.F.E. Marine litter and microplastic pollution on mangrove soils of the Ciénaga Grande de Santa Marta, Colombian Caribbean. Mar. Pollut. Bull. 2019, 145, 455–462. [Google Scholar] [CrossRef]
- Garcés-Ordóñez, O.; Mejía-Esquivia, K.A.; Sierra-Labastidas, T.; Patiño, A.; Blandón, L.M.; Díaz, L.F.E. Prevalence of microplastic contamination in the digestive tract of fishes from mangrove ecosystem in Cispata, Colombian Caribbean. Mar. Pollut. Bull. 2020, 154, 111085. [Google Scholar] [CrossRef]
- Elagami, H.; Frei, S.; Boos, J.P.; Trommer, G.; Gilfedder, B.S. Quantifying microplastic residence times in lakes using mesocosm experiments and transport modelling. Water Res. 2023, 229, 119463. [Google Scholar] [CrossRef] [PubMed]
- Biginagwa, F.J.; Mayoma, B.S.; Shashoua, Y.; Syberg, K.; Khan, F.R. First evidence of microplastics in the African Great Lakes: Recovery from Lake Victoria Nile perch and Nile tilapia. J. Great Lakes Res. 2016, 42, 146–149. [Google Scholar] [CrossRef]
- Roch, S.; Walter, T.; Ittner, L.D.; Friedrich, C.; Brinker, A. A systematic study of the microplastic burden in freshwater fishes of south-western Germany—Are we searching at the right scale? Sci. Total Environ. 2019, 689, 1001–1011. [Google Scholar] [CrossRef]
- Wu, J.; Yin, X.; Liu, Y.; Chen, X.; Xie, C.; Liang, Y.; Li, J.; Jiang, Z. Seasonal variation and ecological risk assessment of microplastics ingested by economic fishes in Lake Chao, China. Sci. Total Environ. 2022, 833, 155181. [Google Scholar] [CrossRef]
- Yin, X.; Wu, J.; Liu, Y.; Chen, X.; Xie, C.; Liang, Y.; Li, J.; Jiang, Z. Accumulation of microplastics in fish guts and gills from a large natural lake: Selective or non-selective? Environ. Pollut. 2022, 309, 119785. [Google Scholar] [CrossRef]
- Yuan, W.; Liu, X.; Wang, W.; Di, M.; Wang, J. Microplastic abundance, distribution and composition in water, sediments, and wild fish from Poyang Lake, China. Ecotoxicol. Environ. Saf. 2019, 170, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Faure, F.; Demars, C.; Wieser, O.; Kunz, M.; de Alencastro, L.F. Plastic pollution in Swiss surface waters: Nature and concentrations, interaction with pollutants. Environ. Chem. 2015, 12, 582–591. [Google Scholar] [CrossRef]
- Weinstein, J.E.; Ertel, B.M.; Gray, A.D. Accumulation and depuration of microplastic fibers, fragments, and tire particles in the eastern oyster, Crassostrea virginica: A toxicokinetic approach. Environ. Pollut. 2022, 308, 119681. [Google Scholar] [CrossRef] [PubMed]
- Turner, S.; Horton, A.A.; Rose, N.L.; Hall, C. A temporal sediment record of microplastics in an urban lake, London, UK. J. Paleolimnol. 2019, 61, 449–462. [Google Scholar] [CrossRef]
- Okamoto, K.; Nomura, M.; Horie, Y.; Okamura, H. Color preferences and gastrointestinal-tract retention times of microplastics by freshwater and marine fishes. Environ. Pollut. 2022, 304, 119253. [Google Scholar] [CrossRef]
- Morales, J.; García-Alzate, C.A. Trophic structure of river fish from Corral de San Luis, Magdalena river basin, Colombia Caribbean. Rev. Biol. Trop. 2016, 64, 715–732. [Google Scholar] [CrossRef] [Green Version]
- Rios-Fuster, B.; Arechavala-Lopez, P.; García-Marcos, K.; Alomar, C.; Compa, M.; Álvarez, E.; Julià, M.M.; Solomando Martíd, A.; Sureda, A.; Deudero, S. Experimental evidence of physiological and behavioral effects of microplastic ingestion in Sparus aurata. Aquat. Toxicol. 2021, 231, 105737. [Google Scholar] [CrossRef]
- Vethaak, A.D.; Legler, J. Microplastics and human health. Science 2021, 371, 672–674. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for Drinking-Water Quality: First Addendum to the Fourth Edition. 2017. Available online: https://apps.who.int/iris/handle/10665/254636 (accessed on 1 April 2023).
- Das, R.; Krishnakumar, A.; Kumar, M.R.; Thulseedharan, D. Water quality assessment of three tropical freshwater lakes of Kerala, SW India, with special reference to drinking water potential. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100588. [Google Scholar] [CrossRef]




| Species | Feeding Groups | N | Total Length (mm) * | Weight (g) *** | K Index | ||||
|---|---|---|---|---|---|---|---|---|---|
| Wet | Dry | Wet | Dry | Wet | Dry | Wet | Dry | ||
| Andinoacara latifrons | OV | 54 | 12 | 118.4 (50.2) | 59.8 (4.7) | 42.0 (44.5) | 3.5 (1.2) | 0.6 (0.8) | 1.4 (0.5) |
| Astyanax magdalenae | OI | 14 | 0 | 112.0 (46.6) | NA ** | 40.2 (66) | NA | 0.4 (0.3) | NA |
| Caquetaia kraussii | OC | 23 | 16 | 106.3 (41.2) | 57.8 (5.1) | 34.9 (30) | 3.1 (1.3) | 0.6 (0.9) | 1.3 (0.5) |
| Mugil liza | DM | 13 | 7 | 119.7 (49.5) | 61.8 (8.8) | 39.0 (35.1) | 4.2 (1.7) | 0.3 (0.3) | 1.7 (0.7) |
| Oreochromis niloticus | OV | 23 | 4 | 92.7 (55.1) | 51.7 (11.9) | 26.4 (33.2) | 2.5 (1.8) | 0.6 (0.6) | 1.0 (0.7) |
| Poecilia gillii | HB | 56 | 6 | 115.6 (44) | 60.6 (6.3) | 39.9 (38.2) | 4.0 (1.3) | 0.3 (0.3) | 1.6 (0.5) |
| 183 | 45 | ||||||||
| Season | Station | Total | Fiber | Fragment | Foam |
|---|---|---|---|---|---|
| Wet | S1 | 1.11 | 0.97 | 0.12 | 0.03 |
| S2 | 0.74 | 0.63 | 0.09 | 0.03 | |
| S3 | 0.97 | 0.92 | 0.05 | 0.00 | |
| S4 | 1.38 | 1.00 | 0.35 | 0.03 | |
| S5 | 0.88 | 0.81 | 0.07 | 0.01 | |
| S6 | 1.17 | 1.00 | 0.14 | 0.03 | |
| Dry | S1 | 1.52 | 1.24 | 0.30 | 0.18 |
| S2 | 0.64 | 0.59 | 0.05 | 0.00 | |
| S3 | 0.75 | 0.50 | 0.25 | 0.00 | |
| S4 | 1.47 | 1.19 | 0.25 | 0.03 | |
| S5 | 0.83 | 0.53 | 0.28 | 0.00 | |
| S6 | 0.10 | 0.10 | 0.00 | 0.00 |
| Species | Ni | NMPs | %FO | Average (±SD) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Wet | Dry | Wet | Dry | Max. | Wet | Dry | Wet | Dry | |
| Andinoacara latifrons | 50 | 10 | 181 | 38 | 7–12 | 92.6 | 83.3 | 3.4 (2.9) | 3.2 (3.9) |
| Astyanax magdalenae | 13 | 0 | 50 | 0 | 5–0 | 92.9 | 0.0 | 3.6 (1.8) | 0.0 (0.0) |
| Caquetaia krausii | 19 | 13 | 42 | 49 | 4–20 | 82.6 | 81.2 | 1.8 (1.5) | 3.1 (4.9) |
| Mugil liza | 10 | 4 | 39 | 6 | 7–2 | 76.9 | 57.1 | 3.0 (3.1) | 0.9 (0.9) |
| Oreochromis niloticus | 17 | 1 | 81 | 2 | 9–1 | 73.9 | 25.0 | 3.5 (3.5) | 0.5 (1.0) |
| Poecilia gilli | 49 | 5 | 151 | 9 | 6–3 | 87.5 | 83.3 | 2.7 (2.0) | 1.5 (1.2) |
| Total | 158 | 33 | 544 | 104 | |||||
| Type of Diets | Wet | Dry | |||
|---|---|---|---|---|---|
| Total | Fibers | Fragments | Fibers | Fragments | |
| Detritivorous–microalgae | 0.00064 | 0.00015 | 0.00007 | 0.00042 | 0 |
| Herbivorous | 0.00708 | 0.00137 | 0.0035 | 0.00221 | 0 |
| Omnivorous | 0.00116 | 0.00026 | 0.00042 | 0.00007 | 0.00041 |
| Omnivorous–carnivorous | 0.00052 | 0.00013 | 0 | 0.00011 | 0.00028 |
| Omnivorous–insectivorous | 0.00406 | 0.00406 | 0 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miranda-Peña, L.; Urquijo, M.; Arana, V.A.; García-Alzate, R.; García-Alzate, C.A.; Trilleras, J. Microplastics Occurrence in Fish from Tocagua Lake, Low Basin Magdalena River, Colombia. Diversity 2023, 15, 821. https://doi.org/10.3390/d15070821
Miranda-Peña L, Urquijo M, Arana VA, García-Alzate R, García-Alzate CA, Trilleras J. Microplastics Occurrence in Fish from Tocagua Lake, Low Basin Magdalena River, Colombia. Diversity. 2023; 15(7):821. https://doi.org/10.3390/d15070821
Chicago/Turabian StyleMiranda-Peña, Lindys, Milena Urquijo, Victoria A. Arana, Roberto García-Alzate, Carlos A. García-Alzate, and Jorge Trilleras. 2023. "Microplastics Occurrence in Fish from Tocagua Lake, Low Basin Magdalena River, Colombia" Diversity 15, no. 7: 821. https://doi.org/10.3390/d15070821
APA StyleMiranda-Peña, L., Urquijo, M., Arana, V. A., García-Alzate, R., García-Alzate, C. A., & Trilleras, J. (2023). Microplastics Occurrence in Fish from Tocagua Lake, Low Basin Magdalena River, Colombia. Diversity, 15(7), 821. https://doi.org/10.3390/d15070821








