Sea Ice Dynamics and Planktonic Adaptations: A Study of Terra Nova Bay’s Mesozooplanktonic Community during the Austral Summer
Abstract
:1. Introduction
2. Materials and Methods
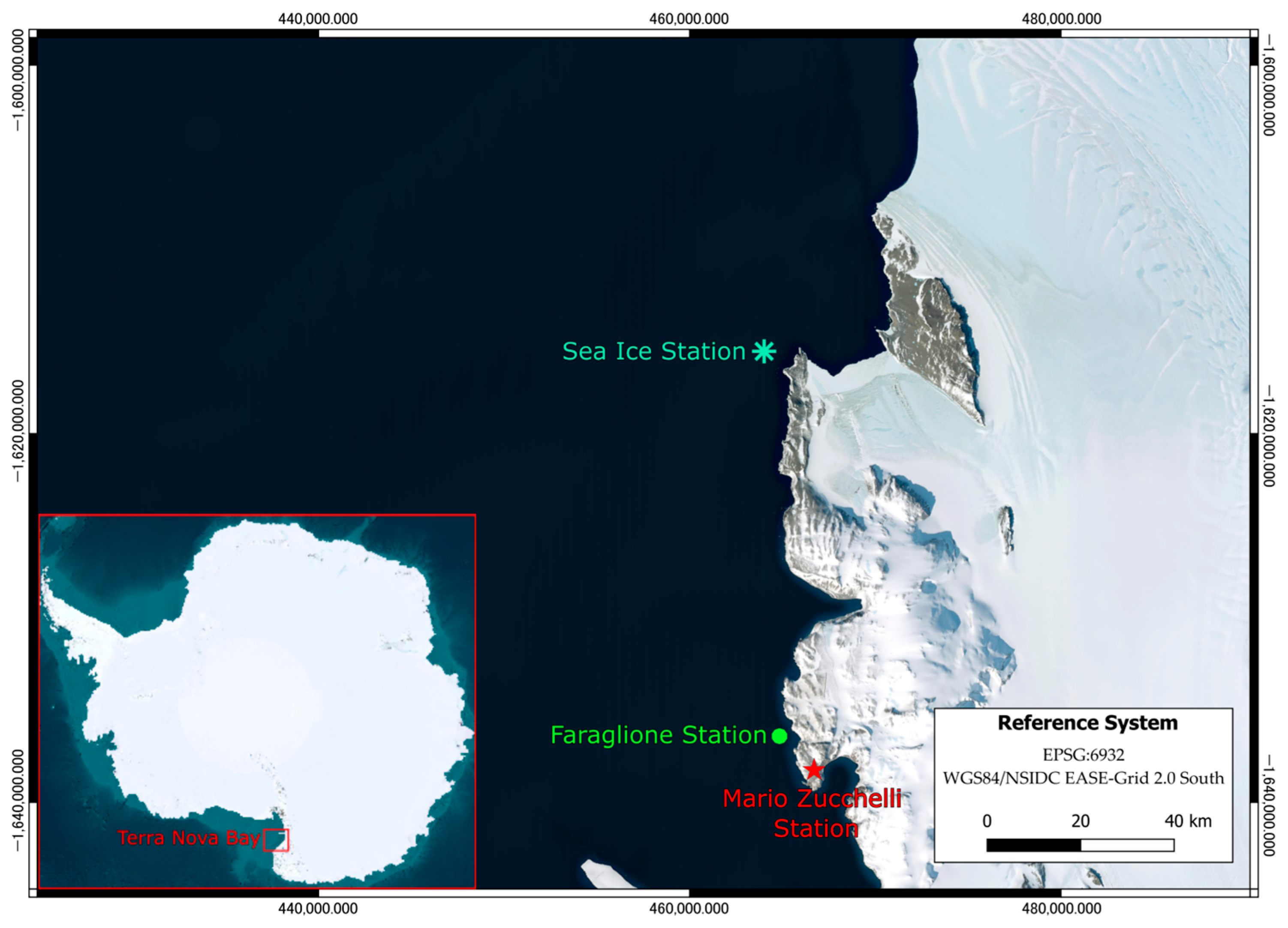
2.1. Study Area, Field and Laboratory Analysis
2.2. Data Processing
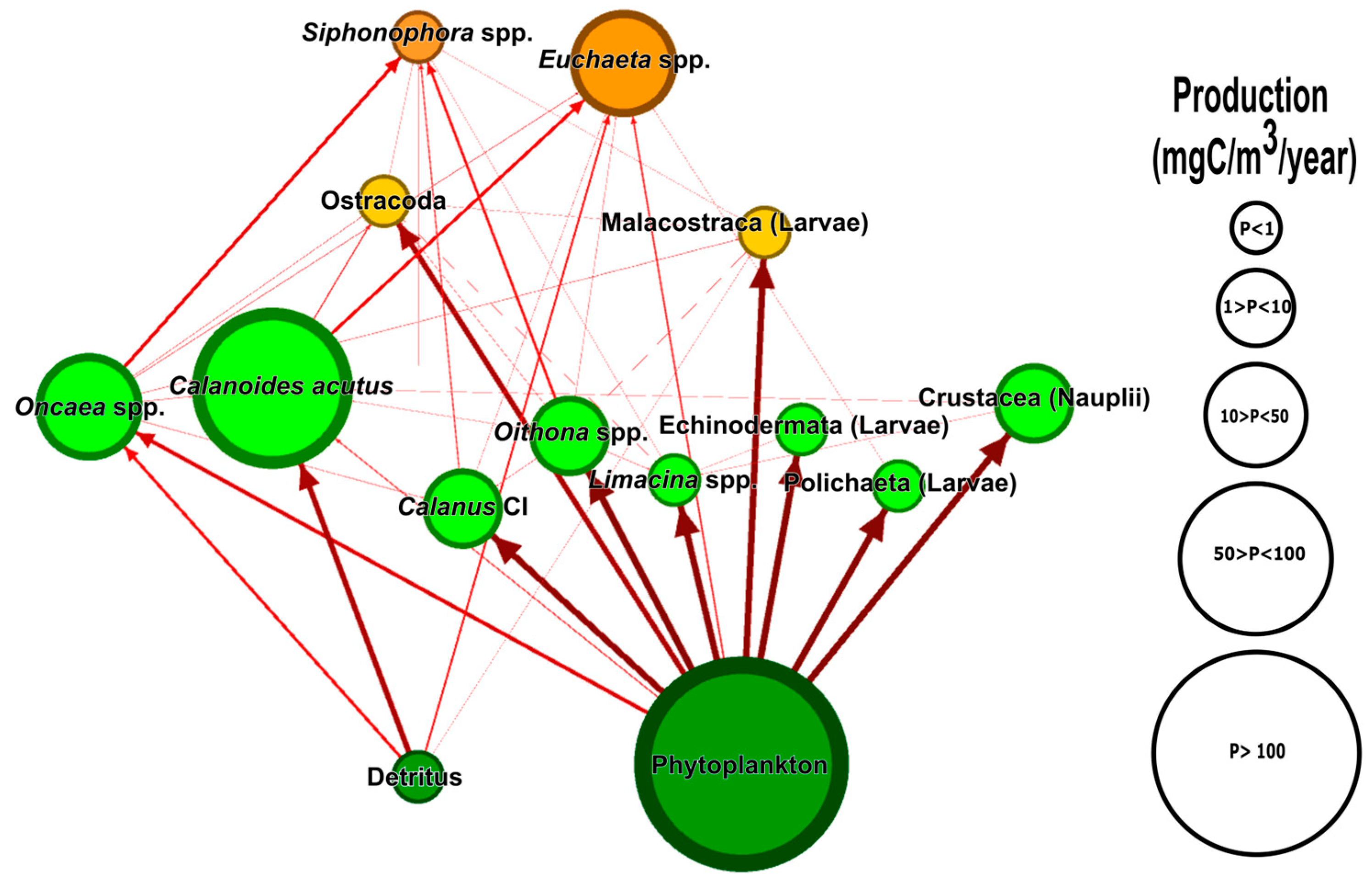
2.2.1. Mass Balanced Models
2.2.2. Ecological Indicators
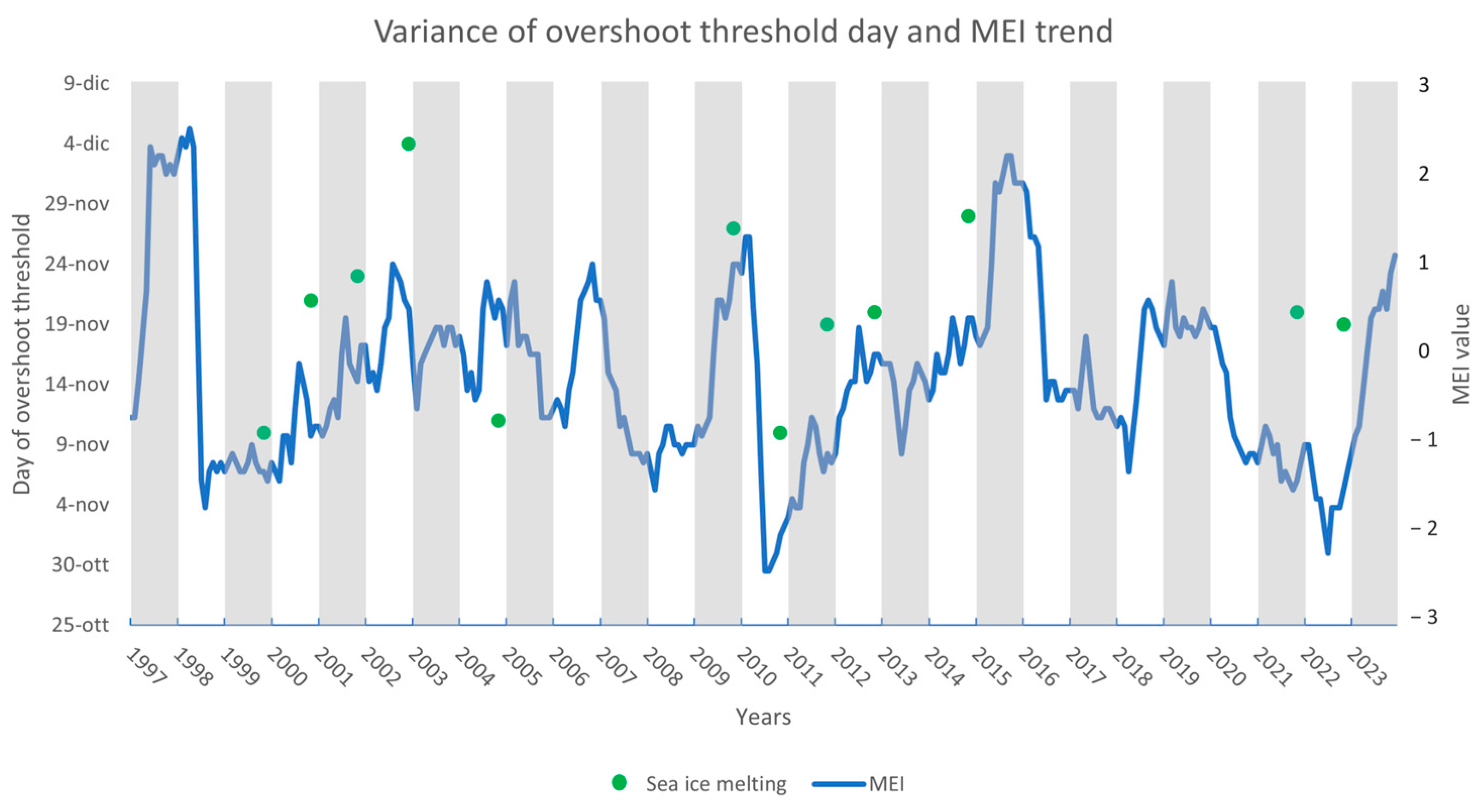
2.2.3. Days since Sea Ice Melting (DaSIM)
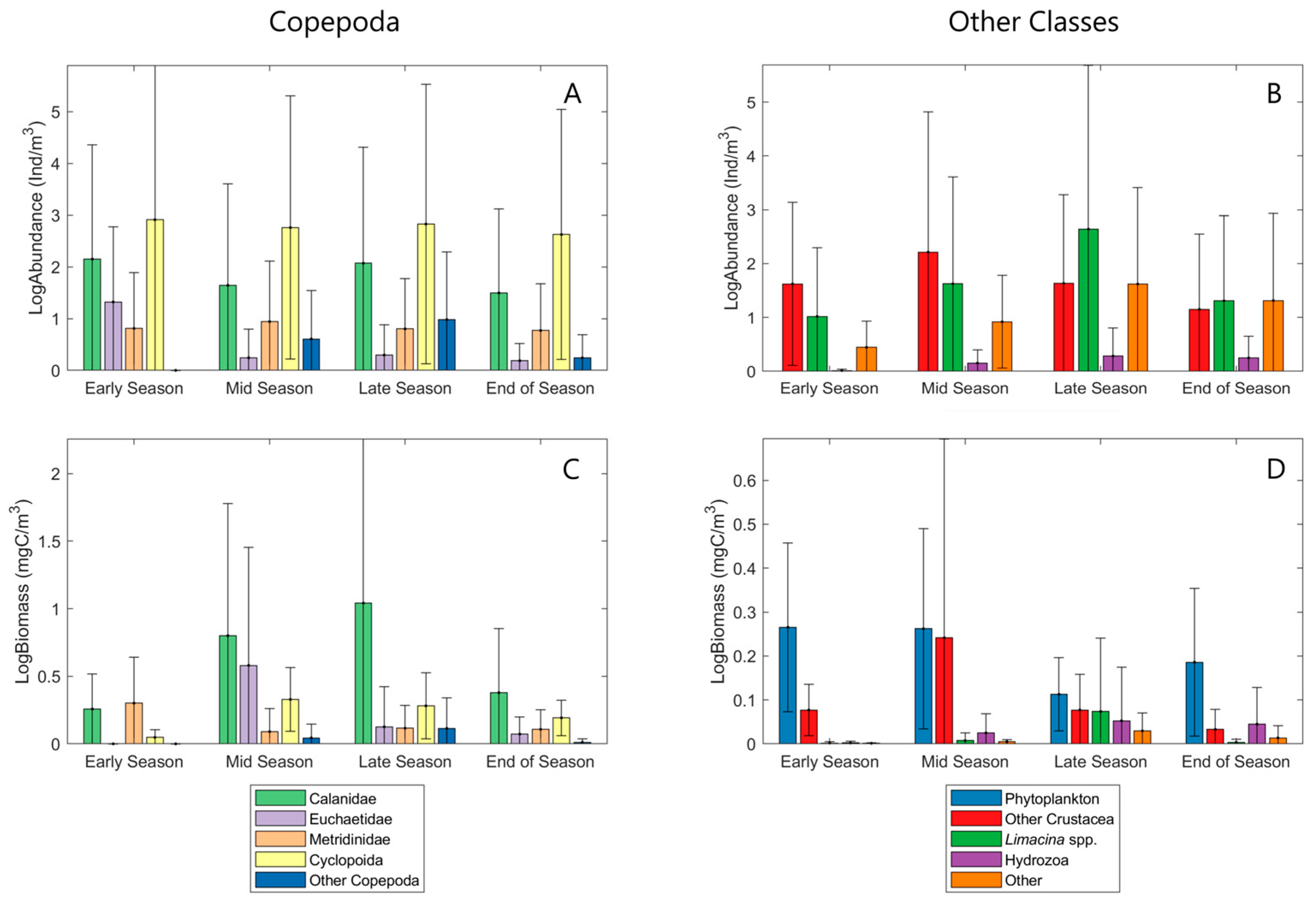
- Early Season: This is the initial phase of a season, characterized by the transition from the previous season. Weather patterns and environmental conditions begin to shift. For example, temperatures start to rise, ice coverage gets lower and the inner production is high due to solar radiation reaching the water body.
- Mid-Season: This stage marks the full development of the season’s typical characteristics. It represents the peak conditions of the season. For example, mid-summer is characterized by the hottest temperatures and longest days, and ice coverage is at a minimum.
- Late Season: This stage represents the gradual winding down of the season. The defining characteristics of the season start to weaken, and signs of the upcoming season begin to emerge. In late summer, for instance, temperatures warm up further while primary production is expected to decrease.
- End of Season: The final phase where the conditions transition into the next season. By the end of the summer, most of its distinct traits have faded. For instance, cooler temperatures signal the onset of fall.
2.2.4. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Deppeler, S.L.; Davidson, A.T. Southern Ocean Phytoplankton in a Changing Climate. Front. Mar. Sci. 2017, 4, 40. [Google Scholar] [CrossRef]
- Catalano, G.; Povero, P.; Fabiano, M.; Benedetti, F.; Goffart, A. Nutrient Utilisation and Particulate Organic Matter Changes during Summer in the Upper Mixed Layer (Ross Sea, Antarctica). Deep Sea Res. Part I Oceanogr. Res. Pap. 1997, 44, 97–112. [Google Scholar] [CrossRef]
- Smith, W.O.; Nelson, D.M. Phytoplankton Bloom Produced by a Receding Ice Edge in the Ross Sea: Spatial Coherence with the Density Field. Science 1985, 227, 163–166. [Google Scholar] [CrossRef] [PubMed]
- Kasyan, V.V. Recent Changes in Composition and Distribution Patterns of Summer Mesozooplankton off the Western Antarctic Peninsula. Water 2023, 15, 1948. [Google Scholar] [CrossRef]
- Johnston, N.M.; Murphy, E.J.; Atkinson, A.; Constable, A.J.; Cotté, C.; Cox, M.; Daly, K.L.; Driscoll, R.; Flores, H.; Halfter, S.; et al. Status, Change, and Futures of Zooplankton in the Southern Ocean. Front. Ecol. Evol. 2022, 9, 624692. [Google Scholar] [CrossRef]
- Stevens, C.J.; Pakhomov, E.A.; Robinson, K.V.; Hall, J.A. Mesozooplankton Biomass, Abundance and Community Composition in the Ross Sea and the Pacific Sector of the Southern Ocean. Polar Biol. 2015, 38, 275–286. [Google Scholar] [CrossRef]
- Lin, Y.; Yang, Q.; Mazloeff, M.; Wu, X.; Tian-Kunze, X.; Kaleschke, L.; Yu, L.; Chen, D. Transiting Consolidated Ice Strongly Influenced Polynya Area during a Shrink Event in Terra Nova Bay in 2013. Commun. Earth Environ. 2023, 4, 54. [Google Scholar] [CrossRef]
- Jendersie, S.; Williams, M.J.M.; Langhorne, P.J.; Robertson, R. The Density-Driven Winter Intensification of the Ross Sea Circulation. J. Geophys. Res. Oceans 2018, 123, 7702–7724. [Google Scholar] [CrossRef]
- Orsi, A.H.; Wiederwohl, C.L. A Recount of Ross Sea Waters. Deep Sea Res. II Top. Stud. Oceanogr. 2009, 56, 778–795. [Google Scholar] [CrossRef]
- Miller, U.K.; Zappa, C.J.; Gordon, A.L.; Yoon, S.T.; Stevens, C.; Lee, W.S. High Salinity Shelf Water Production Rates in Terra Nova Bay, Ross Sea from High-Resolution Salinity Observations. Nat. Commun. 2024, 15, 373. [Google Scholar] [CrossRef]
- Semprucci, F.; Appolloni, L.; Grassi, E.; Donnarumma, L.; Cesaroni, L.; Tirimberio, G.; Chianese, E.; Di Donato, P.; Russo, G.F.; Balsamo, M.; et al. Antarctic Special Protected Area 161 as a Reference to Assess the Effects of Anthropogenic and Natural Impacts on Meiobenthic Assemblages. Diversity 2021, 13, 626. [Google Scholar] [CrossRef]
- Pane, L.; Feletti, M.; Francomacaro, B.; Mariottini, G.L. Summer Coastal Zooplankton Biomass and Copepod Community Structure near the Italian Terra Nova Base (Terra Nova Bay, Ross Sea, Antarctica). J. Plankton Res. 2004, 26, 1479–1488. [Google Scholar] [CrossRef]
- Sertorio, T.Z.; Licandro, P.; Ossola, C.; Artegiani, A. Copepod Communities in the Pacific Sector of the Southern Ocean in Early Summer. In Ross Sea Ecology; Springer: Berlin/Heidelberg, Germany, 2000; pp. 291–307. [Google Scholar]
- Povero, P.; Chiantore, M.; Misic, C.; Budillon, G.; Cattaneo-Vietti, R. Land Forcing Controls Pelagic-Benthic Coupling in Adelie Cove (Terra Nova Bay, Ross Sea). Polar Biol. 2001, 24, 875–882. [Google Scholar] [CrossRef]
- Povero, P.; Castellano, M.; Ruggieri, N.; Monticelli, L.S.; Saggiomo, V.; Chiantore, M.; Guidetti, M.; Cattaneo-Vietti, R. Water Column Features and Their Relationship with Sediments and Benthic Communities along the Victoria Land Coast, Ross Sea, Summer 2004. Antarct. Sci. 2006, 18, 603–613. [Google Scholar] [CrossRef]
- Minutoli, R.; Bonanno, A.; Guglielmo, L.; Bergamasco, A.; Grillo, M.; Schiaparelli, S.; Barra, M.; Bergamasco, A.; Remirens, A.; Genovese, S.; et al. Biodiversity and Functioning of Mesozooplankton in a Changing Ross Sea. Deep Sea Res. Part II Top. Stud. Oceanogr. 2024, 217, 105401. [Google Scholar] [CrossRef]
- LTER Italy—MBT-IT17-005-M. Available online: https://deims.org/7fb8e2c6-b11f-41a7-b494-44ceeb3bed2d (accessed on 26 July 2024).
- Arrigo, K.R.; McClain, C.R. Spring Phytoplankton Production in the Western Ross Sea. Science 1994, 266, 261–263. [Google Scholar] [CrossRef]
- Smith, W.O.; Gordon, L.I. Hyperproductivity of the Ross Sea (Antarctica) Polynya during Austral Spring. Geophys. Res. Lett. 1997, 24, 233–236. [Google Scholar] [CrossRef]
- Mangoni, O.; Saggiomo, M.; Bolinesi, F.; Castellano, M.; Povero, P.; Saggiomo, V.; DiTullio, G.R. Phaeocystis Antarctica Unusual Summer Bloom in Stratified Antarctic Coastal Waters (Terra Nova Bay, Ross Sea). Mar. Environ. Res. 2019, 151, 104733. [Google Scholar] [CrossRef]
- Nuccio, C.; Innamorati, M.; Lazzara, L.; Mori, G.; Massi, L. Spatial and Temporal Distribution of Phytoplankton Assemblages in the Ross Sea. In Ross Sea Ecology, Italian Antarctic Expeditions (1987–1995); Faranda, F.M., Guglielmo, L., Ianora, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2000; pp. 231–245. [Google Scholar]
- Smith, W.O.; Ainley, D.G.; Arrigo, K.R.; Dinniman, M.S. The Oceanography and Ecology of the Ross Sea. Ann. Rev. Mar. Sci. 2014, 6, 469–487. [Google Scholar] [CrossRef]
- Mangoni, O.; Saggiomo, V.; Bolinesi, F.; Margiotta, F.; Budillon, G.; Cotroneo, Y.; Misic, C.; Rivaro, P.; Saggiomo, M. Phytoplankton Blooms during Austral Summer in the Ross Sea, Antarctica: Driving Factors and Trophic Implications. PLoS ONE 2017, 12, e0176033. [Google Scholar] [CrossRef]
- Knox, G.A. The Biology of the Southern Ocean; Cambridge University Press: Cambridge, UK, 1994. [Google Scholar]
- Hopkins, T.L.; Torres, J.J. Midwater Food Web in the Vicinity of a Marginal Ice Zone in the Western Weddell Sea. Deep Sea Res. Part A Oceanogr. Res. Pap. 1989, 36, 543–560. [Google Scholar] [CrossRef]
- Carli, A.; Pane, L.; Stocchino, C. Planktonic Copepods in Terra Nova Bay (Ross Sea): Distribution and Relationship with Environmental Factors. In Ross Sea Ecology; Springer: Berlin/Heidelberg, Germany, 2000. [Google Scholar]
- Christensen, V. A Model of Trophic Interactions in the North Sea in 1981, the Year of the Stomach. Dana 2003, 11, 1–18. [Google Scholar]
- Vidussi, F.; Claustre, H.; Bustillos-Guzmàn, J.; Cailliau, C.; Marty, J.C. Determination of Chlorophylls and Carotenoids of Marine Phytoplankton: Separation of Chlorophyll a from Divinylchlorophyll a and Zeaxanthin from Lutein. J. Plankton Res. 1996, 18, 2377–2382. [Google Scholar] [CrossRef]
- Holm-Hansen, O.; Lorenzen, C.J.; Holmes, R.W.; Strickland, J.D.H. Fluorometric Determination of Chlorophyll. ICES J. Mar. Sci. 1965, 30, 3–15. [Google Scholar] [CrossRef]
- Frontier, S. Calcul de l’erreur Sur Un Comptage de Zooplancton. J. Exp. Mar. Biol. Ecol. 1972, 8, 121–132. [Google Scholar] [CrossRef]
- Hunt, B.P.V.; Pakhomov, E.A.; Hosie, G.W.; Siegel, V.; Ward, P.; Bernard, K. Pteropods in Southern Ocean Ecosystems. Prog. Oceanogr. 2008, 78, 193–221. [Google Scholar] [CrossRef]
- Boysen-Ennen, E.; Hagen, W.; Hubold, G.; Piatkowski, U. Zooplankton Biomass in the Ice-Covered Weddell Sea, Antarctica. Mar. Biol. 1991, 111, 227–235. [Google Scholar] [CrossRef]
- Schnack-Schiel, S.B.; Hagen, W.; Mizdalski, E. Seasonal Comparison of Calanoides Acutus and Calanus Propinquus (Copepoda: Calanoida) in the Southeastern Weddel Sea, Antarctica. Mar. Ecol. Prog. Ser. 1991, 70, 17–27. [Google Scholar] [CrossRef]
- Vassallo, P.; Bellardini, D.; Castellano, M.; Dapueto, G.; Povero, P. Structure and Functionality of the Mesozooplankton Community in a Coastal Marine Environment: Portofino Marine Protected Area (Liguria). Diversity 2022, 14, 19. [Google Scholar] [CrossRef]
- Bednaršek, N.; Mozina, J.; Vogt, M.; O’Brien, C.; Tarling, G.A. The Global Distribution of Pteropods and Their Contribution to Carbonate and Carbon Biomass in the Modern Ocean. Earth Syst. Sci. Data 2012, 4, 167–186. [Google Scholar] [CrossRef]
- Bednaršek, N.; Tarling, G.A.; Fielding, S.; Bakker, D.C.E. Population Dynamics and Biogeochemical Significance of Limacina helicina Antarctica in the Scotia Sea (Southern Ocean). Deep Sea Res. II Top. Stud. Oceanogr. 2012, 59–60, 105–116. [Google Scholar] [CrossRef]
- Pond, D.W.; Ward, P. Importance of Diatoms for Oithona in Antarctic Waters. J. Plankton Res. 2011, 33, 105–118. [Google Scholar] [CrossRef]
- Garcia, M.D.; Hoffmeyer, M.S.; Abbate, M.C.L.; Barría de Cao, M.S.; Pettigrosso, R.E.; Almandoz, G.O.; Hernando, M.P.; Schloss, I.R. Micro- and Mesozooplankton Responses during Two Contrasting Summers in a Coastal Antarctic Environment. Polar Biol. 2016, 39, 123–137. [Google Scholar] [CrossRef]
- Kerkar, A.U.; Venkataramana, V.; Tripathy, S.C. Assessing the Trophic Link between Primary and Secondary Producers in the Southern Ocean: A Carbon-Biomass Based Approach. Polar Sci. 2022, 31, 100734. [Google Scholar] [CrossRef]
- Brey, T. Estimating Productivity of Macrobenthic Invertebrates from Biomass and Mean Individual Weight. Meeresforsch 1990, 32, 329–343. [Google Scholar]
- Misic, C.; Bavestrello, G.; Bo, M.; Borghini, M.; Castellano, M.; Covazzi Harriague, A.; Massa, F.; Spotorno, F.; Povero, P. The “Seamount Effect” as Revealed by Organic Matter Dynamics around a Shallow Seamount in the Tyrrhenian Sea (Vercelli Seamount, Western Mediterranean). Deep Sea Res. Part I Oceanogr. Res. Pap. 2012, 67, 1–11. [Google Scholar] [CrossRef]
- Paoli, C.; Morten, A.; Bianchi, C.N.; Morri, C.; Fabiano, M.; Vassallo, P. Capturing Ecological Complexity: OCI, a Novel Combination of Ecological Indices as Applied to Benthic Marine Habitats. Ecol. Indic. 2016, 66, 86–102. [Google Scholar] [CrossRef]
- Vassallo, P.; Paoli, C.; Fabiano, M. Ecosystem Level Analysis of Sandy Beaches Using Thermodynamic and Network Analyses: A Study Case in the NW Mediterranean Sea. Ecol. Indic. 2012, 15, 10–17. [Google Scholar] [CrossRef]
- Vassallo, P.; Paoli, C.; Schiavon, G.; Albertelli, G.; Fabiano, M. How Ecosystems Adapt to Face Disruptive Impact? The Case of a Commercial Harbor Benthic Community. Ecol. Indic. 2013, 24, 431–438. [Google Scholar] [CrossRef]
- Heymans, J.J.; Coll, M.; Link, J.S.; Mackinson, S.; Steenbeek, J.; Walters, C.; Christensen, V. Best Practice in Ecopath with Ecosim Food-Web Models for Ecosystem-Based Management. Ecol. Model. 2016, 331, 173–184. [Google Scholar] [CrossRef]
- Ulanowicz, R.E.; Scharler, U.M. Least-Inference Methods for Constructing Networks of Trophic Flows. Ecol. Model. 2008, 210, 278–286. [Google Scholar] [CrossRef]
- Vassallo, P.; D’Onghia, G.; Fabiano, M.; Maiorano, P.; Lionetti, A.; Paoli, C.; Sion, L.; Carlucci, R. A Trophic Model of the Benthopelagic Fauna Distributed in the Santa Maria Di Leuca Cold-Water Coral Province (Mediterranean Sea). Energy Ecol. Environ. 2017, 2, 114–124. [Google Scholar] [CrossRef]
- Christensen, V.; Walters, C.J. Ecopath with Ecosim: Methods, Capabilities and Limitations. Ecol. Model. 2004, 172, 109–139. [Google Scholar] [CrossRef]
- Odum, W.E.; Heald, E.J. The Detritus-Based Food Web of an Estuarine Mangrove Community. Estuar. Res. 1975, 1, 265–286. [Google Scholar]
- Odum, E.P. The Strategy of Ecosystem Development. Science 1969, 164, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Ulanowicz, R.E. Growth and Development: Ecosystem Phenomenology; Springer: New York, NY, USA, 1986. [Google Scholar]
- Scharler, U.M. Ecological Network Analysis, Ascendency. In Ecosystem Ecology; Jorgensen, S.E., Ed.; Elsevier B.V.: Amsterdam, The Netherlands, 2009; pp. 57–64. [Google Scholar]
- Gilbert, A.J. Connectance Indicates the Robustness of Food Webs When Subjected to Species Loss. Ecol. Indic. 2009, 9, 72–80. [Google Scholar] [CrossRef]
- Copernicus Climate Change Service (C3S) Climate Data Store (CDS). Copernicus Climate Change Service (C3S) (2024): Sea Ice Concentration Daily Gridded Data from 1979 to Present Derived from Satellite Observations. Available online: https://cds.climate.copernicus.eu/cdsapp#!/dataset/satellite-sea-ice-concentration?tab=form (accessed on 10 September 2024).
- The MathWorks Inc. Optimization Toolbox, Version: R2023b (23.2.0.2428915); The MathWorks Inc.: Natick, MA, USA, 2022. [Google Scholar]
- Hastie, T.; Tibshirani, R. Generalized Additive Models: Some Applications. J. Am. Stat. Assoc. 1987, 82, 371–386. [Google Scholar] [CrossRef]
- Wood, S.N. Fast Stable Restricted Maximum Likelihood and Marginal Likelihood Estimation of Semiparametric Generalized Linear Models. J. R. Stat. Soc. Ser. B Stat. Methodol. 2011, 73, 3–36. [Google Scholar] [CrossRef]
- Lecomte, O.; Goosse, H.; Fichefet, T.; De Lavergne, C.; Barthélemy, A.; Zunz, V. Vertical Ocean Heat Redistribution Sustaining Sea-Ice Concentration Trends in the Ross Sea. Nat. Commun. 2017, 8, 258. [Google Scholar] [CrossRef]
- Arrigo, K.R.; Van Dijken, G.L. Annual Changes in Sea-Ice, Chlorophyll a, and Primary Production in the Ross Sea, Antarctica. Deep Sea Res. Part II Top. Stud. Oceanogr. 2004, 51, 117–138. [Google Scholar] [CrossRef]
- Wolter, K.; Timlin, M.S. El Niño/Southern Oscillation Behaviour since 1871 as Diagnosed in an Extended Multivariate ENSO Index (MEI.Ext). Int. J. Climatol. 2011, 31, 1074–1087. [Google Scholar] [CrossRef]
- NOAA Physical Sciences Laboratory. Available online: https://psl.noaa.gov/enso/mei/ (accessed on 25 July 2024).
- Turner, J. The El Niño-Southern Oscillation and Antarctica. Int. J. Climatol. 2004, 24, 1–31. [Google Scholar] [CrossRef]
- Robinson, N.J.; Williams, M.J.M. Iceberg-Induced Changes to Polynya Operation and Regional Oceanography in the Southern Ross Sea, Antarctica, from in Situ Observations. Antarct. Sci. 2012, 24, 514–526. [Google Scholar] [CrossRef]
- Martin, S.; Drucker, R.S.; Kwok, R. The Areas and Ice Production of the Western and Central Ross Sea Polynyas, 1992–2002, and Their Relation to the B-15 and C-19 Iceberg Events of 2000 and 2002. J. Mar. Syst. 2007, 68, 201–214. [Google Scholar] [CrossRef]
- Harangozo, S.A.; Connolley, W.M. The Role of the Atmospheric Circulation in the Record Minimum Extent of Open Water in the Ross Sea in the 2003 Austral Summer. Atmosphere-Ocean 2006, 44, 83–97. [Google Scholar] [CrossRef]
- Brunt, K.M.; Sergienko, O.; MacAyeal, D.R. Observations of Unusual Fast-Ice Conditions in the Southwest Ross Sea, Antarctica: Preliminary Analysis of Iceberg and Storminess Effects. Ann. Glaciol. 2006, 44, 183–187. [Google Scholar] [CrossRef]
- Arrigo, K.R.; van Dijken, G.L. Impact of Iceberg C-19 on Ross Sea Primary Production. Geophys. Res. Lett. 2003, 30, 1836. [Google Scholar] [CrossRef]
- Smith, K.L. Free-Drifting Icebergs in the Southern Ocean: An Overview. Deep Sea Res. Part II Top. Stud. Oceanogr. 2011, 58, 1277–1284. [Google Scholar] [CrossRef]
- Rossi, L.; Sporta Caputi, S.; Calizza, E.; Careddu, G.; Oliverio, M.; Schiaparelli, S.; Costantini, M.L. Antarctic Food Web Architecture under Varying Dynamics of Sea Ice Cover. Sci. Rep. 2019, 9, 12454. [Google Scholar] [CrossRef]
- Saggiomo, V.; Carrada, G.C.; Mangoni, O.; D’alcalà, M.R.; Russo, A. Spatial and Temporal Variability of Size-Fractionated Biomass and Primary Production in the Ross Sea (Antarctica) during Austral Spring and Summer. J. Mar. Syst. 1998, 17, 115–127. [Google Scholar] [CrossRef]
- Arrigo, K.R.; Weiss, A.M.; Smith, W.O. Physical Forcing of Phytoplankton Dynamics in the Southwestern Ross Sea. J. Geophys. Res. Oceans 1998, 103, 1007–1021. [Google Scholar] [CrossRef]
- Saggiomo, M.; Escalera, L.; Bolinesi, F.; Rivaro, P.; Saggiomo, V.; Mangoni, O. Diatom Diversity during Two Austral Summers in the Ross Sea (Antarctica). Mar. Micropaleontol. 2021, 165, 101993. [Google Scholar] [CrossRef]
- Cau, A.; Ennas, C.; Moccia, D.; Mangoni, O.; Bolinesi, F.; Saggiomo, M.; Granata, A.; Guglielmo, L.; Swadling, K.M.; Pusceddu, A. Particulate Organic Matter Release below Melting Sea Ice (Terra Nova Bay, Ross Sea, Antarctica): Possible Relationships with Zooplankton. J. Mar. Syst. 2021, 217, 103510. [Google Scholar] [CrossRef]
- Granata, A.; Weldrick, C.K.; Bergamasco, A.; Saggiomo, M.; Grillo, M.; Bergamasco, A.; Swadling, K.M.; Guglielmo, L. Diversity in Zooplankton and Sympagic Biota during a Period of Rapid Sea Ice Change in Terra Nova Bay, Ross Sea, Antarctica. Diversity 2022, 14, 425. [Google Scholar] [CrossRef]
- Monti-Birkenmeier, M.; Diociaiuti, T.; Bolinesi, F.; Saggiomo, M.; Mangoni, O. Microzooplankton and Phytoplankton of Ross Sea Polynya Areas and Potential Linkage among Functional Traits. Deep Sea Res. II Top. Stud. Oceanogr. 2024, 216, 105393. [Google Scholar] [CrossRef]
- Monti, M.; Zoccarato, L.; Fonda Umani, S. Microzooplankton Composition under the Sea Ice and in the Open Waters in Terra Nova Bay (Antarctica). Polar Biol. 2017, 40, 891–901. [Google Scholar] [CrossRef]


| DaSIM | Temperature | Salinity | |
|---|---|---|---|
| Total Biomass | <0.001 | 0.067 | |
| Primary Production | <0.001 | ||
| Mean Trophic Level | 0.002 | 0.006 | |
| Total system throughput | 0.010 | ||
| Average mutual information | 0.003 | 0.049 | 0.033 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guida, A.; Povero, P.; Castellano, M.; Magozzi, S.; Paoli, C.; Novellino, A.; Donnarumma, L.; Appolloni, L.; Vassallo, P. Sea Ice Dynamics and Planktonic Adaptations: A Study of Terra Nova Bay’s Mesozooplanktonic Community during the Austral Summer. Diversity 2024, 16, 600. https://doi.org/10.3390/d16100600
Guida A, Povero P, Castellano M, Magozzi S, Paoli C, Novellino A, Donnarumma L, Appolloni L, Vassallo P. Sea Ice Dynamics and Planktonic Adaptations: A Study of Terra Nova Bay’s Mesozooplanktonic Community during the Austral Summer. Diversity. 2024; 16(10):600. https://doi.org/10.3390/d16100600
Chicago/Turabian StyleGuida, Alessandro, Paolo Povero, Michela Castellano, Sarah Magozzi, Chiara Paoli, Antonio Novellino, Luigia Donnarumma, Luca Appolloni, and Paolo Vassallo. 2024. "Sea Ice Dynamics and Planktonic Adaptations: A Study of Terra Nova Bay’s Mesozooplanktonic Community during the Austral Summer" Diversity 16, no. 10: 600. https://doi.org/10.3390/d16100600








