Congruent and Hierarchical Intra-Lake Subdivisions from Nuclear and Mitochondrial Data of a Lake Baikal Shoreline Amphipod
Abstract
1. Introduction
2. Materials and Methods
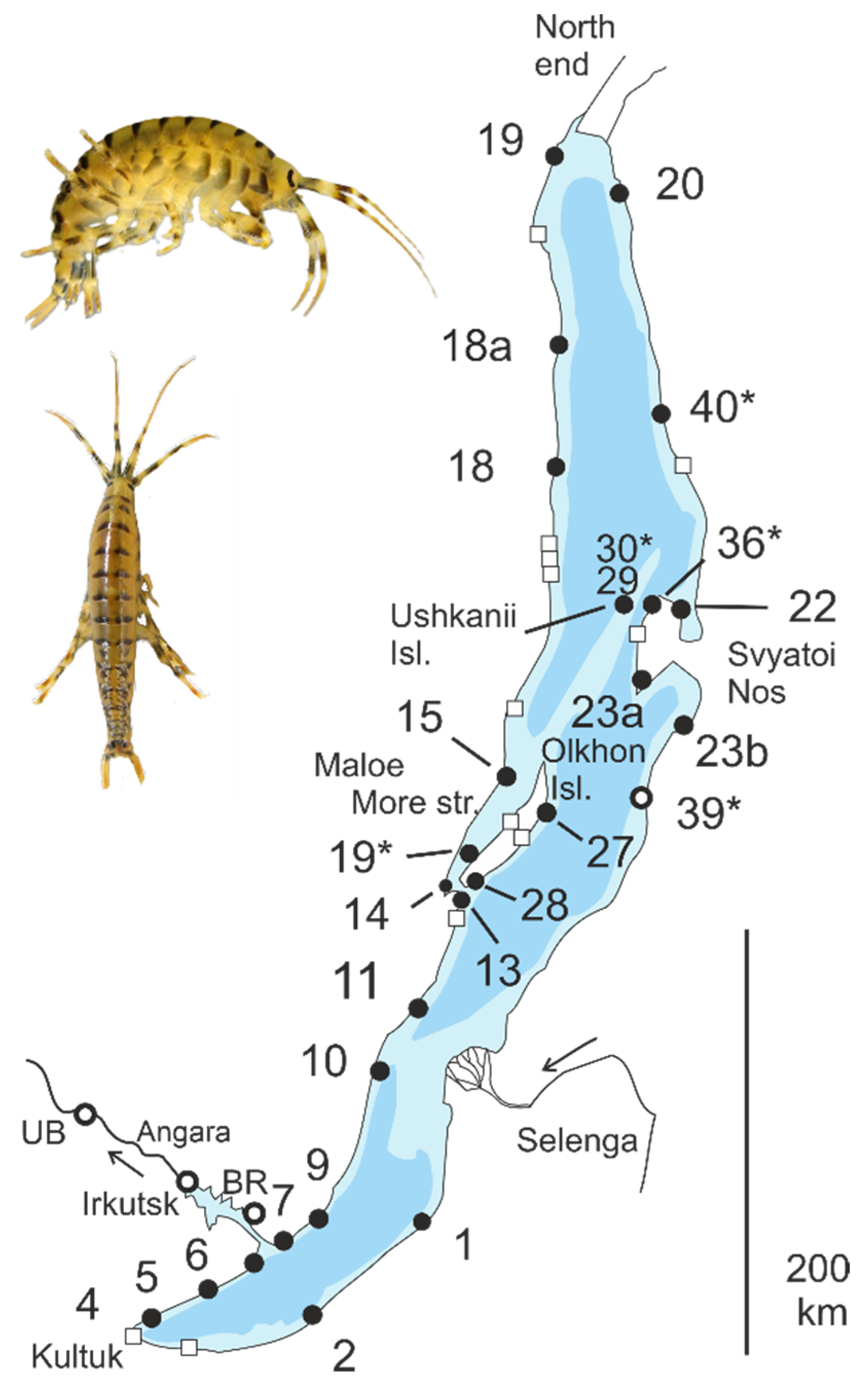
2.1. Samples
2.2. Molecular Data
2.3. Data Analysis
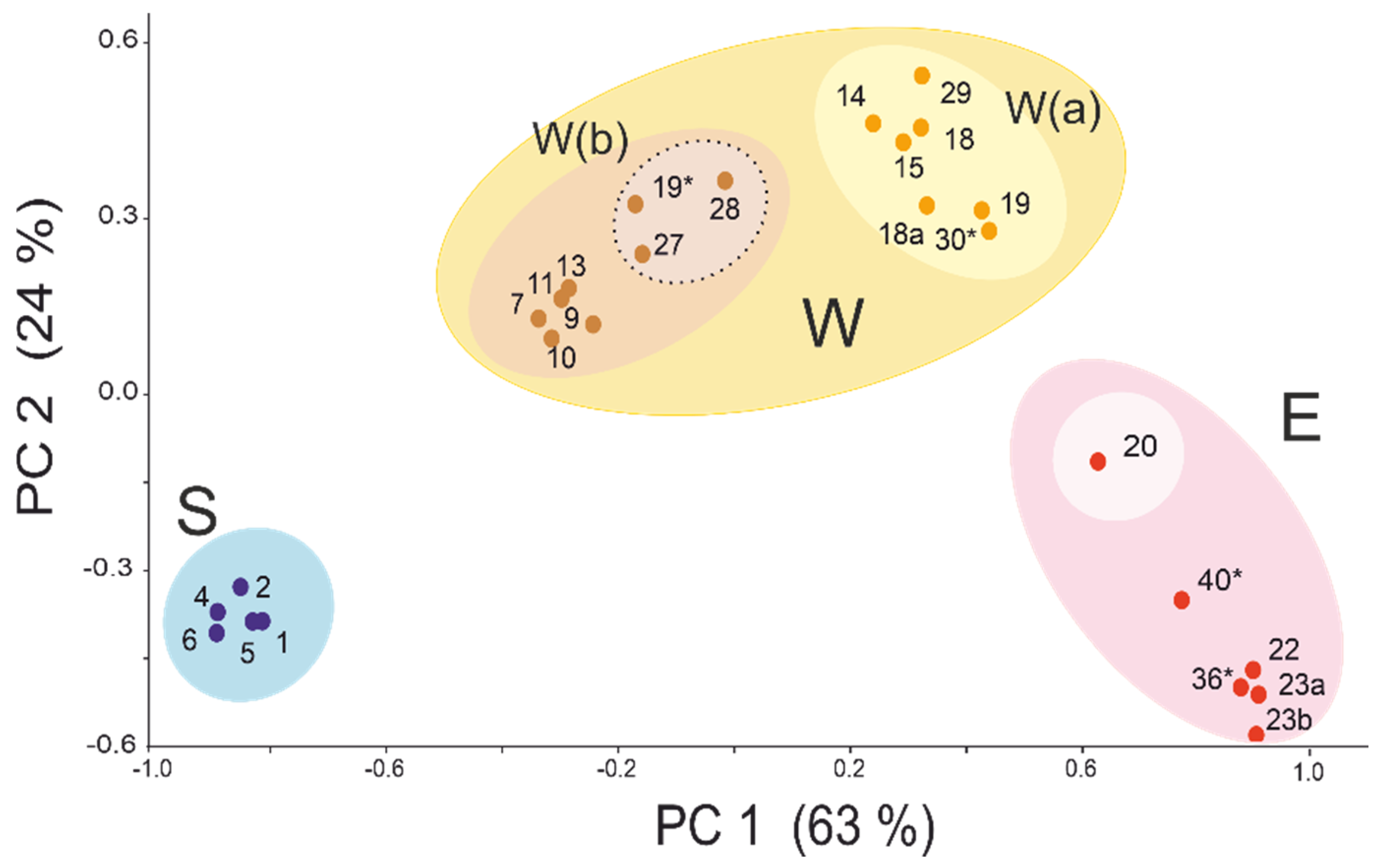
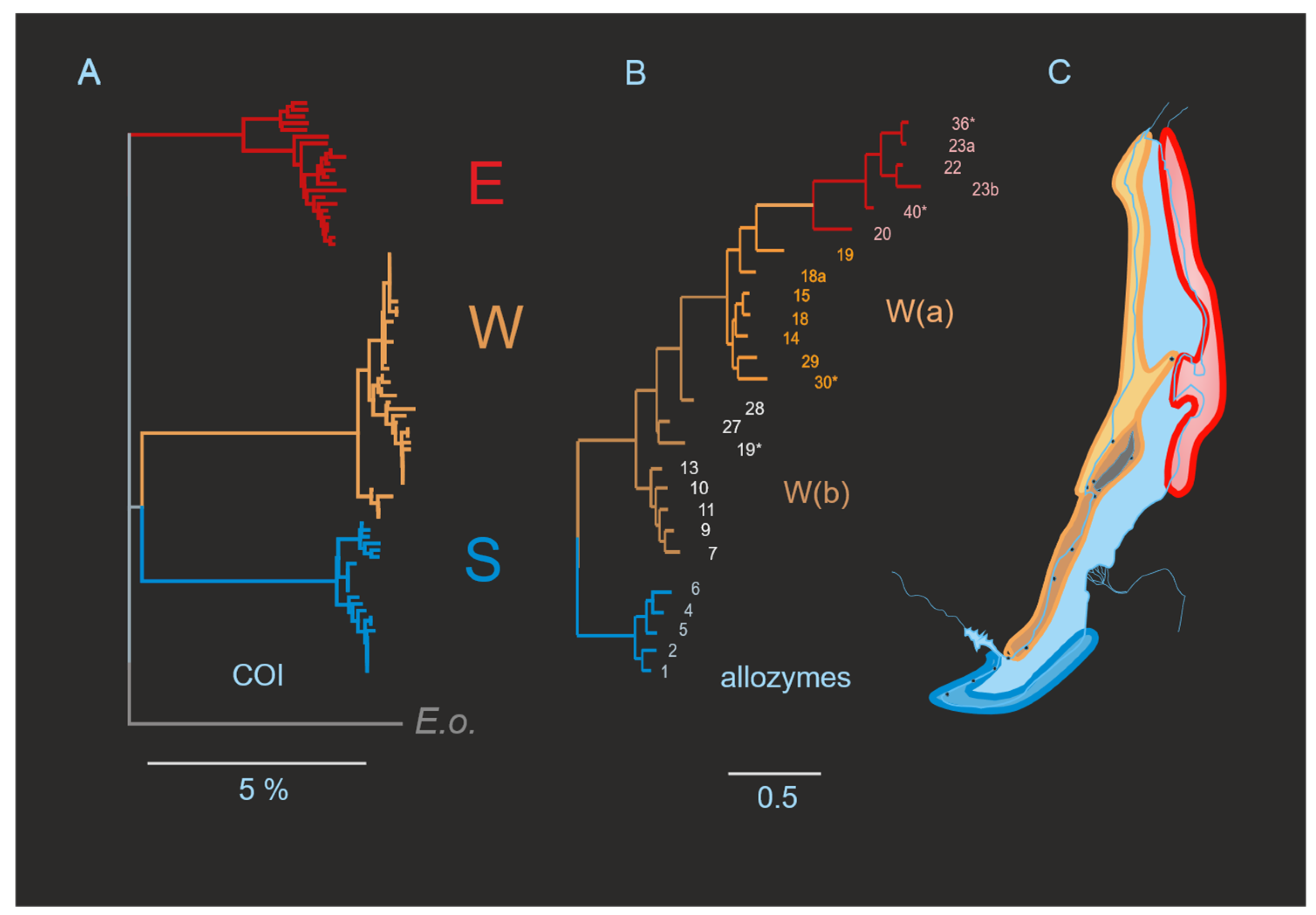
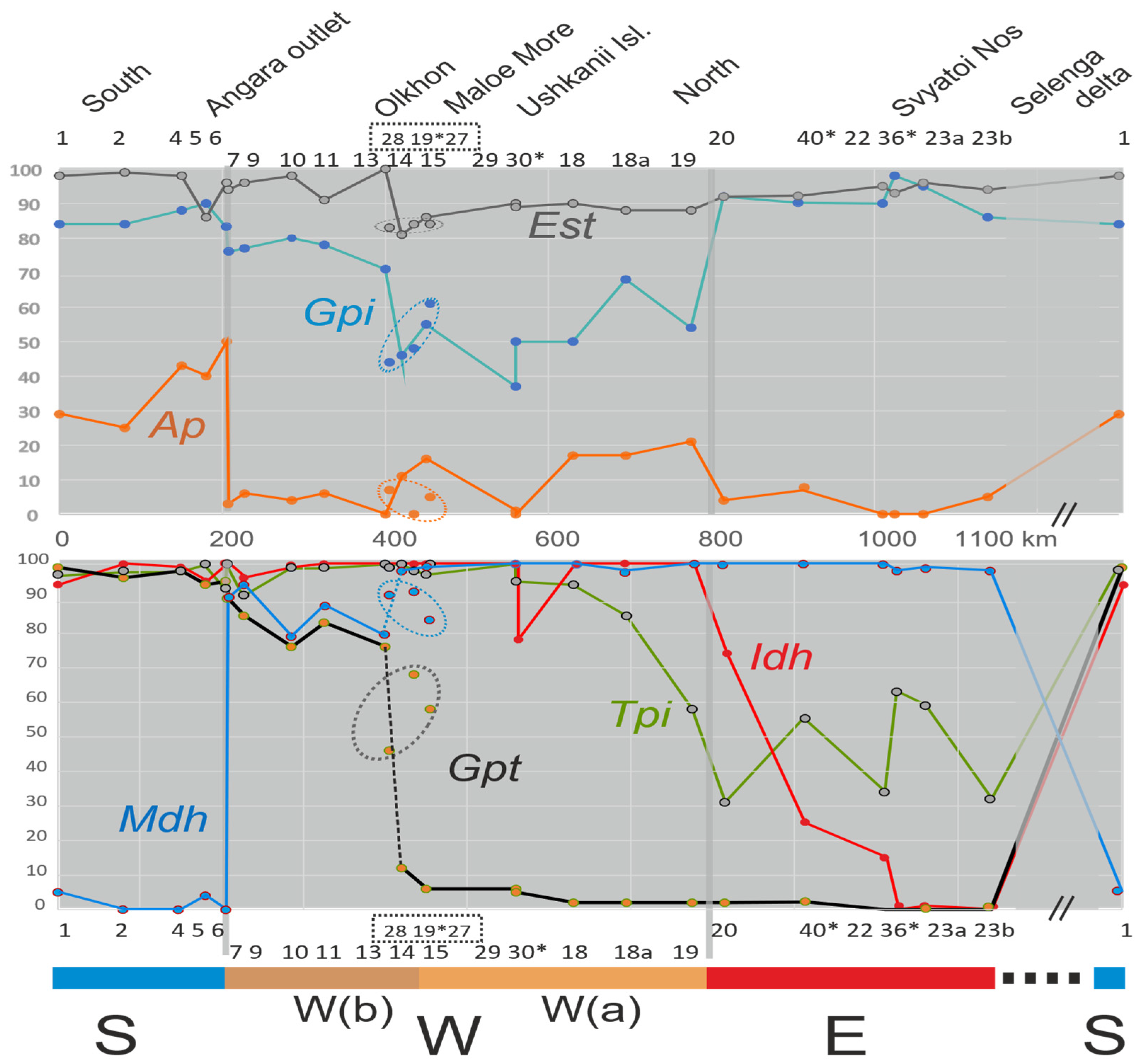
3. Results
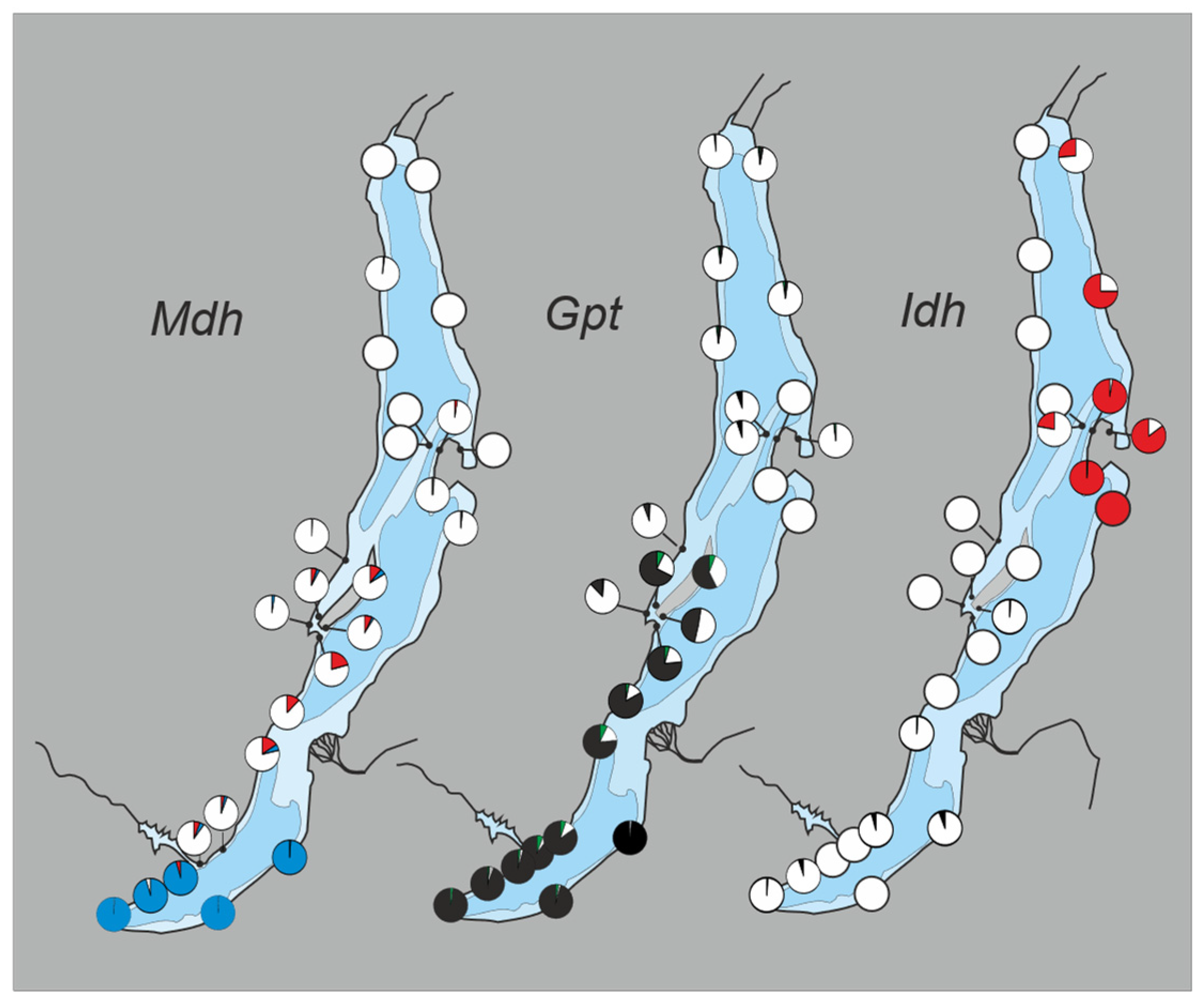
3.1. Subdivision by Nuclear Characters
3.2. Contact Zones
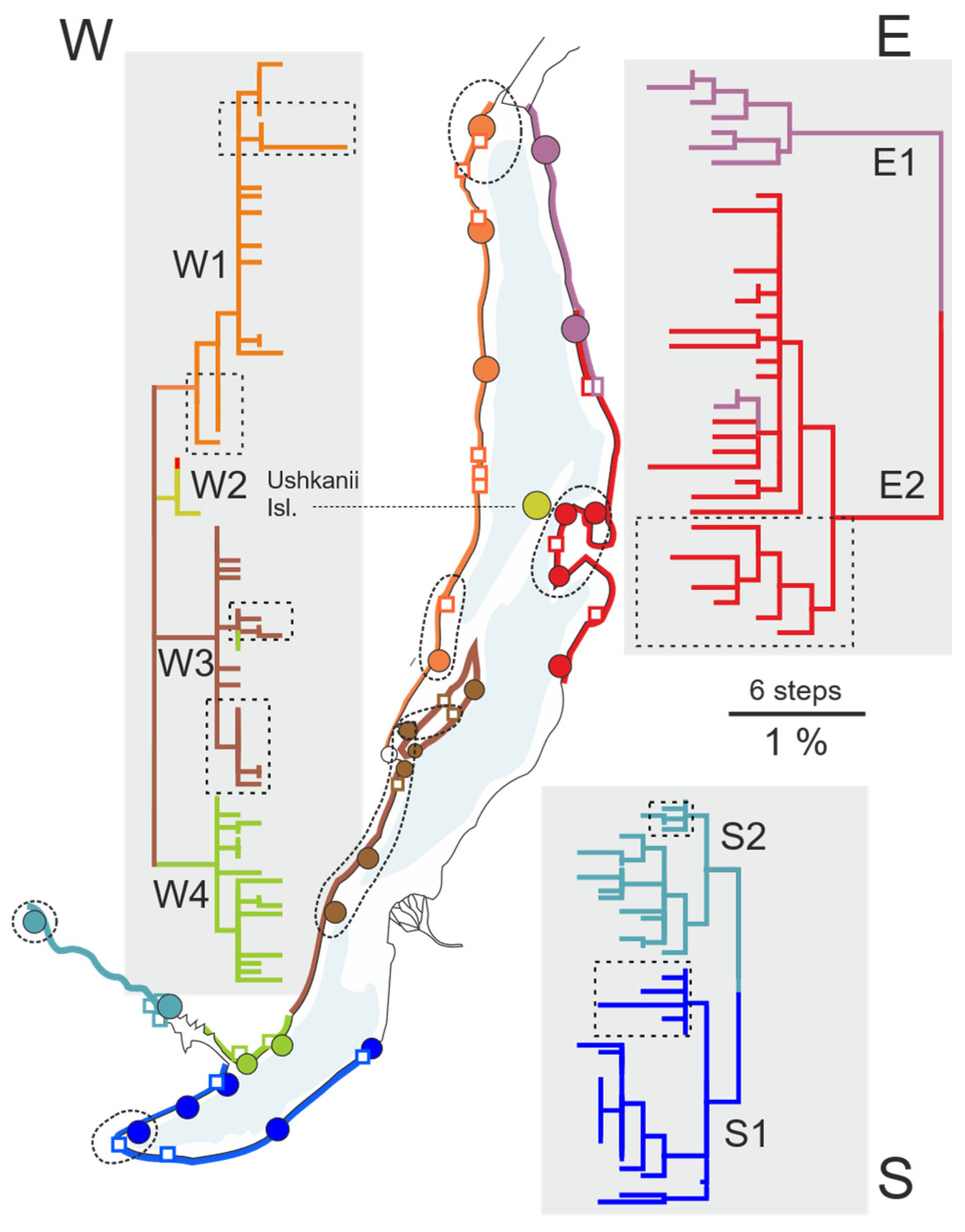
3.3. Hierarchical Parapatry of Mitochondrial Variation
4. Discussion
4.1. Systematic Significance and Taxonomy
4.2. Divergence and Diversification
4.3. Eulimnogammarus oligacanthus, the Angara River “E. verrucosus A” and Associated Variation
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kozhova, O.M.; Izmesteva, L.R. Lake Baikal: Evolution and Biodiversity; Backhuys: Leiden, The Netherlands, 1998. [Google Scholar]
- Kamaltynov, R.M. Amfipody (Amphipoda: Gammaroidea). In Annotirovannyi Spisok Fauny Ozera Baikal i Ego Vodosbornogo Basseina. Tom I: Ozero Baikal. Kniga 1; Timoshkin, O.A., Ed.; Nauka: Novosibirsk, Russia, 2001; pp. 572–831. [Google Scholar]
- Takhteev, V.V.; Berezina, N.A.; Sidorov, D.A. Checklist of the Amphipoda (Crustacea) from continental waters of Russia, with data on alien species. Arthropoda Sel. 2015, 24, 335–370. [Google Scholar] [CrossRef]
- Kamaltynov, R.M. Vysshie rakoobraznye (Amphipoda: Gammaroidea) Angary i Eniseya. In Annotirovannyi Spisok Fauny Ozera Baikal i Ego Vodosbornogo Basseina. Tom II: Vodoemy i Vodotoki Yuga Vostochnoi Sibiri i Severnoi Mongolii. Kniga 1; Timoshkin, O.A., Ed.; Nauka: Novosibirsk, Russia, 2009; pp. 297–329. [Google Scholar]
- Macdonald, K.S., III; Yampolsky, L.; Duffy, J.E. Molecular and morphological evolution of the amphipod radiation of Lake Baikal. Mol. Phylogenet. Evol. 2005, 35, 323–343. [Google Scholar] [CrossRef] [PubMed]
- Hou, Z.; Sket, B.; Li, S. Phylogenetic analyses of Gammaridae crustacean reveal different diversification patterns among sister lineages in the Tethyan region. Cladistics 2014, 30, 352–365. [Google Scholar] [CrossRef] [PubMed]
- Naumenko, S.A.; Logacheva, M.D.; Popova, N.V.; Klepikova, A.V.; Penin, A.A.; Bazykin, G.A.; Etingova, A.E.; Mugue, N.S.; Kondrashov, A.S.; Yampolsky, L.Y. Transcriptome-based phylogeny of endemic Lake Baikal amphipod species flock: Fast speciation accompanied by frequent episodes of positive selection. Mol. Ecol. 2017, 26, 536–553. [Google Scholar] [CrossRef] [PubMed]
- Drozdova, P.B.; Madyarova, E.V.; Gurkov, A.N.; Saranchina, A.E.; Romanova, E.V.; Petunina, J.V.; Peretolchina, T.E.; Sherbakov, D.Y.; Timofeyev, M.A. Lake Baikal amphipods and their genomes, great and small. Vavilovskii Zhurnal Genet. Sel. 2024, 28, 317–325. [Google Scholar] [CrossRef]
- Bazikalova, A.Y. Amfipoda ozera Baikala. Tr. Baikalskoi Limnol. Stantsii 1945, 11, 1–440. [Google Scholar]
- Takhteev, V. On the current state of taxonomy of the Baikal Lake amphipods (Crustacea, Amphipoda) and the typological ways of constructing their system. Arthropoda Sel. 2019, 28, 374–402. [Google Scholar] [CrossRef]
- Väinölä, R.; Kamaltynov, R.M. Species diversity and speciation in the endemic amphipods of Lake Baikal: Molecular evidence. Crustaceana 1999, 72, 945–955. [Google Scholar]
- Yampolsky, L.Y.; Kamaltynov, R.M.; Ebert, D.; Filatov, D.A.; Chernykh, V.I. Variation of allozyme loci in endemic gammarids of Lake Baikal. Biol. J. Linn. Soc. 1994, 53, 309–323. [Google Scholar] [CrossRef]
- Mashiko, K.; Kamaltynov, R.; Shervakov, D.Y.; Morino, H. Genetic separation of gammarid (Eulimnogammarus cyaneus) populations by localized topographic changes in ancient Lake Baikal. Arch. Hydrobiol. 1997, 139, 379–387. [Google Scholar] [CrossRef]
- Mashiko, K.; Kamaltynov, R.; Morino, H.; Sherbakov, D.Y. Genetic differentiation among gammarid (Eulimnogammarus cyaneus) populations in Lake Baikal, East Siberia. Arch. Hydrobiol. 2000, 148, 249–261. [Google Scholar] [CrossRef]
- Gomanenko, G.V.; Kamaltynov, R.M.; Kuzmenkova, Z.V.; Berenos, K.; Sherbakov, D.Y. Population structure of the Baikalian amphipod Gmelinoides fasciatus (Stebbing). Russ. J. Genet. 2005, 41, 907–912. [Google Scholar] [CrossRef]
- Bukin, Y.S.; Petunina, J.V.; Sherbakov, D.Y. The mechanisms for genetic diversity of Baikal endemic amphipod Gmelinoides fasciatus: Relationships between the population processes and paleoclimatic history of the lake. Russ. J. Genet. 2018, 54, 1059–1068. [Google Scholar] [CrossRef]
- Daneliya, M.E.; Kamaltynov, R.M.; Kontula, T.; Väinölä, R. Systematics of the Baikalian Babr (Crustacea: Amphipoda: Pallaseidae). Zootaxa 2009, 2276, 49–68. [Google Scholar] [CrossRef]
- Daneliya, M.E.; Väinölä, R. Five subspecies of the Dorogostaiskia parasitica complex (Dybowsky) (Crustacea: Amphipoda: Acanthogammaridae), epibionts of sponges in Lake Baikal. Hydrobiologia 2014, 739, 95–117. [Google Scholar] [CrossRef]
- Gurkov, A.; Rivarola-Duarte, L.; Bedulina, D.; Fernández Casas, I.; Michael, H.; Drozdova, P.; Nazarova, A.; Govorukhina, E.; Timofeyev, M.; Stadler, P.F.; et al. Indication of ongoing amphipod speciation in Lake Baikal by genetic structures within endemic species. BMC Evol. Biol. 2019, 19, 138. [Google Scholar] [CrossRef]
- Saranchina, A.; Mutin, A.; Govorukhina, E.; Rzhechitskiy Ya Gurkov, D.; Timofeyev, M.; Drozdova, P. Genetic diversity in a Baikal species complex Eulimnogammarus verrucosus (Amphipoda: Gammaroidea) in the Angara River, the only outflow of Lake Baikal. Zool. Scr. 2024, 53, 867–879. [Google Scholar] [CrossRef]
- Ballard, J.W.O.; Whitlock, M.C. The incomplete natural history of mitochondria. Mol. Ecol. 2004, 13, 729–744. [Google Scholar] [CrossRef]
- Rubinoff, D.; Holland, B.S. Between Two Extremes: Mitochondrial DNA is neither the Panacea nor the Nemesis of Phylogenetic and Taxonomic Inference. Syst. Biol. 2005, 54, 952–961. [Google Scholar] [CrossRef]
- Väinölä, R.; Kontula, T.; Mashiko, K.; Kamaltynov, R.M. Extensive geographical structuring of ”intraspecific” molecular diversity in endemic Lake Baikal amphipods. In Abstracts of the Third International Symposium “Ancient Lakes: Speciation, Development in Time and Space, Natural History”, Irkutsk, Russia, 2–7 September 2002; Nauka: Novosibirsk, Russia, 2002; p. 198. Available online: https://www.marinespecies.org/aphia.php?p=sourcedetails&id=492598 (accessed on 25 September 2024).
- Horton, T.; Lowry, J.; De Broyer, C.; Bellan-Santini, D.; Copilaş-Ciocianu, D.; Daneliya, M.E.; Dauvin, J.C.; Fišer, C.; Gasca, R.; Grabowski, M.; et al. World Amphipoda Database. 2024. Available online: https://www.marinespecies.org/amphipoda (accessed on 3 November 2024). [CrossRef]
- Murphy, R.W.; Sites, J.W.; Buth, D.G.; Haufler, C.H. Proteins: Isozyme electrophoresis. In Molecular Systematics; Hillis, D.M., Moritz, C., Mable, B.K., Eds.; Sinauer Associates: Sunderland, MA, USA, 1996; pp. 51–120. [Google Scholar]
- Väinölä, R.; Varvio, S.L. Molecular divergence and evolutionary relationships in Pontoporeia (Crustacea: Amphipoda). Can. J. Fish. Aquat. Sci. 1989, 46, 1705–1713. [Google Scholar] [CrossRef]
- Väinölä, R.; Vainio, J.K.; Palo, J.U. Phylogeography of “glacial relict” Gammaracanthus (Crustacea, Amphipoda) from boreal lakes and the Caspian and White seas. Can. J. Fish. Aquat. Sci. 2001, 58, 2247–2257. [Google Scholar] [CrossRef]
- Audzijonytė, A.; Damgaard, J.; Varvio, S.L.; Vainio, J.K.; Väinölä, R. Phylogeny of Mysis (Crustacea, Mysida): History of continental invasions inferred from molecular and morphological data. Cladistics 2005, 21, 575–596. [Google Scholar] [CrossRef] [PubMed]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 1–9. Available online: https://www.nhm.uio.no/english/research/resources/past/ (accessed on 1 August 2023).
- Tamura, K.; Stecher, G.; Kumar, S. MEGA 11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Delić, T.; Švara, V.; Coleman, C.O.; Trontelj, P.; Fišer, C. The giant cryptic amphipod species of the subterranean genus Niphargus (Crustacea, Amphipoda). Zool. Scr. 2017, 46, 740–752. [Google Scholar] [CrossRef]
- Audzijonyte, A.; Väinölä, R. Mysis nordenskioldi n. sp. (Crustacea, Mysida), a circumpolar coastal mysid separated from the NE Pacific M. litoralis (Banner, 1948). Polar Biol. 2007, 30, 1137–1157. [Google Scholar] [CrossRef]
- Drozdova, P.; Saranchina, A.; Madyarova, E.; Gurkov, A.; Timofeyev, M. Experimental crossing confirms reproductive isolation between cryptic species within Eulimnogammarus verrucosus (Crustacea: Amphipoda) from Lake Baikal. Int. J. Mol. Sci. 2022, 23, 10858. [Google Scholar] [CrossRef]
- Mats, V.D.; Shcherbakov, D.Y.; Efimova, I.M. Late Cretaceous-Cenozoic history of the Lake Baikal depression and formation of its unique biodiversity. Stratigr. Geol. Correl. 2011, 19, 404–423. [Google Scholar] [CrossRef]
- Daneliya, M.E.; Kamaltynov, R.M.; Väinölä, R. Phylogeography and systematics of Acanthogammarus s. str., giant amphipod crustaceans from Lake Baikal. Zool. Scr. 2011, 40, 623–637. [Google Scholar] [CrossRef]
- Kochanova, E.; Mayor, T.; Väinölä, R. Cryptic diversity and speciation in an endemic copepod crustacean Harpacticella inopinata within Lake Baikal. Ecol. Evol. 2024, 14, e11471. [Google Scholar] [CrossRef]
- Crandall, E.D.; Sbrocco, E.J.; DeBoer, T.S.; Barber, P.H.; Carpenter, K.E. Expansion dating: Calibrating molecular clocks in marine species from expansions onto the Sunda shelf following the last glacial maximum. Mol. Biol. Evol. 2012, 29, 707–719. [Google Scholar] [CrossRef] [PubMed]
- Audzijonyte, A.; Väinölä, R. Phylogeographic analyses of a circumarctic coastal and a boreal lacustrine mysid crustacean, and evidence of fast postglacial mtDNA rates. Mol. Ecol. 2006, 15, 3287–3301. [Google Scholar] [CrossRef] [PubMed]
- Loehr, J.; Sundell, J.; Immonen, M.; Väinölä, R. Patterns in antipredator armature reduction and maintenance in isolated spring populations of an amphipod crustacean. Ecol. Evol. 2023, 13, e10423. [Google Scholar] [CrossRef] [PubMed]
| Lineage | Total π Within Lineage (%) | Average π Within Phylogroups (%) | Average π Within Sites (%) | Between Phylogroups Net Distance (%) | Average Between-Phylogroup Distance (%) | Differentiation Among Sites ΦSP |
|---|---|---|---|---|---|---|
| S | 1.09 | 0.64 | 0.54 | 0.92 | 1.56 | 0.20 |
| W | 0.98 | 0.27 | 0.21 | 0.88 | 1.16 | 0.27 |
| E | 1.64 | 0.95 | 0.87 | 2.21 | 3.16 | 0.10 |
| Average | 1.23 | 0.62 | 0.54 | 1.34 | 1.96 | 0.19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Väinölä, R.; Kontula, T.; Mashiko, K.; Kamaltynov, R.M. Congruent and Hierarchical Intra-Lake Subdivisions from Nuclear and Mitochondrial Data of a Lake Baikal Shoreline Amphipod. Diversity 2024, 16, 706. https://doi.org/10.3390/d16110706
Väinölä R, Kontula T, Mashiko K, Kamaltynov RM. Congruent and Hierarchical Intra-Lake Subdivisions from Nuclear and Mitochondrial Data of a Lake Baikal Shoreline Amphipod. Diversity. 2024; 16(11):706. https://doi.org/10.3390/d16110706
Chicago/Turabian StyleVäinölä, Risto, Tytti Kontula, Kazuo Mashiko, and Ravil M. Kamaltynov. 2024. "Congruent and Hierarchical Intra-Lake Subdivisions from Nuclear and Mitochondrial Data of a Lake Baikal Shoreline Amphipod" Diversity 16, no. 11: 706. https://doi.org/10.3390/d16110706
APA StyleVäinölä, R., Kontula, T., Mashiko, K., & Kamaltynov, R. M. (2024). Congruent and Hierarchical Intra-Lake Subdivisions from Nuclear and Mitochondrial Data of a Lake Baikal Shoreline Amphipod. Diversity, 16(11), 706. https://doi.org/10.3390/d16110706





