Change in the Ecological Stoichiometry of Carex thunbergii in Response to Seasonal Dynamics and Environmental Factors in Shengjin Lake, China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Experimental Communities and Sampling
2.3. Laboratory Analysis
2.4. Data Analysis
3. Results
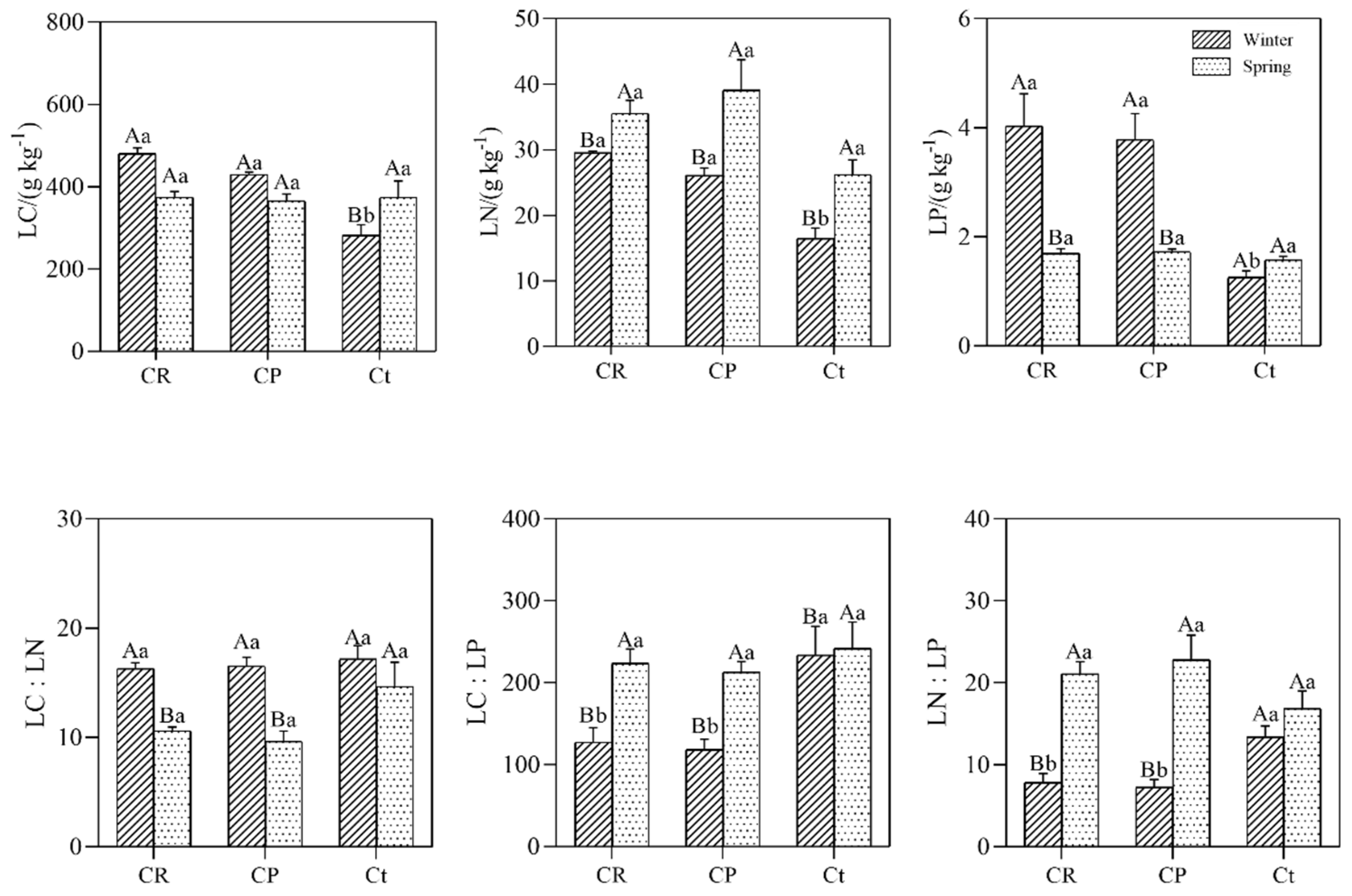
3.1. Leaf Nutrients Stoichiometry and Seasonal Variation Characteristics among Different Plant Communities
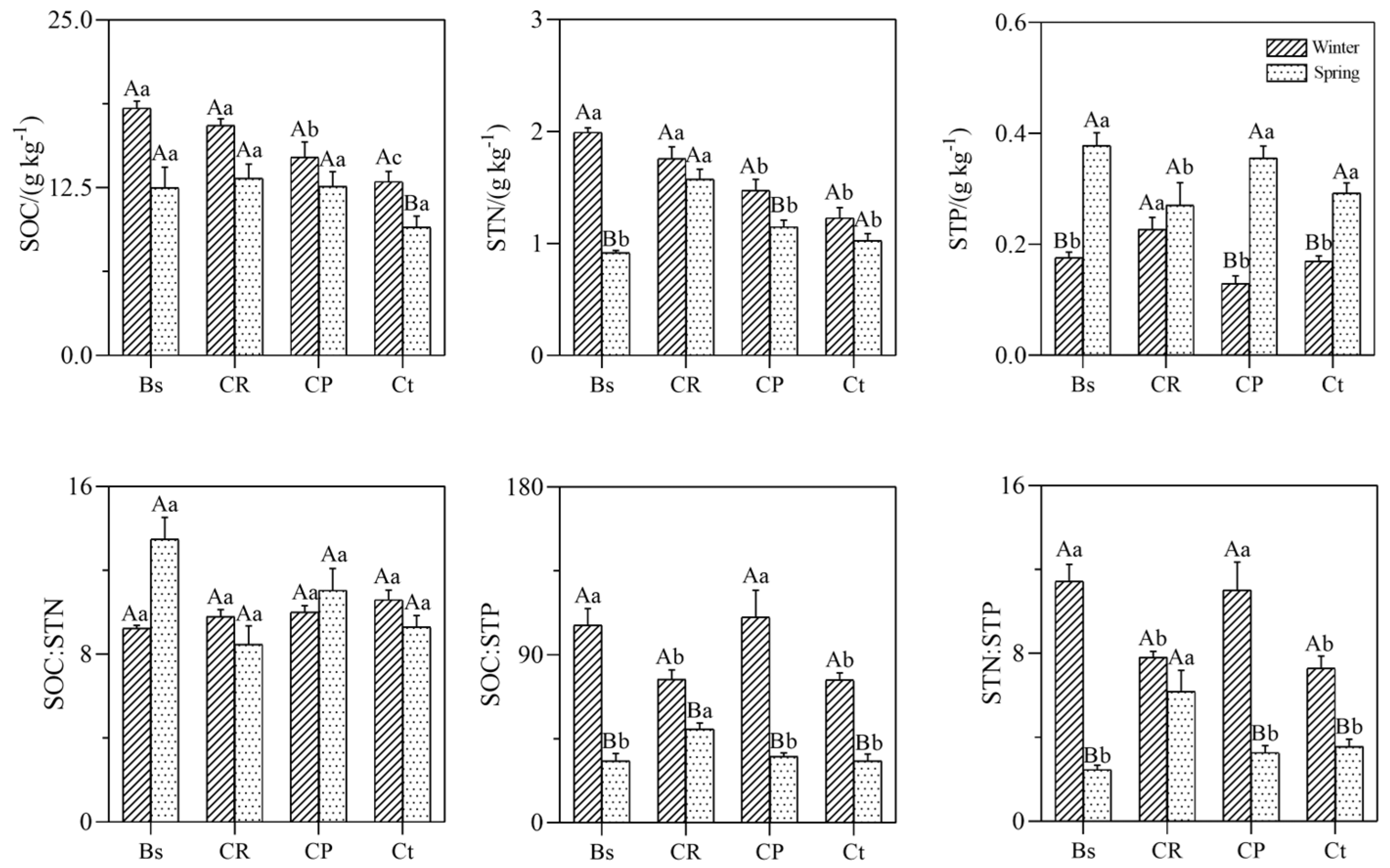
3.2. Soil Stoichiometry and Seasonal Variation Characteristics among Different Plant Communities
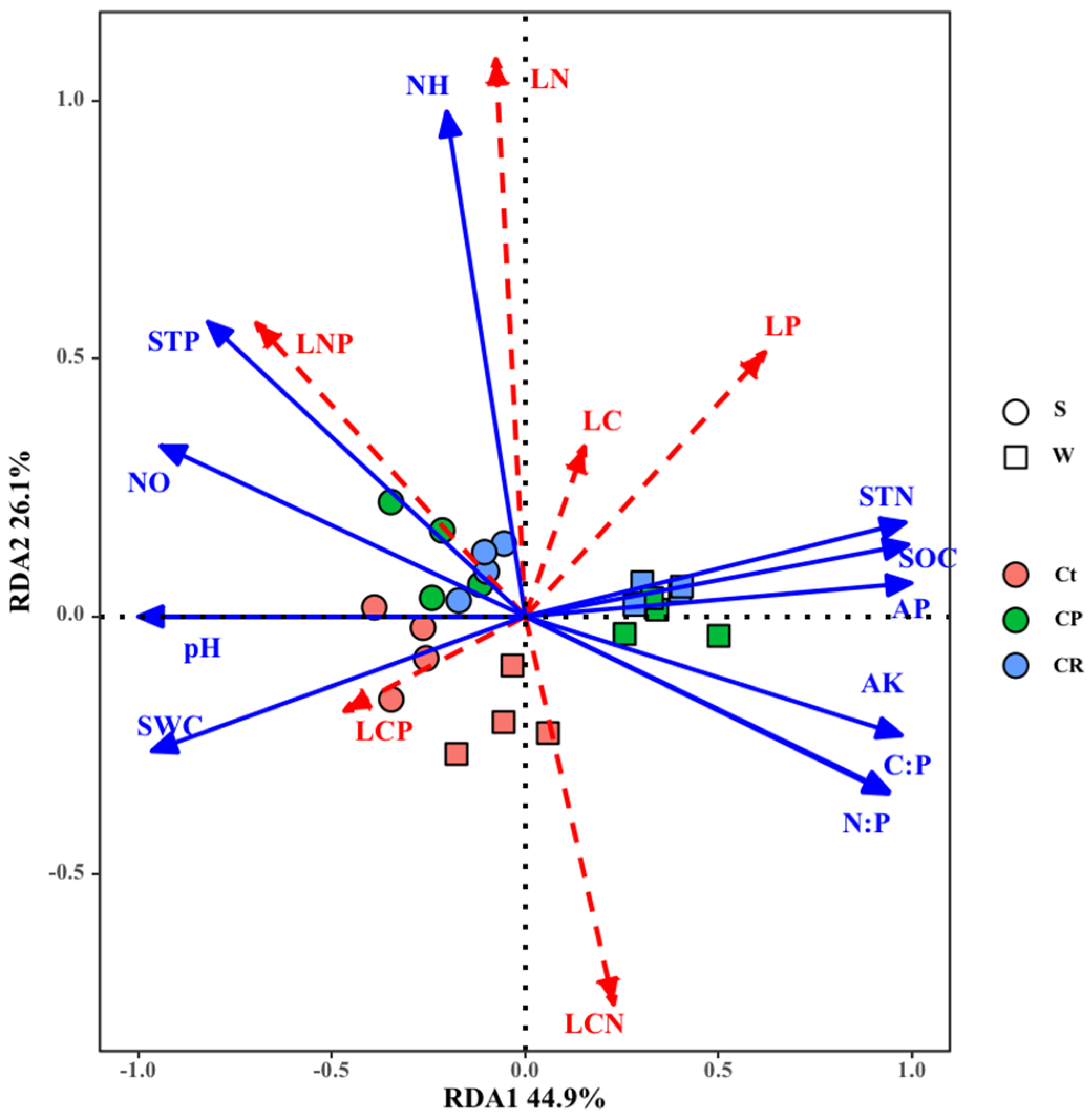
3.3. Correlations between Soil Physicochemical Properties
3.4. Correlations between Soil Physicochemical Properties and Leaf Stoichiometry
4. Discussion
4.1. Patterns and Seasonal Variation in Leaf C, N, and P Stoichiometry
4.2. Soil Stoichiometry Patterns and the Relation with Soil Physicochemical Properties
4.3. Correlations between Soil Stoichiometry and Plant Stoichiometry
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Demars, B.O.L.; Edwards, A.C. Tissue nutrient concentrations in freshwater aquatic macrophytes: High inter-taxon differences and low phenotypic response to nutrient supply. Freshw. Biol. 2007, 52, 2073–2086. [Google Scholar] [CrossRef]
- Yu, M.-F.; Tao, Y.; Liu, W.; Xing, W.; Liu, G.; Wang, L.; Ma, L. C, N, and P stoichiometry and their interaction with different plant communities and soils in subtropical riparian wetlands. Environ. Sci. Pollut. Res. 2019, 27, 1024–1034. [Google Scholar] [CrossRef]
- Tockner, K.; Stanford, J.A. Riverine flood plains: Present state and future trends. Environ. Conserv. 2002, 29, 308–330. [Google Scholar] [CrossRef]
- Strayer, D.L.; Findlay, S.E.G. Ecology of freshwater shore zones. Aquat. Sci. 2010, 72, 127–163. [Google Scholar] [CrossRef]
- Bedford, B.L.; Walbridge, M.R.; Aldous, A. Patterns in Nutrient Availability and Plant Diversity of Temperate North American Wetlands. Ecology 1999, 80, 2151–2169. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, L.; Tang, Y.; Li, Y.; Chen, J.; Xi, X.; Zhang, Y.; Fu, X.; Wu, J.; Sun, Y. Variability in soil microbial community and activity between coastal and riparian wetlands in the Yangtze River estuary—Potential impacts on carbon sequestration. Soil Biol. Biochem. 2014, 70, 221–228. [Google Scholar] [CrossRef]
- Bernot, R.J.; Poulin, R. Ecological Stoichiometry for Parasitologists. Trends Parasitol. 2018, 34, 928–933. [Google Scholar] [CrossRef]
- Sardans, J.; Rivas−Ubach, A.; Peñuelas, J. The elemental stoichiometry of aquatic and terrestrial ecosystems and its relationships with organismic lifestyle and ecosystem structure and function: A review and perspectives. Biogeochemistry 2011, 111, 1–39. [Google Scholar] [CrossRef]
- Koerselman, W.; Meuleman, A.F.M. The Vegetation N:P Ratio: A New Tool to Detect the Nature of Nutrient Limitation. J. Appl. Ecol. 1996, 33, 1441–1450. [Google Scholar] [CrossRef]
- Gusewell, S. N:P ratios in terrestrial plants: Variation and functional significance. New Phytol 2004, 164, 243–266. [Google Scholar] [CrossRef]
- Venterink, H.O.; Güsewell, S. Competitive interactions between two meadow grasses under nitrogen and phosphorus limitation. Funct. Ecol. 2010, 24, 877–886. [Google Scholar] [CrossRef]
- Shen, R.; Lan, Z.; Chen, Y.; Leng, F.; Jin, B.; Fang, C.; Chen, J. The effects of flooding regimes and soil nutrients on lakeshore plant diversity in a pristine lake and a human managed lake in subtropical China. J. Freshw. Ecol. 2019, 34, 757–769. [Google Scholar] [CrossRef]
- Mao, R.; Chen, H.M.; Zhang, X.H.; Shi, F.X.; Song, C.C. Effects of P addition on plant C:N:P stoichiometry in an N−limited temperate wetland of Northeast China. Sci. Total Environ. 2016, 559, 1–6. [Google Scholar] [CrossRef]
- Engelhardt, K.A.; Ritchie, M.E. Effects of macrophyte species richness on wetland ecosystem functioning and services. Nature 2001, 411, 687–689. [Google Scholar] [CrossRef]
- Liu, F.; Liu, Y.; Wang, G.; Song, Y.; Liu, Q.; Li, D.; Mao, P.; Zhang, H. Seasonal Variations of C: N: P Stoichiometry and Their Trade−Offs in Different Organs of Suaeda salsa in Coastal Wetland of Yellow River Delta, China. PLoS ONE 2015, 10, e0138169. [Google Scholar] [CrossRef]
- Sato, S.; Comerford, N.B. Influence of soil pH on inorganic phosphorus sorption and desorption in a humid brazilian Ultisol. Rev. Bras. Ciência Do Solo 2005, 29, 685–694. [Google Scholar] [CrossRef]
- Sun, Z.; Mou, X.; Song, H.; Jiang, H. Sulfur biological cycle of the different Suaeda salsa marshes in the intertidal zone of the Yellow River estuary, China. Ecol. Eng. 2013, 53, 153–164. [Google Scholar] [CrossRef]
- Shang, Z.H.; Feng, Q.S.; Wu, G.L.; Ren, G.H.; Long, R.J. Grasslandification has significant impacts on soil carbon, nitrogen and phosphorus of alpine wetlands on the Tibetan Plateau. Ecol. Eng. 2013, 58, 170–179. [Google Scholar] [CrossRef]
- Li, L.; Chang, Y.; Li, X.; Qiao, X.; Luo, Q.; Xu, Z.; Xu, Z.; Yang, X. Carbon sequestration potential of cropland reforestation on the northern slope of the Tianshan Mountains. Can. J. Soil Sci. 2016, 96, 461–471. [Google Scholar] [CrossRef]
- Fischer, J.M.; Reed−Andersen, T.; Klug, J.L.; Chalmers, A.G. Spatial Pattern of Localized Disturbance along a Southeastern Salt Marsh Tidal Creek. Estuaries 2000, 23, 565–571. [Google Scholar] [CrossRef]
- Liu, J.-j.; Zhou, Z.-z.; Tian, H.-x.; Wan, Y.; Zhao, L.-n. Vascular plant community types and flora in Shengjin Lake National Nature reserve. J. Biol. 2016, 33. [Google Scholar]
- Rong, Q.; Liu, J.; Cai, Y.; Lu, Z.; Zhao, Z.; Yue, W.; Xia, J. Leaf carbon, nitrogen and phosphorus stoichiometry of Tamarix chinensis in the Laizhou Bay coastal wetland, China. Ecol. Eng. 2015, 76, 57–65. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, H.; Yu, Q.; Wang, Z.; Wei, C.; Long, M.; Kattge, J.; Smith, M.; Han, X. Sampling date, leaf age and root size: Implications for the study of plant C:N:P stoichiometry. PLoS ONE 2013, 8, e60360. [Google Scholar] [CrossRef]
- Bao, S.D. Soil and Agricultural Chemistry Analysis; China Agricultural Press: Beijing, China, 2000. [Google Scholar]
- Zhang, W.; Zhao, J.; Pan, F.; Li, D.; Chen, H.; Wang, K. Changes in nitrogen and phosphorus limitation during secondary succession in a karst region in southwest China. Plant Soil 2015, 391, 77–91. [Google Scholar] [CrossRef]
- Maccherini, S.; Marignani, M.; Gioria, M.; Renzi, M.; Rocchini, D.; Santi, E.; Torri, D.; Tundo, J.; Honnay, O. Determinants of plant community composition of remnant biancane badlands: A hierarchical approach to quantify species−environment relationships. Appl. Veg. Sci. 2011, 14, 378–387. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, B.-R.; An, S.-S. Ecological stoichiometry in leaves, roots, litters and soil among different plant communities in a desertified region of Northern China. Catena 2018, 166, 328–338. [Google Scholar] [CrossRef]
- Yuan, Z.-Q. Factors affecting lucerne−rich vegetation under revegetation in a semi−arid environment. Ecol. Eng. 2017, 108, 249–254. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, B.; Wang, B.; Zhang, G.; Zhang, W.; Zhang, B.; Chang, S.; Chen, T.; Liu, G. Leaf elemental stoichiometry of Tamarix Lour. species in relation to geographic, climatic, soil, and genetic components in China. Ecol. Eng. 2017, 106, 448–457. [Google Scholar] [CrossRef]
- Baldwin, D.S.; Rees, G.N.; Mitchell, A.M.; Watson, G.; Williams, J. The short−term effects of salinization on anaerobic nutrient cycling and microbial community structure in sediment from a freshwater wetland. Wetlands 2006, 26, 455–464. [Google Scholar] [CrossRef]
- Li, W.; Cao, T.; Ni, L.; Zhang, X.; Zhu, G.; Xie, P. Effects of water depth on carbon, nitrogen and phosphorus stoichiometry of five submersed macrophytes in an in situ experiment. Ecol. Eng. 2013, 61, 358–365. [Google Scholar] [CrossRef]
- Kerkhoff, A.J.; Fagan, W.F.; Elser, J.J.; Enquist, B.J. Phylogenetic and growth form variation in the scaling of nitrogen and phosphorus in the seed plants. Am. Nat. 2006, 168, E103–E122. [Google Scholar] [CrossRef]
- Li−ying, X.; Shou−biao, Z.; Shou−feng, L.; He−quan, Z. Ecological Characteristics and Dynamics of Above−Ground Nutritions of Carex Thunbergii Steud in Wuhu Wetland; Anhui Polytechnic University: Wuhu, China, 2015; Volume 30. [Google Scholar]
- Zhang, J.; Zhao, N.; Liu, C.; Yang, H.; Li, M.; Yu, G.; Wilcox, K.; Yu, Q.; He, N.; Niu, S. C:N:P stoichiometry in China’s forests: From organs to ecosystems. Funct. Ecol. 2017, 32, 50–60. [Google Scholar] [CrossRef]
- Reich, P.B.; Oleksyn, J.; Wright, I.J.; Niklas, K.J.; Hedin, L.; Elser, J.J. Evidence of a general 2/3−power law of scaling leaf nitrogen to phosphorus among major plant groups and biomes. Proc. Biol. Sci. 2010, 277, 877–883. [Google Scholar] [CrossRef]
- Berman−Frank, I.; Dubinsky, Z. Balanced Growth in Aquatic Plants: Myth or Reality? Phytoplankton use the imbalance between carbon assimilation and biomass production to their strategic advantage. BioScience 1999, 49, 29–37. [Google Scholar] [CrossRef]
- Feng, Y.L. Photosynthesis, nitrogen allocation and specific leaf area in invasive Eupatorium adenophorum and native Eupatorium japonicum grown at different irradiances. Physiol. Plant. 2008, 133, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Saibo, Y.; Zhendong, Y.; Xueqin, L.; Hongzhu, W. Water level requirements of a Carex hygrophyte in Yangtze floodplain lakes. Ecol. Eng. 2019, 129, 29–37. [Google Scholar] [CrossRef]
- Galmes, J.; Flexas, J.; Keys, A.J.; Cifre, J.; Mitchell, R.A.C.; Madgwick, P.J.; Haslam, R.P.; Medrano, H.; Parry, M.A.J. Rubisco specificity factor tends to be larger in plant species from drier habitats and in species with persistent leaves. Plant Cell Environ. 2005, 28, 571–579. [Google Scholar] [CrossRef]
- Barbosa, E.R.; Tomlinson, K.W.; Carvalheiro, L.G.; Kirkman, K.; de Bie, S.; Prins, H.H.; van Langevelde, F. Short−term effect of nutrient availability and rainfall distribution on biomass production and leaf nutrient content of savanna tree species. PLoS ONE. 2014, 9, e92619. [Google Scholar] [CrossRef]
- Jin−Sheng, H.; Xing−Guo, H. Ecological stoichiometry: Searching for unifying principles from individuals to ecosystems. Chin. J. Plant Ecol. 2010, 34, 2–6. [Google Scholar]
- Rong, Y.; Na, S.; Liang, S.; Haijun, S.; Yonghong, L.; Yongsheng, G. Ecological stoichiometry characteristics of soil carbon, nitrogen and phosphorus of the Yellow River wetland in Baotou, Inner Mongolia. Acta Ecol. Sin. 2020, 40. [Google Scholar]
- Jian, Z.; Li, S.; Liping, W.; Yalan, B.; Jingwen, L.; Xueli, G.; Tao, C.; Jianjun, C. The effect of vegetation cover on ecological stoichiometric ratios of soil carbon, nitrogen and phosphorus: A case study of the Dunhuang Yangguan wetland. Acta Ecol. Sin. 2019, 39, 580–589. [Google Scholar] [CrossRef]
- Gao, Z.; Fang, H.; Bai, J.; Jia, J.; Lu, Q.; Wang, J.; Chen, B. Spatial and seasonal distributions of soil phosphorus in a short−term flooding wetland of the Yellow River Estuary, China. Ecol. Inform. 2016, 31, 83–90. [Google Scholar] [CrossRef]
- Lu, Q.; Bai, J.; Zhang, G.; Zhao, Q.; Wu, J. Spatial and seasonal distribution of carbon, nitrogen, phosphorus, and sulfur and their ecological stoichiometry in wetland soils along a water and salt gradient in the Yellow River Delta, China. Phys. Chem. Earth Parts A/B/C 2018, 104, 9–17. [Google Scholar] [CrossRef]
- Maire, V.; Wright, I.J.; Prentice, I.C.; Batjes, N.H.; Bhaskar, R.; van Bodegom, P.M.; Cornwell, W.K.; Ellsworth, D.; Niinemets, Ü.; Ordonez, A.; et al. Global effects of soil and climate on leaf photosynthetic traits and rates. Glob. Ecol. Biogeogr. 2015, 24, 706–717. [Google Scholar] [CrossRef]
- Xian−xiang, L.; Meng, D.; Qin, Y. Dynamic Distribution and Influence Factors of Soil Phosphorus in Yellow River Estuary Wetland. J. Soil Water Conserv. 2011, 5, 034. [Google Scholar]
- Matzek, V.; Vitousek, P.M. N:P stoichiometry and protein:RNA ratios in vascular plants: An evaluation of the growth−rate hypothesis. Ecol. Lett. 2009, 12, 765–771. [Google Scholar] [CrossRef]
- Yu, Q.; Wu, H.; He, N.; Lu, X.; Wang, Z.; Elser, J.J.; Wu, J.; Han, X. Testing the growth rate hypothesis in vascular plants with above− and below−ground biomass. PLoS ONE 2012, 7, e32162. [Google Scholar] [CrossRef]
- Craft, C.B. Dynamics of nitrogen and phosphorus retention during wetland ecosystem succession. Wetl. Ecol. Manag. 1997, 4, 177–187. [Google Scholar] [CrossRef]
- Gao, Y.; Yu, G.; He, N. Equilibration of the terrestrial water, nitrogen, and carbon cycles: Advocating a health threshold for carbon storage. Ecol. Eng. 2013, 57, 366–374. [Google Scholar] [CrossRef]
- Mi, Z.; Huang, Y.; Gan, H.; Zhou, W.; Flynn, D.F.; He, J.S. Leaf P increase outpaces leaf N in an Inner Mongolia grassland over 27 years. Biol. Lett. 2015, 11, 20140981. [Google Scholar] [CrossRef]
- Li, L.; Zerbe, S.; Han, W.; Thevs, N.; Li, W.; He, P.; Schmitt, A.O.; Liu, Y.; Ji, C. Nitrogen and phosphorus stoichiometry of common reed (Phragmites australis) and its relationship to nutrient availability in northern China. Aquat. Bot. 2014, 112, 84–90. [Google Scholar] [CrossRef]
- Hirel, B.; Le Gouis, J.; Ney, B.; Gallais, A. The challenge of improving nitrogen use efficiency in crop plants: Towards a more central role for genetic variability and quantitative genetics within integrated approaches. J. Exp. Bot. 2007, 58, 2369–2387. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Y.; Hsu, P.K.; Tsay, Y.F. Uptake, allocation and signaling of nitrate. Trends Plant Sci. 2012, 17, 458–467. [Google Scholar] [CrossRef]
- Krapp, A.; David, L.C.; Chardin, C.; Girin, T.; Marmagne, A.; Leprince, A.S.; Chaillou, S.; Ferrario−Mery, S.; Meyer, C.; Daniel−Vedele, F. Nitrate transport and signalling in Arabidopsis. J. Exp. Bot. 2014, 65, 789–798. [Google Scholar] [CrossRef]
- Ruffel, S.; Gojon, A.; Lejay, L. Signal interactions in the regulation of root nitrate uptake. J. Exp. Bot. 2014, 65, 5509–5517. [Google Scholar] [CrossRef]
- Vidal, E.A.; Moyano, T.C.; Canales, J.; Gutierrez, R.A. Nitrogen control of developmental phase transitions in Arabidopsis thaliana. J. Exp. Bot. 2014, 65, 5611–5618. [Google Scholar] [CrossRef]
- von Wirén, N.; Gazzarrini, S.; Gojon, A.; Frommer, W.B. The molecular physiology of ammonium uptake and retrieval. Curr. Opin. Plant Biol. 2000, 3, 254–261. [Google Scholar] [CrossRef]
- O’Brien, J.A.; Vega, A.; Bouguyon, E.; Krouk, G.; Gojon, A.; Coruzzi, G.; Gutierrez, R.A. Nitrate Transport, Sensing, and Responses in Plants. Mol. Plant 2016, 9, 837–856. [Google Scholar] [CrossRef]
- Jahan, S.J.L.S.; Wahocho, N.; Laghari, G.; Laghari, A.; Bhabhan, G.; HussainTalpur, K.; Ahmed, T.; Wahocho, S.; Lashari, A. Role of Nitrogen for Plant Growth and Development: A review. Adv. Environ. Biol. 2016, 10, 209–218. [Google Scholar]
- Li−Xia, Z.; Yong−Fei, B.; Xing−Guo, H. Application of N:P Stoichiometry to Ecology Studies. Acta Bot. Sin. 2003, 45, 1009–1018. [Google Scholar]

| LC | LN | LP | LC: LN | LC: LP | LN: LP | |
|---|---|---|---|---|---|---|
| LC | 1 | |||||
| LN | 0.336 | 1 | ||||
| LP | 0.678 ** | 0.018 | 1 | |||
| LC: LN | 0.250 | −0.784 ** | 0.327 | 1 | ||
| LC: LP | −0.299 | 0.037 | −0.834 ** | −0.091 | 1 | |
| LN: LP | −0.290 | 0.639 ** | −0.715 ** | −0.731 ** | 0.714 ** | 1 |
| PH | SWC | NO3−−N | NH4+−N | AP | AK | SOC | STN | STP | SOC: STN | SOC: STP | STN: STP | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PH | 1 | |||||||||||
| SWC | 0.516 ** | 1 | ||||||||||
| NO3−−N | 0.570 ** | 0.565 ** | 1 | |||||||||
| NH4+−N | 0.309 | 0.133 | 0.551 ** | 1 | ||||||||
| AP | −0.561 ** | −0.626 ** | −0.444 * | −0.203 | 1 | |||||||
| AK | −0.518 ** | −0.472 * | −0.540 ** | −0.277 | 0.376 | 1 | ||||||
| SOC | −0.800 ** | −0.612 ** | −0.533 ** | 0.007 | 0.600 ** | 0.460 * | 1 | |||||
| STN | −0.515 * | −0.501 * | −0.39 | 0.185 | 0.233 | 0.374 | 0.751 ** | 1 | ||||
| STP | 0.406 * | 0.396 | 0.620 ** | 0.625 ** | −0.394 | −0.483 * | −0.312 | −0.277 | 1 | |||
| SOC: STN | −0.131 | −0.142 | −0.338 | −0.304 | 0.227 | −0.15 | 0.17 | −0.117 | −0.284 | 1 | ||
| SOC: STP | −0.625 ** | −0.577 ** | −0.745 ** | −0.448 * | 0.551 ** | 0.558** | 0.654 ** | 0.557 ** | −0.884 ** | 0.323 | 1 | |
| STN: STP | −0.446 * | −0.526 ** | −0.650 ** | −0.35 | 0.378 | 0.539** | 0.517 ** | 0.639 ** | −0.874 ** | 0.199 | 0.925 ** | 1 |
| Name | Contribution % | Pseudo−F | p |
|---|---|---|---|
| STP | 10.54 | 38.89 | 0.017 |
| NH4+−N | 7.17 | 8.05 | 0.01 |
| NO3–−N | 7.26 | 1.00 | 0.016 |
| AK | 11.35 | 2.58 | 0.021 |
| AP | 3.63 | 4.46 | 0.684 |
| SOC | 7.01 | 5.94 | 0.363 |
| pH | 10.3 | 4.91 | 0.001 |
| SWC | 10.33 | 0.42 | 0.003 |
| LC | LN | LP | LC: LN | LC: LP | LN: LP | |
|---|---|---|---|---|---|---|
| pH | −0.411 | −0.235 | −0.569 | −0.422 | 0.607 * | 0.684 * |
| SWC | 0.619 * | 0.717 ** | 0.509 | −0.384 | −0.379 | −0.288 |
| NO3–−N | −0.159 | −0.132 | −0.104 | −0.140 | 0.040 | 0.064 |
| NH4+−N | 0.892 ** | 0.910 ** | 0.781 ** | −0.156 | −0.608 * | −0.559 |
| AP | 0.356 | 0.350 | 0.220 | −0.053 | −0.191 | −0.168 |
| AK | 0.587 * | 0.562 | 0.428 | −0.081 | −0.310 | −0.284 |
| SOC | 0.627 * | 0.460 | 0.747 ** | 0.438 | −0.648 * | −0.728 ** |
| STN | 0.685 * | 0.477 | 0.750 ** | 0.523 | −0.612 * | −0.712 ** |
| STP | −0.017 | −0.038 | −0.169 | −0.002 | 0.102 | 0.120 |
| SOC: STN | −0.721 ** | −0.487 | −0.528 | −0.568 | 0.272 | 0.389 |
| SOC: STP | 0.304 | 0.213 | 0.469 | 0.261 | −0.390 | −0.453 |
| STN: STP | 0.352 | 0.244 | 0.494 | 0.299 | −0.397 | −0.467 |
| LC | LN | LP | LC: LN | LC: LP | LN: LP | |
|---|---|---|---|---|---|---|
| pH | −0.707** | −0.747 ** | −0.765 ** | −0.708 ** | −0.748 ** | −0.754 ** |
| SWC | 0.547 * | 0.583 * | 0.671 ** | 0.547 * | 0.569 * | 0.566 * |
| NO3–−N | −0.287 | −0.321 | −0.244 | −0.254 | −0.28 | −0.293 |
| NH4+−N | 0.781 ** | 0.829 ** | 0.860 ** | 0.762 ** | 0.809 ** | 0.818 ** |
| AP | 0.197 | 0.336 | 0.147 | 0.1 | 0.176 | 0.231 |
| AK | −0.329 | −0.337 | −0.324 | −0.328 | −0.336 | −0.331 |
| SOC | −0.061 | 0.044 | −0.085 | −0.165 | −0.12 | −0.08 |
| STN | 0.516 * | 0.499 * | 0.622 * | 0.505 * | 0.505 * | 0.487 |
| STP | −0.425 | −0.483 | −0.399 | −0.462 | −0.510 * | −0.532 * |
| SOC: STN | −0.558 * | −0.454 | −0.638 ** | −0.622 * | −0.590 * | −0.545 * |
| SOC: STP | 0.332 | 0.502* | 0.262 | 0.275 | 0.374 | 0.44 |
| STN: STP | 0.259 | 0.402 | 0.202 | 0.203 | 0.284 | 0.339 |
| Season | Type | Soil pH | SWC(%) | NO3−−N (mg kg−1) | NH4+−N (mg kg−1) | AP (mg kg−1) | AK (mg kg−1) |
|---|---|---|---|---|---|---|---|
| winter | Bs | 5.68 ± 0.05 Ca | 22.23 ± 1.00 Ac | 6.39 ± 0.30 Ba | 3.25 ± 0.43 Bd | 30.15 ± 7.34 Ab | 45.47 ± 4.90 Ba |
| CR | 5.23 ± 0.17 Bb | 26.17 ± 0.32 Cb | 4.88 ± 0.87 Ab | 15.83 ± 1.51 ABa | 34.97 ± 8.07 Aab | 40.79 ± 5.76 Ba | |
| CP | 5.18 ± 0.39 Ab | 27.31 ± 0.90 ABab | 5.06 ± 0.67 Bb | 10.23 ± 1.75 Bb | 41.64 ± 1.94 Aa | 48.58 ± 7.62 Ba | |
| Ct | 5.42 ± 0.16 Bab | 28.60 ± 1.10 Aa | 5.20 ± 0.63 Bb | 5.86 ± 0.95 Cc | 32.67 ± 2.52 Aab | 23.07 ± 7.28 Bb | |
| spring | Bs | 7.07 ± 0.46 Aa | 23.38 ± 0.91 Ac | 6.49 ± 0.06 Ba | 5.81 ± 0.73 Ac | 26.67 ± 2.92 Ab | 32.77 ± 3.12 Ca |
| CR | 5.63 ± 0.39 Bb | 29.31 ± 0.69 Bab | 6.44 ± 0.14 Aa | 20.55 ± 1.81 Aa | 27.26 ± 4.46 ABab | 22.90 ± 3.98 Bb | |
| CP | 5.66 ± 0.18 Ab | 28.62 ± 0.92 Ab | 6.45 ± 0.08 Ba | 20.07 ± 2.83 Aa | 33.73 ± 5.51 Ba | 15.77 ± 4.97 Cc | |
| Ct | 6.13 ± 0.34 Ab | 30.62 ± 1.52 Aa | 6.42 ± 0.08 ABa | 12.96 ± 2.54 Bb | 24.77 ± 4.00 Bb | 29.40 ± 4.52 Ba |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, W.; Wang, X.; Ren, Y.; Ye, X. Change in the Ecological Stoichiometry of Carex thunbergii in Response to Seasonal Dynamics and Environmental Factors in Shengjin Lake, China. Diversity 2024, 16, 198. https://doi.org/10.3390/d16040198
Xu W, Wang X, Ren Y, Ye X. Change in the Ecological Stoichiometry of Carex thunbergii in Response to Seasonal Dynamics and Environmental Factors in Shengjin Lake, China. Diversity. 2024; 16(4):198. https://doi.org/10.3390/d16040198
Chicago/Turabian StyleXu, Wenjing, Xin Wang, Yujing Ren, and Xiaoxin Ye. 2024. "Change in the Ecological Stoichiometry of Carex thunbergii in Response to Seasonal Dynamics and Environmental Factors in Shengjin Lake, China" Diversity 16, no. 4: 198. https://doi.org/10.3390/d16040198




