Seeking a Hideout: Caves as Refuges for Various Functional Groups of Bryophytes from Terceira Island (Azores, Portugal)
Abstract
1. Introduction
2. Materials and Methods
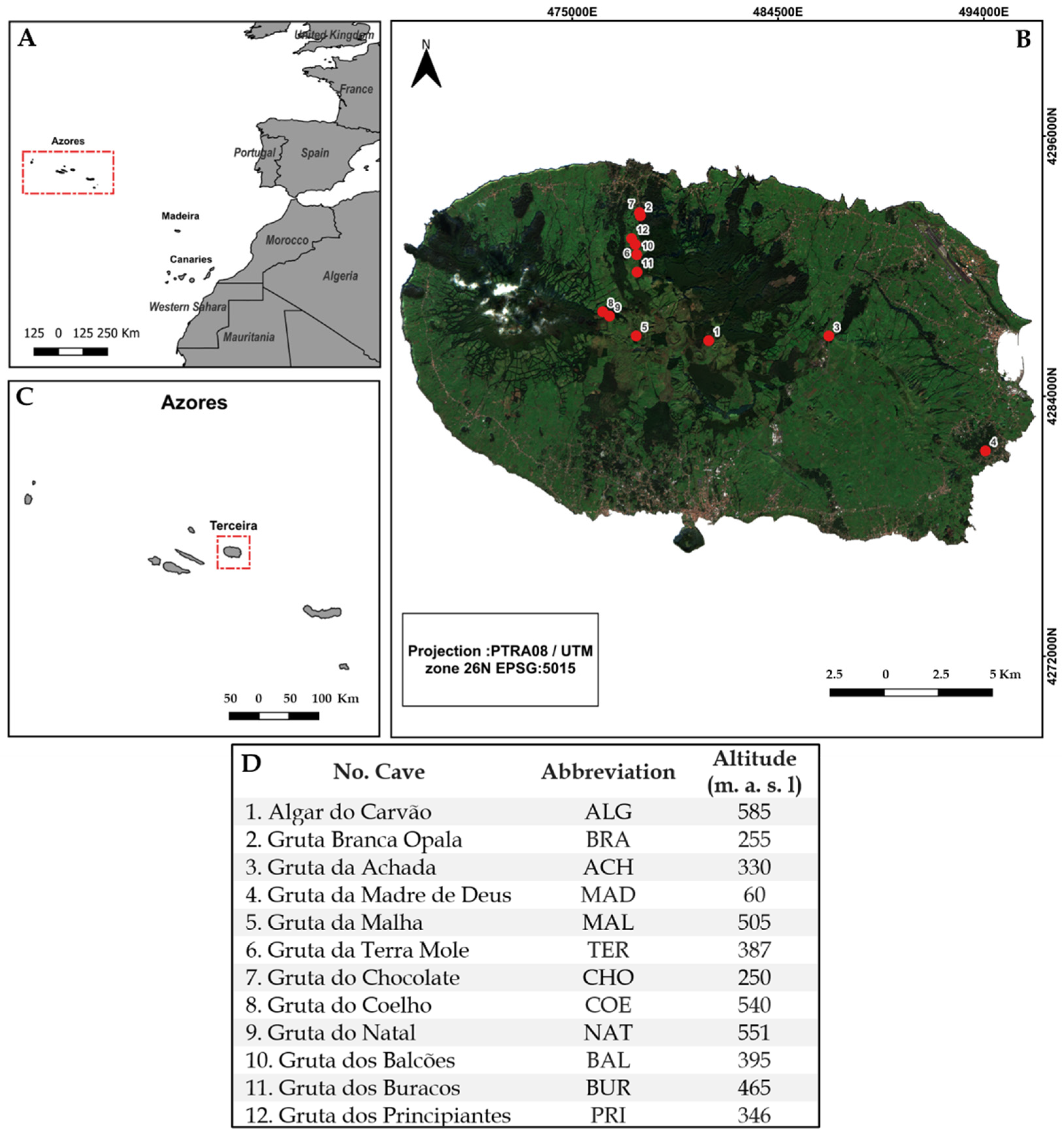
2.1. Study Area
2.2. Sampling Method and Functional Group Categorisation
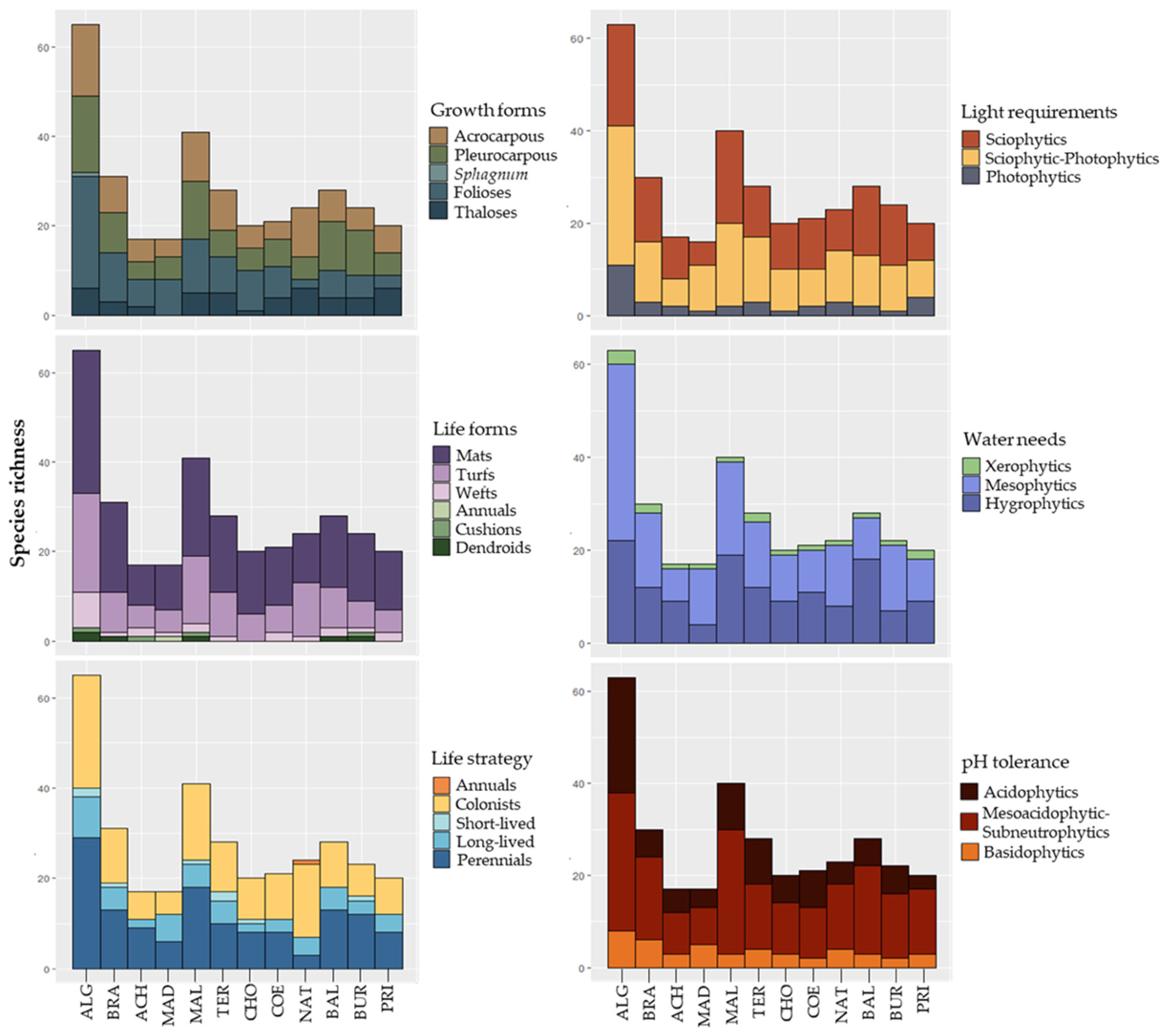
2.3. Studied Variables
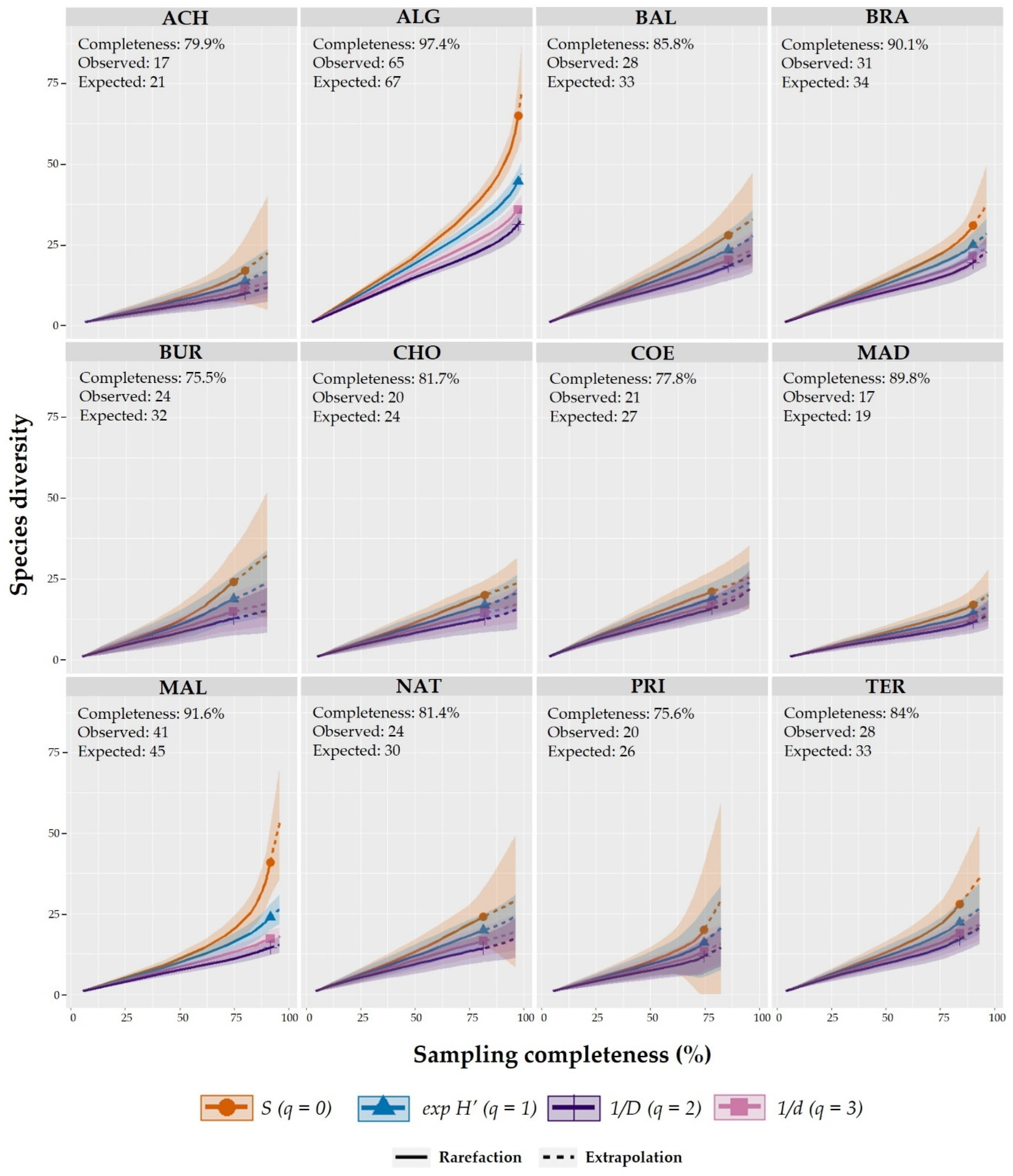
2.4. Alpha (α) and Beta (β) Diversity and Sampling Completeness
2.5. Data Analysis
3. Results
3.1. Species Inventory
3.2. Sampling Completeness, Alpha (α), and Beta (β) Diversity Richness across Caves
3.3. Influence of Drivers on the Richness and Abundance of Bryophyte Species
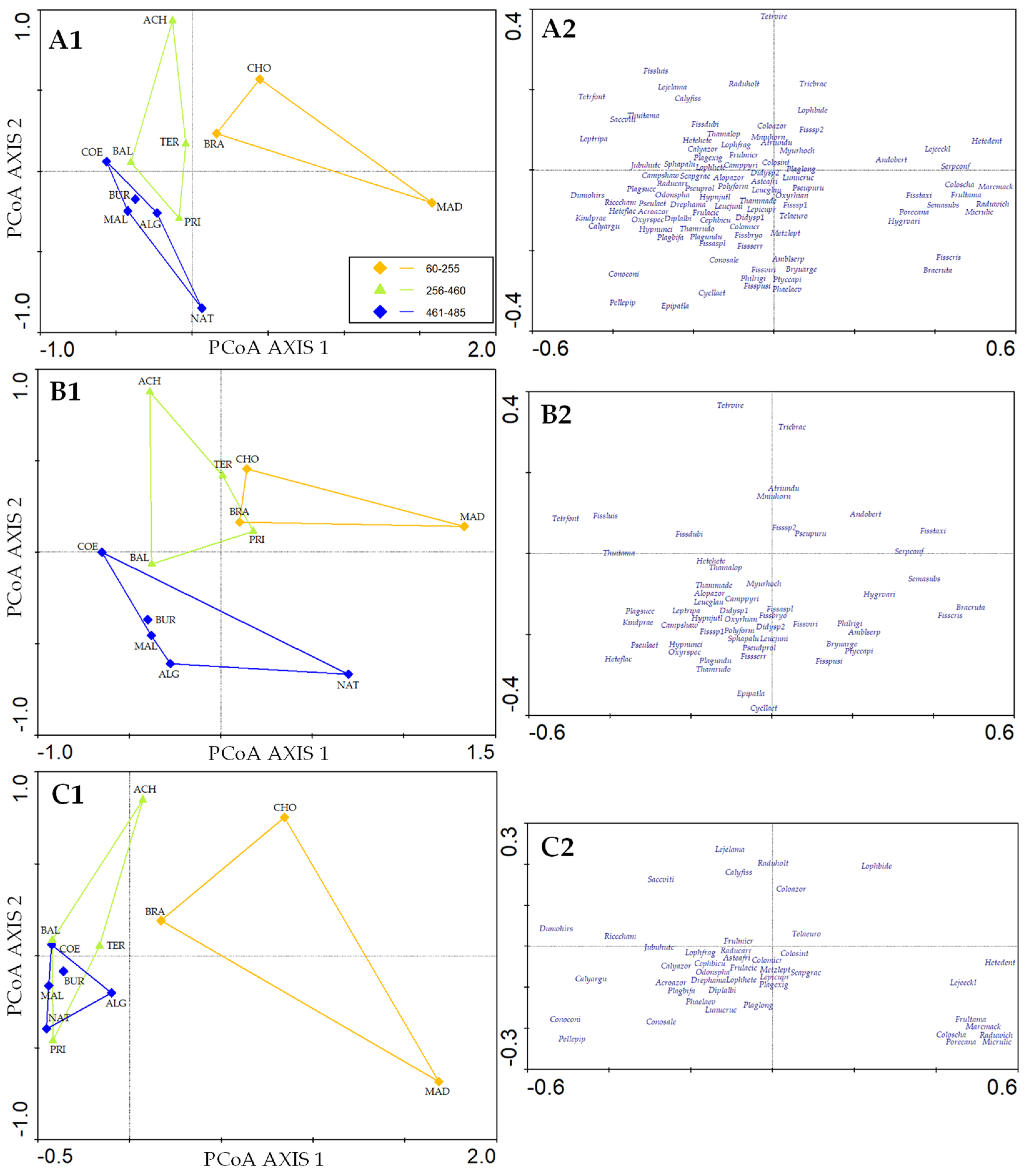
3.4. Species Composition
4. Discussion
4.1. Bryophyte Richness Patterns in Caves on Terceira Island
4.2. Bryophyte Composition Patterns in Caves on Terceira Island
4.3. Influence of Drivers on the Bryophyte Species
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Occurence | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Family | Life Strategy | Distribution | Abbreviation | TOTAL | ALG | BRA | ACH | MAD | MAL | TER | CHO | COE | NAT | BAL | BUR | PRI | |
| Div. Antocerophyta | |||||||||||||||||
| Phaeoceros laevis (L.) Prosk. | Notothyladaceae | Annual shuttle | COSM | Phaelaev | 1 | 1 | |||||||||||
| Div. Marchantiophyta | |||||||||||||||||
| Acrobolbus azoricus (Grolle & Perss.) Briscoe | Acrobolbaceae | Perennial | AZOR | Acroazor | 6 | 4 | 1 | 1 | |||||||||
| Asterella africana (Mont.) Underw. ex A.Evans | Aytoniaceae | Short-lived shuttle | EUR-AFR | Asteafri | 1 | 1 | |||||||||||
| Calypogeia arguta Nees & Mont. | Calypogeiaceae | Colonist | COSM | Calyargu | 35 | 1 | 6 | 4 | 5 | 1 | 1 | 2 | 4 | 6 | 5 | ||
| Calypogeia azorica Bischl. | Calypogeiaceae | Colonist | MAC | Calyazor | 17 | 10 | 2 | 2 | 1 | 1 | 1 | ||||||
| Calypogeia fissa (L.) Raddi | Calypogeiaceae | Colonist | COSM | Calyfiss | 37 | 16 | 3 | 3 | 5 | 6 | 1 | 3 | |||||
| Cephalozia bicuspidate (L.) Dumort. | Cephaloziaceae | Colonist | COSM | Cephbici | 1 | 1 | |||||||||||
| Cololejeunea azorica V.Allorge & Jovet-Ast | Lejeuneaceae | Short-lived shuttle | MAC | Coloazor | 4 | 1 | 1 | 2 | |||||||||
| Cololejeunea microscopica (Taylor) Schiffn. | Lejeuneaceae | Short-lived shuttle | COSM | Colomicr | 3 | 3 | |||||||||||
| Cololejeunea schaeferi Grolle | Lejeuneaceae | Long-lived shuttle | MAC | Colosche | 3 | 3 | |||||||||||
| Cololejeunea sintenisii (Steph.) Pócs | Lejeuneaceae | Short-lived shuttle | COSM | Colosint | 4 | 2 | 2 | ||||||||||
| Conocephalum conicum (L.) Dumort. | Conocephalaceae | Long-lived shuttle | COSM | Conoconi | 45 | 16 | 5 | 8 | 5 | 2 | 2 | 3 | 1 | 3 | |||
| Conocephalum salebrosum Szweyk., Buczk. & Odrzyk. | Conocephalaceae | Long-lived shuttle | COSM | Conosale | 21 | 7 | 7 | 2 | 2 | 3 | |||||||
| Diplophyllum albicans (L.) Dumort. | Scapaniaceae | Colonist | COSM | Diplalba | 5 | 4 | 1 | ||||||||||
| Drepanolejeunea hamatifolia (Hook.) Schiffn. | Lejeuneaceae | Long-lived shuttle | COSM | Draphama | 1 | 1 | |||||||||||
| Dumortiera hirsute (Sw.) Nees | Dumortieraceae | Long-lived shuttle | COSM | Dumohirs | 47 | 15 | 4 | 4 | 13 | 1 | 2 | 3 | 2 | 2 | 1 | ||
| Frullania acicularis Hentschel & von Konrat | Frullaniaceae | Long-lived shuttle | MAC | Frulacic | 5 | 5 | |||||||||||
| Frullania microphylla (Gottsche) Pearson | Frullaniaceae | Long-lived shuttle | EUR | Frulmicr | 3 | 2 | 1 | ||||||||||
| Frullania tamarisci (L.) Dumort. | Frullaniaceae | Long-lived shuttle | COSM | Frultama | 1 | 1 | |||||||||||
| Heteroscyphus denticulatus (Mitt.) Schiffn. | Lophocoleaceae | Long-lived shuttle | MAC | Hetedent | 11 | 6 | 2 | 3 | |||||||||
| Jubula hutchinsiae (Hook.) Dumort. | Jubulaceae | Perennial | COSM | Jubahutc | 60 | 20 | 7 | 24 | 3 | 2 | 4 | ||||||
| Lejeunea eckloniana Lindenb. | Lejeuneaceae | Perennial | EUR | Lejeecko | 16 | 7 | 2 | 6 | 1 | 1 | |||||||
| Lejeunea lamacerina (Steph.) Schiffn. | Lejeuneaceae | Long-lived shuttle | COSM | Lejelama | 25 | 8 | 5 | 4 | 2 | 2 | 1 | 1 | 2 | ||||
| Lepidozia cupressina (Sw.) Lindenb. | Lepidoziaceae | Perennial | COSM | Lepicups | 6 | 6 | |||||||||||
| Lophocolea bidentata (L.) Dumort. | Lophocoleaceae | Perennial | COSM | Lophbide | 4 | 1 | 1 | 2 | |||||||||
| Lophocolea fragrans (Moris & De Not.) Gottsche, Lindenb. & Nees | Lophocoleaceae | Perennial | COSM | Lophfrag | 8 | 1 | 4 | 1 | 1 | 1 | |||||||
| Lophocolea heterophylla (Schrad.) Dumort. | Lophocoleaceae | Colonist | COSM | Lophheter | 2 | 1 | 1 | ||||||||||
| Lunularia cruciata (L.) Dumort ex. Lindb. | Lunulariaceae | Perennial | COSM | Lunucruc | 1 | 1 | |||||||||||
| Marchesinia mackaii (Hook.) Gray | Lejeuneaceae | Perennial | EUR | Marcmack | 6 | 6 | |||||||||||
| Metzgeria leptoneura Spruce | Metzgeriaceae | Long-lived shuttle | COSM | Metzlept | 3 | 3 | |||||||||||
| Microlejeunea ulicina (Taylor) Steph. | Lejeuneaceae | Long-lived shuttle | COSM | Micrulic | 1 | 1 | |||||||||||
| Odontoschisma sphagni (Dicks.) Dumort. | Cephaloziaceae | Colonist | COSM | Odonsphg | 9 | 7 | 2 | ||||||||||
| Pellia epiphylla (L.) Corda | Pelliaceae | Colonist | COSM | Pellepip | 42 | 10 | 11 | 4 | 2 | 6 | 5 | 2 | 2 | ||||
| Plagiochila bifaria (Sw.) Lindenb. | Plagiochilaceae | Perennial | COSM | Plagbifr | 9 | 7 | 1 | 1 | |||||||||
| Plagiochila exigua (Taylor) Taylor | Plagiochilaceae | Perennial | COSM | Plagexiq | 6 | 6 | |||||||||||
| Plagiochila longispina Lindenb. & Gottsche | Plagiochilaceae | Perennial | AMER-AZOR | Plaglong | 12 | 12 | |||||||||||
| Porella canariensis (F.Weber) Underw. | Porellaceae | Long-lived shuttle | IBER-MAC | Porecana | 5 | 1 | 4 | ||||||||||
| Radula carringtonii J.B.Jack | Radulaceae | Long-lived shuttle | EUR | Radicarr | 4 | 3 | 1 | ||||||||||
| Radula holtii Spruce | Radulaceae | Colonist | EUR | Radiholt | 4 | 3 | 1 | ||||||||||
| Radula wichurae Steph. | Radulaceae | Long-lived shuttle | MAC | Radiwich | 5 | 5 | |||||||||||
| Riccardia chamedryfolia (With.) Grolle | Aneuraceae | Colonist | COSM | Ricccham | 59 | 27 | 5 | 1 | 13 | 4 | 1 | 3 | 2 | 2 | 1 | ||
| Saccogyna viticulosa (L.) Dumort. | Saccogynaceae | Perennial | EUR | Saccvita | 19 | 9 | 2 | 1 | 2 | 1 | 1 | 2 | 1 | ||||
| Scapania gracilis Lindb. | Scapaniaceae | Perennial | EUR | Scapgrac | 1 | 1 | |||||||||||
| Telaranea europaea J.J.Engel & G.L.Merr. | Lepidoziaceae | Colonist | EUR | Telaeuro | 5 | 3 | 1 | 1 | |||||||||
| Div. Bryophyta | |||||||||||||||||
| Alophosia azorica (Renauld & Cardot) Cardot | Polytrichaceae | NA | MAC | Alopazor | 1 | 1 | |||||||||||
| Amblystegium serpens (Hedw.) Schimp. | Amblystegiaceae | Perennial | COSM | Amblsere | 2 | 2 | |||||||||||
| Andoa berthelotiana (Mont.) Ochyra | Hypnaceae | Perennial | MAC | Andobert | 14 | 3 | 2 | 1 | 2 | 3 | 1 | 1 | 1 | ||||
| Atrichum undulatum (Hedw.) P.Beauv. | Polytrichaceae | Short-lived shuttle | COSM | Atriundu | 1 | 1 | |||||||||||
| Brachythecium rutabulum (Hedw.) Schimp. | Brachytheciaceae | Colonist | COSM | Bracruta | 4 | 3 | 1 | ||||||||||
| Bryum argenteum Hedw. | Bryaceae | Colonist | COSM | Bryuarge | 1 | 1 | |||||||||||
| Campylopus pyriformis (Schultz) Brid. | Leucobryaceae | Colonist | COSM | Camppyri | 4 | 3 | 1 | ||||||||||
| Campylopus shawii Wilson | Leucobryaceae | Perennial | MEX-AZ-UK | Campshaw | 11 | 9 | 1 | 1 | |||||||||
| Cyclodictyon laetevirens (Hook. & Taylor) Mitt. | Pilotrichaceae | Colonist | EUR | Cycllaet | 12 | 6 | 3 | 2 | 1 | ||||||||
| Didymodon sp. 1 | Pottiaceae | Colonist | COSM | Didysp.1 | 1 | 1 | |||||||||||
| Didymodon sp. 2 | Pottiaceae | Colonist | COSM | Didysp.2 | 1 | 1 | |||||||||||
| Epipterygium atlanticum Hanusch | Mniaceae | Colonist | EUR | Epiptatla | 22 | 2 | 7 | 1 | 1 | 4 | 3 | 3 | 1 | ||||
| Fissidens asplenioides Hedw. | Fissidentaceae | Colonist | COSM | Fissaspl | 31 | 11 | 1 | 2 | 3 | 2 | 1 | 1 | 4 | 6 | |||
| Fissidens bryoides Hedw. | Fissidentaceae | Colonist | COSM | Fissbryo | 18 | 5 | 2 | 5 | 1 | 2 | 2 | 1 | |||||
| Fissidens crispus Mont. | Fissidentaceae | Colonist | COSM | Fisscris | 4 | 1 | 2 | 1 | |||||||||
| Fissidens dubius P.Beauv. | Fissidentaceae | Perennial | COSM | Fissdoub | 3 | 1 | 2 | ||||||||||
| Fissidens luisierii P. de la Varde | Fissidentaceae | Colonist | MAC | Fissluis | 57 | 17 | 9 | 1 | 9 | 5 | 6 | 3 | 2 | 5 | |||
| Fissidens pusillus (Wilson) Milde | Fissidentaceae | Colonist | COSM | Fisspusi | 2 | 1 | 1 | ||||||||||
| Fissidens serrulatus Brid. | Fissidentaceae | Colonist | COSM | Fissserr | 9 | 2 | 1 | 4 | 1 | 1 | |||||||
| Fissidens sp. 1 | Fissidentaceae | Colonist | Fisssp.1 | 1 | 1 | ||||||||||||
| Fissidens sp. 2 | Fissidentaceae | Colonist | Fisssp.2 | 1 | 1 | ||||||||||||
| Fissidens taxifolius Hedw. | Fissidentaceae | Colonist | COSM | Fisstaxi | 14 | 2 | 1 | 2 | 4 | 2 | 2 | 1 | |||||
| Fissidens viridulus (Sw.) Wahlenb. | Fissidentaceae | Colonist | COSM | Fissviri | 38 | 9 | 3 | 1 | 2 | 6 | 2 | 3 | 6 | 1 | 2 | 3 | |
| Heterocladium flaccidum (Schimp.) A.J.E.Sm. | Lembophyllaceae | Perennial | EUR | Heteflac | 63 | 23 | 5 | 22 | 1 | 2 | 2 | 1 | 4 | 1 | 2 | ||
| Heterocladium heteropterum (Brid.) Schimp. | Lembophyllaceae | Perennial | COSM | Heteheter | 1 | 1 | |||||||||||
| Hygroamblystegium varium (Hedw.) Mönk. | Amblystegiaceae | Perennial | COSM | Hygrvari | 5 | 3 | 1 | 1 | |||||||||
| Hypnum jutlandicum Holmen & E.Warncke | Hypnaceae | Perennial | COSM | Hypnjutl | 1 | 1 | |||||||||||
| Hypnum uncinulatum Jur. | Hypnaceae | Perennial | EUR | Hypnunci | 10 | 8 | 1 | 1 | |||||||||
| Kindbergia praelonga (Hedw.) Ochyra | Brachytheciaceae | Perennial | COSM | Kindprae | 59 | 22 | 5 | 4 | 7 | 6 | 2 | 3 | 5 | 1 | 4 | ||
| Leptodictyum riparium (Hedw.) Warnst. | Amblystegiaceae | Perennial | COSM | Leptripa | 3 | 2 | 1 | ||||||||||
| Leucobryum glaucum (Hedw.) Ångstr. | Leucobryaceae | Perennial | COSM | Leucglau | 2 | 2 | |||||||||||
| Leucobryum juniperoideum (Brid.) Müll.Hal. | Leucobryaceae | Perennial | COSM | Leucjuni | 7 | 7 | |||||||||||
| Mnium hornum Hedw. | Mniaceae | Long-lived shuttle | COSM | Mniumhorn | 1 | 1 | |||||||||||
| Myurium hochstetteri (Schimp.) Kindb. | Myuriaceae | Perennial | EUR | Myurhoch | 10 | 9 | 1 | ||||||||||
| Oxyrrhynchium hians (Hedw.) Loeske | Brachytheciaceae | Colonist | COSM | Oxyrhian | 2 | 2 | |||||||||||
| Oxyrrhynchium speciosum (Brid.) Warnst. | Brachytheciaceae | Perennial | COSM | Oxyrspec | 22 | 15 | 5 | 2 | |||||||||
| Philonotis rigida Brid. | Bartramiaceae | Long-lived shuttle | EUR | Philrigi | 7 | 4 | 2 | 1 | |||||||||
| Plagiomnium undulatum (Hedw.) T.J.Kop. | Mniaceae | Perennial | COSM | Plagundu | 11 | 10 | 1 | ||||||||||
| Plagiothecium succulentum (Wilson) Lindb. | Plagiotheciaceae | Perennial | COSM | Plagsucc | 47 | 23 | 11 | 1 | 1 | 5 | 6 | ||||||
| Polytrichum formosum Hedw. | Polytrichaceae | Perennial | COSM | Polyform | 5 | 5 | |||||||||||
| Pseudoscleropodium purum (Hedw.) M.Fleisch. | Brachytheciaceae | Perennial | COSM | Pseupuru | 1 | 1 | |||||||||||
| Pseudisothecium prolixum (Mitt.) Ignatova, Fedosov & Ignatov | Lembophyllaceae | Perennial | MAC | Pseuproli | 1 | 1 | |||||||||||
| Pseudotaxiphyllum laetevirens (Dixon & Luisier ex F.Koppe & Düll) Hedenäs | Plagiotheciaceae | Colonist | IBER-MAC | Pseulaet | 16 | 11 | 1 | 2 | 1 | 1 | |||||||
| Ptychostomum capillare (Hedw.) Holyoak & N.Pedersen | Bryaceae | Colonist | COSM | Ptyccapi | 2 | 2 | |||||||||||
| Sematophyllum substrumulosum (Hampe) E.Britton | Sematophyllaceae | Colonist | COSM | Semasubstr | 3 | 2 | 1 | ||||||||||
| Serpoleskea confervoides (Brid.) Schimp. | Amblystegiaceae | Perennial | COSM | Serpconf | 5 | 2 | 1 | 1 | 1 | ||||||||
| Sphagnum palustre L. | Sphagnaceae | Long-lived shuttle | COSM | Sphapalu | 13 | 13 | |||||||||||
| Tetrastichium fontanum (Mitt.) Cardot | Leucomiaceae | Perennial | IBER-MAC | Tetrfont | 90 | 27 | 5 | 7 | 23 | 5 | 4 | 4 | 6 | 5 | 4 | ||
| Tetrastichium virens (Cardot) S.P.Churchill | Leucomiaceae | Perennial | IBER-MAC | Tetrvire | 13 | 5 | 1 | 1 | 4 | 2 | |||||||
| Thamnobryum alopecurum (Hedw.) Gangulee | Neckeraceae | Perennial | COSM | Thamalop | 2 | 1 | 1 | 1 | |||||||||
| Thamnobryum maderense (Kindb.) Hedenäs | Neckeraceae | Perennial | MAC | Thammade | 2 | 2 | |||||||||||
| Thamnobryum rudolphianum Mastracci | Neckeraceae | Perennial | AZOR | Thamrudo | 21 | 19 | 2 | ||||||||||
| Thuidium tamariscinum (Hedw.) Schimp. | Thuidiaceae | Perennial | COSM | Thuitama | 13 | 7 | 3 | 1 | 1 | 1 | |||||||
| Trichostomum brachydontium Bruch | Pottiaceae | Perennial | COSM | Tricbrac | 7 | 1 | 1 | 1 | 3 | 1 | 1 | ||||||
Appendix B
| Hill | ALL | ALG | BRA | ACH | MAD | MAL | TER | CHO | COE | NAT | BAL | BUR | PRI | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S (q = 0) | Total | 98 | 65 | 31 | 17 | 17 | 41 | 28 | 20 | 21 | 24 | 28 | 24 | 20 |
| Mosses | 55 | 34 | 17 | 9 | 9 | 24 | 15 | 10 | 10 | 16 | 18 | 15 | 11 | |
| Liverworts | 43 | 31 | 14 | 8 | 8 | 17 | 13 | 10 | 11 | 8 | 10 | 9 | 9 | |
| exp H’ (q = 1) | Total | 50.46 | 37.70 | 21.05 | 10.72 | 13.44 | 19.40 | 20.22 | 12.22 | 16.33 | 17.22 | 20.89 | 17.44 | 14.55 |
| Mosses | 27.48 | 20.26 | 10.56 | 5.61 | 7.68 | 11.47 | 10.53 | 7.13 | 7.28 | 11.87 | 13.33 | 10.66 | 7.91 | |
| Liverworts | 23.01 | 17.48 | 10.50 | 5.74 | 6.26 | 8.04 | 9.95 | 8.22 | 9.16 | 5.57 | 7.63 | 7.23 | 6.73 | |
| 1/D (q = 2) | Total | 35.10 | 28.86 | 16.19 | 7.32 | 11.56 | 13.33 | 16.49 | 8.29 | 13.84 | 13.52 | 16.73 | 13.33 | 11.94 |
| Mosses | 18.57 | 16.08 | 7.52 | 3.99 | 6.92 | 7.27 | 8.63 | 5.50 | 5.96 | 9.69 | 10.33 | 8.27 | 6.48 | |
| Liverworts | 16.71 | 12.79 | 8.74 | 4.31 | 5.56 | 6.06 | 8.36 | 6.75 | 8.26 | 4.31 | 6.77 | 5.86 | 5.64 | |
| 1/d (q = 3) | Total | 29.81 | 24.93 | 13.61 | 5.80 | 10.48 | 11.15 | 14.60 | 6.55 | 12.36 | 11.50 | 14.28 | 11.20 | 10.74 |
| Mosses | 15.53 | 14.23 | 6.07 | 3.30 | 6.49 | 5.90 | 7.76 | 4.65 | 5.29 | 8.56 | 8.72 | 7.11 | 5.86 | |
| Liverworts | 14.42 | 10.72 | 7.82 | 3.58 | 5.20 | 5.25 | 7.43 | 5.79 | 7.76 | 3.69 | 6.38 | 4.99 | 5.12 |
| All | Liverworts | Mosses | |||||||
|---|---|---|---|---|---|---|---|---|---|
| SC (%) | Observed | Expected | SC (%) | Observed | Expected | SC (%) | Observed | Expected | |
| ALG | 97.36 | 65 | 67 | 97.24 | 31 | 32 | 97.47 | 34 | 35 |
| BRA | 90.1 | 31 | 34 | 98.22 | 13 | 13 | 82.77 | 18 | 22 |
| ACH | 79.88 | 17 | 21 | 84.55 | 8 | 9 | 76.66 | 9 | 12 |
| MAD | 89.84 | 17 | 19 | 92.88 | 7 | 8 | 87.55 | 10 | 11 |
| MAL | 91.61 | 41 | 45 | 90.03 | 17 | 19 | 92.95 | 24 | 26 |
| TER | 83.97 | 28 | 33 | 89.62 | 13 | 15 | 78.67 | 15 | 19 |
| CHO | 81.73 | 20 | 24 | 54.17 | 9 | 17 | 94.14 | 11 | 12 |
| COE | 77.83 | 21 | 27 | 82.33 | 11 | 13 | 74.8 | 10 | 13 |
| NAT | 81.35 | 24 | 30 | 91.39 | 8 | 9 | 76.49 | 16 | 21 |
| BAL | 85.84 | 28 | 33 | 88.92 | 10 | 11 | 84.6 | 18 | 21 |
| BUR | 75.48 | 24 | 32 | 74.64 | 9 | 12 | 76.31 | 15 | 20 |
| PRI | 75.6 | 20 | 26 | 74.86 | 9 | 12 | 76.33 | 11 | 14 |
References
- Cong, M.; Zhu, T.; Li, Y.; Yang, W.; Wei, Y. Ancient Ecological Disaster Site Is Now a Refuge: Bryophyte Diversity in Volcanic Lava Caves of Jingpo Lake World Geopark. Diversity 2023, 15, 842. [Google Scholar] [CrossRef]
- Kosznik-Kwaśnicka, K.; Golec, P.; Jaroszewicz, W.; Lubomska, D.; Piechowicz, L. Into the unknown: Microbial communities in caves, their role, and potential use. Microorganisms 2022, 10, 222. [Google Scholar] [CrossRef]
- Medellin, R.A.; Wiederholt, R.; Lopez-Hoffman, L. Conservation relevance of bat caves for biodiversity and ecosystem services. Biol. Conserv. 2017, 211, 45–50. [Google Scholar] [CrossRef]
- Rabelo, L.M.; Souza-Silva, M.; Ferreira, R.L. Priority caves for biodiversity conservation in a key karst area of Brazil: Comparing the applicability of cave conservation indices. Biodivers. Conserv. 2018, 27, 2097–2129. [Google Scholar] [CrossRef]
- Ficetola, G.F.; Canedoli, C.; Stoch, F. The Racovitzan impediment and the hidden biodiversity of unexplored environments. Conserv. Biol. 2019, 33, 214–216. [Google Scholar] [CrossRef] [PubMed]
- Whitten, T. Applying ecology for cave management in China and neighboring countries. J. Appl. Ecol. 2009, 46, 520–523. [Google Scholar] [CrossRef]
- Elliot, W.R. Biological dos and don’ts for cave restoration and conservation. In Cave Conservation and Restoration; National Speleological Society: Huntsville, AL, USA, 2006; pp. 33–46. [Google Scholar]
- Oromí, P.; Socorro, S. Biodiversity in the Cueva del Viento lava tube system (Tenerife, Canary Islands). Diversity 2021, 13, 226. [Google Scholar] [CrossRef]
- Riquelme, C.; Marshall Hathaway, J.J.; Enes Dapkevicius, M.D.L.; Miller, A.Z.; Kooser, A.; Northup, D.E.; Jurado, V.; Fernandez, O.; Saiz-Jimenez, Z.; Cheeptham, N. Actinobacterial diversity in volcanic caves and associated geomicrobiological interactions. Front. Microbiol. 2015, 6, 1342. [Google Scholar] [CrossRef]
- Ammons, N. Bryophytes of McKinney’s cave. Bryologist 1933, 36, 16–19. [Google Scholar] [CrossRef]
- Thatcher, E.P. Bryophytes of an artificially illuminated cave. Bryologist 1949, 52, 212–214. [Google Scholar] [CrossRef]
- Mason-Williams, M.; Benson-Evans, K. Summary of results obtained during a preliminary investigation into the bacterial and botanical flora of caves in south Wales. Int. J. Speleol. 1967, 2, 397–402. [Google Scholar] [CrossRef][Green Version]
- Zhang, Z.; Peng, T.; Li, X.; Zhao, C. A study on the bryophytes of karst cave threshold at Kunming area in Yunnan province, P.R. China. Carsologica Sin. 2004, 23, 229–233. (In Chinese) [Google Scholar]
- Monro, A.K.; Bystriakova, N.; Fu, L.; Wen, F.; Wei, Y. Discovery of a diverse cave flora in China. PLoS ONE 2018, 13, e0190801. [Google Scholar] [CrossRef]
- Laenen, B.; Shaw, B.; Schneider, H.; Goffinet, B.; Paradis, E.; Désamoré, A.; Shaw, A.J. Extant diversity of bryophytes emerged from successive post-Mesozoic diversification bursts. Nat. Commun. 2014, 5, 5134. [Google Scholar] [CrossRef] [PubMed]
- Gensel, P.G. The earliest land plants. Annu. Rev. Ecol. Evol. Syst. 2008, 39, 459–477. [Google Scholar] [CrossRef]
- Mishler, B.D. The Biology of Bryophytes—Bryophytes aren’t just small tracheophytes. Am. J. Bot. 2001, 88, 2129–2131. [Google Scholar] [CrossRef]
- Shou-Qin, S.; Yan-Hong, W.; Gen-Xu, W.; Jun, Z.; Dong, Y.; Hai-Jian, B.; Ji, L. Bryophyte species richness and composition along an altitudinal gradient in Gongga Mountain, China. PLoS ONE 2013, 8, e58131. [Google Scholar] [CrossRef]
- Andrew, N.R.; Rodgerson, L.; Dunlop, M. Variation in invertebrate-bryophyte community structure at different spatial scales along altitudinal gradients. J. Biogeogr. 2003, 30, 731–746. [Google Scholar] [CrossRef]
- Frego, K.A. Bryophytes as potential indicators of forest integrity. For. Ecol. Manag. 2007, 242, 65–75. [Google Scholar] [CrossRef]
- Halpern, C.B.; Dovčiak, M.; Urgenson, L.S.; Evans, S.A. Substrates mediate responses of forest bryophytes to a gradient in overstory retention. Can. J. For. Res. 2014, 44, 855–866. [Google Scholar] [CrossRef]
- Vanderpoorten, A.; Goffinet, B. Introduction to Bryophyte Biology; Cambridge University Press: Cambridge, UK, 2009. [Google Scholar] [CrossRef]
- Baldwin, L.K.; Bradfield, G.E. Bryophyte responses to fragmentation in temperate coastal rainforests: A functional group approach. Biol. Conserv. 2007, 136, 408–422. [Google Scholar] [CrossRef]
- Cedrés-Perdomo, R.D.; Hernández-Hernández, R.; Emerson, B.C.; González-Mancebo, J.M. Multiple responses of bryophytes in a chronosequence of burnt areas in non-fire prone subtropical cloud forests. Perspect. Plant Ecol. Evol. Syst. 2023, 58, 125702. [Google Scholar] [CrossRef]
- Vieira, C.; Séneca, A.; Sérgio, C.; Ferreira, M.T. Bryophyte taxonomic and functional groups as indicators of fine scale ecological gradients in mountain streams. Ecol. Indic. 2012, 18, 98–107. [Google Scholar] [CrossRef]
- Hernández-Hernández, R.; Borges, P.A.; Gabriel, R.; Rigal, F.; Ah-Peng, C.; González-Mancebo, J.M. Scaling α-and β-diversity: Bryophytes along an elevational gradient on a subtropical oceanic Island (La Palma, Canary Islands). Appl. Veg. Sci. 2017, 28, 1209–1219. [Google Scholar] [CrossRef]
- Puglisi, M.; Privitera, M.; Minissale, P.; Costa, R. Diversity and ecology of the bryophytes in the cave environment: A study on the volcanic and karstic caves of Sicily. Plant Biosyst. 2019, 153, 134–146. [Google Scholar] [CrossRef]
- Singh, P.; Těšitel, J.; Plesková, Z.; Peterka, T.; Hájková, P.; Dítě, D.; Hájek, M. The ratio between bryophyte functional groups impacts vascular plants in rich fens. Appl. Veg. Sci. 2019, 22, 494–507. [Google Scholar] [CrossRef]
- Lett, S.; Jónsdóttir, I.S. Can bryophyte groups increase functional resolution in tundra ecosystems? Arct. Sci. 2021, 8, 609–637. [Google Scholar] [CrossRef]
- La Farge-England, C. Growth form, branching pattern, and perichaetial position in mosses: Cladocarpy and pleurocarpy redefined. Bryologist 1996, 99, 170–186. [Google Scholar] [CrossRef]
- Mägdefrau, K. Life-forms of bryophytes. In Bryophyte Ecology; Springer: Dordrecht, The Netherlands, 1982; pp. 45–58. [Google Scholar] [CrossRef]
- During, H.J. Life strategies of bryophytes: A preliminary review. Lindbergia 1979, 5, 2–18. [Google Scholar]
- Mulec, J.; Kubešova, S. Diversity of bryophytes in show caves in Slovenia and relation to light intensities. Acta Carsologica 2010, 39, 587–596. [Google Scholar] [CrossRef]
- Gaspar, C.; Borges, P.A.; Gaston, K.J. Diversity and Distribution of Arthropods in Native Forests of the Azores Archipelago; Universidade dos Açores: Ponta Delgada, Portugal, 2008. [Google Scholar]
- Triantis, K.A.; Borges, P.A.; Ladle, R.J.; Hortal, J.; Cardoso, P.; Gaspar, C.; Whittaker, R.J. Extinction debt on oceanic islands. Ecography 2010, 33, 285–294. [Google Scholar] [CrossRef]
- Elias, R.B.; Connor, S.E.; Góis-Marques, C.A.; Schaefer, H.; Silva, L.; Sequeira, M.M.; Moura, M.; Borges, P.A.V.; Gabriel, R. Is there solid evidence of widespread landscape disturbance in the Azores before the arrival of the Portuguese? Proc. Natl. Acad. Sci. USA 2022, 119, e2119218119. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, P.; Arnedo, M.A.; Triantis, K.A.; Borges, P.A. Drivers of diversity in Macaronesian spiders and the role of species extinctions. J. Biogeogr. 2010, 37, 1034–1046. [Google Scholar] [CrossRef]
- Gabriel, R.; Bates, J.W. Bryophyte community composition and habitat specificity in the natural forests of Terceira, Azores. Plant Ecol. 2005, 177, 125–144. [Google Scholar] [CrossRef]
- Borges, P.A.; Pereira, F.E.; Constância, J.P. Indicators of Conservation Value of Azorean Caves Based on Its Arthropod Fauna. In Proceedings of the XI International Symposium on Vulcanospeleology, Pico Island, Portugal, 12–18 May 2004; Espinasa-Pereña, R., Pint, J., Eds.; pp. 109–113. [Google Scholar]
- Reboleira, A.S.; Borges, P.A.; Gonçalves, F.; Serrano, A.R.; Oromí, P. The subterranean fauna of a biodiversity hotspot region-Portugal: An overview and its conservation. Int. J. Speleol. 2011, 40, 23–37. [Google Scholar] [CrossRef]
- Hathaway, J.J.M.; Garcia, M.G.; Balasch, M.M.; Spilde, M.N.; Stone, F.D.; Dapkevicius, M.D.L.N.; Northup, D.E. Comparison of bacterial diversity in Azorean and Hawai’ian lava cave microbial mats. Geomicrobiol. J. 2014, 31, 205–220. [Google Scholar] [CrossRef] [PubMed]
- Borges, P.A.; Oromí, P.; Serrano, A.R.; Amorim, I.R.; Pereira, F. Biodiversity patterns of cavernicolous ground-beetles and their conservation status in the Azores, with the description of a new species: Trechus isabelae n. sp. (Coleoptera: Carabidae: Trechinae). Zootaxa 2007, 1478, 21–31. [Google Scholar] [CrossRef]
- Borges, P.A.V.; Lamelas-Lopez, L.; Amorim, I.R.; Danielczak, A.; Boieiro, M.; Rego, C.; Hochkirch, A. Species conservation profiles of cave-dwelling arthropods from Azores, Portugal. Biodivers. Data J. 2019, 7, e32530. [Google Scholar] [CrossRef]
- Borges, P.V.; Serrano, A.R.; Amorim, I.R. New species of cave-dwelling beetles (Coleoptera: Carabidae: Trechinae) from the Azores. J. Nat. Hist. 2004, 38, 1303–1313. [Google Scholar] [CrossRef][Green Version]
- Stock, J.H. A new genus and species of Talitridae (Amphipoda) from a cave in Terceira, Azores. J. Nat. Hist. 1989, 23, 1109–1118. [Google Scholar] [CrossRef]
- Gabriel, R.; Dias, E. First approach to the study of the Algar do Carvão flora (Terceira, Azores). In Actas do 3° Congresso Nacional de Espeleologia e do 1° Encontro Internacional de Vulcanoespeleologia das Ilhas Atlânticas 1994 (30 de Setembro a 4 de Outubro de 1992); pp. 206–213.
- González-Mancebo, J.M.; Losada-Lima, A.; Hérnandez-Garcia, C.D. A contribution to the floristic knowledge of caves on the Azores. Mémoires Biospéologie 1991, 18, 219–226. [Google Scholar]
- Gabriel, R.; Pereira, F.; Borges, P.A.V.; Constância, J.P. Indicators of conservation value of Azorean caves based on its bryophyte flora at cave entrances. In Proceedings of the XI International Symposia on Vulcanospeleology, Pico Island, Azores, Portugal, 12–18 May 2004; pp. 114–118. [Google Scholar]
- Gabriel, R.; Pereira, F.; Câmara, S.; Homem, N.; Sousa, E.; Henriques, M.I. Bryophytes of lava tubes and volcanic pits from Graciosa Island (Azores, Portugal). In Proceedings of the XII International Symposium on Vulcanospeleology, Tepóztlan, Mexico, 2–7 July 2006; pp. 260–263. [Google Scholar]
- Gabriel, R.; Homem, N.; Couto, A.B.; Aranda, S.C.; Borges, P.A. Azorean bryophytes: A preliminary review of rarity patterns. Açoreana Rev. Estud. Açoreanos 2011, (Suppl. S7), 149–206. [Google Scholar]
- Forjaz, V.H. (Ed.) Atlas Básico dos Açores; OVGA: Ponta Delgada, Portugal, 2004; 112p, ISBN 9729746648. [Google Scholar]
- Nunes, J.C.; Barcelos, P.; Pereira, F.E.; Forjaz, V.H.; Borges, P.A. Monumento Natural Regional do Algar do Carvão (Ilha Terceira): Biodiversidade e Geodiversidade. Atlântida 2004, 49, 279–286. [Google Scholar]
- Hodgetts, N.G.; Söderström, L.; Blockeel, T.L.; Caspari, S.; Ignatov, M.S.; Konstantinova, N.A.; Lockhart, N.; Papp, B.; Schröck, C.; Sim-Sim, M.; et al. An annotated checklist of bryophytes of Europe, Macaronesia and Cyprus. J. Bryol. 2020, 42, 1–116. [Google Scholar] [CrossRef]
- Van Zuijlen, K.; Nobis, M.P.; Hedenäs, L.; Hodgetts, N.; Calleja Alarcón, J.A.; Albertos, B.; Bernhardt-Römermann, M.; Gabriel, R.; Garilleti, R.; Lara, F.; et al. Bryophytes of Europe Traits (BET) data set: A fundamental tool for ecological studies. J. Veg. Sci. 2023, 34, e13179. [Google Scholar] [CrossRef]
- Dierssen, K. Distribution, ecological amplitude and phyto-sociological characterization of European bryophytes. Bryophyt. Bibl. 2001, 56, 1–289. [Google Scholar]
- Azevedo, E.B. Modelação do Clima Insular à Escala Local. Modelo CIELO Aplicado à ilha Terceira. Ph.D. Thesis, University of the Azores, Angra do Heroísmo, Portugal, 1996. [Google Scholar]
- Azevedo, E.B.; Pereira, L.S.; Itier, B. Modelling the local climate in island environments: Water balance applications. Agric. Water Manag. 1999, 40, 393–403. [Google Scholar] [CrossRef]
- Azevedo, E.B.; Pereira, L.S.; Itier, B. Simulation of local climate in islands environments using a GIS integrated model. In Proceedings of the 2nd Inter-Regional Conference on Environment—Water 99: Emerging Technologies for Sustainable land Use and Water Management, Lausanne, Switzerland, 1–3 September 1999. [Google Scholar]
- Ferreira, M.T.; Cardoso, P.; Borges, P.A.; Gabriel, R.; de Azevedo, E.B.; Reis, F.; Araújo, M.B.; Elias, R.B. Effects of climate change on the distribution of indigenous species in oceanic islands (Azores). Clim. Chang. 2016, 138, 603–615. [Google Scholar] [CrossRef]
- Magurran, A.E. Species abundance distributions: Pattern or process? Funct. Ecol. 2005, 19, 177–181. [Google Scholar] [CrossRef]
- Chao, A.; Gotelli, N.J.; Hsieh, T.C.; Sander, E.L.; Ma, K.H.; Colwell, R.K.; Ellison, A.M. Rarefaction and extrapolation with Hill numbers: A framework for sampling and estimation in species diversity studies. Ecol. Monogr. 2014, 84, 45–67. [Google Scholar] [CrossRef]
- Hill, M.O. Diversity and evenness: A unifying notation and its consequences. Ecology 1973, 54, 427–432. [Google Scholar] [CrossRef]
- Krebs, C.J. Ecological Methodology, 2nd ed.; Harper Collins Publishers: New York, NY, USA, 1999. [Google Scholar]
- Berger, W.H.; Parker, F.L. Diversity of planktonic foraminifera in deep-sea sediments. Science 1970, 168, 1345–1347. [Google Scholar] [CrossRef] [PubMed]
- Jost, L. Partitioning diversity into independent alpha and beta components. Ecology 2007, 88, 2427–2439. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, P.; Rigal, F.; Carvalho, J.C.; Fortelius, M.; Borges, P.A.; Podani, J.; Schmera, D. Partitioning taxon, phylogenetic and functional beta diversity into replacement and richness difference components. J. Biogeogr. 2014, 41, 749–761. [Google Scholar] [CrossRef]
- Carvalho, J.C.; Cardoso, P.; Gomes, P. Determining the relative roles of species replacement and species richness differences in generating beta-diversity patterns. Glob. Ecol. Biogeogr. 2012, 21, 760–771. [Google Scholar] [CrossRef]
- Podani, J.; Schmera, D. A new conceptual and methodological framework for exploring and explaining pattern in presence-absence data. Oikos 2011, 120, 1625–1638. [Google Scholar] [CrossRef]
- Chao, A.; Kubota, Y.; Zelený, D.; Chiu, C.H.; Li, C.F.; Kusumoto, B.; Colwell, R.K. Quantifying sample completeness and comparing diversities among assemblages. Ecol. Res. 2020, 35, 292–314. [Google Scholar] [CrossRef]
- Hsieh, T.C.; Ma, K.H.; Chao, A.; Hsieh, M.T. ‘iNEXT’: An R package for rarefaction and extrapolation of species diversity (Hill numbers). Methods Ecol. Evol. 2016, 7, 1451–1456. [Google Scholar] [CrossRef]
- Chao, A.; Jost, L. Coverage-based rarefaction and extrapolation: Standardizing samples by completeness rather than size. Ecology 2012, 93, 2533–2547. [Google Scholar] [CrossRef]
- Chao, A.; Chiu, C.H.; Jost, L. Unifying species diversity, phylogenetic diversity, functional diversity, and related similarity and differentiation measures through hill numbers. Annu. Rev. Ecol. Evol. Syst. 2014, 45, 297–324. [Google Scholar] [CrossRef]
- The Jamovi Project. Jamovi, Version 2.3. 2022. Available online: https://www.jamovi.org (accessed on 23 November 2023).
- R Core Team. R: A Language and Environment for Statistical Computing, Version 4.1; R Packages Retrieved from MRAN Snapshot 2022-01-01. 2021. Available online: https://cran.r-project.org (accessed on 23 November 2023).
- De Caceres, M.; Jansen, F. Package ‘Indicspecies’. Relationship between Species and Groups of Sites. R Package Version. Available online: https://cran.r-project.org/web/packages/indicspecies/index.html (accessed on 3 September 2020).
- Oksanen, J. Vegan: Ecological Diversity. R Proj. 2013, 368, 1–11. Available online: https://mirror.linux.duke.edu/cran/web/packages/vegan/vignettes/diversity-vegan.pdf (accessed on 23 November 2023).
- Simpson, G.L.; Team, R.C.; Bates, D.M.; Oksanen, J.; Simpson, M.G.L. Package ‘Permute’: Functions for Generating Restricted Permutations of Data. Version 0.9-7. 2022, pp. 1–29. Available online: https://vps.fmvz.usp.br/CRAN/web/packages/permute/permute.pdf (accessed on 23 November 2023).
- Cardoso, P.; Rigal, F.; Carvalho, J.C. BAT–Biodiversity Assessment Tools, an R package for the measurement and estimation of alpha and beta taxon, phylogenetic and functional diversity. Methods Ecol. Evol. 2015, 6, 232–236. [Google Scholar] [CrossRef]
- Wickham, H.; Chang, W.; Wickham, M.H. Package ‘ggplot2’. Create Elegant Data Visualisations Using the Grammar of Graphics. Version 2023, 2, 1–304. Available online: https://CRAN.R-project.org/package=ggplot2 (accessed on 23 November 2023).
- Mills, B. MetBrewer: Color Palettes Inspired by Works at the Metropolitan Museum of Art. 2022. Available online: https://CRAN.R-project.org/package=MetBrewer (accessed on 23 November 2023).
- Ter Braak, C.J.; Smilauer, P. CANOCO Reference Manual and CanoDraw for Windows User’s Guide: Software for Canonical Community Ordination, Version 4.5. 2002. Available online: www.canoco.com (accessed on 10 November 2023).
- Lepš, J.; Šmilauer, P. Multivariate Analysis of Ecological Data Using CANOCO; Cambridge University Press: Cambridge, UK, 2003. [Google Scholar] [CrossRef]
- Gabriel, R.; Sjögren, E.; Schumacker, R.; Sérgio, C.; Frahm, J.; Sousa, E. Lista dos Briófitos (Bryophyta). In A List of the Terrestrial and Marine Biota from the Azores; Princípia Editora Lda: Cascais, Portugal, 2010; pp. 117–130. [Google Scholar] [CrossRef]
- IUCN. The IUCN Red List of Threatened Species. Version 2022-2. Available online: https://www.iucnredlist.org (accessed on 17 October 2023).
- Ah-Peng, C.; Wilding, N.; Kluge, J.; Descamps-Julien, B.; Bardat, J.; Chuah-Petiot, M.; Strasberg, D.; Hedderson, T.A.J. Bryophyte diversity and range size distribution along two altitudinal gradients: Continent vs. island. Acta Oecologica 2012, 42, 58–65. [Google Scholar] [CrossRef]
- Boch, S.; Martins, A.; Ruas, S.; Fontinha, S.; Carvalho, P.; Reis, F.; Sim-Sim, M. Bryophyte and macrolichen diversity show contrasting elevation relationships and are negatively affected by disturbances in laurel forests of Madeira Island. J. Veg. Sci. 2019, 30, 1122–1133. [Google Scholar] [CrossRef]
- Coelho, M.C.; Gabriel, R.; Hespanhol, H.; Borges, P.A.; Ah-Peng, C. Bryophyte diversity along an elevational gradient on Pico Island (Azores, portugal). Diversity 2021, 13, 162. [Google Scholar] [CrossRef]
- Polaíno-Martín, C.P.; Gabriel, R.; Jennings, L.; Amorin, I.R.; Henar-Sández, M.; Prieto, A.R. Crescendo na obscuridade: Briófitos da ilha Terceira em ambientes cavernícolas. Pingo Lava 2019, 43, 48–58. [Google Scholar]
- Henriques, D.S.; Borges, P.A.; Ah-Peng, C.; Gabriel, R. Mosses and liverworts show contrasting elevational distribution patterns in an oceanic island (Terceira, Azores): The influence of climate and space. J. Bryol. 2016, 38, 183–194. [Google Scholar] [CrossRef]
- Losada Lima, A.; Beltran Tejera, E. Study of the bryological flora of Monte de Agua García and Cerro del Lomo (Tenerife, Canary Islands). An. Jard. Bot. Madr. 1987, 44, 233–254. [Google Scholar]
- Cedrés-Perdomo, R.D.; Losada-Lima, A.; González-Montelongo, C.; Arencibia, M.C.L.; Pérez-Vargas, I. Flora briológica del Bosque del Adelantado (El Rosario, Tenerife). Vieraea 2017, 45, 313–322. [Google Scholar] [CrossRef]
- González-Mancebo, J.M.; Hernández-García, C.D. Bryophyte life strategies along an altitudinal gradient in El Canal y los Tiles (La Palma, Canary Islands). J. Bryol. 1996, 19, 243–255. [Google Scholar] [CrossRef]
- Luís, L.; Bergamini, A.; Figueira, R.; Sim-Sim, M. Riparian bryophyte communities on Madeira: Patterns and determinants of species richness and composition. J. Bryol. 2010, 32, 32–45. [Google Scholar] [CrossRef]
- Ren, H.; Wang, F.; Ye, W.; Zhang, Q.; Han, T.; Huang, Y.; Chu, G.; Hui, D.; Guo, Q. Bryophyte diversity is related to vascular plant diversity and microhabitat under disturbance in karst caves. Ecol. Indic. 2021, 120, 106947. [Google Scholar] [CrossRef]
- Belnap, J. Biological Soil Crusts: Structure, Function, and Management; Lange, O.L., Ed.; Springer: Berlin/Heidelberg, Germany, 2001; Volume 150, pp. 241–261. [Google Scholar]
- Cao, W.; Xiong, Y.; Zhao, D.; Tan, H.; Qu, J. Bryophytes and the symbiotic microorganisms, the pioneers of vegetation restoration in karst rocky desertification areas in southwestern China. Appl. Microbiol. Biotechnol. 2020, 104, 873–891. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Zhang, Z.; Shen, J.; Wang, Z. Bryophyte diversity in karst sinkholes affected by different degrees of human disturbance. Acta Soc. Bot. Pol. 2019, 88, 362. [Google Scholar] [CrossRef]
| Species | IUCN | Distribution |
|---|---|---|
| Liverworts | ||
| Acrobolbus azoricus | EN | AZOR |
| Asterella africana | VU | EUR-AFR |
| Calypogeia azorica | EN | MAC |
| Cololejeunea azorica | LC | MAC |
| Cololejeunea schaeferi | VU | MAC |
| Cololejeunea sintenisii | EN | COSM |
| Dumortiera hirsuta | NT | COSM |
| Frullania acicularis | NT | AZOR |
| Heteroscyphus denticulatus | NT | MAC |
| Lejeunea eckloniana | LC | EUR |
| Marchesinia mackaii | LC | EUR |
| Plagiochila longispina | EN | AMER-AZOR |
| Porella canariensis | LC | IBER-MAC |
| Radula carringtonii | NT | EUR |
| Radula holtii | NT | EUR |
| Radula wichurae | NT | MAC |
| Scapania gracilis | LC | EUR |
| Telaranea europaea | LC | EUR |
| Mosses | ||
| Alophosia azorica | NT | MAC |
| Andoa berthelotiana | VU | MAC |
| Cyclodictyon laetevirens | LC | EUR |
| Epipterygium atlanticum | LC | EUR |
| Fissidens luisierii | LC | MAC |
| Heterocladium flaccidum | LC | EUR |
| Hypnum uncinulatum | LC | EUR |
| Myurium hochstetteri | LC | EUR |
| Philonotis rigida | VU | EUR |
| Pseudisothecium prolixum | VU | MAC |
| Pseudotaxiphyllum laetevirens | NT | IBER-MAC |
| Tetrastichium fontanum | VU | IBER-MAC |
| Tetrastichium virens | NT | IBER-MAC |
| Thamnobryum maderense | NT | MAC |
| Thamnobryum rudolphianum | EN | AZOR |
| ALG | BRA | ACH | MAD | MAL | TER | CHO | COE | NAT | BAL | BUR | PRI | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BRA | 0.86 | |||||||||||
| ACH | 0.93 | 0.75 | ||||||||||
| MAD | 0.97 | 0.93 | 0.96 | |||||||||
| MAL | 0.66 | 0.74 | 0.88 | 0.99 | ||||||||
| TER | 0.87 | 0.61 | 0.74 | 0.94 | 0.76 | |||||||
| CHO | 0.94 | 0.68 | 0.81 | 0.89 | 0.90 | 0.70 | ||||||
| COE | 0.92 | 0.73 | 0.74 | 0.99 | 0.83 | 0.60 | 0.73 | |||||
| NAT | 0.93 | 0.84 | 0.90 | 0.96 | 0.85 | 0.74 | 0.89 | 0.77 | ||||
| BAL | 0.89 | 0.67 | 0.77 | 0.98 | 0.73 | 0.60 | 0.79 | 0.65 | 0.71 | |||
| BUR | 0.93 | 0.77 | 0.83 | 0.97 | 0.82 | 0.69 | 0.77 | 0.67 | 0.83 | 0.66 | ||
| PRI | 0.93 | 0.75 | 0.81 | 0.97 | 0.85 | 0.62 | 0.80 | 0.68 | 0.72 | 0.62 | 0.73 | |
| Total | 0.89 | 0.76 | 0.83 | 0.96 | 0.82 | 0.72 | 0.81 | 0.76 | 0.83 | 0.73 | 0.79 | 0.77 |
| TEMP | REHU | PREC | ELEV | SLOP | BRIG | ROUG | EVAP | MOIS | |
|---|---|---|---|---|---|---|---|---|---|
| Total richness | −0.39 | - | 0.200 | 0.241 | −0.268 | 0.355 | - | 0.326 | - |
| Liverworts | −0.371 | - | - | - | −0.202 | 0.309 | - | 0.329 | - |
| Mosses | −0.338 | - | 0.238 | 0.285 | −0.265 | 0.315 | −0.161 | 0.280 | - |
| Foliose | −0.310 | −0.278 | - | - | - | 0.367 | −0.194 | 0.431 | - |
| Thallose | −0.165 | 0.312 | 0.293 | 0.281 | −0.258 | - | 0.184 | - | - |
| Acrocarpous | - | - | - | - | −0.225 | 0.337 | - | 0.156 | - |
| Pleurocarpous | −0.445 | - | 0.241 | 0.322 | −0.207 | - | - | 0.223 | - |
| Colonist | - | - | - | - | −0.308 | 0.239 | - | - | - |
| Short-lived | - | - | - | - | - | 0.211 | - | 0.162 | - |
| Long-lived | - | −0.219 | - | - | - | 0.174 | - | 0.229 | - |
| Perennial | −0.550 | - | 0.235 | 0.334 | - | 0.301 | −0.206 | 0.372 | - |
| Mat | - | 0.163 | 0.170 | - | 0.183 | −0.240 | - | −0.254 | 0.169 |
| Turf | −0.173 | - | 0.270 | 0.236 | −0.215 | 0.335 | −0.218 | 0.214 | - |
| Weft | −0.251 | 0.193 | 0.215 | 0.242 | −0.178 | 0.378 | −0.222 | 0.312 | - |
| Cushion | - | - | - | 0.173 | - | 0.189 | −0.201 | 0.165 | - |
| Dendroid | −0.357 | - | 0.324 | 0.383 | - | - | - | 0.278 | 0.193 |
| Mean cover | −0.481 | 0.204 | 0.394 | 0.904 | −0.256 | 0.585 | −0.182 | −0.495 | 0.329 |
| Liverworts | −0.428 | 0.191 | 0.274 | 0.243 | - | 0.223 | - | −0.267 | 0.274 |
| Mosses | −0.296 | - | 0.242 | 0.270 | −0.270 | 0.290 | −0.215 | −0.250 | - |
| Foliose | −0.381 | −0.232 | - | - | - | 0.258 | −0.206 | 0.312 | - |
| Thallose | -0.202 | 0.308 | 0.349 | 0.299 | - | - | 0.172 | - | 0.266 |
| Acrocarpous | - | −0.172 | - | - | −0.206 | 0.313 | −0.176 | - | - |
| Pleurocarpous | −0.431 | 0.190 | 0.257 | 0.330 | −0.225 | - | −0.188 | 0.199 | - |
| Colonist | - | - | 0.190 | - | −0.236 | 0.238 | −0.236 | - | - |
| Short-lived | - | - | - | - | - | 0.208 | - | 0.158 | - |
| Long-lived | - | −0.184 | - | - | - | 0.160 | 0.160 | 0.186 | - |
| Perennial | −0.553 | 0.162 | 0.278 | 0.377 | - | 0.236 | −0.242 | 0.303 | - |
| Mat | −0.337 | - | - | - | - | - | - | - | −0.180 |
| Turf | −0.173 | - | 0.285 | 0.243 | −0.239 | 0.298 | −0.219 | - | - |
| Weft | −0.220 | 0.182 | 0.185 | 0.223 | −0.183 | 0.371 | −0.232 | 0.335 | - |
| Cushion | - | - | - | 0.174 | - | 0.186 | −0.198 | 0.162 | - |
| Dendroid | −0.345 | - | 0.316 | 0.374 | - | - | - | 0.276 | 0.184 |
| Cave | Species | Taxonomic Group | Life Strategy | Indicator Value |
|---|---|---|---|---|
| Algar do Carvão | Thamnobryum rudolphianum | Moss | Perennial | 0.710 *** |
| Sphagnum palustre * | Moss | Long-lived | 0.672 ** | |
| Plagiochila longispina * | Liverwort | Perennial | 0.648 ** | |
| Plagiomnium undulatum | Moss | Perennial | 0.570 ** | |
| Leucobryum juniperoideum * | Moss | Perennial | 0.508 * | |
| Lepidozia cupressina * | Liverwort | Perennial | 0.475 * | |
| Plagiochila exigua * | Liverwort | Perennial | 0.475 * | |
| Gruta Branca Opala | Frullania microphylla | Liverwort | Long-lived | 0.527 ** |
| Cololejeunea sintenisii | Liverwort | Short-lived | 0.508 ** | |
| Fissidens sp. 2 * | Moss | Colonist | 0.471 ** | |
| Gruta da Achada | Non-significant species | |||
| Gruta da Madre de Deus | Marchesinia mackaii * | Liverwort | Perennial | 1.00 *** |
| Radula wichurae * | Liverwort | Long-lived | 0.926 *** | |
| Porella canariensis | Liverwort | Long-lived | 0.831 *** | |
| Lejeunea eckoniana | Liverwort | Perennial | 0.807 *** | |
| Cololejeunea schaeferi * | Liverwort | Long-lived | 0.756 *** | |
| Hygroamblystegium varium | Moss | Perennial | 0.706 *** | |
| Brachythecium rutabulum | Moss | Colonist | 0.681 *** | |
| Fissidens crispus | Moss | Colonist | 0.590 ** | |
| Frullania tamarisci * | Liverwort | Long-lived | 0.535 ** | |
| Microlejeunea ulicina * | Liverwort | Long-lived | 0.535 ** | |
| Gruta da Malha | Non-significant species | |||
| Gruta da Terra Mole | Atrichum undulatum * | Moss | Colonist | 0.426 ** |
| Mnium hornum * | Moss | Long-lived | 0.426 ** | |
| Gruta do Chocolate | Lophocolea bidentata | Liverwort | Perennial | 0.696 *** |
| Cololejeunea azorica | Liverwort | Short-lived | 0.677 *** | |
| Gruta do Coelho | Odontoschisma sphagni | Liverwort | Colonist | 0.502 * |
| Gruta do Natal | Amblystegium serpens * | Moss | Perennial | 0.548 ** |
| Ptychostomum capillare * | Moss | Colonist | 0.548 ** | |
| Bryum argenteum * | Moss | Colonist | 0.447 ** | |
| Phaeoceros laevis * | Hornwort | Annual | 0.447 ** | |
| Gruta dos Balcões | Heterocladium heteropterum | Moss | Perennial | 0.577 ** |
| Leptodictyum riparium | Moss | Perennial | 0.471 * | |
| Radula carringtonii | Liverwort | Long-lived | 0.459 * | |
| Gruta dos Buracos | Leucobryum glaucum * | Moss | Perennial | 0.548 ** |
| Thamnobryum maderense * | Moss | Perennial | 0.548 ** | |
| Alophosia azorica * | Moss | - | 0.447 * | |
| Asterella africana * | Liverwort | Short-lived | 0.447 * | |
| Gruta dos Principiantes | Non-significant species | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cedrés-Perdomo, R.D.; Polaíno-Martín, C.; Jennings, L.; Gabriel, R. Seeking a Hideout: Caves as Refuges for Various Functional Groups of Bryophytes from Terceira Island (Azores, Portugal). Diversity 2024, 16, 58. https://doi.org/10.3390/d16010058
Cedrés-Perdomo RD, Polaíno-Martín C, Jennings L, Gabriel R. Seeking a Hideout: Caves as Refuges for Various Functional Groups of Bryophytes from Terceira Island (Azores, Portugal). Diversity. 2024; 16(1):58. https://doi.org/10.3390/d16010058
Chicago/Turabian StyleCedrés-Perdomo, Ruymán David, Clara Polaíno-Martín, Laura Jennings, and Rosalina Gabriel. 2024. "Seeking a Hideout: Caves as Refuges for Various Functional Groups of Bryophytes from Terceira Island (Azores, Portugal)" Diversity 16, no. 1: 58. https://doi.org/10.3390/d16010058
APA StyleCedrés-Perdomo, R. D., Polaíno-Martín, C., Jennings, L., & Gabriel, R. (2024). Seeking a Hideout: Caves as Refuges for Various Functional Groups of Bryophytes from Terceira Island (Azores, Portugal). Diversity, 16(1), 58. https://doi.org/10.3390/d16010058









