A Subsurface Stepping Stone Hypothesis for the Conquest of Land by Arthropods
Abstract
:1. Introduction
1.1. The Terrestrialization Problem
1.2. The Earliest Terrestrial Ecosystems
1.3. Arthropod Terrestrialization
1.4. Previous Hypotheses
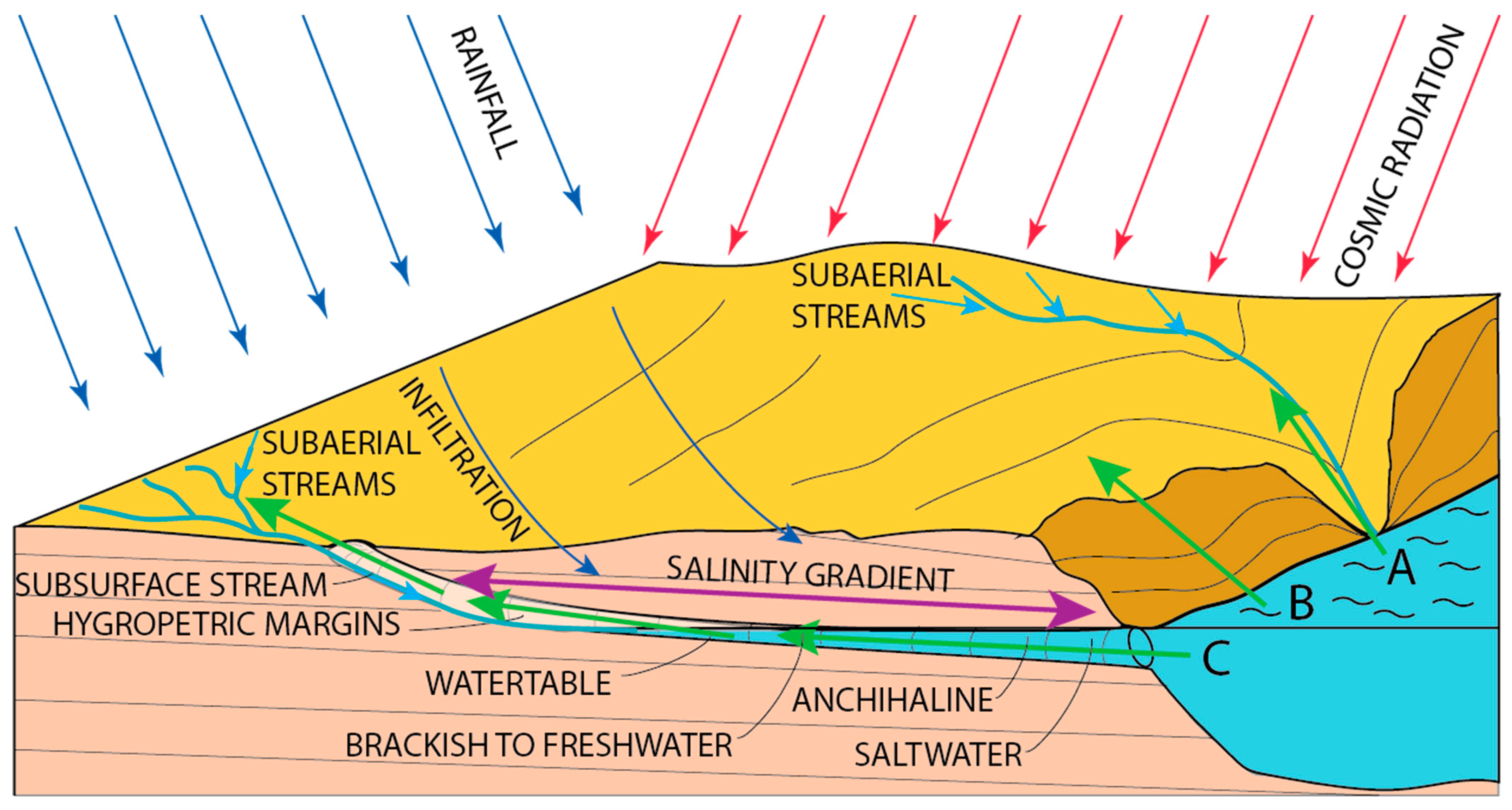
1.5. Advances in the Study of Chemoautotrophic Caves
2. Discussion
3. Conclusions
- Look for geological and fossil evidence of suitable ancient coastal/cave/paleokarst habitats existing in the Cambrian through Ordovician periods when molecular data suggests early arthropod diversification on land. The recovery of early Paleozoic fossils representing crown group or upper-stem group terrestrial arthropod taxa with identifiable adaptations to cave life or to bacterial mat feeding would help to substantiate the new hypothesis.
- Identify fossils with signs of incipient troglomorphy (eye/pigment loss, sensory elongation) that do not show full adaptation to caves. Transitional forms would be expected.
- Use modeling approaches to determine if subsurface habitats could have supported arthropod nutritional and respiratory needs before surface habitats developed. Energetic feasibility modeling could be informative.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Little, C. The Terrestrial Invasion—An Ecophysiological Approach to the Origins of Land Animals; Cambridge University Press: Cambridge, UK, 1990. [Google Scholar]
- Lozano-Fernandez, J.; Carton, R.; Tanner, A.R.; Puttick, M.N.; Blaxter, M.; Vinther, J.; Olesen, J.; Giribet, G.; Edgecombe, G.D.; Pisani, D. A molecular palaeobiological exploration of arthropod terrestrialization. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 20150133. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.P. Chelicerates and the conquest of land: A view of arachnid origins through an evo-devo spyglass. Integr. Comp. Biol. 2017, 57, 510–522. [Google Scholar] [CrossRef] [PubMed]
- Edwards, D.; Selden, P.A. The development of early terrestrial ecosystems. Bot. J. Scotl. 1992, 46, 337–366. [Google Scholar] [CrossRef]
- Rota-Stabelli, O.; Daley, A.C.; Pisani, D. Molecular timetrees reveal a Cambrian colonization of land and a new scenario for ecdysozoan evolution. Curr. Biol. 2013, 23, 392–398. [Google Scholar] [CrossRef]
- Wolfe, J.M.; Daley, A.C.; Legg, D.A.; Edgecombe, G.D. Fossil calibrations for the arthropod Tree of Life. Earth-Sci. Rev. 2016, 160, 43–110. [Google Scholar] [CrossRef]
- Howard, R.J.; Edgecombe, G.D.; Legg, D.A.; Pisani, D.; Lozano-Fernandez, J. Exploring the evolution and terrestrialization of scorpions (Arachnida: Scorpiones) with rocks and clocks. Org. Divers. Evol. 2019, 19, 71–86. [Google Scholar] [CrossRef]
- Kendall, B. Recent advances in geochemical paleo-oxybarometers. Annu. Rev. Earth Planet. Sci. 2021, 49, 399–433. [Google Scholar] [CrossRef]
- Gregory, B.S.; Claire, M.W.; Rugheimer, S. Photochemical modelling of atmospheric oxygen levels confirms two stable states. Earth Planet. Sci. Lett. 2021, 561, 116818. [Google Scholar] [CrossRef]
- Krause, A.J.; Mills, B.J.; Zhang, S.; Planavsky, N.J.; Lenton, T.M.; Poulton, S.W. Stepwise oxygenation of the Paleozoic atmosphere. Nat. Commun. 2018, 9, 4081. [Google Scholar] [CrossRef]
- Steemans, P.; Hérissé, A.L.; Melvin, J.; Miller, M.A.; Paris, F.; Verniers, J.; Wellman, C.H. Origin and radiation of the earliest vascular land plants. Science 2009, 324, 353. [Google Scholar] [CrossRef]
- Lüttge, U. Terrestrialization: The conquest of dry land by plants. In Progress in Botany; Springer International Publishing: Cham, Switzerland, 2020; Volume 83, pp. 65–89. [Google Scholar]
- Wellman, C.H.; Osterloff, P.L.; Mohiuddin, U. Fragments of the earliest land plants. Nature 2003, 425, 282–285. [Google Scholar] [CrossRef] [PubMed]
- Buschmann, H.; Holzinger, A. Understanding the algae to land plant transition. J. Exp. Bot. 2020, 71, 3241–3246. [Google Scholar] [CrossRef] [PubMed]
- Gan, T.; Luo, T.; Pang, K.; Zhou, C.; Zhou, G.; Wan, B.; Li, G.; Yi, Q.; Czaja, A.D.; Xiao, S. Cryptic terrestrial fungus-like fossils of the early Ediacaran Period. Nat. Commun. 2021, 12, 641. [Google Scholar] [CrossRef] [PubMed]
- Gan, T.; Zhou, G.; Luo, T.; Pang, K.; Zhou, M.; Luo, W.; Wang, S.; Xiao, S. Earliest Ediacaran speleothems and their implications for terrestrial life after the Marinoan snowball Earth. Precambrian Res. 2022, 376, 106685. [Google Scholar] [CrossRef]
- Clarke, J.T.; Warnock, R.C.M.; Donoghue, P.C.J. Establishing a time-scale for plant evolution. New Phytol. 2011, 192, 266–301. [Google Scholar] [CrossRef]
- Morris, J.L.; Puttick, M.N.; Clark, J.W.; Edwards, D.; Kenrick, P.; Pressel, S.; Wellman, C.H.; Yang, Z.H.; Schneider, H.; Donoghue, P.C.J. The timescale of early land plant evolution. Proc. Natl. Acad. Sci. USA 2018, 115, E2274–E2283. [Google Scholar] [CrossRef]
- Donoghue, P.C.J.; Harrison, C.J.; Paps, J.; Schneider, H. The evolutionary emergence of land plants. Curr. Biol. 2021, 31, R1281–R1298. [Google Scholar] [CrossRef]
- Lozano-Fernandez, J.; Tanner, A.R.; Puttick, M.N.; Vinther, J.; Edgecombe, G.D.; Pisani, D. A Cambrian–Ordovician terrestrialization of arachnids. Front. Genet. 2020, 11, 182. [Google Scholar] [CrossRef]
- Wheat, C.W.; Wahlberg, N. Phylogenomic insights into the Cambrian explosion, the colonization of land and the evolution of flight in Arthropoda. Syst. Biol. 2013, 62, 93–109. [Google Scholar] [CrossRef]
- MacNaughton, R.B.; Cole, J.M.; Dalrymple, R.W.; Braddy, S.J.; Briggs, D.E.; Lukie, T.D. First steps on land: Arthropod trackways in Cambrian-Ordovician eolian sandstone, southeastern Ontario, Canada. Geology 2002, 30, 391–394. [Google Scholar] [CrossRef]
- Briggs, D.; Wright, J.; Suthren, R. Forum Comment—Death near the shoreline, not life on land: Ordovician arthropod trackways in the Borrowdale Volcanic Group, UK. Geology 2019, 47, e464. [Google Scholar] [CrossRef]
- Shillito, A.P.; Davies, N.S. Death near the shoreline, not life on land: Ordovician arthropod trackways in the Borrowdale Volcanic Group, UK. Geology 2019, 47, 55–58. [Google Scholar] [CrossRef]
- Lozano-Fernandez, J.; Giacomelli, M.; Fleming, J.F.; Chen, A.; Vinther, J.; Thomsen, P.F.; Glenner, H.; Palero, F.; Legg, D.A.; Iliffe, T.M.; et al. Pancrustacean evolution illuminated by taxon-rich genomic-scale data sets with an expanded remipede sampling. Genome Biol. Evol. 2019, 11, 2055–2070. [Google Scholar] [CrossRef] [PubMed]
- Schwentner, M.; Combosch, D.J.; Nelson, J.P.; Giribet, G. A phylogenomic solution to the origin of insects by resolving crustacean-hexapod relationships. Curr. Biol. 2017, 27, 1818–1824. [Google Scholar] [CrossRef]
- Haas, F.; Waloszek, D.; Hartenberger, R. Devonohexapodus bocksbergensis, a new marine hexapod from the Lower Devonian Hunsrück Slates, and the origin of Atelocerata and Hexapoda. Org. Divers. Evol. 2003, 3, 39–54. [Google Scholar] [CrossRef]
- Willmann, R. Reinterpretation of an alleged marine hexapod stem-group representative. Org. Divers. Evol. 2005, 5, 199–202. [Google Scholar] [CrossRef]
- Garrouste, R.; Clément, G.; Nel, P.; Engel, M.S.; Grandcolas, P.; D’Haese, C.; Lagebro, L.; Denayer, J.; Gueriau, P.; Lafaite, P.; et al. A complete insect from the Late Devonian period. Nature 2012, 488, 82–85. [Google Scholar] [CrossRef]
- Haug, C.; Haug, J.T. The presumed oldest flying insect: More likely a myriapod? PeerJ 2017, 5, e3402. [Google Scholar] [CrossRef]
- Ballesteros, J.A.; Sharma, P.P. A critical appraisal of the placement of Xiphosura (Chelicerata) with account of known sources of phylogenetic error. Syst. Biol. 2019, 68, 896–917. [Google Scholar] [CrossRef]
- Jeram, A.J.; Selden, P.A.; Edwards, D. Land animals in the Silurian: Arachnids and myriapods from Shropshire, England. Science 1990, 250, 658–661. [Google Scholar] [CrossRef]
- Dunlop, J.A.; Scholtz, G.; Selden, P.A. Water-to-land transitions. In Arthropod Biology and Evolution; Springer: Berlin/Heidelberg, Germany, 2013; pp. 417–439. [Google Scholar]
- Ben-Yakir, D. Direct and indirect effects of UV radiation. In Optical Manipulation of Arthropod Pests and Beneficials; Ben-Yakir, D., Ed.; CABI Digital Library: Vancouver, BC, Canada, 2020; pp. 49–59. [Google Scholar]
- Ben-Yakir, D.; Fereres, A. The effects of UV radiation on arthropods: A review of recent publications (2010–2015). Acta Hortic. 2016, 1134, 335–342. [Google Scholar] [CrossRef]
- Ward, P. Out of Thin Air: Dinosaurs, Birds, and Earth’s Ancient Atmosphere; National Academies Press: Cambridge, MA, USA, 2006. [Google Scholar]
- Selden, P.A.; Edwards, D. Colonisation of the land. Evol. Foss. Rec. 1989, 6, 122–152. [Google Scholar]
- Little, C. The Colonisation of Land: Origins and Adaptations of Terrestrial Animals; Cambridge University Press: Cambridge, MA, USA, 1983. [Google Scholar]
- Dunlop, J.A. Geological history and phylogeny of Chelicerata. Arthropod Struct. Dev. 2010, 39, 124–142. [Google Scholar] [CrossRef] [PubMed]
- Labandeira, C.C. The origin of herbivory on land: Initial patterns of plant tissue consumption by arthropods. Insect Sci. 2007, 14, 259–275. [Google Scholar] [CrossRef]
- De Deckker, P. Terrestrial ostracods in Australia. Aust. Mus. Mem. 1983, 18, 87–100. [Google Scholar] [CrossRef]
- Diesel, R.; Schubart, C.D.; Schuh, M. A reconstruction of the invasion of land by Jamaican crabs (Grapsidae: Sesarminae). J. Zool. 2000, 250, 141–160. [Google Scholar] [CrossRef]
- Retallack, G.J. Ordovician-Devonian lichen canopies before evolution of woody trees. Gondwana Res. 2022, 106, 211–223. [Google Scholar] [CrossRef]
- Delwiche, C.F.; Graham, L.E.; Thomson, N. Lignin-like compounds and sporopollen in Coleochaete, an algal model for land plant ancestry. Science 1989, 245, 399–401. [Google Scholar] [CrossRef]
- Graham, L.E.; Cook, M.E.; Busse, J.S. The origin of plants: Body plan changes contributing to a major evolutionary radiation. Proc. Natl. Acad. Sci. USA 2000, 97, 4535–4540. [Google Scholar] [CrossRef]
- Martin, M.W. Early Evolution of Terrestrial Arthropods—Paleontological and Molecular Evidence. Ph.D. Thesis, University of Toronto, Toronto, ON, Canada, 1999. [Google Scholar]
- Schram, F.R. Cladistic analysis of metazoan phyla and the placement of fossil problematica. In The Early Evolution of Metazoa and the Significance of Problematic Taxa; Cambridge University Press: Cambridge, UK, 1991; pp. 177–197. [Google Scholar]
- Ghilarov, M.S. The Peculiarities of the Soil as an Environment and Its Significance in Insect Evolution; Academic Science Publishing House: Moscow, Russia, 1949; 279p. [Google Scholar]
- van Straalen, N.M. Evolutionary terrestrialization scenarios for soil invertebrates. Pedobiologia 2021, 87, 150753. [Google Scholar] [CrossRef]
- Labandeira, C. Early history of arthropod and vascular plant associations. Annu. Rev. Earth Planet. Sci. 1998, 26, 329–377. [Google Scholar] [CrossRef]
- Tsurnamal, M.; Por, F.D. The subterranean fauna associated with the blind palaemonid prawn Typhlocaris galilea Calman. Int. J. Speleol. 1968, 3, 219–223. [Google Scholar] [CrossRef]
- Sarbu, S.M.; Kane, T.C.; Kinkle, B.K. A chemoautotrophically based cave ecosystem. Science 1996, 272, 1953–1955. [Google Scholar] [CrossRef] [PubMed]
- Engel, A.S. Microbial diversity of cave ecosystems. In Geomicrobiology: Molecular and Environmental Perspective; Barton, L.L., Mandl, M., Loy, A., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 219–238. [Google Scholar]
- Thurber, A.R.; Levin, L.A.; Rowden, A.A.; Sommer, S.; Linke, P.; Kröger, K. Microbes, macrofauna, and methane: A novel seep community fueled by aerobic methanotrophy. Limnol. Oceanogr. 2013, 58, 1640–1656. [Google Scholar] [CrossRef]
- Frumkin, A.; Chipman, A.D.; Naaman, I. An Isolated Chemolithoautotrophic ecosystem deduced from environmental isotopes: Ayyalon Cave (Israel). Front. Ecol. Evol. 2023, 10, 1040385. [Google Scholar] [CrossRef]
- Sarbu, S.M. Movile Cave: A chemoautotrophically based groundwater ecosystem. In Subterranean Ecosystems; Wilkens, H., Culver, D.C., Humphreys, W.F., Eds.; Elsevier: Amsterdam, The Netherlands, 2000; pp. 319–343. [Google Scholar]
- Por, F.D.; Dimentman, C.; Frumkin, A.; Naaman, I. Animal life in the chemoautotrophic ecosystem of the hypogenic groundwater cave of Ayyalon (Israel): A summing up. Nat. Sci. 2013, 5, 7–13–13. [Google Scholar] [CrossRef]
- Porter, M.L.; Engel, A.S.; Kane, T.C.; Kinkle, B.K. Productivity-diversity relationships from chemolithoautotrophically based sulfidic karst systems. Int. J. Speleol. 2009, 38, 27–40. [Google Scholar] [CrossRef]
- Guy-Haim, T.; Simon-Blecher, N.; Frumkin, A.; Naaman, I.; Achituv, Y. Multiple transgressions and slow evolution shape the phylogeographic pattern of the blind cave-dwelling shrimp Typhlocaris. PeerJ 2018, 11, e16690. [Google Scholar] [CrossRef]
- Guy-Haim, T.; Kolodny, O.; Frumkin, A.; Achituv, Y.; Velasquez, X.; Morov, A.R. Shedding light on the Ophel biome: The Trans-Tethyan phylogeography of the sulfide shrimp Tethysbaena (Peracarida: Thermosbaenacea) in the Levant. PeerJ 2023, in press. [Google Scholar] [CrossRef]
- Frumkin, A.; Dimentman, C.; Naaman, I. Biogeography of living fossils as a key for geological reconstruction of the East Mediterranean: Ayyalon-Nesher Ramla system, Israel. Quat. Int. 2022, 624, 168–180. [Google Scholar] [CrossRef]
- Rasmussen, B. Filamentous microfossils in a 3235-million-year-old volcanogenic massive sulphide deposit. Nature 2000, 405, 676–679. [Google Scholar] [CrossRef] [PubMed]
- Galvez, M.E.; Fischer, W.W.; Jaccard, S.L.; Eglinton, T.I. Materials and pathways of the organic carbon cycle through time. Nat. Geosci. 2020, 13, 535–546. [Google Scholar] [CrossRef]
- Lepot, K. Signatures of early microbial life from the Archean (4 to 2.5 Ga) eon. Earth-Sci. Rev. 2020, 209, 103296. [Google Scholar] [CrossRef]
- Hourdez, S.; Lallier, F.H. Adaptations to hypoxia in hydrothermal-vent and cold-seep invertebrates. Rev. Environ. Sci. Biotechnol. 2007, 6, 143–159. [Google Scholar] [CrossRef]
- Burmester, T. Origin and evolution of arthropod hemocyanins and related proteins. J. Comp. Physiol. B 2002, 172, 95–107. [Google Scholar] [PubMed]
- Bilandžija, H.; Hollifield, B.; Steck, M.; Meng, G.; Ng, M.; Koch, A.D.; Gračan, R.; Ćetković, H.; Porter, M.L.; Renner, K.J.; et al. Phenotypic plasticity as a mechanism of cave colonization and adaptation. eLife 2020, 9, e51830. [Google Scholar] [CrossRef] [PubMed]
- Ćurčić, B.P.M. Ayyalonia dimentmani ng, n. sp. (Ayyaloniini n. trib., Chthoniidae, Pseudoscorpiones) from a cave in Israel. Arch. Biol. Sci. 2008, 60, 331–339. [Google Scholar] [CrossRef]
- Fet, V.; Soleglad, M.E.; Zonstein, S.L. The genus Akrav Levy, 2007 (Scorpiones: Akravidae) revisited. Euscorpius 2011, 134, 1–49. [Google Scholar] [CrossRef]
- Santibáñez-López, C.E.; Francke, O.F.; Prendini, L. Shining a light into the world’s deepest caves: Phylogenetic systematics of the troglobiotic scorpion genus Alacran Francke, 1982 (Typhlochactidae: Alacraninae). Invertebr. Syst. 2014, 28, 643–664. [Google Scholar] [CrossRef]
- Harfoot, M.B.; Beerling, D.J.; Lomax, B.H.; Pyle, J.A. A two-dimensional atmospheric chemistry modeling investigation of Earth’s Phanerozoic O3 and near-surface ultraviolet radiation history. J. Geophys. Res. Atmos. 2007, 112, D07308. [Google Scholar] [CrossRef]
- Lenton, T.M.; Dahl, T.W.; Daines, S.J.; Mills, B.J.; Ozaki, K.; Saltzman, M.R.; Porada, P. Earliest land plants created modern levels of atmospheric oxygen. Proc. Natl. Acad. Sci. USA 2016, 113, 9704–9709. [Google Scholar] [CrossRef] [PubMed]
- Sønderholm, F.; Bjerrum, C.J. Minimum levels of atmospheric oxygen from fossil tree roots imply new plant–oxygen feedback. Geobiology 2021, 19, 250–260. [Google Scholar] [CrossRef] [PubMed]
- Prendini, L.; Francke, O.F.; Vignoli, V. Troglomorphism, trichobothriotaxy and typhlochactid phylogeny (Scorpiones, Chactoidea): More evidence that troglobitism is not an evolutionary dead-end. Cladistics 2010, 26, 117–142. [Google Scholar] [CrossRef] [PubMed]
- Copilaş-Ciocianu, D.; Fišer, C.; Borza, P.; Petrusek, A. Is subterranean lifestyle reversible? Independent and recent large-scale dispersal into surface waters by two species of the groundwater amphipod genus Niphargus. Mol. Phylogenetics Evol. 2018, 119, 37–49. [Google Scholar]
- Chipman, A.D.; Ferrier, D.E.; Brena, C.; Qu, J.; Hughes, D.S.; Schröder, R.; Torres-Oliva, M.; Znassi, N.; Jiang, H.; Almeida, F.C.; et al. The first myriapod genome sequence reveals conservative arthropod gene content and genome organisation in the centipede Strigamia maritima. PLoS Biol. 2014, 12, e1002005. [Google Scholar] [CrossRef] [PubMed]
- James, N.P.; Choquette, P.W. (Eds.) Paleokarst; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1988. [Google Scholar]
- Bosák, P.; Ford, D.C.; Glazek, J.; Horácek, I. (Eds.) Paleokarst: A Systematic and Regional Review; Elsevier: Amsterdam, The Netherlands, 1989. [Google Scholar]
- Sauro, F.; Pozzobon, R.; Massironi, M.; De Berardinis, P.; Santagata, T.; De Waele, J. Lava tubes on Earth, Moon and Mars: A review on their size and morphology revealed by comparative planetology. Earth-Sci. Rev. 2020, 209, 103288. [Google Scholar] [CrossRef]
- Wynne, J.J.; Titus, T.N.; Agha-Mohammadi, A.A.; Azua-Bustos, A.; Boston, P.J.; de León, P.; Demirel-Floyd, C.; De Waele, J.; Jones, H.; Malaska, M.J.; et al. Fundamental science and engineering questions in planetary cave esxploration. J. Geophys. Res. Planets 2021, 127, e2022JE007194. [Google Scholar] [CrossRef]
- Dai, X.; Wang, Y.; Luo, L.; Pfiffner, S.M.; Li, G.; Dong, Z.; Xu, Z.; Dong, H.; Huang, L. Detection of the deep biosphere in metamorphic rocks from the Chinese continental scientific drilling. Geobiology 2021, 19, 278–291. [Google Scholar] [CrossRef]
- D’Angeli, I.M.; Ghezzi, D.; Leuko, S.; Firrincieli, A.; Parise, M.; Fiorucci, A.; Vigna, B.; Addesso, R.; Baldantoni, D.; Carbone, C.; et al. Geomicrobiology of a seawater-influenced active sulfuric acid cave. PLoS ONE 2019, 14, e0220706. [Google Scholar] [CrossRef]
- Northup, D.E.; Melim, L.A.; Spilde, M.N.; Hathaway, J.J.M.; Garcia, M.G.; Moya, M.; Stone, F.D.; Boston, P.J.; Dapkevicius, M.L.N.E.; Riquelme, C.; et al. Lava cave microbial communities within mats and secondary mineral deposits: Implications for life detection on other planets. Astrobiology 2011, 11, 601–618. [Google Scholar] [CrossRef]
- Hathaway, J.J.M.; Garcia, M.G.; Balasch, M.M.; Spilde, M.N.; Stone, F.D.; Dapkevicius, M.D.L.N.; Amorim, I.R.; Gabriel, R.; Borges, P.A.; Northup, D.E. Comparison of bacterial diversity in Azorean and Hawai’ian lava cave microbial mats. Geomicrobiol. J. 2014, 31, 205–220. [Google Scholar] [CrossRef] [PubMed]
- Retallack, G.J. Ordovician land plants and fungi from Douglas Dam, Tennessee. J. Palaeosci. 2019, 68, 173–205. [Google Scholar] [CrossRef]
- Sendi, H.; Vršanský, P.; Podstrelena, L.; Hinkelman, J.; Kudelova, T.; Kúdela, M.; Vidlička, Ľ.; Ren, X.; Quicke, D.L. Nocticolid cockroaches are the only known dinosaur age cave survivors. Gondwana Res. 2020, 82, 288–298. [Google Scholar] [CrossRef]
- Osborne, A.R. The world’s oldest Caves: How did they survive and what can they tell us? Acta Carsologica 2007, 36, 133–142. [Google Scholar] [CrossRef]
- Schram, F.R. Crustacea; Oxford University Press: New York, NY, USA, 1986. [Google Scholar]
- Culver, D.C.; Pipan, T. The Biology of Caves and Other Subterranean Habitats; Oxford University Press: New York, NY, USA, 2019. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frumkin, A.; Chipman, A.D. A Subsurface Stepping Stone Hypothesis for the Conquest of Land by Arthropods. Diversity 2024, 16, 6. https://doi.org/10.3390/d16010006
Frumkin A, Chipman AD. A Subsurface Stepping Stone Hypothesis for the Conquest of Land by Arthropods. Diversity. 2024; 16(1):6. https://doi.org/10.3390/d16010006
Chicago/Turabian StyleFrumkin, Amos, and Ariel D. Chipman. 2024. "A Subsurface Stepping Stone Hypothesis for the Conquest of Land by Arthropods" Diversity 16, no. 1: 6. https://doi.org/10.3390/d16010006
APA StyleFrumkin, A., & Chipman, A. D. (2024). A Subsurface Stepping Stone Hypothesis for the Conquest of Land by Arthropods. Diversity, 16(1), 6. https://doi.org/10.3390/d16010006








