Geometric Morphometric Analysis of Genus Chaetocnema (Coleoptera: Chrysomelidae: Alticini) with Insights on Its Subgenera Classification and Morphological Diversity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Taxa Examined
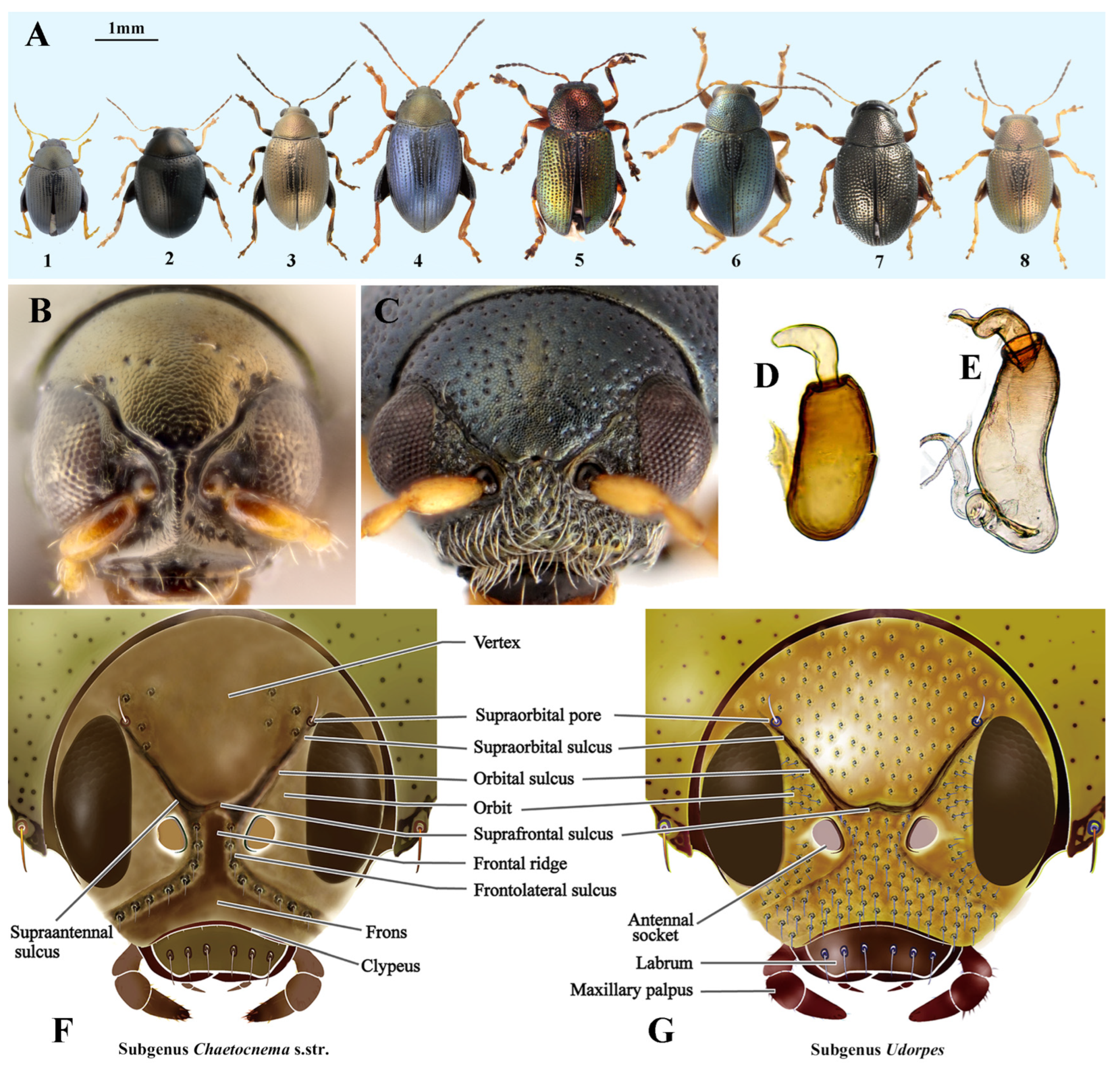
2.2. Morphological Delimitation of the Two Subgenera of Chaetocnema
2.3. Data Analysis
3. Results
3.1. Morphological Differences between Subgenera Chaetocnema s. str. and Udorpes
3.2. Morphological Diversity of Different Characters in Chaetocnema
3.3. Morphological Differences of Oriental, Palearctic, and Nearctic Chaetocnema Species
4. Discussion
4.1. The Subgenera Arrangement and Morphological Diversity of Chaetocnema
4.2. Geometric Morphometrics in the Classification of Higher Taxa
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schlick-Steiner, B.C.; Steiner, F.M.; Seifert, B.; Stauffer, C.; Christian, E.; Crozier, R.H. Integrative taxonomy: A multisource approach to exploring biodiversity. Annu. Rev. Entomol. 2010, 55, 421–438. [Google Scholar] [CrossRef]
- Ibañez, A.L.; Cowx, I.G.; O’Higgins, P. Geometric morphometric analysis of fish scales for identifying genera, species, and local populations within the Mugilidae. Can. J. Fish. Aquat. Sci. 2007, 64, 1091–1100. [Google Scholar] [CrossRef] [Green Version]
- Bookstein, F.L. Morphometric Tools for Landmark Data: Geometry and Biology; Cambridge University Press: Cambridge, UK, 1991; p. 435. [Google Scholar]
- Rohlf, F.J.; Marcus, L.F. A revolution in morphometrics. Trends Ecol. Evol. 1993, 8, 129–132. [Google Scholar] [CrossRef]
- Adam, D.C.; Slice, D.E.; Rohlf, F.J. Geometric morphometrics: Ten years of progress following the ‘revolution’. Ital. J. Zool. 2004, 71, 5–16. [Google Scholar] [CrossRef] [Green Version]
- Zelditch, M.L.; Swiderski, D.L.; Sheets, H.D.; Fink, W.L. Geometric Morphometrics for Biologists: A Primer; Elsevier: San Diego, CA, USA, 2004; p. 428. [Google Scholar]
- Bai, M.; Beutel, R.G.; Liu, W.; Li, S.; Zhang, M.; Lu, Y.; Song, K.; Ren, D.; Yang, X. Description of a new species of Glaresidae (Coleoptera: Scarabaeoidea) from the Jehol Biota of China with a geometric morphometric evaluation. Arthropod Syst. Phylogeny 2014, 72, 223–236. [Google Scholar] [CrossRef]
- Bastir, M.; García-Martínez, D.; Torres-Tamayo, N.; Palancar, C.A.; Beyer, B.; Barash, A.; Villa, C.; Sanchis-Gimeno, J.A.; Riesco-López, A.; Nalla, S.; et al. Rib cage anatomy in Homo erectus suggests a recent evolutionary origin of modern human body shape. Nat. Ecol. Evol. 2020, 4, 1178–1187. [Google Scholar] [CrossRef] [PubMed]
- Leaché, A.D.; Koo, M.S.; Spencer, C.L.; Papenfuss, T.J.; Fisher, R.N.; McGuire, J.A. Quantifying ecological, morphological, and genetic variation to delimit species in the coast horned lizard species complex (Phrynosoma). Proc. Natl. Acad. Sci. USA 2009, 106, 12418–12423. [Google Scholar] [CrossRef] [PubMed]
- Ruane, S. Using geometric morphometrics for integrative taxonomy: An examination of head shapes of milksnakes (genus Lampropeltis). Zool. J. Linn. Soc. 2015, 174, 394–413. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Kubáň, V.; Volkovitsh, M.; Ge, S.; Bai, M.; Yang, X. Morphological variability and taxonomy of Coraebus hastanus Gory & Laporte de Castelnau, 1839 (Coleoptera: Buprestidae: Agrilinae: Coraebini: Coraebina). Zootaxa 2013, 3682, 178–190. [Google Scholar] [CrossRef] [Green Version]
- Zúñiga-Reinoso, A.; Benitez, H.A. The overrated use of the morphological cryptic species concept: An example with Nyctelia dark beetles (Coleoptera: Tenebrionidae) using geometric morphometrics. Zool. Anz. 2015, 255, 47–53. [Google Scholar] [CrossRef]
- Li, S.; Ricchiardi, E.; Bai, M.; Yang, X. A taxonomy review of Oreoderus Burmeister, 1842 from China with a geometric morphometric evaluation (Coleoptera, Scarabaeidae, Valgini). ZooKeys 2016, 552, 67–89. [Google Scholar] [CrossRef]
- Villemant, C.; Simbolotti, G.; Kenis, M. Discrimination of Eubazus (Hymenoptera, Braconidae) sibling species using geometric morphometrics analysis of wing venation. Syst. Biol. 2007, 32, 625–634. [Google Scholar] [CrossRef]
- Konstantinov, A.S.; Baselga, A.; Grebennikov, V.V.; Prena, J.; Lingalfelter, S.W. Revision of the Palearctic Chaetocnema Species. (Coleoptera: Chrysomelidae: Galerucinae: Alticini); Pensoft: Sofia, Bulgaria, 2011; p. 363. [Google Scholar]
- Ruan, Y.; Yang, X.; Konstantinov, A.S.; Prathapan, K.D.; Zhang, M. Revision of the Oriental Chaetocnema species (Coleoptera, Chrysomelidae, Galerucinae, Alticini). In Zootaxa; Magnolia Press: Auckland, New Zealand, 2019; Volume 4699, pp. 1–206. [Google Scholar] [CrossRef]
- Biondi, M. Revision of the species of Chaetocnema from Madagascar (Coleoptera: Chrysomelidae: Alticinae). Eur. J. Entomol. 2001, 98, 233–248. [Google Scholar] [CrossRef] [Green Version]
- Biondi, M.; D’Alessandro, P. Biogeographical analysis of the flea beetle genus Chaetocnema in the Afrotropical region: Distribution patterns and areas of endemism. J. Biogeogr. 2006, 33, 720–730. [Google Scholar] [CrossRef]
- White, R.E. A revision of the genus Chaetocnema of America north of Mexico (Coleoptera: Chrysomelidae). In Contributions of the American Entomological Institute; The American Entomological Institute: Logan, UT, USA, 1996; Volume 29, pp. 1–158. [Google Scholar]
- Stephens, J. Illustrations of British Entomology; or, a Synopsis of Indigenous Insects: Containing Their Generic and Specific Distinctions; with an Account of Their Metamorphoses, Times of Appearance, Localities, Food, and Economy, as far as Practicable. Mandibulata. 4 & 5; Baldwin & Cradock: London, UK, 1831; p. 414. [Google Scholar]
- Motschulsky, V. Remarques sur la collection du Coléoptères Russes. In Bulletin de la Societe Imperiale des Naturalistes de Moscou; Natural History Museum Library: London, UK, 1845; Volume 18, pp. 1–127. [Google Scholar]
- Maulik, S. Coleoptera. Chrysomelidae (Chrysomelinae and Halticinae). In The Fauna of British India Including Ceylon and Burma; Shipley, A.E., Ed.; Taylor and Francis: London, UK, 1926; p. 442. [Google Scholar]
- Heikertinger, F.; Csiki, E. Chysomelidae: Halticinae I. In Coleopterorum Catalogus. Pars 166; Junk, W., Ed.; W. Junk’s: Gravenhage, The Netherlands, 1940; p. 336. [Google Scholar]
- Heikertinger, F. Bestimmungstabelle der paläarktischen Arten der Gattungen Podagrica Foudr., Mantura Steph. und Chaetocnema. Koleopterol. Rundsch. 1951, 32, 133–216. [Google Scholar]
- Gressitt, J.L.; Kimoto, S. The Chrysomelidae (Coleoptera) of China and Korea. Pac. Insects Monogr. 1963, 1, 301–1026. [Google Scholar]
- Samuelson, G.A. Alticinae of Oceania (Coleoptera: Chrysomelidae); Pacific Insects Monograph; Bernice Bishop Museum: Honolulu, HI, USA, 1973; Volume 30, pp. 1–165. [Google Scholar]
- Döberl, M. Alticinae. In Catalogue of Palearctic Coleoptera; Löbl, I., Smetana, A., Eds.; Apollo Books: Stenstrup, Denmark, 2010; Volume 6, pp. 491–563. [Google Scholar]
- Ruan, Y.; Konstantinov, A.S.; Ge, S.; Yang, X. Revision of Chaetocnema semicoerulea species-group (Coleoptera, Chrysomelidae, Galerucinae, Alticini) in China, with descriptions of three new species. Zookeys 2014, 463, 57–74. [Google Scholar] [CrossRef] [Green Version]
- Ruan, Y.; Konstantinov, A.S.; Ge, S.; Yang, X. Revision of the Chaetocnema picipes species–group (Coleoptera, Chrysomelidae, Galerucinae, Alticini) in China, with descriptions of three new species. Zookeys 2014, 387, 11–32. [Google Scholar] [CrossRef]
- Özdikmen, H. A new subgeneric arrangement of the genus Chaetocnema Stephens (Chrysomelidae: Galerucinae: Alticini) with new subgenera based on spermathecal structures. Munis Entomol. Zool. 2021, 16, 41–105. [Google Scholar]
- Rohlf, F.J. tpsDig, Digitize Landmarks and Outlines, Version 2.05; Department of Ecology and Evolution, State University of New York: New York, NY, USA, 2005. [Google Scholar]
- MacLeod, N. Morphometrics: History, development methods and prospects. Zool. Syst. 2017, 42, 4–33. [Google Scholar] [CrossRef]
- Zhang, M.; Ruan, Y.; Wan, X.; Tong, Y.; Yang, X.; Bai, M. Geometric morphometric analysis of the pronotum and elytron in stag beetles: Insight into its diversity and evolution. ZooKeys 2019, 833, 21–40. [Google Scholar] [CrossRef] [Green Version]
- Tong, Y.; Yang, H.; Jenkins, S.J.; Yang, X.; Bai, M. The relationship between genus/species richness and morphological diversity among subfamilies of jewel beetles. Insects 2021, 12, 24. [Google Scholar] [CrossRef] [PubMed]
- Klingenberg, C.P. MorphoJ: An integrated software package for geometric morphometrics. Mol. Ecol. Resour. 2011, 11, 353–357. [Google Scholar] [CrossRef]
- Sherratt, E.; Gower, D.J.; Klingenberg, C.P.; Wilkinson, M. Evolution of cranial shape in caecilians (Amphibia: Gymnophiona). Evol. Biol. 2014, 41, 528–545. [Google Scholar] [CrossRef]
- Klingenberg, C.P.; Monteiro, L.R. Distances and directions in multidimensional shape spaces: Implications for morphometric applications. Syst. Biol. 2005, 54, 678–688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pluot-Sigwalt, D.; Lis, J.A. Morphology of the spermatheca in the Cydnidae (Hemiptera: Heteroptera): Bearing of its diversity on classification and phylogeny. Eur. J. Entomol. 2008, 105, 279. [Google Scholar] [CrossRef] [Green Version]
- López-Guerrero, Y.; Halffter, G. Evolution of the spermatheca in the Scarabaeoidea (Coleoptera). Fragm. Entomol. 2000, 32, 225–285. [Google Scholar]
- Aslam, N.A. An assessment of some internal characters in the higher classification of the Curculionidae s.l. (Coleoptera). Entomol. Soc. 1961, 113, 417–480. [Google Scholar] [CrossRef]
- Schuler, L. La spermatheque chez les Harpalidae et les Pterostichitae de France. Rev. Française D’entomologie 1963, 30, 81–103. [Google Scholar]
- Han, T.; Lee, Y.B.; Park, S.W.; Lee, S.; Park, H.C. A new genus, Ohirathous (Coleoptera, Elateridae, Dendrometrinae) from Taiwan. Elytra 2012, 2, 43–52. [Google Scholar]
- Reid, C.A.M. The Australian species of the tribe Zeugophorini (Coleoptera: Chrysomelidae: Megalopodinae). Gen. Appl. Entomol. 1989, 21, 39–47. [Google Scholar]
- Hernández, J.M. La genitalia femenina en el género Corymbia Des Gozis, 1886 (Coleóptera, Cerambycidae). Elytron 1993, 7, 99–104. [Google Scholar]
- Borowiec, L.; Opalinska, S. The structure of spermathecae of selected genera of Stolaini and Eugenysini (Coleoptera: Chrysomelidae: Cassidinae) and its taxonomic significance. Ann. Zool. 2007, 57, 463–479. [Google Scholar] [CrossRef]
- Borowiec, L.; Pomorska, J. The structure of the spermathecae of the genus Stolas (Coleoptera: Chrysomelidae: Cassidinae: Mesomphaliini) and its taxonomic significance. Ann. Zool. 2009, 59, 201–221. [Google Scholar] [CrossRef]
- Borowiec, L.; Skuza, M. The structure of spermatheca in the genus Chelymorpha Chevrolat, 1837 (Coleoptera: Chrysomelidae: Cassidinae) and its taxonomic significance. Ann. Zool. 2004, 54, 439–451. [Google Scholar] [CrossRef]
- Borowiec, L.; Świętojańska, J. Revision of Cassida litigiosa group from southern Africa (Coleoptera: Chrysomelidae: Cassidinae). Ann. Zool. 2001, 51, 153–184. [Google Scholar]
- Rodríguez-Mirón, G.M.; Zaragoza-Caballero, S.; López-Pérez, S. Comparative morphology of the spermatheca in Megalopodidae (Coleoptera, Chrysomeloidea). ZooKeys 2017, 720, 47–64. [Google Scholar] [CrossRef] [Green Version]
- Arnqvist, G. The evolution of animal genitalia: Distinguishing between hypotheses by single species studies. Biol. J. Linn. Soc. 1997, 60, 365–379. [Google Scholar] [CrossRef]
- Flowers, R.W.; Eberhard, W.G. Fitting together: Copulatory linking in some Neotropical Chrysomeloidea. Rev. Biol. Trop. 2006, 54, 829–842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zunino, M. Cuarenta años de anatomía de las piezas genitales en la taxonomía de los escarabajos (Coleoptera: Scarabaeoidea): El estado del arte. Dugesiana 2012, 18, 197–206. [Google Scholar]
- Eberhard, W.G. Sexual Selection and Animal Genitalia; Cambridge University Press: Cambridge, UK, 1985; p. 256. [Google Scholar]
- Tatsuta, H.; Takahashi, K.H.; Sakamaki, Y. Geometric morphometrics in entomology: Basics and applications. Entomol. Sci. 2018, 21, 164–184. [Google Scholar] [CrossRef] [Green Version]
- Viscosi, V.; Cardini, A. Leaf morphology, taxonomy and geometric morphometrics: A simplified protocol for beginners. PLoS ONE 2011, 6, e25630. [Google Scholar] [CrossRef] [Green Version]
- Karanovic, T.; Djurakic, M.; Eberhard, S.M. Cryptic species or inadequate taxonomy? Implementation of 2D geometric morphometrics based on integumental organs as landmarks for delimitation and description of copepod taxa. Syst. Biol. 2016, 65, 304–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.; Qi, Y.; Yang, Y.; Bai, M. A new species of Falsopodabrus Pic characterized with geometric morphometrics (Coleoptera, Cantharidae). ZooKeys 2016, 614, 97–112. [Google Scholar] [CrossRef] [Green Version]
- White, S.; Pope, M.; Hillson, S.; Soligo, C. Geometric morphometric variability in the supraorbital and orbital region of Middle Pleistocene hominins: Implications for the taxonomy and evolution of later Homo. J. Hum. Evol. 2022, 162, 103095. [Google Scholar] [CrossRef]
- Humphreys, A.; Barraclough, T. The evolutionary reality of higher taxa in mammals. Proc. R. Soc. B Biol. Sci. 2014, 281, 20132750. [Google Scholar] [CrossRef] [PubMed]
- Kemp, T.S. The origin of higher taxa: Macroevolutionary processes, and the case of the mammals. Acta. Zool. 2007, 88, 3–22. [Google Scholar] [CrossRef]
- de Oliveira, S.S.; Ortega, J.C.; Ribas, L.; Lopes, V.; Bini, L. Higher taxa are sufficient to represent biodiversity patterns. Ecol. Indic. 2020, 111, 105994. [Google Scholar] [CrossRef]
- Simpson, G.G. The Major Features of Evolution; Columbia University Press: New York, NY, USA, 1953; p. 434. [Google Scholar]
- Alroy, J. Geographical, environmental and intrinsic biotic controls on Phanerozoic marine diversification. Palaeontol. 2010, 53, 1211–1235. [Google Scholar] [CrossRef]
- Maruvka, Y.E.; Shnerb, N.M.; Kessler, D.A.; Ricklefs, R.E. Model for macroevolutionary dynamics. Proc. Natl. Acad. Sci. USA 2013, 110, E2460–E2469. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Tong, Y.; Zhao, Y.; Sun, Z.; Wang, X.; Ma, F.; Bai, M. Study on the relationship between richness and morphological diversity of higher taxa in the darkling beetles (Coleoptera: Tenebrionidae). Diversity 2022, 14, 60. [Google Scholar] [CrossRef]







| Pronotum | Elytron | Head | Aedeagus in Lateral View | Aedeagus in Ventral View | Spermathecal Receptacle | Spermathecal Pump | |
|---|---|---|---|---|---|---|---|
| p-values for MD | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| p-values for PD | <0.0001 | 0.3499 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Pronotum | Elytron | Head | Aedeagus in Lateral View | Aedeagus in Ventral View | Spermathecal Receptacle | Spermathecal Pump | ||
|---|---|---|---|---|---|---|---|---|
| OR vs. PA | p-values for MD | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| p-values for PD | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.0002 | 0.6871 | 0.0030 | |
| OR vs. NE | p-values for MD | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | - | - |
| p-values for PD | <0.0001 | <0.0001 | 0.0003 | 0.0797 | 0.0773 | - | - | |
| PA vs. NE | p-values for MD | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | - | - |
| p-values for PD | <0.0001 | 0.3282 | 0.0015 | 0.0001 | 0.0003 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Ruan, Y.; Bai, M.; Chen, X.; Li, L.; Yang, X.; Meng, Z.; Liu, Y.; Du, X. Geometric Morphometric Analysis of Genus Chaetocnema (Coleoptera: Chrysomelidae: Alticini) with Insights on Its Subgenera Classification and Morphological Diversity. Diversity 2023, 15, 918. https://doi.org/10.3390/d15080918
Zhang M, Ruan Y, Bai M, Chen X, Li L, Yang X, Meng Z, Liu Y, Du X. Geometric Morphometric Analysis of Genus Chaetocnema (Coleoptera: Chrysomelidae: Alticini) with Insights on Its Subgenera Classification and Morphological Diversity. Diversity. 2023; 15(8):918. https://doi.org/10.3390/d15080918
Chicago/Turabian StyleZhang, Mengna, Yongying Ruan, Ming Bai, Xiaoqin Chen, Lixia Li, Xingke Yang, Ziye Meng, Yang Liu, and Xinyan Du. 2023. "Geometric Morphometric Analysis of Genus Chaetocnema (Coleoptera: Chrysomelidae: Alticini) with Insights on Its Subgenera Classification and Morphological Diversity" Diversity 15, no. 8: 918. https://doi.org/10.3390/d15080918





