Effects of Non-Native Annual Plant Removal on Native Species in Mediterranean-Climate Shrub Communities
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Soil Composition of Study Sites
| Region | Restoration Site | Soil Composition |
|---|---|---|
| Coastal | Cattle Crest | 71.3% Anaheim loam, 28.7% Cieneba-Rock outcrop complex |
| Coastal | Cut Across | 92.4% Bosanko clay, 7.6 Cieneba sandy loam |
| Coastal | Laguna Laurel | 100% Yorba cobbly sandy loam |
| Coastal | Moro Ridge | 100% Myford sandy loam |
| Coastal | Strawberry Farms | 100% Alo clay |
| Coastal | Veeh Creek | 87.9% Alo variant clay, 6.4% Capistrano sandy loam, 5.8% Yorba cobbly sandy loam |
| Coastal | West Canyon | 97.6% Cieneba sandy loam, 2.4% Myford sandy loam |
| Inland | Agua Chinon | 100% Balcom clay loam |
| Inland | Blackstar | 100% Soper gravelly loam |
| Inland | Gypsum | 100% Cieneba-Rock outcrop complex |
| Inland | Shoestring | 100% Calleguas clay loam |
| Inland | Upper Weir | 100% Alo clay |
| Inland | West Loma | 100% Anaheim loam |
Appendix B. Tukey Post Hoc Results for Native and Non-Native Seedling Density
| Effect | Dependent Variable | Season | Treatment | Estimate | SE | Letter | |
| Treatment | ln(x + 1) native density | Winter | Weeded | 1.6622 | 0.2446 | A | |
| Winter | Control | 1.5918 | 0.2446 | A | |||
| Spring | Weeded | 0.9912 | 0.133 | A | |||
| Spring | Control | 0.7566 | 0.133 | B | |||
| Effect | Dependent Variable | Season | Native Cover Class | Treatment | Estimate | SE | Letter |
| Treatment x native cover class | ln(x + 1) native density | Winter | Medium-low | Weeded | 1.8268 | 0.3 | A |
| Winter | High | Control | 1.7696 | 0.3 | A | ||
| Winter | Low | Weeded | 1.7183 | 0.3 | A | ||
| Winter | Medium-high | Control | 1.6833 | 0.3 | A | ||
| Winter | High | Weeded | 1.6571 | 0.3 | A | ||
| Winter | Low | Control | 1.5132 | 0.3 | A | ||
| Winter | Medium-high | Weeded | 1.4464 | 0.3 | A | ||
| Winter | Medium-low | Control | 1.401 | 0.3 | A | ||
| Spring | Medium-low | Weeded | 1.2168 | 0.1687 | A | ||
| Spring | Low | Weeded | 1.144 | 0.1687 | AB | ||
| Spring | High | Control | 0.9414 | 0.1687 | ABC | ||
| Spring | Medium-high | Weeded | 0.8223 | 0.1703 | ABC | ||
| Spring | Medium-high | Control | 0.8185 | 0.1687 | ABC | ||
| Spring | High | Weeded | 0.7817 | 0.1687 | ABC | ||
| Spring | Medium-low | Control | 0.68 | 0.1703 | BC | ||
| Spring | Low | Control | 0.5865 | 0.1687 | C | ||
| Effect | Dependent Variable | Season | Region | Estimate | SE | Letter | |
| Region | ln(x + 1) native density | Winter | Inland | 2.0823 | 0.3436 | A | |
| Winter | Coastal | 1.1716 | 0.3181 | A | |||
| Spring | Inland | 1.3612 | 0.1849 | A | |||
| Spring | Coastal | 0.3866 | 0.171 | B | |||
| Effect | Dependent Variable | Season | Treatment | Estimate | SE | Letter | |
| Treatment | ln(x + 1) native shrub density | Winter | Weeded | 1.0694 | 0.1523 | A | |
| Winter | Control | 0.8016 | 0.1523 | B | |||
| Spring | Weeded | 0.5142 | 0.08285 | A | |||
| Spring | Control | 0.219 | 0.08285 | B | |||
| Effect | Dependent Variable | Season | Region | Estimate | SE | Letter | |
| Region | ln(x + 1) native shrub density | Winter | Inland | 1.1067 | 0.2102 | A | |
| Winter | Coastal | 0.7643 | 0.1946 | A | |||
| Spring | Inland | 0.5145 | 0.1024 | A | |||
| Spring | Coastal | 0.2187 | 0.09457 | B | |||
| Effect | Dependent Variable | Season | Region | Estimate | SE | Letter | |
| Region | ln(x + 1) native forb density | Winter | Inland | 1.6502 | 0.3158 | A | |
| Winter | Coastal | 0.7178 | 0.2924 | B | |||
| Spring | Inland | 0.9825 | 0.169 | A | |||
| Spring | Coastal | 0.1589 | 0.1563 | B | |||
| Effect | Dependent Variable | Season | Region | Native Cover Class | Estimate | SE | Letter |
| Interaction between region and initial native cover class | ln(x + 1) native forb density | Winter | Inland | High | 1.8096 | 0.3654 | A |
| Winter | Inland | Medium-low | 1.7847 | 0.3654 | A | ||
| Winter | Inland | Low | 1.5315 | 0.3654 | A | ||
| Winter | Inland | Medium-high | 1.475 | 0.3654 | A | ||
| Winter | Coastal | Low | 0.9536 | 0.3383 | A | ||
| Winter | Coastal | Medium-high | 0.7928 | 0.3383 | A | ||
| Winter | Coastal | High | 0.7569 | 0.3383 | A | ||
| Winter | Coastal | Medium-low | 0.3681 | 0.3383 | A | ||
| Spring | Inland | Medium-low | 1.2256 | 0.1894 | A | ||
| Spring | Inland | High | 0.9492 | 0.1884 | AB | ||
| Spring | Inland | Medium-high | 0.9221 | 0.1894 | AB | ||
| Spring | Inland | Low | 0.8332 | 0.1884 | ABC | ||
| Spring | Coastal | Medium-high | 0.2384 | 0.1744 | BC | ||
| Spring | Coastal | High | 0.2137 | 0.1744 | BC | ||
| Spring | Coastal | Low | 0.1195 | 0.1744 | C | ||
| Spring | Coastal | Medium-low | 0.06407 | 0.1744 | C | ||
| Effect | Dependent Variable | Season | Treatment | Estimate | SE | Letter | |
| Treatment | ln(x + 1) non-native density | Winter | Control | 3.6099 | 0.2253 | A | |
| Winter | Weeded | 2.6637 | 0.2253 | B | |||
| Spring | Control | 2.6157 | 0.207 | A | |||
| Spring | Weeded | 1.8246 | 0.207 | B | |||
| Effect | Dependent Variable | Season | Native Cover Class | Estimate | SE | Letter | |
| Initial native cover class | ln(x + 1) non-native density | Winter | Medium-low | 3.5293 | 0.2721 | A | |
| Winter | Low | 3.3051 | 0.2721 | A | |||
| Winter | High | 2.8946 | 0.2721 | A | |||
| Winter | Medium-high | 2.8182 | 0.2721 | A | |||
| Spring | Medium-low | 2.5256 | 0.2384 | A | |||
| Spring | Low | 2.4608 | 0.2376 | AB | |||
| Spring | High | 2.015 | 0.2376 | AB | |||
| Spring | Medium-high | 1.8791 | 0.2384 | B |
Appendix C. Tukey Post Hoc Results for Native and Non-Native Species Richness
| Effect | Dependent Variable | Season | Native Cover Class | Treatment | Estimate | SE | Letter |
| Treatment x initial native cover class | sqrt (x + 1) native species richness | Winter | High | Control | 1.7302 | 0.1206 | A |
| Winter | Medium-low | Weeded | 1.6614 | 0.1206 | A | ||
| Winter | Low | Weeded | 1.645 | 0.1206 | A | ||
| Winter | Medium-high | Control | 1.6201 | 0.1206 | A | ||
| Winter | Medium-high | Weeded | 1.5932 | 0.1206 | A | ||
| Winter | Medium-low | Control | 1.56 | 0.1206 | A | ||
| Winter | Low | Control | 1.5307 | 0.1206 | A | ||
| Winter | High | Weeded | 1.5255 | 0.1206 | A | ||
| Spring | Medium-low | Weeded | 1.4318 | 0.0686 | A | ||
| Spring | Low | Weeded | 1.4287 | 0.0686 | AB | ||
| Spring | High | Control | 1.4042 | 0.0686 | ABC | ||
| Spring | Medium-high | Control | 1.3753 | 0.0686 | ABC | ||
| Spring | Medium-high | Weeded | 1.2951 | 0.06909 | ABC | ||
| Spring | Medium-low | Control | 1.2941 | 0.06909 | ABC | ||
| Spring | Low | Control | 1.2524 | 0.0686 | BC | ||
| Spring | High | Weeded | 1.2416 | 0.0686 | C | ||
| Effect | Dependent Variable | Season | Region | Estimate | SE | Letter | |
| Region | sqrt (x + 1) native species richness | Winter | Inland | 1.7882 | 0.1481 | A | |
| Winter | Coastal | 1.4283 | 0.1371 | A | |||
| Spring | Inland | 1.5409 | 0.08366 | A | |||
| Spring | Coastal | 1.1399 | 0.0774 | B | |||
| Effect | Dependent Variable | Season | Region | Treatment | Estimate | SE | Letter |
| Region x Treatment | sqrt (x + 1) native species richness | Winter | Inland | Control | 1.8205 | 0.1526 | A |
| Winter | Inland | Weeded | 1.7559 | 0.1526 | A | ||
| Winter | Coastal | Weeded | 1.4566 | 0.1412 | A | ||
| Winter | Coastal | Control | 1.3999 | 0.1412 | A | ||
| Spring | Inland | Control | 1.5731 | 0.08635 | A | ||
| Spring | Inland | Weeded | 1.5087 | 0.08635 | A | ||
| Spring | Coastal | Weeded | 1.1899 | 0.07985 | B | ||
| Spring | Coastal | Control | 1.0899 | 0.07985 | B | ||
| Effect | Dependent Variable | Season | Treatment | Estimate | SE | Letter | |
| Treatment | sqrt (x + 1) non-native species richness | Winter | Control | 1.8419 | 0.05725 | A | |
| Winter | Weeded | 1.7391 | 0.05725 | B | |||
| Spring | Control | 1.6285 | 0.06289 | A | |||
| Spring | Weeded | 1.5494 | 0.06289 | A | |||
| Effect | Dependent Variable | Season | Region | Estimate | SE | Letter | |
| Region | sqrt (x + 1) non-native species richness | Winter | Inland | 1.9098 | 0.07674 | A | |
| Winter | Coastal | 1.6712 | 0.07105 | B | |||
| Spring | Inland | 1.669 | 0.08582 | A | |||
| Spring | Coastal | 1.5089 | 0.07937 | A | |||
| Effect | Dependent Variable | Season | Region | Native Cover Class | Estimate | SE | Letter |
| Region x initial native cover class | sqrt (x + 1) non-native species richness | Winter | Inland | Medium-low | 2.0247 | 0.09697 | A |
| Winter | Inland | Low | 1.8993 | 0.09697 | AB | ||
| Winter | Inland | Medium-high | 1.8841 | 0.09697 | AB | ||
| Winter | Inland | High | 1.831 | 0.09697 | AB | ||
| Winter | Coastal | Medium-low | 1.7201 | 0.08978 | AB | ||
| Winter | Coastal | High | 1.7143 | 0.08978 | AB | ||
| Winter | Coastal | Low | 1.6554 | 0.08978 | AB | ||
| Winter | Coastal | Medium-high | 1.5951 | 0.08978 | B | ||
| Spring | Inland | Medium-high | 1.7364 | 0.1045 | A | ||
| Spring | Inland | Medium-low | 1.7081 | 0.1045 | A | ||
| Spring | Inland | Low | 1.6992 | 0.104 | A | ||
| Spring | Coastal | Medium-low | 1.592 | 0.09624 | A | ||
| Spring | Coastal | Low | 1.592 | 0.09624 | A | ||
| Spring | Inland | High | 1.5323 | 0.104 | A | ||
| Spring | Coastal | High | 1.5005 | 0.09624 | A | ||
| Spring | Coastal | Medium-high | 1.3513 | 0.09624 | A |
Appendix D. ANOVA Results for Established Shrub Metrics
| Response Variable | Effect | Num DF | Den DF | F Value | p Value |
|---|---|---|---|---|---|
| Mean shrub volume | Treatment | 1 | 135 | 3.28 | 0.0723 |
| Native cover class | 3 | 135 | 1.2 | 0.3108 | |
| Native cover class × treatment | 3 | 135 | 1.06 | 0.3682 | |
| Region | 1 | 135 | 17.5 | <0.0001 | |
| Region × treatment | 1 | 135 | 1.49 | 0.2248 | |
| Region × native cover class | 3 | 135 | 0.07 | 0.9747 | |
| Region × native cover Class × treatment | 3 | 135 | 0.76 | 0.5166 | |
| Total shrub volume | Treatment | 1 | 135 | 3.12 | 0.0796 |
| Native cover class | 3 | 135 | 8.64 | <0.0001 | |
| Native cover class × treatment | 3 | 135 | 0.19 | 0.9031 | |
| Region | 1 | 135 | 0.73 | 0.3953 | |
| Region × treatment | 1 | 135 | 0.59 | 0.4455 | |
| Region × native Cover Class | 3 | 135 | 2.39 | 0.0718 | |
| Region × native Cover Class × treatment | 3 | 135 | 0.21 | 0.888 | |
| Shrub number | Treatment | 1 | 135 | 9.95 | 0.002 |
| Native cover class | 3 | 135 | 1.74 | 0.1626 | |
| Native cover class × treatment | 3 | 135 | 0.85 | 0.468 | |
| Region | 1 | 135 | 16.71 | <0.0001 | |
| Region × treatment | 1 | 135 | 1.01 | 0.316 | |
| Region × native cover class | 3 | 135 | 0.94 | 0.4244 | |
| Region × native cover Class × treatment | 3 | 135 | 1.76 | 0.157 |
Appendix E. Tukey Post Hoc Results for Established Shrub Metrics
| (a) Tukey–Kramer post hoc results for ln(x) mean established shrub volume Effect of Region | |||
| Region | Estimate | SE | Letter |
| Coastal | 5.5534 | 0.2199 | A |
| Inland | 4.253 | 0.2197 | B |
| (b) Tukey–Kramer post hoc results for total established shrub volume Effect of Initial Native Cover Class | |||
| Native Cover Class | Estimate | SE | Letter |
| High | 24.6762 | 1.9698 | A |
| Medium-high | 21.6437 | 1.9698 | AB |
| Medium-low | 17.5053 | 1.9698 | B |
| Low | 17.4724 | 1.9767 | B |
| (c) Tukey–Kramer post hoc results for ln(x) number of established shrubs Effect of Treatment | |||
| Treatment | Estimate | SE | Letter |
| Weeded | 2.7857 | 0.1437 | A |
| Control | 2.4356 | 0.1435 | B |
| Effect of Region | |||
| Region | Estimate | SE | Letter |
| Inland | 3.152 | 0.1872 | A |
| Coastal | 2.0694 | 0.1873 | B |
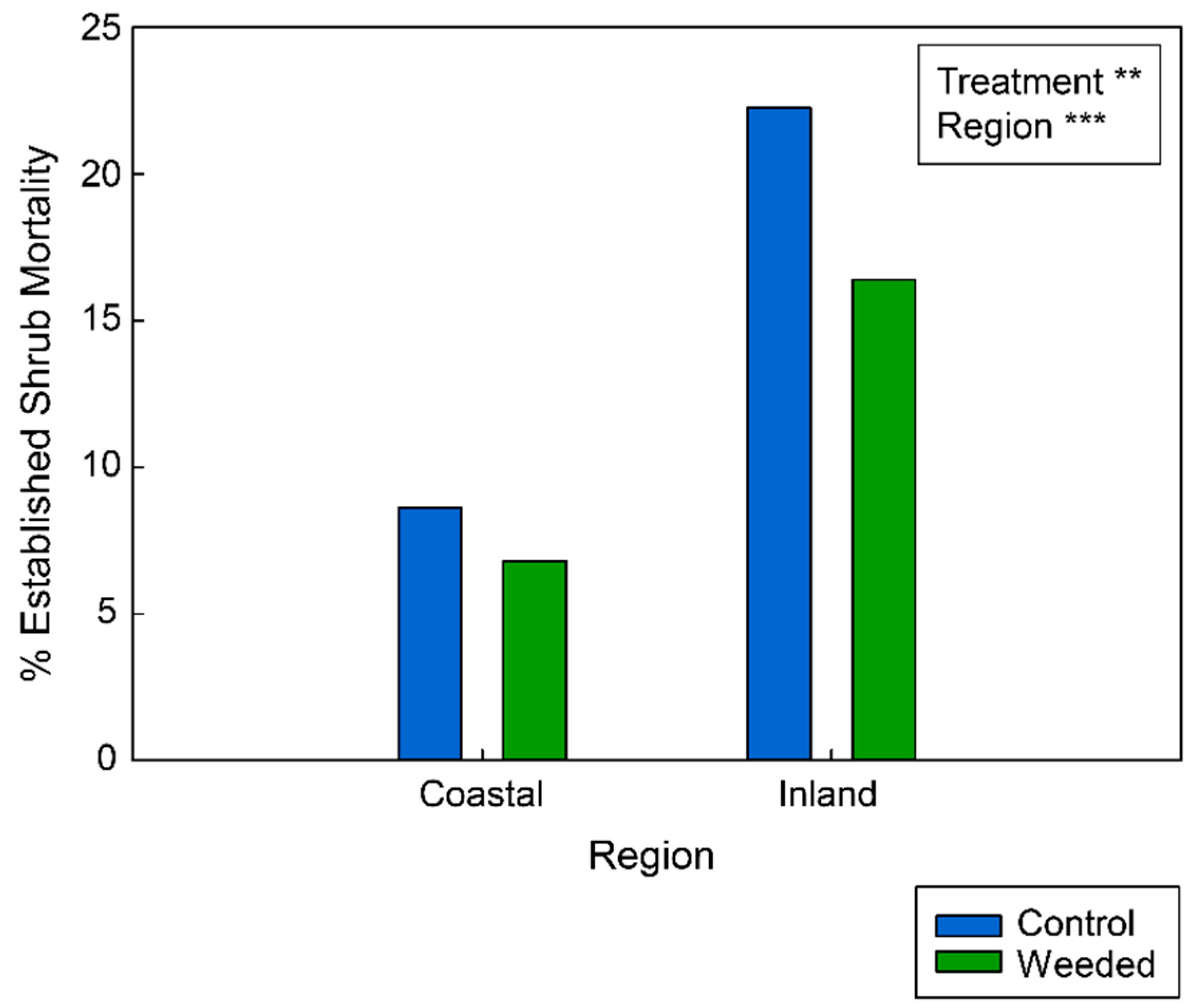
Appendix F. Logistic Regression Results for Established Shrub Mortality
| Response Variable | Effect | Estimate | SE | Z Value | p Value |
|---|---|---|---|---|---|
| Shrub mortality | (Intercept) | −1.22329 | 0.17106 | −7.1511 | 8.61 × 10−13 |
| Treatment | −0.35182 | 0.12274 | −2.8663 | 0.00415 | |
| Native cover class | −0.01446 | 0.5546 | −0.2607 | 0.79431 | |
| Region | −1.05432 | 0.14839 | −7.105 | 1.2 × 10−12 |
References
- Cowling, R.M.; Rundel, P.W.; Lamont, B.B.; Arroyo, M.K.; Arianoutsou, M. Plant diversity in mediterranean-climate regions. Trends Ecol. Evol. 1996, 11, 362–366. [Google Scholar] [CrossRef]
- Medail, F.; Quezel, P. Hot-Spots Analysis for Conservation of Plant Biodiversity in the Mediterranean Basin. Ann. Mo. Bot. Gard. 1997, 84, 112–127. [Google Scholar] [CrossRef]
- Sala, O.E.; Chapin, F.S.; Armesto, J.J.; Berlow, E.; Bloomfield, J.; Dirzo, R.; Huber-Sanwald, E.; Huenneke, L.F.; Jackson, R.B.; Kinzig, A.; et al. Global Biodiversity Scenarios for the Year 2100. Science 2000, 287, 1770–1774. [Google Scholar] [CrossRef] [PubMed]
- Talluto, M.V.; Suding, K.N. Historical change in coastal sage scrub in southern California, USA in relation to fire frequency and air pollution. Landsc. Ecol. 2008, 23, 803–815. [Google Scholar] [CrossRef]
- Kimball, S.; Goulden, M.L.; Suding, K.N.; Parker, S. Altered water and nitrogen input shifts succession in a southern California coastal sage community. Ecol. Appl. 2014, 24, 1390–1404. [Google Scholar] [CrossRef] [PubMed]
- Syphard, A.D.; Brennan, T.J.; Keeley, J.E. Extent and drivers of vegetation type conversion in Southern California chaparral. Ecosphere 2019, 10, 14. [Google Scholar] [CrossRef]
- Cione, N.K.; Padgett, P.E.; Allen, E.B. Restoration of a native shrubland impacted by exotic grasses, frequent fire, and nitrogen deposition in southern California. Restor. Ecol. 2002, 10, 376–384. [Google Scholar] [CrossRef]
- Kimball, S.; Lulow, M.; Sorenson, Q.; Balazs, K.; Fang, Y.; Davis, S.J.; O’Connell, M.; Huxman, T.E. Cost-effective ecological restoration. Restor. Ecol. 2015, 23, 800–810. [Google Scholar] [CrossRef]
- Schmidt, K.T.; Maltz, M.; Ta, P.; Khalili, B.; Weihe, C.; Phillips, M.; Aronson, E.; Lulow, M.; Long, J.; Kimball, S. Identifying Mechanisms for Successful Ecological Restoration with Salvaged Topsoil in Coastal Sage Scrub Communities. Diversity 2020, 12, 150. [Google Scholar] [CrossRef]
- De Steven, D.; Sharitz, R.R.; Singer, J.H.; Barton, C.D. Testing a passive revegetation approach for restoring coastal plain depression wetlands. Restor. Ecol. 2006, 14, 452–460. [Google Scholar] [CrossRef]
- Crouzeilles, R.; Prevedello, J.A.; de Souza Lima Figueiredo, M.; Lorini, M.L.; Grelle, C.E.V. The effect of the number, size and isolation of patches along a gradient of native vegetation cover: How can we increment habitat availability? Landsc. Ecol. 2014, 29, 479–489. [Google Scholar] [CrossRef]
- Eliason, S.A.; Allen, E.B. Exotic grass competition in suppressing native shrubland re-establishment. Restor. Ecol. 1997, 5, 245–255. [Google Scholar] [CrossRef]
- Shono, L.; Cadaweng, E.A.; Durst, P.B. Application of Assisted Natural Regeneration to Restore Degraded Tropical Forestlands. Restor. Ecol. 2007, 15, 620–626. [Google Scholar] [CrossRef]
- de Mesquita, C.P.B.; Solon, A.J.; Barfield, A.; Mastrangelo, C.F.; Tubman, A.J.; Vincent, K.; Porazinska, D.L.; Hufft, R.A.; Shackelford, N.; Suding, K.N.; et al. Adverse impacts of Roundup on soil bacteria, soil chemistry and mycorrhizal fungi during restoration of a Colorado grassland. Appl. Soil Ecol. 2023, 185, 104778. [Google Scholar] [CrossRef]
- Morrison, E.; Lindell, C. Active or Passive Forest Restoration? Assessing Restoration Alternatives with Avian Foraging Behavior. Restor. Ecol. 2010, 19, 170–177. [Google Scholar] [CrossRef]
- Holl, K.; Aide, T. When and where to actively restore ecosystems? For. Ecol. Manag. 2011, 261, 1558–1563. [Google Scholar] [CrossRef]
- Ruwanza, S.; Gaertner, M.; Esler, K.; Richardson, D. The effectiveness of active and passive restoration on recovery of indigenous vegetation in riparian zones in the Western Cape, South Africa: A preliminary assessment. S. Afr. J. Bot. 2013, 88, 132–141. [Google Scholar] [CrossRef]
- Bell, C.E.; Allen, E.B.; Weathers, K.A.; McGiffen, M. Simple Approaches to Improve Restoration of Coastal Sage Scrub Habitat in Southern California. Nat. Areas J. 2016, 36, 20–28. [Google Scholar] [CrossRef]
- Dyer, A.R.; Rice, K.J. Effects of competition on resource availability and growth of a California bunchgrass. Ecology 1999, 80, 2697–2710. [Google Scholar] [CrossRef]
- Clary, J.; Save, R.; Biel, C.; De Herralde, F. Water relations in competitive interactions of Mediterranean grasses and shrubs. Ann. Appl. Biol. 2004, 144, 149–155. [Google Scholar] [CrossRef]
- Wainwright, C.E.; Wolkovich, E.M.; Cleland, E.E. Seasonal priority effects: Implications for invasion and restoration in a semi-arid system. J. Appl. Ecol. 2012, 49, 234–241. [Google Scholar] [CrossRef]
- Balshor, B.J.; Garrambone, M.S.; Austin, P.; Balazs, K.R.; Weihe, C.; Martiny, J.B.; Huxman, T.E.; McCollum, J.R.; Kimball, S. The effect of soil inoculants on seed germination of native and invasive species. Botany 2016, 95, 469–480. [Google Scholar] [CrossRef]
- Esch, E.; Ashbacher, A.; Kopp, C.; Cleland, E. Competition reverses the response of shrub seedling mortality and growth along a soil moisture gradient. J. Ecol. 2018, 106, 2096–2108. [Google Scholar] [CrossRef]
- Morgan, J.W. Patterns of invasion of an urban remnant of a species-rich grassland in southeastern Australia by non-native plant species. J. Veg. Sci. 1998, 9, 181–190. [Google Scholar] [CrossRef]
- Molinari, N.A.; D’Antonio, C.M. Where have all the wildflowers gone? The role of exotic grass thatch. Biol. Invasions 2020, 22, 957–968. [Google Scholar] [CrossRef]
- Chen, B.M.; D’Antonio, C.M.; Molinari, N.; Peng, S.L. Mechanisms of influence of invasive grass litter on germination and growth of coexisting species in California. Biol. Invasions 2018, 20, 1881–1897. [Google Scholar] [CrossRef]
- Molinari, N.A.; D’Antonio, C.M. Structural, compositional and trait differences between native- and non-native-dominated grassland patches. Funct. Ecol. 2014, 28, 745–754. [Google Scholar] [CrossRef]
- Uebel, K.; Wison, K.A.; Shoo, L.P. Assisted natural regeneration accelerates recovery of highly disturbed rainforest. Ecol. Manag. Restor. 2017, 18, 231–238. [Google Scholar] [CrossRef]
- D’Antonio, C.M.; Hughes, R.F.; Mack, M.; Hitchcock, D.; Vitousek, P.M. The response of native species to removal of invasive exotic grasses in a seasonally dry Hawaiian woodland. J. Veg. Sci. 1998, 9, 699–712. [Google Scholar] [CrossRef]
- Baylis, A.D. Why glyphosate is a global herbicide: Strengths, weaknesses and prospects. Pest Manag. Sci. 2000, 56, 299–308. [Google Scholar] [CrossRef]
- Reid, A.M.; Morin, L.; Downey, P.O.; French, K.; Virtue, J.G. Does invasive plant management aid the restoration of natural ecosystems? Biol. Conserv. 2009, 142, 2342–2349. [Google Scholar] [CrossRef]
- Westman, W.E. Factors Influencing the Distribution of Species of Californian Coastal Sag Scrub. Ecology 1981, 62, 439–455. [Google Scholar] [CrossRef]
- Kimball, S.; Lulow, M.E.; Balazs, K.R.; Huxman, T.E. Predicting drought tolerance from slope aspect preference in restored plant communities. Ecol. Evol. 2017, 7, 3123–3131. [Google Scholar] [CrossRef]
- Davis, M.A.; Pelsor, M. Experimental support for a resource-based mechanistic model of invasibility. Ecol. Lett. 2001, 4, 421–428. [Google Scholar] [CrossRef]
- Blumenthal, D.; Mitchell, C.E.; Pysek, P.; Jarosík, V. Synergy between pathogen release and resource availability in plant invasion. Proc. Natl. Acad. Sci. USA 2009, 106, 7899–7904. [Google Scholar] [CrossRef]
- Vilà, M.; Espinar, J.L.; Hejda, M.; E Hulme, P.; Jarošík, V.; Maron, J.L.; Pergl, J.; Schaffner, U.; Sun, Y.; Pyšek, P. Ecological impacts of invasive alien plants: A meta-analysis of their effects on species, communities and ecosystems. Ecol. Lett. 2011, 14, 702–708. [Google Scholar] [CrossRef]
- Funk, J.L.; Vitousek, P.M. Resource-use efficiency and plant invasion in low-resource systems. Nature 2007, 446, 1079–1081. [Google Scholar] [CrossRef]
- Kimball, S.; Gremer, J.R.; Barron-Gafford, G.A.; Angert, A.L.; Huxman, T.E.; Venable, D.L. High water-use efficiency and growth contribute to success of non-native Erodium cicutarium in a Sonoran Desert winter annual community. Conserv. Physiol. 2014, 2, cou006. [Google Scholar] [CrossRef]
- Dudney, J.; Hallett, L.M.; Larios, L.; Farrer, E.C.; Spotswood, E.N.; Stein, C.; Suding, K.N. Lagging behind: Have we overlooked previous-year rainfall effects in annual grasslands? J. Ecol. 2017, 105, 484–495. [Google Scholar] [CrossRef]
- Mazzochini, G.G.; Lira-Martins, D.; de Barros, F.V.; Oliveira, A.C.C.; Xavier, R.O.; Furtado, M.N.; Verona, L.S.; Viani, R.A.G.; Rowland, L.; Oliveira, R.S. Effects of grass functional diversity on invasion success by exotic grasses in Cerrado grasslands. J. Appl. Ecol. 2023. early view. [Google Scholar] [CrossRef]
- Hobbs, R.; Norton, D. Towards a Conceptual Framework for Restoration Ecology. Restor. Ecol. 1996, 4, 93–110. [Google Scholar] [CrossRef]
- Suding, K.N.; Gross, K.L.; Houseman, G.R. Alternative states and positive feedbacks in restoration ecology. Trends Ecol. Evol. 2004, 19, 46–53. [Google Scholar] [CrossRef]
- Marchante, H.; Freitas, H.; Hoffmann, J.H. The potential role of seed banks in the recovery of dune ecosystems after removal of invasive plant species. Appl. Veg. Sci. 2011, 14, 107–119. [Google Scholar] [CrossRef]
- Conlisk, E.; Swab, R.; Martinez-Berdeja, A.; Daugherty, M.P. Post-Fire Recovery in Coastal Sage Scrub: Seed Rain and Community Trajectory. PLoS ONE 2016, 11, e0162777. [Google Scholar] [CrossRef]
- Cox, R.; Allen, E. Composition of soil seed banks in southern California coastal sage scrub and adjacent exotic grassland. Plant Ecol. 2008, 198, 37–46. [Google Scholar] [CrossRef]
- Young, S.L.; Hamerlynch, E.P. Patterning ecological restoration after weeds. Restor. Ecol. 2022, 31, e13841. [Google Scholar] [CrossRef]
- Griffoul, E. The Effectiveness of Passive Restoration: Influence of Initial Cover on Success. Master’s Thesis, University of California, Irvine, CA, USA, 2017. [Google Scholar]
- Allen, E.B.; Cox, R.D.; Tennant, T.; Kee, S.N.; Deutschman, D.H. Landscape restoration in southern California forblands: Response of abandoned farmland to invasive annual grass control. Isr. J. Plant Sci. 2005, 53, 237–245. [Google Scholar] [CrossRef]
- Medrano, H.; Flexas, J.; Galmes, J. Variability in water use efficiency at the leaf level among Mediterranean plants with different growth forms. Plant Soil 2009, 317, 17–29. [Google Scholar] [CrossRef]
- Galmés, J.; Cifre, J.; Medrano, H.; Flexas, J. Modulation of relative growth rate and its components by water stress in Mediterranean species with different growth forms. Oecologia 2005, 145, 21–31. [Google Scholar] [CrossRef]
- Seastedt, T.R.; Hobbs, R.J.; Suding, K.M. Management of novel ecostystems: Are novel approaches required? Front. Ecol. Environ. 2008, 6, 547–553. [Google Scholar] [CrossRef]
- Kimball, S.; Principe, Z.; Deutschman, D.; Strahm, S.; Huxman, T.; Lulow, M.; Balazs, K. Resistance and resilience: Ten years of monitoring shrub and prairie communities in Orange County, CA, USA. Ecosphere 2018, 9, e02212. [Google Scholar] [CrossRef]
- Keeley, J.E.; Baer-Keeley, M.; Fotheringham, C.J. Alien plant dynamics following fire in Mediterranean-climate California shrublands. Ecol. Appl. 2005, 15, 2109–2125. [Google Scholar] [CrossRef]
- Goldstein, L.J.; Suding, K.N. Applying competition theory to invasion: Resource impacts indicate invasion mechanisms in California shrublands. Biol. Invasions 2014, 16, 191–203. [Google Scholar] [CrossRef]
- Wakefield, Z.R.; Cavalcanti, A.R.O.; Driessen, L.; Jaramillo, A.; Crane, E.J.; Richetta, G.; Meyer, W.M. Effects of Mustard Invasions on Soil Microbial Abundances and Fungal Assemblages in Southern California. Diversity 2023, 15, 50. [Google Scholar] [CrossRef]
- Oduor, A.M.O.; van Kleunen, M.; Stift, M. Allelopathic effects of native and invasive Brassica nigra do not support the novel-weapons hypothesis. Am. J. Bot. 2020, 107, 1106–1113. [Google Scholar] [CrossRef]
- Dewees, S.L.; D’Antonio, C.M.; Molinari, N. Determining potential drivers of vegetation change in a Mediterranean environment. Ecosphere 2022, 13, e4313. [Google Scholar] [CrossRef]
- Kimball, S.; Lulow, M.E.; Mooney, K.A.; Sorenson, Q.M. Establishment and Management of Native Functional Groups in Restoration. Restor. Ecol. 2014, 22, 81–88. [Google Scholar] [CrossRef]
- USDA. Soil Quality Test Kit Guide; USDA: Washington, DC, USA, 1999.
- Kirkpatrick, J.B.; Hutchinson, C.F. The Environmental Relationships of Californian Coastal Sage Scrub and Some of its Component Communities and Species. J. Biogeogr. 1980, 7, 23–28. [Google Scholar] [CrossRef]
- Dave Bramlett & Jones & Stokes Associates, Inc. Methods Used to Survery the Vegetation of Orange County Parks and Open Space Areas and The Irvine Company Property; Unpublished report prepared for County of Orange; Environmental Management Agency: Santa Ana, CA, USA, 1993. [Google Scholar]
- SAS Institute 2012 Software; Version 94; S.A.S. Institute Inc.: Cary, NC, USA, 2012.
- Thomson, D.M.; Meyer, W.M.; Whitcomb, I.F. Non-native plant removal and high rainfall years promote post-fire recovery of Artemisia californica in southern California sage scrub. PLoS ONE 2021, 16, e0254398. [Google Scholar] [CrossRef]
- O’Loughlin, L.S.; Panetta, F.D.; Gooden, B. Identifying thresholds in the impacts of an invasive groundcover on native vegetation. Sci. Rep. 2021, 11, 20512. [Google Scholar] [CrossRef]
- DeMeester, J.; Richter, D. Restoring restoration: Removal of the invasive plant Microstegium vimineum from a North Carolina wetland. Biol. Invasions 2010, 12, 781–793. [Google Scholar] [CrossRef]
- HilleRisLambers, J.; Yelenik, S.; Colman, B.; Levine, J. California annual grass invaders: The drivers or passengers of change? J. Ecol. 2010, 98, 1147–1156. [Google Scholar] [CrossRef]
- Leege, L.; Kilgore, J. Recovery of Foredune and Blowout Habitats in a Freshwater Dune Following Removal of Invasive Austrian Pine (Pinus nigra). Restor. Ecol. 2014, 22, 641–648. [Google Scholar] [CrossRef]
- DiVittorio, C.; Corbin, J.; D’Antonio, C. Spatial and Temporal Patterns of Seed Dispersal: An Important Determinant of Grassland Invasion. Ecol. Appl. 2007, 17, 311–316. [Google Scholar] [CrossRef]
- Padilla, F.; Pugnaire, F. Rooting depth and soil moisture control Mediterranean woody seedling survival during drought. Funct. Ecol. 2007, 21, 489–495. [Google Scholar] [CrossRef]
- Biggerstaff, M.S.; Beck, C.W. Effects of Method of English Ivy Removal and Seed Addition on Regeneration of Vegetation in a Southeastern Piedmont Forest. Am. Midl. Nat. 2007, 158, 206–220. [Google Scholar] [CrossRef]
- Flory, S.L.; Clay, K. Invasive plant removal method determines native plant community responses. J. Appl. Ecol. 2009, 46, 434–442. [Google Scholar] [CrossRef]
- Castro-Díez, P.; Vaz, A.S.; Silva, J.S.; Loo, M.; Alonso, A.; Aponte, C.; Bayón, A.; Bellingham, P.J.; Chiuffo, M.C.; DiManno, N.; et al. Global effects of non-native tree species on multiple ecosystem services. Biol. Rev. 2019, 94, 1477–1501. [Google Scholar] [CrossRef]
- Fehr, V.; Buitenwerf, R.; Svenning, J.C. Non-native palms (Arecaceae) as generators of novel ecosystems: A global assessment. Divers. Distrib. 2020, 26, 1523–1538. [Google Scholar] [CrossRef]
- Arrington, A. Urban foraging of five non-native plants in NYC: Balancing ecosystem services and invasive species management. Urban For. Urban Green. 2021, 58, 126896. [Google Scholar] [CrossRef]
- DiTomaso, J.M. Invasive Weeds in Rangelands: Species, Impacts, and Management. Weed Sci. 2000, 48, 255–265. [Google Scholar] [CrossRef]
- Seabloom, E.W.; Borer, E.T.; Boucher, V.L.; Burton, R.S.; Cottingham, K.L.; Goldwasser, L.; Gram, W.K.; Kendall, B.E.; Micheli, F. Competition, seed limitation, disturbance, and reestablishment of California native annual forbs. Ecol. Appl. 2003, 13, 575–592. [Google Scholar] [CrossRef]
- Crone, E.E.; Marler, M.; Pearson, D.E. Non-target effects of broadcast leaf herbicide on a native perennial forb; a demographic framework for assessing and minimizing impacts. J. Appl. Ecol. 2009, 46, 673–682. [Google Scholar] [CrossRef]
- McLellan, A.J.; Fitter, A.H.; Law, R. On Decaying Roots, Mycorrhizal Colonization and the Design of Removal Experiments. J. Ecol. 1995, 83, 225. [Google Scholar] [CrossRef]
- Burke, M.J.W.; Grime, J.P. An experimental study of plant community invasibility. Ecology 1996, 77, 776–790. [Google Scholar] [CrossRef]
- Kyle, G.P.; Beard, K.H.; Kulmatiski, A. Reduced soil compaction enhances establishment of non-native plant species. Plant Ecol. 2007, 193, 223–232. [Google Scholar] [CrossRef]
- Goldstein, L.J.; Suding, K.N. Intra-annual rainfall regime shifts competitive interactions between coastal sage scrub and invasive grasses. Ecology 2014, 95, 425–435. [Google Scholar] [CrossRef]
- Llorens, L.; Penuelas, J.; Estiarte, M. Ecophysiological responses of two Mediterranean shrubs, Erica multiflora and Globularia alypum, to experimentally drier and warmer conditions. Physiol. Plant. 2003, 119, 231–243. [Google Scholar] [CrossRef]
- Okin, G.S.; Dong, C.; Willis, K.S.; Gillespie, T.W.; MacDonald, G.M. The Impact of Drought on Native Southern California Vegetation: Remote Sensing Analysis Using MODIS-Derived Time Series. J. Geophys. Res. Biogeosci. 2018, 123, 1927–1939. [Google Scholar] [CrossRef]
- Llorens, L.; Penuelas, J.; Estiarte, M.; Bruna, P. Contrasting Growth Changes in Two Dominant Species of a Mediterranean Shrubland Submitted to Experimental Drought and Warming. Ann. Bot. 2004, 94, 843–853. [Google Scholar] [CrossRef]
- Dawson, W.; Rohr, R.P.; van Kleunen, M.; Fischer, M. Alien pant species with a wider global distribution are better able to capitalize on increased resource availability. New Phytol. 2012, 194, 859–867. [Google Scholar] [CrossRef]
- Winkler, D.E.; Belnap, J.; Hoover, D.; Reed, S.C.; Duniway, M.C. Shrub persistence and increased grass mortality in response to drought in dryland systems. Glob. Change Biol. 2019, 25, 3121–3135. [Google Scholar] [CrossRef]
- Adams, H.D.; Guardiola-Claramonte, M.; Barron-Gafford, G.A.; Villegas, J.C.; Breshears, D.D.; Zou, C.B.; Troch, P.A.; Huxman, T.E. Temperature sensitivity of drought-induced tree mortality portends increased regional die-off under global-change-type drought. Proc. Natl. Acad. Sci. USA 2009, 106, 7063–7066. [Google Scholar] [CrossRef]
- Seabloom, E.W.; Harpole, W.S.; Reichman, O.J.; Tilman, D. Invasion, competitive dominance, and resource use by exotic and native California grassland species. Proc. Natl. Acad. Sci. USA 2003, 100, 13384–13389. [Google Scholar] [CrossRef]
- Cox, R.D.; Allen, E.B. The roles of exotic grasses and forbs when restoring native species to highly invaded southern California annual grassland. Plant Ecol. 2011, 212, 1699–1707. [Google Scholar] [CrossRef]
- Marrs, R.H.; Williams, C.T.; Frost, A.J.; Plant, R.A. Assessment of the effects of herbicide spray drift on a range of plant species of conservation interest. Environ. Pollut. 1989, 59, 71–86. [Google Scholar] [CrossRef]
- Ferreira, M.F.; Torres, C.; Bracamonte, E.; Galetto, L. Glyphosate affects the susceptibility of non-target native plant species according to their stage of development and degree of exposure in the landscape. Sci. Total Environ. 2023, 865, 161091. [Google Scholar] [CrossRef]
- Dai, A.G. Increasing drought under global warming in observations and models. Nat. Clim. Change 2013, 3, 52–58. [Google Scholar] [CrossRef]




| Response Variable | Season | Effect | Num DF | Den DF | F Value | p Value |
|---|---|---|---|---|---|---|
| Native density | Winter | Treatment | 1 | 285 | 0.25 | 0.6198 |
| Native cover class | 3 | 285 | 0.19 | 0.9014 | ||
| Native cover class × treatment | 3 | 285 | 1.13 | 0.3374 | ||
| Region | 1 | 285 | 3.78 | 0.0527 | ||
| Region × treatment | 1 | 285 | 1.96 | 0.1623 | ||
| Region × native cover class | 3 | 285 | 0.84 | 0.4713 | ||
| Region × native cover class × treatment | 3 | 285 | 0.82 | 0.4856 | ||
| Spring | Treatment | 1 | 283 | 7.57 | 0.0063 | |
| Native cover class | 3 | 283 | 0.39 | 0.7583 | ||
| Native cover class × treatment | 3 | 283 | 4.65 | 0.0035 | ||
| Region | 1 | 283 | 14.97 | 0.0001 | ||
| Region × treatment | 1 | 283 | 1.59 | 0.2081 | ||
| Region × native cover class | 3 | 283 | 1.47 | 0.224 | ||
| Region × native cover class × treatment | 3 | 283 | 0.41 | 0.7439 | ||
| Native shrub density | Winter | Treatment | 1 | 285 | 6.73 | 0.01 |
| Native cover class | 3 | 285 | 2.12 | 0.098 | ||
| Native cover class × treatment | 3 | 285 | 1.32 | 0.2669 | ||
| Region | 1 | 285 | 1.43 | 0.233 | ||
| Region × treatment | 1 | 285 | 0.79 | 0.3743 | ||
| Region × native cover class | 3 | 285 | 0.33 | 0.8033 | ||
| Region × native cover class × treatment | 3 | 285 | 2.26 | 0.0818 | ||
| Spring | Treatment | 1 | 283 | 10.86 | 0.0011 | |
| Native cover class | 3 | 283 | 0.36 | 0.7832 | ||
| Native cover class × treatment | 3 | 283 | 1.14 | 0.332 | ||
| Region | 1 | 283 | 4.5 | 0.0347 | ||
| Region × treatment | 1 | 283 | 0.12 | 0.7328 | ||
| Region × native cover class | 3 | 283 | 0.12 | 0.9472 | ||
| Region × native cover class × treatment | 3 | 283 | 0.77 | 0.5101 | ||
| Native forb density | Winter | Treatment | 1 | 285 | 0.47 | 0.493 |
| Native cover class | 3 | 285 | 0.44 | 0.727 | ||
| Native cover class × treatment | 3 | 285 | 1.09 | 0.3529 | ||
| Region | 1 | 285 | 4.69 | 0.0311 | ||
| Region × treatment | 1 | 285 | 0.14 | 0.7105 | ||
| Region × native cover class | 3 | 285 | 1.4 | 0.1583 | ||
| Region × native cover class × treatment | 3 | 285 | 0.6 | 0.6148 | ||
| Spring | Treatment | 1 | 283 | 0.01 | 0.9049 | |
| Native cover class | 3 | 283 | 1.12 | 0.3428 | ||
| Native cover class × treatment | 3 | 283 | 1.6 | 0.1889 | ||
| Region | 1 | 283 | 12.8 | 0.0004 | ||
| Region × treatment | 1 | 283 | 1.88 | 0.1718 | ||
| Region × native cover class | 3 | 283 | 2.91 | 0.0351 | ||
| Region × native cover class × treatment | 3 | 283 | 1.53 | 0.2074 | ||
| Non-native density | Winter | Treatment | 1 | 285 | 19.21 | <0.0001 |
| Native cover class | 3 | 285 | 2.45 | 0.0638 | ||
| Native cover class × treatment | 3 | 285 | 0.78 | 0.5057 | ||
| Region | 1 | 285 | 0.03 | 0.872 | ||
| Region × treatment | 1 | 285 | 1.09 | 0.2967 | ||
| Region × native cover class | 3 | 285 | 0.31 | 0.8198 | ||
| Region × native cover class × treatment | 3 | 285 | 0.19 | 0.903 | ||
| Spring | Treatment | 1 | 283 | 22.66 | <0.0001 | |
| Native cover class | 3 | 283 | 3.73 | 0.0118 | ||
| Native cover class × treatment | 3 | 283 | 1.64 | 0.1804 | ||
| Region | 1 | 283 | 0.05 | 0.816 | ||
| Region × treatment | 1 | 283 | 2.72 | 0.1002 | ||
| Region × native cover class | 3 | 283 | 2.32 | 0.0754 | ||
| Region × native cover class × treatment | 3 | 283 | 0.49 | 0.6888 |
| Response Variable | Season | Effect | Num DF | Den DF | F Value | p Value |
|---|---|---|---|---|---|---|
| Native species richness | Winter | Treatment | 1 | 285 | 0.01 | 0.9366 |
| Native cover class | 3 | 285 | 0.11 | 0.9551 | ||
| Native cover class × treatment | 3 | 285 | 2.21 | 0.0873 | ||
| Region | 1 | 285 | 3.18 | 0.0756 | ||
| Region × treatment | 1 | 285 | 1.48 | 0.2249 | ||
| Region × native cover class | 3 | 285 | 0.38 | 0.7703 | ||
| Region × native cover class × treatment | 3 | 285 | 0.49 | 0.6927 | ||
| Spring | Treatment | 1 | 283 | 0.38 | 0.54 | |
| Native cover class | 3 | 283 | 0.33 | 0.802 | ||
| Native cover class × treatment | 3 | 283 | 8.1 | <0.0001 | ||
| Region | 1 | 283 | 12.38 | 0.0005 | ||
| Region × treatment | 1 | 283 | 8.02 | 0.005 | ||
| Region × native cover class | 3 | 283 | 1.36 | 0.2564 | ||
| Region × native cover class × treatment | 3 | 283 | 0.18 | 0.9104 | ||
| Non-native species richness | Winter | Treatment | 1 | 285 | 4.85 | 0.0284 |
| Native cover class | 3 | 285 | 1.5 | 0.2148 | ||
| Native cover class × treatment | 3 | 285 | 0.51 | 0.6756 | ||
| Region | 1 | 285 | 5.2 | 0.0233 | ||
| Region × treatment | 1 | 285 | 1.17 | 0.2795 | ||
| Region × native cover class | 3 | 285 | 0.83 | 0.4757 | ||
| Region × native cover class × treatment | 3 | 285 | 0.55 | 0.6478 | ||
| Spring | Treatment | 1 | 283 | 2.91 | 0.0893 | |
| Native cover class | 3 | 283 | 2.21 | 0.0874 | ||
| Native cover class × treatment | 3 | 283 | 0.09 | 0.9638 | ||
| Region | 1 | 283 | 1.88 | 0.172 | ||
| Region × treatment | 1 | 283 | 0.37 | 0.5426 | ||
| Region × native cover class | 3 | 283 | 2.77 | 0.042 | ||
| Region × native cover class × treatment | 3 | 283 | 1.18 | 0.3186 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ta, P.M.; Griffoul, E.; Sorenson, Q.; Schmidt, K.T.; Ostmann, I.; Huxman, T.E.; Long, J.J.; Balazs, K.R.; Burger, J.C.; Lulow, M.; et al. Effects of Non-Native Annual Plant Removal on Native Species in Mediterranean-Climate Shrub Communities. Diversity 2024, 16, 115. https://doi.org/10.3390/d16020115
Ta PM, Griffoul E, Sorenson Q, Schmidt KT, Ostmann I, Huxman TE, Long JJ, Balazs KR, Burger JC, Lulow M, et al. Effects of Non-Native Annual Plant Removal on Native Species in Mediterranean-Climate Shrub Communities. Diversity. 2024; 16(2):115. https://doi.org/10.3390/d16020115
Chicago/Turabian StyleTa, Priscilla M., Emily Griffoul, Quinn Sorenson, Katharina T. Schmidt, Isaac Ostmann, Travis E. Huxman, Jennifer J. Long, Kathleen R. Balazs, Jutta C. Burger, Megan Lulow, and et al. 2024. "Effects of Non-Native Annual Plant Removal on Native Species in Mediterranean-Climate Shrub Communities" Diversity 16, no. 2: 115. https://doi.org/10.3390/d16020115





