Abstract
Epiphytic orchids comprise 68% of vascular epiphytes globally; nevertheless, many are endangered. One such epiphytic orchid is Phalaenopsis japonica, which is widely used in the floricultural industry. This study aimed to identify the mycorrhizal fungi of adult P. japonica and their roles in seed germination and seedling development. Root samples were collected from 32 adults across 4 sites in southern Japan, and mycorrhizal fungi were identified using Sanger and high-throughput sequencing (HTS). The results show phylogenetically diverse mycobionts, mainly Ceratobasidiaceae (CE) and Tulasnellaceae (TU), with dominant OTUs designated CE6 and CE22. Sanger sequencing found 9 OTUs, 4 CE, and 5 TU; HTS detected 22 OTUs, 4 CE, 16 TU, and 2 Serendipitaceae. Seeds inoculated with CE6 improved germination and protocorm development compared with other strains. In addition, asymbiotic seedlings inoculated with CE6, CE22, and TU18 displayed varying effects in growth, with CE6 being the most notable. While TU18 did not promote seed germination, it effectively promoted leaf development in seedlings. Overall, Ceratobasidiaceae was predominantly associated with seed germination, seedling growth, and the adult stages, with CE6 and CE22 becoming the primary partners throughout the life history of P. japonica. Our findings illuminate mycorrhizal symbiosis in epiphytic habitats, offering conservation and commercial production insights.
1. Introduction
Orchidaceae is one of the largest and most diverse angiosperm families, with more than 28,000 species [1]. Epiphytic orchids account for 69% of all orchid species and 68% of all vascular epiphytes [2]. At present, numerous orchid species face the threat of extinction due to factors such as overcollection, habitat loss, and environmental changes [3,4]. Consequently, the conservation of these species has emerged as a critical global priority. The symbiotic relationship between orchids and mycorrhizal fungi is essential for orchids to thrive in their habitats [5]. Orchids have an unusual reproductive strategy, producing thousands of dust-like seeds within a single seed capsule. However, these seeds lack sufficient nutrients for independent germination. Therefore, successful germination and development of orchid seeds depend heavily on the presence of specific mycorrhizal fungi that provide carbon resources [6,7,8]. Colonization by orchid mycorrhizal fungi (OMF) begins during seed germination and persists throughout the entire orchid life cycle [9]. While orchids can establish associations with various fungal partners, not all associations have equal impacts on orchid growth and development [10,11]. Since mycorrhizal fungi vary significantly among orchid species [12,13], thorough elucidation of the diversity and function of the OMF associated with each orchid species is vital.
The genus Phalaenopsis, commonly known as the moth orchid, is a prominent and extensively cultivated group within the family Orchidaceae. This genus includes approximately 80 accepted species [14] that are native to tropical and subtropical regions from Asia to northeastern Australia [14,15]. This genus also has significant popularity in the floricultural industry, and numerous hybrid varieties are available on the market [16]. Despite their importance, several species have become endangered or rare in their natural habitats [17]. Hence, it is crucial to elucidate the mycorrhizal associations of Phalaenopsis orchids in order to prioritize conservation efforts. Furthermore, orchid mycorrhizal associations can lead to various beneficial outcomes for orchid cultivars, including improved plant growth and flower quality, and enhanced disease resistance [18,19]. Two previous studies identified the primary mycorrhizal associates of Phalaenopsis species, P. cornu-cervi [20] and P. wilsonii [21]. However, knowledge about the mycorrhizal associations of specific Phalaenopsis species remains limited. Therefore, gaining better information about the OMF of Phalaenopsis species is essential to advance both basic research and the conservation and horticultural industries.
The mycorrhizal fungi of epiphytic orchids are mainly members of the Ceratobasidiaceae, Serendipitaceae, Tulasnellaceae, and Atractiellomycetes groups [22,23,24,25]. Molecular identification of OMF relies primarily on the internal transcribed spacer (ITS) region. Most OMF families can be identified using universal fungal primers; however, Tulasnellaceae species are difficult to detect with universal primers due to sequence mismatches [26]. Such mismatches can introduce bias in the descriptions of fungal communities, potentially affecting the outcomes of analysis. Thus, the use of Tulasnella-specific primers is necessary to avoid the underrepresentation of Tulasnellaceae fungi. According to Rammitsu et al. (2021) [27], the Tulasnellaceae lineage consists of four distinct groups, known as Tulasnellaceae groups (TGs) 1–4, which are differentiated based on primer mismatch. In high-throughput sequencing (HTS), the fungal universal primer pair ITS86F/ITS4 [28,29] is suitable for fungi within TG4, while the Tulasnella-specific primers 5.8S-Tulngs/ITS4-Tul2 [30,31] can effectively identify the respective TG1 and TG2 Tulasnellaceae fungi associated with orchids. However, the detection of fungi within TG3 requires an additional forward primer due to numerous mismatches with both ITS86F and 5.8S-Tulngs.
Fungal community associations in orchids often change during the orchid’s ontogeny [32]. Therefore, identification of the mycorrhizal fungi involved in seed germination, seedling development, and the adult stages of a particular orchid species is essential, as the most effective fungal partner may vary among developmental stages. For example, the epiphytic orchid Vanda falcata is predominantly associated with a single Tulasnellaceae fungus in adulthood, although seed germination is strongly induced by fungi in Ceratobasidiaceae [33]. Comprehensive elucidation of mycorrhizal associations throughout the orchid life cycle is crucial to unravel the complexities of orchid mycorrhizal associations and establish effective strategies for orchid conservation and cultivation.
In this study, we assessed the mycorrhizal community of adult P. japonica using both Sanger sequencing and HTS methods. For HTS techniques targeting the ITS2 region, we employed Tulasnellaceae-specific primers to reveal the entire Tulasnellaceae groups. To understand the fungal impacts at different growth stages, we separately evaluated seed germination and seedling growth by in vitro symbiotic culture.
2. Materials and Methods
2.1. Study Species and Sampling
Phalaenopsis japonica (Rchb.f.) Kocyan & Schuit. (synonyms: Sedirea japonica, Hygrochilus japonicus, Aerides japonica) is a monopodial epiphytic orchid that typically grows on trees or rocks in open subtropical forests or among scrub on cliffs and valleys at low to moderate elevations (Figure 1) [34]. Its distribution range includes central to southern Japan, China, Taiwan, and Korea [35]. Renowned for its fragrant, light-greenish-white flowers and attractive foliage, this species has found widespread application in orchid breeding programs [36].

Figure 1.
Blooming mature Phalaenopsis japonica (photograph by NPO Shima-niji of Tokunoshima).
In total, 32 individuals of P. japonica were sampled from 4 sites in their natural habitat or among cultivated plants in gardens in the southern part of Japan (Table 1). To examine the effect of host tree species on the mycorrhizal association, samples were collected from multiple host tree species. Sampling sites 1 to 3 are characterized by warm temperate climates, whereas site 4 has a subtropical climate. The collected root fragments were washed with tap water and hand-sliced sections were observed under a microscope to check for fungal colonization. Then, mycorrhizal root segments were cut into 1–2 cm fragments and stored in Tris-ethylenediaminetetraacetic acid (TE) buffer at −20 °C for molecular identification of fungi. Root fragments containing living hyphal coils were used for fungal isolation.

Table 1.
Details of the sampling sites, host tree species, number of individuals, and number of DNA samples collected at each site.
2.2. Isolation of Mycorrhizal Fungi
Mycorrhizal fungi were isolated from seven individual orchids (Table 1) using the method described by Rammitsu et al. (2021) [31] with slight modifications. Root fragments containing living hyphal coils (pelotons) were washed in sterile distilled water (SDW) and we released individual pelotons from the cortex cells into fresh SDW. The pelotons were collected and rinsed with SDW and cultured in 1.5% water agar medium (10 mL) with 50 ppm each of streptomycin and tetracycline. The plates were incubated at 25 ± 1 °C for 1 week, and fungal colonies that formed from individual pelotons were transferred to fresh potato dextrose agar (PDA) plates for subculturing. Pure cultures of OMF were obtained and identified using molecular methods, as described in the following section. Isolates were stored at −80 °C until use for in vitro symbiosis experiments and deposited with the National Institute of Technology and Evaluation (NITE) Biological Resource Center in Japan.
2.3. Molecular Identification of Mycorrhizal Fungi through Sanger Sequencing
Colonized root fragments were crushed with forceps to disperse the hyphal coils into the fresh buffer. Pelotons (100–300 per root fragment) were collected using a micropipette, then rinsed twice in fresh buffer and homogenized with a 20 µL buffer using a BioMasher II homogenizer (Nippi Inc., Tokyo, Japan). For fungal isolates, hyphae growing on the culture medium were collected using a sterilized toothpick and suspended in 50 µL TE buffer. DNA extraction was conducted as described by Izumitsu et al. (2012) [37]. The supernatant obtained from the extraction process was used as the template for polymerase chain reaction (PCR).
PCR was performed to amplify the ITS sequence of nuclear ribosomal DNA. For amplification, we utilized the universal primer pairs ITS1F/ITS4 [28,38] and ITS1F/ITS4B [38]. We also employed the Tulasnella-specific primer pairs ITS5/ITS4-Tul2 [28] and 5.8S-Tulngs/ITS4-Tul2. Mighty Amp DNA polymerase Ver. 3 (TaKaRa, Shiga, Japan) was used for PCR amplification as described by Rammitsu et al. (2021) [31]. The resulting PCR amplicons were purified using the Fast Gene Gel/PCR Extraction Kit (Nippon Genetics, Tokyo, Japan) and subsequently sequenced using the BigDye Terminator v3.1 Cycle Sequencing Kit (Thermo Fisher Scientific Baltics, Vilnius, Lithuania) and the 3130 Genetic Analyzer (Applied Biosystems, Tokyo, Japan), following the manufacturer’s instructions. For amplicons that presented challenges in direct sequencing, the pGEM-T Vector System II (Promega, Madison, MI, USA) was employed for cloning. All ITS sequences were searched using the Basic Local Alignment Search Tool (BLAST) with the GenBank database of the National Center for Biotechnology Information (NCBI). The fungi were assigned to operational taxonomic units (OTUs) based on 97% sequence similarity, and OTU numbers were allocated based on the OMF database in our laboratory. Subsequently, ITS sequences were deposited in the DNA Data Bank of Japan (DDBJ) under the following accession numbers: LC746356–LC746365.
2.4. Phylogenetic Analysis
Putative mycorrhizal fungi, Ceratobasidiaceae, were subjected to phylogenetic analysis utilizing whole ITS sequences obtained from Sanger sequencing. Representative fungal ITS sequences from GenBank showing sequence similarity > 90% to the OTUs were included in the analysis. The ITS sequences were aligned using the MAFFT online server [39,40]. Positions with less than 80% site coverage were removed, i.e., fewer than 20% alignment gaps, missing data, and ambiguous bases were allowed at any position. The GTR + G was estimated to be the best-fit model in MEGA11. The phylogenetic tree was constructed employing the maximum-likelihood method in MEGA 11 [41]. To estimate the relative robustness of branches in the phylogenetic tree, bootstrap analysis was performed with 1000 replicates [42].
2.5. High-Throughput Sequencing
To create simulated communities for Illumina NovaSeq 6000 sequencing, we mixed 2–6 DNA samples (1 µL) from each population that had undergone Sanger sequencing. The ITS2 region of fungal nuclear ribosomal DNA was amplified using the tailed primer pairs ITS86F/ITS4, along with two Tulasnellaceae-specific primer pairs: 5.8S-Tulngs/ITS4-Tul2 and 5.8S-Tulngsg3 (5′-GCTGCGAAATGTGATGTGAA-3′)/ITS4-Tul2. The 5.8S-Tulngsg3 primer was newly designed for the study, targeting TG3. PCR was performed in triplicate using a 1 µL sample of DNA and 5 µL 2× KAPA HiFi HotStart ReadyMix (Kapa Biosystems, Wilmington, MA, USA), as described by Rammitsu et al. (2021) [27]. The purified PCR products were quantified with a Qubit dsDNA HS Assay kit and equimolar amounts of each sample were pooled to prepare sequencing libraries. Then, the libraries were sequenced using Illumina NovaSeq paired-end sequencing with 2 × 250-bp reads following standard protocols by Novogene Co., Ltd. (Singapore).
The data quality was first checked using FastQC [43]. Then, the reads were cleaned and demultiplexed into corresponding samples using barcode labels with the process_shortreads program of STACKS version 2.61 [44]. The demultiplexed sequences were further processed using the Qiime2 (version 2022.11.1) software package [45]. In Qiime2, the q2-cutadapt plugin [46] was utilized to trim barcodes and primers from the raw sequencing data. Following this process, the q2-dada2 plugin [47] was employed to remove noisy sequences, singleton sequences, and chimeras. OTU clustering was conducted at a 97% similarity threshold using the VSEARCH algorithm [48]. To create a comprehensive and customized reference database, we added fungal reference sequences downloaded from the UNITE database (version 9.0) [49], as well as OTU sequences obtained through Sanger sequencing. Subsequently, the pre-trained classifier was employed for the taxonomic classification of the query OTUs, which in turn was completed using the q2-classify-sklearn plugin [50]. To ensure accuracy, OTUs with less than 100 total reads were removed before the construction of the OTU table, as such reads may be due to sequencing errors or contaminants. Each OTU was annotated to the lowest possible taxonomic rank, reaching the genus and species levels.
2.6. Symbiotic Culture
2.6.1. In Vitro Symbiotic Germination
Six fungal isolates were used for the in vitro symbiotic germination experiment (Table 2). All fungal isolate OTUs, except for TU27, were detected from adult P. japonica. Four Ceratobasidiaceae isolates were obtained from P. japonica roots. To assess the effect of Tulasnellaceae fungi, we utilized two Tulasnellaceae isolates, TU18 and TU27. Because the isolation of Tulasnellaceae fungi from P. japonica proved unsuccessful, we utilized TU18 isolates obtained from another epiphytic orchid. Seeds from five mature capsules collected from three individual orchids were mixed and used for the symbiotic culture. Seed viability was evaluated using the 2,3,5-triphenyl tetrazolium chloride (TTC) test to ensure high viability (>80%). Then, the seeds were sterilized with 1% sodium hypochlorite solution for 1 min, sowed on water agar medium (agar, 15 g/L), and maintained at 25 °C for 1 week to check for contamination. After 1 week of contamination-free culturing, 1 cm × 1 cm discs (5–10 seeds per disc) were removed and transferred to oatmeal agar (OMA) medium. On each plate containing fresh medium, four discs containing about 20–40 seeds were placed. Each treatment contained six replicates with a total of 120–240 seeds. A 6 mm plug of pre-cultured fungal isolates was inoculated onto the OMA medium, and the plates were cultured under a 12 h/12 h light/dark photoperiod at 25 ± 1 °C. As a control, plates with no fungal inoculum were also prepared. After 4 months of culturing, the seeds were counted under a stereomicroscope, and seed germination and protocorm development were scored on a scale of 0–6 as follows: stage 0, no germination; stage 1, seed with swollen embryo; stage 2, enlarged embryo with ruptured seed coat; stage 3, appearance of the rhizoid; stage 4, appearance of the proto-meristem; stage 5, emergence of the first leaf; and stage 6, emergence of the first root and further development (Supplementary Figure S1) [11]. Seed germination (%) per stage was calculated using the following formula: percentage seed germination = (number of seeds per germination stage/total number of viable seeds) × 100. The germination stage corresponded to stages 2 and above for this calculation. The data were analyzed using one-way analysis of variance (ANOVA), followed by Tukey’s test using the R statistical package (version 4.2.1) [51].

Table 2.
Fungal isolates of Phalaenopsis japonica and other epiphytic orchids used for symbiotic culturing.
2.6.2. In Vitro Symbiotic Culture of Seedlings
Four fungal isolates (CE6–2, CE22, TU18, and TU27) were cultured with seedlings obtained through asymbiotic culturing for the in vitro seedling growth experiment. Seeds were sterilized with 1% sodium hypochlorite solution for 1 min and sown in New Dogashima Medium [52]. The fungal isolates were pre-cultured on PDA in the dark at 25 ± 10 °C for 7 days. When the seedlings reached the 1–2 leaf stage, 4 seedlings per bottle were transplanted into transparent bottles containing modified oatmeal agar medium (ONY; 0.38 g/L NH4NO3, 0.2 g/L KH2PO4, 0.1 g/L MgSO4·7H2O, 0.1 g/L KCl, 0.1 g/L yeast extract, 2.5 g/L oatmeal, and 15 g/L agar). Each glass bottle (450 mL) contained 50 mL ONY medium, and five bottles were used per treatment. Colonized agar plugs (diameter = 6 mm) were transferred to the OMA medium. As a control, bottles with no fungal inoculum were prepared. The cultures were grown at 25 ± 1 °C under a 12 h/12 h light/dark photoperiod. After 4.5 months, the fresh weight, dry weight, number of leaves, and number of roots were calculated. The seedlings were dried at 50 °C for 48 h for dry weight measurement [53]. The resulting data were subjected to one-way ANOVA followed by Tukey’s test (p < 0.05) for pairwise comparisons using the R statistical package (version 4.2.1) [51].
3. Results
3.1. Molecular Identification of Mycorrhizal Fungi through Sanger Sequencing
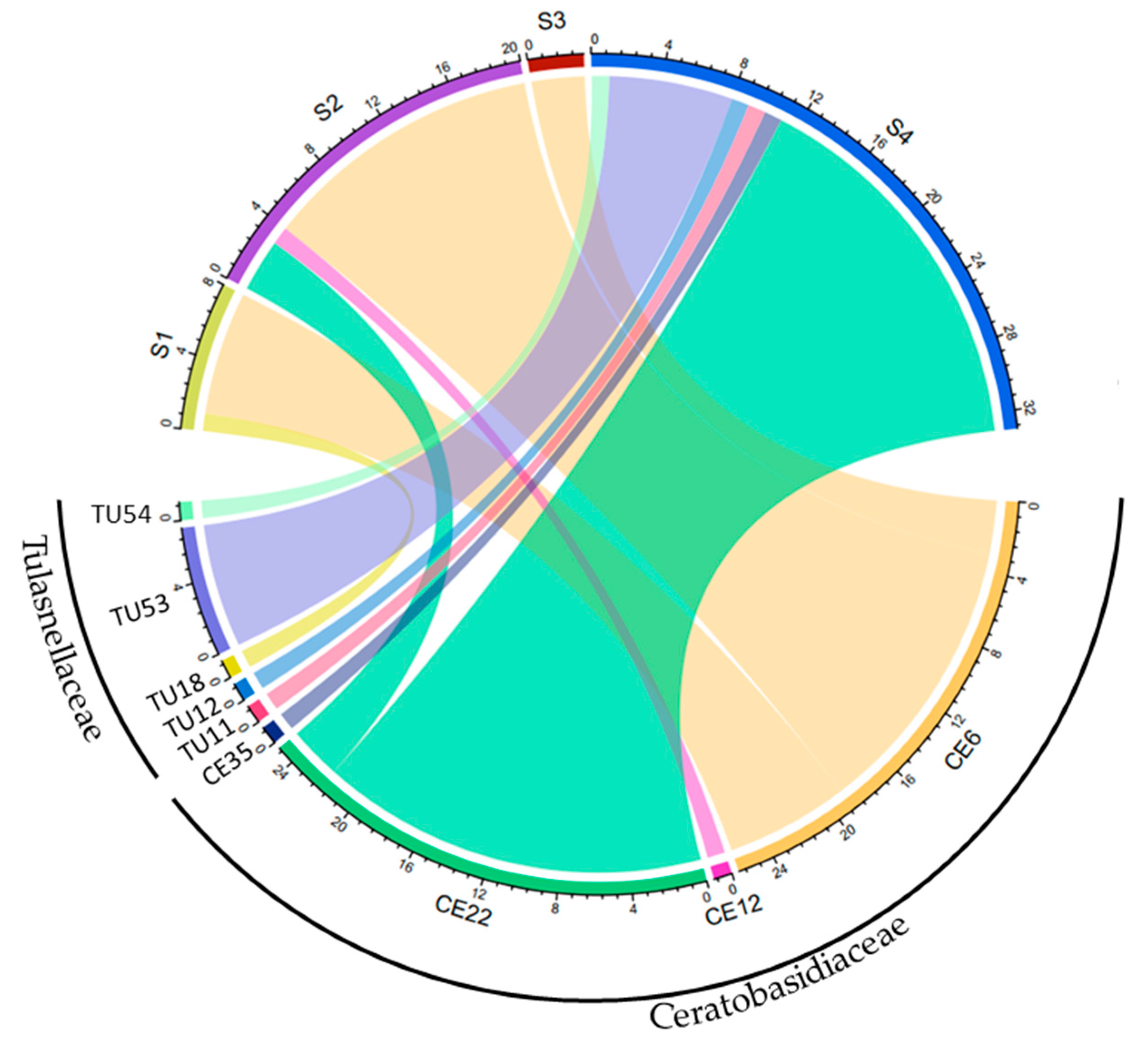
In total, 58 root samples and 16 fungal isolates collected from 32 individuals at 4 sites were analyzed (Table 1). All fungal isolates belonged to the Ceratobasidiaceae family. Specifically, 13, 2, and 1 OTUs were identified as CE6, CE22, and CE12, respectively. In all, 78 fungal sequences were obtained from these samples, of which 82% were OMF, including 68% Ceratobasidiaceae and 14% Tulasnellaceae. The OMF was assigned to nine OTUs, including four Ceratobasidiaceae (CE) and five Tulasnellaceae (TU) (Figure 2). Mycorrhizal fungi were compared among sites. The dominant mycorrhizal fungi varied among sites, with CE6 being the most frequently detected OTU in 26 root samples from 3 sites (S1, S2, and S3), accounting for 41% of all OMF sequences. CE22 was the second-most frequently detected OTU, found in 25 root samples from 2 sites (S2 and S4) and accounting for 39% of fungal sequences. Tulasnellaceae was detected in 11 root samples from 2 sites (S1 and S4), accounting for 17% of total fungal sequences.
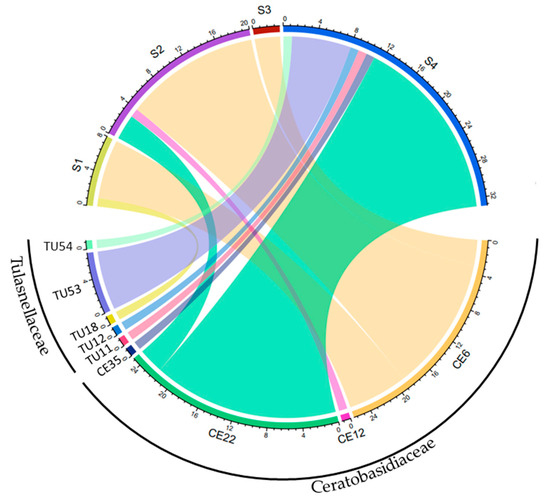
Figure 2.
Relationship between the sampling sites and the abundance of detected OTUs obtained through Sanger sequencing. The width of each strip in the diagram represents the number of OTU taxa detected in different sites. Identical sequences derived from a single sample using different primer pairs or cloning were discarded. CE; Ceratobasidiaceae, TU: Tulasnellaceae.
3.2. Phylogenetic Analysis
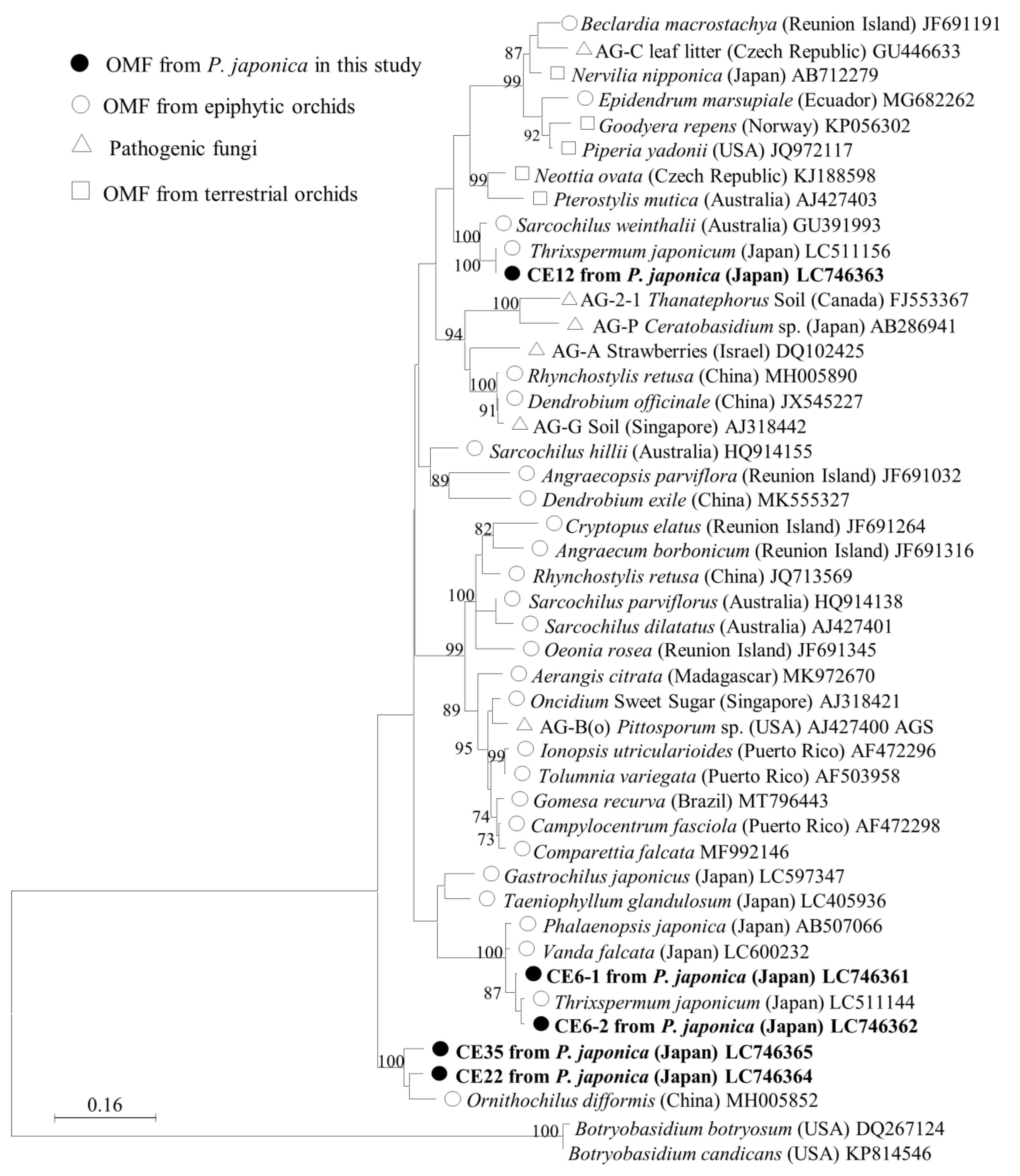
Phylogenetic analysis of putative mycorrhizal fungi in the family Ceratobasidiaceae was conducted using four OTUs identified in this study (Figure 3). One of the dominant mycorrhizal fungus, CE6, has 2 ITS types, designated CE6–1 and CE6–2, which differ in 15 of 600 bp. This OTU formed a monophyletic clade with Ceratobasidiaceae sequences from the epiphytic orchids Thrixspermum japonicum (Japan, LC511144) and Vanda falcata (Japan, LC600232), sharing 96.0–98.2% ITS sequence similarity. The CE12 sequence was closely related to two mycorrhizal fungal sequences from the epiphytic orchid T. japonicum (Japan, LC511156) and Sarcochilus weinthalii (Australia, GU391993), sharing 95.1–99.8% similarity. CE22 and CE35 were more distantly related, forming a separate group that clustered with fungi detected from the epiphytic orchid Ornithochilus difformis (China, MH005852). Aside from this sequence, no other sequences were found with >90% similarity during the BLAST search. These results provide insights into the evolutionary relationships among these fungi and their potential ecological roles.
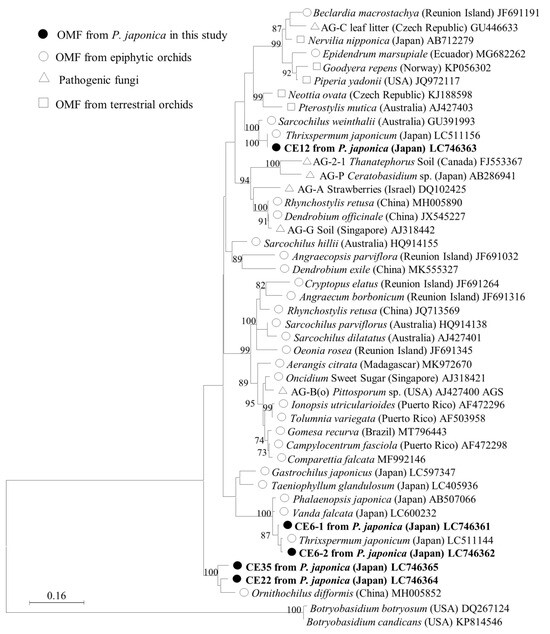
Figure 3.
Maximum-likelihood tree of Ceratobasidiaceae for the ITS1, 5.8S, and ITS2 regions, including the four OTUs identified in this study. The tree is drawn to scale, with branch lengths reflecting the number of substitutions per site. Only bootstrap values greater than 70% are shown. ITS sequences of Botryobasidium were used as the outgroup. The final dataset was 575 bp.
3.3. High-Throughput Sequencing
Three different primer combinations were employed to detect the potential OMF of P. japonica, yielding a total of 31,142,712 raw sequence reads. After filtering out ambiguous and low-quality reads, a total of 2,580,818 high-quality reads were retained. Clustering of those sequences based on a 97% similarity threshold produced 645 OTUs. After removing contaminants, non-fungal OTUs, and OTUs with fewer than 100 sequences, a final count of 135 fungal OTUs (comprising 886,543 sequences) was obtained as our working dataset. The ITS86F/ITS4 primer pair detected a total of 120 OTUs, while the 5.8S-Tulngs/ITS4-Tul2 and 5.8S-Tulngsg3/ITS4-Tul2 primer pairs detected 15 and 10 OTUs, respectively.
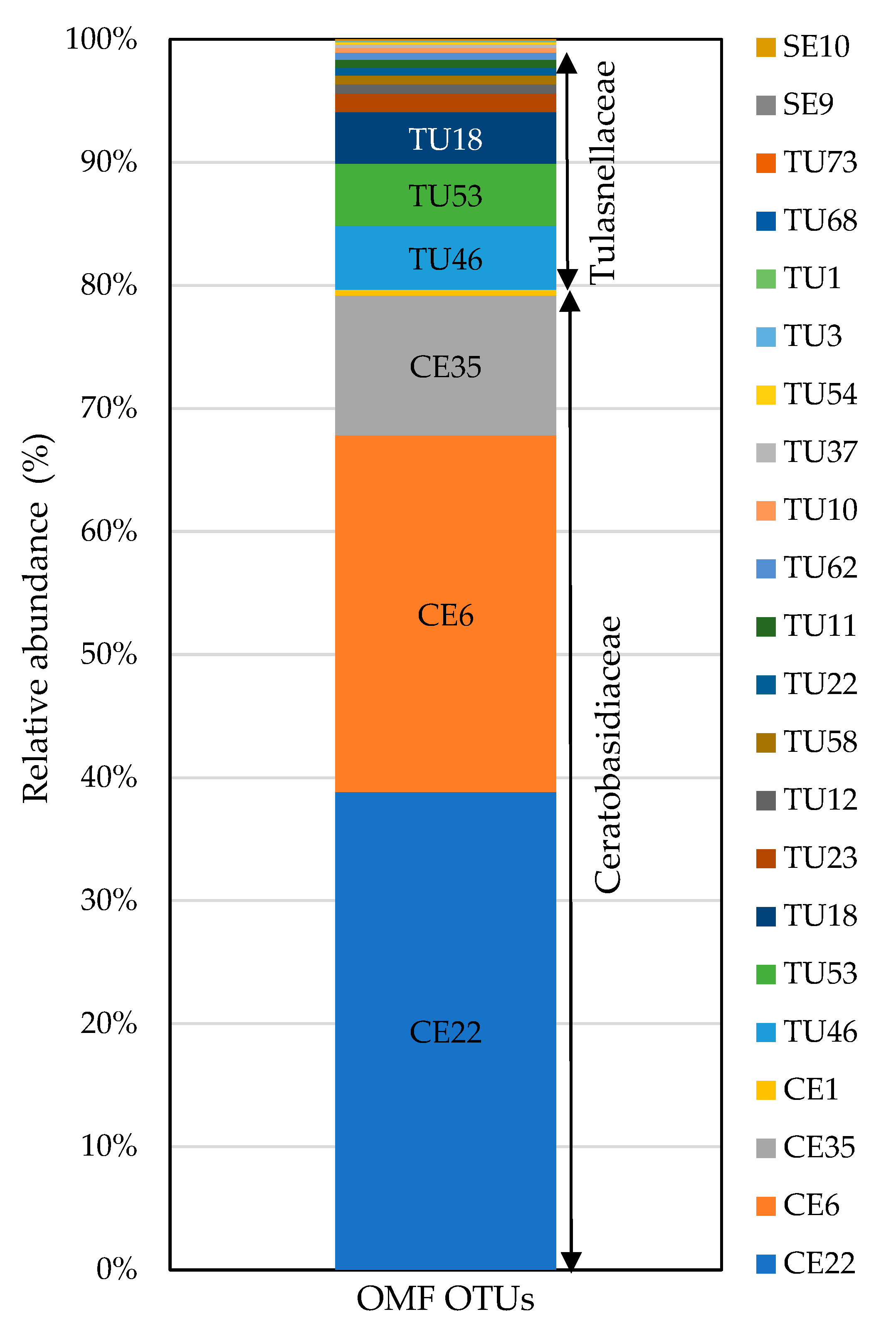
Of all OTU sequences analyzed, 22 (826,325 sequences) were classified into families of OMF, specifically Ceratobasidiaceae and Tulasnellaceae in the order Cantharellales and Serendipitaceae in the order Sebacinales (Table 3). Other putative OMFs, such as Atractiellales (represented by one OTU), Fusarium (represented by one OTU), and Peziza (represented by one OTU) were identified in the sample, but their low frequency precluded their inclusion as key OMFs in our analysis. All OTUs detected through Sanger sequencing, except CE12 (which was an isolate), were also detected with HTS. In addition, HTS revealed the presence of additional OTUs belonging to both Ceratobasidiaceae and Tulasnellaceae, as well as fungi in Serendipitaceae considered OMF species. Tulasnellaceae (16 OTUs), Ceratobasidiaceae (4 OTUs), and Serendipitaceae (2 OTUs) were the fungal groups with the highest numbers of putative OMF OTUs (Table 3 and Figure 4). Among these, Ceratobasidiaceae was the most prevalent fungal group, representing 79.7% (658,332 reads) of the total OMF reads obtained. The variations in the relative abundances of OTUs across three different primer combinations were compared (Supplementary Figure S2).

Table 3.
OMF OTUs detected through Sanger sequencing and high-throughput sequencing (HTS). Frequently detected OTUs are indicated in bold.
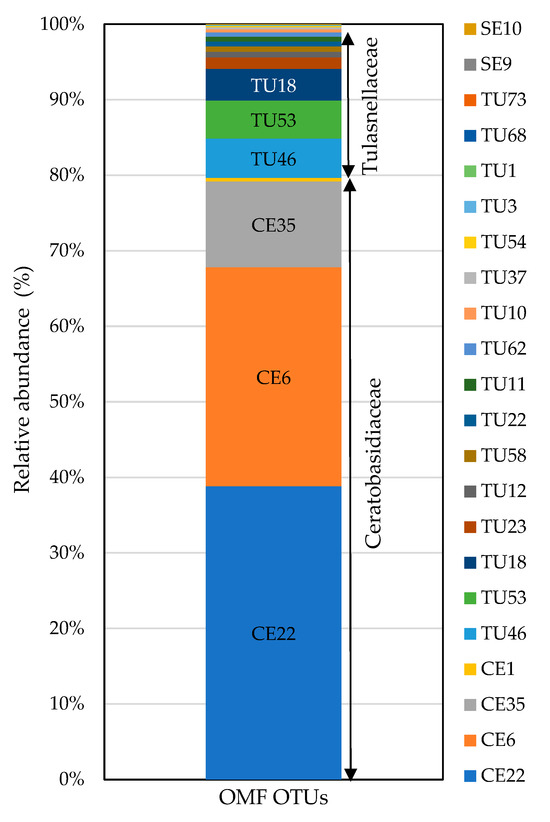
Figure 4.
Relative abundances of OMF OTUs obtained from P. japonica using fungal universal (ITS86F/ITS4) and Tulasnellaceae-specific (5.8S-Tulngs/ITS4-Tul2 and 5.8S-Tulngsg3/ITS4-Tul2) primer pairs. OTUs with fewer than 100 total reads were excluded. TU: Tulasnellaceae, CE: Ceratobasidiaceae, SE: Serendipitaceae. Sequences were assigned to OTUs at 97% sequence similarity. In total, 826,325 reads were obtained for OMF fungi.
3.4. Symbiotic Culture
3.4.1. Effects of Fungal Strains on Symbiotic Seed Germination
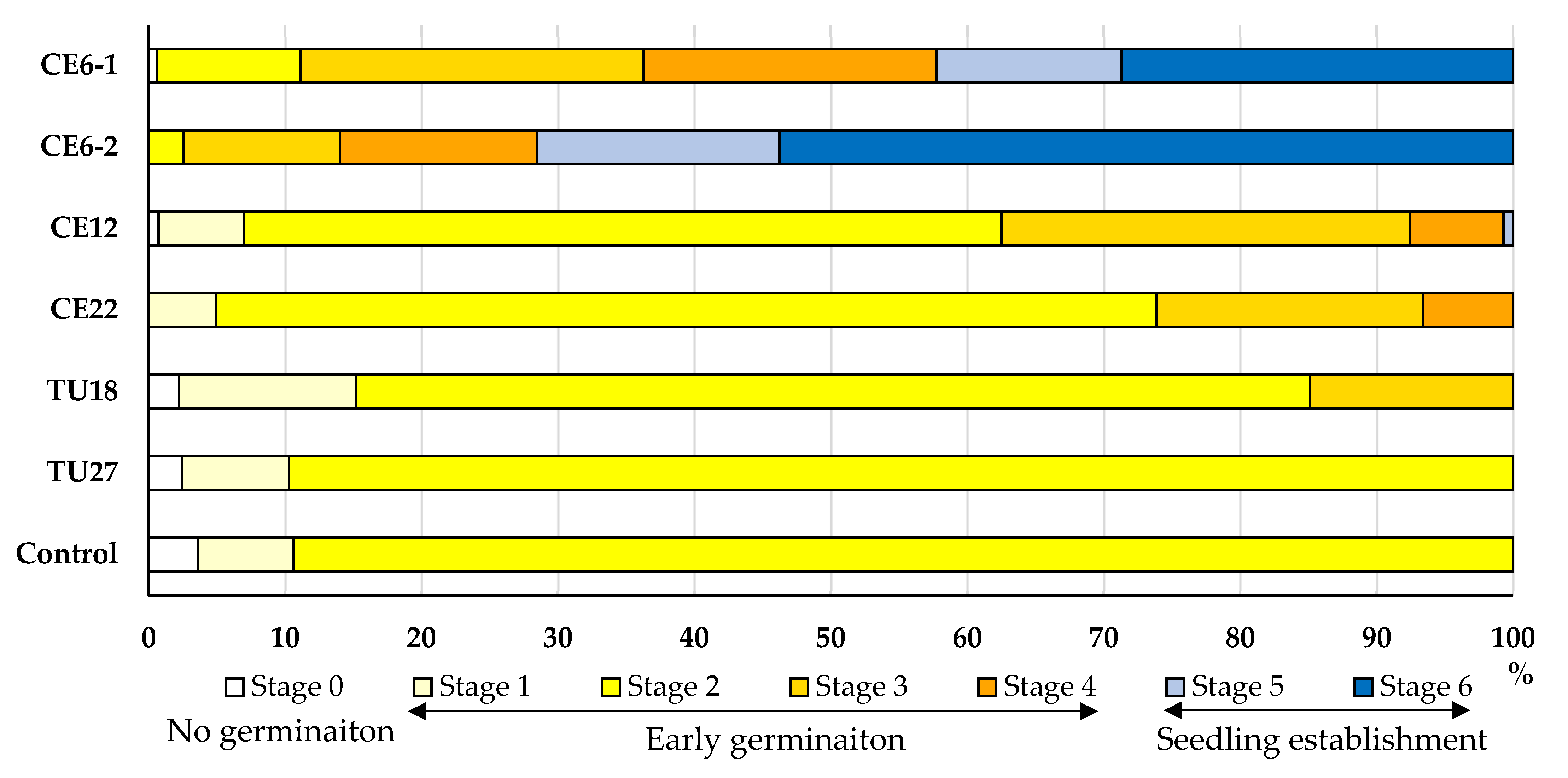
Seeds of P. japonica were cultured with six fungal isolates (five OTUs), four of which belonged to Ceratobasidiaceae and two to Tulasnellaceae (Table 2). After four months of culture, all isolates except TU27 promoted seed germination to varying degrees relative to the control (Figure 5; Supplementary Figure S1). CE6 significantly enhanced seed germination up to stage 6, which was the highest stage among all of the OTUs tested. Significant differences were observed between the two ITS types of CE6 (CE6–1 and CE6–2), with CE6–2 reaching stage 6 earlier than CE6–1 (Supplementary Table S1). In addition, CE12 and CE22 stimulated seed germination through stages 5 and 4, respectively. Over the same period, seeds cultured with TU18 slowly reached the rhizoid formation stage (stage 3). By contrast, TU27 and the un-inoculated control led to swollen seeds that failed to progress beyond stage 2 of germination.
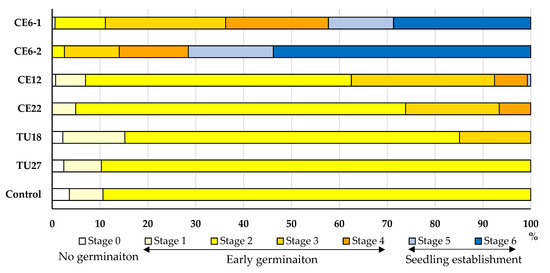
Figure 5.
Effects of fungal isolates on P. japonica seed germination and protocorm development after four months of culturing. Seed development was assigned to six stages. Control indicates seeds that were not inoculated with fungi.
3.4.2. Effects of Fungal Strains on Seedling Growth
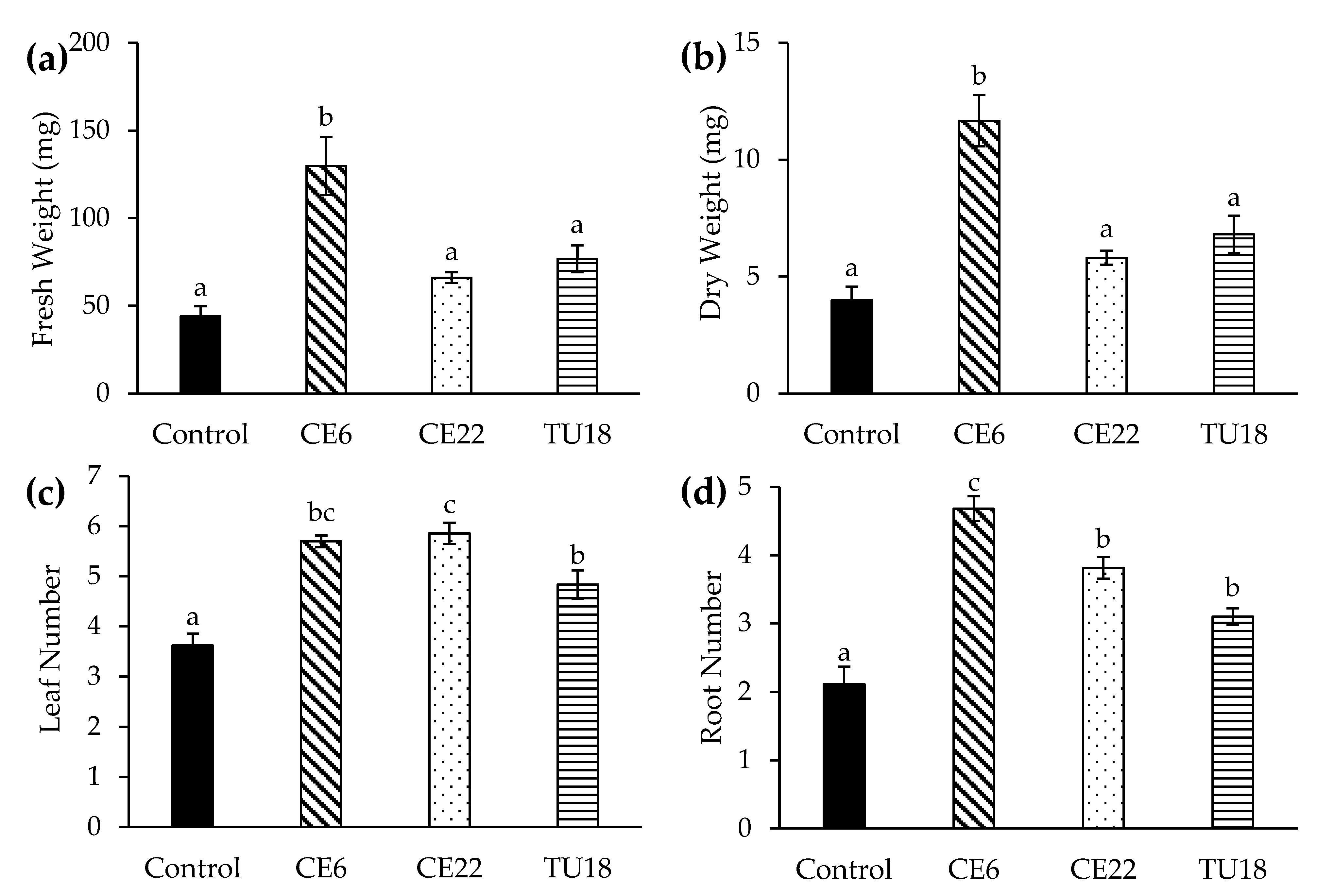
The growth of P. japonica seedlings significantly varied among fungal inoculation treatments (Figure 6). In all of the treatments except TU27, the seedlings were alive after 4.5 months of cultivation. Seedlings inoculated with CE6 displayed the highest fresh and dry matter yields, which were significantly higher than the yields of other treatments. Fresh and dry matter yields of seedlings inoculated with CE22 and TU18 were also relatively high, although not significantly different from each other or the control. In terms of leaf number, all three treatments (excluding TU27) showed significant increases relative to the control, with CE6 and CE22 having the highest leaf numbers. Similarly, the number of roots also significantly differed among treatments, with CE6 producing the highest root number, followed by CE22 and TU18. Overall, CE6 showed the strongest seedling growth, while CE22 and TU18 had significantly higher leaf and root numbers despite relatively little fresh and dry matter accumulation. By contrast, seedlings inoculated with TU27 did not survive beyond 2–3 weeks after inoculation.
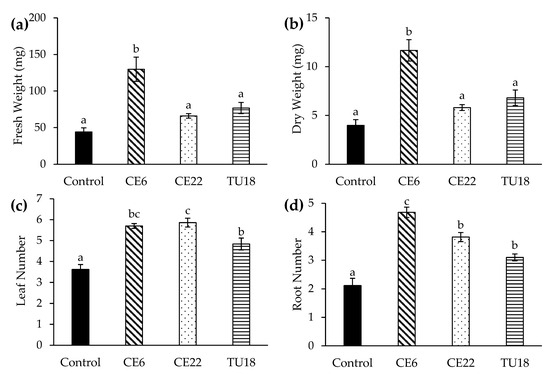
Figure 6.
Effect of fungal isolates on the growth of asymbiotic P. japonica seedlings at 4.5 months after symbiotic culturing. Fresh weight (a), dry weight (b), leaf number (c), and root number (d). Seedlings inoculated with TU27 did not survive and were excluded from the figure. Bars show standard errors (n = 20 plants per treatment). Mean values with different letters are significantly different at p < 0.05 (Tukey test).
4. Discussion
4.1. Mycorrhizal Fungal Composition of Phalaenopsis japonica
Both Sanger and HTS results reveal that the epiphytic orchid P. japonica is associated with diverse mycobionts belonging to mainly two families of Basidiomycota, Ceratobasidiaceae and Tulasnellaceae. Two Ceratobasidiaceae OTUs, CE6 and CE22 were dominant sequences in the adult stage. Thus, P. japonica exhibits diversity in its mycobiont associations within the Ceratobasidiaceae and Tulasnellaceae and tends to establish particularly strong symbioses with these two fungal groups. Previous studies have shown that several Phalaenopsis species, such as P. cornu-cervi and P. wilsonii, have shared associations with various mycorrhizal fungi, including members of the Ceratobasidiaceae, Serendipitaceae, and Tulasnellaceae, but are mainly associated with Ceratobasidiaceae [20,21]. Furthermore, some studies have reported the isolation of Ceratobasidiaceae fungi from Phalaenopsis species, including P. mannii [54], P. pulcherrima [55], and P. amabilis [56,57]. These findings imply that Ceratobasidiaceae is an important fungal partner of the genus Phalaenopsis.
All OMF OTUs identified through Sanger sequencing, except CE12 isolated from P. japonica roots, were also successfully identified via HTS. The dominant presence of CE6 and CE22 in Sanger sequencing was further supported by their relatively high abundances in HTS (68%), confirming that they are the main fungal partners of P. japonica (Figure 4). A greater number of OMF OTUs was identified through HTS (22) than Sanger sequencing (9). While Sanger sequencing identified only two OMF families (Ceratobasidiaceae and Tulasnellaceae), HTS detected three families, including Serendipitaceae, which includes commonly found OMFs. The HTS approach revealed additional OTUs, and greater phylogenetic divergence compared with those detected through Sanger sequencing.
In HTS, while the universal primer pair successfully detected all Ceratobasidiaceae, Serendipitaceae, and one OTU of Tulasnellaceae, the remaining Tulasnellaceae OTUs were detected only with Tulasnella-specific primer pairs (Supplementary Figure S2). All Sanger-sequenced OMF OTUs from the root samples were also detected using the three primer sets, confirming the effective evaluation of the OMF community without primer bias in HTS. Although the universal primer pair was able to detect some Tulasnellaceae OTUs, Tulasnella-specific primer pairs demonstrated greater capacity for the detection of Tulasnellaceae. This finding emphasizes the importance of incorporating Tulasnella-specific primers for a comprehensive assessment of OMF diversity. TU46, the only OTU belonging to lineage TG3, was exclusively detected with the TG3 primer. This OTU was not detected through Sanger sequencing. Other Tulasnella OTUs detected with the TG3 primer were also detected with the primer pair 5.8S-Tulngs/ITS4-Tul2. Epiphytic orchids are mainly associated with TG1 and TG2 [27], implying that TG3 has a rare association with P. japonica.
The OMF communities of P. japonica varied among sites and host tree species (Figure 2 and Table 1). The dominant OTU, CE6, was frequently detected at sites 1–3, implying that P. japonica shares a common fungal partner of CE6 at all of these sites. Moreover, the same OTU was previously observed in P. japonica [58]. At site 4, CE22 was more prevalent, and orchids exhibited fungal associations with multiple Tulasnellaceae fungi, which was not observed at other sites. Environmental conditions and host tree differences may explain this variation. For example, sites 1–3 are characterized by warm temperate climates, while site 4 has a subtropical climate. Furthermore, the host tree at site 4, Pinus luchuensis, differed from those at other sites. A previous study on the epiphytic orchid Dendrobium sinense indicated that host tree species influence the OMF community of orchids [59], and this pattern was also apparent in P. japonica in the present study. However, we cannot definitively identify the host tree effect since each site has distinct hosts. Further analysis is necessary to elucidate this aspect.
The results of the phylogenetic analysis indicate that the Ceratobasidiaceae OTUs identified in this study are phylogenetically diverse and distributed across multiple clades. This finding implies that P. japonica has a wide range of mycobiont partners within the family Ceratobasidiaceae, including established orchid mycobionts. CE6 shares a sequence identity > 96% with mycobionts associated with two other epiphytic orchids collected in Japan, Thrixspermum japonicum and Vanda falcata (Figure 3), implying that it is a common fungal partner of epiphytic orchids. Moreover, OTU CE12 formed a monophyletic clade with fungi associated with two epiphytic orchids, T. japonicum (Japan) and Sarcochilus weinthalii (Australia), with sequence similarities of 99.8% and 95.0%, respectively. These findings imply that CE12 is a common OMF of epiphytic orchids, with a global distribution. OTUs CE22 and CE35 shared high sequence similarities, 96.0%, and 93.4%, respectively, with an OTU from the epiphytic orchid Ornithochilus difformis in China, and no other sequences were identified in the BLAST search with a similarity greater than 90%. These two OTUs were found to be distantly related to other members of the family Ceratobasidiaceae in the phylogenetic tree. This unusual genetic distance may be attributed to the rarity of these OTUs compared with CE6 and CE12 and their unique relationships with epiphytic orchids.
4.2. Fungal Effects on Seed Germination and Seedling Growth
All tested OMFs except TU27 had the potential to promote seed germination but the effectiveness varied significantly among OTUs. CE6, CE12, and CE22 induced seed germination through stages 6, 5, and 4, respectively, while TU18 reached only stage 3 (Figure 5). Therefore, the in vitro symbiotic germination test revealed that the primary inducers of seed germination and early development in P. japonica were fungi in the family Ceratobasidiaceae. Three Ceratobasidiaceae OTUs used in our in vitro study were dispersed throughout the phylogenetic tree, implying that phylogenetically diverse Ceratobasidiaceae play roles in seed germination. Both Ceratobasidiaceae and Tulasnellaceae were associated with orchids during the adult stage but only Ceratobasidiaceae was found to promote seed germination; TU18 and TU27 did not effectively promote seed germination. A similar study conducted by Qin et al. (2021) [21] found that seed germination in P. wilsonii was successfully induced by Ceratobasidiaceae fungi (2 OTUs), while Tulasnellaceae fungi (3 OTUs) failed to do so. This implies that the fungi of Ceratobasidiaceae more effectively promote germination in Phalaenopsis compared with Tulasnellaceae. In that study, Tulasnellaceae fungi were isolated from other epiphytic orchids. Future investigations using Tulasnella isolated directly from the host plant may provide more conclusive evidence.
Symbiotic culturing of asymbiotic seedlings demonstrated that both Ceratobasidiaceae and Tulasnellaceae had significant positive impacts on seedling growth. The OTU CE6 showed the most promising results, as it increased the yields of fresh and dry matter and greater numbers of leaves and roots were formed compared with the other treatments (Figure 6). CE22 and TU18 also significantly contributed to increases in the numbers of roots and leaves. Moreover, seedlings inoculated with these exhibited greater fresh weight and dry weights compared with the control seedlings, although this difference was not statistically significant. CE6 and CE22 played crucial roles in both seed germination and seedling growth, indicating that they are highly important fungi for P. japonica. Two previous studies found that inoculating Phalaenopsis cultivars with mycorrhizal fungi of Rhizoctonia sp., including Ceratobasidium AG-A (R02) and Rhizoctonia solani AG-6 (R04), promoted vegetative and reproductive growth [18,19]. This indicates that Ceratobasidiaceae fungi more effectively promote the growth and development of Phalaenopsis species. TU18 did not effectively promote seed germination but did improve seedling growth. The primary inducers of seed germination and early development in P. japonica were CE6, with CE22 and TU18 also significantly contributing to increased root and leaf numbers. Therefore, OMF bottlenecks in seed germination might be prevalent for P. japonica. Orchid seed germination relies on mycorrhizal fungi for carbon resources. However, once leaves have developed, the plants obtain carbon through photosynthesis. As the physiological requirements of plants vary after leafing, the fungi necessary for their development may also change [60]. This type of divergence in mycorrhizal fungi among early developmental stages, including seed germination, protocorm growth, and seedling development, has been reported in other epiphytic orchid species, such as Dendrobium okinawense [11]. By contrast, TU27 did not exhibit any significant positive impact on seedling growth. Seedlings inoculated with TU27 did not survive beyond 2–3 weeks, implying that this strain may act as a parasite or opportunist and not be suitable for symbiotic culturing with P. japonica.
5. Conclusions
Two OTUs of Ceratobasidiaceae, CE6, and CE22, were the most prevalent fungal OTUs associated with adult P. japonica individuals, and additional OTUs of Tulasnellaceae were also detected. The findings were consistent across both Sanger and HTS analyses. Our results indicate that Ceratobasidiaceae fungal partners, specifically CE6 and CE22, play crucial roles in inducing seed germination and promoting seedling growth and development. These fungal partners are associated with orchids in the adult stage and maintain their association throughout the life cycle of P. japonica. Furthermore, TU18, a minor partner, also promoted seedling growth, implying that minor fungal partners associate with P. japonica after leafing. The primary fungal partners are mainly responsible for producing seedlings, while minor partners contribute significantly to seedling growth. As a result, these fungi may significantly contribute to the establishment and survival of P. japonica populations in their natural habitat and may support mass propagation in the conservation field and horticultural industry. In addition, P. japonica adults were associated with both Ceratobasidiaceae and Tulasnellaceae simultaneously, indicating the potential for a synergistic effect of double association, which could be more effective than a single association. Thus, future research regarding P. japonica should prioritize the evaluation of the combined effect of Ceratobasidiaceae and Tulasnellaceae. Through this comprehensive approach, greater elucidation of the interactions between OMF partners can be achieved for all stages of their symbiosis under natural conditions.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/d16040218/s1, Figure S1: the six developmental stages of seed germination and protocorm development in P. japonica seeds; Figure S2: Relative abundances of OMF OTUs obtained from P. japonica using fungal universal (ITS86F/ITS4) and Tulasnellaceae-specific (5.8S-Tulngs/ITS4-Tul2 and 5.8S-Tulngsg3/ITS4-Tul2) primer pairs; Table S1: Effects of fungal isolates on P. japonica seed germination and protocorm development after four months of culturing.
Author Contributions
Y.O.-T. conceived and designed the study. K.R., M.M., A.K., N.K., T.Y. and Y.O.-T. conducted the sampling. R.M.S.R.C. and K.R. conducted fungal isolation. R.M.S.R.C. performed DNA analysis, conducted experiments, collected, and analyzed the data, and performed bioinformatics analysis. The manuscript was written by R.M.S.R.C. and then revised by Y.O.-T. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Environment Research and Technology Development Fund of the Environmental Restoration and Conservation Agency of Japan (JPMEERF20184004 to N.K.) and by JSPS KAKENHI (21K06306 to Y.O.-T. and 23K05914 to T.Y.).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The sequence reads have been deposited into the DNA Data Bank of Japan (DDBJ) under the accession numbers LC746356–LC746365. All other data and materials supporting this article are available from the corresponding author, Y.O.-T., upon reasonable request.
Acknowledgments
We thank K. Tanaka, K. Tetsuka, and T. Tetsuka for collecting samples. The Sanger sequencing analyses were made using a Genetic Analyzer spectrometer at the Analytical Research Center for Experimental Sciences, Saga University. This research was part of the dissertation submitted by the first author in partial fulfillment of a Ph.D. degree. All authors have given their consent.
Conflicts of Interest
The authors declare no conflict of interests.
References
- Christenhusz, M.J.M.; Byng, J.W. The number of known plant species in the world and its annual increase. Phytotaxa 2016, 261, 201–217. [Google Scholar] [CrossRef]
- Zotz, G. The systematic distribution of vascular epiphytes—A critical update. Bot. J. Linn. Soc. 2013, 171, 453–481. [Google Scholar] [CrossRef]
- Gale, S.W.; Fischer, G.A.; Cribb, P.J.; Fay, M.F. Orchid conservation: Bridging the gap between science and practice. Bot. J. Linn. Soc. 2018, 186, 425–434. [Google Scholar] [CrossRef]
- Hinsley, A.; De Boer, H.J.; Fay, M.F.; Gale, S.W.; Gardiner, L.M.; Gunasekara, R.S.; Kumar, P.; Masters, S.; Metusala, D.; Roberts, D.L.; et al. A review of the trade in orchids and its implications for conservation. Bot. J. Linn. Soc. 2018, 186, 435–455. [Google Scholar] [CrossRef]
- Li, T.; Wu, S.; Yang, W.; Selosse, M.A.; Gao, J. How mycorrhizal associations influence the orchid distribution and population dynamics. Front. Plant Sci. 2021, 12, 647114. [Google Scholar] [CrossRef] [PubMed]
- Dearnaley, J.D.; Martos, F.; Selosse, M.A. Orchid mycorrhizas: Molecular ecology, physiology, evolution, and conservation aspects. In Fungal Associations, 2nd ed.; Hock, B., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 207–230. [Google Scholar] [CrossRef]
- Smith, S.E.; Read, D. Mycorrhizal Symbiosis, 3rd ed.; Academic Press: San Diego, CA, USA, 2008. [Google Scholar]
- Yeh, C.M.; Chung, K.; Liang, C.K.; Tsai, W.C. New insights into the symbiotic relationship between orchids and fungi. Appl. Sci. 2019, 9, 585. [Google Scholar] [CrossRef]
- Rasmussen, H.N.; Rasmussen, F.N. Orchid mycorrhiza: Implications of a mycophagous lifestyle. Oikos 2009, 118, 334–345. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.Y.; Chen, X.M.; Guo, S.X.; Lee, Y.I. Effect of different mycobionts on symbiotic germination and seedling growth of Dendrobium officinale, an important medicinal orchid. Bot. Stud. 2020, 61, 2. [Google Scholar] [CrossRef]
- Zhang, L.; Rammitsu, K.; Kinoshita, A.; Tokuhara, K.; Yukawa, T.; Ogura-Tsujita, Y. Symbiotic culture of three closely related Dendrobium species reveals a growth bottleneck and differences in mycorrhizal specificity at early developmental stages. Diversity 2022, 14, 1119. [Google Scholar] [CrossRef]
- Jacquemyn, H.; Brys, R.; Merckx, V.S.; Waud, M.; Lievens, B.; Wiegand, T. Coexisting orchid species have distinct mycorrhizal communities and display strong spatial segregation. New Phytol. 2014, 202, 616–627. [Google Scholar] [CrossRef]
- Waud, M.; Busschaert, P.; Lievens, B.; Jacquemyn, H. Specificity and localized distribution of mycorrhizal fungi in the soil may contribute to co-existence of orchid species. Fungal Ecol. 2016, 20, 155–165. [Google Scholar] [CrossRef]
- Govaerts, R.; Bernet, P.; Kratochvil, K.; Gerlach, G.; Carr, G.; Alrich, P.; Pridgeon, A.M.; Pfahl, J.; Campacci, M.A.; Holland, B.D.; et al. World checklist of Orchidaceae. Facilitated by the Royal Botanic Gardens, Kew. 2021. Available online: http://wcsp.science.kew.org/ (accessed on 4 July 2023).
- Christenson, E.A. Phalaenopsis—A Monograph; Timber Press: Portland, OR, USA, 2001; pp. 458–465. [Google Scholar]
- Yuan, S.C.; Bolaños-Villegas, P.; Tsao, C.Y.; Chen, F.C. The breeding of Phalaenopsis hybrids. In The Orchid Genome. Compendium of Plant Genomes; Chen, F.C., Chin, S.W., Eds.; Springer: Cham, Switzerland, 2021; pp. 29–40. [Google Scholar] [CrossRef]
- IUCN. IUCN Red List of Threatened Species. Version 2022-2. 2022. Available online: https://www.iucnredlist.org (accessed on 15 July 2023).
- Chang, D.C. The screening of orchid mycorrhizal fungi (OMF) and their applications. In Orchid Biotechnology; Chen, W.H., Chen, H.H., Eds.; World Scientific: Singapore, 2007; pp. 77–98. [Google Scholar]
- Wu, P.H.; Huang, D.D.; Chang, D.C. Mycorrhizal symbiosis enhances Phalaenopsis orchid’s growth and resistance to Erwinia chrysanthemi. Afr. J. Biotechnol. 2011, 10, 10095–10100. [Google Scholar] [CrossRef]
- Izuddin, M.; Srivathsan, A.; Lee, A.L.; Yam, T.W.; Webb, E.L. Availability of orchid mycorrhizal fungi on roadside trees in a tropical urban landscape. Sci. Rep. 2019, 9, 19528. [Google Scholar] [CrossRef]
- Qin, J.; Zhang, W.; Feng, J.Q.; Zhang, S.B. Leafless epiphytic orchids share Ceratobasidiaceae mycorrhizal fungi. Mycorrhiza 2021, 31, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Suárez, J.P.; Weiß, M.; Abele, A.; Garnica, S.; Oberwinkler, F.; Kottke, I. Diverse tulasnelloid fungi form mycorrhizas with epiphytic orchids in an Andean cloud forest. Mycol. Res. 2006, 110, 1257–1270. [Google Scholar] [CrossRef]
- Suárez, J.P.; Weiß, M.; Abele, A.; Oberwinkler, F.; Kottke, I. Members of Sebacinales subgroup B form mycorrhizae with epiphytic orchids in a neotropical mountain rain forest. Mycol. Prog. 2008, 7, 75–85. [Google Scholar] [CrossRef]
- Kottke, I.; Suárez, J.P.; Herrera, P.; Cruz, D.; Bauer, R.; Haug, I.; Garnica, S. Atractiellomycetes belonging to the ‘rust’ lineage (Pucciniomycotina) form mycorrhizae with terrestrial and epiphytic neotropical orchids. Proc. R. Soc. B Biol. 2010, 277, 1289–1298. [Google Scholar] [CrossRef] [PubMed]
- Valadares, R.B.D.S.; Otero, J.T.; Pereira, M.C.; Cardoso, E.J.B.N. The epiphytic orchids Ionopsis utricularioides and Psygmorchis pusilla associate with different Ceratobasidium lineages at Valle del Cauca, Colombia. Acta Bot. Bras. 2015, 29, 40–44. [Google Scholar] [CrossRef]
- Waud, M.; Busschaert, P.; Ruyters, S.; Jacquemyn, H.; Lievens, B. Impact of primer choice on characterization of orchid mycorrhizal communities using 454 pyrosequencing. Mol. Ecol. Resour. 2014, 14, 679–699. [Google Scholar] [CrossRef]
- Rammitsu, K.; Kajita, T.; Imai, R.; Ogura-Tsujita, Y. Strong primer bias for Tulasnellaceae fungi in metabarcoding: Specific primers improve the characterization of the mycorrhizal communities of epiphytic orchids. Mycoscience 2021, 62, 356–363. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.J.W.T.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press, Inc.: New York, NY, USA, 1990; pp. 315–322. [Google Scholar]
- Turenne, C.Y.; Sanche, S.E.; Hoban, D.J.; Karlowsky, J.A.; Kabani, A.M. Rapid identification of fungi by using the ITS2 genetic region and an automated fluorescent capillary electrophoresis system. J. Clin. Microbiol. 1999, 37, 1846–1851. [Google Scholar] [CrossRef] [PubMed]
- Oja, J.; Kohout, P.; Tedersoo, L.; Kull, T.; Kõljalg, U. Temporal patterns of orchid mycorrhizal fungi in meadows and forests as revealed by 454 pyrosequencing. New Phytol. 2015, 205, 1608–1618. [Google Scholar] [CrossRef] [PubMed]
- Rammitsu, K.; Abe, S.; Abe, T.; Kotaka, N.; Kudaka, M.; Kudaka, N.; Kinoshita, A.; Ogura-Tsujita, Y. The endangered epiphytic orchid Dendrobium okinawense has a highly specific mycorrhizal association with a single Tulasnellaceae fungus. J. For. Res. 2021, 26, 215–221. [Google Scholar] [CrossRef]
- McCormick, M.; Burnett, R.; Whigham, D. Protocorm-supporting fungi are retained in roots of mature Tipularia discolor orchids as mycorrhizal fungal diversity increases. Plants 2021, 10, 1251. [Google Scholar] [CrossRef]
- Rammitsu, K.; Yamamoto, N.; Chamara, R.M.S.R.; Minobe, M.; Kinoshita, A.; Kotaka, N.; Ogura-Tsujita, Y. The epiphytic orchid Vanda falcata is predominantly associated with a single Tulasnellaceae fungus in adulthood, and Ceratobasidiaceae fungi strongly induce its seed germination in vitro. Plant Species Biol. 2023, 38, 306–318. [Google Scholar] [CrossRef]
- Alrich, P.; Higgins, W. Phalaenopsis japonica. Phytotaxa 2014, 161, 67. [Google Scholar]
- Shim, Y.J.; Park, Y.S.; Jang, R.H.; Yoon, Y.J.; Kim, S.R.; Han, S.H. The development of habitat suitability index model of class I endangered wildlife, Sedirea japonica. J. Korean Isl. 2020, 32, 153–172. [Google Scholar] [CrossRef]
- Kim, Y.K.; Jo, S.J.; Kim, K.J. Phylogenetic position of Neofinetia and Sedirea (Orchidaceae) and their species identification using the chloroplast matK and the nuclear ITS sequences. Korean J. Pl. Taxon. 2014, 44, 39–50. [Google Scholar] [CrossRef][Green Version]
- Izumitsu, K.; Hatoh, K.; Sumita, T.; Kitade, Y.; Morita, A.; Tanaka, C.; Gafur, A.; Ohta, A.; Kawai, M.; Yamanaka, T.; et al. Rapid and simple preparation of mushroom DNA directly from colonies and fruiting bodies for PCR. Mycoscience 2012, 53, 396–401. [Google Scholar] [CrossRef]
- Gardes, M.; Bruns, T.D. ITS primers with enhanced specificity for basidiomycetes—Application to the identification of mycorrhizae and rusts. Mol. Ecol. 1993, 2, 113–118. [Google Scholar] [CrossRef]
- Kuraku, S.; Zmasek, C.M.; Nishimura, O.; Katoh, K. aLeaves facilitates on-demand exploration of metazoan gene family trees on MAFFT sequence alignment server with enhanced interactivity. Nucleic Acids Res. 2013, 41, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Rozewicki, J.; Yamada, K.D. MAFFT online service: Multiple sequence alignment, interactive sequence choice, and visualization. Brief. Bioinform. 2019, 20, 1160–1166. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evol. 1985, 39, 783–791. [Google Scholar] [CrossRef]
- Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data. 2010. Available online: http://www.bioinformatics.babraham.ac.uk/projects/fastqc (accessed on 5 May 2023).
- Catchen, J.; Hohenlohe, P.A.; Bassham, S.; Amores, A.; Cresko, W.A. Stacks: An analysis tool set for population genomics. Mol. Ecol. 2013, 22, 3124–3140. [Google Scholar] [CrossRef] [PubMed]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahé, F. VSEARCH: A versatile open-source tool for metagenomics. PeerJ 2016, 4, 2584. [Google Scholar] [CrossRef]
- Abarenkov, K.; Zirk, A.; Piirmann, T.; Pöhönen, R.; Ivanov, F.; Nilsson, R.H.; Kõljalg, U. UNITE QIIME release for fungi. Version 16.10.2022. UNITE Community 2022. [Google Scholar] [CrossRef]
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Dubourg, V.; et al. Scikit-learn: Machine learning in Python. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023. [Google Scholar]
- Tokuhara, K.; Mii, M. Micropropagation of Phalaenopsis and Doritaenopsis by culturing shoot tips of flower stalk buds. Plant Cell Rep. 1993, 13, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.M.; Wang, C.L.; Chen, X.M.; Zhou, Y.Q.; Wang, Y.Q.; Luo, A.X.; Liu, Z.H.; Guo, S.X. In vitro seed germination and seedling growth of an endangered epiphytic orchid, Dendrobium officinale, endemic to China using mycorrhizal fungi (Tulasnella sp.). Sci. Hortic. 2014, 165, 62–68. [Google Scholar] [CrossRef]
- Saha, D.; Rao, A.N. Studies on endophytic mycorrhiza of some selected orchids of Arunachal Pradesh–1. Isolation and identification. Bull. Arunachal Forest Res. 2006, 22, 9–16. [Google Scholar]
- Idris, N.A.; Zuhir, Z.M.; Radzuan, N.A.M.; Muda, N.S.; Rosli, R.I. In vitro response of fungi isolated from orchids in BRIS, Setiu wetland and mangrove in Morib, to different concentrations of lead. Malays. Appl. Biol. 2019, 48, 229–233. [Google Scholar]
- Situmorang, N.; Kasiamdari, R.S. Isolation and identification of Rhizoctonia associated with Phalaenopsis amabilis (L.) Blume roots. In Proceedings of the ICBB, The International Conference on Bioscience and Biotechnology, Yogyakarta, Indonesia, 11–12 October 2011; Volume 1, pp. 39–44. [Google Scholar]
- Warcup, J.H. The mycorrhizal relationships of Australian orchids. New Phytol. 1981, 87, 371–381. [Google Scholar] [CrossRef]
- Ogura-Tsujita, Y.; Yokoyama, J.; Miyoshi, K.; Yukawa, T. Shifts in mycorrhizal fungi during the evolution of autotrophy to mycoheterotrophy in Cymbidium (Orchidaceae). Am. J. Bot. 2012, 99, 1158–1176. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Song, X.; Meng, Q.; Zhu, J.; Zhao, Y.; Yu, W. Influence of host tree species on isolation and communities of mycorrhizal and endophytic fungi from roots of a tropical epiphytic orchid, Dendrobium sinense (Orchidaceae). Mycorrhiza 2017, 27, 709–718. [Google Scholar] [CrossRef]
- Ventre Lespiaucq, A.; Jacquemyn, H.; Rasmussen, H.N.; Mendez, M. Temporal turnover in mycorrhizal interactions: A proof of concept with orchids. New Phytol. 2021, 230, 1690–1699. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).