Abstract
Glomalin-related soil protein (GRSP), an important arbuscular mycorrhizal (AM) fungal by-product, plays a key role in preserving or sequestrating soil organic carbon (C). Silver nanoparticles (AgNPs) have become an emerging contaminant and their impacts on soil ecosystems attract increasing concerns. The dynamics of AM fungi and GRSP could therefore form the basis for an in-depth exploration of the influences of AgNPs on soil ecosystems. This study investigated the effects of AgNPs on mycorrhizal growth and AM fungal communities, as well as the GRSP contents in maize (Zea mays L.) soils, with a pot experiment. The contributions of GRSP to soil organic C and the correlations of GRSP with soil organic C were also evaluated. The results indicated that AgNPs decreased the mycorrhizal colonization, AM fungal biomass, and diversity indices, and strongly shifted the community composition of AM fungi with a reduction in Acaulosporaceae and an enrichment in Glomeraceae. Additionally, AgNPs also decreased the soil’s easily extractable (EE) GRSP and total (T) GRSP contents, resulting in lower contributions of EE-GRSP-C and T-GRSP-C to the soil organic C. Linkage analyses revealed that AM fungal abundances have positive correlations with EE- and T-GRSP, and EE- and T-GRSP also positively correlated with soil organic C, indicating that the negative effects of AgNPs on AM fungal abundances and communities were extended to AM-fungal-associated C processes. Altogether, our study found that AgNPs decreased the AM fungal abundances shaped AM fungal communities, and reduced the soil GRSP content, which might subsequently be unfavorable for soil C storage.
1. Introduction
Glomalin-related soil protein (GRSP) is an important component of soil carbon (C) pools. It can promote soil aggregate formation and soil C accumulation, thus benefiting soil fertility and plant productivity [1,2]. GRSP is a complex mixture of glomalin, lipids, and humic matter or other heat-stable proteins [3]. Glomalin has long been recognized as a glycoprotein produced by arbuscular mycorrhizal (AM) fungi [4]. AM fungi can form mutual associations with more than 85% of land plants that utilize approximately 4–20% of plant-derived C and then excrete recalcitrant C products such as chitin and glomalin to soils as well as providing C to the microbial community [5]. Therefore, AM fungi are well known to participate in soil C cycles [1,6]. Environmental changes could alter plant growth and thus substantially affect AM fungi growth and community, which might further influence soil GRSP contents.
Engineered nanoparticles are becoming potential environmental contaminants due to the increasing usage of nanoparticle products in a series of technical applications [7]. Silver nanoparticles (AgNPs) are one of three commonly used engineered nanoparticles in the world [8]. Previous studies have found that the biological effects of AgNPs were complex and depended on the AgNP dose, the treatment duration, and the soil type [9,10,11]. For example, AgNPs at appropriate doses were found to prime biological defense pathways and thus stimulate the growth of plants and microbes [12,13]. Alternatively, when present at high doses, AgNPs can exert oxidative stresses on plants and microbes, resulting in decreases in plant biomasses [10,14], reductions in microbial biomasses and activities [9], and variations in microbial community composition [15,16]. Changes in plant and AM fungal growth might affect the contents of soil GRSP by altering glomalin production. Previous studies have found that heavy metals (e.g., molybdenum, cadmium) decreased the GRSP contents, and the reduction was positively related to heavy metal levels [17,18]. Other works have observed that cadmium stimulated the secretion of GRSP and increased soil GRSP contents [19,20]. These inconsistent responses of GRSP to heavy metals might be attributed to the different heavy metal levels and soil conditions. AgNPs are also expected to influence soil GRSP contents directly via AM fungi–nanoparticle interactions or indirectly through plant–AM fungi interactions as AM fungi are an important C route from plants to soils. However, few investigations have focused on the effects of AgNPs on GRSP contents and AM-fungal-associated soil C processes.
Maize is one of the most important agricultural crops in the world and can form strong mutual interactions with AM fungi [21]. In this study, a pot experiment was conducted to explore the effects of AgNPs on AM fungal abundances, communities, and GRSP contents in the rhizosphere of maize seedlings. Previous studies found that AgNPs significantly decreased plant biomasses [9,22] and mycorrhizal colonization [15,23]. Because decreased plant growth might lead to a lower allocation of photosynthates to belowground mycorrhizas, it was hypothesized that AgNPs would decrease the soil GRSP contents. Moreover, fungal-associated soil C accumulation has been found to closely correlate with AM fungal communities since AM fungal species possess different abilities to produce glomalin [1,6]. Thus, we further assumed that the effects of AgNPs on AM fungal abundances and communities would extend to their associated soil C processes. The results will aid in systematic evaluations of the ecological effects of nanoparticles on soil systems, which are important for scientific applications of nanoproducts. The correlations between GRSP and soil C contents were also estimated.
2. Materials and Methods
2.1. Soil and AgNPs Preparation
Soil was collected from an agricultural field at the Fengqiu Agro-ecological experimental station of the Chinese Academy of Sciences, Fengqiu County (35°00′ N, 114°24′ E), Henan Province, northern China. The crop succession has been winter wheat and summer maize since 1989. The soil is categorized as an Aquic Inceptisol (a calcareous fluvo-aquic soil) with sandy loam texture. Soil was air-dried, sieved (2 mm), and then stored for the pot experiment. Commercial AgNPs were purchased from Shanghai Aladdin Biochemical Technology Co., Ltd. (Shanghai, China). The particle’s average diameter was approximately 20.6 ± 3.1 nm.
2.2. Pot Experiment and Sample Collection
Previous studies have set up wide ranges of AgNP concentrations to evaluate the effects on soil ecosystems due to the increasing usage and inevitable release of AgNPs [15,24]. Therefore, nanoparticle-amended soils were prepared by adding 1, 2.5, 5, and 10 mg AgNPs to 500 g soil to reach a wide range of NP levels of 1, 5, 10, and 20 mg kg−1 air-dried soil. Soil without AgNPs was used as the control. AgNP solution was diluted in water and then added drop by drop to the soil surface [25]. There were five treatments with three replicates, resulting in a total of 15 pots.
Maize seeds were sterilized with sodium hypochlorite, washed with distilled water, and germinated at a temperature of 28 °C for 2 days. Two germinated seeds of uniform size were sowed into each pot (10 cm diameter × 8 cm depth) filled with AgNP-amended soil. All maize seedlings were grown in a natural and full-sunlight greenhouse. During the entire pot experiment period, maize seedlings were irrigated to maintain 70% of the field water-holding capacity. After 30 days, a time at which the impacts of AgNPs on plants were visible, maize seedlings were harvested and soil samples were collected as previously reported [26]. Soil samples were separated into two subsamples and stored at 4 °C and −20 °C for soil property determination and DNA extraction, respectively.
2.3. Soil Property Determination
Soil pH and electrical conductivity (EC) were measured using a pH meter and EC meter, respectively, with a soil/water suspension (1:5, w/v). Dissolved organic carbon (DOC) was measured using an elemental analyzer (Skalar, The Netherlands) after extraction with potassium sulfate. Nitrate (NO3−-N) and ammonium (NH4+-N) contents were measured by a continuous-flow analyzer (San++, Breda, Netherlands). Available phosphorus (AP) was extracted with sodium bicarbonate and determined using a spectrophotometer with the molybdenum method [27]. Available potassium (AK) was extracted using ammonium acetate and tested using a flame photometer [27]. Soluble Ag content was analyzed with an inductively coupled plasma atomic emission spectrophotometer (ICP-AES) after extraction by diethylene triamine penta-acetic acid (DTPA) [28].
2.4. Mycorrhizal Colonization and AM Fungal Biomass Analyses
Fresh roots were washed with water, cleared with potassium hydroxide and hydrochloric acid, and stained with trypan blue for mycorrhizal colonization determination. One hundred root fragments were randomly selected and counted to determine the proportion of roots colonized by AM fungi via the gridline intersect technique with a stereomicroscope [29].
According to the protocols described by Frostegård et al., [30], lipids were extracted using a chloroform–methanol–citrate buffer from soils. The phospholipid fatty acids (PLFAs) were separated from extracted lipids using silica acid columns and then quantified using gas chromatography (6890N, Agilent, Santa Clara, CA, USA) using C19:0 as an internal reference. The 16:1ω5c was used to evaluate the biomass of AM fungi [31,32].
2.5. GRSP and SOC Contents Determination
GRSP can be categorized into easily extractable (EE) GRSP, difficult to extract (DE) GRSP, and total (T) GRSP [33]. In brief, 1.0 g soils were used to extract EE-GRSP and T-GRSP with sodium citrate (pH 7.0 or pH 8.0) at 121 °C for 30 min and at 121 °C for 60 min, respectively. The extraction program of T-GRSP was repeated until the supernatant was straw-colored. The soil GRSP amount was determined by the Bradford protein method using bovine serum albumin as the standard [34]. DE-GRSP content was calculated as T-GRSP minus EE-GRSP. Moreover, the C contribution of GRSP was evaluated using a coefficient of 45% C [35,36]. Soil organic carbon (SOC) content was determined after oxidizing by K2CrO7 with an oil bath [37].
2.6. Soil DNA Extraction and AM Fungal Community Sequencing
Soil genomic DNA was extracted with a FastDNA SPIN Kit for soil (MP Biomedicals, Santa Ana, CA, USA) and then stored at −20 °C until further amplification. The primer pairs of AMV4.5NF and AMDGR were used to amplify the target AM fungal gene fragment [38]. The PCR was conducted with 50 μL reaction mixtures containing 2 U Taq DNA polymerase, 20 μmol L−1 each primer, DNA template, and sterile deionized water. The program of PCR amplifications was performed at 94 °C for 5 min, and then 35 cycles of 94 °C for 30 s, 55 °C for 30 s, and 72 °C for 30 s, and finally at 72 °C for 7 min on a thermal cycler (Bio-Rad, Hercules, CA, USA). All PCR products were purified and mixed at equimolar concentrations. Finally, sequencing was performed on an Illumina MiSeq PE250 platform (Illumina, San Diego, CA, USA).
All generated raw reads were processed by the QIIME pipeline [39]. First, low-quality sequences with ambiguous nucleotides, lower quality scores (<20), or short lengths (<200 bp) were removed with USEARCH. The high-quality sequences were then clustered into Operational Taxonomic Units (OTUs) at a 97% similarity level with UPARSE. All singletons and chimeras were detected and removed. Taxonomy was assigned by blasting the most abundant sequence in each OTU against the MaarjAM database [40]. To correct the sampling effort, the smallest sample size was selected for downstream analyses [41]. Sequences were deposited in the NCBI (SRP071033).
2.7. Statistical Data Analysis
Data were displayed as means with standard deviation (SD). The statistical analyses were conducted based on Tukey’s multiple range tests at the p < 0.05 using SPSS 18.0 (SPSS Inc., Chicago, IL, USA). Linkages between GRSP and mycorrhizal colonization or PLFA, as well as between GRSP and SOC, were determined using linear regression analyses. The phylogenetic diversity (PD), Chao1, and observed OTUs were estimated to test the diversity of AM fungal communities. Principal coordinates analysis was used to analyze the community composition of AM fungi based on the Unifrac distance matrix in R [42]. The response ratio method was used to analyze the changes in AM fungal abundances at the OTU level under AgNPs [43]. The mantel test was used to investigate the correlations between soil properties and the diversity and community composition of AM fungi under AgNPs [44]. The variations in potential markers in AM fungal communities under AgNPs were analyzed with the randomForest package in R [45].
3. Results
3.1. Mycorrhizal Colonization Rate, AM Fungal Biomass, and GRSP Content
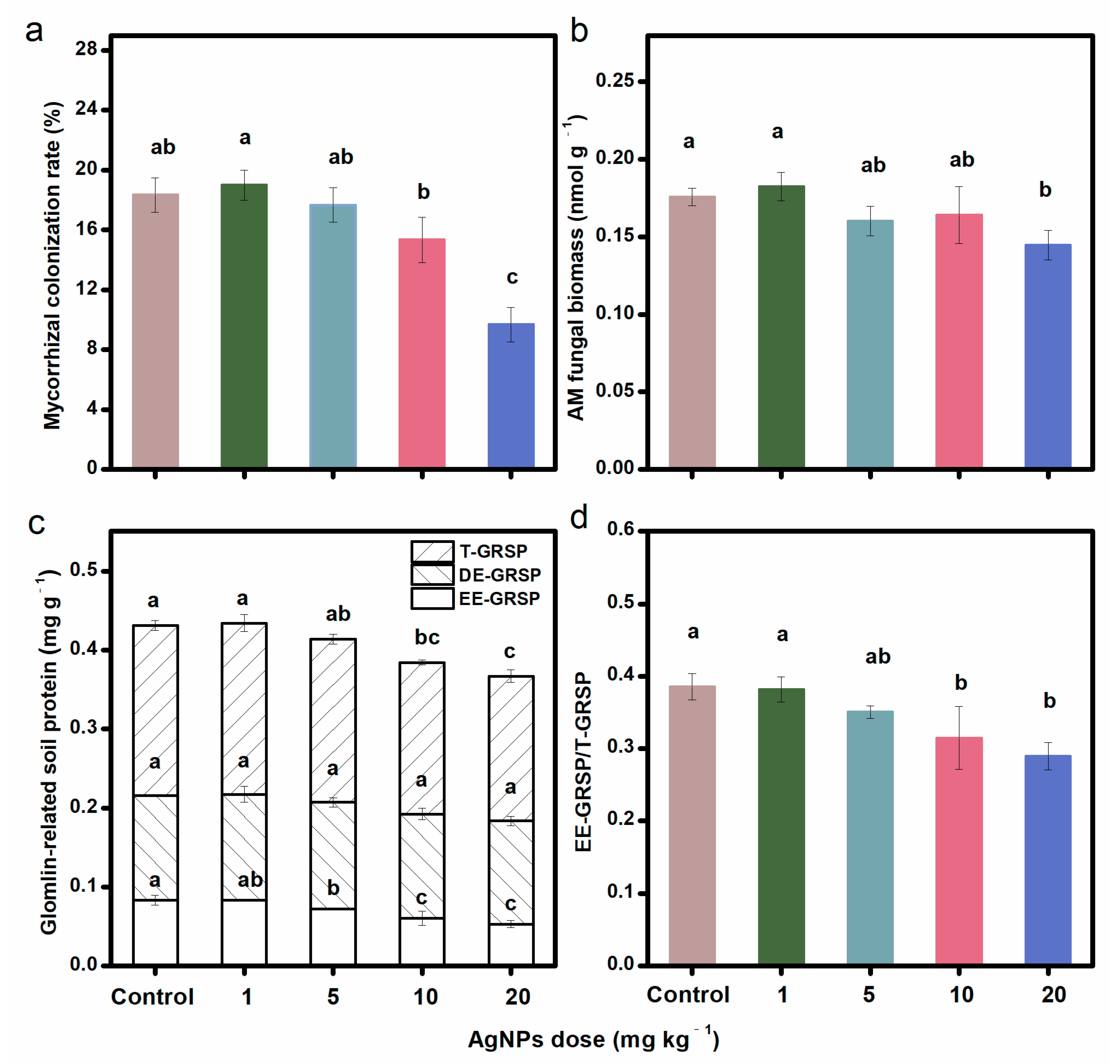
Compared to the control, AgNPs at different concentrations decreased the mycorrhizal colonization rate (Figure 1a), AM fungal biomass (as indicated by PLFA 16:1ω5c) (Figure 1b), EE-GRSP, and T-GRSP (Figure 1c), with greater decreases occurring under higher concentrations of AgNPs. AgNPs at 20 mg kg−1 significantly decreased the mycorrhizal colonization rate, AM fungal biomass, EE-GRSP, and T-GRSP contents. AgNPs at 20 mg kg−1 also decreased the EE-GRSP/T-GRSP ratio (Figure 1d). However, regardless of the doses, the soil DE-GRSP content was not changed by AgNPs. AgNPs also had a negative influence on the SOC content. AgNPs at 20 mg kg−1 significantly decreased the SOC content (Figure S1).
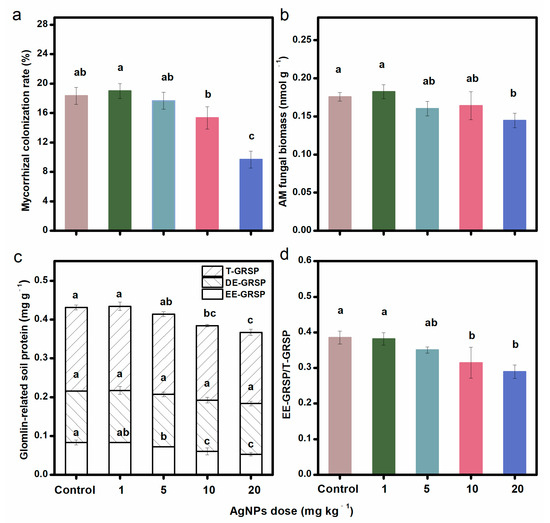
Figure 1.
Mycorrhizal colonization rate (a), AM fungal biomass (b), GRSP contents (c), and EE-GRSP/T-GRSP ratio (d) under different concentrations of AgNPs. Bars with different letters indicated significant differences among treatments (p < 0.05).
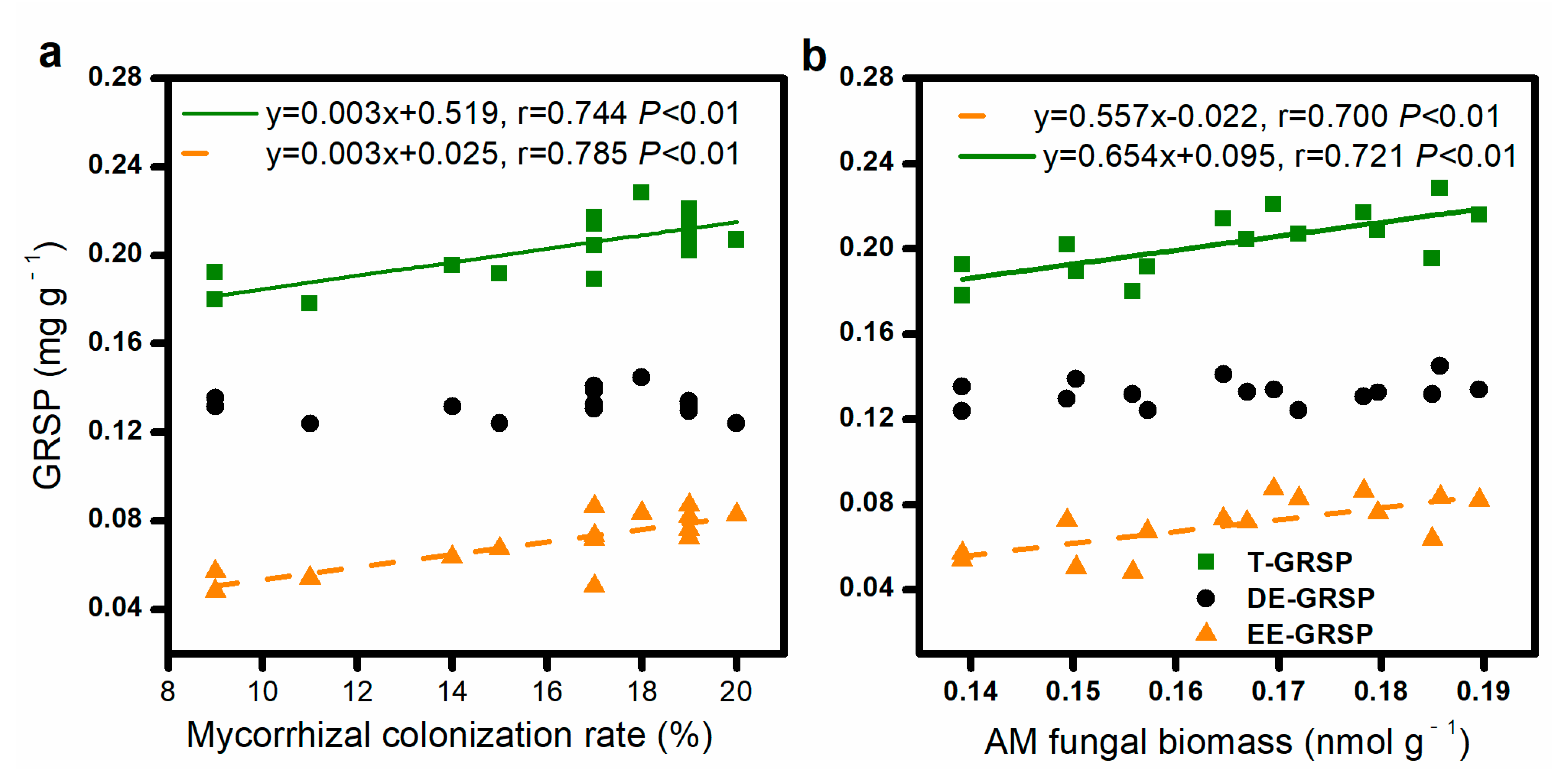
The mycorrhizal colonization and AM fungal biomass were positively correlated with the soil EE-GRSP and T-GRSP contents but did not significantly correlate with the soil DE-GRSP content (Figure 2).
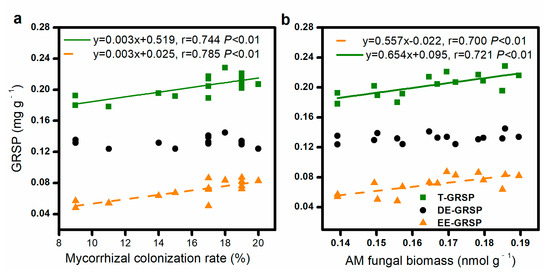
Figure 2.
Relationship between mycorrhizal colonization rate and concentration of glomalin-related soil protein (GRSP) (a), AM fungal biomass, and GRSP contents (b).
3.2. Contribution of GRSP to SOC
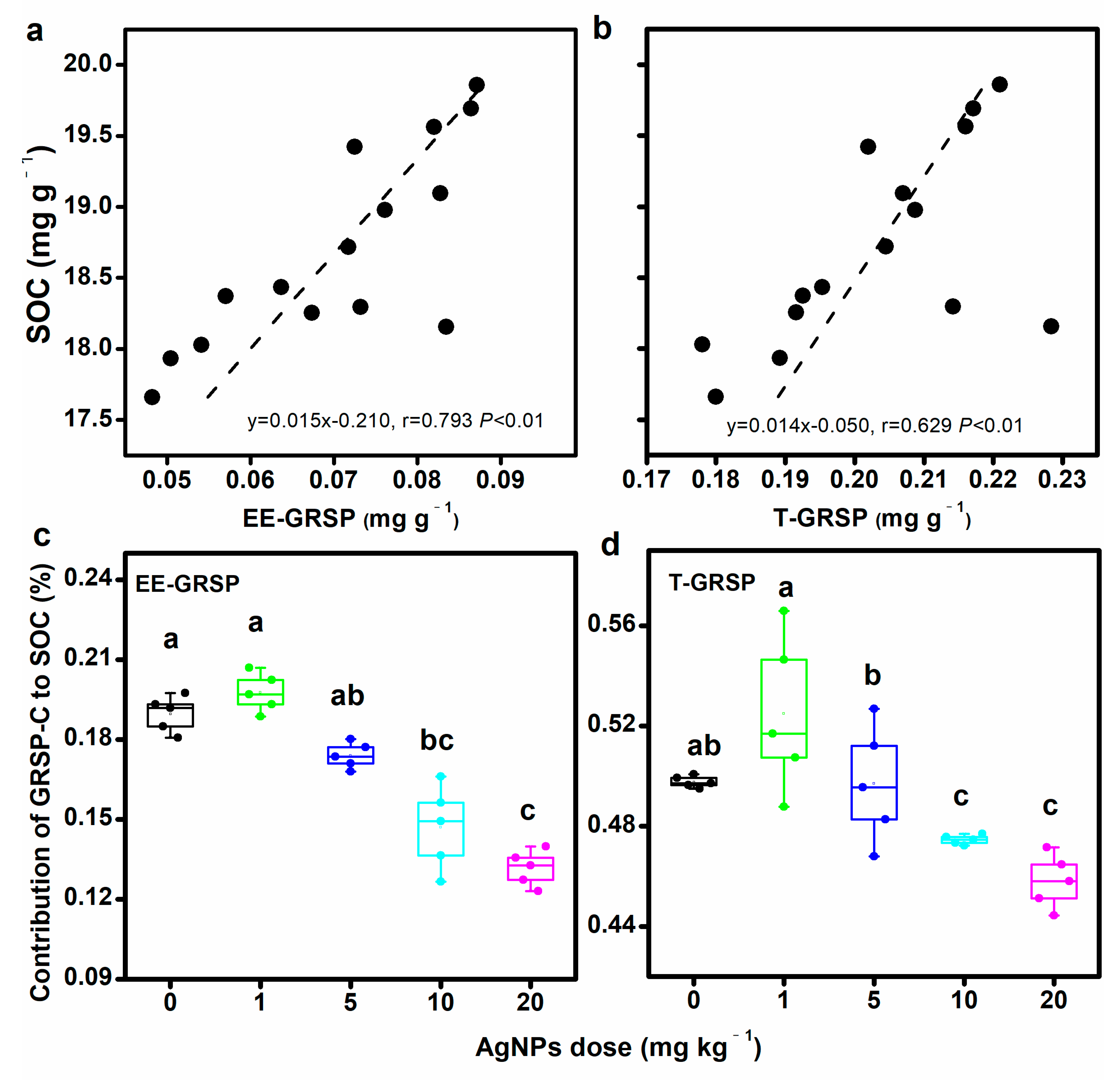
Compared to the control, the EE-GRSP/SOC ratio was decreased with the increase in AgNP concentrations. AgNPs at 10 mg kg−1 and 20 mg kg−1 significantly decreased the EE-GRSP/SOC ratio by 22.2% and 30.9%. The highest concentration of AgNPs significantly decreased the T-GRSP/SOC ratio. In contrast, AgNPs at either concentration did not show any influence on the DE-GRSP/SOC ratio (Table 1).

Table 1.
Ratios of EE-GRSP, DE-GRSP, and T-GRSP to soil organic C (SOC) under different concentrations of AgNPs.
Both the soil EE-GRSP and T-GRSP contents were positively correlated with soil organic C (Figure 3a,b). However, no significant correlation between DE-GRSP and SOC was observed (Figure S2a). Compared to the control, AgNPs showed negative effects on GRSP-C’s contribution to SOC, decreasing the EE-GRSP–C from 0.19% ± 0.01% to 0.13% ± 0.01% and T-GRSP-C from 0.50% ± 0.003% to 0.46% ± 0.01% (Figure 3c,d). AgNPs did not show any influences on the DE-GRSP-C (Figure S2b).
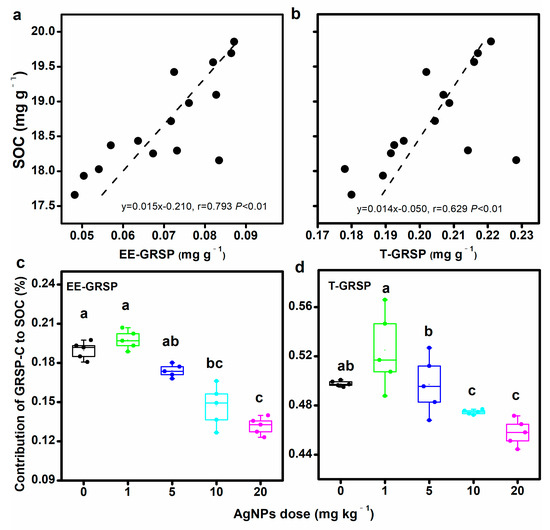
Figure 3.
Correlation of glomalin-related soil protein (GRSP) with soil organic carbon (SOC) content (a,b), and contribution of GRSP to SOC (c,d). Bars with different letters indicate significant differences among treatments (p < 0.05).
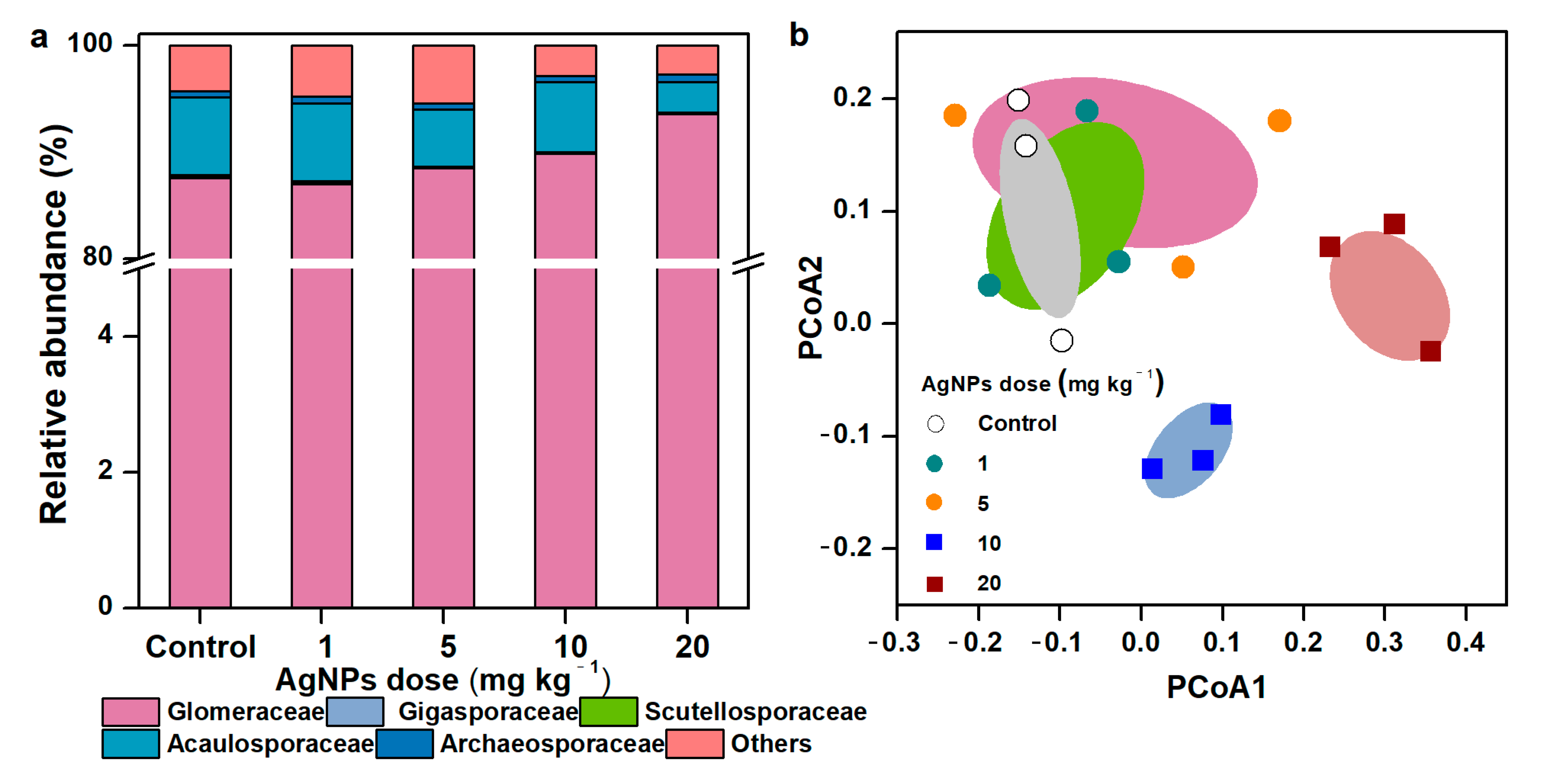
3.3. Taxonomic Distribution and Community Composition of AM Fungal Communities
In general, the rarefaction curves across all treatments achieved smooth stages (Figure S3), suggesting that the number of sequenced reads was enough to analyze the fungal communities. Most sequences were assigned to the family of Glomeraceae (89.3% of all reads) at 97% similarity. Acaulosporaceae and Gigasporaceae accounted for 5.87% and 0.15% of all sequences, respectively (Figure 4a). Compared to the control, the differences in AM fungal profiles increased along with the increase in AgNP concentrations. AgNPs strongly shifted the community composition of AM fungi. The AM fungal communities in the control and in soil with 1 mg kg−1 and 5 mg kg−1 of AgNPs were clustered into one group, apart from the communities in soil with 10 mg kg−1 and 20 mg kg−1 of AgNPs (Figure 4b).
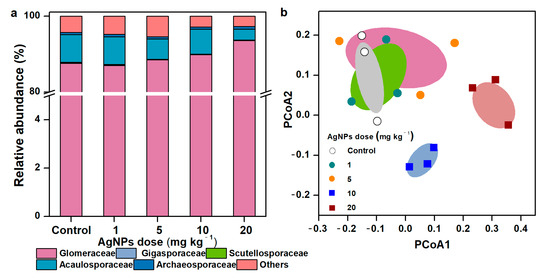
Figure 4.
Taxonomic composition of soil AM fungal communities at the family level (a), PCoA plot of AM fungal community dissimilarities (b) in in the rhizosphere of maize seedlings under different concentrations of AgNPs.
Compared to the control, AgNPs at 20 mg kg−1 significantly decreased the phylogenetic diversity, Chao1, and observed OTUs. The PD, Chao1, and observed OTUs were marginally decreased by 5 mg kg−1 and 10 mg kg−1 of AgNPs. AgNPs at 1 mg kg−1 also marginally decreased the Chao1 (Table 2).

Table 2.
The AM fungal diversity indices in in the rhizosphere of maize seedlings under AgNP treatments.
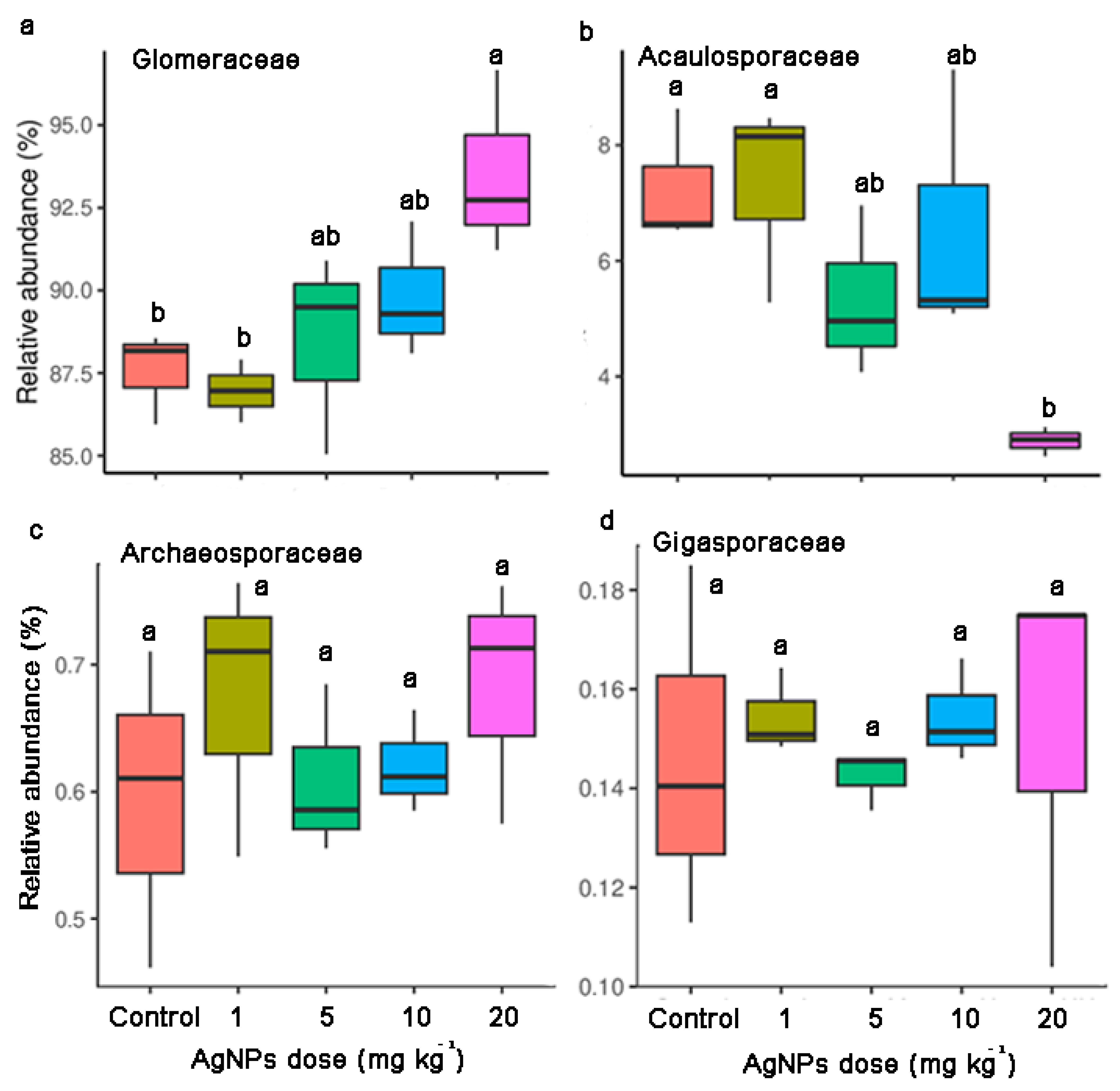
Compared to the control, the relative abundance of Glomeraceae increased and the relative abundance of Acaulosporaceae decreased along with the increase in AgNP concentrations (Figure 5). AgNPs at 20 mg kg−1 significantly increased Glomeraceae abundance and decreased Acaulosporaceae abundance. AgNPs at 5 mg kg−1 and 10 mg kg−1 marginally influenced the relative abundances of Glomeraceae and Acaulosporaceae. In contrast, AgNPs at 1 mg kg−1 did not lead to significant changes in Glomeraceae and Acaulosporaceae (Figure 5). Scutellosporaceae was only detected in the control and the soil with 1 mg kg−1 of AgNPs (Table S1).
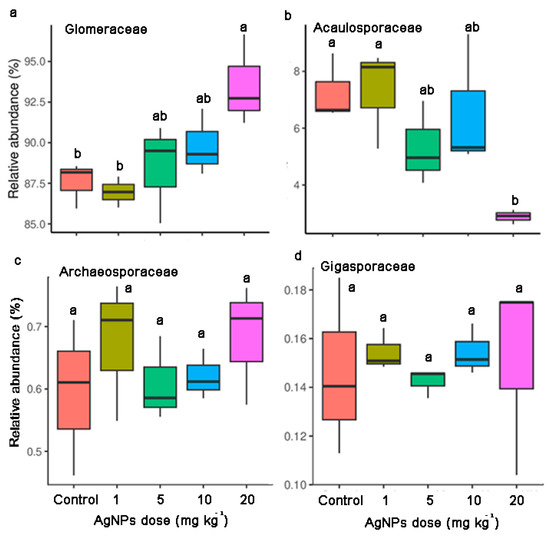
Figure 5.
Relative abundances of Glomeraceae (a), Acaulosporaceae (b), Archaeosporaceae (c) and Gigasporaceae (d) in the rhizosphere of maize seedlings under different concentrations of AgNPs.
Moreover, correlation analyses found that the AM fungal community composition, the diversity, and the relative abundance of Glomeraceae were positively correlated with soil EE-GRSP and T-GRSP contents. The relative abundances of Acaulosporaceae and Scutellosporaceae were negatively related to soil EE-GRSP and T-GRSP contents. In contrast, there was no significant correlation between the DE-GRSP and AM fungal community composition, the diversity, and the abundance of AM fungal families. The relative abundance of Gigasporaceae did not significantly correlate with the soil GRSP contents (Table 3).

Table 3.
Correlations between soil GRSP contents and the AM fungal community composition and abundance of AM fungal families.
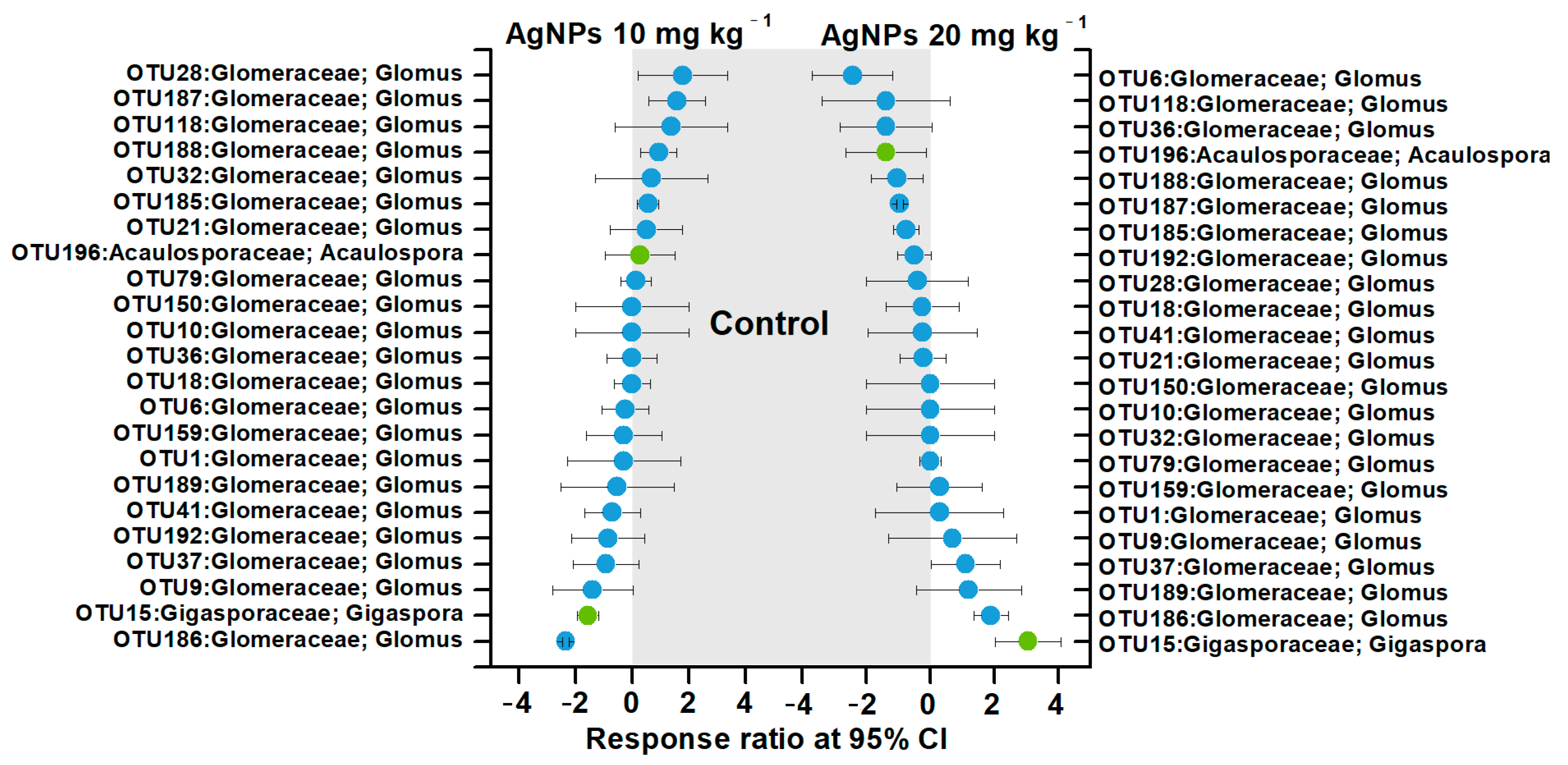
Due to the lack of influence of 1 mg kg−1 and 5 mg kg−1 AgNPs on AM fungal communities, the effects of 10 mg kg−1 and 20 mg kg−1 AgNPs on the dominant AM fungal OTUs were further evaluated according to the response ratio method (Figure 6). AgNPs at 10 mg kg−1 decreased the relative abundances of four OTUs in Glomeraceae and increased three OTUs in Glomeraceae and one OTU in Gigasporaceae. AgNPs at 20 mg kg−1 decreased the relative abundances of six OTUs in Glomeraceae and one OTU in Acaulosporaceae and increased two OTUs in Glomeraceae and one OTU in Gigasporaceae. In contrast, AM fungal OTUs in Glomeraceae or Acaulosporaceae families were not significantly influenced by AgNPs (Figure 6).
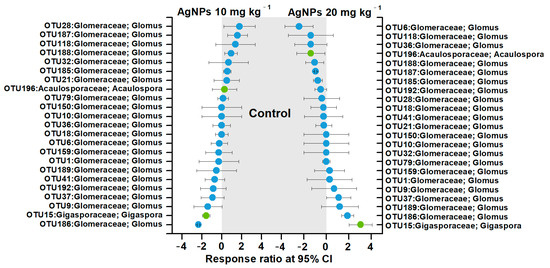
Figure 6.
Significant changes in the dominant AM fungal OTUs under different concentrations of AgNPs using the response ratio method.
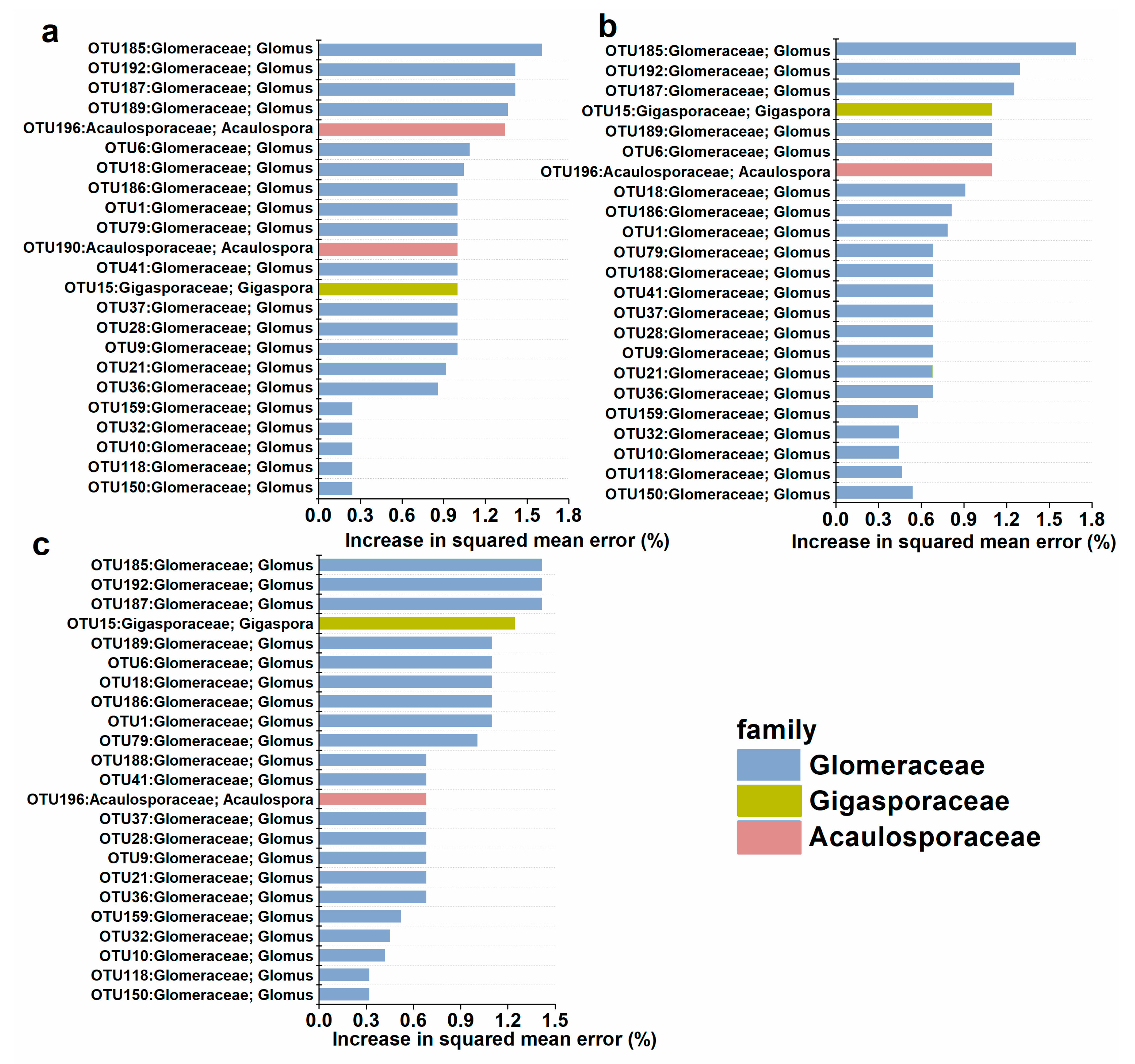
The potential AM fungal biomarkers were changed by AgNPs. The top 25 AM fungal members were selected to represent the important biomarker taxa. In the control, there were twenty OTUs in Glomeraceae, two OTUs in Acaulosporaceae, and one OTU in Gigasporaceae as biomarkers. In contrast, there were twenty-one Glomeraceae OTUs, one Acaulosporaceae OTU, and one Gigasporaceae OTU as biomarkers in 10 mg kg−1 and 20 mg kg−1 of AgNPs. The importance of the OTU in Acaulosporaceae decreased under AgNPs, especially in 20 mg kg−1 of AgNPs. Moreover, the Acaulosporaceae was less important in AgNPs. In contrast, Glomeraceae was more important under AgNPs (Figure 7).
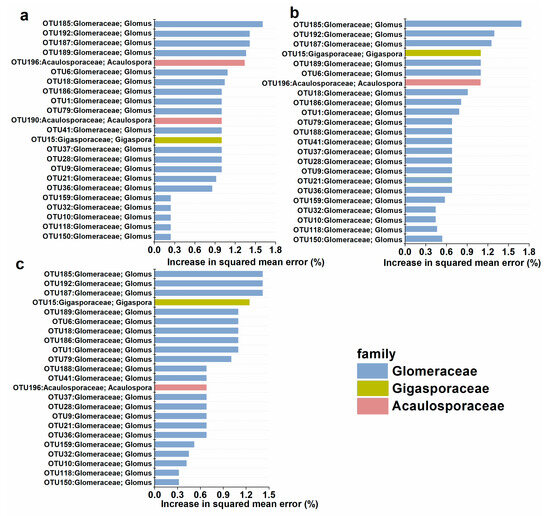
Figure 7.
Predictor importance of the top 20 AM fungal taxonomic biomarkers in the control (a), AgNPs at 10 mg kg−1 (b), and 20 mg kg−1 (c).
3.4. Correlations between AM Fungal Communities and Soil Properties and Plant Biomasses
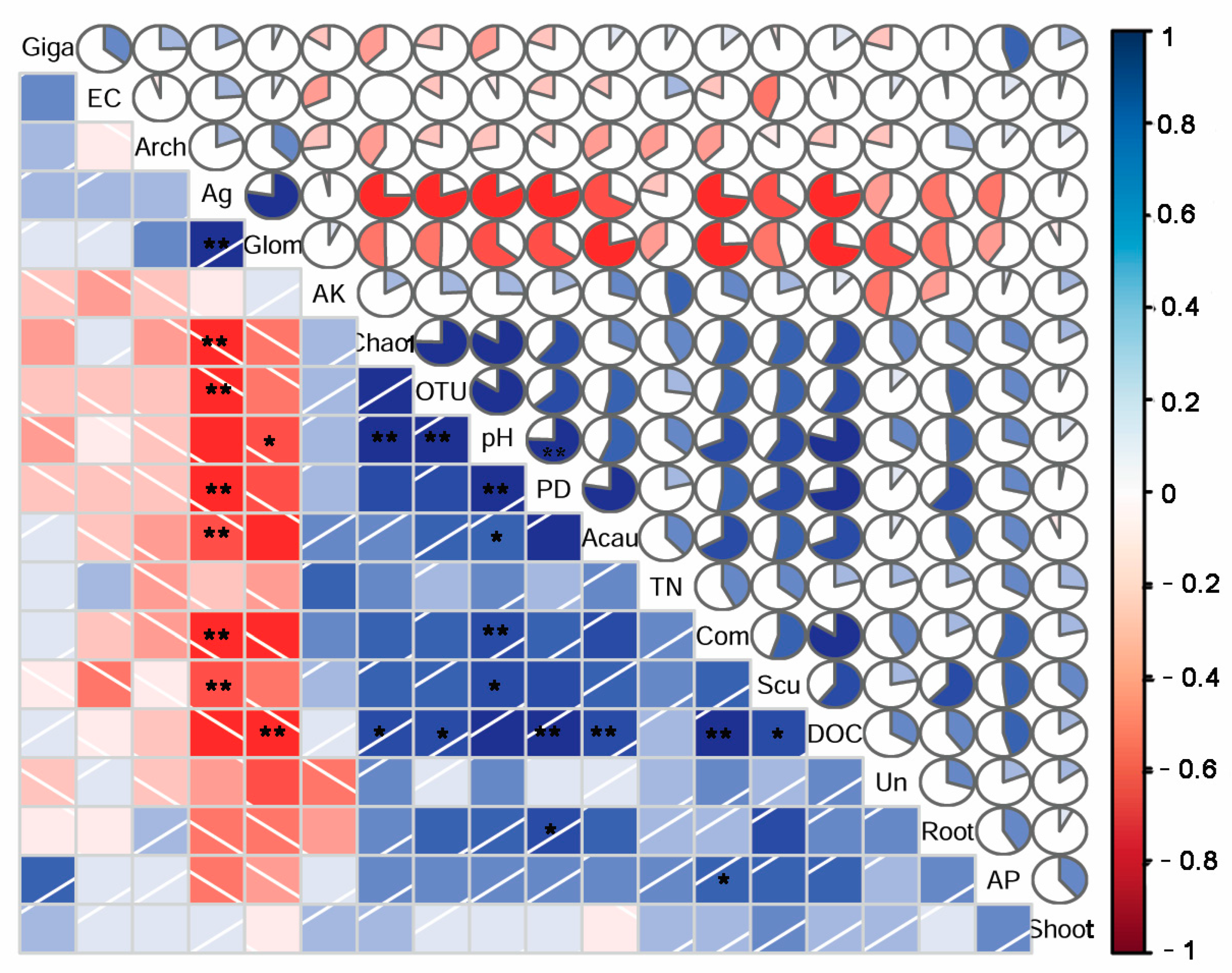
Soil DOC, pH, and soluble Ag have significant correlations with the community composition and diversity indices of AM fungi (Figure 8). The relative abundances of Glomeraceae and Acaulosporaceae were also significantly related to the soil DOC, pH, and soluble Ag. Specifically, the AM fungal community’s composition, the diversity indices, and the relative abundances of Acaulosporaceae and Scutellosporaceae were positively correlated with the soil pH and DOC, but negatively related to the DTPA-Ag. In contrast, the relative abundance of Glomeraceae was negatively related to the soil pH and DOC, but positively related to the soil-soluble Ag. Moreover, the AM fungal community composition was also positively related to the soil AP content. However, the soil TN, AK, EC, and shoot biomass did not show significant influences on the community composition and diversity indices of AM fungi, or on the relative abundances of AM fungal families (Figure 8).
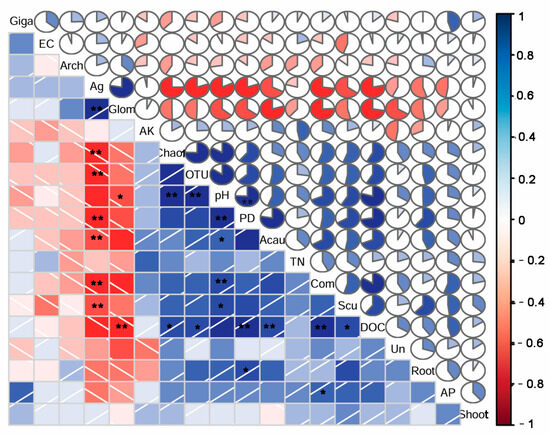
Figure 8.
Correlations between the soil AM fungal community composition, diversity, and the relative abundance of the dominant AM fungal families and soil properties and plant biomasses. Com: community composition; OTU: OTU richness; Ag: soluble Ag; Shoot: shoot biomass; root: root biomass; Acau: Acaulosporaceae; Glom: Glomeraceae; Arch: Archaeosporaceae; Giga: Gigasporaceae; Scu: Scutellosporaceae. *, p < 0.05; **, p < 0.01.
4. Discussion
Previous studies have shown that AgNPs not only adversely affect the growth and production of aboveground plants but also negatively influence the activities and community composition of belowground microbes [7,9]. In this study, we observed that AgNPs decreased the mycorrhizal colonization rate and AM fungal biomass. Correspondingly, the AM fungal diversity was also lower under AgNPs. The toxicity of AgNPs in AM fungi was consistent with that of those in bacteria [10,46] due to the excellent antimicrobial activity of AgNPs [47]. There were negative correlations between the AM fungal diversity indices and soil-soluble Ag content. Moreover, AgNPs also strongly shifted the soil AM fungal community’s composition. Specifically, AgNPs significantly increased the relative abundance of Glomeraceae, implying that Glomeraceae might have a relative tolerance to AgNPs. This is not surprising because the relative abundance of Glomeraceae was also found to increase under heavy metal stresses [48], climate changes [49], and fertilizer enrichments [50] in forest and grassland ecosystems. Glomeraceae could reproduce through root or mycelium fragments rather than spores [51,52]. They also can grow faster via the more easily utilized recently fixed C in the plant and thus might outcompete other fungi like Acaulosporaceae [53,54]. Therefore, the Glomeraceae abundance increased and the Acaulosporaceae abundance decreased under AgNPs, similar to what was reported in previous works [15]. As possible evidence of this, the soluble Ag was shown to have a significant influence on the relative abundances of Glomeraceae and Acaulosporaceae. At a higher resolution, AgNPs increased, decreased, or had no influence on the AM fungal OTUs in the Glomeraceae family, implying that survival abilities vary among OTUs within a fungal family.
The variations in soil conditions under AgNPs may also contribute to the shifts in AM fungal community structure, since AM fungal groups usually have different niches and distinct growing strategies. A significant correlation was recorded between the soil AM fungal community composition and diversity and DOC content, as well as between the AM fungal diversity and root biomass. It has been reported that plants might allocate 4–20% of their photosynthetic products to AM fungi to maintain mycorrhizal growth and survival [55]. Thus, decreases in plant biomasses might lead to a decrease in DOC, which might be unfavorable for AM fungal growth, resulting in a reduction in AM fungal diversity indices and changes in AM fungal community composition. It has been documented that AM fungal families possess different abilities to utilize recently fixed plant carbohydrates; for example, Glomeraceae can more easily obtain plant carbohydrates [53,54]. Previous studies have also observed that changes in the plant growth or DOC content have significant correlations with the bacterial and AM fungal communities [56,57]. Moreover, soil pH significantly influenced both the AM fungal community composition and diversity. It is likely that a higher soil pH may lead to the selection of AM fungal taxa, forming more mycelia networks [58]. The oxidization of Ag can consume H ions, thus changing soil pH [59]. Changes in soil pH or pH-driven properties can influence the composition and diversity of the soil AM fungal community under heavy-metal-contaminated conditions [60,61] or climate changes [62]. In natural ecosystems, the variability in soil pH also plays an important role in influencing the structure and composition of AM fungal communities [63,64]. Collectively, our results emphasize that AgNPs might directly, via their antimicrobial ability, and indirectly, through plant growth and soil properties, influence the mycorrhizal fungal communities.
The negative effects of AgNPs are not limited to belowground microorganisms but also extend to soil biochemical processes. It is well known that AM fungi have different abilities to form hyphal networks [65,66] and secrete hyphal products like glomalin [4,34]. Thus, it is speculated that the variation in the community composition of AM fungi induced by AgNPs could induce changes in AM fungal-associated C processes [6,64]. The present study found that both the mycorrhizal colonization rate and biomass were positively correlated with the soil EE-GRSP and T-GRSP contents, and there was also a strong linkage of community composition with soil EE-GRSP and T-GRSP. This result supports our initial speculation that changes in the abundances and community composition of AM fungi induced by AgNPs could extend to their related C sequestration functions. Previous studies also found that the AM fungal biomass and community composition significantly correlated with soil GRSP contents [64,67]. It was demonstrated that Acaulosporaceae members could produce more glomalin relative to Glomeraceae [4]. The decrease in the relative abundance of Acaulosporaceae and the increase in the relative abundance of Glomeraceae under AgNPs may be a possible reason for the reduction in the soil GRSP contents. Similarly, Lee et al. [68] found that Acaulospora inoculation exerted a greater increase in soil GRSP contents than Glomus. Another study also reported that mycorrhizal colonization increased soil GRSP contents and found that the increases in GRSP contents were varied among AM fungal species [69,70].
Soil GRSP has long been recognized as an important composite of soil C pools [1,71]. Our study found that soil GRSP-C represented about 0.56% of SOC, similar to that found in tropical orange forests (~0.27%) [72]. However, other studies found that soil GRSP contributed to approximately 4.75% of SOC in tropical broad-leaved forests [36] or 9.9% in tropical rainforests [1]. The discrepancy in the C contribution of soil GRSP is possibly due to the differences in plants and soil environments, as well as in climate conditions [73]. The C contributions of EE-GRSP and T-GRSP decreased along with increases in AgNP concentrations. Moreover, AgNPs also decreased the EE-GRSP/T-GTSP ratio, indicating a higher reduction or decomposition of EE-GRSP under AgNPs. This result is consistent with the previous finding showing that the soil GRSP contents and EE-GRSP/T-GRSP ratio reduced in cadmium-contaminated soils [19]. Soil EE-GRSP is newly secreted by mycorrhizas and more active than DE-GRSP [74], which means that EE-GRSP contents would be more closely related to the C allocated by plants to mycorrhizas. AgNPs have been documented to inhibit plant growth and decrease plant biomasses [9,46]; thus, AgNPs would reduce the C allocation from plants to soils. Correlation analyses observed that both EE-GRSP and T-GRSP contents were positively correlated with SOC contents, suggesting that the decreased soil GRSP contents could be unfavorable for soil C accumulation. Moreover, GRSP also can provide physical protection for soil C by stimulating soil aggregation [75]. Many studies have observed positive correlations among GRSP, soil aggregation, and SOC contents [67]. Therefore, the negative effects of AgNPs on soil GRSP contents might be extended to soil C processes. Given the relatively long residence time of GRSP in soil, a lower accumulation could unfavorable for soil C storage under AgNPs.
5. Conclusions
The study reveals that AgNPs negatively influenced AM fungal abundances and communities, decreasing the mycorrhizal colonization, biomass, and diversity, and shifting the community composition of AM fungi. Moreover, AgNPs reduced the soil EE-GRSP and T-GRSP contents, which were positively correlated with soil organic C. AgNPs also decreased the C contribution of GRSP to soil organic C. Together, it seems that the adverse effects of AgNPs on AM fungi could be extended to soil C accumulation. These findings have deepened our knowledge of the ecological toxicity of AgNPs to AM fungal growth and function and provided a scientific basis for a systematic evaluation of the effects of AgNPs on soil ecosystems. It should be noted that the studies described in this paper were conducted in both a short-term pot experiment and in a specific soil type. Considering the continuously increased concentration of NPs, the complex interactions between NPs and soil components, and the host-specific nature of the plant–AM fungi symbiosis, future investigations need to take into account plant and soil physico-chemical diversities and include long-term experiments to produce an integrative evaluation of the effects of AgNPs on soil ecosystems.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/d16050273/s1, Figure S1: Soil organic carbon content under different concentrations of AgNPs; Figure S2: Relationship between soil difficult extract glomalin-related soil protein (DE-GRSP) and soil organic carbon (SOC) (a) and contribution of DE-GRSP to SOC (b); Figure S3: Rarefaction curves of observed soil arbuscular mycorrhizal fungal OTUs under different concentrations of AgNPs; Table S1: Relative abundances of AM fungi at family level under different concentrations of AgNPs; Table S2: Correlations between soil GRSP contents and the AM fungal community composition and AM fungal groups.
Author Contributions
Conceptualization and methodology, H.Z. and Z.L.; software and formal analysis, Z.L. and Y.H.; validation and investigation, H.Z. and Y.H.; data curation, H.Z. and J.C.; Resources, H.Z. and J.C.; writing—original draft preparation, H.Z. and J.C.; writing—review and editing, J.C.; visualization, Z.L.; supervision, J.C.; project administration, J.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Science and Technology Development Plan Project of Jilin Province (No. YDZJ202201ZYTS512), the Scientific Research Project of Jilin Provincial Department of Education (No. JJKH20240649KJ), and the National Natural Science Foundation of China (No.32360303).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Rillig, M.C.; Wright, S.F.; Nichols, K.A.; Schmidt, W.F.; Torn, M.S. Large contribution of arbuscular mycorrhizal fungi to soil carbon pools in tropical forest soils. Plant Soil. 2001, 233, 167–177. [Google Scholar] [CrossRef]
- Wright, S.F.; Upadhyaya, A. A survey of soils for aggregate stability and glomalin, a glycoprotein produced by hyphae of arbuscular mycorrhizal fungi. Plant Soil. 1998, 198, 97–107. [Google Scholar] [CrossRef]
- Gillespie, A.W.; Farrell, R.E.; Walley, F.L.; Ross, A.R.S.; Leinweber, P.; Eckhardt, K.U. Glomalin-related soil protein contains non-mycorrhizal-related heat-stable proteins, lipids and humic materials. Soil. Biol. Biochem. 2011, 43, 766–777. [Google Scholar] [CrossRef]
- Lovelock, C.E.; Wright, S.F.; Nichols, K.A. Using glomalin as an indicator for arbuscular mycorrhizal hyphal growth: An example from a tropical rain forest soil. Soil. Biol. Biochem. 2004, 36, 1009–1012. [Google Scholar] [CrossRef]
- Smith, S.E.; Read, D.J. Mycorrhizal symbiosis; Academic Press: New York, NY, USA, 2008. [Google Scholar]
- Wilson, G.W.T.; Rice, C.W.; Rillig, M.C.; Springer, A.; Hartnett, D.C. Soil aggregation and carbon sequestration are tightly correlated with the abundance of arbuscular mycorrhizal fungi: Results from long-term field experiments. Ecol. Lett. 2009, 12, 452. [Google Scholar] [CrossRef] [PubMed]
- Madanayake, N.H.; Perera, N.; Adassooriya, N.M. Engineered nanomaterials: Threats, releases, and concentrations in the environment. In Emerging Contaminants in the Environment; Sarma, H., Dominguez, D.C., Lee, W.Y., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 225–240. [Google Scholar]
- Vance, M.E.; Kuiken, T.; Vejerano, E.P.; McGinnis, S.P.; Hochella, M.F.J.; Rejeski, D.; Hull, M.S. Nanotechnology in the real world: Redeveloping the nanomaterial consumer products inventory. Beilstein J. Nanotechnol. 2015, 6, 1769–1780. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Pan, Z.; Chen, S. Rice exposure to silver nanoparticles in a life cycle study: Effect of dose responses on grain metabolomic profile, yield, and soil bacteria. Environ. Sci. Nano 2022, 9, 2195. [Google Scholar] [CrossRef]
- Kwak, J.I.; Nam, S.H.; An, Y.J. Assessing the risks of silver nanoparticle-concentrated matrix application in agricultural soil: Implications for plant and soil enzymes. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2023, 269, 109631. [Google Scholar] [CrossRef]
- Lee, W.M.; Kwak, J.I.; An, Y.J. Effect of silver nanoparticles in crop plants Phaseolus radiatus and Sorghum bicolor: Media effect on phytotoxicity. Chemosphere 2012, 86, 491–499. [Google Scholar] [CrossRef]
- Gupta, S.D.; Agarwal, A.; Pradhan, S. Phytostimulatory effect of silver nanoparticles (AgNPs) on rice seedling growth: An insight from antioxidative enzyme activities and gene expression patterns. Ecotoxicol. Environ. Saf. 2018, 161, 624–633. [Google Scholar] [CrossRef]
- Shen, H.Y.; Liu, Y.Y.; Liu, Y.N.; Duan, Z.M.; Wu, P.P.; Lin, Z.F.; Sun, H.Y. Hormetic dose-responses for silver antibacterial compounds, quorum sensing inhibitors, and their binary mixtures on bacterial resistance of Escherichia coli. Sci. Total Environ. 2021, 786, 147464. [Google Scholar] [CrossRef] [PubMed]
- Falco, W.F.; Scherer, M.D.; Oliveira, S.L.; Wender, H.; Colbeck, I.; Lawson, T.; Caires, A.R. Phytotoxicity of silver nanoparticles on Vicia faba: Evaluation of particle size effects on photosynthetic performance and leaf gas exchange. Sci. Total Environ. 2020, 701, 134816. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Xiao, Y. Silver nanoparticles and arbuscular mycorrhizal fungi influence Trifolium repen root-associated AMF community structure and its co-occurrence pattern. Sci. Hortic. 2023, 320, 112232. [Google Scholar] [CrossRef]
- Cao, J.L.; Feng, Y.Z.; He, S.Y.; Lin, X.G. Silver nanoparticles deteriorate the mutual interaction between maize (Zea mays L.) and arbuscular mycorrhizal fungi: A soil microcosm study. Applied Soil. Ecology 2017, 119, 307–316. [Google Scholar] [CrossRef]
- Yin, K.J.; Cao, L.B.; Xu, X.F.; Shi, Z.Y. Effects of molybdenum pollution on arbuscular mycorrhizal fungi and GRSP. Soil. Fertil. Sci. China 2022, 1, 180–187. [Google Scholar]
- Aalipour, H.; Nikbakht, A.; Etemadi, N. Physiological response of Arizona cypress to Cd-contaminated soil inoculated with arbuscular mycorrhizal fungi and plant growth promoting rhizobacteria. Rhizosphere 2021, 18, 100354. [Google Scholar] [CrossRef]
- Jia, X.; Zhao, Y.; He, Y. Glomalin-related soil protein in the rhizosphere of Robinia pseudoacacia L. seedlings under higher air temperature combined with Cd-contaminated soil. Eur. J. Soil. Sci. 2018, 69, 634–645. [Google Scholar] [CrossRef]
- Vodnik, D.; Grčman, H.; Maček, I.; van Elteren, J.T.; Kovačevič, M. The contribution of glomalin-related soil protein to Pb and Zn sequestration in polluted soil. Sci. Total Environ. 2008, 392, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Requejo, R.; Tena, M. Proteome analysis of maize roots reveals that oxidative stress a main contributing factor to plant arsenic toxicity. Phytochemistry 2005, 66, 1519–1528. [Google Scholar] [CrossRef]
- Cao, J.L.; Feng, Y.Z.; Lin, X.G.; Wang, J.H. A beneficial role of arbuscular mycorrhizal fungi in influencing the effects of silver nanoparticles on plant-microbe systems in a soil matrix. Environ. Sci. Pollut. Res. 2020, 27, 11782–11796. [Google Scholar] [CrossRef]
- Noori, A.; White, J.C.; Newman, L.A. Mycorrhizal fungi influence on silver uptake and membrane protein gene expression following silver nanoparticle exposure. J. Nanoparticle Res. 2017, 19, 66. [Google Scholar] [CrossRef]
- Giese, B.; Klaessig, F.; Park, B.; Kagegi, R.; Steinfeldt, M.; Wigger, H.; Gleich, A.V.; Gottschalk, F. Risks, Release and Concentrations of Engineered Nanomaterial in the Environment. Sci. Rep. 2018, 8, 1565. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.J.; Conrad, R. Bacteria rather than Archaea dominate microbial ammonia oxidation in an agricultural soil. Environ. Microbiol. 2009, 11, 1658–1671. [Google Scholar] [CrossRef] [PubMed]
- Philippot, L.; Raaijmakers, J.M.; Lemanceau, P.; van der Putten, W.H. Going back to the roots: The microbial ecology of the rhizosphere. Nat. Rev. Microbiol. 2013, 11, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Bray, R.H.; Kurtz, L.T. Determination of total, organic, and available forms of phosphorus in soils. Soil. Sci. 1945, 59, 39–45. [Google Scholar] [CrossRef]
- Lindsay, W.L.; Norvell, W.A. Development of a DTPA soil test for zinc, iron, manganese, and copper. Soil. Sci. Soc. Am. J. 1978, 42, 421–428. [Google Scholar] [CrossRef]
- Giovannetti, M.; Mosse, B. An evaluation of techniques for measuring vesicular-arbuscular mycorrhizal infection in roots. New Phytol. 1980, 84, 489–500. [Google Scholar] [CrossRef]
- Frostegård, Å.; Tunlid, A.; Bååth, E. Microbial biomass measured as total lipid phosphate in soils of different organic content. J. Microbiol. Methods 1991, 14, 151–163. [Google Scholar] [CrossRef]
- Olsson, P.A. Signature fatty acids provide tools for determination of the distribution and interactions of mycorrhizal fungi in soil. FEMS Microbiol. Ecol. 1999, 29, 303–310. [Google Scholar] [CrossRef]
- Olsson, P.A.; Bååth, E.; Jakobsen, I.; Söderström, B. The use of phospholipid and neutral lipid fatty acids to estimate biomass of arbuscular mycorrhizal fungi in soil. Mycol. Res. 1995, 99, 623–629. [Google Scholar] [CrossRef]
- Treseder, K.K.; Turner, K.M. Glomalin in ecosystems. Soil. Sci. Soc. Am. J. 2007, 71, 1257–1266. [Google Scholar] [CrossRef]
- Wright, S.F.; Upadhyaya, A. Extraction of an abundant and unusual protein from soil and comparison with hyphal protein of arbuscular mycorrhizal fungi. Soil. Sci. 1996, 161, 575–586. [Google Scholar] [CrossRef]
- Schindler, F.V.; Mercer, E.J.; Rice, J.A. Chemical characteristics of glomalinrelated soil protein (GRSP) extracted from soils of varying organic matter content. Soil. Biol. Biochem. 2007, 39, 320–329. [Google Scholar] [CrossRef]
- Zhang, J.; Tang, X.L.; He, X.H.; Liu, J.X. Glomalin-related soil protein responses to elevated CO2 and nitrogen addition in a subtropical forest: Potential consequences for soil carbon accumulation. Soil. Biol. Biochem. 2015, 83, 142–149. [Google Scholar] [CrossRef]
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon and organic matter. In Methods of Soil Analysis Part 2; American Society of Agronomy: Madison, WI, USA, 1982; pp. 539–580. [Google Scholar]
- Biddle, J.F.; Fitz-Gibbon, S.; Schuster, S.C.; Brenchley, J.E.; House, C.H. Metagenomic signatures of the Peru Margin subseafloor biosphere show a genetically distinct environment. Proc. Natl. Acad. Sci. USA 2008, 105, 10583–10588. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Pena, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of highthroughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Opik, M.; Vanatoa, A.; Vanatoa, E.; Moora, M.; Davison, J.; Kalwij, J.M.; Reier, U.; Zobel, M. The online database MaarjAM reveals global and ecosystemic distribution patterns in arbuscular mycorrhizal fungi (Glomeromycota). New Phytol. 2010, 188, 223–241. [Google Scholar] [CrossRef]
- Colwell, R.K. EstimateS: Statistical Estimation of Species Richness and Shared Species from Samples. 2013. Available online: https://purl.oclc.org/estimates (accessed on 1 April 2024).
- Paradis, E.; Claude, J.; Strimmer, K. APE: Analyses of phylogenetics and evolution in R language. Bioinformatics 2004, 20, 289–290. [Google Scholar] [CrossRef]
- Luo, Y.Q.; Hui, D.F.; Zhang, D.Q. Elevated CO2 stimulates net accumulations of carbon and nitrogen in land ecosystems: A meta-analysis. Ecology 2006, 87, 53–63. [Google Scholar] [CrossRef]
- Hauke, J.; Kossowski, T. Comparison of values of Pearson’s and Spearman’s correlation coefficients on the same sets of data. Quaest. Geogr. 2011, 30, 87–93. [Google Scholar] [CrossRef]
- Liaw, A.; Wiener, M. Classification and regression by randomForest. R News 2002, 2, 18–22. [Google Scholar]
- Das, P.; Barua, S.; Sarkar, S.; Chatterjee, S.K.; Mukherjee, S.; Goswami, L.; Das, S.; Bhattacharya, S.; Karak, N.; Bhattacharya, S.S. Mechanism of toxicity and transformation of silver nanoparticles: Inclusive assessment in earthworm-microbe-soil-plant system. Geoderma 2018, 314, 73–84. [Google Scholar] [CrossRef]
- Kim, J.S.; Kuk, E.; Yu, K.N.; Kim, J.H.; Park, S.J.; Lee, H.J.; Kim, S.H.; Park, Y.K.; Park, Y.H.; Hwang, C.Y.; et al. Antimicrobial effects of silver nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2007, 3, 95–101. [Google Scholar] [CrossRef]
- Parvin, S.; Geel, M.V.; Yeasmin, T.; Lievens, B.; Honnay, O. Variation in arbuscular mycorrhizal fungal communities associated with lowland rice (Oryza sativa) along a gradient of soil salinity and arsenic contamination in Bangladesh. Sci. Total Environ. 2019, 686, 546–554. [Google Scholar] [CrossRef]
- Zheng, J.Q.; Cui, M.M.; Wang, C.; Wang, J.; Wang, S.L.; Sun, Z.J.; Ren, F.R.; Wan, S.Q.; Han, S.J. Elevated CO2, warming, N addition, and increased precipitation affect different aspects of the arbuscular mycorrhizal fungal community. Sci. Total Environ. 2022, 806, 150522. [Google Scholar] [CrossRef]
- Chen, Y.L.; Xu, Z.W.; Xu, T.L.; Veresoglou, S.D.; Yang, G.W.; Chen, B.D. Nitrogen deposition and precipitation induced phylogenetic clustering of arbuscular mycorrhizal fungal communities. Soil. Biol. Biochem. 2017, 115, 233–242. [Google Scholar] [CrossRef]
- Hart, M.M.; Reader, R.J. Taxonomic basis for variation in the colonization strategy of arbuscular mycorrhizal fungi. New Phytol. 2002, 153, 335–344. [Google Scholar] [CrossRef]
- Daniell, T.J.; Husband, R.; Fitter, A.H.; Young, J.P.W. Molecular diversity of arbuscular mycorrhizal fungi colonising arable crops. Fems Microbiol. Ecol. 2001, 36, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Maček, I.; Clark, D.R.; Šibanc, N.; Moser, G.; Vodnik, D.; Müller, C.; Dumbrell, A.J. Impacts of long-term elevated atmospheric CO2 concentrations on communities of arbuscular mycorrhizal fungi. Mol. Ecol. 2019, 28, 3445–3458. [Google Scholar] [CrossRef]
- Cotton, T.A.; Fitter, A.H.; Miller, R.M.; Dumbrell, A.J.; Helgason, T. Fungi in the future: Interannual variation and effects of atmospheric change on arbuscular mycorrhizal fungal communities. New Phytol. 2015, 205, 1598–1607. [Google Scholar] [CrossRef]
- Johnson, D.; Leake, J.R.; Read, D.J. Transfer of recent photosynthate into mycorrhizal mycelium of an upland grassland: Short-term respiratory losses and accumulation of C-14. Soil. Biol. Biochem. 2002, 34, 1521–1524. [Google Scholar] [CrossRef]
- Angelard, C.; Tanner, C.J.; Fontanillas, P.; Niculita-Hirzel, H.; Masclaux, F.; Sanders, I.R. Rapid genotypic change and plasticity in arbuscular mycorrhizal fungi is caused by a host shift and enhanced by segregation. Isme J. 2014, 8, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Priester, J.H.; Van De Werfhorst, L.C.; Walker, S.L.; Nisbet, R.M.; An, Y.-J.; Schimel, J.P.; Gardea-Torresdey, J.L.; Holden, P.A. Soybean plants modify metal oxide nanoparticle effects on soil bacterial communities. Environ. Sci. Technol. 2014, 48, 13489–13496. [Google Scholar] [CrossRef] [PubMed]
- Dumbrell, A.J.; Nelson, M.; Helgason, T.; Dytham, C.; Fitter, A.H. Relative roles of niche and neutral processes in structuring a soil microbial community. ISME J. 2010, 4, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Xiu, Z.M.; Zhang, Q.B.; Puppala, H.L.; Colvin, V.L.; Alvarez, P.J.J. Negligible particle-specific antibacterial activity of silver nanoparticles. Nano Lett. 2012, 12, 4271–4275. [Google Scholar] [CrossRef] [PubMed]
- Bainard, L.D.; Bainard, J.D.; Hamel, C.; Gan, Y. Spatial and temporal structuring of arbuscular mycorrhizal communities is differentially influenced by abiotic factors and host crop in a semi-arid prairie agroecosystem. Fems Microbiol. Ecol. 2014, 88, 333–344. [Google Scholar] [CrossRef]
- Yang, Y.R.; Song, Y.Y.; Scheller, H.V.; Ghosh, A.; Ban, Y.H.; Chen, H.; Tang, M. Community structure of arbuscular mycorrhizal fungi associated with Robinia pseudoacacia in uncontaminated and heavy metal contaminated soils. Soil. Biol. Biochem. 2015, 86, 146–158. [Google Scholar] [CrossRef]
- Zheng, Z.; Ma, X.; Zhang, Y.; Liu, Y.J.; Zhang, S.H. Soil properties and plant community-level traits mediate arbuscular mycorrhizal fungal response to nitrogen enrichment and altered precipitation. Appl. Soil. Ecol. 2022, 169, 104245. [Google Scholar] [CrossRef]
- Dumbrell, A.J.; Ashton, P.D.; Aziz, N.; Feng, G.; Nelson, M.; Dytham, C.; Fitter, A.H.; Helgason, T. Distinct seasonal assemblages of arbuscular mycorrhizal fungi revealed by massively parallel pyrosequencing. New Phytol. 2011, 190, 794–804. [Google Scholar] [CrossRef]
- Qin, H.; Wu, Q.F.; Chen, J.H.; Li, Y.C.; Liang, C.F.; Xu, Q.F.; Fuhrmann, J.J.; Shen, Y. Bamboo forest expansion increases soil organic carbon through its effect on soil arbuscular mycorrhizal fungal community and abundance. Plant Soil. 2017, 420, 407–421. [Google Scholar] [CrossRef]
- Voets, L.; De La Providencia, I.; Declerck, S. Glomeraceae and Gigasporaceae differ in their ability to form hyphal networks. New Phytol. 2006, 172, 185–188. [Google Scholar] [CrossRef] [PubMed]
- Rillig, M.C.; Mummey, D.L. Mycorrhizas and soil structure. New Phytol. 2006, 171, 41–53. [Google Scholar] [CrossRef]
- Ren, A.T.; Mickan, B.S.; Li, J.Y.; Zhou, R.; Zhang, X.C.; Ma, M.S.; Wesly, K.; Xiong, Y.C. Soil labile organic carbon sequestration is tightly correlated with the abundance and diversity of arbuscular mycorrhizal fungi in semiarid maize fields. Land. Degrad. Dev. 2021, 32, 1224–1236. [Google Scholar] [CrossRef]
- Lee, J.E.; Lee, E.H.; Eom, A.H. Effects of arbuscular mycorrhizal fungal inoculation on the growth of red pepper and soil glomalin content. Korean J. Mycol. 2021, 4, 517–524. [Google Scholar]
- Chi, G.G.; Wu, Q.S. Effects of mycorrhizal fungi on plant and growth soil properties in trifoliate orange seedlings grown in a root–box. Philipp. Agric. Sci. 2017, 3, 271–277. [Google Scholar]
- Zhang, F.; Wang, P.; Zou, Y.N.; Wu, Q.S.; Kuča, K. Effects of mycorrhizal fungi on root-hair growth and hormone levels of taproot and lateral roots in trifoliate orange under drought stress. Arch. Agron. Soil. Sci. 2019, 65, 1316–1330. [Google Scholar] [CrossRef]
- Zhang, J.; Tang, X.L.; Zhong, S.Y.; Yin, G.C.; Gao, Y.F.; He, X.H. Recalcitrant carbon components in glomalin-related soil protein facilitate soil organic carbon preservation in tropical forests. Sci. Rep. 2017, 7, 2391. [Google Scholar] [CrossRef] [PubMed]
- He, J.D.; Chi, G.G.; Zou, Y.N.; Shu, B.; Wu, Q.S.; Strivastava, A.K.; Kuča, K. Contribution of glomalin-related soil proteins to soil organic carbon in trifoliate orange. Appl. Soil. Ecol. 2020, 154, 103592. [Google Scholar] [CrossRef]
- Wang, Q.; Li, L.W.; Chen, J.Y.; Hong, H.L.; Lu, H.L.; Liu, J.C.; Dong, Y.W.; Yan, C.L. Glomalin-related soil protein deposition and carbon sequestration in the old Yellow River delta. Sci. Total Environ. 2018, 625, 619–626. [Google Scholar] [CrossRef]
- Wu, Q.S.; Cao, M.Q.; Zou, Y.N.; He, X.H. Direct and indirect effects of glomalin, mycorrhizal hyphae, and roots on aggregate stability in rhizosphere of trifoliate orange. Sci. Rep. 2014, 4, 5823. [Google Scholar] [CrossRef]
- Treseder, K.K.; Turner, K.M.; Mack, M.C. Mycorrhizal responses to nitrogen fertilization in boreal ecosystems: Potential consequences for soil carbon storage. Glob. Chang. Biol. 2007, 13, 78–88. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).