Abstract
Phenotypic characterization of the variability present within bottle gourd has been limited to morpho-agronomic traits, and this evaluation is a prerequisite for a bottle gourd breeding program. Despite playing an important role in the phenotypic variation in plants, the root system has limited use in studies of morphological diversity. Thus, the objective of this study was to characterize the morphological diversity present in bottle gourd accessions of different countries based on roots and morpho-agronomic traits. The magnitude of morphological diversity and divergence among nineteen bottle gourd accessions that represent nine different countries of origin were evaluated with analysis of variance, principal component analysis, and an agglomerative hierarchical clustering (AHC) analysis. ANOVA for morpho-agronomic and root traits revealed significant morphological effects among the accessions, suggesting substantial differences among the bottle gourd accessions. The nineteen accessions were grouped into three clusters, and while these were not grouped according to the country of origin, clear differences among the roots and flowering traits were observed between the L. siceraria var. hispida with L. siceraria var. siceraria accessions, which were confirmed with the AHC analysis, revealing the divergence between these varieties and the opportunities for rootstock breeding programs.
1. Introduction
Bottle gourd (Lagenaria siceraria (Molina) Standl.), commonly known as calabash or white-flowered gourd, is a species belonging to the genus Lagenaria of the Cucurbitaceae family. It is a diploid (2n = 2x = 22), monoecious, and cross-pollinating self-fertile vegetable crop widely grown in rural communities in Africa, including South Africa [1] and Zimbabwe [2]. In Asia (i.e., India and China), the crop is an important commercial vegetable crop [3]. Lagenaria siceraria is the only cultivated species with significant economic value grown worldwide for a wide array of uses such as food, medicine, decoration, the crafting of household utensils, and even musical instruments [1,2,3,4,5]. Particularly in the realm of medicine, fresh bottle gourd fruit juice is harnessed as a medicine for various diseases such as flatulence, diabetes mellitus, hypertension, and liver diseases and is used as a diuretic [6]. Additionally, the seeds of this crop offer a rich source of essential amino acids and oil, which is why certain varieties of bottle gourd are specifically cultivated for their seeds [1,7,8]. Beyond its medicinal and culinary applications, bottle gourd also serves as a rootstock in watermelon breeding to control soil-borne diseases and the management of low soil temperature stress [9,10,11]. Notably, recent advancements have even led to successful crosses between watermelon and bottle gourd as pollen parents, resulting in the development of seedless watermelon varieties [12].
Bottle gourd is one of the oldest crops cultivated by humans, with at least 12,000 years since its first collection and use [13]. Archeological evidence suggests that bottle gourd originated in Africa and subsequently diffused to other parts of the world through trans-oceanic drift or human transportation [2,13]. In this sense, the discovery of wild indigenous bottle gourd species in Zimbabwe [2] and the knowledge that American bottle gourds are most closely related to African gourds [14] support the hypothesis that Africa could be the center of origin for bottle gourd. However, based on seed morphology and DNA analysis, Erickson et al. [15] reported that American bottle gourd germplasm showed a genetic lineage closely linked to bottle gourds originating from Eastern Asia, which suggests that bottle gourd was likely introduced to the American continent from Asia. Hence, the evolutionary origins of bottle gourd remain unresolved and are still subject to debate.
Bottle gourd has been widely grown worldwide due to its rich genetic and morphological diversity, which allows it to adapt to a wide variety of growing conditions [16]. Particularly, the wide genetic diversity of bottle gourd has led to notable variations in the size, length, width, and shape of the fruit, shell thickness, seed morphology, root system architecture, and other morphological characteristics [17,18,19]. These characteristics are economically important traits for the development of bottle gourd cultivars and play a key role in domestic and agro-industrial applications of this crop [1]. This diversity arose from extensive selection processes carried out by farmers over generations, often influenced by sociocultural preferences, cultural practices, and the local environment. The progress achieved through such selection relies on the availability of genotypes possessing favorable alleles for desired traits, underlining the critical role of conserving and studying the genetic resources and available genetic diversity of bottle gourd [1].
Traditionally, diversity has been estimated by evaluating variations in phenotypic or qualitative traits such as fruit and seed characteristics (type, color, size) and plant type along with quantitative agronomic traits such as flowering initiation or maturity timing [20,21,22]. Key agronomic traits that can be targeted in bottle gourd improvement include high male and female flowering capacity, profuse branching (i.e., high number of primary and secondary branches), and high fruit load. These attributes support the high-yield potential of the crop. Knowledge of phenotypic variations and their relationships with genotypes is of great importance for breeders seeking to formulate effective breeding strategies and develop highly adaptable and productive cultivars. The study of the variation in morphological, phenological, and agronomic traits among landraces from various locations around the world offers insights into the genetic diversity of bottle gourd and helps to develop new varieties with enhanced adaptation to biotic and abiotic stressors as well as greater yield potential for diverse production environments [1,23].
Previous research has extensively explored the genetic diversity in bottle gourd genotypes based on quantitative and qualitative phenotypic traits. These traits have emerged as valuable tools for characterizing bottle gourd genetic resources [1,17,19,22]. In this sense, Yildiz et al. [20] and Yetisir et al. [18] classified bottle gourds into distinct genetic sub-populations defined primarily by the morphological diversity of their traits, such as fruit size and shape. Similarly, Mashilo et al. [19] and Koffi et al. [24] showed that traits associated with the fruit, seed, and flower account for approximately 50% of the genetic variability within bottle gourd. These findings suggest the utility of phenotypic traits as useful attributes for breeding and genetic analysis of bottle gourd germplasm. To date, the phenotypic characterization of the variability present within bottle gourd has been limited to fruit, seed, and flower traits and to specific geographical regions. However, the genetic variation of this crop remains largely unexplained, so the inclusion of other characteristics would help to improve the explanation of the genetic variation of bottle gourd. Particularly, the root system, despite playing an important role in nutrient acquisition, anchorage, and water transport [25], has not been considered in studies of genetic variability due to the difficulty of studying the roots because they are hidden in the soil matrix [26]. The traits associated with the root system present a high potential for physiological and morphological variation [27], which highlights its potential use in genetic diversity studies. Therefore, the objective of this study was to characterize the morphological diversity and divergence present in bottle gourd accessions with different countries based on phenotypic traits. This study represents the first characterization of the morphological diversity of L. siceraria based on the collection of germplasm from four continents (African, American, European, and Asiatic) and the use of root system traits.
2. Materials and Methods
2.1. Plant Materials
The plant material used in this study consisted of nineteen landrace accessions of bottle gourd representing nine different countries of origin (Supplementary Table S1). The accessions were sourced from the Limpopo Department of Agriculture and Rural Development (Towoomba Research Station) of South Africa, the Genetic Resource Center of Japan (specifically from the National Agriculture and Food Research Organization; NARO), and two regions of Chile (North and South). Details about the bottle gourd accessions are shown in Supplementary Table S1.
2.2. Experimental Design
The nineteen accessions were sown in the field and grown under normal irrigated conditions from December 2022 to May 2023 in a complete randomized design with three replications on a total area of 20 m (mt) × 19.8 mt. Plants were transplanted when they reached the two-leaf stage into an open field assay. The plots were 10 m long with eight plants in each experimental unit, the distance between the plants was 1 mt within the lane and 3 mt within the row in order to avoid competition among neighboring plants. The trial was conducted in San Fernando Province (34°36′42.2″ S 70°59′26.1″ W), Chile, with an average temperature of 18.62 °C, a relative humidity of 50.6%, and a solar radiation level of 18.27 Mj/m2. The incorporation of nutrients was performed by fertigation, following the recommendation described by Crawford and Abarca [28], with minor modifications as mentioned by Morales et al. [11]. Three plants from each plot or replicate were sampled at the center of each experimental unit for quantitative scoring. Plants were evaluated for ten morpho-agronomic traits at various stages of development.
2.3. Evaluated Traits
The morpho-agronomic traits consist of 5 radical and 5 aerial traits. The number of leaves (NL), plant height (PH), number of primary branches (NPrB), and days to first female and male flowering (DFF, DFM) corresponded to aerial traits. A measuring tape was used to measure the PH from the stem base to the leaf apex. Following the same guides that were used to measure the length, the number of branches (NPrB) and number of leaves (NL) were also counted. DFF and DFM were measured in each sample as the number of days from sowing until the appearance of the first female and male flower, respectively. On the other hand, the root length (RL), root surface area (SA), mean root diameter (AvD), number of tips (NT), and root volume (RV) were determined using WinRhizo 2019a software (Regent Instruments Inc., Quebec City, QC, Canada).
For root evaluation, the plants were sampled at the end of the growth cycle using the shovelomics protocol [29]. The roots were cleaned by washing them over a sieve. For root traits characterization, a prototype for the automated root traits phenotyping using an array of cameras was used. The methodology involves the acquisition of a set of images of the root and the generation of a 2D geometrical root traits calculation using the WinRhizo software.
2.4. Data Analysis
An analysis of variance (ANOVA) was performed after testing the homogeneity of variances and the normality of the residuals using the Bartlett and Shapiro–Wilk tests, respectively. A one-way ANOVA was performed for morpho-agronomic and root traits.
The mean values of morpho-agronomic and root traits were used to compute Pearson’s linear correlation coefficients using the “chart. Correlation” function of the PerformanceAnalytics package [30] in R 4.0.5 software [31].
A principal component analysis (PCA) based on the correlation matrix was performed using the “princomp” function in R. The PCA biplot was then generated using the “ggbiplot” function of the ggbiplot2 package in R-software [32].
To measure the similarity and the morphological divergence among the bottle gourd accessions, an agglomerative hierarchical clustering (AHC) analysis was conducted according to Ward’s method using the squared Euclidean distance. The groups were established using a horizontal cut-off value of the distance means for each group formed according to Mojena [33]. The analysis was performed using the R 4.0.5 software with the vegan package [34].
3. Results
3.1. Morpho-Agronomical Variability among Bottle Gourd Accessions
Analysis of variance showed significant morphological differences (p < 0.01) for most of the root and morpho-agronomic traits among the nineteen bottle gourd accessions, except for days to first female and male flowering (DFF and DFM), which did not show statistical differences (Table 1). These significant differences indicate the existence of high morphological variation among the evaluated bottle gourd accessions (Table 2).

Table 1.
Significance of the source of variation from the Type III analysis of genotype effect on root and plant traits of 19 bottle gourd accessions of diverse geographical origins under field conditions in Chile.

Table 2.
Average performance and standard error of morpho-agronomic and root traits of each bottle gourd accession.
3.2. Correlation among Root and Morpho-Agronomic Traits
The Pearson correlation coefficients among morpho-agronomic and root traits are presented in Figure 1. Morpho-agronomic and root traits did not show a significant correlation. However, root traits showed a high positive and significant (p < 0.001) correlation between RL and SA (0.79), RL and NT (0.91), RV and SA (0.91), and NT and SA (0.71). Similarly, RV was significantly and positively associated with AvD (0.63).
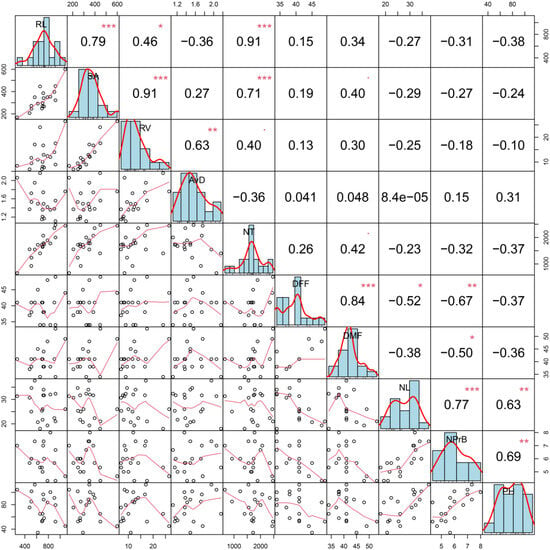
Figure 1.
Plot representing phenotypic distribution and correlation for root length (RL), root surface area (SA), mean root diameter (AvD), root volume (RV), number of tips (NT), days to first female and male flowering (DFF, DFM), number of leaves (NL), number of primary branches (NPrB), and plant height (PH). The diagonal line of the plot shows the histograms and the distribution of the observed phenotype values. The lower off-diagonal is the scatterplot between the traits, whereas the upper off-diagonal represents the correlation value between traits. *, **, and *** denote the level of significance at 5%, 1%, and 0.1%, respectively.
For morpho-agronomic traits, a high positive and significant (p < 0.001) correlation was observed between DFF and DFM (0.84) and between NL and NPrB (0.75). Also, a moderate positive and significant (p < 0.01) correlation was observed between PH and both NL (0.61) and NPrB (0.62). On the other hand, a negative and significant correlation was observed between NPrB and DFF (−0.67; p < 0.01) and between NPrB and DFM (−0.53; p < 0.05).
3.3. Principal Component Analysis
A principal component analysis was carried out to discover the most contributing morpho-agronomic and root traits for the differentiation of the bottle gourd accessions. Principal components explained 65% of the cumulative variance observed, with 44% at the first and 21% at the second axes (Figure 2).
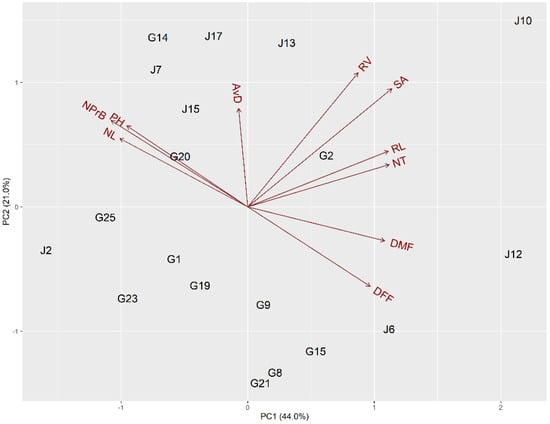
Figure 2.
Principal components analysis (PCA) biplot for bottle gourd accessions based on morpho-agronomic traits (DFF, days to first female flowering; DMF, days to first male flowering; NL, number of leaves; NPrB, number of primary branches; PH, plant height) and root traits (RL, root length; AvD, mean root diameter; NT, number of tips; RV, root volume; SA, surface area).
The parameters that contributed to positive loading of the first principal component (PC1) were RL, SA, RV, NT, DMF, and DFF. For the second component (PC2), the parameters were all the root traits, PH, NL, and NPrB. In contrast, AvD, PH, NL, and NPrB contributed with negative loading to the first component, while DMF and DFF contributed with negative loading to the second component (Figure 2).
The accessions G2, G14, G20, J7, J10, J13, J15, and J17 were grouped with high values of root traits and PH, NL, and NPrB. In contrast, the accessions G1, G8, G9, G15, G19, G21, G23, G25, J2, J6, and J12 were grouped with low values of flowering traits (DFF and DFM; Figure 2).
The agglomerative hierarchical clustering (AHC) analysis for the means of morpho-agronomic and root traits classified the nineteen bottle gourd accessions into three groups (Figure 3). The first cluster was the most differentiated group and only included L. siceraria var. hispida accessions with origins from Switzerland (J6), Taiwan (J10), and Thailand (J12). The second group was admixed because accessions of different origins and varieties were grouped according to all the root and some morpho-agronomic traits, similar to the PCA results. In the same manner, as PCA results, the third group was associated with flowering traits, including most of the accessions of L. siceraria var. siceraria of South African origin (G1, G8, G9, G15, G21, G23, G25, and J2) (Figure 3; Supplementary Table S1).

Figure 3.
Dendrogram of agglomerative hierarchical clustering analysis based on ten morpho-agronomic and root traits using Ward method among the nineteen bottle gourd accessions of diverse origins. The blue dotted line indicates the cutoff for group formation according to Mojena [33].
4. Discussion
Bottle gourd is a species widely distributed around the world, which is cultivated in most of the regions using genetically unimproved varieties that are phenotypically and genotypically well-adapted for diverse purposes. However, according to a revision by Mkhize et al. [35], there is generally limited data available regarding diverse germplasm of Lagenaria species for rootstock breeding purposes. Information on the nature and degree of genetic and morphological divergence would help plant breeders select suitable parents for hybridization [3,18,19]. Based on this assumption, the present study determined the morphological variation and divergence between nineteen bottle gourd accessions collected from nine different countries using quantitative traits, and according to our knowledge, this is the first study that used morpho-agronomic and root traits to determine the magnitude of variability and classify a collection of bottle gourd genotypes into breeding clusters on the basis of these quantitative parameters.
Analysis of variance for morpho-agronomic and roots traits revealed significant morphological effects among the accessions that represent different geographical origins, suggesting substantial differences among the bottle gourd accessions (Table 1). Previous studies reported that bottle gourd is a morphologically diverse crop with variations in different morpho-agronomic traits, which can be used for variety and rootstock design [3,36]. There is high genetic and morphological variation for some qualitative fruit traits, including fruit shape and color; seed size, color, and shape [2,3,19]; and quantitative traits, such as plant architecture, flowering date, fruit, and seed traits [17]. These reports demonstrate that bottle gourds possess extensive phenotypic and genetic variation that can be exploited for breeding and cultivar development.
On the other hand, it is widely known that bottle gourd serves as a rootstock for watermelon and other cucurbits [18]. In that context, previous studies confirmed that root system architecture traits are used for the selection and identification of high-performing rootstocks [37,38]. However, there is generally limited data available regarding root system architecture (RSA) traits aiming to select and identify high-performing Lagenaria rootstocks [16,35,39]. In the present study, the high RSA variation observed for root traits revealed a significant genotypic effect, which can be explored for selecting desirable genotypes for rootstock development.
According to the correlation analysis, the morpho-agronomic traits did not show a significant correlation with the root traits. However, these traits explained 65% of the cumulative variance observed, and the nineteen bottle gourd accessions were classified into three groups, which did not necessarily coincide with the country of origin of the accessions. Accessions from the same country of origin were grouped into separate clusters, indicating wide morphological diversity among this bottle gourd collection. This result agrees with previous studies conducted on bottle gourds, which reported that the clustering of different landraces was independent of geographical location [3,18,19,21,40]. In fact, in a previous study, we reported two genetically differentiated groups comprising South African accessions and an admixed group with accessions of Asian and Chilean origin [16].
In the present study, interestingly, one cluster only grouped L. siceraria var. hispida accessions (J6, J10, and J12), which were associated with high values of root and flowering traits (Supplementary Table S2). Particularly, the root traits of this cluster were between 2% and 87% higher in comparison with the other clusters, while flowering traits were between 16% and 30% higher. Additionally, according to the agglomerative hierarchical clustering analysis, this cluster was the most differentiated group, probably because it corresponds only with the hispida variety accessions. In this sense, the accessions of L. siceraria var. hispida showed higher values in roots (between 6% and 25%) and flowering (between 2% and 8%) traits when compared with L. siceraria var. siceraria accessions, according to the mean values but without statistical analysis (Supplementary Table S3). Similarly, a second cluster (G21, J2, G1, G15, G23, G25, G8, and G9), which included most of the accessions of L. siceraria var. siceraria (75%) with South African and Chilean origin was associated with low values of root and flowering traits, confirming the differentiation with var. hispida based on these traits (Supplementary Tables S2 and S3). Likewise, the third cluster (G19, J13, J15, J7, G14, G2, G20, and J17) grouped accessions of mixed origin (South Africa, India, Japan, and Bangladesh) and variety (50% var. siceraria and 50% var. hispida). In this way, this cluster obtained average values of roots and flowering. It should be noted that there is little information about the morphological relationship between L. siceraria var. hispida and L. siceraria var. siceraria, which reveals the great opportunity to consider L. siceraria var. hispida in breeding purposes, mainly thinking about root and morpho-agronomic traits.
5. Conclusions
Wide variation exists in morpho-agronomic and root traits of the studied Lagenaria siceraria accessions. The accessions were not grouped according to the country of origin; however, clear differences among roots and flowering traits were observed between the L. siceraria var. hispida and L. siceraria var. siceraria accessions, which were confirmed using the agglomerative hierarchical clustering analysis, revealing the divergence between these varieties and the opportunities for rootstock breeding programs.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/d16030136/s1, Supplementary Table S1: List of the nineteen bottle gourd accessions used in the study with description of collection sites: origin, region, and location. Supplementary Table S2: Averages for each cluster for root and morpho-agronomic traits. Supplementary Table S3: Averages for each variety (hispida and siceraria) for root and morpho-agronomic traits.
Author Contributions
Conceptualization, R.C.-S.; methodology, C.M. and R.C.-S.; software, C.M. and R.C.-S.; formal analysis, C.M. and R.C.-S.; investigation, S.F.-C., G.C., C.M. and R.C.-S.; resources R.C.-S.; data curation, S.F.-C. and G.C.; writing—original draft preparation, C.M. and R.C.-S.; writing—review and editing, C.M. and R.C.-S.; supervision, R.C.-S.; project administration, R.C.-S.; funding acquisition, R.C.-S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Universidad de O’Higgins through “Proyecto Puente FP16825704”.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in this published paper and Supplementary Materials.
Acknowledgments
R.C.-S. thanks the National Agricultural and Food Research Organization (NARO) and the Limpopo Department of Agriculture and Rural Development of South Africa for providing the plant material.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mashilo, J.; Shimelis, H.; Odindo, A.; Amelework, B. Genetic differentiation of bottle gourd [Lagenaria siceraria (Molina) Standl.] landraces assessed by fruit qualitative traits and simple sequence repeat markers. Sci. Hortic. 2017, 216, 1–11. [Google Scholar] [CrossRef]
- Decker-Walters, D.; Wilkins-Ellert, M. Discovery and genetic assessment of wild bottle gourd from Zimbabwe. Econ. Bot. 2004, 58, 501–508. [Google Scholar] [CrossRef]
- Mahapatra, S.; Sureja, A.K.; Behera, T.K.; Verma, M. Assessment of genetic diversity of ninety-one bottle gourd [Lagenaria siceraria (Mol.) Standl.] genotypes from fourteen different agro-climatic zones of India using agro-morphological traits and SSR markers. Mol. Biol. Rep. 2022, 49, 6367–6383. [Google Scholar] [CrossRef] [PubMed]
- Ellsworth, P.Z.; Feldman, M.J.; Baxter, I.; Cousins, A.B. A genetic link between leaf carbon isotope composition and whole-plant water use efficiency in the C4 grass Setaria. Plant J. 2020, 102, 1234–1248. [Google Scholar] [CrossRef] [PubMed]
- Payus, C.; Huey, L.A.; Adnan, F.; Rimba, A.B.; Mohan, G.; Chapagain, S.K.; Roder, G.; Gasparatos, A.; Fukushi, K. Impact of Extreme Drought Climate on Water Security in North Borneo: Case Study of Sabah. Water 2020, 12, 1135. [Google Scholar] [CrossRef]
- Ghule, B.V.; Ghante, M.H.; Yeole, P.G.; Saoji, A.N. Diuretic activity of Lagenaria siceraria fruit extracts in rats. Indian J. Pharm. Sci. 2007, 69, 817–819. [Google Scholar] [CrossRef]
- Chung, H.; Choi, Y.; Shin, Y.; Youn, S. Chemical composition, quality evaluation and characteristics of immature fruits of Korean native bottle gourd (Lagenaria siceraria). J. Hortic. Sci. 2000, 41, 319–328. [Google Scholar]
- Ogunbusola, M.E.; Fagbemi, T.N.; Osundahunsi, O.F. Amino acid composition of Lagenaria siceraria seed flour and protein fractions. J. Food Sci. Technol. 2010, 47, 656–661. [Google Scholar] [CrossRef]
- Aslam, W.; Noor, R.S.; Hussain, F.; Ameen, M.; Ullah, S.; Chen, H. Evaluating Morphological Growth, Yield, and Postharvest Fruit Quality of Cucumber (Cucumis sativus L.) Grafted on Cucurbitaceous Rootstocks. Agriculture 2020, 10, 101. [Google Scholar] [CrossRef]
- Ulas, A.; Doganci, E.; Ulas, F.; Yetisir, H. Root-growth characteristics contributing to genotypic variation in nitrogen efficiency of bottle gourd and rootstock potential for watermelon. Plants 2019, 8, 77. [Google Scholar] [CrossRef]
- Morales, C.; Riveros-Burgos, C.; Espinoza Seguel, F.; Maldonado, C.; Mashilo, J.; Pinto, C.; Contreras-Soto, R.I. Rootstocks Comparison in Grafted Watermelon under Water Deficit: Effects on the Fruit Quality and Yield. Plants 2023, 12, 509. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, K.; Kami, D.; Muro, T. Induction of parthenocarpic fruit set in watermelon by pollination with bottle gourd (Lagenaria siceraria (Molina) Standl.) pollen. Sci. Hortic. 2014, 171, 1–5. [Google Scholar] [CrossRef]
- Whitaker, T.W. Endemism and pre-columbian migration of the bottle gourd, Lagenaria siceraria (Mol.) Standl. In Man Across the Sea; Kelley, J.C., Pennington, C.W., Eds.; University of Texas Press: Austin, TX, USA, 1971; pp. 320–337. [Google Scholar]
- Kistler, L.; Montenegro, A.; Smith, B.D.; Gifford, J.A.; Greene, R.E.; Newsom, L.A.; Shapiro, B. Transoceanic drift and the domestication of African bottle gourds in the Americas. Proc. Natl. Acad. Sci. USA 2014, 111, 2937–2941. [Google Scholar] [CrossRef] [PubMed]
- Erickson, D.L.; Smith, B.D.; Clarke, A.C.; Sandweiss, D.H.; Tuross, N. An Asian origin for a 10,000-year-old domesticated plant in the Americas. Proc. Natl. Acad. Sci. USA 2005, 102, 18315–18320. [Google Scholar] [CrossRef]
- Contreras-Soto, R.; Salvatierra, A.; Maldonado, C.; Mashilo, J. The genetic diversity and population structure of different geographical populations of bottle gourd (Lagenaria siceraria) accessions based on genotyping-by-sequencing. Agronomy 2021, 11, 1677. [Google Scholar] [CrossRef]
- Morimoto, Y.; Maundu, P.; Fujimaki, H.; Morishima, H. Diversity of landraces of the white-flowered gourd (Lagenaria siceraria) and its wild relatives in Kenya: Fruit and seed morphology. Gen. Res. Crop Evol. 2005, 52, 737–747. [Google Scholar] [CrossRef]
- Yetişir, H.; Şakar, M.; Serçe, S. Collection and morphological characterization of Lagenaria siceraria germplasm from the Mediterranean region of Turkey. Genet. Resour. Crop. Evol. 2008, 55, 1257–1266. [Google Scholar] [CrossRef]
- Mashilo, J.; Shimelis, H.; Odindo, A. Genetic diversity of bottle gourd (Lagenaria siceraria (Molina) Standl.) landraces of South Africa assessed by morphological traits and simple sequence repeat markers. S. Afr. J. Plant Soil 2015, 33, 113–124. [Google Scholar] [CrossRef]
- Yildiz, M.; Cuevas, H.E.; Sensoy, S.; Erdinc, C.; Baloch, F.S. Transferability of Cucurbita SSR markers for genetic diversity assessment of Turkish bottle gourd (Lagenaria siceraria) genetic resources. Biochem. Syst. Ecol. 2015, 59, 45–53. [Google Scholar] [CrossRef]
- Gürcan, K.; Say, A.; Yetişir, H.; Denli, H. A study of genetic diversity in bottle gourd [Lagenaria siceraria (Molina) Standl.] population, and implication for the historical origins on bottle gourds in Turkey. Genet. Resour. Crop. Evol. 2015, 62, 321–333. [Google Scholar] [CrossRef]
- Xu, P.; Xu, S.; Wu, X.; Tao, Y.; Wang, B.; Wang, S.; Li, G. Population genomic analyses from low-coverage RAD-Seq data: A case study on the non-model cucurbit bottle gourd. Plant J. 2014, 77, 430–442. [Google Scholar] [CrossRef] [PubMed]
- Ngompe-Deffo, T.; Kouam, E.B.; Beyegue-Djonko, H.; Anoumaa, M. Evaluation of the genetic variation of cowpea landraces (Vigna unguiculata) from Western Cameroon using qualitative traits. Not. Sci. Biol. 2017, 9, 508–514. [Google Scholar] [CrossRef]
- Koffi, K.K.; Anzera, G.K.; Malice, M.; Dey, Y.; Bertin, P.; Baudoin, J.P.; Zoro Bi, I.A. Morphological and allozyme variation in a collection of Lagenaria siceraria (Molina) Standl. from Cote D’Ivoire. Biotechnol. Agron. 2009, 13, 257–270. [Google Scholar]
- Contreras-Soto, R.I.; Zacarias Rafael, D.; Domingos Moiana, L.; Maldonado, C.; Mora-Poblete, F. Variation in root-related traits is associated with water uptake in Lagenaria siceraria genotypes under water-deficit conditions. Front. Plant Sci. 2022, 49, 9453–9463. [Google Scholar] [CrossRef] [PubMed]
- Erktan, A.; McCormack, M.L.; Roumet, C. Frontiers in root ecology: Recent advances and future challenges. Plant Soil 2018, 424, 1–9. [Google Scholar] [CrossRef]
- Hong, J.; Ma, X.; Yan, Y.; Zhang, X.; Wang, X. Which root traits determine nitrogen uptake by alpine plant species on the Tibetan Plateau? Plant Soil 2018, 424, 63–72. [Google Scholar] [CrossRef]
- Crawford, L.H.; Abarca, P. Manual de Manejo Agronómico Para Cultivo de Sandia (Citrullus lanatus (Thunb.) Matsum. et Nakai.); Instituto de Investigaciones Agropecuarias (INIA): Rengo, Chile, 2017. [Google Scholar]
- Trachsel, S.; Kaeppler, S.M.; Brown, K.M.; Lynch, J.P. Shovelomics: High throughput phenotyping of maize (Zea mays L.) root architecture in the field. Plant Soil 2011, 341, 75–87. [Google Scholar] [CrossRef]
- Peterson, B.G.; Carl, P.; Boudt, K.; Bennett, R.; Ulrich, J.; Zivot, E.; Cornilly, D.; Hung, E.; Lestel, M.; Balkissoon, K. Package ‘Performanceanalytics’; R Team Cooperation: Vienna, Austria, 2018; Volume 3, pp. 13–14. [Google Scholar]
- R Core Team. R Core Team: A Language and Environment for Statistical Computing; Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Vu, V.Q. ggbiplot: A ggplot2 Based Biplot. R Package, Version 0.55. 2011. Available online: https://github.com/vqv/ggbiplot (accessed on 7 July 2023).
- Mojena, R. Hierarchical grouping methods and stopping rules: An evaluation. Comput. J. 1977, 20, 359–363. [Google Scholar] [CrossRef]
- Oksanen, J. Vegan: Ecological diversity. R Proj. 2013, 1, 12. [Google Scholar]
- Mkhize, P.; Mashilo, J.; Shimelis, H. Progress on genetic improvement and analysis of bottle gourd [Lagenaria siceraria (Molina) Standl.] for agronomic traits, nutrient compositions, and stress tolerance: A review. Front. Sustain. Food Syst. 2021, 5, 683635. [Google Scholar] [CrossRef]
- Mashilo, J.; Shimelis, H.; Ngwepe, R.M. Genetic resources of bottle gourd (Lagenaria siceraria (Molina) Standl.] and citron watermelon (Citrullus lanatus var. citroides (L.H. Bailey) Mansf. ex Greb.): Implications for genetic improvement, product development and commercialization: A review. S. Afr. J. Bot. 2021, 145, 28–47. [Google Scholar] [CrossRef]
- Bertucci, M.B.; Suchoff, D.; Jennings, K.; Monks, D.W.; Gunter, C.C.; Schultheis, J.R.; Louws, F. Comparison of Root System Morphology of Cucurbit Rootstocks for Use in Watermelon Grafting. HortTechnology 2018, 28, 625–636. [Google Scholar] [CrossRef]
- Katuuramu, D.N.; Wechter, W.P.; Washington, M.L.; Horry, M.; Cutulle, M.A.; Jarret, R.L.; Levi, A. Phenotypic diversity for root traits and identification of superior germplasm for root breeding in watermelon. HortScience 2020, 5, 1272–1279. [Google Scholar] [CrossRef]
- Mashilo, J.; Shimelis, H.; Contreras-Soto, R.I.; Ngwepe, R.M. A meta-analysis on rootstock-induced effects in grafted watermelon (Citrullus lanatus var. lanatus). Sci. Hortic. 2023, 319, 112158. [Google Scholar] [CrossRef]
- Sarao, N.K.; Pathak, M.; Kaur, N.; Kaur, K. Microsatellite-based DNA fingerprinting and genetic diversity of bottle gourd genotypes. Plant Genet. Resour. 2014, 12, 156–159. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).