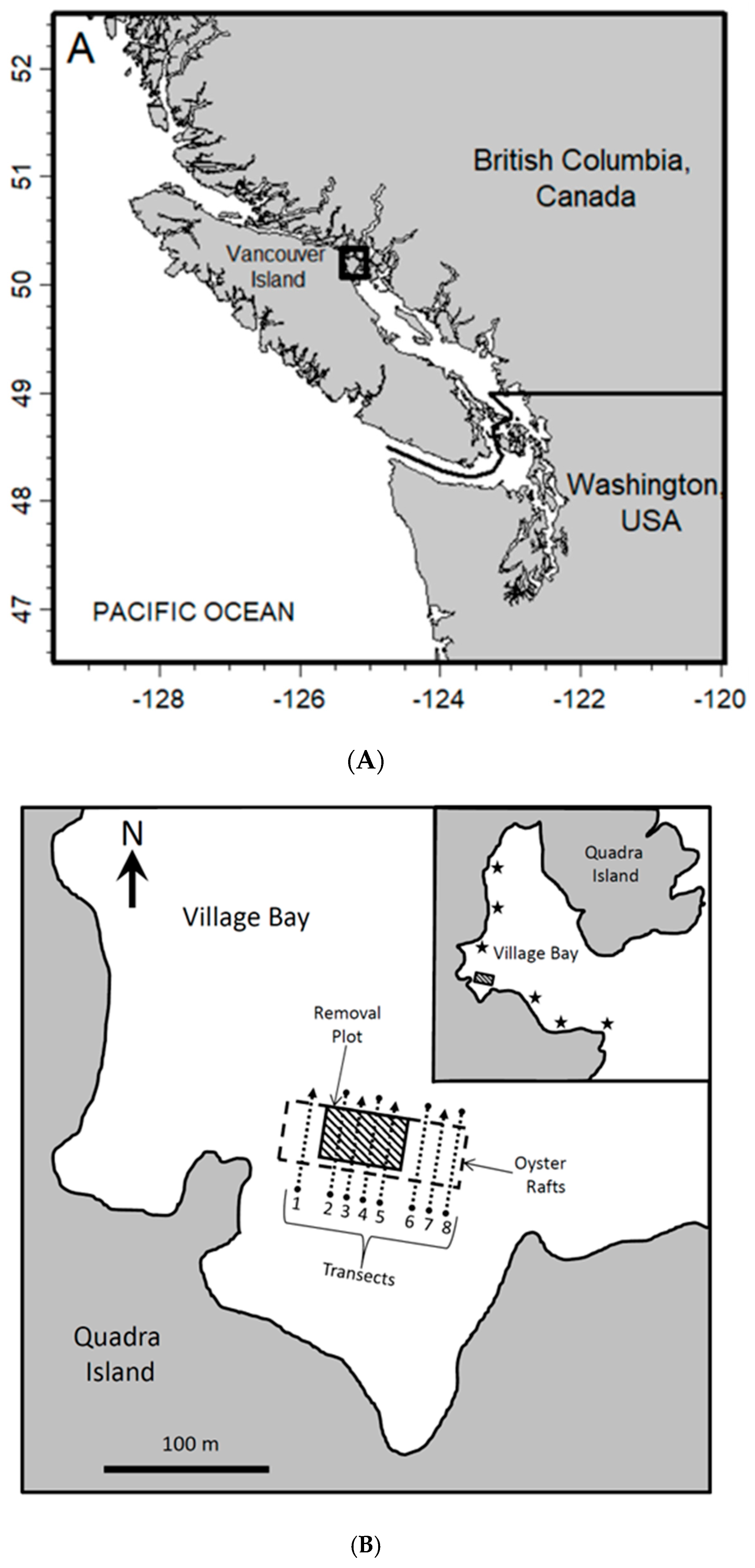
1. Introduction
Aquaculture of the Pacific oyster (
Crassostrea gigas) in British Columbia (BC), Canada is a highly valuable industry, 7,997,000 kg, worth CAD 16,044,000, being produced in 2021 (DFO statistics:
https://www.dfo-mpo.gc.ca/stats/aqua/aqua20-eng.htm, accessed on 3 August 2023). Wild fishery of the California sea cucumber (
Apostichopus (
Parastichopus)
californicus) in BC is also a lucrative industry, 605,546 kg worth about CAD 12,300,000 being fished in the same year [
1]. Presently, though, there is no commercial sea cucumber aquaculture industry in BC. However, recent increases in the price of wild-caught California sea cucumbers, along with worldwide concern for the conservation of many species of highly valuable tropical sea cucumbers [
2] have led to increased interest in the culture and/or sea ranching of this species in BC and Washington, USA [
3,
4,
5]. Interest is primarily driven by shellfish and finfish aquaculture proponents who are thinking of farming California sea cucumbers at existing suspended shellfish culture sites or finfish net-pen sites. That concept fits well with the principles of integrated multitrophic aquaculture (IMTA) [
6], whereby cocultured deposit feeders (e.g., sea cucumbers) may help to ameliorate increased organic loading associated with aquaculture via their feeding activity, which recycles nutrients and bioturbates sediments [
7,
8,
9,
10,
11]. The first objective of this study was therefore to determine if high densities of California sea cucumbers on shellfish aquaculture sites were able to fully or partially mitigate the increased organic loading associated with farming activities.
While previous studies have suggested that the use of sea cucumbers may be an effective means of mitigating organic loading at aquaculture sites (e.g., [
3,
4,
5,
7,
8,
11]), coculture with such deposit feeders is not without concern. Some proponents believe that no fencing or caging is required to keep cultured sea cucumbers within the boundaries of the aquaculture tenure, the premise being that the animals have a vested interest in remaining in areas of high food concentration [
12,
13]. Even if emigration was minimal, however, there is also concern about the immigration of wild sea cucumbers that may be attracted to the increased sediment organic levels at aquaculture sites. For one species of sea cucumber, there is evidence that the mixing of cultured and wild stocks is negligible: Slater and Carton (2010) [
12] observed a stable isotope signature in
Australostichopus mollis collected beneath a mussel farm that was consistent with that of farm-impacted sediment and distinct from the stable isotope signature of
A. mollis collected from natural reefs nearby. It was concluded that the shellfish farm site demonstrated a high sea cucumber retention and that negligible mixing occurred between sea cucumbers at the farm and those at natural reefs nearby [
12].
Our work has shown that although California sea cucumbers are not attracted to sites of high organic content per se, if they randomly encounter such areas, they alter their foraging behaviour, which may serve to retain them there for an indefinite period [
14]. Since the conditions of licence allow BC shellfish farmers to retain wild animals on aquaculture tenures when they are harvested along with cultivated individuals for which they are licensed [
15]—and there is difficulty in discriminating between wild and cultured stocks—there is the potential for wild sea cucumbers to be harvested along with cultured individuals. That would ultimately reduce the number of sea cucumbers available to the wild fishery and increase the fishing pressure on the wild stock if these removals were not included in the wild fishery’s total allowable catch (although sea cucumber larvae, produced by wild and/or cultured individuals, settling on oyster culture gear could eventually recruit into the benthos as juveniles, once they are dislodged from the gear, and bolster wild populations). The second objective of our study was therefore to determine if there was immigration or emigration of sea cucumbers to or from the area of increased organic loading associated with an active commercial shellfish aquaculture site.
Another reason for the increased interest in the culture of
A. californicus at existing shellfish farms is the high densities of individuals that are often already found on the seafloor at those sites. The likely reason is the settling of larval sea cucumbers on the suspended shellfish (primarily Pacific oysters) and gear, which provide a highly complex matrix where juveniles are able to grow essentially free from most predators. Those juveniles can be knocked off the suspended shellfish by storms or during shellfish harvest events every 2–5 years. Once on the seafloor, shellfish aquaculture sites, which typically have a thick layer of shell hash, provide refuge for typically cryptic juvenile California sea cucumbers [
16] and create a hard substrate where they can selectively feed on high-organic-content faeces and pseudofaeces deposited by the shellfish [
17]. That provides a unique mechanism whereby shellfish farms are essentially “self-seeding” for California sea cucumbers. To appreciate the impact aquaculture may have on wild sea cucumbers, it is important to understand the potential contribution of those wild-set individuals to the population on/off the shellfish farms. The third objective of this study was therefore to determine the approximate rate at which wild-set juvenile
A. californicus drop from suspended cultured shellfish/gear onto the seafloor.
If sea cucumber immigration and emigration occur on shellfish farms, it will be necessary to determine effective means of containment. That is particularly pertinent given the logistical difficulties associated with attempting to contain a large-size range of animals that can scale on vertical or inverted surfaces and whose only prominent hard part is their calcareous oral ring [
18]. Therefore, the fourth and final objective of our study was to determine, through a series of laboratory experiments, if California sea cucumbers could be contained using fencing, rather than sealed cages, and what mesh types and sizes were effective at doing so.
4. Discussion
During times of high primary productivity and when high densities of oysters were stocked at the farm (August 2013 and March 2014), the farm site showed higher total sediment deposition rates than the control site, findings in harmony with those of [
4] who found that peak sedimentation rates at the same farm site in 2004 occurred in April and July. Similar results were also shown by [
26], who found that the contribution of biodeposits from mussels (
Mytilus edulis) to the total sediment deposition was greatest during times of high primary productivity. In the current study, despite differences in the total sedimentation rate between the farm and the control site during times of high primary productivity (spring and summer, when total sediment deposition was higher at the farm site), the TOC deposition rate at both sites was similar. That result was due to higher levels of carbonate carbon deposition at the farm site, likely from deposited shell debris. Sedimentary material being deposited at the farm site showed a consistently lower C:N ratio than that deposited at the control site throughout the study, indicating that the former was of higher nutritional value than the latter (see [
22,
23]). Thus, although there was no additional organic carbon being deposited at the farm site during periods of high productivity, the material deposited was of higher nutritional value. Previous work has shown that the nutritional quality of available food as well as the volume of food available both play an important role in the growth of deposit-feeding sea cucumbers [
27]. In addition, the hard substrate created by the layer of shell hash beneath the oyster farm likely facilitates selective feeding on such high-nutritional-quality biodeposits and provides cryptic spaces for juveniles to hide in. Therefore, it may be the quality and availability of food that allows some oyster farms to support high densities of sea cucumbers rather than the total amount of sediment deposition. In January 2014 and January 2015, the total sediment deposition rate at the control site was higher than at the farm. During those times, the TOC deposition rate was also higher at the control site than at the farm, but there was no significant difference in the TN deposition rate, resulting in higher C:N ratios at the former. That indicates that there was an abundance of low-nutritional-quality organic material being deposited at the control site. Inspection confirmed that the material collected in the sediment traps at the control site contained a high proportion of fine woody debris. That was likely benthic material that was resuspended during storm events. An extensive study of Village Bay by [
19] had similar findings during winter months. In that investigation, the benthic sediments in the area near the control site had a high proportion of woody debris that became more prominent in sediment traps during the winter.
Numerous benthic processes contribute to the breakdown and trophic transfer of deposited organic material. Although not as dramatic as at finfish farms, if left unchecked, the deposition of organic material beneath shellfish farms can eventually result in an anoxic state dominated by bacterial mats (e.g., [
28]). The effects of epibenthic grazers on sediment organic content was qualitatively, and to some extent quantitatively, evident beneath the oyster farm at Village Bay. When all epibenthic grazers were completely excluded from small areas of the benthos, mats of
Beggiatoa (a bacterium associated with high levels of organic loading and anoxia) quickly formed. When grazers were allowed back, the mats disappeared (Curtis, unpublished observations). That extreme shift was not observed, however, when California sea cucumbers were the only epibenthic grazers removed. If California sea cucumbers mitigate the accumulation of organic material beneath the farm, it would be expected that the organic content of the sediment would be linked to the density of sea cucumbers. Accordingly, there was a weak trend towards increased densities of California sea cucumbers and a decreased organic content of the sediment over the course of the study, but the decrease in organic content was not significant. Additionally, there was a sharp decrease in sediment organic content within the removal plot in March 2014 that was associated with a sharp increase in California sea cucumber density. One possible explanation for such a phenomenon is that changes in density were linked to seasonal changes in sediment deposition rate: during the winter months when the TOC deposition rate was low, the California sea cucumber density was also relatively low, and during the spring and summer, when the TOC deposition rate was high, their density was also higher. Further complicating that scenario is the fact that California sea cucumbers are likely preferentially feeding on biodeposits that settle on the hard substrate created by the shell hash beneath the farm and those materials are consumed or carried away by currents before they can become part of the organic content of the benthic sediment. Alternatively, a large number of green sea urchins (
Strongylocentrotus droebachiensis) remained in the removal plot following the harvest of sea cucumbers, and previous work has shown that sea urchins actively consume biodeposits produced by aquaculture [
29]. Therefore, such activity may have masked the relationship between the sediment organic content and California sea cucumber density when the sea cucumbers were removed. While the direct influence of sea cucumbers on sediment organic content is unclear, it is certain that epibenthic deposit feeders play a key role in mitigating the accumulation of organic matter in the sediments beneath shellfish farms. In future studies it may be possible to further refine the contribution of different members of the epibenthic community through more in-depth exclusion experiments.
The density of adult California sea cucumbers within the removal plot was slow to recover in the fall and winter months following their harvest. In addition, over the course of the study, the density of adult sea cucumbers beneath the shellfish farm showed a clear seasonal pattern that was overlaid by a general increase that was probably associated with the harvesting of oysters at the site. The failure of the sea cucumbers to redistribute beneath the farm between sea cucumber removal (September 2012) and when the area beneath the farm was first resurveyed (January 2013) is consistent with other studies that suggest that California sea cucumbers show little movement in the fall and winter (e.g., [
30]). During that time, they resorb their internal organs, and it has been suggested that they enter a state of hibernation [
4,
30,
31]. The fact that adult sea cucumbers moved less within the site during that time, combined with no change in overall density, also makes it unlikely that they were moving off the site. Between winter and late summer during each year of the study, there was a net increase in the density of adult sea cucumbers in all areas of the study site. That increase was most pronounced between winter and spring, followed by a more gradual increase over the summer. Although those increases are likely the result of juvenile sea cucumbers falling from the oyster strings, being retained at the site, and growing up to become adults, the potential contribution of sea cucumbers moving onto the site from the surrounding area is unclear. Work by [
12] on
Australostichopus mollis in New Zealand, using stable isotopes to analyze their diet, suggests that sea cucumbers beneath shellfish aquaculture sites show a high site fidelity with minimal movement away from the site; however, the authors were unable to demonstrate that sea cucumbers from the surrounding area were moving into the site. In the current study, we were unable to show that sea cucumbers were retained at the site. Between late summer and early winter in the second and third years of the study, there was a clear decline in the density of sea cucumbers beneath the shellfish farm, with approximately 90 and 50 sea cucumbers per day leaving the site in 2013 and 2014, respectively. That decline was not evident in the first year. However, in 2012, sampling occurred in early September, which was later than in following years. Given the lack of dispersal within the site in the first year, it is probable that movements away from it occur in late summer/early fall, rates of movement being high during late summer/early fall and minimal thereafter. The window for observing movements away from the site may, therefore, have been missed in 2012.
Laboratory work has shown that the organic content of the sediment (and therefore available food) can alter sea cucumber foraging behaviour [
14]. When the sea cucumber density is low and/or the food availability is high, they display a random foraging pattern. When resources become scarce, however, they display directed movements. In the current study, decreases in adult sea cucumber density were associated with decreases in total sediment, TOC, and TN deposition rates. Those changes were not significantly reflected in the sediment organic content, but that may have been masked by selective feeding on the hard substrate created by the layer of shell hash beneath the farm [
4]. If our laboratory observations are applicable to a field setting, one possible explanation for the decrease in adult density is that in the late summer/early fall, sea cucumbers switch from a random foraging behaviour that would retain them at the site to more directed movements that could potentially lead them away from it. The number of sea cucumbers and the distance at which they were observed on the farm transects tended to be greater in August 2013 than during the other survey periods. The fact that a similar pattern was not observed at midsummer in July 2014 provides additional evidence that sea cucumbers may move away from the site in late summer/early fall (late August/early September). There were no clear patterns in the density of sea cucumbers in the shoreline transects located away from the farm, though that is not surprising given the amount of available habitat over which the sea cucumbers could disperse. Interestingly, despite anecdotal evidence that sea cucumbers seasonally migrate to deeper waters during winter, there was no clear seasonal change in the average depth of occurrence of individuals observed on the transects located away from the farm. While it is clear that California sea cucumbers move away from the farm site, likely in the late summer or early fall, it remains uncertain where they are going. It is also apparent that there is a net input of adults to the farm site in the spring and early summer. However, it was not possible to identify the relative contributions of movements onto the site and juveniles being knocked off from the overhanging oyster gear. Given the low density of adult California sea cucumbers in the surrounding area relative to the density of those at the farm site, it is likely that juveniles falling from the oyster strings were the main contributor.
In contrast to the density of adult sea cucumbers, the density of juveniles within the removal plot when first resurveyed (January 2013) was similar to preremoval values (September 2012), and the total density (inside and outside the removal plot combined) of juveniles more than doubled. A possible explanation is that very small and cryptic juvenile sea cucumbers on the seafloor had grown to such a size that they could be detected in surveys or to a size where they could no longer be hidden within layers of shell hash. That is unlikely, however, since sea cucumbers show little growth during that time of the year and often actually get smaller [
32]. A more plausible explanation is that the net input of juvenile sea cucumbers resulted from individuals being knocked off the oyster strings during fall and winter storms. When the density of juvenile sea cucumbers observed on the oyster strings (
Figure 7) is extrapolated to the entire farm, it represents approximately 85,000 to 96,000 ind. During the summer months, we observed a drop-off rate of 0.087 ind string
−1 d
−1, which when extrapolated to the entire farm equates to approximately 780 ind d
−1 or 30,380 ind in the 39-day period of observation. Although this drop-off rate likely varies temporally with a variety of factors, the contribution of sea cucumbers recruited to the farm equipment/oysters to the benthic population could be sizeable. The rate of increase in density of adult sea cucumbers beneath the farm was highest in all years between the winter (January) and spring (either March or April) and was approximately 100, 180, and 350 ind d
−1 for 2013, 2014, and 2015, respectively. It is important to note that oyster harvesting began in February 2014 and continued for the duration of the study, the effects of which are unknown. Nevertheless, it is apparent that the contribution of juvenile sea cucumbers from the oyster strings due to the natural drop-off alone could easily account for the observed differences in adult density when oyster harvesting is not occurring. In terms of the total recruitment of sea cucumbers to the farm, one must not only consider the immigration of wild juveniles/adults and drop-off of juveniles recruited to the oyster strings, but also the natural settlement of wild larvae leading to juvenile recruitment. Pulses of recruits during “good” versus “bad” years for sea cucumber reproduction would likely have some impact on total recruitment within the farm.
The results of our study have shown that California sea cucumbers move away from the site and that they may also move onto it. Therefore, if there is a desire to prevent the mixing of wild and cultured individuals, some type of containment will be required. With regard to the fence height and type experiments, when fences did not extend to the surface of the water, more sea cucumbers escaped from fences made of nylon netting than from the stiffer plastic Vexar
TM mesh. Although that seems counterintuitive, since one would assume that a stiffer substrate is easier to scale, there are a few possible explanations. The upper edge of the Vexar
TM mesh creates a sharp edge, which may be an adverse stimulus. The nylon net is flexible and when sea cucumbers attempt to push themselves through, they distort the mesh, creating a larger effective opening, making it easier for them to escape. Lastly, in that and other experiments, we have observed California sea cucumbers displaying a strong thigmotaxis, whereby they press themselves against hard objects and minimize their movement; the stiffer Vexar
TM mesh may have provided a better substrate for that behaviour. With regard to fences that extended above the surface of the water, there were no observed escapes from either type. That indicates that distortion of the nylon mesh is not the reason for the increased number of escapes since the sea cucumbers would be able to push themselves through the mesh at both fence heights and the maximum possible dimension of the collapsed netting was <20 mm. The lack of escapes when fencing reached the water’s surface also indicates that sea cucumbers are unlikely to aerially expose themselves to scale over a fence. Although California sea cucumbers are occasionally observed in the intertidal, due to their reliance on hydrostatic pressure for locomotion, it is unlikely that they are able to move when out of the water. Therefore, in order to contain California sea cucumbers, it is probable that fences that protrude above the surface of the water or full enclosures will be required. The other issue with containing sea cucumbers relates to their lack of prominent hard parts [
18] and subsequent ability to squeeze through small openings. In mesh-size experiments using a range of sizes, sea cucumbers were able to squeeze through stiff mesh ranging from 32 to 55% of their contracted width. Accordingly, it is recommended that mesh openings be no greater than 30% of the smallest sea cucumber’s contracted width. In order to maintain optimal water and nutrient flow when culturing sea cucumbers, it may therefore be necessary to increase the size of the mesh used for containment structures as the sea cucumbers grow. The size of mesh that would be required to ensure no movement of wild sea cucumber larvae (<1 mm) into enclosures would likely be too small to be used effectively in the field, as the very small mesh openings would likely become quickly constricted due to biofouling, hence restricting water flow. Thus, some wild larvae are likely to recruit into culture enclosures and be lost to the natural population and/or wild fishery. Conversely, adult sea cucumbers kept in enclosures are likely to spawn before they are harvested, and “cultured” larvae can cross the mesh barrier and be recruited back into the wild population.
The results of the present study have shown that through the deposition of faeces and pseudofaeces during times of high primary productivity, deep-water shellfish farms may provide a high-quality food source for deposit-feeding animals living beneath them. Additionally, the hard substrate created by shell debris beneath the farms may increase access to nutrients by facilitating selective feeding. In some cases, the combination of those factors likely helps to support higher densities of deposit feeders, which in turn may help to mitigate the increased organic deposition associated with shellfish farming. In our study, seasonal changes in the density of California sea cucumbers beneath the farm were observed, the density decreasing in the late summer/early fall and increasing in the spring/summer. Increases in the density resulted from either the addition of wild-set California sea cucumbers falling from the oyster strings and/or the immigration of adult sea cucumbers to the farm, while decreases in the density resulted from emigration of individuals away from the farm. These findings suggest that if shellfish farms are seeded with California sea cucumbers, some form of containment will be required to prevent the mixing of wild and cultured stocks. If the mixing of stocks is to be prevented, either full containment or fences reaching above the surface of the water will be required, the mesh size being determined by the size of the sea cucumbers to be enclosed.