Redescription of Euscorpius studentium Based on Adult Specimens; Updated Classification of Cavernicolous Euscorpiidae; and Review of Cavernicolous Scorpions in the Balkans
Abstract
:1. Introduction
2. Materials and Methods
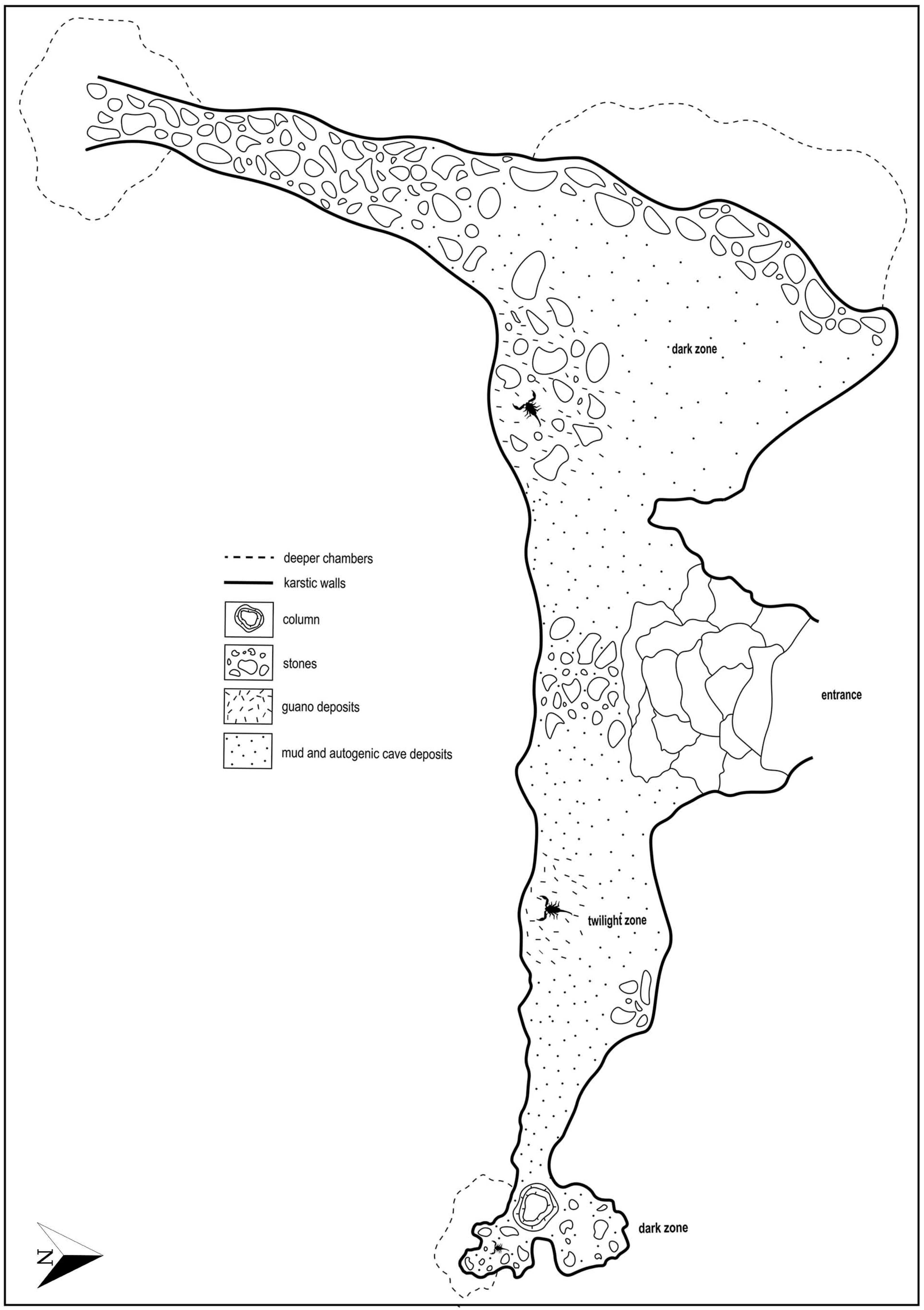

3. Key to Identification of Cavernicolous Scorpions in the Dinaric Karst
- Pedipalp patella retrolateral surface with three trichobothria in em series ………………………………………………………………...…….. Alpiscorpius liburnicus
- -
- Pedipalp patella retrolateral surface with four trichobothria in em series …...…….... 2
- 2.
- Median ocelli absent, lateral ocelli reduced ……………….....…. Euscorpius studentium
- -
- Median ocelli present, lateral ocelli well-developed …………………………...……… 3
- 3.
- Pedipalp patella retrolateral surface with four trichobothria in eb series and four trichobothria in eba series ……………………………………………………...….………. 4
- -
- Pedipalp patella retrolateral surface with five trichobothria in eb series and 6–8 trichobothria in eba series ………………………………………...…… Euscorpius lagostae
- 4.
- Pedipalp patella retrolateral surface with six trichobothria in et series; ventral surface with 7–9 trichobothria in v series ………………………………..……………….…...…. 5
- -
- Pedipalp patella retrolateral surface with more than six trichobothria in et series; ventral surface with 11–12 trichobothria in v series ………………….…....... Euscorpius feti
- 5.
- Metasomal segment V, ventrolateral and ventromedian carinae obsolete, intercarinal surfaces smooth …………………………………………...……..… Euscorpius biokovensis
- -
- Metasomal segment V, ventrolateral and ventromedian carinae distinct, intercarinal surfaces finely granular .…………………………………….………….. Euscorpius sulfur
4. Systematics
5. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barr, T.C. Cave Ecology and the Evolution of Troglobites. In Evolutionary Biology; Dobzhansky, T., Hecht, M.K., Steere, W.C., Eds.; Springer: Boston, MA, USA, 1968; pp. 35–102. [Google Scholar]
- Poulson, T.L.; White, W.B. The cave environment. Science 1969, 165, 971–981. [Google Scholar] [CrossRef] [PubMed]
- Culver, D.C.; Sket, B. Hotspots of subterranean biodiversity in caves and wells. J. Cave Karst Stud. 2000, 62, 11–17. [Google Scholar]
- Prendini, L. Substratum specialization and speciation in southern African scorpions: The Effect Hypothesis revisited. In Scorpions 2001: In Memoriam Gary A. Polis; Fet, V., Selden, P.A., Eds.; British Arachnological Society: Burnham Beeches, UK, 2001; pp. 113–138. [Google Scholar]
- Volschenk, E.S.; Prendini, L. Aops oncodactylus gen. et sp. nov., the first troglobitic urodacid (Urodacidae: Scorpiones), with a re-assessment of cavernicolous, troglobitic and troglomorphic scorpions. Invertebr. Syst. 2008, 22, 235–257. [Google Scholar] [CrossRef]
- Vignoli, V.; Prendini, L. Systematic revision of the troglomorphic North American scorpion family Typhlochactidae (Scorpiones: Chactoidea). Bull. Am. Mus. Nat. Hist. 2009, 326, 1–94. [Google Scholar] [CrossRef]
- Prendini, L.; Francke, O.F.; Vignoli, V. Troglomorphism, trichobothriotaxy and typhlochactid phylogeny (Scorpiones, Chactoidea): More evidence that troglobitism is not an evolutionary dead-end. Cladistics 2010, 26, 117–142. [Google Scholar] [CrossRef]
- Prendini, L.; Ehrenthal, V.L.; Loria, S.F. Systematics of the relictual Asian scorpion family Pseudochactidae Gromov, 1998, with a review of cavernicolous, troglobitic, and troglomorphic scorpions. Bull. Am. Mus. Nat. Hist. 2021, 453, 1–149. [Google Scholar] [CrossRef]
- Schiner, J.R. Fauna der Adelsberger-, Lueger- und Magdalenen Grotte. In Zur Höhlenkunde des Karstes. Die Grotten und Höhlen von Adelsberg, Lueg, Planina und Laas; Schmidl, A., Ed.; Braumüller: Vienna, Austria, 1854; pp. 231–272. [Google Scholar]
- Racovitza, E.G. Essai sur les problèmes biospéologiques. Arch. Zool. Exp. Gén. 1907, 6, 371–488. [Google Scholar]
- Christiansen, K. Proposition pour la classification des animaux cavernicoles. Spelunca 1962, 2, 76–78. [Google Scholar]
- Poulson, T.L. Cave adaptation in ambliopsid fishes. Am. Midl. Nat. 1963, 70, 257–290. [Google Scholar] [CrossRef]
- Sket, B. Can we agree on an ecological classification of subterranean animals? J. Nat. Hist. 2008, 42, 1549–1563. [Google Scholar] [CrossRef]
- Trajano, E. Ecological Classification of Subterranean Organisms. In Encyclopedia of Caves; White, W.B., Culver, D.C., Eds.; Academic Press: Waltham, MA, USA, 2012; pp. 275–277. [Google Scholar]
- Trajano, E.; de Carvalho, M.R. Towards a biologically meaningful classification of subterranean organisms: A critical analysis of the Schiner-Racovitza system from a historical perspective, difficulties of its application and implications for conservation. Subterr. Biol. 2017, 22, 1–26. [Google Scholar] [CrossRef]
- Mammola, S. Finding answers in the dark: Caves as models in ecology fifty years after Poulson and White. Ecography 2019, 42, 1331–1351. [Google Scholar] [CrossRef]
- Hamann, O. Europäische Höhlenfauna; Hermann Costenoble: Jena, Germany, 1896; 319p. [Google Scholar]
- Ćurčić, B.P.M. The Cave Fauna in Serbia: Origin, Historical Development, and Diversification. In Speleological Atlas of Serbia; Ðurović, P., Ed.; Geographical Institute “Jovan Cvijić”, Serbian Academy of Sciences and Arts: Belgrade, Serbia, 1998; pp. 75–83. [Google Scholar]
- Ćurčić, B.P.M.; Jovanović, V.M. The cave-dwelling fauna of the Balkan Peninsula: Its origin and diversification. Acta Entomol. Slov. 2004, 12, 35–56. [Google Scholar]
- Griffiths, H.I.; Kryštufek, B.; Reed, J.M. Balkan Biodiversity: Pattern and Process in the European Hotspot; Springer: Dordrecht, The Netherlands, 2004; 358p. [Google Scholar]
- Sket, B. Diversity Patterns in the Dinaric Karst. In Encyclopedia of Caves; White, W.B., Culver, D.C., Eds.; Academic Press: Waltham, MA, USA, 2012; pp. 228–238. [Google Scholar]
- Zagmajster, M.; Malard, F.; Eme, D.; Culver, D.C. Subterranean Biodiversity Patterns from Global to Regional Scales. In Ecological Studies Series: Cave Ecology; Moldovan, O.T., Kováč, L., Halse, S., Eds.; Springer: Cham, Switzerland, 2018; pp. 195–227. [Google Scholar]
- Hlebec, D.; Podnar, M.; Kučinić, M.; Harms, D. Molecular analyses of pseudoscorpions in a subterranean biodiversity hotspot reveal cryptic diversity and microendemism. Sci. Rep. 2023, 13, 430. [Google Scholar] [CrossRef] [PubMed]
- Deltshev, C. A faunistic and zoogeographical review of the spiders (Araneae) of the Balkan Peninsula. J. Arachnol. 1999, 25, 255–261. [Google Scholar]
- Lukić, M.; Fišer, C.; Delić, T.; Bilandžija, H.; Pavlek, M.; Komerički, A.; Dražina, T.; Jalžić, B.; Ozimec, R.; Slapnik, R.; et al. Subterranean fauna of the Lukina Jama–Trojama Cave System in Croatia: The deepest cave in the Dinaric Karst. Diversity 2023, 15, 726. [Google Scholar] [CrossRef]
- Vesović, N.; Deltshev, C.; Mitov, P.; Antić, D.; Stojanović, D.Z.; Stojanović, D.V.; Stojanović, K.; Božanić, M.; Ignjatović-Ćupina, A.; Ćurčić, S. The diversity of subterranean terrestrial arthropods in Resava Cave (eastern Serbia). Diversity 2024, 16, 234. [Google Scholar] [CrossRef]
- Deeleman-Reinhold, C.L. Revision of the cave-dwelling and related spiders of the genus Troglohyphantes Joseph (Linyphiidae), with special reference to the Yugoslav species. In Academia Scientiarum et Artium Slovenica, Classis IV: Historia Naturalis; Opera; The Academy: Ljubljana, Yugoslavia, 1978; Volume 23, pp. 1–221. [Google Scholar]
- Ćurčić, B.P.M. Cave-dwelling pseudoscorpions of the Dinaric Karst. In Academia Scientiarum et Artium Slovenica, Classis IV: Historia Naturalis; Opera; The Academy: Ljubljana, Yugoslavia, 1988; Volume 26, pp. 1–191. [Google Scholar]
- Savić, I.R. Diversification of the Balkan fauna: Its origin, historical development and present status. Adv. Arachnol. Dev. Biol. 2008, 12, 57–78. [Google Scholar]
- Apfelbeck, V. Sur la faune des cavernes de Bosnie et Herzégovine. Spelunca 1895, 1, 23–24. [Google Scholar]
- Tropea, G. A new species of Euscorpius Thorell, 1876 from the western Balkans (Scorpiones: Euscorpiidae). Euscorpius 2013, 174, 1–10. [Google Scholar] [CrossRef]
- Tropea, G.; Ozimec, R. Another new species of Euscorpius Thorell, 1876 from the caves of Croatia and Bosnia-Herzegovina (Scorpiones: Euscorpiidae) with notes on biogeography and cave ecology. Euscorpius 2020, 308, 1–13. [Google Scholar]
- Karaman, I. A new Euscorpius species (Scorpiones: Euscorpiidae) from a Dinaric cave—The first record of troglobite scorpion in European fauna. Biol. Serb. 2020, 42, 14–31. [Google Scholar]
- Podnar, M.; Grbac, I.; Tvrtković, N.; Hörweg, C.; Haring, E. Hidden diversity, ancient divergences, and tentative Pleistocene microrefugia of European scorpions (Euscorpiidae: Euscorpiinae) in the eastern Adriatic region. Zool. Syst. Evol. Res. 2021, 59, 1824–1849. [Google Scholar] [CrossRef]
- Podnar, M.; Tvrtković, N.; Vuković, M.; Rebrina, F.; Grbac, I.; Hörweg, C. Alpiscorpius liburnicus sp. n. with a note on the “Alpiscorpius croaticus group” (Scorpiones: Euscorpiidae) in Croatia. Nat. Croat. 2022, 31, 265–282. [Google Scholar] [CrossRef]
- Kovařík, F.; Audy, M.; Sarbu, S.M.; Fet, V. Euscorpius sulfur sp. n. (Scorpiones: Euscorpiidae), a new cave scorpion from Albania and northwestern Greece. Euscorpius 2023, 376, 1–14. [Google Scholar]
- Juberthie, C.; Delay, B.; Bouillon, M. Sur l’existence d’un milieu souterrain superficiel en zone non calcaire. C. R. De L’académie Des Sci. 1980, 290, 49–52. [Google Scholar]
- Mammola, S.; Giachino, P.M.; Piano, E.; Jones, A.; Barberis, M.; Badino, G.; Isaia, M. Ecology and sampling techniques of an understudied subterranean habitat: The Milieu Souterrain Superficiel (MSS). Sci. Nat. 2016, 103, 88. [Google Scholar] [CrossRef]
- Ficetola, G.F.; Canedoli, C.; Stoch, F. The Racovitzan impediment and the hidden biodiversity of unexplored environments. Conserv. Biol. 2019, 33, 214–216. [Google Scholar] [CrossRef]
- Potočnik, F. Alpioniscus (Illyrionethes) christiani spec. nov., eine neue Trichoniscinae-Art (Isopoda terrestrial) aus Jugoslawien. Ann. Naturhist. Mus. Wien. 1983, 84, 389–395. [Google Scholar]
- Christian, E.; Potočnik, F. Ein Beitrag zur Kenntnis der Höhlenfauna der Insel Krk. Biološki Vestn. 1985, 33, 13–19. [Google Scholar]
- Graham, M.R.; Webber, M.M.; Blagoev, G.; Ivanova, N.; Fet, V. Elevation of Euscorpius germanus croaticus Di Caporiacco, 1950. Rev. Iber. Aracnol. 2012, 21, 41–50. [Google Scholar]
- Fet, V.; Graham, M.R.; Blagoev, G.; Karataş, A.; Karataş, A. DNA barcoding indicates hidden diversity of Euscorpius (Scorpiones: Euscorpiidae) in Turkey. Euscorpius 2016, 216, 1–12. [Google Scholar] [CrossRef]
- Tropea, G. Concerning some Balkan Euscorpiidae populations (Scorpiones: Euscorpiidae). Biol. Serb. 2021, 43, 22–54. [Google Scholar]
- Boldori, L. Cavernicola Italica. I. Dalle Alpi Occidentali alla Valle del Brenta, a nord del Po. Parte 1. Dai Protozoa ai Crustacea. Ann. Mus. Civ. Stor. Nat. Brescia 1977, 14, 127–172. [Google Scholar]
- Tropea, G. Reconsideration of the taxonomy of Euscorpius tergestinus (Scorpiones: Euscorpiidae). Euscorpius 2013, 162, 1–23. [Google Scholar] [CrossRef]
- Tropea, G.; Fet, V. Two new Euscorpius species from central-western Greece (Scorpiones: Euscorpiidae). Euscorpius 2015, 199, 1–16. [Google Scholar] [CrossRef]
- Tropea, G.; Ozimec, R. Description of the adult male of Euscorpius feti Tropea, 2013 (Scorpiones: Euscorpiidae), with notes on cave ecology of this species. Euscorpius 2019, 291, 1–10. [Google Scholar] [CrossRef]
- Blasco-Aróstegui, J.; Prendini, L. Glacial relicts? A new scorpion from Mount Olympus, Greece (Euscorpiidae: Euscorpius). Am. Mus. Novit. 2023, 4003, 1–36. [Google Scholar] [CrossRef]
- Fet, V.; Soleglad, M.E.; Parmakelis, A.; Kotsakiozi, P.; Stathi, I. Two new species of Euscorpius from Euboea Island, Greece (Scorpiones: Euscorpiidae). Arthropoda Sel. 2014, 23, 111–126. [Google Scholar] [CrossRef]
- Fet, V.; Parmakelis, A.; Stathi, I.; Tropea, G.; Kotsakiozi, P.; Kardaki, L.; Nikolakakis, M. Fauna and Zoogeography of Scorpions in Greece. In Biogeography and Biodiversity of the Aegean; Sfenthourakis, S., Pafilis, P., Parmakelis, A., Poulakakis, N., Triantis, K.A., Eds.; Broken Hill Publishers Ltd.: Nicosia, Cyprus, 2018; pp. 123–134. [Google Scholar]
- Tropea, G.; Onnis, C. A remarkable discovery of a new scorpion genus and species from Sardinia (Scorpiones: Chactoidea: Belisariidae). Arachn. Riv. Aracnol. Ital. 2020, 26, 3–25. [Google Scholar]
- Wolf, B. Ordnung Scorpiones. In Animalium Cavernarum Catalogus; Dr. W. Junk: The Hague, The Netherlands, 1937; Volume III, p. 534. [Google Scholar]
- di Caporiacco, L. Aracnidi cavernicoli Liguri. Ann. Mus. Civ. Stor. Nat. Giacomo Doria 1950, 64, 101–110. [Google Scholar]
- Bologna, M.A.; Taglianti, A.V. Fauna Cavernicola Delle Alpi Liguri; Museo Civico di Storia Naturale “G. Doria”: Genova, Italy, 1985; pp. 1–389. [Google Scholar]
- Guéorguiev, V.; Beron, P. Essai sur la faune cavernicole de Bulgarie. Ann. Spéléol. 1962, 17, 285–441. [Google Scholar]
- Fet, V.; Graham, M.R.; Webber, M.M.; Blagoev, G. Two new species of Euscorpius (Scorpiones: Euscorpiidae) from Bulgaria, Serbia, and Greece. Zootaxa 2014, 3894, 83–105. [Google Scholar] [CrossRef] [PubMed]
- di Caporiacco, L. Le Specie e Sottospecie del Genre “Euscorpius” Viventi in Italia ed in Alcune Zone Confinanti; Accademia Nazionale dei Lincei: Rome, Italy, 1950; Volume 2, pp. 159–230. [Google Scholar]
- Stahnke, H.L. UV light, a useful field tool. BioScience 1972, 22, 604–607. [Google Scholar] [CrossRef]
- Stahnke, H.L. Scorpion nomenclature and mensuration. Entomol. News 1970, 81, 297–316. [Google Scholar]
- Sissom, W.D.; Polis, G.A.; Watt, D.D. Field and laboratory methods. In The Biology of Scorpions; Polis, G.A., Ed.; Stanford University Press: Stanford, CA, USA, 1990; pp. 215–221. [Google Scholar]
- Prendini, L.; Crowe, T.M.; Wheeler, W.C. Systematics and biogeography of the family Scorpionidae (Chelicerata: Scorpiones), with a discussion on phylogenetic methods. Invertebr. Syst. 2003, 17, 185–259. [Google Scholar] [CrossRef]
- Loria, S.F.; Prendini, L. Homology of the lateral eyes of Scorpiones: A six-ocellus model. PLoS ONE 2014, 9, e112913. [Google Scholar] [CrossRef] [PubMed]
- Vachon, M. Études sur Les Scorpions; Institut Pasteur d’Algérie: Algiers, Algeria, 1952; 482p. [Google Scholar]
- Prendini, L. Phylogeny and classification of the superfamily Scorpionoidea Latreille 1802 (Chelicerata, Scorpiones): An exemplar approach. Cladistics 2000, 16, 1–78. [Google Scholar] [CrossRef]
- Vachon, M. De l’utilité, en systématique, d’une nomenclature des dents des chélicères chez les scorpions. Bull. Mus. Natl. Hist. Nat. 1963, 35, 161–166. [Google Scholar]
- Prendini, L.; Loria, S.F. Systematic revision of the Asian forest scorpions (Heterometrinae Simon, 1879), revised suprageneric classification of Scorpionidae Latreille, 1802, and revalidation of Rugodentidae Bastawade et al., 2005. Bull. Am. Mus. Nat. Hist. 2020, 442, 1–480. [Google Scholar] [CrossRef]
- Vachon, M. Remarques sur la classification sous-spécifique des espèces appartenant au genre Euscorpius Thorell, 1876 (Scorpionida, Chactidae). Atti Soc. Tosc. Sci. Nat. Mem. Ser. B 1981, 88, 193–203. [Google Scholar]
- Stockwell, S.A. Revision of the Phylogeny and Higher Classification of Scorpions (Chelicerata). Ph.D. Dissertation, University of California, Berkeley, CA, USA, 1989; 413p. [Google Scholar]
- Prendini, L. The systematics of southern African Parabuthus Pocock (Scorpiones: Buthidae): Revisions to the taxonomy and key to the species. J. Arachnol. 2004, 32, 109–186. [Google Scholar] [CrossRef]
- Dollo, L. Les lois de l’evolution. Bull. Soc. Belge Géol. Paléontol. Hydrol. 1893, 7, 164–166. [Google Scholar]
- Cope, E.D. The Primary Factors of Organic Evolution; Open Court Publishing: Chicago, IL, USA, 1896; 555p. [Google Scholar]
- Conway Morris, S. Ecology in deep time. Trends Ecol. Evol. 1995, 10, 290–294. [Google Scholar] [CrossRef]
- Humphreys, W.F. Background and glossary. In Ecosystems of the World: Subterranean Ecosystems; Wilkens, H., Culver, D.C., Humphreys, W.F., Eds.; Elsevier: Amsterdam, The Netherlands, 2000; Volume 30, pp. 3–14. [Google Scholar]
- Ashcroft, M.B.; Gollan, J.R. Moisture, thermal inertia, and the spatial distributions of near-surface soil and air temperatures: Understanding factors that promote microrefugia. Agric. For. Meteorol. 2013, 176, 77–89. [Google Scholar] [CrossRef]
- Keppel, G.; Anderson, S.; Williams, C.; Kleindorfer, S.; O’Connell, C. Microhabitats and canopy cover moderate high summer temperatures in a fragmented Mediterranean landscape. PLoS ONE 2017, 12, e0183106. [Google Scholar] [CrossRef]
- Hewitt, G.M. Some genetic consequences of ice ages, and their role in divergence and speciation. Biol. J. Linn. Soc. 1996, 58, 247–276. [Google Scholar] [CrossRef]
- Hewitt, G.M. Post-glacial re-colonization of European biota. Biol. J. Linn. Soc. 1999, 68, 87–112. [Google Scholar] [CrossRef]
- Schmitt, T. Molecular biogeography of Europe: Pleistocene cycles and postglacial trends. Front. Zool. 2007, 4, 11. [Google Scholar] [CrossRef] [PubMed]
- Darwin, C. On the Origin of Species by Means of Natural Selection, or the Preservation of Favoured Races in the Struggle of Life; John Murray: London, UK, 1859; 502p. [Google Scholar]
- Toscano-Gadea, C.A. Euscorpius flavicaudis (DeGeer, 1778) in Uruguay: First record from the New World. Newsl. Br. Arachnol. Soc. 1998, 81, 6. [Google Scholar]
- Fet, V.; Kovařík, F. First record of Euscorpius (Polytrichobothrius) italicus (Scorpiones: Euscorpiidae) from Iraq. Acta Soc. Zool. Bohem. 2003, 67, 179–181. [Google Scholar]
- Fet, V.; Gantenbein, B.; Karataş, A.; Karataş, A. An extremely low genetic divergence across the range of Euscorpius italicus (Scorpiones, Euscorpiidae). J. Arachnol. 2006, 34, 248–253. [Google Scholar] [CrossRef]
- Hampe, A.; Jump, A.S. Climate relicts: Past, present, future. Annu. Rev. Ecol. Evol. Syst. 2011, 42, 313–333. [Google Scholar] [CrossRef]
- Loria, S.F.; Ehrenthal, V.L.; Nguyen, A.D.; Prendini, L. Climate relicts: Asian scorpion family Pseudochactidae survived Miocene aridification in caves of the Annamite Mountains. Insect Syst. Divers. 2022, 6, 3. [Google Scholar] [CrossRef]
- Murienne, J.; Karaman, I.; Giribet, G. Explosive evolution of an ancient group of Cyphophthalmi (Arachnida: Opiliones) in the Balkan Peninsula. J. Biogeogr. 2010, 37, 90–102. [Google Scholar] [CrossRef]
- Njunjić, I.; Perreau, M.; Hendriks, K.; Schilthuizen, M.; Deharveng, L. The cave beetle genus Anthroherpon is polyphyletic: Molecular phylogenetics and description of Graciliella n. gen. (Leiodidae, Leptodirini). Contrib. Zool. 2016, 85, 337–359. [Google Scholar] [CrossRef]
- Bedek, J.; Taiti, S.; Bilandžija, H.; Ristori, E.; Baratti, M. Molecular and taxonomic analyses in troglobiotic Alpioniscus (Illyrionethes) species from the Dinaric Karst (Isopoda: Trichoniscidae). Zool. J. Linn. Soc. 2019, 187, 539–584. [Google Scholar] [CrossRef]
- Ribera, C.; Dimitrov, D. A molecular phylogeny of the European nesticid spiders (Nesticidae, Araneae): Implications for their systematics and biogeography. Mol. Phylogenet. Evol. 2023, 180, 107685. [Google Scholar] [CrossRef]
- Nanni, V.; Piano, E.; Cardoso, P.; Isaia, M.; Mammola, S. An expert-based global assessment of threats and conservation measures for subterranean ecosystems. Biol. Conserv. 2023, 283, 110136. [Google Scholar] [CrossRef]
- Mammola, S.; Cardoso, P.; Culver, D.C.; Deharveng, L.; Ferreira, R.L.; Fišer, C.; Zagmajster, M. Scientists’ warning on the conservation of subterranean ecosystems. BioScience 2019, 69, 641–650. [Google Scholar] [CrossRef]
- Mammola, S.; Piano, E.; Cardoso, P.; Vernon, P.; Domínguez-Villar, D.; Culver, D.C.; Isaia, M. Climate change going deep: The effects of global climatic alterations on cave ecosystems. Anthr. Rev. 2019, 6, 98–116. [Google Scholar] [CrossRef]


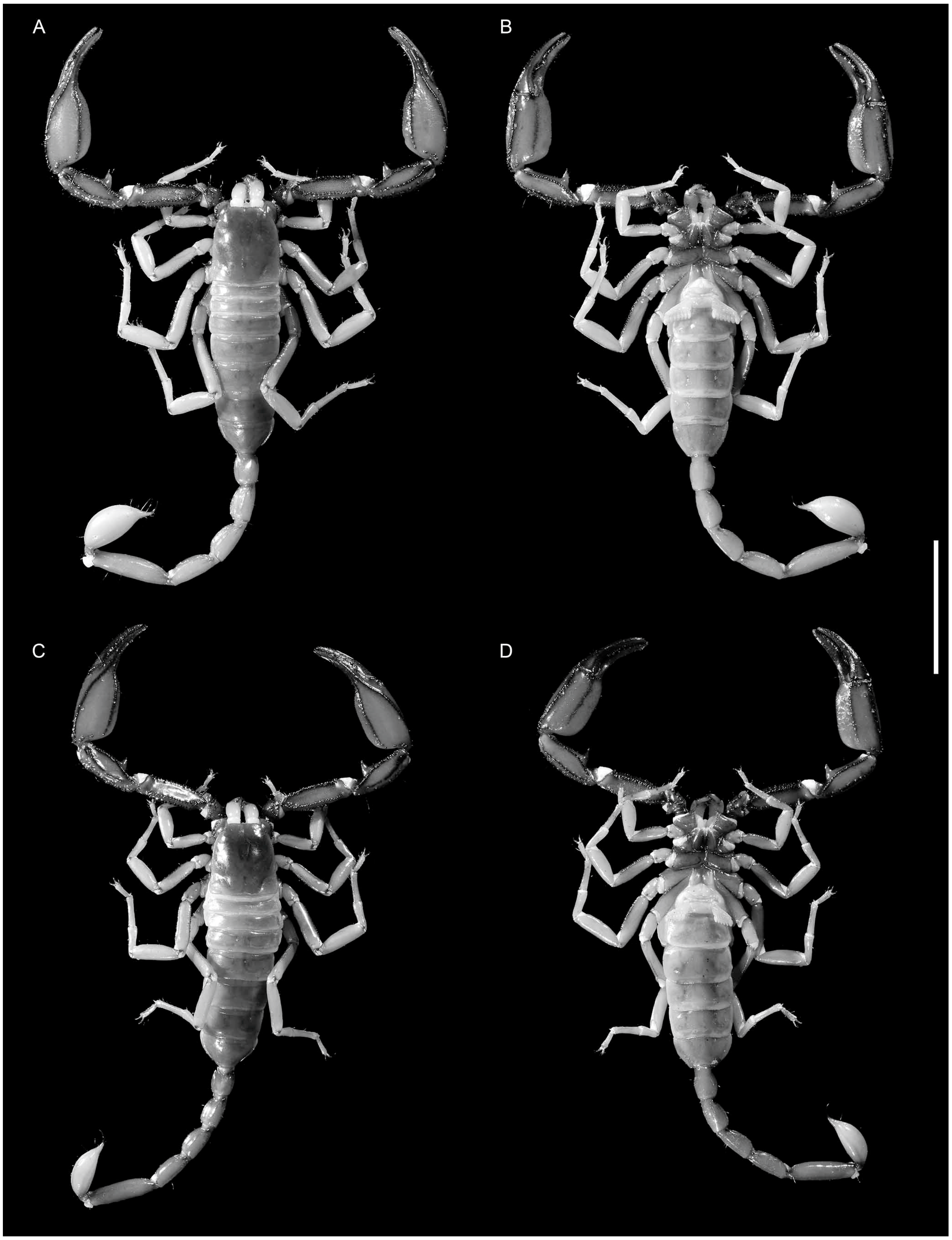
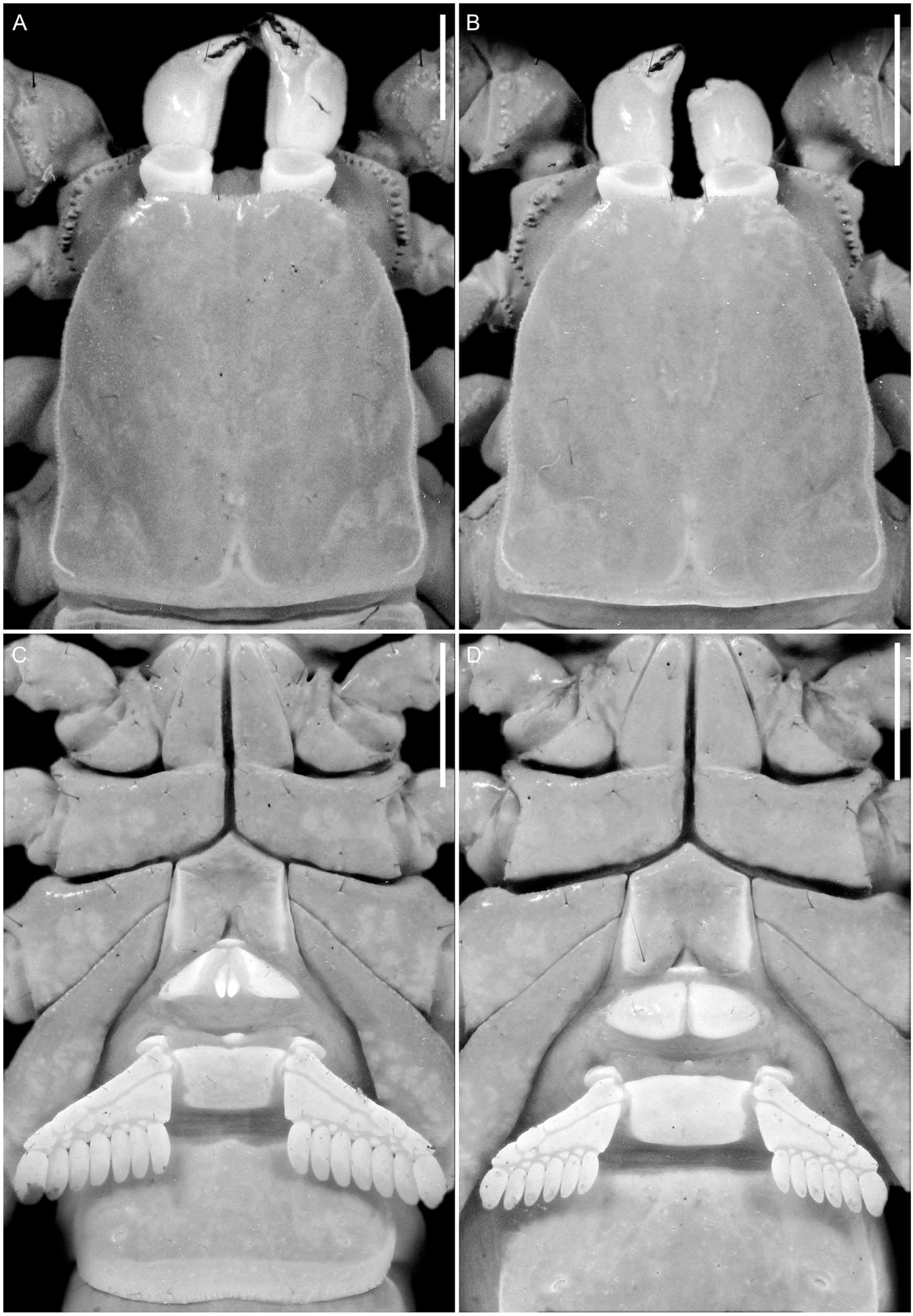
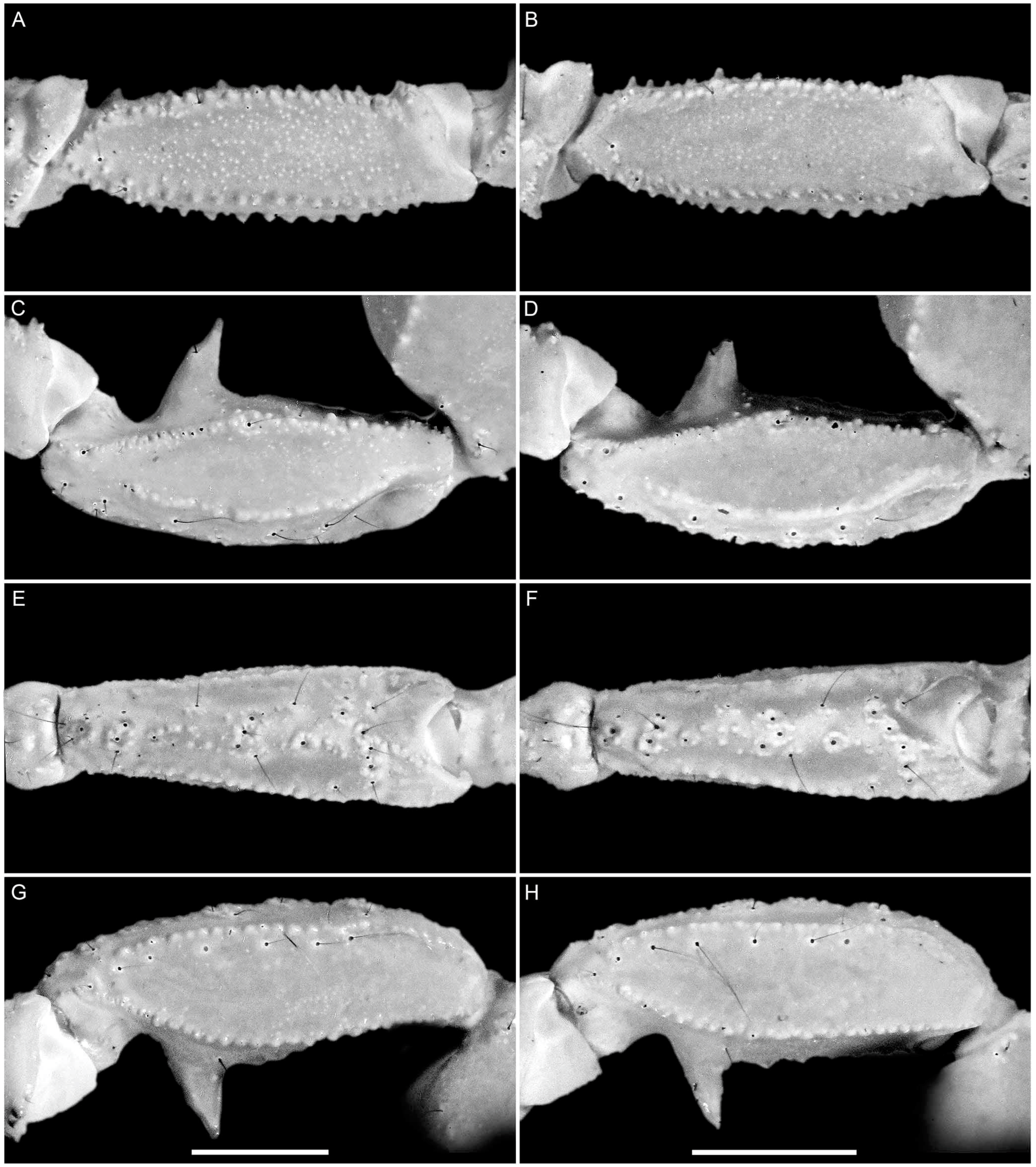
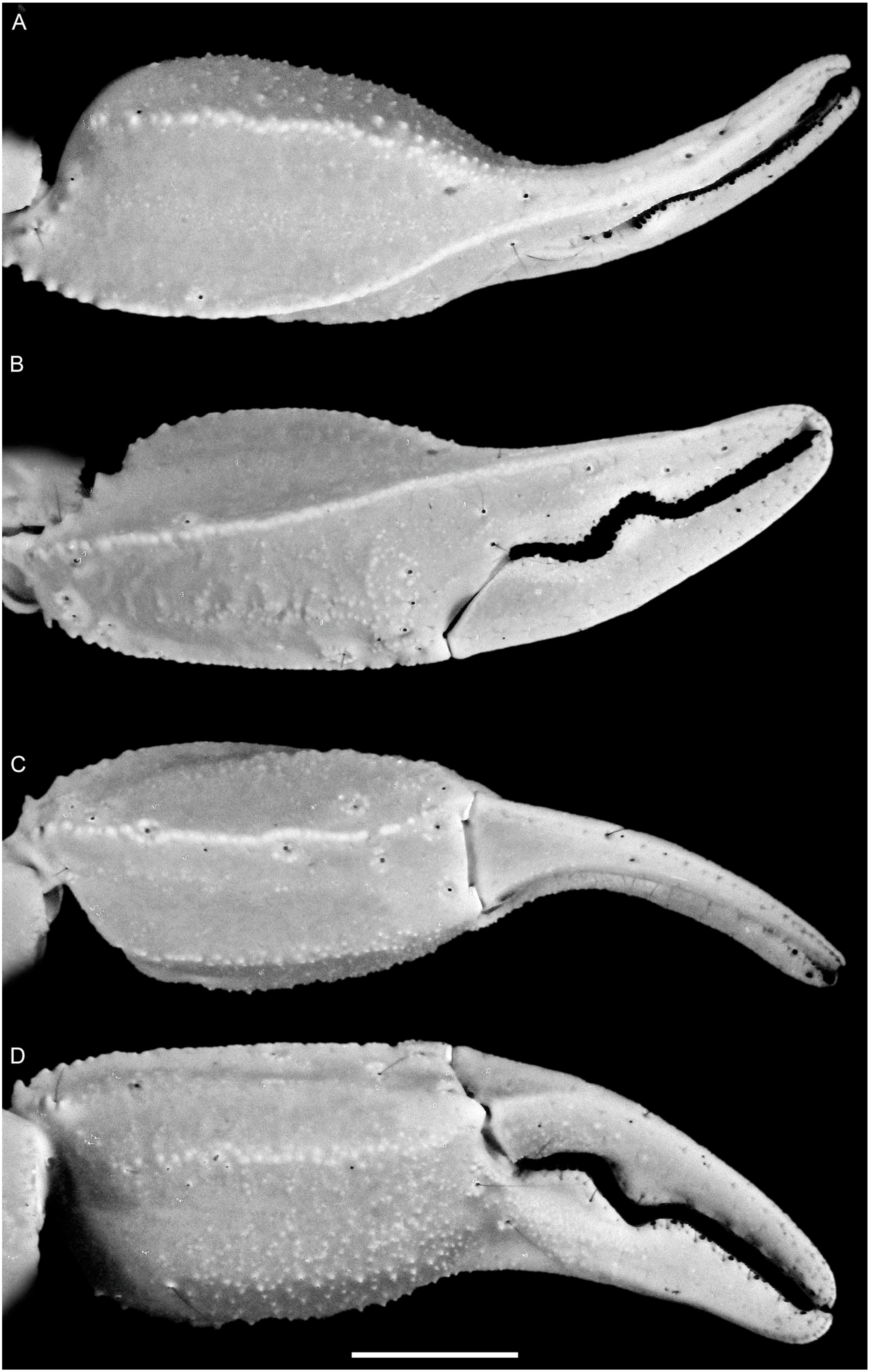


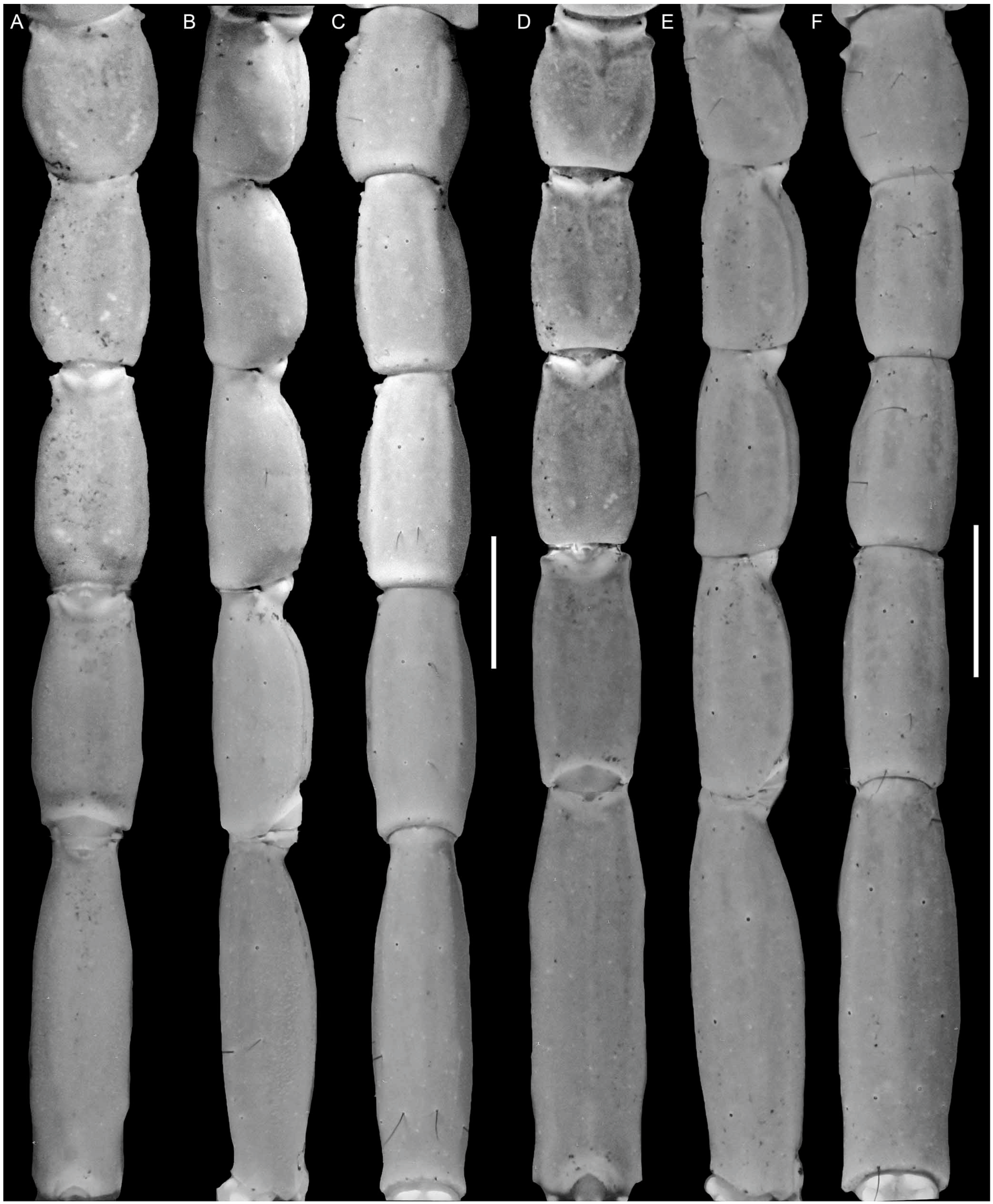
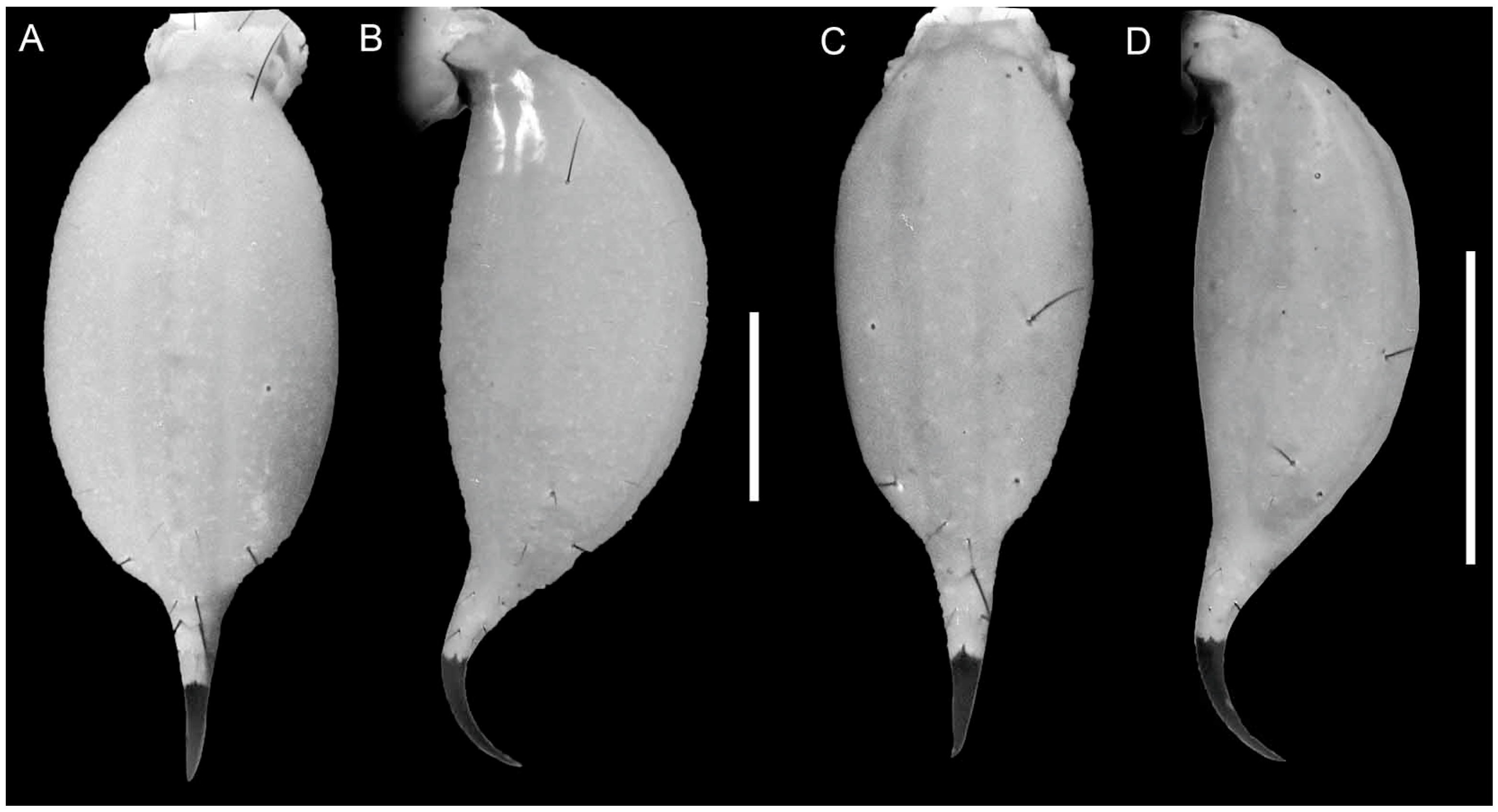
| Alpiscorpius liburnicus Tvrtković & Rebrina, 2022: CROATIA. Habitat: inside cave. Troglomorphies: pigmentation and sclerotization reduced; pedipalps, legs, and metasoma attenuate; telson vesicle enlarged. Classification: hypogean: troglophile. Citations: [8,33,34,35,40,41,42,43]. Alpiscorpius pavicevici Tropea, 2021: SERBIA. Habitat: inside cave; surface habitats, in leaf litter or under stones. Classification: endogean: accidental. Troglomorphies: none. Citations: [44]. Euscorpius aquilejensis (C.L. Koch, 1837): CROATIA, ITALY, SLOVENIA. Habitat: inside cave; surface habitats, in rock crevices or under tree bark. Troglomorphies: pedipalps, legs, and metasoma attenuate. Classification: epigean, hypogean: accidental. Citations: [8,32,34,35,36,45,46,47,48,49]. Euscorpius biokovensis Tropea & Ozimec, 2020: BOSNIA AND HERZEGOVINA, CROATIA. Habitat: inside caves. Troglomorphies: pigmentation and sclerotization reduced; pedipalps, legs, and metasoma attenuate; telson vesicle enlarged. Classification: hypogean: troglophile. Citations: [8,32,33,34,35,36,49]. Euscorpius birulai Fet et al., 2014: GREECE. Habitat: inside cave. Troglomorphies: median ocelli reduced; pigmentation and sclerotization reduced; pedipalps, legs, and metasoma attenuate; telson vesicle enlarged. Classification: hypogean: troglophile. Citations: [8,32,33,36,48,50,51]. Euscorpius canestrinii (Fanzago, 1872): ITALY. Habitat: inside cave; surface habitats, in rock crevices. Troglomorphies: none. Classification: epigean, hypogean: accidental. Citations: [52]. Euscorpius concinnus (C.L. Koch, 1837): ITALY. Habitat: inside cave; surface habitats, in rock crevices, leaf litter, or under stones. Troglomorphies: none. Classification: epigean: accidental. Citations: [8,45,53,54,55]. Euscorpius deltshevi Fet et al., 2014: BULGARIA, SERBIA. Habitat: inside cave; surface habitats, in rock crevices, leaf litter or under stones. Troglomorphies: none. Classification: epigean: accidental. Citations: [8,56,57]. Euscorpius feti Tropea, 2013: BOSNIA AND HERZEGOVINA, CROATIA, MONTENEGRO. Habitat: both inside and outside cave. Troglomorphies: pigmentation and sclerotization slightly reduced; pedipalps, legs, and metasoma attenuate. Classification: epigean, hypogean: trogloxene. Citations: [8,31,32,33,34,35,36,47,48,49]. Euscorpius giachinoi Tropea & Fet, 2015: GREECE. Habitat: both inside caves and outside. Troglomorphies: median ocelli reduced; pigmentation and sclerotization reduced; pedipalps, legs, and metasoma attenuate; telson vesicle enlarged. Classification: epigean, hypogean: trogloxene. Citations: [8,33,36,47,51]. Euscorpius lagostae Caporiacco, 1950: CROATIA. Habitat: both inside and outside caves. Troglomorphies: pedipalps, legs, and metasoma slightly attenuate. Classification: epigean, hypogean: trogloxene. Citations: [34,58]. Euscorpius sulfur Kovařík et al., 2023: ALBANIA, GREECE. Habitat: inside cave. Troglomorphies: pigmentation and sclerotization slightly reduced. Classification: hypogean: troglophile. Citations: [36]. Euscorpius studentium Karaman, 2020: MONTENEGRO. Habitat: inside cave. Troglomorphies: median and lateral ocelli reduced; pigmentation and sclerotization reduced; pectinal teeth count reduced; pedipalps, legs, and metasoma attenuate; telson vesicle enlarged. Classification: hypogean: troglobite. Citations: [8,33,34,35,36]. |
| AMNH | AMNH | ||||||
|---|---|---|---|---|---|---|---|
| ♂ | ♀ | ♂ | ♀ | ||||
| Total length 1 | 41.9 | 40.28 | Telson vesicle | L | 4.5 | 3.62 | |
| Carapace | L | 5.53 | 5.47 | W | 2.56 | 1.65 | |
| Anterior W | 3.87 | 3.63 | H | 2.22 | 1.93 | ||
| Posterior W | 5.07 | 4.9 | Aculeus | L | 0.88 | 1.2 | |
| Mesosoma | L 2 | 13.03 | 12.67 | Pedipalp | L 4 | 23.24 | 21.6 |
| Metasoma | L 3 | 23.34 | 22.14 | Femur | L | 5.55 | 5.26 |
| Segment I | L | 2.62 | 2.49 | W | 1.79 | 1.75 | |
| W | 1.96 | 1.64 | H | 1.12 | 1.1 | ||
| H | 1.38 | 1.22 | Patella | L | 5.94 | 5.62 | |
| Segment II | L | 3.08 | 2.96 | W | 1.9 | 1.85 | |
| W | 1.78 | 1.49 | H | 1.64 | 1.61 | ||
| H | 1.37 | 1.29 | Chela | L 5 | 11.75 | 10.72 | |
| Segment III | L | 3.22 | 3.12 | Manus | L | 6.7 | 6.18 |
| W | 1.64 | 1.43 | W | 3.38 | 3.21 | ||
| H | 1.38 | 1.25 | H | 2.67 | 2.49 | ||
| Segment IV | L | 3.82 | 3.46 | Fixed finger | L | 5.05 | 4.54 |
| W | 1.57 | 1.34 | W 6 | 0.61 | 0.65 | ||
| H | 1.38 | 1.25 | H 6 | 0.94 | 0.89 | ||
| Segment V | L | 6.1 | 5.29 | Movable finger | L | 5.93 | 5.48 |
| W | 1.51 | 1.26 | W 7 | 0.58 | 0.58 | ||
| H | 1.45 | 1.39 | H 7 | 1.1 | 0.81 | ||
| Telson | L | 5.38 | 4.82 | Pectines | L | 2.45 | 1.84 |
| Holotype | Paratype | ||||||
|---|---|---|---|---|---|---|---|
| ♂ | ♀ | subad. ♂ | juv. ♂ | juv. ♀ | |||
| AMNH | AMNH | ZZDBE | AMNH | ZZDBE | |||
| SC1/01 | SC2/02 | ||||||
| Pedipalp chela | Fixed finger subrows | 7/7 | 7/7 | 7/7 | 7/7 | -/- | |
| Fixed finger PAD | 11/11 | 11/11 | -/11 | 11/11 | -/- | ||
| Fixed finger RAD | 7/7 | 7/7 | 7/7 | 7/7 | -/- | ||
| Movable finger subrows | 7/7 | 7/7 | 7/7 | 7/7 | -/- | ||
| Movable finger PAD | 11/11 | 11/11 | -/11 | 11/11 | -/- | ||
| Movable finger RAD | 7/7 | 7/7 | -//7 | 7/7 | -/- | ||
| External trichobothria | Et | 5/5 | 5/5 | 5/5 | 5/5 | 5/5 | |
| Est | 1/1 | 1/1 | 1/1 | 1/1 | 1/1 | ||
| Esb | 1/1 | 1/1 | 1/1 | 1/1 | 1/1 | ||
| Eb | 3/3 | 3/3 | 3/3 | 3/3 | 3/3 | ||
| et | 1/1 | 1/1 | 1/1 | 1/1 | 1/1 | ||
| est | 1/1 | 1/1 | 1/1 | 1/1 | 1/1 | ||
| esb | 1/1 | 1/1 | 1/1 | 1/1 | 1/1 | ||
| eb | 1/1 | 1/1 | 1/1 | 1/1 | 1/1 | ||
| Ventral trichobothria | V | 4/4 | 4/4 | 4/4 | 4/4 | 4/4 | |
| Pedipalp patella | External trichobothria | et | 6/6 | 6/6 | 6/6 | 6/6 | 6/6 |
| est | 4/4 | 4/4 | 4/4 | 4/4 | 4/4 | ||
| em | 4/4 | 4/4 | 4/4 | 4/4 | 4/4 | ||
| esb | 2/2 | 2/2 | 2/2 | 2/2 | 2/2 | ||
| esba | 4/4 | 4/4 | 4/4 | 4/4 | 4/4 | ||
| eb | 4/4 | 4/4 | 4/4 | 4/4 | 4/4 | ||
| Ventral trichobothria | v | 7/7 | 7/7 | 7/7 | 7/7 | 7/7 | |
| Telotarsal VMS | Leg I | 7/7 | 6/6 | -/- | -/- | -/- | |
| Leg II | 7/6 | 7/7 | -/- | -/- | -/- | ||
| Leg III | 8/7 | 5/7 | -/7 | -/- | -/- | ||
| Leg IV | 9/9 | -/8 | -/- | -/- | -/- | ||
| Pectines | Teeth | 7/7 | 6/6 | 7/7 | 7/7 | 7/7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blasco-Aróstegui, J.; Prendini, L. Redescription of Euscorpius studentium Based on Adult Specimens; Updated Classification of Cavernicolous Euscorpiidae; and Review of Cavernicolous Scorpions in the Balkans. Diversity 2024, 16, 737. https://doi.org/10.3390/d16120737
Blasco-Aróstegui J, Prendini L. Redescription of Euscorpius studentium Based on Adult Specimens; Updated Classification of Cavernicolous Euscorpiidae; and Review of Cavernicolous Scorpions in the Balkans. Diversity. 2024; 16(12):737. https://doi.org/10.3390/d16120737
Chicago/Turabian StyleBlasco-Aróstegui, Javier, and Lorenzo Prendini. 2024. "Redescription of Euscorpius studentium Based on Adult Specimens; Updated Classification of Cavernicolous Euscorpiidae; and Review of Cavernicolous Scorpions in the Balkans" Diversity 16, no. 12: 737. https://doi.org/10.3390/d16120737
APA StyleBlasco-Aróstegui, J., & Prendini, L. (2024). Redescription of Euscorpius studentium Based on Adult Specimens; Updated Classification of Cavernicolous Euscorpiidae; and Review of Cavernicolous Scorpions in the Balkans. Diversity, 16(12), 737. https://doi.org/10.3390/d16120737









