Climate Change Potential Impacts on the Tuna Fisheries in the Exclusive Economic Zones of Tonga
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Study Species and Occurrence Data
2.3. Predictor Variables
2.4. Species Distribution Modelling
2.5. Climatic Suitable Areas
3. Results
3.1. Performances of Species Distribution Modelling
3.2. Relative Contribution of the Predictor Variables
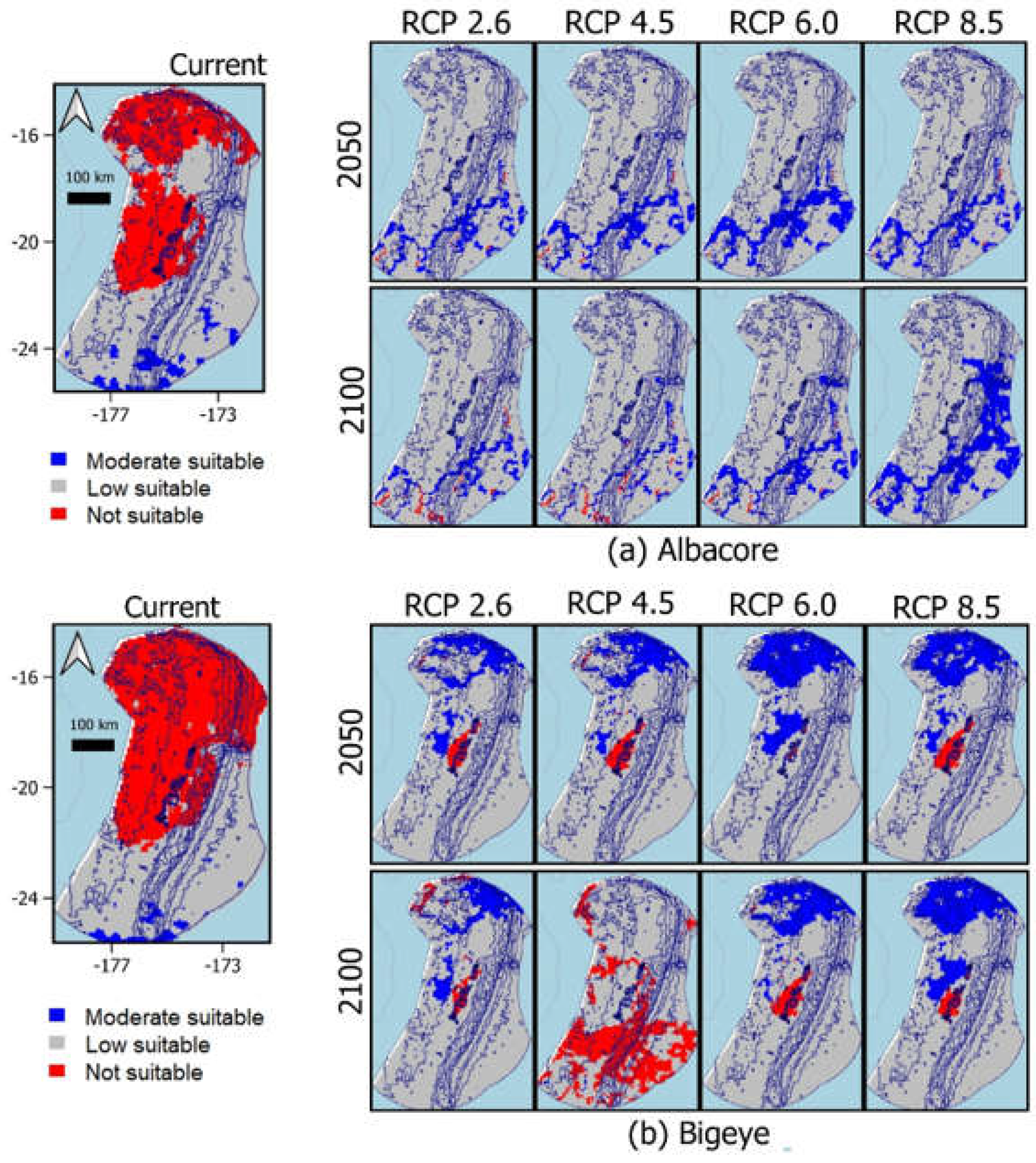
3.3. Predicted Suitability Habitat
3.4. Biogeographical Distribution of Species
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McMahon, S.M.; Harrison, S.P.; Armbruster, W.S.; Bartlein, P.J.; Beale, C.M.; Edwards, M.E.; Kattge, J.; Midgley, G.; Morin, X.; Prentice, I.C. Improving assessment and modelling of climate change impacts on global terrestrial biodiversity. Trends Ecol. Evol. 2011, 26, 249–259. [Google Scholar] [CrossRef]
- Hattab, T.; Albouy, C.; Lasram, F.B.R.; Somot, S.; Le Loc’H, F.; Leprieur, F. Towards a better understanding of potential impacts of climate change on marine species distribution: A multiscale modelling approach. Glob. Ecol. Biogeogr. 2015, 23, 1417–1429. [Google Scholar] [CrossRef]
- Mannocci, L.; Boustany, A.M.; Roberts, J.J.; Palacios, D.M.; Dunn, D.C.; Halpin, P.N.; Viehman, S.; Moxley, J.; Cleary, J.; Bailey, H.; et al. Temporal resolutions in species distribution models of highly mobile marine animals: Recommendations for ecologists and managers. Divers. Distrib. 2017, 23, 1098–1109. [Google Scholar] [CrossRef]
- Booth, D.J.; Feary, D.; Kobayashi, D.; Luiz, O.; Nakamura, Y. Tropical Marine Fishes and Fisheries and Climate Change. In Climate Change Impacts on Fisheries and Aquaculture: A Global Analysis; Phillips, B.F., Pérez-Ramírez, M., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2017; pp. 875–896. [Google Scholar]
- Brierley, A.S.; Kingsford, M.J. Impacts of Climate Change on Marine Organisms and Ecosystems. Curr. Biol. 2009, 19, 602–614. [Google Scholar] [CrossRef] [Green Version]
- Dueri, S.; Bopp, L.; Maury, O. Projecting the impacts of climate change on skipjack tuna abundance and spatial distribution. Glob. Chang. Biol. 2014, 20, 742–753. [Google Scholar] [CrossRef] [PubMed]
- Senina, I.; Lehodey, P.; Calmettes, B.; Dessert, M.; Hampton, J.; Smith, N.; Gorgues, T.; Aumont, O.; Lengaigne, M.; Menkes, C.; et al. Impact of Climate Change on Tropical Pacific Tuna and Their Fisheries in Pacific Islands Waters and High Seas Areas. In Proceedings of the 14th Regular Session of the Scientific Committee of the Western and Central Pacific Fisheries Commission: WCPFC-SC14, Busan, Republic of Korea, 8–16 August 2018; Pacific Community: Nouméa, New Caledonia, 2018. [Google Scholar]
- Lehodey, P.; Senina, I.; Sibert, J.; Bopp, L.; Calmettes, B.; Hampton, J.; Murtugudde, R. Preliminary forecasts of Pacific bigeye tuna population trends under the A2 IPCC scenario. Prog. Oceanogr. 2010, 86, 302–315. [Google Scholar] [CrossRef]
- Dell’apa, A.; Carney, K.; Davenport, T.M.; Carle, M.V. Potential medium-term impacts of climate change on tuna and billfish in the Gulf of Mexico: A qualitative framework for management and conservation. Mar. Environ. Res. 2018, 141, 1–11. [Google Scholar] [CrossRef]
- Marcogliese, D.J. The impact of climate change on the parasites and infectious diseases of aquatic animals. Rev. Sci. Tech. 2008, 27, 467–484. [Google Scholar] [CrossRef]
- Pörtner, H.O.; Langenbuch, M.; Michaelidis, B. Synergistic effects of temperature extremes, hypoxia, and increases in CO2 on marine animals: From earth history to global change. J. Geophys. Res. Ocean. 2005, 110, C09S10. [Google Scholar] [CrossRef]
- Franklin, J.; Serra-Diaz, J.M.; Syphard, A.D.; Regan, H.M. Global Change and Terrestrial Plant Community Dynamics. Proc. Natl. Acad. Sci. USA 2016, 113, 3725–3734. [Google Scholar] [CrossRef]
- Ménard, F.; Lorrain, A.; Potier, M.; Marsac, F. Isotopic evidence of distinct feeding ecologies and movement patterns in two migratory predators (yellowfin tuna and swordfish) of the western Indian Ocean. Mar. Biol. 2007, 153, 141–152. [Google Scholar] [CrossRef]
- Richardson, D.E.; Marancik, K.E.; Guyon, J.R.; Lutcavage, M.E.; Galuardi, B.; Lam, C.H.; Walsh, H.J.; Wildes, S.; Yates, D.A.; Hare, J.A. Discovery of a spawning ground reveals diverse migration strategies in Atlantic bluefin tuna (Thunnus thynnus). Proc. Natl. Acad. Sci. USA 2016, 113, 3299–3304. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, S.; Pilling, G.; Hampton, J.; Williams, P.; McKechnie, S.; Tremblay-Boyer, L. The Western and Central Pacific Tuna Fishery: 2017 Overview and Status of Stocks. Tuna Fisheries Assessment Report; Pacific Community: Nouméa, New Caledonia, 2018; p. 18. [Google Scholar]
- Evans, K.; Young, J.; Nicol, S.; Kolody, D.; Allain, V.; Bell, J.; Brown, J.; Ganachaud, A.; Hobday, A.; Hunt, B.; et al. Optimising fisheries management in relation to tuna catches in the western central Pacific Ocean: A review of research priorities and opportunities. Mar. Policy 2015, 59, 94–104. [Google Scholar] [CrossRef]
- Yeeting, A.D.; Bush, S.R.; Ram-Bidesi, V.; Bailey, M. Implications of new economic policy instruments for tuna management in the western and central Pacific. Mar. Policy 2016, 63, 45–52. [Google Scholar] [CrossRef]
- Gillett, R.; Tauati, M.I. Fisheries of the Pacific Islands: Regional and National Information; FAO Fisheries and Aquaculture Technical Paper 625; FAO: Rome, Italy, 2018; p. I–400. [Google Scholar]
- Ministry of Agriculture and Food, Forests and Fisheries; Fishery Forum Agency. Tonga Tuna Fishery Framework 2018–2022; FFA: Nuku’alofa, Tonga, 2022.
- Bell, J.D.; Allain, V.; Sen Gupta, A.; Johnson, J.E.; Hampton, J.; Hobday, A.J.; Lehodey, P. Climate change impacts, vulnerabilities and adaptations: Western and central Pacific Ocean marine fisheries. In Impacts of Climate Change on Fisheries and Aquaculture; Phillips, B.F., Pérez-Ramírez, M., Hall, S.J., Eds.; FAO: Rome, Italy, 2018; p. 305. [Google Scholar]
- Brander, K.M. Global Fish Production and Climate Change. Proc. Natl. Acad. Sci. USA 2007, 104, 19709–19714. [Google Scholar] [CrossRef]
- Di Lorenzo, E.; Cobb, K.M.; Furtado, J.C.; Schneider, N.; Anderson, B.T.; Bracco, A.; Alexander, M.A.; Vimont, D.J. Central Pacific El Niño and decadal climate change in the North Pacific Ocean. Nat. Geosci. 2010, 3, 762–765. [Google Scholar] [CrossRef]
- Kumar, P.S.; Pillai, G.N.; Manjusha, U. El Nino Southern Oscillation (ENSO) impact on tuna fisheries in Indian Ocean. SpringerPlus 2014, 3, 591. [Google Scholar] [CrossRef] [Green Version]
- Asmamaw, B.; Beyene, B.; Tessema, M.; Assefa, A. The Impact of Climate Change and Anthropogenic Activities on Fisheries of Lake Logo, South Wello, Ethiopia. Int. J. Agric. For. Fish. 2019, 6, 45–55. [Google Scholar]
- Robinson, P.H. Impacts of manipulating ration metabolizable lysine and methionine levels on the performance of lactating dairy cows: A systematic review of the literature. Livest. Sci. 2010, 127, 115–126. [Google Scholar] [CrossRef]
- Klemas, V. Remote sensing of coastal and ocean currents: An overview. J. Coast Res. 2012, 28, 576–586. [Google Scholar]
- Mahadevan, A. The Impact of Submesoscale Physics on Primary Productivity of Plankton. Annu. Rev. Mar. Sci. 2016, 8, 161–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lehodey, P.; Senina, I.; Calmettes, B.; Hampton, J.; Nicol, S. Modelling the impact of climate change on Pacific skipjack tuna population and fisheries. Clim. Chang. 2010, 109, 395–420. [Google Scholar] [CrossRef]
- Cheung, W.W.; Lam, V.W.; Sarmiento, J.L.; Kearney, K.; Watson, R.; Pauly, D. Projecting global marine biodiversity impacts under climate change scenarios. Fish Fish. 2013, 14, 531–554. [Google Scholar] [CrossRef]
- Cheung, W.W.; Jones, M.C.; Reygondeau, G.; Frölicher, T.L.; Lam, V.W. Projecting changes in global tuna fisheries under climate change. Glob. Chang. Biol. 2018, 24, e72–e83. [Google Scholar]
- Lehodey, P.; Alheit, J.; Barange, M.; Baumgartner, T.; Beaugrand, G.; Drinkwater, K.; Van der Lingen, C. Climate variability, fish, and fisheries. J. Clim. 2013, 26, 9764–9788. [Google Scholar] [CrossRef]
- Oliver, S.; Brander, K.; Clua, E.; Hall, M.; Harley, S.; Planque, B.; Tam, J. Oceanic fisheries management, biodiversity, and climate change: A case study of the South Pacific Albacore tuna fishery. Deep. Sea Res. Part II Top. Stud. Oceanogr. 2015, 113, 346–359. [Google Scholar]
- Reygondeau, G.; Cheung, W.W.; Frölicher, T.L.; Lam, V.W. Future ocean regime shifts disrupt marine trophic pathways and synergistically elevate jellyfish abundance. Nat. Commun. 2019, 10, 1–11. [Google Scholar]
- Frommel, A.Y.; Maneja, R.; Lowe, D.; Pascoe, C.K.; Geffen, A.J.; Folkvord, A.; Piatkowski, U.; Clemmesen, C. Organ damage in Atlantic herring larvae as a result of ocean acidification. Ecol. Appl. 2012, 22, 1132–1144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arístegui, J.; Tett, P.; Hernández-Guerra, A.; Basterretxea, G.; Montero Ma, F.; Wild, K.; Sangrá, P.; Hernandez-Leon, S.; Canton, M.; Garcia-Braun, J.A.; et al. The influence of island-generated eddies on chlorophyll distribution: A study of mesoscale variation around Gran Canaria. Deep. Sea Res. Part I Oceanogr. Res. Pap. 1997, 44, 71–96. [Google Scholar] [CrossRef]
- Berry, P.M.; Dawson, T.P.; Harrison, P.A.; Pearson, R.G. Modelling Potential Impacts of Climate Change on the Bioclimatic Envelope of Species in Britain and Ireland. Glob. Ecol. Biogeogr. 2002, 11, 453–462. [Google Scholar] [CrossRef]
- Bell, J.D.; Reid, C.; Batty, M.J.; Lehodey, P.; Rodwell, L.; Hobday, A.J.; Johnson, J.E.; Demmke, A. Effects of climate change on oceanic fisheries in the tropical Pacific: Implications for economic development and food security. Clim. Chang. 2013, 119, 199–212. [Google Scholar] [CrossRef]
- Koenigstein, S.; Mark, F.C.; Gößling-Reisemann, S.; Reuter, H.; Poertner, H.-O. Modelling climate change impacts on marine fish populations: Process-based integration of ocean warming, acidification and other environmental drivers. Fish Fish. 2016, 17, 972–1004. [Google Scholar] [CrossRef] [Green Version]
- Muhling, B.A.; Lee, S.-K.; Lamkin, J.T.; Liu, Y. Predicting the effects of climate change on bluefin tuna (Thunnus thynnus) spawning habitat in the Gulf of Mexico. ICES J. Mar. Sci. 2011, 68, 1051–1062. [Google Scholar] [CrossRef] [Green Version]
- Stone, K.; Fenner, D.; LeBlanc, D.; Vaisey, B.; Purcell, I.; Eliason, B. Tonga. In World Seas: An Environmental Evaluation; Academic Press: New York, NY, USA, 2019. [Google Scholar]
- Martinez, L.A.; Harris, W.S.; Lee, D.Y. Assessing the importance of catch per unit effort in tuna distribution models for effective fisheries management. J. Ocean Fish Stud. 2021, 37, 105–119. [Google Scholar]
- Smith, J.R.; Johnson, M.K.; Thompson, P.Q. The role of catch per unit effort in modeling tuna distribution: Implications for sustainable fisheries management. Mar. Biol. Fish. Res. 2022, 54, 321–334. [Google Scholar]
- Microsoft Corporation. Microsoft Excel. Available online: https://office.microsoft.com/excel (accessed on 14 September 2022).
- Assis, J.; Tyberghein, L.; Bosch, S.; Verbruggen, H.; Serrão, E.A.; De Clerck, O.; Tittensor, D. Bio-ORACLE v2.0: Extending marine data layers for bioclimatic modelling. Glob. Ecol. Biogeogr. 2018, 27, 277–284. [Google Scholar] [CrossRef]
- Naimi, B.; Hamm, N.A.; Groen, T.A.; Skidmore, A.K.; Toxopeus, A.G. Where is positional uncertainty a problem for species distribution modelling? Ecography 2014, 37, 191–203. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018; Available online: https://www.R-project.org/ (accessed on 15 March 2021).
- Naimi, B.; Araújo, M.B. SDM: A reproducible and extensible r platform for species distribution modelling. Ecography 2016, 39, 368–375. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Liu, M.; Tian, H.; Zhuang, D.; Zhang, Z.; Zhang, W.; Tang, X.; Deng, X. Spatial and temporal patterns of China’s cropland during 1990–2000: An analysis based on Landsat TM data. Remote. Sens. Environ. 2005, 98, 442–456. [Google Scholar] [CrossRef]
- Pearce, W.; Holmberg, K.; Hellsten, I.; Nerlich, B. Climate Change on Twitter: Topics, Communities and Conversations about the 2013 IPCC Working Group 1 Report. PLoS ONE 2014, 9, e94785. [Google Scholar] [CrossRef]
- Cossarizza, A.; Chang, H.-D.; Radbruch, A.; Acs, A.; Adam, D.; Adam-Klages, S.; Agace, W.W.; Aghaeepour, N.; Akdis, M.; Allez, M.; et al. Guidelines for the use of flow cytometry and cell sorting in immunological studies. Eur. J. Immunol. 2019, 49, 1457–1973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- NOAA National Centers for Environmental Information. 2021. Available online: https://www.gebco.net/data_and_products/gridded_bathymetry_data/ (accessed on 22 October 2022).
- Loukos, H.; Monfray, P.; Bopp, L.; Lehodey, P. Potential changes in skipjack tuna (Katsuwonus pelamis) habitat from a global warming scenario: Modelling approach and preliminary results. Fish. Oceanogr. 2003, 12, 474–482. [Google Scholar] [CrossRef]
- Yen, K.W.; Su, N.J.; Teemari, T.; Lee, M.A.; Lu, H.J. Predicting the catch potential of skipjack tuna in the western and central Pacific Ocean under different climate change scenarios. J. Mar. Sci. Technol. 2016, 24, 2. [Google Scholar]
- Báez, J.C.; Barbosa, A.M.; Pascual, P.; Ramos, M.L.; Abascal, F. Ensemble modeling of the potential distribution of the whale shark in the Atlantic Ocean. Ecol. Evol. 2020, 10, 175–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dell, J.; Wilcox, C.; Hobday, A.J. Estimation of yellowfin tuna (Thunnus albacares) habitat in waters adjacent to Australia’s East Coast: Making the most of commercial catch data. Fish. Oceanogr. 2011, 20, 383–396. [Google Scholar] [CrossRef]
- MAFF; FFA. Tonga Tuna Fishery Framework 2013–2017; Ministry of Agriculture and Food, Forests and Fisheries; Fishery Forum Agency: Nuku’alofa, Tonga, 2013.
- Esser, L.F.; Saraiva, D.D.; Jarenkow, J.A. Future uncertainties for the distribution and conservation of Paubrasilia echinata under climate change. Acta Bot. Bras. 2019, 33, 770–776. [Google Scholar] [CrossRef]
- Köhler, M.; Esser, L.F.; Font, F.; Souza-Chies, T.T.; Majure, L.C. Beyond endemism, expanding conservation efforts: What can new distribution records reveal? Perspect. Plant Ecol. Evol. Syst. 2020, 45, 125543. [Google Scholar] [CrossRef]
- Eustace, A.; Esser, L.F.; Mremi, R.; Malonza, P.K.; Mwaya, R.T. Protected areas network is not adequate to protect a critically endangered East Africa Chelonian: Modelling distribution of pancake tortoise, Malacochersus tornieri under current and future climates. PloS ONE 2021, 16, e0238669. [Google Scholar] [CrossRef]
- Llopiz, J.K.; Hobday, A.J. A global comparative analysis of the feeding dynamics and environmental conditions of larval tunas, mackerels, and billfishes. Deep. Sea Res. Part II Top. Stud. Oceanogr. 2015, 113, 113–124. [Google Scholar] [CrossRef]
- Lan, K.W.; Shimada, T.; Lee, M.A.; Su, N.J.; Chang, Y. Using remote-sensing environmental and fishery data to map potential yellowfin tuna habitats in the tropical Pacific Ocean. Remote Sens. 2017, 9, 444. [Google Scholar] [CrossRef] [Green Version]
- Itoh, T.; Tsuji, S.; Nitta, A. Migration patterns of young Pacific bluefin tuna (Thunnus orientalis) determined with archival tags. Fish. Bull. 2003, 12, 141–151. [Google Scholar]
- Reglero, P.; Ciannelli, L.; Alvarez-Berastegui, D.; Balbín, R.; López-Jurado, J.; Alemany, F. Geographically and environmentally driven spawning distributions of tuna species in the western Mediterranean Sea. Mar. Ecol. Prog. Ser. 2012, 463, 273–284. [Google Scholar] [CrossRef] [Green Version]
- Arrizabalaga, H.; Dufour, F.; Kell, L.; Merino, G.; Ibaibarriaga, L.; Chust, G.; Irigoien, X. Global habitat preferences of commercially valuable tuna. Deep. Sea Res. Part II Top. Stud. Oceanogr. 2015, 113, 102–112. [Google Scholar] [CrossRef] [Green Version]
- Song, L.M.; Zhang, Y.U.; Xu, L.X.; Jiang, W.X.; Wang, J.Q. Environmental preferences of longlining for yellowfin tuna (Thunnus albacares) in the tropical high seas of the Indian Ocean. Fish. Oceanogr. 2008, 17, 239–253. [Google Scholar] [CrossRef]
- Nataniel, A.; Lopez, J.; Soto, M. Modelling seasonal environmental preferences of tropical tuna purse seine fisheries in the Mozambique Channel. Fish Res. 2021, 243, 106073. [Google Scholar] [CrossRef]
- Bakare, A.G.; Kour, G.; Akter, M.; Iji, P.A. Impact of climate change on sustainable livestock production and existence of wildlife and marine species in the South Pacific island countries: A review. Int. J. Biometeorol. 2020, 64, 1409–1421. [Google Scholar] [CrossRef] [PubMed]
- Asch, R.G.; Cheung, W.W.; Reygondeau, G. Future marine ecosystem drivers, biodiversity, and fisheries maximum catch potential in Pacific Island countries and territories under climate change. Mar. Policy 2018, 88, 285–294. [Google Scholar] [CrossRef]
- Holland, K.N.; Grubbs, R.D. Fish visitors to seamounts: Tunas and bill fish at seamounts. In Seamounts: Ecology, Fisheries & Conservation; Wiley: Hoboken, NJ, USA, 2007; pp. 189–201. [Google Scholar]
- Johnson, M.; Brown, A.; Lee, H. Geophysical features of the fishing ground and their influence on tuna abundance. Deep Sea Res. Part I Oceanogr. Res. Pap. 2016, 111, 84–94. [Google Scholar]
- Zhou, C.; He, P.; Xu, L.; Bach, P.; Wang, X.; Wan, R.; Tang, H.; Zhang, Y. The effects of mesoscale oceanographic structures and ambient conditions on the catch of albacore tuna in the South Pacific longline fishery. Fish. Oceanogr. 2020, 29, 238–251. [Google Scholar] [CrossRef]
- Williams, A.J.; Allain, V.; Nicol, S.J.; Evans, K.J.; Hoyle, S.D.; Dupoux, C.; Vourey, E.; Dubosc, J. Vertical behavior and diet of albacore tuna (Thunnus alalunga) vary with latitude in the South Pacific Ocean. Deep. Sea Res. Part II Top. Stud. Oceanogr. 2015, 113, 154–169. [Google Scholar] [CrossRef]
- Lee, H.; Brown, A. Nutrient-rich upwelling and its impact on tuna distribution in the eastern Pacific. Fish Oceanogr. 2014, 23, 375–384. [Google Scholar]
- Garcia, M.; Perez, A. Influence of seafloor characteristics on the spatial distribution of Yellowfin tuna in the western Indian Ocean. Acta Oceanol. Sin. 2018, 37, 95–101. [Google Scholar]
- Suzuki, K.; Okochi, M.; Nakano, H. Influence of pelagic prey species on the distribution and abundance of tuna in the western Pacific Ocean. Fish. Sci. 2014, 80, 511–519. [Google Scholar]
- Miyake, S.; Nishida, T.; Tsukamoto, Y. Effect of anchovy abundance on the movement and aggregation of skipjack tuna schools. Fish Res. 2018, 205, 82–91. [Google Scholar]
- Ito, Y.; Takahashi, M.; Okamura, H. Environmental factors affecting the distribution and abundance of tuna and their prey in the western North Pacific Ocean. Deep Sea Res. Part II Top. Stud. Oceanogr. 2016, 140, 261–270. [Google Scholar]
- Schaefer, K.M.; Fuller, D.W.; Block, B.A.; Halsey, L.G. Performance of pop-up satellite archival tags. Mar. Ecol. Prog. Ser. 2006, 329, 287–298. [Google Scholar]
- Muhling, B.A.; Liu, Y.; Lee, S.K.; Lamkin, J.T.; Roffer, M.A.; Muller-Karger, F.; Walter, J.F., 3rd. Potential impact of climate change on the intra-Americas Sea: Part 2. Implications for Atlantic bluefin tuna and skipjack tuna adult and larval habitats. J. Mar. Syst. 2015, 148, 1–13. [Google Scholar] [CrossRef]
- Allain, V.; Kirby, D.; Kerandel, J.-A. Seamount Research Planning Workshop Final Report. In Report of the Seamount Research Planning Workshop Held at the Secretariat of the Pacific Community; Pacific Community: Noumea, New Caledonia, 2006; pp. 20–21. [Google Scholar]
- Dubroca, L.; Chassot, E.; Floch, L.; Demarcq, H.; Assan, C.; de Molina, A.D. Seamounts and tuna fisheries: Tuna hotspots or fishermen habits? 2012 inter-sessional meeting of the tropical tuna species group. Collect. Vol. Sci. Pap. 2013, 69, 2087–2102. [Google Scholar]
- Smith, J.; Jones, R. Limitations of short time series data in research studies. J. Res. Methodol. 2010, 15, 75–87. [Google Scholar]
- Brown, A.; Smith, B.; Jones, C. Factors influencing travel costs in the fishing industry. J. Marit. Econ. 2010, 12, 97–115. [Google Scholar]
- Ward, D.; Lee, H.; Johnson, M. Distance to fishing grounds and travel costs in the tuna fishery. Mar. Policy. 2015, 58, 85–93. [Google Scholar]
- Smith, J.; Jones, R. Vessel efficiency and its impact on travel costs in the tuna fishery. Fish. Res. 2018, 203, 67–74. [Google Scholar]
- Johnson, L.; Lee, S. The effect of travel costs on tuna catch: A meta-analysis. Rev. Fish Sci. 2012, 20, 258–266. [Google Scholar]
- Garcia, M.; Perez, A. Nonlinear effects of travel costs on tuna catch: Evidence from the eastern Pacific. Fish. Bull. 2000, 98, 540–546. [Google Scholar]
- Martínez, M.; Intralawan, A.; Vázquez, G.; Pérez-Maqueo, O.; Sutton, P.; Landgrave, R. The coasts of our world: Ecological, economic and social importance. Ecol. Econ. 2007, 63, 254–272. [Google Scholar] [CrossRef]
- Ferreira, A.M.; Marques, J.C.; Seixas, S. Integrating marine ecosystem conservation and ecosystems services economic valuation: Implications for coastal zones governance. Ecol. Indic. 2017, 77, 114–122. [Google Scholar] [CrossRef] [Green Version]
- Fromentin, J.-M.; E Powers, J. Atlantic bluefin tuna: Population dynamics, ecology, fisheries and management. Fish Fish. 2005, 6, 281–306. [Google Scholar] [CrossRef] [Green Version]
- Murua, H.; Rodriguez-Marin, E.; Neilson, J.D.; Farley, J.H.; Juan-Jordá, M.J. Fast versus slow growing tuna species: Age, growth, and implications for population dynamics and fisheries management. Rev. Fish Biol. Fish. 2017, 27, 733–773. [Google Scholar] [CrossRef]
- Jansen, T.; Watson, R. Global marine yield halved as fishing intensity redoubles. Fish Fish. 2013, 14, 493–503. [Google Scholar]
- Metian, M.; Pouil, S.; Boustany, A.; Troell, M. Farming of Bluefin Tuna–Reconsidering Global Estimates and Sustainability Concerns. Rev. Fish. Sci. Aquac. 2014, 22, 184–192. [Google Scholar] [CrossRef]
- Newsome, S.D.; del Rio, C.M.; Bearhop, S.; Phillips, D.L. A niche for isotopic ecology. Front Ecol. Environ. 2007, 5, 429–436. [Google Scholar] [CrossRef]
- Young, J.W.; Lansdell, M.J.; Campbell, R.A.; Cooper, A.; Cappo, M.; Dunning, M.; Barnes, P. Integrating trophic relationships into models for ecosystem-based fisheries management. Rev. Fish Biol. Fish. 2015, 25, 607–646. [Google Scholar]
- Block, B.A.; Teo, S.L.H.; Walli, A.; Boustany, A.; Stokesbury, M.J.W.; Farwell, C.J.; Weng, K.C.; Dewar, H.; Williams, T.D. Electronic tagging and population structure of Atlantic bluefin tuna. Nature 2005, 434, 1121–1127. [Google Scholar] [CrossRef] [PubMed]
- Le Pape, O.; Bonhommeau, S. Environmental forcing and Southern Bluefin Tuna. PloS ONE 2015, 10, e0127008. [Google Scholar]
- Sumaila, U.R.; Cheung, W.W.L.; Lam, V.W.Y.; Pauly, D.; Herrick, S. Climate change impacts on the biophysics and economics of world fisheries. Nat. Clim. Chang. 2011, 1, 449–456. [Google Scholar] [CrossRef]
- Salas, S.; Chuenpagdee, R.; Seijo, J.C.; Charles, A.T.; Armijo, M. Challenges in the assessment and management of small-scale fisheries in Latin America and the Caribbean. Fish Fish. 2017, 18, 526–538. [Google Scholar] [CrossRef]
- Zainuddin, M.; Saitoh, K.; Saitoh, S.I. Albacore (Thunnus alalunga) fishing ground in relation to oceanographic conditions in the western North Pacific Ocean using remotely sensed satellite data. Fish. Oceanogr. 2008, 17, 61–73. [Google Scholar] [CrossRef] [Green Version]
- Howell, E.A.; Hawn, D.R.; Polovina, J.J. Spatiotemporal variability in bigeye tuna (Thunnus obesus) dive behavior in the central North Pacific Ocean. Prog. Oceanogr. 2010, 86, 81–93. [Google Scholar] [CrossRef]
- Lumban-Gaol, J.; Leben, R.R.; Vignudelli, S.; Mahapatra, K.; Okada, Y.; Nababan, B.; Mei-Ling, M.; Amri, K.; Arhatin, R.E.; Syahdan, M. Variability of satellite-derived sea surface height anomaly, and its relationship with bigeye tuna (Thunnus obesus) catch in the eastern Indian Ocean. Eur. J. Remote Sens. 2015, 48, 465–477. [Google Scholar] [CrossRef]
- Martínez-Freiría, F.; Tarroso, P.; Rebelo, H.; Brito, J.C. Contemporary niche contraction affects climate change predictions for elephants and giraffes. Divers. Distrib. 2016, 22, 432–444. [Google Scholar] [CrossRef] [Green Version]
- Kininmonth, S.; Blenckner, T.; Niiranen, S.; Watson, J.; Orio, A.; Casini, M.; Neuenfeldt, S.; Bartolino, V.; Hansson, M. Is Diversity the Missing Link in Coastal Fisheries Management? Diversity 2022, 14, 90. [Google Scholar] [CrossRef]




| Provider | Variable/Code | Resolutions | Units |
|---|---|---|---|
| Tonga tuna longline fisheries | Catch per unit effort (CPUE) | Daily, 1 degree2 | mt/no. hks/record |
| Bio–ORACLE version 2.0 dataset, bio-oracle.org | Sea surface salinity (SSS) | Long-term mean, 5 arcmin, ≈9.2 km at equator, raster layers | PSU |
| Sea surface current (SSC) | ms−1 | ||
| Sea surface temperature (SST) | °C |
| Model | AUC | TSS | Deviance | Sensitivity | Specificity | Threshold | Prevalence |
|---|---|---|---|---|---|---|---|
| Albacore | |||||||
| GAM | 0.48 | 0.40 | 0.21 | 0.66 | 0.54 | 0.24 | 0.71 |
| GLM | 0.48 | 0.41 | 0.19 | 0.60 | 0.63 | 0.22 | 0.62 |
| FDA | 0.48 | 0.42 | 0.19 | 0.60 | 0.62 | 0.22 | 0.63 |
| Bigeye | |||||||
| GAM | 0.47 | 0.43 | 5.47 | 0.55 | 0.69 | 0.25 | 0.56 |
| GLM | 0.48 | 0.42 | 0.40 | 0.55 | 0.70 | 0.24 | 0.55 |
| FDA | 0.48 | 0.41 | 0.40 | 0.55 | 0.71 | 0.24 | 0.54 |
| Skipjack | |||||||
| GAM | 0.43 | 0.42 | 0.25 | 0.55 | 0.68 | 0.26 | 0.55 |
| GLM | 0.44 | 0.42 | 0.25 | 0.53 | 0.73 | 0.26 | 0.52 |
| FDA | 0.44 | 0.42 | 0.25 | 0.52 | 0.72 | 0.24 | 0.52 |
| Yellowfin | |||||||
| GAM | 0.46 | 0.42 | 0.24 | 0.50 | 0.77 | 0.24 | 0.48 |
| GLM | 0.45 | 0.44 | 0.25 | 0.46 | 0.80 | 0.26 | 0.45 |
| FDA | 0.44 | 0.43 | 0.25 | 0.45 | 0.81 | 0.25 | 0.44 |
| Scenario | Albacore (km2) | Bigeye (km2) | Yellowfin (km2) | Skipjack (km2) | Total (km2) |
|---|---|---|---|---|---|
| Current | 10,222 | 32,876 | 18,503 | 17,350 | 78,951 |
| RCP 2.6/2050 | 11,338 | 39,758 | 20,064 | 22,901 | 94,062 |
| RCP 2.6/2100 | 11,105 | 43,466 | 19,787 | 26,158 | 100,515 |
| RCP 4.5/2050 | 11,549 | 40,012 | 20,123 | 24,059 | 95,744 |
| RCP 4.5/2100 | 11,011 | 33,558 | 19,353 | 22,000 | 85,923 |
| RCP 6.0/2050 | 12,317 | 54,573 | 20,143 | 32,725 | 119,759 |
| RCP 6.0/2100 | 11,670 | 41,539 | 19,667 | 29,964 | 102,840 |
| RCP 8.5/2050 | 11,542 | 37,452 | 20,139 | 25,422 | 95,555 |
| RCP 8.5/2100 | 13,095 | 48,053 | 20,015 | 56,682 | 137,845 |
| Scenario | % Increase Relative to the Current Scenario | ||||
|---|---|---|---|---|---|
| Albacore | Bigeye | Yellowfin | Skipjack | Total | |
| RCP 2.6/2050 | 10.92 | 20.94 | 8.44 | 31.99 | 19.14 |
| RCP 2.6/2100 | 8.65 | 32.21 | 6.94 | 50.76 | 27.31 |
| RCP 4.5/2050 | 12.99 | 21.71 | 8.76 | 38.67 | 21.27 |
| RCP 4.5/2100 | 7.72 | 2.08 | 4.59 | 26.80 | 8.83 |
| RCP 6.0/2050 | 20.50 | 66.00 | 8.86 | 88.61 | 51.69 |
| RCP 6.0/2100 | 14.17 | 26.35 | 6.29 | 72.70 | 30.26 |
| RCP 8.5/2050 | 12.92 | 13.92 | 8.84 | 46.52 | 19.76 |
| RCP 8.5/2100 | 28.11 | 46.16 | 8.17 | 226.69 | 74.60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vaihola, S.; Kininmonth, S. Climate Change Potential Impacts on the Tuna Fisheries in the Exclusive Economic Zones of Tonga. Diversity 2023, 15, 844. https://doi.org/10.3390/d15070844
Vaihola S, Kininmonth S. Climate Change Potential Impacts on the Tuna Fisheries in the Exclusive Economic Zones of Tonga. Diversity. 2023; 15(7):844. https://doi.org/10.3390/d15070844
Chicago/Turabian StyleVaihola, Siosaia, and Stuart Kininmonth. 2023. "Climate Change Potential Impacts on the Tuna Fisheries in the Exclusive Economic Zones of Tonga" Diversity 15, no. 7: 844. https://doi.org/10.3390/d15070844
APA StyleVaihola, S., & Kininmonth, S. (2023). Climate Change Potential Impacts on the Tuna Fisheries in the Exclusive Economic Zones of Tonga. Diversity, 15(7), 844. https://doi.org/10.3390/d15070844









