Abstract
Local ecological knowledge has been shown to convey key information about elusive mammal species. Many of Africa’s nocturnal mammals are not yet considered globally threatened, yet behavioural ecology and population trends across their diverse ranges remain effectively unknown. We conducted semi-structured oral and visual interviews with eight groups of Ba’Aka in three villages (n = 53 males; n = 17 females) using trigger cards, to gain insights into beliefs about nocturnal mammals in the Central African Republic (CAR). We aimed to (1) explore the presence and local knowledge of nocturnal mammals; (2) determine cultural attitudes regarding nocturnal species; and (3) report on Traditional Ecological Knowledge (TEK) in the CAR. Using trigger cards, interviewees identified nine nocturnal mammals. Frequency of key words was measured and presented in word clouds, depicting that angwantibos (Arctocebus aureus) (n = 14), genets (Genetta spp.) (n = 11) and tree hyrax (Dendrohyrax dorsalis) (n = 6) were strongly associated with the supernatural (witchcraft; spiritual protection). The traditional uses of cryptic nocturnal mammals in Ba’Aka culture, including as meat and medicine, may affect the conservation of these species. We suggest a need to protect and include nocturnal mammals with unknown or decreasing populations in wildlife management strategies and community conservation programmes.
Keywords:
indigenous; culture; taboo; tradition; cultural consensus; ecological knowledge; freelisting 1. Introduction
Indigenous knowledge passed down through generations influences different cultures in various ways, and certain animals can be perceived as good or bad luck [1]. Beliefs and supernatural representations are a major part of indigenous tribal culture and can influence attitudes towards some species, while reasons for selecting specific species are often unclear [2,3,4]. Acknowledging the diverse use of terminology reflecting beliefs and perceptions, in this study, we consider belief systems in the following ways. Traditional Ecological Knowledge (TEK) is the overall body of indigenous cultural practices, beliefs and ecological knowledge, linking humans to their environment and handed down through generations [5], whilst local ecological knowledge (LEK) is on a smaller, local scale [6,7,8]. Beliefs are accepted to be true based on the values, faith or integrity of a culture, which influence behaviour, attitudes and thoughts that provide satisfaction, regardless of evidence [9]. Taboos are customary beliefs that prohibit association with an object, person or place due to perceived negative consequences or harm. Taboos gain strength from the number of people who observe them [10,11].
Nocturnal species, particularly primates, owls and reptiles, are frequently considered to be negative omens within countries, regions and communities due to their curious appearance, cryptic behaviour and perturbing vocalisations. Furthermore, the nighttime activity of these animals may highlight associations with evil and danger. In Madagascar, aye-ayes (Daubentonia madagascariensis) and night snakes (Ithycyphus spp.) are often considered to signify early death, whether to individuals, families, communities or their crops and livestock, leading to deliberate targeting and destruction of these species throughout most of the country [12,13,14,15,16]. In parts of Africa, a range of nocturnal animals, including owls, snakes, hyaenas and small cats, are associated with the occult and are subject to fear and persecution [16]. Conversely, research has shown that beliefs about the same species vary between communities; for example, in south-east Madagascar, aye-ayes are believed to bring good luck in some villages [13]. In parts of the Democratic Republic of the Congo (DRC), meat from the East African potto (Perodicticus ibeanus), an animal known for the vice-like grip of its hands and feet, is consumed for medicinal purposes as it is believed to make pregnant women strong, while in the north-eastern DRC, potto meat is believed to cause a perilous childbirth because the baby grasps on to the womb [17,18]. In Ciptalegar, West Java, Javan slow lorises (Nycticebus javanicus) can bring misfortune, especially when brought into a home, whilst 400 km away in Pangandaran, inhabitants displayed indifference towards the species [19].
Negative associations may offer species protection due to people’s avoidance; however, some species are deliberately pursued for personal spiritual protection. It is claimed that animism is the oldest attribute of religion, and in some cultures, rituals are followed where natural objects are believed to possess souls, deliberately taking on specific traits of targeted animals in order to gain strength or courage [3]. Previous studies offer evidence that nocturnal animals have spiritual and medicinal uses [19,20,21,22,23]. Throughout Africa and Asia, pangolin parts are used for many ailments ranging from skin infections, wounds, rickets, breathing difficulties and impotence, but may also be used in spiritual practices [23]. In Vietnam, body parts of slow and pygmy lorises (Nycticebus spp., Xanthonycticebus spp.) are used to heal open wounds; they are consumed in wine in Cambodia to reduce childbirth pain [21]. Slender lorises (Loris tardigradus) are used in Sri Lankan and Indian medicine to cure ailments, such as leprosy, or are believed to bring misfortune to travellers. They are even used as living voodoo dolls to bring spiritual harm to targets [24]. Javan slow lorises (Nycticebus javanicus) are consumed to provide healing after childbirth and to alleviate stomach ailments but are also sold as talismans against the evil eye [19,21,25,26]. In Central Africa, burned skins and remains of golden angwantibo (Arctocebus aureus) and Milne-Edwards’s potto (Perodicticus edwardsi) were observed in the Republic of Congo for use in traditional practices and potto or angwantibo hearts have been used for protection against misfortune in the CAR [22]. The anecdotal evidence for animals used as medicine is so widespread that some species’ populations are declining at such a rate that it is imperative that the understanding of traditional practices and the impacts must be improved to conserve LEK in a way that does not exploit wildlife species to a great extent [27,28].
The urban demand for animals as bushmeat, in traditional medicine and for witchcraft practices, is causing a rapid decline in African mammal populations, mirroring the decline in mammals in Asia [18,22,26,28,29]. Poverty is a main driver behind the increased use of mammals for bushmeat and medicine [30,31]. African minority forest groups (Mbenga) are threatened by increased commercial logging, mining and protection of natural land resulting in the displacement of communities to other areas [32]. In the Central African Republic (CAR) and the Republic of Congo, hunter-gatherer Ba’Aka (or Aka) people inhabit the Dzanga-Sangha Protected Areas (APDS) and the larger Sangha Tri-National landscape (TNS) [33]. The rights of the Ba’Aka are protected by the World Wildlife Fund (WWF), which permits traditional hunting in areas outside the national park boundary. Though prey choice is opportunistic, favoured mammal prey species for the Ba’Aka include blue duiker (Philantomba monticola) (90%) and primates of the genera Cercocebus, Cercopithecus, Colobus and Lophocebus, all of which have shown population declines in their ranges [22,33,34,35].
There has been a neglect of studies of nocturnal animals in TEK studies, despite hunting being a known conservation threat for many nocturnal species. Such information can help researchers to explore the evolution of traditions within societies, which can help to leverage global support, social standing and resources for the environment and conservation organisations [5,36,37]. Moreover, there is an overriding need to determine the effect on populations of species that are used for these practices. We conducted this study to examine the importance of nocturnal mammals in Ba’Aka culture, with a focus on nocturnal primates, and to investigate whether any species are actively avoided or deliberately hunted and how LEK affects their conservation. Avoidance may offer protection to some species’ populations, though the requirement for meat may override the traditions [38]. On this basis, our research set out to find out whether the Ba’Aka (1) are familiar with certain species; (2) believe nocturnal mammals have spiritual properties; (3) attitudes towards some species were positively or negatively affected by beliefs; and (4) there is a possibility that cultural beliefs are affecting hunting pressure on nocturnal mammals. By gaining knowledge on cultural beliefs, conservationists can gauge hunting interest and purposes, attitudes towards specific species and increase conservation awareness to highlight unsustainable hunting threats on nocturnal mammals in the APDS.
2. Materials and Methods
2.1. Study Site
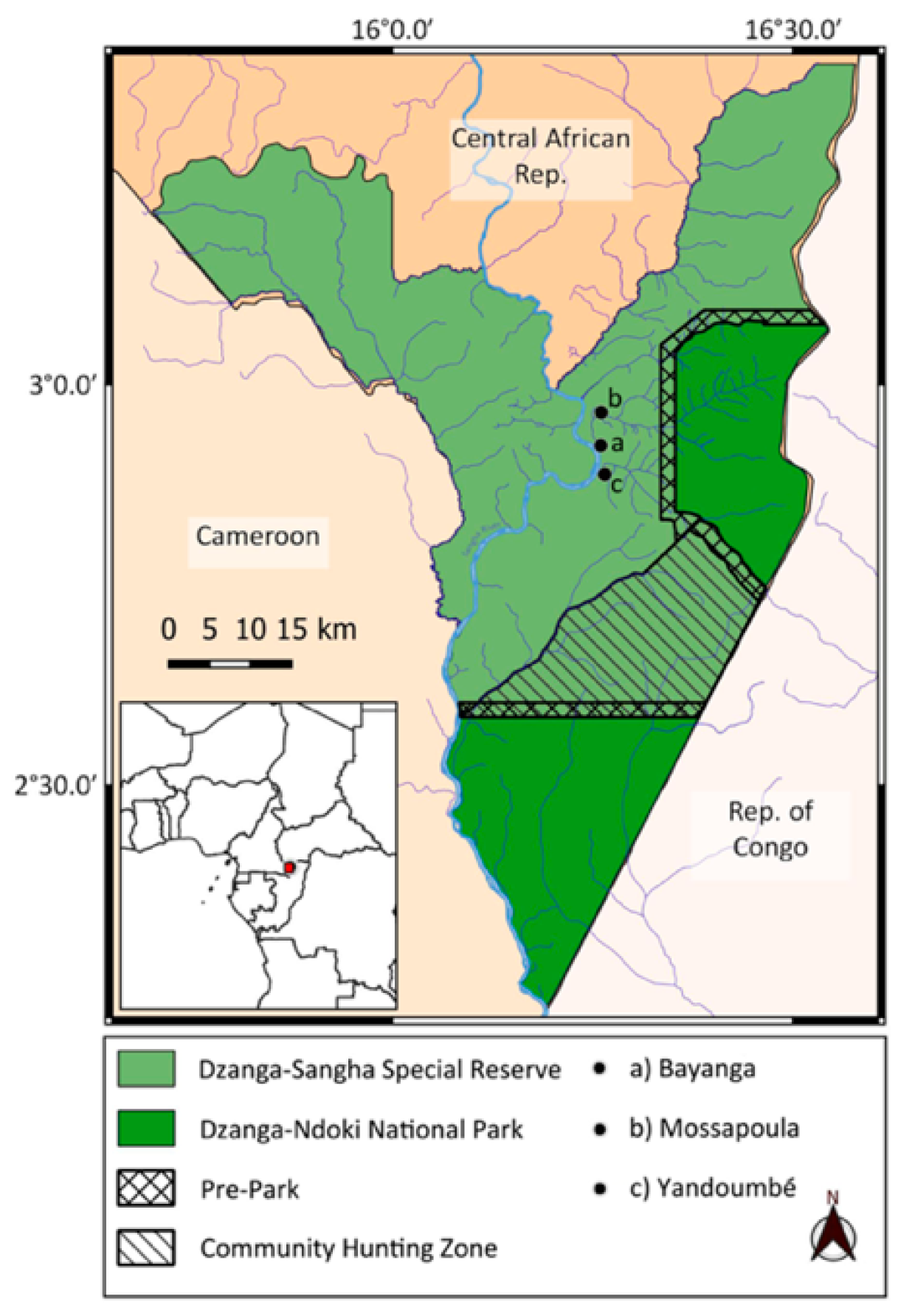
The Dzanga-Sangha Protected Areas (APDS) (4200 km2, 28°13′26 N, 16°11′26 E) were formally established in 1990; some parts are a UNESCO World Heritage Site due to the rich biodiversity [34,39]. The APDS are situated within the Sangha River TNS in the south-western Sangha-Mbaéré prefecture of the CAR. The TNS covers 7542 km2, encompassing three national parks: Lobeke in Cameroon, Dzanga-Ndoki in the CAR and Nouabale-Ndoki in the Republic of Congo and 36,000 km2 of buffer zones around these parks. The Dzanga-Sangha Dense Forest Special Reserve (Figure 1) is 3359 km2, comprising the Dzanga and Ndoki national parks, which contain a combination of intact primary and secondary mixed and monodominant habitats, with large herbaceous undergrowth particularly in secondary patches near villages [40]. Since 1990, the APDS have been managed by the Ministry of Water and Forest Resources and Tourism Department of the CAR government and the WWF, working closely with the International Union for the Conservation of Nature (IUCN) to protect the flora and fauna.
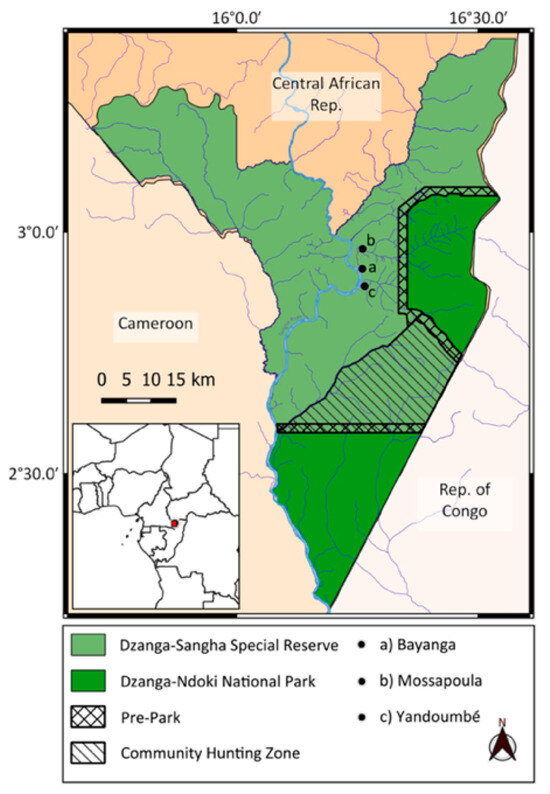
Figure 1.
Map showing location of villages where interviews were conducted regarding perceptions of nocturnal mammals within Dzanga-Sangha Protected Areas in the Central African Republic.
2.2. Data Collection
Gunn presented laminated ‘trigger’ cards depicting images of 22 mammal species (Figure S1) to eight different groups of Ba’Aka villagers. An in-country 50% adult illiteracy rate meant visual stimulants were important tools for the interviews [39]. Trigger cards are used for promoting interest, reducing cross-cultural language difficulties, increasing self-confidence and engagement and exploring local opinions [41,42,43,44].
Interview permission was obtained from village chiefs, and we included them for their authority and to present the project for their understanding. The interviewees totalled 70 adults (53 males; 17 females) across three villages, Bayanga, Mossapoula and Yandoumbé (Figure 1), with local language interpretation by one of the authors [TFN]. The villages differed as follows: Bayanga featured mostly the Ba’Aka and the WWF Gorilla Guardian trackers who spent considerable time in the national park and surrounding forest. Mossapoula is situated closest to the national park and consists of both the Ba’Aka and Bantu, and Yandoumbé is a typical Ba’Aka village where most residents are hunter-gatherers, with many families spending time in the forest.
The cards showed eight nocturnal and six diurnal mammal species that are present in the APDS, as well as six mammal species that occur in the CAR but not in the APDS. Two cards showed reptiles—snake and crocodile. This inclusion was because, as identified prior to the interviews, many cultures, such as in Brazilian and European folklore, have traditional beliefs about reptiles regarding disease, medicinal properties or taboos [45,46]. Species that were not directly from the area (i.e., Calabar angwantibo and West African dwarf crocodile (Osteolaemus tetraspis)) were used to determine if respondents (a) recognised the Family and (b) recognised that the animals looked different from those they had personal experience of in the area. Unfortunately, we could not acquire a good-quality photograph of a golden angwantibo—the resident species—to use as a trigger card, and at the time of the study, crocodiles had not yet been seen at the study site, making selecting a trigger card with the correct species difficult.
We adapted qualitative interview methods following Miard et al. [20] for interview approach, interviewee selection and word frequency analysis. Interviewees willingly participated without payment. We designed six structured questions specifically for informal oral interviews [44] (Table S1). Interviews were mostly opportunistic, and questions were both open and closed to reduce entrapment and encourage opportunities for freelisting (discussing anything that came to mind) [47]. To encourage open thinking, it was important that interviewees had not seen the cards beforehand. No questions were directly related to hunting as participants can become resistant to participation if they do not know the interviewer and may have adapted their answers if they felt vulnerable [31,48]. Many of the interviewees knew Fuh Neba, enabling trust and more rapid collaborative participation [41]. We obtained oral consent, and interviewees could participate or withdraw at any moment.
Each interview group was assessed using a Likert scale of attitude measurement depending on their participation using the trigger cards: 1 = active, 5 = did not take part [49]. We observed which animals they pointed to or spoke about and repeated their words, which encouraged elaboration and engagement. We observed diligently, noting down descriptive gestures and sounds that symbolised recognition and understanding of either appearance or behaviour of the species in question, and key words and sentences were translated into English. The recorded notes described actions, such as ‘crossing arms in front of face’ to show the defensive pose of Arctocebus and ‘nodding in agreement’ to verify that a comment said was true. No specific key words were highlighted prior to interview because there was little known to researchers about how much LEK was present regarding many nocturnal species in this area at the time. Discussions allowed for an exchange of information and engagement to lengthen the interviews. We aimed to build good rapport with the interviewees so did not record interviews or take photographs that might have made them less confident to engage.
2.3. Data Analyses
We ran word frequency queries in NVivo qualitative data analysis software (QSR International, Version 12, 2018) to identify the weight of each key word relating to species per interview group. Word similarity groupings for key words were not required in NVivo as we had categorised them previously into ten appropriate categories, combining words associated with specific factors, such as hunting, taboos and medicine. Word clouds were made to display overall nocturnal animals and nocturnal primates depicting the most frequent words used by villagers in the largest font size and the least in the smallest font. Cultural consensus theory (CCT) is a means of measuring common knowledge to estimate or reach an agreement on the beliefs of the same topic across different parties, through techniques such as saliency (the frequency and weight of certain words) and freelisting: allowing interviewees to speak words associated with the topic [47]. We conducted cultural domain analyses in UCINET 6 for Windows [50] for each location where interviews took place (Bayanga, Mossapoula and Yandoumbé) to compare key words mentioned regarding beliefs about nocturnal species in each village. We used the minimum residual factor loadings using the first groupings, which held the strongest weight. A consensus was achieved when the eigenvalue ratio was 3 to 1 or higher and factor loading percentages were over 50% [51]. Finally, in SPSS V29, we ran a chi-square cross-tabulation with adjusted residuals to examine if frequencies of the categories discussed about nocturnal animals differed across species, with p ≤ 0.05 set as significance.
3. Results
3.1. Overview
A total of 70 (male: n = 53; female: n = 17) interviewees studied and picked up the cards, pointed at and discussed the animal species. Local names were spoken in either Ba’Aka or Sangho (Bantu). More males were interviewed (n = 53) and expressed more species knowledge (Table 1), yet women did name Gorilla gorilla and Pan troglodytes correctly. There were fewer females involved because most Ba’Aka who entered the forest at night and at a greater distance from the village were male. Females do occasionally visit the adjacent forest during the day to find fruits, nuts and various vegetation, and those who felt confident enough to participate were warmly welcomed. One group mentioned the most key words (n = 41) despite there only being three interviewees, indicating that they were very knowledgeable about the species. Interviewees recognised most species and acknowledged their presence or absence in the APDS. Villagers in Mossapoula did not recognise three nocturnal species, despite all of the species inhabiting the APDS. All groups engaged, with three groups (50%) being ‘Hesitant, leading to active’, displaying more hesitance to engage when in a smaller group, followed by three groups rating ‘Active’ (37.5%). The groups were not a set size each time, depending on how many people were available at that moment and wanted to participate, resulting in some groups of 3 to 4 males, and up to 20 male and female villagers. The more that were involved, the more information and personal experience with nocturnal species we were able to discover.

Table 1.
Table showing interview results: species identification, location of interviews and gender ratio. Location codes: Bayanga (BA); Yandoumbé (YN) and Mossapoula (MS) in Dzanga-Sangha Protected Areas, Central African Republic.
In total, interviewees expressed 53 key words (Table S2) when freelisting about seven nocturnal species: potto; angwantibo; Southern needle-clawed galago (Euoticus elegantulus); genet (Genetta spp.); African civet (Civettictus civetta); flying squirrel (Anomolurus spp.) and Western tree hyrax Dendrohyrax dorsalis. We presented the categorized key words according to species (Figure 2). The words ‘village’ and ‘night’ were the most frequent key words used by villagers when asked about nocturnal mammals, as depicted in word clouds using NVIVO 12 data analysis software (Figure 2). All nocturnal species were correctly associated with night.

Figure 2.
Word clouds of most frequent terms describing (a) nocturnal mammals and (b) nocturnal primates by 70 interviewees when freelisting across eight groups of Ba’Aka villagers in Dzanga-Sangha Protected Areas, Central African Republic.
3.2. Species Identification and Names
The potto trigger card caused confusion, with some interviewees believing it was a tree hyrax, later confirmed to be ‘yukka’ in Sangho and information on this species was added to the results because hyraxes are nocturnal. There was no differentiation between potto and angwantibo in Sangho (‘ndindiki’), whereas the Ba’Aka had different names for each (potto = ‘kota’; angwantibo = ‘likiti’). Furthermore, there appeared not to be any differentiation between Southern needle-clawed galago and other Galago or Galagoides spp., using the same name ‘pollo’ and this was agreed by all interviewees, even when confirming with an additional photograph of a lesser bushbaby (Galago spp.). Other mentioned species included the clarification between tree pangolins (Phataginus spp.) and giant ground pangolins (Smutsia gigantea)—the Ba’Aka differentiated between Phataginus spp. (‘kokolo’) and Smutsia spp. (‘ikadi’) and African palm civet (Nandinia binotata) in comparison with genet or African civet. Some individuals recognised that the crocodile pictured was not the same species as was present in the APDS and that the pictured angwantibo was red and not golden.
Pangolins and bats were rarely discussed after recognition, but identification was clarified by imitations of bat vocalisations and one individual mentioned that pangolins are hunted for meat. Due to the small sample size, these species were omitted from the main data analysis.
3.3. Frequency of Species Mentions and Their Associations
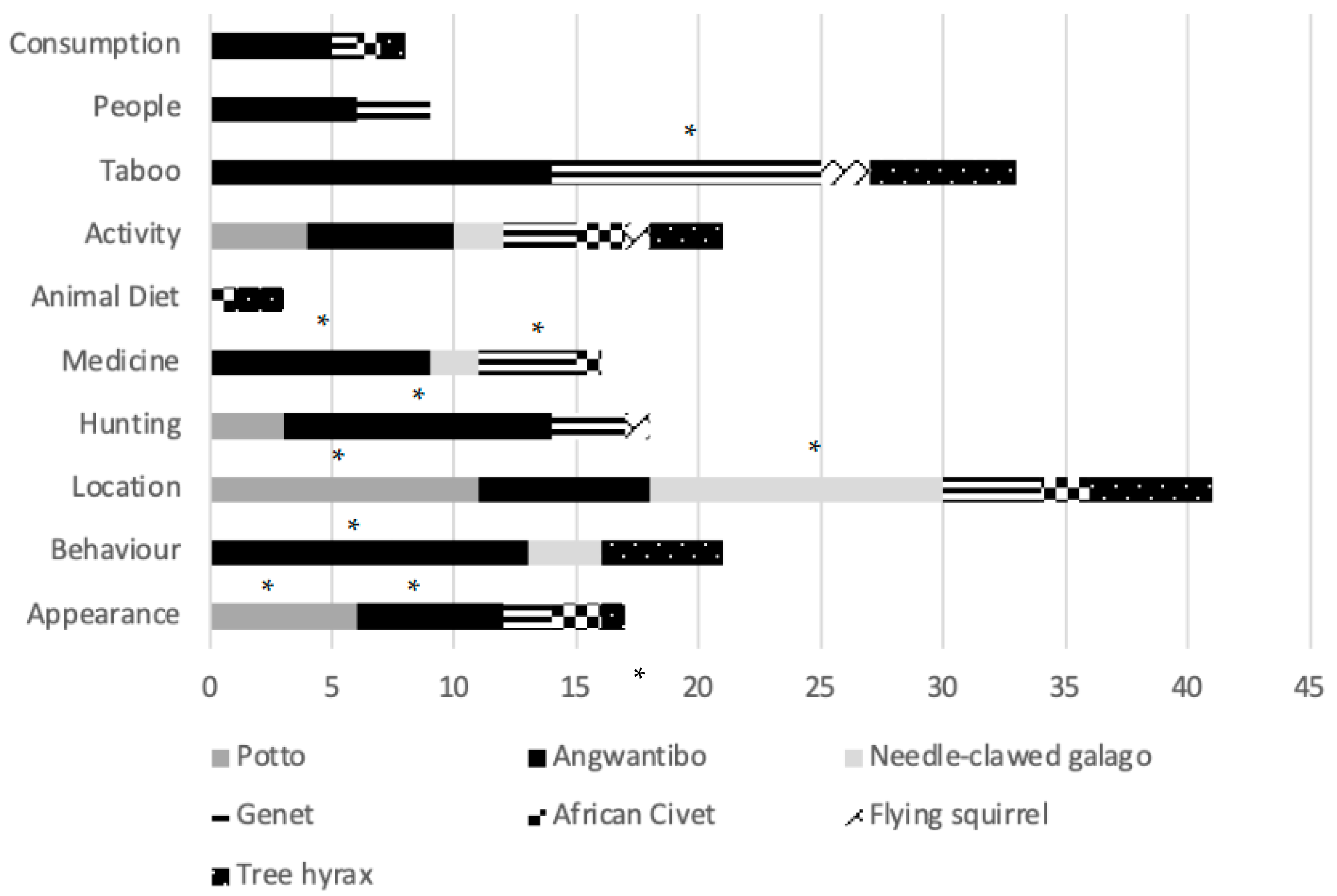
A chi-square test revealed that some species had significantly different associations (x2 = 121.872, df = 54, p ≤ 0.001). Pottos (n = 11) and needle-clawed galagos (n = 12) were most frequently mentioned regarding their location, with interviewees agreeing they see these species most often (Figure 3). Villagers described the appearance of pottos and angwantibos the most (n = 6 each). Angwantibos scored significantly highest in three other categories, being mentioned more regarding Behaviour (n = 13), Hunting (n = 11) and Medicine (n = 9). Genets were significantly mentioned in association with Taboo (n = 11).
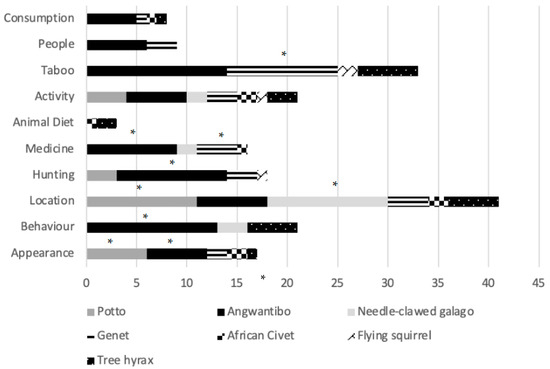
Figure 3.
Bar chart depicting the frequency of a category regarding a certain nocturnal mammal species from group interviews with Ba’Aka villagers across three villages in Dzanga-Sangha Protected Areas, Central African Republic. Species described significantly more in a category using a chi-score cross-tabulation are indicated with an *.
Pottos were connected with village the most (75%), followed by angwantibos, needle-clawed galagos and genets (50% each). Needle-clawed galagos (62.5%) and hyrax (37.5%) were stated to be in trees, while angwantibos were hard to find (50%) or hiding (25%). Angwantibos were slow (37.5%) and showed a defence posture (12.5%). Galagos were seen jumping (37.5%) and pottos had dorsal spines (25%)—villagers reached to the back of their necks to demonstrate. Hyraxes make sounds (25%) and are known as the timekeepers (25%) by calling at the top of the hour.
Animals associated with meat were angwantibos (62.5%), genets, civets and hyrax (12.5%). Hyrax intestines and faeces were considered dangerous (12.5%) or poisonous (37.5%), and personal revenge within the Ba’Aka community could lead to the death of a whole village. Not everyone can kill a hyrax because it would not be safe in some hands. Hyrax meat is eaten, but intestines must be buried first. Hunting pressure did not appear to affect hyraxes significantly due to avoidance in part due to these cultural beliefs.
Angwantibos offered spiritual protection (37.5%) though this belief was even more strongly associated with genets (62.5%) because the Ba’Aka believe genets are their ancestors, watching them in the forest at night. Shaman-like people used skin (angwantibos = 50%; genets = 25%) to improve [spiritual] sight (genets = 12.5%). Skin is wrapped around the wrist (angwantibo = 37.5%) for strength (angwantibo = 50%) and 37.5% of villagers said that if a pregnant woman or her husband eats angwantibo meat, bones or drinks angwantibo in wine, they will receive a strong child who has a good grip (angwantibo = 37.5%). The Ba’Aka can tell if a child’s mother drank or ate angwantibo if a child is fighting in the street.
Decoration (angwantibo = 25%; genets = 37.5%) either included animal skin or genet tail (25%) for charms (angwantibos = 25%; genets = 25%). Flying squirrels and angwantibos (12.5%) were used in rituals, including piercing the sole of the foot with tree hyrax claws to gain the best climbers in the village.
Pottos (37.5%) and angwantibos (50%) could deliver a painful bite but there was a suspicion towards angwantibo bites leading to illness (25%) when bitten. Many people did not know further details because they were careful when hunting them. Hunting was carried out using machetes but angwantibos (12.5%) and genets (25%) were difficult to kill but did not suggest that TEK was significantly affecting hunting pressure on these species.
3.4. Cultural Consensus
Key word frequency data were run in UCINET 6 for each village and compared. Ratios of the eigenvalues show that a cultural consensus was achieved between the villagers in Bayanga and Yandoumbé, condensing at 4.06 and 4.45 (Table 2). The eigenvalue ratio for Mossapoula was only at 2.20 but fewer overall key words were offered from this village. Bayanga had the highest eigenvalue percentage in the first rotated factor loadings (n = 61.5%), but strength in results also occurred in both Mossapoula (58.3%) and Yandoumbé (56.5%).

Table 2.
Highest factor loadings and ratio of key words from interviews with the Ba’Aka about traditional beliefs regarding nocturnal mammals in the Central African Republic, using UCINET 6.
4. Discussion
This study adds to the growing body of literature for the uses of nocturnal mammals, especially primates, for traditional practices, especially in Ba’Aka cultural traditions in the APDS. Our preliminary data show that nocturnal mammals have a place in the Ba’Aka culture, with several species having cultural significance. Their informed knowledge on the identity, behaviours and locations of the focal species was shown when using the trigger cards. The traditional taboos and beliefs regarding aspects of these species, such as the angwantibo morphology (strong grip) and being watched by genets, held in high regard by associating them with ancestors for spiritual protection, reflect the Ba’Aka appreciation of the environment that have been incorporated into their culture.
Unlike some cultures that actively kill animals that are believed to be bad omens, such as aye-ayes in Madagascar, the Ba’Aka simply avoid them. The only unfavourable species in the interviews was tree hyrax, perceived to be very dangerous or ‘poisonous’. For other species, the Ba’Aka were indifferent and did not display overly positive attitudes about any particular species. They did react positively to the trigger card depicting G. gorilla, a key species of importance in the DSNP due to the livelihoods that research and tourism provide to the Ba’Aka. The male interviewees were more knowledgeable than females [1] and were very amenable when discussing uses of nocturnal mammals for traditional medicine and spiritual protection, of which the necessity for hunting also appeared opportunistic. The fewest key words were mentioned in Mossapoula (Group H, n = 13; Group F, n = 18), indicating hesitancy to participate or lack of knowledge in this village. Despite this, a strong cultural consensus was reached between villages regarding the use of angwantibos associated with gaining strength, genet parts used by the local (Shaman-like) doctor to gain spiritual protection and that the species were hard to find.
Past interviews with hunters and data from wildlife research in the APDS have shown an increase in the human population in Dzanga-Sangha causing an increase in bushmeat hunting, especially by the Bilo people (farmers) [33,52,53,54]. Adaptations in animal behaviour can occur due to hunting pressure, such as a recent change in duikers refraining from alarm calling when confronted by humans [55]. Aside from pottos in Nigeria [18,56], golden angwantibos and pottos are frequently hunted in Cameroon, the Democratic Republic of Congo and the Republic of Congo [22]. Ba’Aka hunters are known to be opportunistic [34] although they sometimes also hunt selectively, such as avoiding hunting pregnant duikers to protect the next generation [52,53]. Although hunted in other countries, Euoticus spp. and Galago spp. are not actively hunted in the CAR as the reward is not worth the catching effort [34]. We could not discover how often the villagers went into the forest at night, but women went daily to pick fruits and leaves. Woman appeared to be knowledgeable on the medicinal use and preparation of some species for cooking, which is likely to have been a skill passed down generations, and they did not express desire to be in the forest at night. Interviewees in this study only mentioned the local names of pangolins but they are known to be hunted in the area [34,57]. Demand for pangolin scales in international trade is likely to reduce populations in the APDS as rapidly as those in West Africa [58], as poachers are increasingly snaring and capturing these species in the CAR.
Hunting to satisfy traditional beliefs may occur on an ‘as-needed’ basis, for example, for rituals with hyrax claws to enable boys to become good climbers or pregnant women requiring angwantibo meat. Expanding human populations in remote areas and integration of other cultures, including more availability to Western medicine, may decrease opportunities for older Ba’Aka to pass on traditions and skills to the next generation(s), affecting the preservation of traditional Ba’Aka society [57]. The knowledge gained from learning the traditional beliefs of indigenous cultures is valuable to create a better understanding of remote and understudied environments [4,21,59]. Some Ba’Aka hinted towards receiving painful bites from angwantibos and that there could be a link with illness. Asian lorisids possess venom and it is unknown if angwantibo and pottos, which are evolutionarily closely related, also possess venom [60,61]. This suggests future research on possible toxicity in these primates. We also encourage further research into LEK in the Ba’Aka culture in Central Africa using larger sample sizes for interviews to establish information for a broader range of species and investigate any comparisons or similarities.
With this study, we have gained a deeper understanding of traditional beliefs in the Ba’Aka culture, specifically relating to nocturnal mammals. Our preliminary data show that further research in this field is necessary across a wide variety of factors. Future research should be carried out by interviewing hunters in relation to their distribution to gain a larger-scale overview of changes within the APDS, which can be incorporated into management strategies [55], and to determine whether hunting pressure or lack of tradition may affect nocturnal mammal populations [56]. As human populations rise in the APDS, it is likely that habitat alteration, local demand for bushmeat and ethnozoological customs will put unsustainable pressure on nocturnal mammals. We suggest a need to protect and include nocturnal mammals with unknown populations in wildlife management strategies [56]. Gorillas and elephants (Loxodonta africanus) are considered the conservation umbrella species in DSNP, as funders being willing to fund smaller or lesser-known species has been locally challenging. Funds raised for charismatic megafauna and through increased tourism in the area do go towards community and conservation development and protection of all biodiversity within the park, particularly through the presence of funded park rangers. A WWF study on population density and distribution of key larger species was carried out using camera traps to determine the effectiveness of conservation measures. This research discovered high volumes of human activity, informing the anti-poaching patrols in the APDS. Research of this kind, adapted for different species, would provide detailed management strategies for nocturnal mammals affected by taboo, medicine, consumption and more, not only in the study site but also in and around similar habitats across West Africa.
The WWF permits the Ba’Aka to hunt sustainably but regulations and laws exist to restrict illegal wildlife hunting that satisfies high urban demand. Quotas have been fixed for the most hunted species (e.g., duikers) to ensure sustainability. The protected area is a multi-use zone consisting of the park (no access or hunting allowed) and the outer reserve, where the local community is allowed to gather non-timber forest products and hunt (except protected species). In addition, there is a community hunting zone strategically placed between the two blocks of the park for use by the Ba’Aka. The benefits of conservation in the area are clearly visible and appreciated by the Ba’Aka to continue exercising their traditional practices. The Bantu are indigenous to parts of Central Africa but are not forest people like the Ba’Aka. The Bantu use illegal and unsustainable hunting methods (guns, snares, etc.) that destroy the forest and its biodiversity, but it is known that even the legal hunters overexploit resources by not respecting the hunting quotas.
Hunting at night is illegal in the CAR, based on the fact that accurate species identification is more difficult at night. Whilst nocturnal species may be protected within the DSNP itself, without further research on TEK, LEK and population density, it is unknown how hunting for various uses, and equally, conservation and law enforcement across the whole APDS are specifically affecting nocturnal mammals.
High-value species that are deliberately sought out for specific medicines, bushmeat or LEK should be given higher-priority conservation protection to enable more sustainable populations, along with widespread efforts to reduce demand [62]. Norms of law enforcement perception and knowledge and use of sacred resources differ between communities both locally and across Africa; therefore, local-scale regulations need to reflect hunting of local species and financial rewards for selling resources are often greater than the risk of prosecution [11,31]. Viewing nocturnal species at night is somewhat possible in the APDS, so it is likely that tourism may expand to include night walking experiences that will help increase education about these species. Nocturnal mammals are generally more elusive and can be harder to observe, but with increased interest, we hope that they can gain a boost with additional management and funding to conserve them for the future and provide opportunities for the Ba’Aka to further share their LEK that helps drive conservation.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/d16110654/s1, Figure S1: species depicted in trigger cards; Table S1: list of all key words mentioned by respondents regarding nocturnal mammals during interviews and their assigned categories; Table S2: list of all key words mentioned by respondents regarding nocturnal mammals during interviews and their assigned categories.
Author Contributions
Conceptualization, K.A.I.N. and A.S.G.; methodology, K.A.I.N. and A.S.G.; software, K.A.I.N. and A.S.G.; validation, T.F.N. and K.A.I.N.; formal analysis, A.S.G.; investigation, A.S.G.; resources, A.S.G. and K.A.I.N.; data curation, A.S.G.; writing—original draft preparation, A.S.G.; writing—review and editing, A.S.G., T.F.N. and K.A.I.N.; visualization, A.S.G.; supervision, K.A.I.N.; project administration, A.S.G., T.F.N. and K.A.I.N.; funding acquisition, A.S.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by a conservation grant from the Conservation Working Party of the Primate Society of Great Britain (PSGB) and crowd funding was kindly obtained from personal donors.
Institutional Review Board Statement
The study was conducted in accordance with Oxford Brookes University’s Code of Practice on Ethical Standards for Research involving Human Participants and approved by the Institutional Review Board (or Ethics Committee) of Oxford Brookes University (HSS E3, 30 June 2019).
Informed Consent Statement
Ethical approval for interviews was in accordance with the Faculty of Humanities and Social Sciences, Oxford Brookes University, and cleared prior to arrival at the field site. Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Acknowledgments
Thanks to the Ministry of Science and Research in the Central African Republic and to WWF for permitting this study and use of their field assistants, in particular, Mboussa Matthieu. Thanks to Janika Wenderfuerer for QGIS assistance, Magdalena Svensson and to the Loris Lab focus group at Oxford Brookes University. We thank three anonymous reviewers and the Editor for their comments and suggestions.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Rose, N. Animal, Insect, and Bird Omens and their Meanings, Animal Guides, Exemplore. Available online: https://exemplore.com/spirit-animals/Omens-Oracles-And-Signs-Are-You-Superstitious (accessed on 12 April 2019).
- Kideghesho, J.R. The potentials of traditional African cultural practices in mitigating overexploitation of wildlife species and habitat loss: Experience of Tanzania. Int. J. Biodivers. Sci. Manag. 2009, 5, 83–94. [Google Scholar] [CrossRef]
- Peoples, H.C.; Duda, P.; Marlowe, F.W. Hunter-Gatherers and the Origins of Religion. Hum. Nat. 2016, 27, 261–282. [Google Scholar] [CrossRef] [PubMed]
- Walsh, M. Birds of omen and little flying animals with wings. East Afr. Nat. Hist. Soc. Bull. 1992, 22, 2–9. [Google Scholar]
- Martin, J.F.; Roy, E.D.; Diemont, S.A.W.; Ferguson, B.G. Traditional Ecological Knowledge (TEK): Ideas, inspiration and designs for ecological engineering. Ecol. Eng. 2010, 36, 839–849. [Google Scholar] [CrossRef]
- Ruddle, K.; Davis, A. Local Ecological Knowledge (LEK) in Interdisciplinary Research and Application: A Critical Review. Asian Fish. Sci. 2013, 26, 79–100. [Google Scholar] [CrossRef]
- Joa, B.; Winkel, G.; Primmer, E. The unknown known—A review of local ecological knowledge in relation to forest biodiversity conservation. Land Use Policy 2018, 79, 520–530. [Google Scholar] [CrossRef]
- Quarles, L.F.; Dechanupong, J.; Gibson, N.; Nekaris, K.A.I. Knowledge, beliefs, and experience regarding slow lorises in Southern Thailand: Coexistence in a developed landscape. Animals 2023, 13, 3285. [Google Scholar] [CrossRef]
- Cooks-Campbell, A. Belief or Value? Learn the Difference and Set Ourself Free. BetterUp. 2022. Available online: https://www.betterup.com/blog/beliefs-vs-values (accessed on 10 September 2024).
- Fershtman, C.; Gneezy, U.; Hoffman, M. Taboos and Identity: Considering the Unthinkable. Am. Econ. J. Microecon. 2011, 3, 139–164. [Google Scholar] [CrossRef]
- Schwartz, M.W. Conservation lessons from taboos and trolley problems. Conserv. Biol. 2021, 35, 794–803. [Google Scholar] [CrossRef]
- Tingle, J.L. Snakes in Madagascar’s Folklore, A Crawling Shape Intruder. Available online: https://jleetingle.wordpress.com/2012/12/04/snakes-in-madagascars-folklore/ (accessed on 12 April 2019).
- Sefczek, T.M.; Farris, Z.; Wrtight, P. Diurnal Evidence of a Nocturnal Feeder: Using Feeding Traces to Understand Aye-Ayes’ Feeding Strategy in Ranomafana National Park, Madagascar. Master’s Thesis, San Diego State University, San Diego, CA, USA, 2009. [Google Scholar] [CrossRef]
- Simons, E.L.; Meyers, D.M. Folklore and beliefs about the aye-aye (Daubentonia madagascariensis). Lemur News 2001, 6, 11–16. [Google Scholar]
- Schüßler, D.; Richter, T.; Contreras, J.M. Educational approaches to encourage pro-environmental behaviors in Madagascar. Sustainability 2019, 11, 3148. [Google Scholar] [CrossRef]
- Williams, S.T.; Williams, K.S.; Constant, N.; Swanepoel, L.; Taylor, P.J.; Belmain, S.R.; Evans, S.W. Low-intensity environmental education can enhance perceptions of culturally taboo wildlife. Ecosphere 2021, 12, e03482. [Google Scholar] [CrossRef]
- Carpaneto, G.; Germi, F. The mammals in the zoological culture of the Mbuti pygmies in north-eastern Zaire. Hystrix 1989, 1, 1–83. [Google Scholar] [CrossRef]
- Svensson, M.S.; Friant, S.C. Threats from trading and hunting pottos and angwantibos in Africa resemble those faced by slow lorises in Asia. Endanger. Species Res. 2014, 23, 107–114. [Google Scholar] [CrossRef]
- Nijman, V.; Nekaris, K.A.I. Traditions, taboos and trade in slow lorises in Sundanese communities in southern Java, Indonesia. Endanger. Species Res. 2014, 25, 79–88. [Google Scholar] [CrossRef]
- Miard, P.; Nekaris, K.A.I.; Ramlee, H. Hiding in the dark: Local ecological knowledge about slow loris in Sarawak sheds light on relationships between human populations and wild animals. Hum. Ecol. 2017, 45, 823–831. [Google Scholar] [CrossRef]
- Nekaris, K.A.I.; Shepherd, C.; Starr, C.; Nijman, V. Exploring cultural drivers for wildlife trade via an ethnoprimatological approach: A case study of slender and slow lorises (Loris and Nycticebus) in South and Southeast Asia. Am. J. Primatol. 2010, 72, 877–886. [Google Scholar] [CrossRef]
- Svensson, M.S.; Ingram, D.J.; Nekaris, K.A.I.; Nijman, V. Trade and ethnozoological use of African Lorisiforms in the last 20 years. Hystrix Ital. J. Mammol. 2016, 26, 153–161. [Google Scholar] [CrossRef]
- D’Cruze, N.; Assou, D.; Coulthard, E.; Norrey, J.; Megson, D.; Macdonald, D.W.; Harrington, L.A.; Ronfot, D.; Segniagbeto, G.H.; Auliya, M. Snake oil and pangolin scales: Insights into wild animal use at “Marché des Fétiches” traditional medicine market, Togo. Nat. Conserv. 2020, 39, 45–71. [Google Scholar] [CrossRef]
- Gnanaolivu, S.D.; Campera, M.; Nekaris, K.A.I.; Nijman, V.; Satish, R.; Babu, S.; Singh, M. Medicine, black magic and supernatural beings: Cultural rituals as a significant threat to slender lorises in India. People Nat. 2022, 4, 1007–1019. [Google Scholar] [CrossRef]
- Hansel, T.E.; Tizard, R.J. Stripe-backed weasel Mustela strigidorsa for sale as traditional medicine in Lao PDR. Small Carniv. Conserv. 2006, 38, 34–35. [Google Scholar]
- Starr, C.; Nekaris, K.A.I.; Streicher, U.; Leung, L. Traditional use of slow lorises Nycticebus bengalensis and N. pygmaeus in Cambodia: An impediment to their conservation. Endanger. Species Res. 2010, 12, 17–23. [Google Scholar] [CrossRef]
- Alves, R.R.N.; Souto, W.M.S.; Barboza, R.R.D. Primates in traditional folk medicine, a world overview. Mammal Rev. 2010, 40, 155–180. [Google Scholar] [CrossRef]
- Sinthumule, N.I. Traditional ecological knowledge and its role in biodiversity conservation: A systematic review. Front. Environ. Sci. 2023, 11, 1164900. [Google Scholar] [CrossRef]
- Chausson, A.M.; Rowcliffe, J.M.; Escouflaire, L.; Wieland, M.; Wright, J.H. Understanding the sociocultural drivers of urban bushmeat consumption for behaviour change interventions in Pointe Noire, Republic of Congo. Hum. Ecol. 2019, 47, 179–191. [Google Scholar] [CrossRef]
- Alves, R.R.N.; Souto, W.M.S.; Barboza, R.R.D.; Bezerra, D.M.M. Primates in traditional folk medicine: World overview. In Animals in Traditional Folk Medicine: Implications for Conservation; Springer Press: Berlin, Germany, 2013; pp. 135–170. [Google Scholar]
- Bitanyi, S.; Nesje, M.; Kusiluka, L.J.M.; Chenyambuga, S.W.; Kaltenborn, B.P. Awareness and perceptions of local people about wildlife hunting in western Serengeti communities. Trop. Conserv. Sci. 2012, 5, 208–224. [Google Scholar] [CrossRef]
- Ba’Aka and Related Groups. Minority Rights Group. Available online: https://minorityrights.org/minorities/baaka-related-groups/ (accessed on 29 July 2019).
- Remis, M.J.; Jost Robinson, C.A. Nonhuman primates and “others” in the Dzanga Sangha Reserve: The role of anthropology and multispecies approaches in ethnoprimatology. In Ethnoprimatology: A Practical Guide to Research at the Human–Primate Interface; Springer Press: New York, NY, USA, 2017; pp. 190–205. [Google Scholar]
- Mattieu, M. (Dzanga-Sangha Protected Areas, Bayanga, Central African Republic). Personal communication, 2019. [Google Scholar]
- Jost Robinson, C.A.; Zollner, P.A.; Kpanou, J.B. Night and day: Evaluating transect methodologies to monitor duikers in the Dzanga-Sangha Protected Areas, Central African Republic. Afr. J. Ecol. 2016, 55, 222–232. [Google Scholar] [CrossRef]
- Brittain, S.; Ibbet, H.; de Lange, E.; Doward, L.; Hoyte, S.; Marino, A.; Milner-Gulland, E.J.; Newth, J.; Rakotonarivo, S.; Verissimo, D.; et al. Ethical considerations when conservation research involves people, Engaging with Ethics. Conserv. Biol. 2020, 34, 925–933. [Google Scholar] [CrossRef]
- Sandbrook, C. Weak yet strong: The uneven power relations of conservation. Oryx 2018, 51, 379–380. [Google Scholar] [CrossRef]
- Mittermeier, R.A. Effects of hunting on rainforest primates. In Primate Conservation in the Tropical Rain Forest; Marsh, C.W., Mittermeier, R.A., Eds.; Alan R. Liss: New York, NY, USA, 1987; pp. 109–146. [Google Scholar]
- WWF in the Central African Republic. Available online: https://origin-congo.wwf-sites.org/where_we_work/central_africa_republic/ (accessed on 22 January 2024).
- Blom, A.; Cipolletta, C.; Brunsting, A.M.H.; Prins, H.H.T. Behavioural responses of gorillas to habituation in the Dzanga-Ndoki National Park, Central African Republic. Int. J. Primatol. 2004, 25, 179–196. [Google Scholar] [CrossRef]
- Sands, R.G.; Bourjolly, J.N.; Roer-Strier, D. Crossing cultural barriers in research interviewing. Qual. Soc. Work 2007, 6, 353–372. [Google Scholar] [CrossRef]
- Epstein, M.J.; Yuthas, K. Redefining Education in the Developing World, Education, Stanford Social Innovation Review, Stanford University Center for Social Innovation. 2012. Available online: https://pdxscholar.library.pdx.edu/cgi/viewcontent.cgi?article=1009&context=busadmin_fac (accessed on 17 February 2019).
- Horn, M.S.; Leong, Z.; Block, F.; Diamond, J. Of BATS and APES: An Interactive Tabletop Game for Natural History Museums. In Proceedings of the 2012 Annual SIGCHI Conference on Human Factors in Computing Systems, Austin, TX, USA, 5–10 May 2012; pp. 2059–2068. [Google Scholar] [CrossRef]
- Newing, H.S.; Eagle, C.M.; Puri, R.K.; Watson, C.W. Conducting Research in Conservation, Social Science Methods and Practice; Routledge: Oxon, UK, 2010. [Google Scholar] [CrossRef]
- Alves, R.R.N.; Neto, N.A.L.; Santana, G.G.; Vieira, W.L.S.; Almeida, W.O. Reptiles used for medicinal and magic religious purposes in Brazil. Appl. Herpetol. 2009, 6, 257–274. [Google Scholar] [CrossRef]
- Ceríaco, L.M.P.; Marques, M.P.; Maderia, N.C.; Vila-Viçosa, C.M.; Mendes, P. Folklore and traditional ecological knowledge of geckos in Southern Portugal: Implications for conservation and science. J. Ethnobiol. Ethnomed. 2011, 7, 26. [Google Scholar] [CrossRef]
- Weller, S.C. Cultural Consensus Theory: Applications and frequently asked questions. Field Methods 2007, 19, 339–368. [Google Scholar] [CrossRef]
- Jones, J.P.G.; Andriamarovololona, M.M.; Hockey, N.; Gibbons, J.M.; Milner-Gulland, E.J. Testing the use of interviews as a tool for monitoring trends in the harvesting of wild species. J. Appl. Ecol. 2008, 45, 1205–1212. [Google Scholar] [CrossRef]
- Likert Scale. In Encyclopedia of Survey Research Methods; Lavrakas, P.J., Ed.; Sage Publications: Thousand Oaks, CA, USA, 2008; Available online: https://methods.sagepub.com/Reference/encyclopedia-of-survey-research-methods (accessed on 20 March 2019).
- Borgatti, S.P.; Everett, M.G.; Freeman, L.C. UCINET 6 for Windows: Software for Social Network Analysis; Analytic Technologies: Harvard, MA, USA, 2002. [Google Scholar]
- Nekaris, K.A.I.; McCabe, S.; Spaan, D.; Ali, M.I.; Nijman, V. A novel application of cultural consensus models to evaluate conservation education programs. Conserv. Biol. 2018, 32, 466–476. [Google Scholar] [CrossRef]
- Jost Robinson, C.A. Beyond Hunters and Hunted: An Integrative Anthropology of Human-Wildlife Dynamics and Resource Use in a Central African Forest. Ph.D. Thesis, Purdue University, West Lafayette, IN, USA, 2012. Available online: https://www.proquest.com/openview/84d4d2e00cfb4e83f9267f36b46d20ce/1.pdf?pq-origsite=gscholar&cbl=18750&diss=y (accessed on 10 April 2019).
- Noss, A.J. The impacts of cable snare hunting on wildlife populations in the forests of the Central African Republic. Conserv. Biol. 1998, 12, 390–398. [Google Scholar] [CrossRef]
- Sandker, M.; BrunoBokoto-de, S.; Roth, P.; Pellisier, C.; Perez, M.R.; Sayer, J.; Turkalo, A.; Omoze, F.; Campbell, B.M. Logging or Conservation Concession: Exploring Conservation and Development Outcomes in Dzanga-Sangha, Central African Republic. Conserv. Soc. 2011, 9, 299–310. [Google Scholar] [CrossRef]
- Remis, M.J.; Jost Robinson, C.A. Examining short-term nutritional status among BaAka foragers in transitional economies. Am. J. Phys. Anthropol. 2014, 154, 365–375. [Google Scholar] [CrossRef]
- Omifolaji, J.K.; Adedoyin, S.O.; Ikyaagba, E.T.; Khan, T.U.; Ojo, V.A.; Hu, Y.; Alarape, A.A.; Jimoh, S.O.; Hu, H. Population Abundance and Density Estimates of Poorly Documented Near-Threatened Calabar Angwantibo (Arctocebus calabarensis) in Oban Hills Region. Animals 2024, 14, 1374. [Google Scholar] [CrossRef]
- Fuh Neba, T. (WWF-CAR, Bayanga, Central African Republic). Personal communication, 2019. [Google Scholar]
- Aisher, A. Scarcity, alterity and value: Decline of the pangolin, the World’s most trafficked mammal. Conserv. Soc. 2016, 14, 317–329. [Google Scholar] [CrossRef]
- Sõukand, R. Dark local knowledge: The yet-to-be scientifically discovered and locally acknowledged aspects of local knowledge systems. J. Ethnobiol. Ethnomed. 2024, 20, 50. [Google Scholar] [CrossRef]
- Alterman, L. Toxins and Toothcombs: Potential Allospecific Chemical Defenses in Nycticebus and Perodicticus, Creatures of the Dark: The Nocturnal Prosimians; Plenum Press: New York, NY, USA, 1995; pp. 413–424. [Google Scholar]
- Rode-Margono, J.E.; Nekaris, K.A.I. Cabinet of curiosities: Venom systems and their ecological function in mammals, with a focus on primates. Toxins 2015, 7, 2639–2658. [Google Scholar] [CrossRef]
- Bobo, K.S.; Aghomo, F.F.M.; Ntumwel, B.C. Wildlife use and the role of taboos in the conservation of wildlife around the Nkwende Hills Forest Reserve; Southwest Cameroon. J. Ethnobiol. Ethnomed. 2015, 11, 1–23. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).