Abstract
Sunlight and the heat it provides are important ecological resources for reptiles especially for those species living in temperate zones that bask extensively to maximize heat uptake. Sun basking has both benefits and costs for reptiles, giving heat that provides the energy to drive physiology but basking in open patches increases risk of predation due to higher visibility. Prime basking sites are believed to increase benefits for reptiles that include, in addition to open sunlit areas, facilitate detection of predators and prey and escape to nearby refuges. However, if such sites are limited, both inter and intra-specific interference may occur and this kind of competition may impact on a reptile’s ability to access prime basking sites, and as a consequence, its capacity to thermoregulate to optimum body temperatures. This may be especially important for juveniles, for whom rapid growth is a key factor in survivorship. We studied communal basking and interaction events at prime basking sites in the European green lizard, Lacerta bilineata, in a hedgerow in western France. We compared basking behaviour of adults and juveniles with sympatric adult wall lizards Podarcis muralis using non-invasive photographic-mark-recapture. Adult L. bilineata were more evenly distributed across basking sights compared to juveniles but significant differences were only detected between males and juveniles. Juvenile L. bilineata abandoned basking sites at the approach of both adult males and females and were aggressively removed by adult male L. bilineata. We found inter-specific communal basking between both adult and juvenile L. bilineata with adult wall lizards P. muralis. Communal basking was observed between male and female L. bilineata but not between adult males or between adult female L. bilineata. Communal basking was in proportionally greater frequency in juveniles compared to adult L. bilineata.
1. Introduction
The influence of spacing patterns and territoriality in lizards has long been known (e.g., [1,2]). This has shown that heat distribution in complex heterothermic environments in temperate regions will determine spacing patterns and drive lizard habitat selection due to the key influence of temperature on reptile physiology through Q10 effects on reptile physiology [3]. Thus the availability of basking sites and their distribution can determine behaviour by influencing what a reptile does and what it is capable of doing. Inter- and intra-specific interactions at basking sites are part of this behavioural complex since contact events may present both benefits and costs by influencing access to favorable microhabitats that offer suitable thermal mosaics for thermoregulation. If certain behaviors at basking sites increase survivorship and reproductive success by facilitating the capacity to attain optimum body temperatures then natural selection will tend to favor their use [3].
Theory proposes that reptiles by selecting low cost prime basking sites, will increase their rate of heat exchange to optimum body temperatures [3]. This will, among other things, enhance both the reproductive success of adults and enhance the growth of juveniles. Additionally, prime basking sites facilitate detection of approaching predators and prey species, and reduce energy budgets by reducing shuttling frequency between basking sites [3,4,5,6,7]. Compared to tropical regions where heat is more readily available, basking sites in temperate zones are more important especially at sites where dense vegetation can limit basking site availability [8]. Thus, many lizards including non-territorial females [9,10,11] will often defend basking sites against conspecifics [12], or sympatric species ([13], -but see [11] for parthenogenetic females). However, defending basking sites increases energy expenditure [14] and given the extent of basking site defense in different lizard species suggests that this behaviour must be adaptive in evolutionary terms. Therefore, the costs and benefits of thermoregulation in a heliotherm are not solely dependent on Q10 effects and energetics, but are also influenced by habitat heterogeneity, basking site availability and ability to defend such sites [15,16,17,18,19]. When habitats have an abundance of predators [3,19] the shared vigilance and detection of predators includes the dilution effect where several lizards escaping simultaneously create confusion for predators [20].
The western green lizard Lacerta bilineata (formerly Lacerta viridis) is one of the larger Palaearctic lizard species with a distribution that reaches into northern areas of Europe, including northern Germany, the Channel Islands and southern England, the latter where it is an introduced species [21]. It is an example of an ectothermic species that can be found in a wide variety of habitats across Western Europe operating as a heliothermic basker selecting sunlit areas to attain body temperatures of up to 34 °C [22]. It is mainly a sentinel predator ambushing mostly small invertebrates between bouts of basking from low terrestrial microhabitats [21]. Studies of L. bilineata and sympatric Podarcis muralis have been carried out in a hedgerow system and wider area in west France [23,24,25,26,27]. The results indicated that both species primarily selected wood-based materials for basking compared to basking on soil or stones especially in P. muralis. A proposed partial explanation for the differences were the significantly higher levels of communal basking in P. muralis, and hence shared basking resources, compared to L. bilineata, along with frequent intra-specific aggression in L. bilineata where serious injuries or mortalities were found over the wider area around the study site [25]. Additionally, there was evidence that some individual L. bilineata in the hedgerow especially juveniles were constrained by conspecifics from accessing high quality basking sites [27].
How individual lizards adapt to different ecological conditions may provide specific information that is required to develop larger scale ecosystem models that may offer insight into how ecosystems function as a whole [28]. For example, in a previous study numbers of L. bilineata fluctuated widely over a 14-year time period [23], which suggests spacial competition may increase during periods of very high numbers. The present study was prompted by observations of high lizard numbers and changes in the ratios of adult and juvenile L. bilineata numbers in 2023 including high juvenile lizard presence compared to the previous three years [29]. We also noticed an increase in frequency of basking communally at certain sites, especially between adult L. bilineata and adult P. muralis and between juvenile L. bilineata with other juveniles and also with adult P. muralis. In this study we examine the interference hypothesis in L. bilineata [30] focusing on communal basking in adult L. bilineata at prime basking sites with adult P. muralis and juvenile L. bilineata. We expected communal basking between these groups to be minimal due to known predation on both P. muralis and juvenile L. bilineata. To examine this we made new observations of inter and intra-specific basking at prime basking sites defined as such due to their frequency of use in a communal context. We asked the following questions:
- (1)
- What were the factors that determined these prime basking sites and how were they spacially distributed in the hedgerow and how frequently did the lizards at these sites occupy them? This is important because greater movement between basking sites may increase risk of predation and risk levels that may vary for juveniles, males and females.
- (2)
- How frequent was inter and intra-specific communal basking by lizards at such sites and were they used in equal frequencies by adults and juveniles? For example, communal basking may be an adaptive behaviour by increasing detection of approaching predators or, if in physical contact, by reducing heat loss on cooler days. This is important because if prime basking sites are a limited resource many individuals may be constrained to either bask communally with other lizards or operate in less optimal microhabitats.
- (3)
- Aggression between adults and juvenile L. bilineata is known [27] including predation by L. bilineata on P. muralis [31]. This prompts a key question of what is the extent of interference competition and communal basking at prime basking sites, between what groups of lizards and how frequently?
To answer these questions requires identifying prime basking sites. To do this we monitored frequency of use by the lizards of various basking patches along the hedgerow, including those where communal basking was observed, and assumed that the sites with high frequency of use reflected optimum basking microhabitats for the lizards. Lizards were observed basking at other sites but in much less frequency and mostly by solitary individuals. Here we focus on sites where communal basking was most frequent.
2. Methods
2.1. Study Area
The study was carried out in a hedgerow situated on the edge of the village of Chasnais in West France (46°27′38″ N; 1°13′42″ W) that was approximately 5 m above sea level. Historically the area was below sea level (16th century). The hedgerow was bordered by agricultural land but frequent sampling in these areas failed to find lizards presence in these fields, either when it was an open environment when crop growth is minimal as during the study period or when crop growth is underway and the terrain is partially shaded. Field data on L. bilineata were gathered during the main activity period of the species, from March to the end of October 2023. The hedgerow, with a length of approximately 260 m (Figure 1), fits the definition of a low-cost thermal environment in the sense of [3] being structurally relatively simple and formed of trees Fraxinus excelsior (Ash), Quercus robur (Oak) and bush Rubus fruticosus, (Bramble) that with Hedera helix (Ivy) give abundant shaded areas and open sunlit patches (Figure 1).

Figure 1.
Location of prime basking sites along the hedgerow. See text and Table 1 for further details.
When prime basking sites were identified the distances between the sites were measured using a measuring tape. This included measuring heights from the ground and distances from vegetation cover and material (wood, soil etc.). Each basking site was labelled from A to H for simplicity (Figure 1).
When prime basking sites were identified, the distances between the sites were measured using a measuring tape. This included measuring heights from the ground and distances from vegetation cover and material (wood, soil, etc.). Each basking site was labelled from A to H for simplicity (Figure 1).
2.2. Lizard Sampling
Non-invasive photographic-mark-recapture [29,32] was employed to identify individual lizards. This method does not require the capture and handling of individuals and hence minimizes disturbance and stress of handling and potential distortions of behaviour. Photographs were taken using a Lumix DMC-TZ70 camera set to Intelligent-Auto mode for rapid use. Identification was by the varying shades of green, dorsal markings including dorsal lines and other marks presumably due to previous injuries.
Data collection was systematically carried out daily, weather permitting, giving 215 days of sampling each lasting from between 32 to 35 h in each month with daily sampling times of around 45–70 min between 08:30 and 11:00 h. Additional sampling was carried out later in the day but lizards were much more difficult to locate likely due to temperatures being higher and their tendency to spend greater time in vegetation. The lizards tended to bask with all of the body exposed to sun during the earlier part of the day and for longer due to the lower body temperatures when emerging from overnight retreats, which facilitated the identification of individual lizards. Photographic data indicated two size classes of lizards: juveniles with snout to vent lengths of not more than 70 mm and by key body proportions, mainly head and eye dimensions and dorsal patterns. In addition at around 70 mm some individuals started showing aggression to other similar sized individuals—an example of such a lizard is shown in Figure 2G. In this class are included lizards in their first year after birth or early in their second year. Lizards with a snout vent length above 70 mm were classed as adults. Males differed from adult females by their proportionally larger heads and pronounced penile swelling while females had a longer torso. Females often have different colour patterns while males are more uniform with fewer of the unique black patches found in females. All lizards were detected while slowly walking along the hedgerow and being photographed (Figure 1). The above mentioned features enabled individual lizard identification [33].
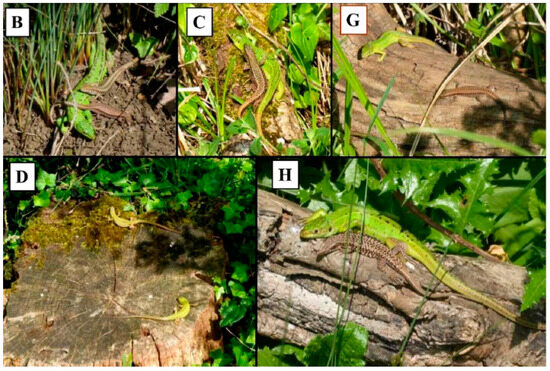
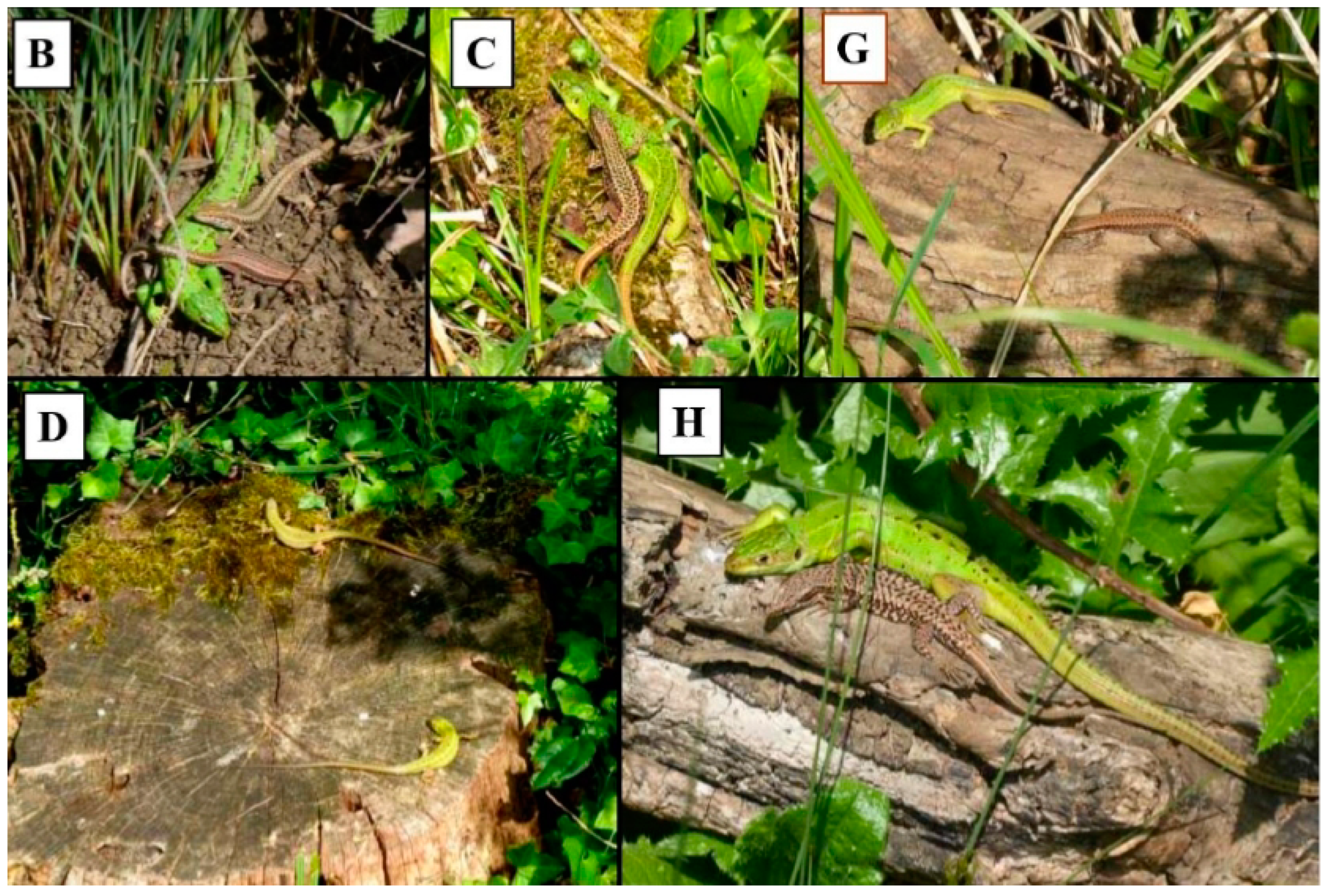
Figure 2.
Examples of communal basking in L. bilineata from selected prime basking sites; (B) adult female with male and female P. muralis, (C) adult male P. muralis with adult male L. bilineata, (G) juvenile L. bilineata with adult female P. muralis, (D) two juvenile L. bilineata and (H) adult female with male P. muralis. Upper case letters refer to site labeling in Figure 1.
Prime basking sites are here defined as the eight most frequently used sites by L. bilineata either during solitary or communally basking. Criteria to define a prime basking site required a lizard to bask for more than 1 min duration at a site and do not include lizards seen in shaded areas or moving across open patches. Communal basking was defined as two or more lizards sharing a basking site. We made no distinction between lizards in physical contact or spacially separated. However we appreciate that during cold weather physical contact may enhance heat conservation but given the warm weather during sampling this may have limited effect. Examples of both are shown in Figure 2.
2.3. Statistical Analysis
To determine whether lizards used basking sites randomly or were selective of basking sites required constructing null models to produce patterns of expected probabilities that would indicate random selection [34]. In this study we have used two sets of null models. The first was based on the assumption that basking site selection was proportional to basking site availability across the 8 sites, which were spaced at different distances along the hedgerow (Figure 1). This gives an expected probability for each cell (n = 1/8) of 0.125. Significant deviations from the null model would be indicative of basking site selection. The test used was the Kolmogorov-Smirnov Goodness of Fit test (Dmax), which is distribution-free, exact and not sensitive to cell counts. It evaluates the maximum difference between the cumulative proportions of the two patterns, here basking site use compared to basking site availability. For analysis, the observed and expected probabilities were converted to decimal fractions since the Kolmogorov- Smirnov test requires that Σn = 1, where n is the decimal proportions. The hypothesis is,
where P is the observed distribution of basking site use and Po the distribution of available basking sites. The second null model utilized frequency of adult male and female basking site use (Padult) as the expected probabilities for comparison against juvenile distributions. This gave null distributions, shown as decimal fractions, of:
Ho: P = Po: H1: P ≠ Po,
Males (n = 53); site A, 0.13, site B, 0.28, site C, 0.07, site D, 0.09 site E, 0.13, site F, 0.09, site G, 0.11, site H, 0.08.
Females (n = 75): site A, 0.16, site B, 0.10, site C, 0.17, site D, 0.09, site E, 0.04, site F, 0.16, site G, 0.15, 0.04.
The expected probabilities of males and females were first compared and found to be in agreement (p > 0.05). These data were then pooled as the null model (see below) and compared with juvenile basking site use. The hypothesis is
Ho: P = Padult: H1: P ≠ Padult,
A Dmax value of 1 in any of the categories (basking sites) in either the equality or adult lizard null model tests indicates observed basking site use at this site is in agreement with expected basking site selection.
To compare individual basking site selection between males, females and juvenile lizards a Kruskal-Wallis multiple comparisons H-test followed by a Dunn’s post hoc was used. This non-parametric analysis compares the ranked data using a χ2 test to ascertain if differences between the ranks is significant or due to random noise with α = 0.05. The data used was the frequency of individual lizard basking at each of the eight sites. Thus the maximum potential n for each lizard in the analysis was 8 (=basking sites). Statistics were carried out using Minitab V17 and online Vasser Stats.
2.4. Defining Q10
Reptiles bask in the sun to raise body temperatures to preferred levels for optimal physiological performance. In this paper we implicitly assume that selecting basking sites that optimize rates of heat gain will enable preferred body temperatures to be attained more rapidly. This involves the well-known Q10 effects on physiology, which is a measure of thermal sensitivity of physiological processes with increases in body temperature of 10 °C. This enables lizards and other ectotherms to optimize rates of digestion, growth, and muscle power and hence speed of movement, and in females embryonic development with increases in body temperature [35]. Muscle power and speed of movement for example impacts on the ability of a reptile to secure prey or escape predators. Most of the Q10 effects on physiological processes in reptiles range between 2 and 3 and are determined from,
where Q10 is a unitless quantity that defines the rate change between reaction rates R2 and R1 between temperatures t2 and t1 in °C. A Q10 of 1 would indicate thermal independence indicating temperature has no effect on physiological processes; values increasingly greater than 1 indicate that physiological processes have increasing thermal dependence.
Q10 = (R2/R1)10C(t2/t1),
3. Results
3.1. Properties of the Prime Basking Sites
Five of the eight prime basking sites were wood based, consisting of a tree stump (D) and four fallen branches (C, E, G, H) with the remaining three sites bare soil (A, B, E) (Figure 1 and Figure 2). Heights of basking sites above ground varied between 0 and 110 cm (mean = 28.5 ± 36.1 cm) and distances between basking sites from 1.4 to 46 m (mean = 20.3 ± 16.4 m). Distances between sites varied, for example, D and E were the closest at around 1.4 m with the longest distance between E and F at around 23 m. All sites were within a few cm of vegetation cover and were situated close to water, at distances of no greater than 1.5 m, and protected from northerly and northwestern winds by the south-facing bank of the hedgerow.
3.2. Basking by Solitary Lizards
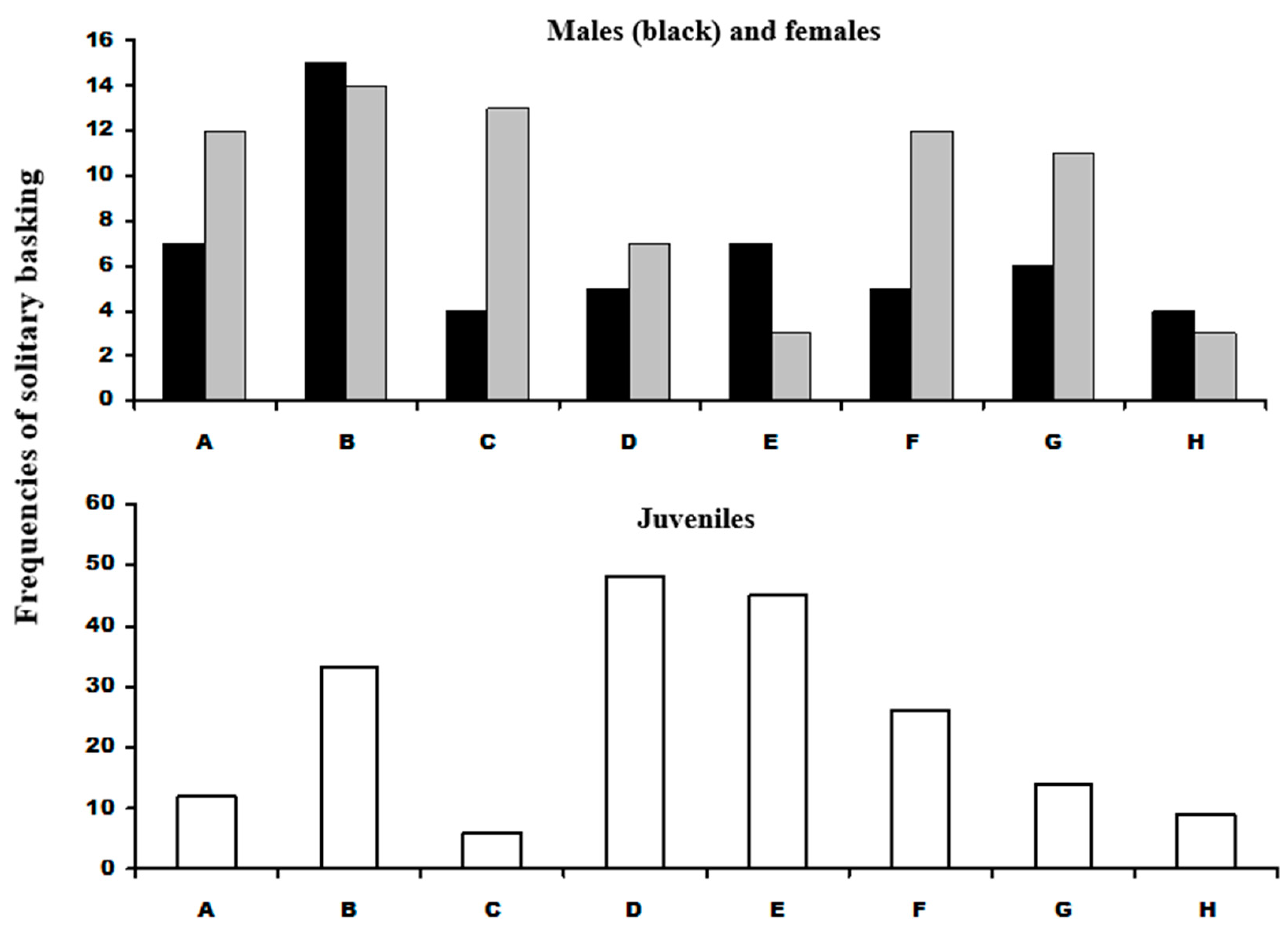
In total, 202 adult L. bilineata sightings were of solitary basking. Of these 87 were of 8 males, 115 observations based on 7 females and 139 observations derived from 17 juveniles. The Kolmogorov-Smirnov test of basking site use indicated no departure from equality of site use in either males or females. A second test of male versus female basking site use with male frequency as the expected probability supported the equality test that both sexes used the sites in approximate equal frequency Dmax = 0.06, p > 0.05 (Figure 3; Table 1). The Dmax value for juveniles differed significantly from equality of basking site use; Site C was significantly less used but D and E were used almost twice as frequently used than the expected use. Given the statistical agreement between the male and female data, the data sets were pooled and comparisons were made between adults and juveniles. This indicated that the adult distribution was significantly different from juveniles, with juveniles basking at up to almost three times more than adults at sites D and E, and over four times less than adults at Site C (Question 1).
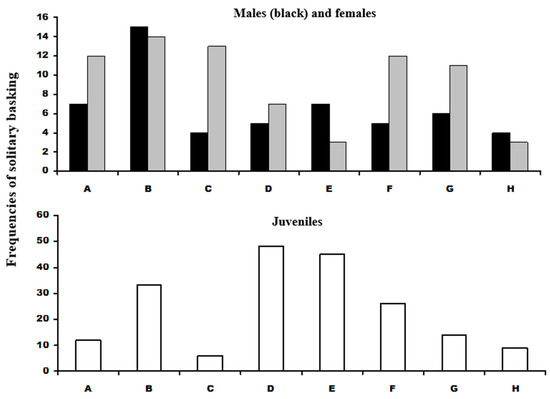
Figure 3.
Male and female L. bilineata solitary basking distributions (top) with males shown as black cells and grey cells as females. (Bottom) graph shows distribution of juvenile L. bilineata solitary basking. Labeling on the x-axis refers to labeling in Figure 1.

Table 1.
Results of the Kolmogorov-Smirnov tests of basking site presence of adult and juvenile L. bilineata. Adult Lb frequencies did not differ from equality of basking site but there was a highly significant difference between adults and juveniles (at sites C and E). Juveniles sightings differed significantly from a null model of equality of distribution but not adults. Comparison of communal basking of adult with juvenile L. bilineata with P. muralis differed significantly (sites B and D). All tests n = 187. Labelling as in Figure 1.
3.3. Basking Site Use by Individual Lizards
The Kruskal-Wallis H-test indicated differences between all groups, χ2(2) = 8.72, p = 0.013 with mean rank scores of 22.5 for males, 19.58 for females and 11.81 for juveniles. The Dunns post-hoc test with Bonferroni-corrected alpha showed a significant difference (p < 0.05) between the mean ranks of males and juveniles.
3.4. Communal Basking
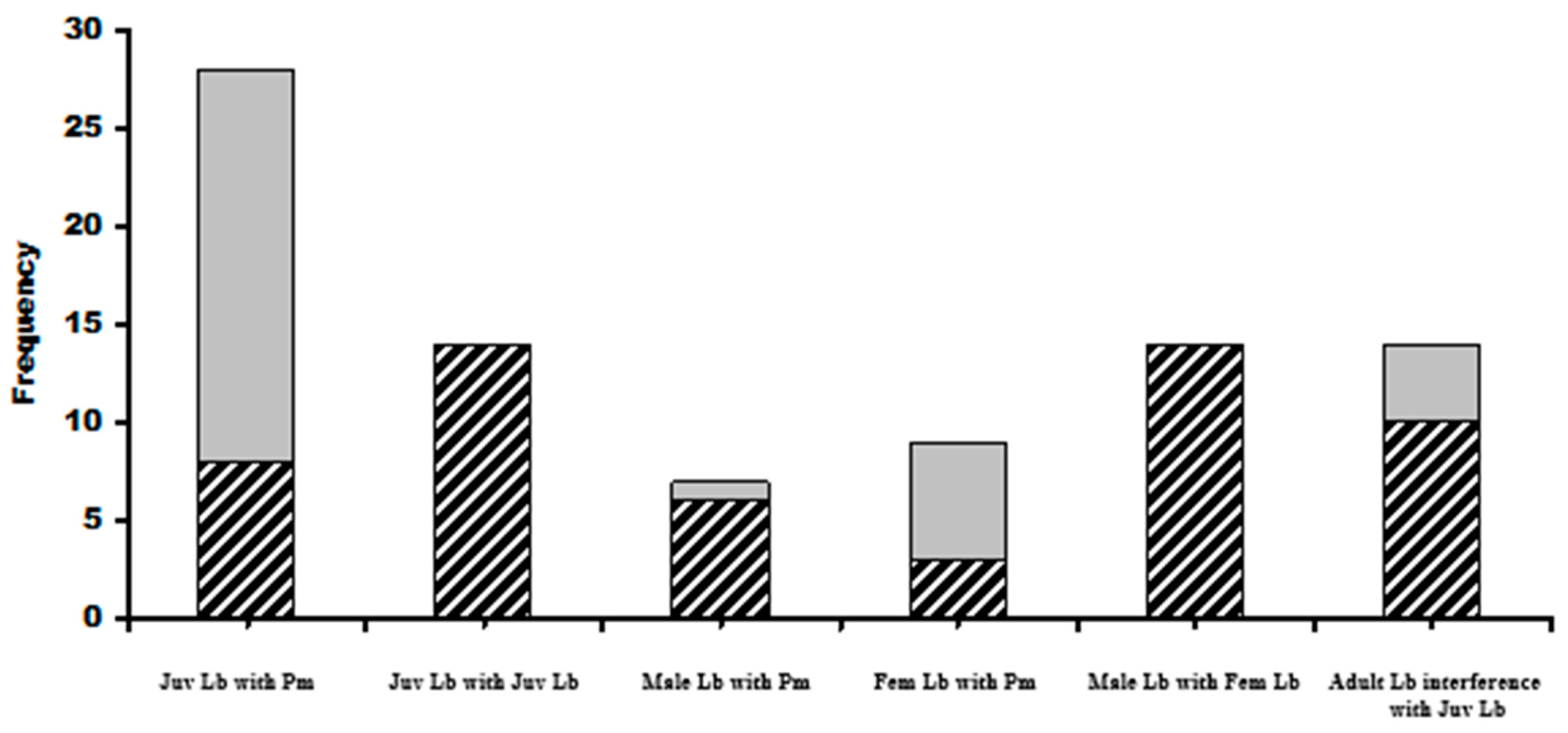
Basking L. bilineata adults were recorded alongside P. muralis on 17 occasions with L. bilineata males more frequently alongside male wall lizards (6 out of 7 times; 85.7%) than L. bilineata females (7 out of 10; 70%). Lacerta bilineata juveniles were seen basking with adult P. muralis on 28 occasions, 11 were alongside male P. muralis (39.3%) and 17 with female P. muralis (60.7%). Lacerta bilineata juveniles were seen basking with other juveniles on 14 occasions but only at sites D (n = 9) and E (n = 5), both wood based sites. Communal basking in adult male and female L. bilineata was recorded on 14 occasions but only at sites B (soil based) (n = 7), C (n = 3) and D (n = 4), C and D were wood based. Communal basking in juveniles represented 30.2% of solitary basking individuals (42 communal versus 139 solitary) whilst in adults, there was a 15.3% frequency of adult solitary basking observations (31 communal versus 202 solitary basking). The differences between adult and juvenile communal basking proportions of total baking observations was significant; two tailed test z = 3.29, p = 0.0009. Wood based sites represented 62.5% of the total identified basking sites with L. bilineata communally basking on these 75% of the time. Soil-based sites represented 37.5% of availability but the lizards were seen on these sites 25% of the time. The differences in proportions were significant (z = 2.36, p = 0.02).
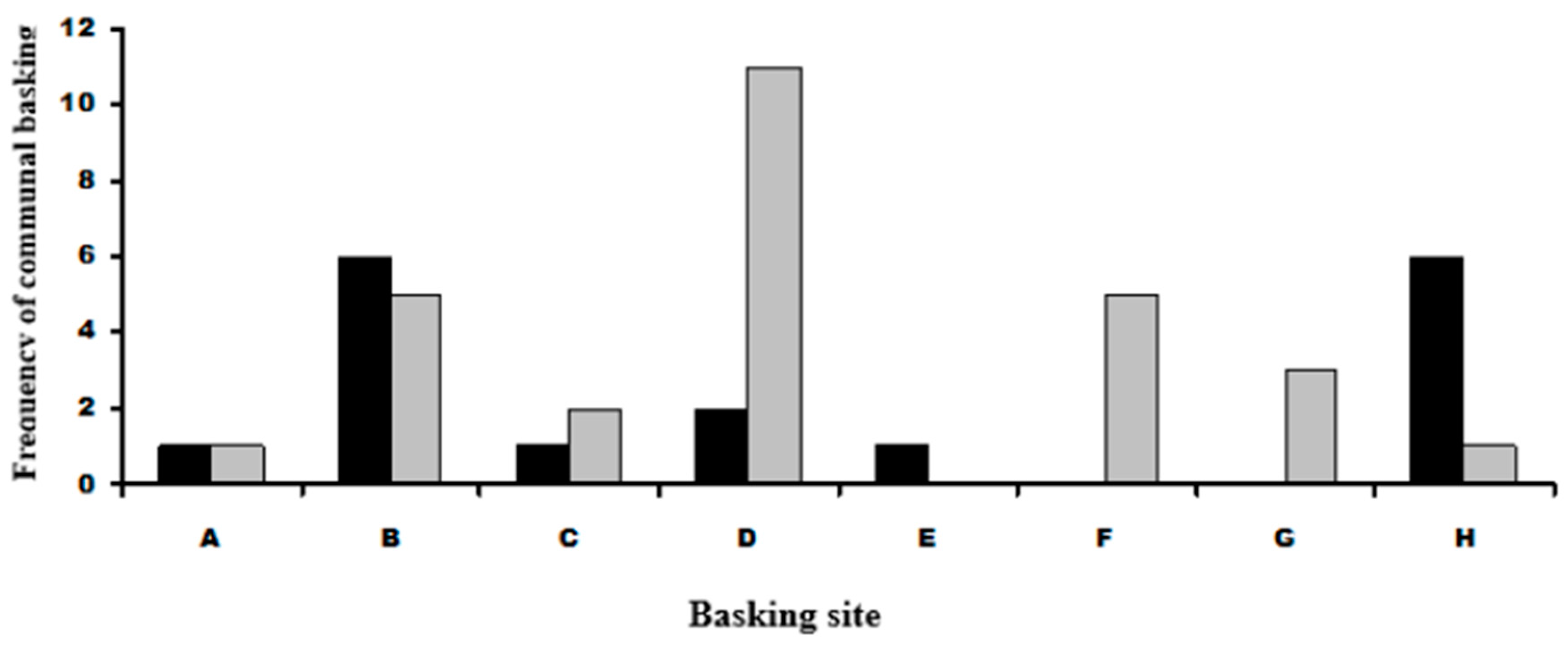
Spacial distributions of adult and juvenile communal basking with P. muralis showed that juveniles basked with P. muralis in greater than expected frequencies at sites D, F and G. Adult L. bilineata basked with P. muralis in greater than expected frequencies at sites B and H. No juvenile L. bilineata were seen basking with adult L. bilineata of either sex or adult females with other adult females or adult males with other adult males. Figure 4 and Figure 5 give graphical summaries of the results and details in Table 1 (Question 2).
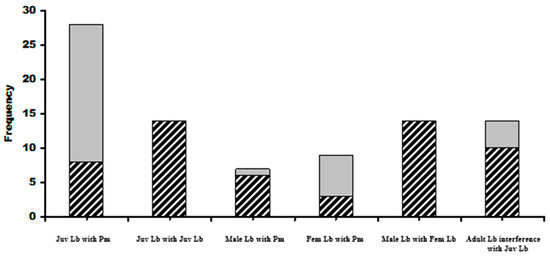
Figure 4.
Frequencies of intra and inter-specific communal basking. Juv = juveniles, cross-hatched cells represent basking with male P. muralis, grey with females. Cross-hatched grey male and female L. bilineata together. Far right histogram shows frequencies of male L bilineata attacks on juveniles (cross-hatched) with grey cells denoting the number of times juveniles abandoned basking sites at the approach of adult females. Symbols: Lb = Lacerta bilineata; Pm = Podarcis muralis.
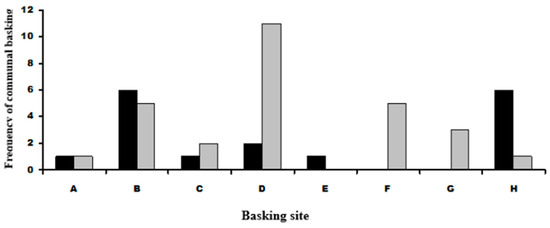
Figure 5.
Frequency distributions of communal basking in juvenile and adult L. bilineata with P. muralis at basking sites. Black cells are adult L. bilineata with P. muralis, grey cells juvenile L. bilineata with P. muralis. Labeling on the x-axis represents labeling in Figure See text for more details.
3.5. Intraspecific Aggression
A total of 14 different individual juvenile L. bilineata abandoned basking sites due to aggression from adults or at the approach of adults (example in Figure 6). Therefore 14 contact events compared to 82 communal basking events was 17.1% (Question 3). Four of the 14 events were at the approach of adult females when no chase was involved with 10 events due to three adult males that conducted aggressive attacks that either removed juveniles from the basking platform or were followed by a chase. No conflicts were observed between adults. This is possibly due to the staggered temporal presence of males and spacing patterns between females but there is also the possibility that conflicts between adult lizards were infrequent. (see Table 2).

Figure 6.
Example of a juvenile L. bilineata being removed from site G by an adult male. The juvenile had been basking for just over 2 min when the adult male appeared on the fallen log. The male then raised the front part of the body and advanced towards the juvenile that quickly fled. In this example there was no chase into the surrounding vegetation. Other contacts between adult males and juveniles involved chases into vegetation, which unfortunately masked the outcomes.

Table 2.
Distributions of individual males M, females F and juveniles J, sighted at the eight basking locations. A–H are locations shown in Figure 1. Large X’s denote five or more sightings; Small x’s denote four or less sightings. Numeric column indicates frequencies that each individual lizard was detected at the 8 sites. For example lizard M1 was seen at all eight sites and M2 at five of the sites. See text for more details.
4. Discussion
The prime basking sites selected by the lizards in this study were mostly wood based (tree stump and fallen branches) that represented five out of the eight sites. In a previous study at the site the higher specific heat capacity of wood (1.17 J/g°C) potentially enabled faster heating rates of body temperatures compared to the other available basking site materials of limestone (0.75 J/g°C) and concrete (0.88 J/g°C) [27]. These differences in heat capacity likely contribute to the high selection of wood by the lizards, since fast heating rates are especially important during morning basking, influencing basking duration and hence reducing the risk of detection by predators, whilst also freeing up time for other activities. However, wind protection especially during spring, when temperatures are much lower, and the visual aspects of the sites probably contributed to their selection (Question 1). Additionally all prime basking sites were situated on a south-facing slope above a water filled ditch (usually dry by June/July) and open to sunshine with protection from cold northern and western winds especially in early spring. The latter is likely a key factor in their selection given that wind is known to impact on lizard body temperatures [36] as was the presence of water at each prime basking site. The presence of water is very likely important in lizard ecology including basking sites being situated in open sunny locations [37,38] along with substrate materials, for example fallen branches have been observed as common basking site features in other species of lizard along with rocky outcrops [39]. Both offer high visual properties. Weather conditions influenced activity in different ways, with the most important being during cloudy weather, although this was infrequent during the study period. The effects were lower levels of locomotory activity and increase basking duration and no sightings of interactions between individuals.
The data from individual lizards showed males in general moved greater distances along the hedgerow than either females or juveniles (Table 2), which would explain their more extensive use of the basking sites. The more limited movements and basking site use in juveniles likely involved avoiding contact with adults or predators (Question 3). However, an unexpected finding was the extent of non-interference communal basking between adult and juvenile L. bilineata with adult P. muralis (Question 2). Given the size differences and known predation on P. muralis by adult L. bilineata [25] this possibly includes the benefit of increased vigilance since communal basking that operates in a similar way to pairing behaviour where shared vigilance and dilution effects enhance escape behaviour when several lizards flee in different directions [40]. Additionally, if adult L. bilineata attempt to aggressively remove P. muralis the rapid locomotion needed to do so would involve higher energy costs, especially during spring and early summer when gravid females in particular experience increases in body mass [41]. Activity would also increase visibility to predators. These observations suggest that communal basking is an adaptive behaviour, including also for P. muralis. However, one of the potential costs is that foraging predators, for example whip snakes Heirophis viridiflavus, were regularly seen foraging in the hedgerow including basking at the prime sites (see Figure 2) potentially learning of their frequent use by the lizards.
Our results offer strong qualitative evidence that juvenile L. bilineata were avoiding adults at basking sites due to the risk of aggression from adults. It should be noted however that these aggression events are difficult to record in a natural environment since they rely on chance encounters and thus may be more frequent than reported here. It is also worth noting that only three individual males of the eight identified were observed removing juveniles aggressively from basking sites. The interactions of juveniles with females were less overtly aggressive and consisted mainly of juveniles retreating at the approach of females. The males involved in aggressive encounters may be examples of individuals that are born with these particular behavioural traits [42,43]. These individuals may have a potential impact on L. bilineata population dynamics, in particular on recruitment levels of juveniles into the adult population that may vary depending on adult numbers in the population [28]. This has been suggested elsewhere in other species of lizard [44].
Intra- and interspecific interference behaviour has been reported in other lizard species, ref. [45,46,47] for example in Leiocephalus schreibersi where the mortality risk for juveniles was due to the behaviour of just three males in a population [48]. The frequency of cannibalistic behaviour might involve population densities and that infanticide may be of ecological and evolutionary significance [49,50]. The notion that if lizards can increase net gain by defending prime basking sites and exclude potential competitors may increase reproductive success [3,50] is not supported here given the extent of communal basking and that both adult, juveniles and P. muralis consume similar prey species (crickets, grasshoppers, spiders, etc. [21]).
The full outcome of interference competition on the population dynamics of lizards is unknown, but during times of high adult numbers, this has been shown to impact juvenile energy budgets and survivorship in other lizard species including through density dependent effects [51,52,53,54]. For example in habitats where basking platforms are limited, competition for thermal resources between adults and juveniles may change in intensity depending on population size and adult/juvenile ratios, which in L. bilineata varies widely over the longer term [24,30]. The results found here for lizards have similarities with other reptile taxa. For example, in snakes where non-interference between individuals at basking sites is common [17,55,56]. It has been suggested [17] that communal basking in snakes might be driven by the need for thermal resources when prime basking sites are scarce, which is supported in the present study. This is a potential area for future synthetic studies to increase understanding of reptile thermal ecology and how it integrates with population ecology, especially in man-made ecotonal habitats.
Author Contributions
R.M. and L.L. conceived the project. R.M. and L.L. wrote the manuscript and agreed the final version. R.M. carried out the fieldwork and data analysis. All authors have read and agreed to the published version of the manuscript.
Funding
No funding was received for this research.
Institutional Review Board Statement
Ethical approval for this study was not required since to animals were trapped, handled or marked in any way.
Data Availability Statement
The data used in this study are part of an ongoing research project.
Conflicts of Interest
The authors declare they have no conflicts of interests.
References
- Tinkle, D.W. Population structure and effective size of a lizard population. Evolution 1965, 18, 569–573. [Google Scholar] [CrossRef]
- Turner, F.B. The dynamics of population of squamates; population response to artificial high densities, crocodilians and rhynchocephalians. In Biology of the Reptilia; Cans, C., Tinkle, D.W., Eds.; Academic Press: London, UK, 1977; pp. 157–264. [Google Scholar]
- Huey, R.B.; Slatkin, M. Costs and benefits of lizard thermoregulation. Q. Rev. Biol. 1976, 51, 363–384. [Google Scholar] [CrossRef] [PubMed]
- Huey, R.B.; Peterson, C.R.; Arnold, S.J.; Porter, W.P. Hot rocks and not so-hot rocks: Retreat-site selection by garter snakes and its thermal consequences. Ecology 1989, 70, 931–944. [Google Scholar] [CrossRef]
- Calsbeek, R.; Sinervo, B. Uncoupling direct and indirect components of female choice in the wild. Proc. Natl. Acad. Sci. USA 2002, 99, 14897–14902. [Google Scholar] [CrossRef] [PubMed]
- Heathcote, R.J.; Bell, E.; d’Ettorre, P.; While, G.M.; Uller, T. The scent of sun worship: Basking experience alters scent mark composition in male lizards. Behav. Ecol. Sociobiol. 2014, 68, 861–870. [Google Scholar] [CrossRef]
- Žagar, A.; Carretero, M.A.; Osojnik, N.; Sillero, N.; Vrezec, A. A place in the sun: Does interspecific interference affect thermoregulation in coexisting lizards. Behav. Ecol. Sociobiol. 2015, 69, 1127–1137. [Google Scholar] [CrossRef]
- Sears, M.W.; Angelletta, M.J., Jr. Costs and benefits of thermoregulation revisited: Both the heterogeneity and spatial structure of temperature drive energetic costs. Am. Nat. 2015, 185, E94–E102. [Google Scholar] [CrossRef]
- Boag, D.A. Spatial relationships among members of a population of wall lizards. Oecologia 1973, 12, 1–13. [Google Scholar] [CrossRef]
- Perry, G.; Garland, T.J. Lizard home ranges revisited: Effects of sex, body size, diet, habitat, and phylogeny. Ecology 2002, 83, 1870–1885. [Google Scholar] [CrossRef]
- Galoyan, E. Joint space use in a parthenogenetic Armenian rock lizard Darevskia armeniaca suggests weak competition among monoclonal females. J. Herpetol. 2013, 47, 97–104. [Google Scholar] [CrossRef]
- Marco, A.; Pérez-Mellado, V. Mate guarding, intrasexual competition and mating success in males of the non-territorial lizard Lacerta schreiberi. Ethol. Ecol. Evol. 1999, 11, 279–286. [Google Scholar] [CrossRef]
- Langkilde, T.; Lance, V.A.; Shine, R. Ecological consequences of agonistic interactions in lizards. Ecology 2005, 86, 1650–1659. [Google Scholar] [CrossRef][Green Version]
- Marler, C.; Walsberg, G.; White, M.; Moore, M.; Marler, C.A. Increased energy expenditure due to increased territorial defence in male lizards after phenotypic manipulation. Behav. Ecol. Sociobiol. 1995, 37, 225–231. [Google Scholar] [CrossRef]
- Basson, C.H.; Levy, O.; Angilletta, M.J., Jr.; Clusella-Trullas, S. Lizards paid a greater opportunity cost to thermoregulate in a less heterogeneous environment. Funct. Ecol. 2016, 31, 856–865. [Google Scholar] [CrossRef]
- Sears, M.W.; Angilletta, M.J.; Schuler, M.S.; Borchert, J.; Dilliplane, K.F.; Stegman, M.; Mitchell, W.A. Configuration of the thermal landscape determines thermoregulatory performance of ectotherms. Proc. Natl. Acad. Sci. USA 2016, 113, 10595–10600. [Google Scholar] [CrossRef]
- Bauwens, D.; Claus, K. Basking aggregations in the adder (Vipera berus): Attraction to conspecific cues or to scarce suitable microhabitats? J. Ethol. 2021, 39, 249–257. [Google Scholar] [CrossRef]
- Brewster, C.L.; Sikes, R.S.; Gifford, M.E. Quantifying the cost of thermoregulation: Thermal and energetic constraints on growth rates in hatchling lizards. Funct. Ecol. 2013, 27, 490–497. [Google Scholar] [CrossRef]
- Roughgarden, J. Competition and theory in community ecology. Am. Nat. 1983, 122, 583–601. [Google Scholar] [CrossRef]
- Burghardt, G.M.; Rand, A.S. Group size and growth rate in hatchling green iguanas (Iguana iguana). Behav. Ecol. Sociobiol. 1985, 18, 101–104. [Google Scholar] [CrossRef]
- Street, D. The Reptiles of Northern and Central Europe; Blanford Press: London, UK, 1979; p. 268. [Google Scholar]
- Rismiller, P.D.; Heldmaier, G. How photoperiod influences body temperature selection in Lacerta viridis. Oecologia 1988, 75, 125–131. [Google Scholar] [CrossRef]
- Meek, R. Temporal distributions, habitat associations and behaviour of the green lizard (Lacerta bilineata) and wall lizard (Podarcis muralis) on roads in a fragmented landscape in Western France. Acta Herpetol. 2014, 9, 179–186. [Google Scholar]
- Meek, R. Temporal trends in Podarcis muralis and Lacerta bilineata populations in a fragmented landscape in western France: Results from a 14-year time series. Herpetol. J. 2020, 30, 19–25. [Google Scholar] [CrossRef]
- Rugiero, L.; Capula, M.; Di Vittorio, M.; Dendi, D.; Meek, R.; Luiselli, L. Ontogenetic habitat use and density of the green lizard (Lacerta bilineata) in contrasted landscapes in France and Italy. Conservation 2021, 1, 1–16. [Google Scholar] [CrossRef]
- Meek, R.; Luiselli, L. Living in patchy habitats: Substrate selection by basking sympatric lizards in contrasted anthropogenic habitats in western France. Russ. J. Herpetol. 2020, 29, 227–236. [Google Scholar] [CrossRef]
- Meek, R.; Luiselli, L. Juveniles are different: Substrate selection in juvenile green lizards Lacerta bilineata. Ethol. Ecol. Evol. 2022, 35, 687–697. [Google Scholar] [CrossRef]
- Mehrabi, Z.; Slade, E.M.; Solis, A.; Mann, D.J. The importance of microhabitat for biodiversity sampling. PLoS ONE 2014, 9, e114015. [Google Scholar] [CrossRef] [PubMed]
- Meek, R.; Luiselli, L. Application of univariate diversity metrics to the study of the population ecology of the lizard Lacerta bilineata in an ecotonal habitat. Diversity 2024, 16, 169. [Google Scholar] [CrossRef]
- Mitchell, J.C. Cannibalism in reptiles: A worldview review. Soc. Stud. Amph. Rept. 1986, 15, 1–23. [Google Scholar]
- Angelici, F.M.; Luiselli, L.; Rugiero, L. Food habits of the green lizard, Lacerta bilineata, in central Italy and a reliability test of faecal pellet analysis. Ital. J. Zool. 1997, 64, 267–272. [Google Scholar] [CrossRef]
- Choo, Y.R.; Kudavidanage, E.P.; Amarasinghe, T.R.; Nimalrathna, T.; Chua, M.A.; Webb, E.L. Best practices for reporting individual identification using camera trap photographs. Global Ecol. Conserv. 2020, 24, e01294. [Google Scholar] [CrossRef]
- Welbourne, D.J.; Claridge, A.W.; Paull, D.J.; Ford, F. Camera-traps are a cost effective method for surveying terrestrial squamates: A comparison with artificial refuges and pitfall traps. PLoS ONE 2020, 15, e0226913. [Google Scholar] [CrossRef] [PubMed]
- Gotelli, N.J.; Ellison, A.M. A Primer of Ecological Statistics; Sinauer Associates: Sunderland, MA, USA, 2004; p. 510. [Google Scholar]
- Bennett, A.F. Thermal dependence of locomotor capacity. Am. J. Physiol. 1990, 250, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Spears, S.; Pettit, C.; Berkowitz, S.; Collier, S.; Colwell, C.; Livingston, E.H.; McQueen, W.; Princeton, V.; Bodensteiner, B.L.; Leos-Barajas, V.; et al. Lizards in the wind: The impact of wind on the thermoregulation of the common wall lizard. J. Therm. Biol. 2024, 121, 103855. [Google Scholar] [CrossRef] [PubMed]
- Sannolo, M.; Carretero, M.A. Dehydration constrains thermoregulation and space use in lizards. PLoS ONE 2019, 14, e0220384. [Google Scholar] [CrossRef] [PubMed]
- Cooper, W.E.; Vitt, L.J. Increased predation risk while mate guarding as a cost of reproduction for male broad-headed skinks (Eumeces laticeps). Acta Ethol. 2002, 5, 19–23. [Google Scholar] [CrossRef]
- Baird, T.A.; Timanus, D.K.; Sloan, C.L. Intra- and intersexual variation in Social behaviour. In Lizard Social Behaviour; John Hopkins University Press: Baltimore, MD, USA, 2003; pp. 7–46. [Google Scholar]
- Beauchamp, G. Animal Vigilance: Monitoring Predators and Competitors; Academic Press: Cambridge, MA, USA, 2015; p. 272. [Google Scholar]
- Schwarzkopf, L.; Shine, R. Costs of reproduction in lizards: Escape tactics and susceptibility to predation. Behav. Ecol. Sociobiol. 1992, 31, 17–25. [Google Scholar] [CrossRef]
- Réale, D.; Reader, S.M.; Sol, D.; McDougall, P.T.; Dingemanse, N.J. Integrating animal temperament within ecology and evolution. Biol. Rev. 2007, 82, 291–318. [Google Scholar] [CrossRef]
- Bajer, K.; Horvath, G.; Molnar, O.; Toeroek, J.; Zsolt Garamszegi, L.; Herczeg, G. European green lizard (Lacerta viridis) personalities: Linking behavioural types to ecologically relevant traits at different ontogenetic stages. Behav. Proc. 2015, 111, 67–74. [Google Scholar] [CrossRef]
- Braun, C.A.; Baird, T.A.; York, J.R. Behavioural plasticity in physically variable microhabitats: A field test of potential adaptive consequences in male collared lizards (Crotaphytus collaris). Biol. J. Linn. Soc. 2018, 125, 37–49. [Google Scholar] [CrossRef]
- Downes, S.; Bauwens, D. Associations between first encounters and ensuing social relations within dyads of two species of lacertid lizards. Behav. Ecol. 2004, 15, 938–945. [Google Scholar] [CrossRef]
- Keren-Rotem, T.; Bouskila, A.; Geffen, E. Ontogenetic habitat shift and risk of cannibalism in the common chameleon (Chamaeleo chamaeleon). Behav. Ecol. Sociobiol. 2006, 59, 723–731. [Google Scholar] [CrossRef]
- Delaney, D.M.; Warner, D.A. Adult male density influences juvenile microhabitat use in a territorial lizard. Ethology 2017, 123, 157–167. [Google Scholar] [CrossRef]
- Jenssen, T.A.; Marcellini, D.L.; Buhlmann, K.A.; Goforth, P.H. Differential infanticide by adult curly-tailed lizards, Leiocephalus schreibersi. Anim. Behav. 1989, 38, 1054–1061. [Google Scholar] [CrossRef]
- Siqueira, C.C.; Rocha, C.F.D. Predation by lizards as a mortality source for juvenile lizards in Brazil. S. Am. J. Herpetol. 2008, 3, 82–87. [Google Scholar] [CrossRef]
- Huntingford, F.A.; Turner, A.K. Animal Conflict; London Chapman Hall: London, UK, 1987; p. 460. [Google Scholar]
- Christian, K.A.; Tracy, C.R. The effect of thermal environment on the ability of hatchling Galapagos land iguanas to avoid predation during dispersal. Oecologia 1981, 49, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Massot, M.; Clobert, J.; Pilorge, T.; LeComte, J.; Barbault, R. Density dependence in the common lizard: Demographic consequences of a density manipulation. Ecology 1992, 73, 1742–1756. [Google Scholar] [CrossRef]
- Martin, J.; Lopez, P. Influence of habitat structure on the escape tactics of the lizard Psammodromus algirus. Can. J. Zool. 1995, 73, 129–132. [Google Scholar] [CrossRef]
- Martin, J.; Lopez, P. Fleeing to unsafe refuges: Effects of conspicuousness and refuge safety on the escape decisions of the lizard Psammodromus algirus. Can. J. Zool. 2000, 78, 265–270. [Google Scholar] [CrossRef][Green Version]
- Przemysław, Z.; Jarmoliński, M. Microhabitat sharing for basking between squamat species in Poland. Herpetozoa 2003, 36, 65–71. [Google Scholar] [CrossRef]
- Hodges, R.J.; Seabrook, C.; Michaels, C.J. Patterns of spatial and temporal association between Zootoca vivipara, Anguis fragilis, Vipera berus and Natrix helvetica at artificial refuges. Herpetol. J. 2024, 34, 145–151. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).