1. Introduction
The Australian monsoonal (seasonal) tropics is not formally recognized as a region of high ant diversity [
1], but there is increasing evidence that it in fact has the world’s richest fauna, likely numbering several thousand species, the great majority of which is undescribed [
2]. One reason why the fauna is so poorly known taxonomically is that sampling has historically been very limited because the region is so remote and sparsely populated, such that relevant material has not been available for taxonomic attention [
3,
4]. However, even when groups have received taxonomic attention it has been extremely cursory, leading to the gross misrepresentation of true diversity. For example, in a relatively recent revision of the ecologically dominant genus
Iridomyrmex Mayr,
I. anceps (Roger) was presented as a single species occurring throughout northern Australia and extending through Southeast Asia to southern China [
5], whereas a subsequent analysis showed that the taxon represents more than a dozen species in Australia alone [
6]. Similarly,
Melophorus rufoniger Heterick, Castalanelli & Shattuck was recently described as a single species occurring throughout mainland Australia when actually it represents dozens of species just in the monsoonal tropics [
7]. Even more remarkably, the similarly widespread
Monomorium ‘
fieldi’ Forel contains well over 100 and possibly 200 species in the region [
8].
Melophorus hirsutipes Heterick, Castalanelli & Shattuck is another recently described ‘species’ that was presented as being highly variable morphologically and occurring throughout most of mainland Australia [
9]. The variable morphology includes eye size, the shape of the head, mesosoma and petiole, and sculpture (
Figure 1). For example, in the type series collected from Broken Hill in southwestern New South Wales, the integument of the head in minor workers is smooth and shiny, whereas in other forms it ranges from shagreenate (
Figure 1d) to scabrid (
Figure 1e) and even rugulose (
Figure 1f). It seems inexplicable that such variation was considered conspecific given that there is no precedent for it in ant taxonomy. The scabrid and rugulose forms occur primarily in the monsoonal north of the continent, and in earlier analyses of the monsoonal ant fauna they were presented as representing a diverse group of species (Group E in [
10,
11]).
Here, we provide an integrated morphological, genetic (CO1) and distributional analysis of diversity within the scabrid and rugulose forms of M. ‘hirsutipes’. Our analysis confirms that such forms do indeed represent a highly diverse group of species.
2. Materials and Methods
Our study is based on pinned specimens of
M. ‘
hirsutipes’ in the ant collection held at the CSIRO laboratory in Darwin (subsequently referred to as the Darwin collection), which holds by far the largest and most comprehensive array of specimens of the taxon. We obtained CO1 sequences from a total of 63 specimens, comprising 56 scabrid and rugulose forms representing their full range of morphological variation and geographic distribution, along with the shiny and shagreenate specimens illustrated in
Figure 1 and four additional shagreenate specimens from the central arid zone (
Supplementary Table S1;
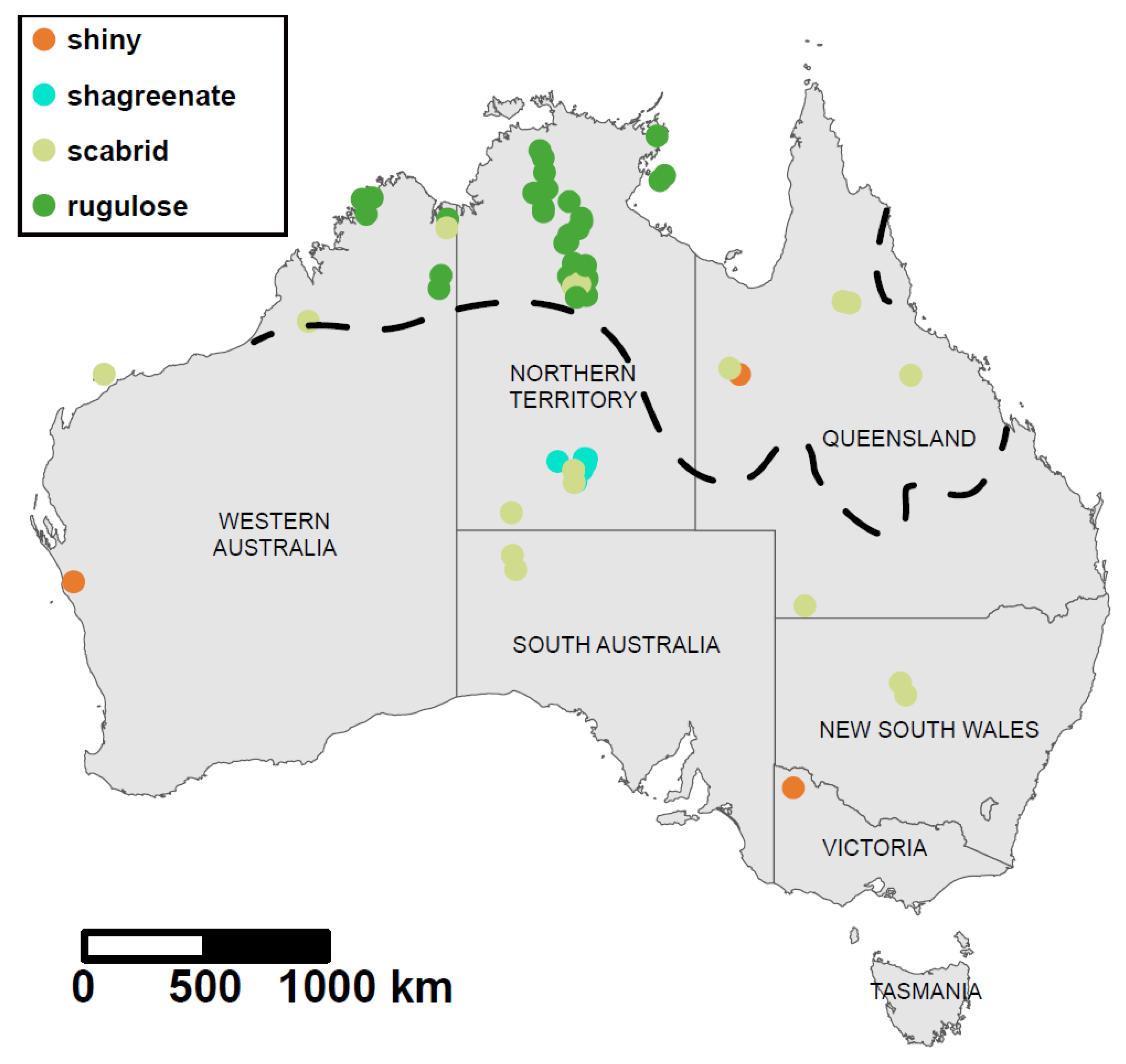
Figure 2). Rugulose specimens are restricted to the western half of the monsoonal zone, whereas scabrid specimens occur throughout the region and extend into southern Australia. We note that geographic coverage of sequenced specimens is extremely patchy, being heavily concentrated in the northern half of Northern Territory (NT) and with relatively few records in the eastern half of the monsoonal zone (
Figure 2). There are only single records from southern Western Australia (WA) and southern Queensland. We also obtained CO1 sequences from three specimens with a shiny integument from widely separated locations (southern WA (
Figure 1a), northwestern Queensland (
Figure 1b), and northwestern Victoria (
Figure 1c)), along with from five specimens from central Australia with shagreenate heads (
Figure 1d).
DNA extraction (from foreleg tissue) and CO1 sequencing were conducted through the Barcode of Life Data (BOLD) System (for extraction details, see
http://ccdb.ca/resources (accessed on 27 July 2023)). Each sequenced specimen was assigned a unique identification code that combines the batch within which it was processed, its number within the batch, and the year of sequencing (e.g., OZBOL1684-21 for the shiny specimen from southern WA (
Figure 1a)). All specimens are sorted into the species documented here and labelled with their respective BOLD identification numbers in the Darwin collection. We checked and edited the DNA sequences in MEGA [
12], which were then aligned using UPGMB clustering in MUSCLE [
13] and translated into (invertebrate) proteins to check for stop codons and nuclear paralogues. Aligned sequences were trimmed accordingly, resulting in 798 base pairs. We used MUSCLE to construct a maximum likelihood tree, using a specimen of the
Melophorus rufoniger group (
Table S1) as the outgroup. We then used FigTree to produce our final figure.
We delimited species based on the integration of CO1 clustering and distance (CO1 divergence is typically 1–3% within a species [
14]), morphological variation and geographic distribution [
15]. We followed a species concept based on reproductive isolation and evolutionary independence, as evidenced by morphological differentiation between sister (most closely related) clades and, when recorded, sympatric distribution. We assigned letter codes (sp. A, etc.) to our recognized species among scabrid and rugulose forms. We imaged a representative specimen of each recognized species using a Leica DMC5400 camera mounted on a Leica M205C dissecting microscope. We took image montages using the Leica Application suite v. 4.13 and stacked them in Zerene stacker.
3. Results
The three shiny specimens are widely divergent genetically and are polyphyletic in the CO1 tree (
Figure 3). They are clearly different species. The shiny species from north-western Queensland (
Figure 1b) is highly divergent genetically from all other sequenced specimens (
Figure 3). Compared with the other shiny species (
Figure 1a,c), it has much larger eyes, a narrower head with a far more convex occipital margin, and a shorter propodeum. The two other shiny specimens sequenced are embedded within CO1 clades of scabrid/rugulose species. The shagreenate specimens form a distinct clade and CO1 clustering is indicative of two species (
Figure 3).
Based on the morphological differentiation of sister CO1 clades, we recognize 16 species (spp. 1–16) among the 56 scabrid/rugulose specimens sequenced. Fourteen of these are clustered within three major clades (clades 1–3 in
Figure 3). These clades have relatively low (<0.65) support values, with scabrid and rugulose species interspersed in each case, and have no obvious morphological characters differentiating them. Clade 1 comprises six species (spp. A–F;
Figure 1e,f and
Figure 4a–d) that have a primarily eastern distribution—in the NT, Queensland and New South Wales (
Figure 5a). Species A has rugulose sculpture (
Figure 1f) and is known only from the NT, where it is widely distributed (
Figure 5a). It is sister to sp. B in the CO1 tree, which has a scabrid head and relatively shiny mesosoma (
Figure 4a). The two species are sympatric in the NT, but the latter is known only from the southern (low rainfall) region of the monsoonal zone and occurs also in WA (
Figure 5a). Species C-F form a separate subclade within clade 1. Both sp. C (
Figure 4b) and sp. E (
Figure 1e) have scabrid sculpture, but the latter is more closely related to rugulose sp. D and sp. F. Compared with sp. D (
Figure 4c), sp. F (
Figure 4d) is more coarsely sculptured, has more protruding eyes and a flattened rather than rounded petiolar dorsum. Species F is sympatric with spp. A and B, all occurring on Powell Creek Station in the NT (
Figure 5a;
Table S1).
Clade 2 also comprises six species (spp. I–N;
Figure 6), but all are known only from northernWA and mostly from the far northern Kimberley region (
Figure 5c). They are all clearly differentiated morphologically; in addition to sculpture, they show marked variation in the occipital margin (strongly convex in spp. J and L, and flattened in the others), eye morphology (protuberant in spp. J, L, and N, not so in spp. I, K, and M), and shape of the petiolar node (in profile, tapering dorsally in spp. J and M, and broadly rounded dorsally in the others) (
Figure 5). Clade 3 includes one rugulose species (sp. O) from the NT, and one scabrid species (sp. P) also from the NT but extending into South Australia (
Figure 5b and
Figure 7); it also includes the shiny species from northwestern Victoria. The two scabrid species from outside the three major clades are from Queensland (sp. G;
Figure 4e and
Figure 5b) and the NT (sp. H;
Figure 4f and
Figure 5b).
4. Discussion
The scabrid and rugulose forms of
Melophorus ‘
hirsutipes’ are clearly not conspecific with the shiny forms and indeed represent a diverse array of species themselves, as previously recognized [
10,
11]. We recognize 16 scabrid/rugulose species among our sequenced specimens, all differentiated both morphologically and genetically. Notably, scabrid and rugulose species are interspersed throughout the CO1 tree and are often sister taxa. Our three primary CO1 clades have relatively low support values and so are not reliably robust phylogenetically, but our common finding of sister scabrid and rugolose species indicates that sculptural coarseness is highly labile from an evolutionary perspective. There was very considerable geographic structure among scabrid/rugulose species in our CO1 tree. The six species in Clade 3 are all known only from northern Western Australia, and the two species in Clade 2 have a central distribution (the Northern Territory and northern South Australia). The six species in Clade 3 have a wide distribution across northern and eastern Australia, but four are known only from the Northern Territory. Overall, there is high species turnover across the monsoonal zone, both from west to east and from north to south.
The shiny specimen sequenced from northwestern Queensland (OZBOL4511-21;
Figure 1b) was so genetically divergent from other sequenced specimens, as well as being morphologically distinctive, that it likely does not belong to the
M. hirsutipes group. It possibly fits under what was described in [
9] as a ‘
pillipes’ form of a different species,
M. turneri Forel.
Melophorus pillipes Santschi was inexplicably synonymized with
M. turneri despite the latter lacking its characteristic clothing of long, silky hairs [
9]. It has previously been described as representing a diverse group of species (the
M.
pillipes group in [
10,
11]). In contrast to specimen OZBOL4511-21, the two other shiny specimens sequenced are embedded within CO1 clades of scabrid/rugulose species and therefore appear to be genuine members of the
M. hirsutipes group. The shagreenate specimens sequenced, apparently representing two species, form a separate clade, but this is sister to clade 1 of scabrid/rugulose species and so can also be considered as belonging to the
M. hirsutipes group.
We have recognized 16 species among the scabrid/rugulose species sequenced, along with four shiny/shagreenate species. Given the highly patchy sampling within their range, and the high rates of species turnover across both latitude and longitude, the total number is likely far higher.
Melophorus ‘
hirsutipes’ is clearly a highly diverse group, probably comprising at least 30 species, and possibly many more. This does not include the ‘forms’ of
Melophorus ‘
hirsutipes’ that lack long, whorled hairs on the legs and antennal scapes [
9]. Such forms do not belong to the
M. hirsutipes group and explain why genetic data indicate that
Melophorus ‘
hirsutipes’, as described, is polyphyletic [
9].
The actual number of species in the
M. hirsutipes group as reported here is several times that of earlier estimates (as
Melophorus Group E in [
10,
11], including the 8 as part of an estimate of 1500 species for the total size of the Australian monsoonal ant fauna [
10]). This is a very substantial increase, but not as high as for other taxa recently examined, such as the
Melophorus rufoniger [
7],
Monomorium nigrius [
8], and
Tetramorium spininode [
4] species groups, where the increase is an order of magnitude.
In conclusion, our findings reinforce previous assessments that the great majority of ant species in monsoonal Australia are undescribed, such that the formal recognition of ant diversity in the region [
1] provides a highly misleading picture of its global significance. The total number of species is very likely in the several thousands, which would make monsoonal Australia the world’s richest known region for ant species.