Abstract
Here I examine the overexploitation of Artemisia granatensis, a narrow endemic medicinal plant species from Sierra Nevada, Spain, and the consequences for its conservation. With over 50,000 flowering plant species used for medicinal purposes worldwide, many species face sustainability issues due to overharvesting and habitat loss. Historical documentation of A. granatensis use dates back to the 13th century, highlighting its significance in traditional medicine. However, this species has suffered extensive overexploitation, particularly in the 19th and 20th centuries, leading to a significant decline in populations. Conservation concerns were first raised in 1909, and despite the species being legally protected since 1982, illegal collection and environmental pressures persist. Today, A. granatensis is critically endangered, with fewer than 2000 individuals remaining in fragmented populations. The study synthesizes the historical and recent literature to understand the long-standing pressures on this species and the limited conservation efforts made. Cultivation of A. granatensis is proposed as a crucial strategy to reduce pressure on wild populations and ensure the survival of this flagship important plant species.
1. Introduction
Worldwide, an estimated 50,000 to 80,000 flowering plant species are used for medicinal purposes [1]. In fact, among the many provisioning services provided by plants, the use of medicinal plants stands out, especially in rural areas where access to conventional drugs is limited [2]. However, the use of these plants is in many cases is far from sustainable. In fact, some 15,000 species worldwide are threatened from overharvesting and habitat depletion [3], with experts estimating that the Earth is losing at least one potential major drug every two years [4]. Also, species decline can have cascading ecological consequences, affecting biodiversity, ecosystem functions, and services. The loss of a species can disrupt food webs, leading to imbalances in predator–prey dynamics, reduced pollination, and altered nutrient cycling [5]. In particular, endemic species decline can severely impact ecosystems affecting specialized interactions, as these species often play critical roles in maintaining habitat structure and ecosystem resilience [6]. For this reason, they are frequently recognized as key indicators in defining conservation priorities [7]. Furthermore, reduced genetic diversity from population declines diminishes the species’ ability to adapt to environmental changes, increasing vulnerability to climate change, disease, and further habitat degradation [8]. These effects collectively threaten the ecosystem’s stability, potentially leading to broader environmental degradation [9].
Mountain ecosystems are proving to be particularly vulnerable to global change [10,11,12], exhibiting strong responses to climate change [11,13], and according to the literature these responses are particularly pronounced in the Mediterranean mountains [14]. Also, human activity has significantly shaped these mountain landscapes over centuries, altering land cover and, through numerous land use changes, fluctuating them depending on socio-economic framework at each time [15]. The Sierra Nevada, like other Mediterranean mountain regions, has been subjected to high anthropic pressure in the last millennia [16]. The interaction between these human-induced changes and climatic change often exacerbates the changing patterns [17]. Disturbing factors in this region can be categorized as follows: (i) traditional and operating nowadays (i.e., livestock grazing or plant harvesting), (ii) traditional but no longer operating, although impacting on the current ecosystems (i.e., management of vegetation with fire or high-mountain agriculture), or (iii) novel impacts (mainly derived from outdoor activities and infrastructure construction).
Here I examine the impact of overexploitation of Artemisia granatensis, a narrow endemic medicinal plant, considered among the most threatened medicinal plant species in Europe [18]. This case could be an outstanding example of overharvesting, exacerbated by the global change drivers acting synergistically, which can push species towards extinction, even a formerly common species [18]. While numerous species have become rare and eventually extinct by overexploitation, well-documented examples remain very rare in the literature. Even the most canonical example, such as the disappearance of “silphium”, which is considered the first extinction of a plant or animal species recorded, has many obscure points [19]. First, it is an unidentified species considered to belong to the Ferula genus (Apiaceae family). Silphium had a remarkably narrow native range, about 201 by 56 km, in the southern steppe of Cyrenaica (present-day eastern Libya). The cause of silphium’s supposed extinction is not fully known, but numerous factors such as overgrazing combined with overharvesting likely played a role [20]. This example is paradigmatic and illustrates how rare is to have detailed historical information on plant species overexploitation and its consequences. In contrast, Artemisia granatensis could offer an outstanding exception, with references to its use and harvest dating back to at least the 13th century [21]. These records provide valuable insights into long-term effects of overexploitation on endemic plant species, offering us an opportunity to explore the process in depth. This species is emblematic for conservation on a local scale. In fact, when the general population is asked about an endemic or endangered plant in the Sierra Nevada, most of the time “Royal Chamomile” is the only species they know. It is also a flagship species for conservation on an international scale, appearing in all the compilations of threatened medicinal species, and even being the cover image of the European red list of medicinal plants [18]. Preserving flagship species is crucial because they serve as symbols for broader conservation efforts, helping to raise awareness and support for protecting entire ecosystems. By focusing on these charismatic species, conservation programs can gain public and political attention, which in turn benefits other species and habitats within their environment [19].
This article aims to examine a paradigmatic case of overuse of an endemic medicinal plant species by a literature review to compile the consequences of overexploitation for the conservation of an endemic and rare species.
2. Studied Species
Artemisia granatensis Boiss. belongs to the large family of Asteraceae. Artemisia is the largest genus in the tribe Anthemideae and one of the largest in the family, with over 500 species [22]. The species is endemic to Sierra Nevada in southeastern Spain (from 36°50′24″ to 37°15′0″ N in latitude, and from 3°44′24″ to 2°35′24″ W in longitude). Sierra Nevada, a small and isolated Mediterranean high-mountain, is the only true alpine region between the North African mountains, the Spanish Central Range, and the Pyrenees, which are all several hundreds of kilometers away [17]. Covering 2100 km2, it has a complex orography and a wide altitudinal range (200 m to 3479 m at Mulhacén peak). The climate is Mediterranean, with cold, wet winters and hot, dry summers [23]. Temperatures above 2000 m range from −10 °C to 10 °C during the snow season with a snowpack that can persist for up to 8 months at the highest elevations (occasionally up to 10 months in small patches of snowbeds) [17,24]. Annual rainfall varies from 220 to 1000 mm per year, influenced by elevation and exposure [25]. Geologically, the core area is composed of siliceous rocks, mainly mica-schists, surrounded by limestone and dolomite [26].
Sierra Nevada marks the southernmost influence of the Quaternary glaciations in Europe, with glaciers only above 2500 m. It served as a refuge for many plant species during glacial ages, leading speciation [27,28]. Today, it hosts 2348 taxa from a total of 756 genera and 146 families, including 95 endemic species [29,30,31]. For this reason, it is considered one of the most important plant hotspots within the Mediterranean region [27,28,32,33,34].
An outstanding example among these endemic species is A. granatensis, appearing in perennial high-mountain pasturelands, screes, and rock crevices on mica-schists, from 2500 m to above 3400 m. Traditionally, its main threat has been the harvesting of complete individuals for medicinal use, leading to significant population decline. Additionally, both wild and domestic ungulates heavily browse the reproductive stems [35], despite the production of sesquiterpenes that make the foliage bitter [36], hence reducing seed set by 20–90%, depending on the population [37]. Due to the significant conservation problems faced by the species, it has been included in different legal protection documents since the first document approved at national level [38]. Also, due to its traditional use, the species is locally very well-known and, together with the conservation issues, has become a clear example of a flagship species [39].
3. Literature Search
I conducted systematic literature searches in Scopus, ISI Web of Science, and PubMed databases. In addition, I retrieved non-indexed but relevant citations from the grey literature and books using Google Scholar, freeFullpdf (https://www.freefullpdf.com, accessed on 30 September 2024), and Biblioteca Digital del Jardín Botánico de Madrid (https://bibdigital.rjb.csic.es, accessed on 29 September 2024). Also, to gather news about the species, I searched in the Hemeroteca Digital, Biblioteca Nacional de España (https://www.bne.es/es, accessed on 28 September 2024). The search terms were “Artemisia granatensis” AND/OR “royal chamomille” AND/OR “wormwood” (both in English and Spanish). I specifically aimed to review the papers dealing with the use and conservation (sensu lato) of the species. For this I subjected all the documents (96) to a manual screening to select the relevant documents to fulfill the aim (including research articles, reviews, commentaries, letters, books, book chapters, and reports). After the screening I selected 61 documents (Table S1) that have been used to illustrate the use and conservation issues of the species for the period 1220–2019.
I have included the 61 documents selected in Table S1. I have also compiled some relevant information for each of them concerning the use, overexploitation, and conservation of the species. Also, I have added a timeline illustrating the main milestones found in the literature (Figure 1).
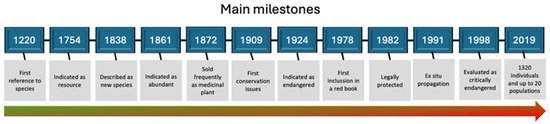
Figure 1.
The most important milestones of the species according to the bibliographic review. Color in the arrow indicates the inferred growing conservation issues.
4. A Long History of Use and Abuse
The first writing references date back to ca. 1220, where Ibn Al-Baytar, a Spanish–Arabic physician and botanist, indicated that “manzanilla real” (Royal Chamomile, Spanish name for A. granatensis) “grows in the colder mountains” [21], which indicates the interest in the species date back even to medieval times.
A reference to the species appeared in [40], an inventory for the cadastre of the Marques de la Ensenada, who indicated that the Veleta summit area was “… producing nothing but Royal Chamomile, polypodium and some amethyst-like points or crystals strongly attached to the surface of the slabs”. It is reasonable to think that its use must have already been widespread, since it is considered one of the few remarkable resources in the high mountain.
The first botanical description of the species was made by Lagasca and Rodríguez in 1802 [41], who assigned the species to the alpine taxon Artemisia rupestris Lamarck; then, other botanists cited the species as A. glacialis L. [42], A. genipi Weber, or A. laxa Fritsch [43]. Afterwards, Webb in 1820 [44] first described the species as new and named it A. basilicata (Webb ex Willk & Lange) but did not formally publish it. Then, Boissier in 1838 [45] finally described the species as A. granatensis, the currently accepted name. This author also commented on its frequency on the summit of the Sierra and the “large quantity of the plant that was observed during the summer in the markets of the city of Granada”.
The rest of the 19th century references indicate the interest in the species, i.e., Madoz [46] in his geographic dictionary commented for Trevelez (a village in the southern slope of Sierra Nevada) when mentioning its products that “… thyme and chamomile grow there”. Other authors noted their abundance [47] or extensive use, as in Willkomm [48]: “… the royal chamomile Artemisia granatensis Boiss., a tiny, silvery herb, existing in very thick grasslands, of the chamomile family or St. John’s wort which, as a medicinal herb, is well known to the inhabitants of the Sierra …” or Rein [49]: “… In the province of Granada the best known and most appreciated plant variety is undoubtedly the royal chamomile (Artemisia granatensis) a very aromatic plant and very popular as a remedy for any stomach illness. This plant, which grows among the schist of the highest peaks, can be easily bought in the streets of Granada”.
It is noteworthy that shortly after these references to its extensive use, a reference first reported the conservation concerns posed by this overuse of the species [50]: “If I mention this species here, it is to point out the war of extermination being waged by the manzanilleros (Spanish name for royal chamomile collectors). It is condemned to disappear: I do not know of any plant in Spain or in any other region where the monomania or social epidemic is more fiercely embodied in the natives of the country …”
The species rapidly became very rare and by 1923 it was indicated [51]: “… the manzanilleros, in search of a special chamomille, exclusive, it seems, of Sierra Nevada, already very rarefied, almost exhausted, preserved only in places almost inaccessible to any interest other than the conquest of bread, the prize of all the sweat”. He also narrated the death of a manzanillero who fell from the Mulhacén peak in the search of the scarce plants growing there. The situation then become even worst; as an example, Font Quer in 1924 [52] wrote: “… it has become so scarce that to collect a few sheets for the herbarium, it is necessary to search a lot”, and “… the main incentive for collectors is not so much the healing virtue of this plant as its price”. He also highlighted the problems that over-harvesting posed for natural regeneration: “Due to the irrational collection that is made of it, it is almost exhausted in Sierra Nevada. As a result of the high price it fetches in the market, it is cut as soon as it is born to prevent other collectors from getting ahead of it, thus not allowing the production of seed and its dissemination to multiply it properly.”
This situation became critical by the 1970s, when some authors considered the species had disappeared from many places [53] and globally “… in the process of disappearing as a consequence of the incessant search to which it has been subjected …” [54].
5. Protection and Conservation Status
Despite some authors’ early consideration that the species was threatened by abusive collection [50], neither was the species protected or its collection regulated [55]. As a result, by the end of the 1970s, Synge indicated “the plant is extremely difficult to find, but there is a still a strong desire to collect and it is not legally protected” [56]. Due to this situation the species was included in the Bern Convention [38], the first attempt to preserve some species at the European scale. However, until 1982, the species was not legally protected in the Spanish legislation, under the category of endangered, with “the collection, cutting and uprooting of this plant or part of it, including its seeds, as well as its commercialization, except for scientific purposes” being prohibited [57]. Since this, the species has been included in all the national and regional legislation for the protection of endangered species.
Despite the legal protection, illegal collection still continued during the 1980s [58] and there was a local black market with a great demand for the species [59,60]. Also, this situation was aggravated by the grazing impact of the Spanish goat (Capra pyrenaica hispanica) [61], whose population grew by this time due to game regulation and the absence of predators (e.g., wolves). Afterwards, even during the 1990s, the situation remained critical [62]. As a result, the species was evaluated as critically endangered (CR sensu [63]), with only six populations (scattered in small patches) and less than 3000 individuals in total [27]. This regressive trend still persisted, with fewer than 2000 individuals by 2003 [64], and it was maintained during the 2000s [29]. Other threat factors can have a localized but strong influence in some populations, such as the hybridization with the closely related alpine species Artemisia umbelliformis [65], present in the area, although rare [29], and the synergistic negative effect of ski slopes’ machine grading and the construction and maintenance of auxiliary infrastructure in the ski area [66].
In addition, it has been found that the low number of individuals in most populations caused a generalized failure in seed set, with a 20–90% decline depending on the populations [37]. An extensive search carried out by the staff of the National Park of Sierra Nevada led to the discovery of more populations (up to 20) but a decreasing estimated number of individuals of 1320 by the end of the 2020s. This estimate is the last one available in the literature. It is therefore considered as critically endangered (CR) and, as a result, is now included in the top ten most endangered species at the regional scale [67].
6. Conservation Efforts
Despite the critical status of the species and the ongoing pressures negatively affecting the populations, existing conservation initiatives remain insufficient to effectively mitigate the continuous population decline. There are many similar examples around the world. In fact, the conservation group United Plant Savers listed 19 medicinal plants native to North America as “endangered”, with a further 22 plant species identified on the list as being “to watch” (https://unitedplantsavers.org/species-at-risk-list/, accessed on 16 November 2024).
From the very first indication of the conservation issues, it was also indicated that a possible measure is the cultivation of the species. Pau (1909) [50] states “… I told the guide to cultivate it to meet the demands and thus avoid its extermination”. Despite this, there was no attempt in this sense until 1979 [68], when it was propagated in a nursery and planted in situ for the first time, but on a small scale (only 40 individuals). Although cultivation of threatened medicinal plants is often cited as the main conservation measure to reduce pressure on wild populations (e.g., [69]), practical successful examples are still scarce [70].
Afterwards, in vitro propagation was tested as an appropriate method to promote its cultivation [71]. However, although in vitro propagation technology allows the production of large numbers of seedlings that can be used to restore or reinforce threatened and endangered species, its applicability is limited by the reduced genetic variability usually provided by this technique [72].
Long after Hernández-Bermejo et al. in 2003 [64] indicated again that, among different conservation measures (such as effective control of illegal harvesting, public awareness campaigns, or ex situ seed conservation in germplasm banks), the promotion of propagation is the only method needed to reinforce populations, and at the same time satisfy the needs of the traditional use of the plant through cultivation. However, to date, reinforcements or re-introductions with a conservation purpose have been very scarce, being limited to about 300 individuals [73]. Nor has a mechanism been established to cultivate it under controlled conditions, although it is acknowledged that it would be a dissuasive element to prevent illegal harvesting, which still occurs at present (author’s personal observation). Paradoxically, it is well known how to propagate the species from seeds and cuttings, as well as maintain it under cultivation conditions, from experiences gained in botanical gardens [73].
7. Conclusions
The case of Artemisia granatensis illustrates very well how overexploitation of a species, especially if it is a narrowly endemic and rare species, can led it to the brink of extinction. It is unusual in the available literature for a given species to have so many references illustrating in detail and over such a long period of time this whole process. We tend to think that the use of resources by rural communities is balanced and sustainable, but current conservation problems are often due to the overexploitation of a resource in the past. In this sense, the example of this species is paradigmatic.
The species was relatively common in the past, but after becoming heavily used, locally traded, and then overexploited, it is one of the most endangered plant species in Spain.
Moreover, despite the status of the species, there is a lack of effective conservation measures to ensure its survival. For this, it is necessary that the environmental administration takes the initiative in conservation and promotes all the proposed conservation measures. In this sense, cultivation could be an interesting way to reduce the pressure on natural populations, while at the same time providing an interesting resource for local farmers. Ultimately, the conservation of this flagship plant species is crucial, not only because of its role in maintaining ecosystem balance, but also to act as a symbol of conservation efforts, favoring entire habitat preservation.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/d16120744/s1, Table S1. Relevant literature including data about use and conservation of Artemisia granatensis.
Funding
This research was funded by Organismo Autónomo Parques Nacionales, Ministerio para la Transición Ecológica y el Reto Demográfico grant number PN30032023.
Acknowledgments
This work was conducted under the framework of the following projects: (1) “Characterisation and conservation of the unique, endemic and/or threatened rock flora of National Parks in a context of global change. (PROROCA)”. Spanish network of National Parks. Organismo Autónomo Parques Nacionales. PN3003/2023. (2) Change Observatory of Sierra Nevada (UGR-Junta de Andalucía), which take part Global Change Observatories network of Andalucia, funded by Consejería de Medio Ambiente y Ordenación del Territorio, Regional Government of Andalucia.
Conflicts of Interest
The author declares no conflict of interest.
References
- Roberson, E.; Mccormack, J.; Medicinal Plants at Risk. Nature’s Pharmacy, Our Treasure Chest: Why We Must Conserve Our Natural Heritage. 2008. Available online: https://www.biologicaldiversity.org/publications/papers/Medicinal_Plants_042008_lores.pdf (accessed on 18 November 2024).
- Millennium Ecosystem Assessment. Ecosystems and Human Well-Being: Synthesis; Island Press: Whashington, DC, USA, 2005; ISBN 1-59726-040-1. [Google Scholar]
- Chen, S.L.; Yu, H.; Luo, H.M.; Wu, Q.; Li, C.F.; Steinmetz, A. Conservation and Sustainable Use of Medicinal Plants: Problems, Progress, and Prospects. Chin. Med. 2016, 11, 37. Available online: https://cmjournal.biomedcentral.com/articles/10.1186/s13020-016-0108-7 (accessed on 16 November 2024).
- Pimm, S.L.; Russell, G.J.; Gittleman, J.L.; Brooks, T.M. The Future of Biodiversity. Science 1995, 269, 347–350. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.; Larsen, T. Ecological Consequences of Extinction. In Lessons in Conservation; American Museum of Natural History, Center for Biodiversity and Conservation: New York, NY, USA, 2010; Volume 3, pp. 25–53. [Google Scholar]
- Hobohm, C. Endemism in Vascular Plants; Springer: Berlin/Heidelberg, Germany, 2014; ISBN 9789400769120. [Google Scholar]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; da Fonseca, G.A.B.; Kent, J. Biodiversity Hotspots for Conservation Priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Frankham, R. Genetics and Extinction. Biol. Conserv. 2005, 126, 131–140. [Google Scholar] [CrossRef]
- Chown, S.L.; Gaston, K.J. Macrophysiology for a Changing World. Proc. R. Soc. Ser. B Biol. Sci. 2008, 275, 1469–1478. [Google Scholar] [CrossRef]
- Nogués-Bravo, D.; Araújo, M.B.; Errea, M.P.; Martínez-Rica, J.P. Exposure of Global Mountain Systems to Climate Warming during the 21st Century. Glob. Environ. Chang. 2007, 17, 420–428. [Google Scholar] [CrossRef]
- Lenoir, J.; Gégout, J.C.; Marquet, P.A.; de Ruffray, P.; Brisse, H. A Significant Upward Shift in Plant Species Optimum Elevation during the 20th Century. Science 2008, 320, 1768–1771. [Google Scholar] [CrossRef]
- Martín-Esquivel, J.L.; Marrero-Gómez, M.; Cubas, J.; González-Mancebo, J.M.; Olano, J.M.; del Arco, M. Climate Warming and Introduced Herbivores Disrupt Alpine Plant Community of an Oceanic Island (Tenerife, Canary Islands). Plant Ecol. 2020, 221, 1117–1131. [Google Scholar] [CrossRef]
- Steinbauer, M.J.; Grytnes, J.-A.; Jurasinski, G.; Kulonen, A.; Lenoir, J.; Pauli, H.; Rixen, C.; Winkler, M.; Bardy-Durchhalter, M.; Barni, E.; et al. Accelerated Increase in Plant Species Richness on Mountain Summits Is Linked to Warming. Nature 2018, 556, 231–234. [Google Scholar] [CrossRef]
- Nogués-Bravo, D.; Araújo, M.B.; Lasanta, T.; López-Moreno, J.I.; Bravo, D.N.; Araújo, M.B.; Lasanta, T.; Moreno, J.I.L. Climate Change in Mediterranean Mountains During the 21st Century. Ambio 2008, 37, 280–285. [Google Scholar] [CrossRef]
- Davis, P. Land Use; McGraw-Hill: New York, NY, USA, 1976. [Google Scholar]
- Jiménez-Olivencia, Y.; Porcel-Rodríguez, L.; Píñar-Álvarez, A. Evolución Histórica de Los Paisajes Del Parque Nacional Sierra Nevada y Su Entorno. In Proyectos de Investigación en Parques Nacionales 2006–2009; Serie Investigación en la Red: Naturaleza y Parques Nacionales; Organismo Autónomo de Parques Nacionales: Madrid, Spain, 2010; pp. 2006–2009. ISBN 9788480147804. [Google Scholar]
- Lamprecht, A.; Pauli, H.; Fernández Calzado, M.R.; Lorite, J.; Molero Mesa, J.; Steinbauer, K.; Winkler, M. Changes in Plant Diversity in a Water-Limited and Isolated High-Mountain Range (Sierra Nevada, Spain). Alp. Bot. 2021, 131, 27–39. [Google Scholar] [CrossRef]
- Allen, D.; Bilz, M.; Leaman, D.J.; Miller, R.M.; Timoshyna, A.; Window, J. European Red List of Medicinal Plants; Publications Office of the European Union: Luxembourg, 2014. [Google Scholar]
- Caro, T.M.; O’Doherty, G. On the Use of Surrogate Species in Conservation Biology. Conserv. Biol. 1999, 13, 805–814. [Google Scholar] [CrossRef]
- Parejko, K. Pliny the Elder’s Silphium: First Recorded Species Extinction. Biology 2003, 17, 925–927. [Google Scholar] [CrossRef]
- Navarro-García, M.A.; Hernández-Bermejo, J.E. Las Manzanillas En Los Autores Andalusíes: Algunos Apuntes Para La Interpretación de Los Textos; Junta de Andalucía: Sevilla, Spain, 1994; Volume 3.
- Martín, J.; Torrell, M.; Korobkov, A.A.; Vallès, J. Palynological Features as a Systematic Marker in Artemisia L. and Related Genera (Asteraceae, Anthemideae)—II: Implications for Subtribe Artemisiinae Delimitation. Plant Biol. 2003, 5, 85–93. [Google Scholar] [CrossRef]
- Gómez-Ortiz, A. Geomorphological Map of Sierra Nevada: Glacial a Periglacial Geo-Morphology; Consejería de Medio Ambiente, Junta de Andalucía y Universidad de Barcelona: Sevilla, Spain, 2002. [Google Scholar]
- Pérez-Palazón, M.; Pimentel, R.; Polo, M. Climate Trends Impact on the Snowfall Regime in Mediterranean Mountain Areas: Future Scenario Assessment in Sierra Nevada (Spain). Water 2018, 10, 720. [Google Scholar] [CrossRef]
- Algarra, J.A.; Cariñanos, P.; Herrero, J.; Delgado-Capel, M.; Ramos-Lorente, M.M.; Díaz de la Guardia, C. Tracking Montane Mediterranean Grasslands: Analysis of the Effects of Snow with Other Related Hydro-Meteorological Variables and Land-Use Change on Pollen Emissions. Sci. Total Environ. 2019, 649, 889–901. [Google Scholar] [CrossRef]
- Jabaloy, A.; Galindo, J.; Sanz, C. Guía Geológica de Granada; Diputación de Granada: Granada, Spain, 2008. [Google Scholar]
- Blanca, G.; Cueto, M.; Martínez-Lirola, M.J.; Molero-Mesa, J. Threatened Vascular Flora of Sierra Nevada (Southern Spain). Biol. Conserv. 1998, 85, 269–285. [Google Scholar] [CrossRef]
- Médail, F.; Diadema, K. Glacial Refugia Influence Plant Diversity Patterns in the Mediterranean Basin. J. Biogeogr. 2009, 36, 1333–1345. [Google Scholar] [CrossRef]
- Lorite, J.; Navarro, F.B.; Valle, F. Estimation of Threatened Orophytic Flora and Priority of Its Conservation in the Baetic Range (S. Spain). Plant Biosyst.—Int. J. Deal. All Asp. Plant Biol. 2007, 141, 1–14. [Google Scholar] [CrossRef]
- Lorite, J. An Updated Checklist of the Vascular Flora of Sierra Nevada (SE Spain). Phytotaxa 2016, 261, 1–57. [Google Scholar] [CrossRef]
- Lorite, J.; Ros-Candeira, A.; Alcaraz-Segura, D.; Salazar-Mendías, C. FloraSNevada: A Trait Database of the Vascular Flora of Sierra Nevada, Southeast Spain. Ecology 2020, 101, ecy.03091. [Google Scholar] [CrossRef] [PubMed]
- Medail, F.; Quezel, P. Biodiversity Hotspots in the Mediterranean Basin: Setting Global Conservation Priorities. Conservation Biology 1999, 13, 1510–1513. [Google Scholar] [CrossRef]
- Cañadas, E.M.; Fenu, G.; Peñas, J.; Lorite, J.; Mattana, E.; Bacchetta, G. Hotspots within Hotspots: Endemic Plant Richness, Environmental Drivers, and Implications for Conservation. Biol. Conserv. 2014, 170, 282–291. [Google Scholar] [CrossRef]
- Peñas, J.; Lorite, J. Biología de La Conservación de Plantas En Sierra Nevada: Principios y Retos Para Su Preservación; Editorial Universidad de Granada: Granada, Spain, 2019; ISBN 9788433865120. [Google Scholar]
- Blanca, G.; López-Onieva, M.; Lorite, J.; Martínez-Lirola, M.; Molero-Mesa, J.; Quintas, S.; Ruiz-Girela, M.; Varo, M.; Vidal, S. Flora Amenazada y Endémica de Sierra Nevada; Universidad de Granada: Granada, Spain, 2001; ISBN 8433827138. [Google Scholar]
- Watson, L.E.; Bates, P.L.; Evans, T.M.; Unwin, M.M.; Estes, J.R. Molecular Phylogeny of Subtribe Artemisiinae (Asteraceae), Including Artemisia and Its Allied and Segregate Genera. BMC Evol. Biol. 2002, 2, 17. [Google Scholar] [CrossRef] [PubMed]
- Peñas, J.; Lorite, J.; Alba, F.; Taisma, M.A. Self-Incompatibility, Floral Parameters, and Pollen Characterization in the Narrow Endemic and Threatened Species Artemisia Granatensis (Asteraceae). An. Jard. Bot. Madr. 2010, 67, 97–105. [Google Scholar] [CrossRef]
- Anonymous. Bern Convention on the Conservation of European Wildlife and Natural Habitats. 1979. Available online: https://www.coe.int/en/web/conventions/full-list?module=treaty-detail&treatynum=104 (accessed on 24 September 2024).
- Jarić, I.; Crowley, S.L.; Veríssimo, D.; Jeschke, J.M. Flagship Events and Biodiversity Conservation. Trends Ecol. Evol. 2024, 39, 106–108. [Google Scholar] [CrossRef]
- Ponz, A. Relación del viaje que desde Granada hizo á Sierra Nevada D. Antonio Ponz a influxo del Excmo. Sr. Marqués de la Ensenada. Mensajero Económico Y Erud. De Granada 1754, 1, 25–30. [Google Scholar]
- Lagasca, M.; Rodríguez, J. Descripción de Algunas Plantas Que Colectó D. Guillermo Thalacker En Sierra Nevada. An. Cienc. Nat. 1802, 5, 263–288. [Google Scholar]
- Rojas-Clemente, S. Viaje a Andalucía. Historia Natural Del Reino de Granada (1804–1809); Transcripción de Antonio Gil Albarracín; G.B.G.: Barcelona, Spain, 2002. [Google Scholar]
- Bory de Saint-Vincent, J.B.G.M. Florule de La Sierra Nevada Ou Catalogue Des Plantes Observées Dans Une Reconnasance Militaire Faite de Grenade Au Sommet Appelé Veleta. Ann. Générales Sci. Phys. 1820, 3, 3–16. [Google Scholar]
- Webb, P.B. Iter Hispaniense or a Synopsis of Plants Collected in the Southern Provinces of Spain and in Portugal, with Geographical Remarks, and Observations on Rare and Undescribed Species; Henry Coxhead: Paris, France; Béthune and Plon: London, UK, 1820. [Google Scholar]
- Boissier, E. Notice Sur l’Abies Pinsapo. Tiré de La Bibliothèque Universelle de Genève; Tiré de la Bibliothèque Universelle de Genève: Genéve, Switzerland, 1838. [Google Scholar]
- Madoz, P. Diccionario Geográfico-Estadístico-Histórico de España y Sus Posesiones de Ultramar; Ámbito de ediciones S.A.: Valladolid, Spain, 1845. [Google Scholar]
- Willkomm, M.; Lange, J. Prodromus Florae Hispanicae: Seu Synopsis Methodica Omnium Plantarum in Hispania, Sponte Nascentium Vel Frequentius Cultarum Quae Innotuerunt/Auctoribus Mauritio Willkomm et Joanni Lange.; E. Schweizerbart: Stuttgartiae, Germany, 1861. [Google Scholar]
- Willkomm, M. Aus Den Hochgebirgen von Granada: Naturschilderungen, Erlebnisse Und Erinnerungen/von Moritz Willkomm, Nebst Granadinischen Volkssagen Und Märchen; Wien Druck und Verlag von Carl Gerold’s Sohn: Wien, Austria, 1882. [Google Scholar]
- Rein, J. Beitrage Zur Kenntnis Der Spanischen Sierra Nevada; Lechner: Wien, Austria, 1899. [Google Scholar]
- Pau, C. Mi Segunda Visita a Sierra Nevada. Boletín Soc. Aragonesa Cienc. Nat. 1909, 8, 104–124. [Google Scholar]
- Bernaldo de Quirós, C. Sierra Nevada; Publicaciones de la Comisaría Regia del Turismo: Madrid, Spain, 1923. [Google Scholar]
- Font Quer, P. Datos Acerca de La Flora Orófila de Sierra Nevada. Boletín Soc. Aragonesa Cienc. Nat. 1924, 24, 238–244. [Google Scholar]
- Prieto, P. Flora de La Tundra de Sierra Nevada; Universidad de Grauada: Granada, Spain, 1975. [Google Scholar]
- Martínez-Parras, J.M.; Molero, J.; Esteve-Chueca, F. Notas Sobre La Provincia de Granada. Lagascalia 1979, 9, 51–64. [Google Scholar]
- Rivas-Goday, S. Algunas Especies Raras o Relícticas Que Deben Protegerse En La España Mediterránea. Rev. Ecol. 1959, 95–101. [Google Scholar]
- Synge, H. The IUCN Plant Red Data Book: Comprising Red Data Sheets on 250 Selected Plants Threatened on a World Scale; Unwin Brothers: London, UK, 1978. [Google Scholar]
- Anonymous. Real Decreto 3091/1982, de 15 de Octubre, Sobre Proteccion de Especies Amenazadas de La Flora Silvestre. 1982. Available online: https://www.boe.es/eli/es/rd/1982/10/15/3091 (accessed on 24 September 2024).
- López-Guadalupe, M.; Sierra, C.; Marín-Calderón, G. Comunidades, Habitat y Tipos de Suelos Sobre Los Que Se Desarrolla La Manzanilla de Sierra Nevada. Ars Pharm. 1985, 24, 255–263. [Google Scholar]
- Losa, J.M.; Giménez, M.A. Especies Medicinales Endémicas de Sierra Nevada. Ars Pharm. 1986, 27, 381–386. [Google Scholar]
- González-Tejero, M.R. Investigaciones Etnobotánicas En La Provincia de Granada. 1989. Available online: https://bibdigital.rjb.csic.es/records/item/1526267-investigaciones-etnobotanicas-en-la-provincia-de-granada?offset=1 (accessed on 28 September 2024).
- Gómez-Campo, C. Libro Rojo de Especies Vegetales Amenazadas de España Peninsular e Islas Baleares; Gómez Campo, C., Ed.; Ministerio de Agricultura, Pesca y Alimentación. ICONA. Serie Técnica: Madrid, Spain, 1987.
- Blanca, G.; Cabezudo, B.; Hernández-Bermejo, E.; Herrera, C.; Muñoz, J.; Valdés, B. Libro Rojo de La Flora Silvestre Amenazada de Andalucía; Consejería de Medio Ambiente, Junta de Andalucía: Sevilla, Spain, 1999; ISBN 89650-75-6.
- IUCN. IUCN Red List Categories and Criteria: Version 3.1., 2nd ed.; IUCN, Ed.; Gland: Cambridge, UK, 2012; ISBN 2831707862. [Google Scholar]
- Hernández-Bermejo, E.; Contreras, P.; Clemente, M.; Prados, J. Artemisia granatensis Boiss. In Atlas y Libro Rojo dela Flora Vascular Amenazada de España; Ministerio de Medio Ambiente: Madrid, Spain, 2003; pp. 124–125. [Google Scholar]
- Gómez, J.M.; González-Megías, A.; Lorite, J.; Abdelaziz, M.; Perfectti, F. The Silent Extinction: Climate Change and the Potential Hybridization-Mediated Extinction of Endemic High-Mountain Plants. Biodivers. Conserv. 2015, 24, 1843–1857. [Google Scholar] [CrossRef]
- Lorite, J.; Molina-Morales, M.; Cañadas, E.M.; Ballesteros, M.; Peñas, J. Evaluating a Vegetation-Recovery Plan in Mediterranean Alpine Ski Slopes: A Chronosequence-Based Study in Sierra Nevada (SE Spain). Landsc. Urban Plan 2010, 97, 92–97. [Google Scholar] [CrossRef]
- Gutierrez Carretero, L.; Fuentes Carretero, J.; Cueto Romero, M.; Blanca López, G. Top Ten de Las Plantas Más Amenazadas de Andalucía Oriental: Taxones Endémicos y No Endémicos. Acta Bot. Malacit. 2019, 44, 5–33. [Google Scholar] [CrossRef]
- Sainz-Ollero, H.; Hernández-Bermejo, J.E. Experimental Reintroductions of Endangered Plant Species in Their Natural Habitats in Spain. Biol. Conserv. 1979, 16, 195–206. [Google Scholar] [CrossRef]
- Khakurel, D.; Uprety, Y.; Karki, S.; Khadka, B.; Poudel, B.D.; Ahn, G.; Cha, J.Y.; Kim, W.Y.; Lee, S.H.; Rajbhandary, S. Assessing the Risks to Valuable Medicinal Plants in Nepal from Human Activities and Environmental Factors. Glob. Ecol. Conserv. 2024, 51, e02860. [Google Scholar] [CrossRef]
- Mateo-Martín, J.; Benítez, G.; Gras, A.; Molina, M.; Reyes-García, V.; Tardío, J.; Verde, A.; Pardo-de-Santayana, M. Cultural Importance, Availability and Conservation Status of Spanish Wild Medicinal Plants: Implications for Sustainability. People Nat. 2023, 5, 1512–1525. [Google Scholar] [CrossRef]
- Clemente, M.; Contreras, P.; Susín, J.; Pliego-Alfaro, F. Micropropagation of Artemisia Granatensis. HortScience 1991, 26, 420. [Google Scholar] [CrossRef]
- Sarasan, V.; Cripps, R.; Ramsay, M.M.; Atherton, C.; McMichen, M.; Prendergast, G.; Rowntree, J.K. Conservation in Vitro of Threatened Plants—Progress in the Past Decade. In Vitro Cell. Dev. Biol. Plant 2006, 42, 206–214. [Google Scholar] [CrossRef]
- Lorite, J.; Ruiz, M.; Plaza, L. Conservación Ex Situ e in Situ. In Biología de la Conservación de Plantas en Sierra Nevada: Principios y Retos para su Preservación; Editorial Universidad de Granada: Granada, Spain, 2019; pp. 225–265. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).