Impact of Silver Nanoparticles on Arbuscular Mycorrhizal Fungi and Glomalin-Related Soil Proteins in the Rhizosphere of Maize Seedlings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil and AgNPs Preparation
2.2. Pot Experiment and Sample Collection
2.3. Soil Property Determination
2.4. Mycorrhizal Colonization and AM Fungal Biomass Analyses
2.5. GRSP and SOC Contents Determination
2.6. Soil DNA Extraction and AM Fungal Community Sequencing
2.7. Statistical Data Analysis
3. Results
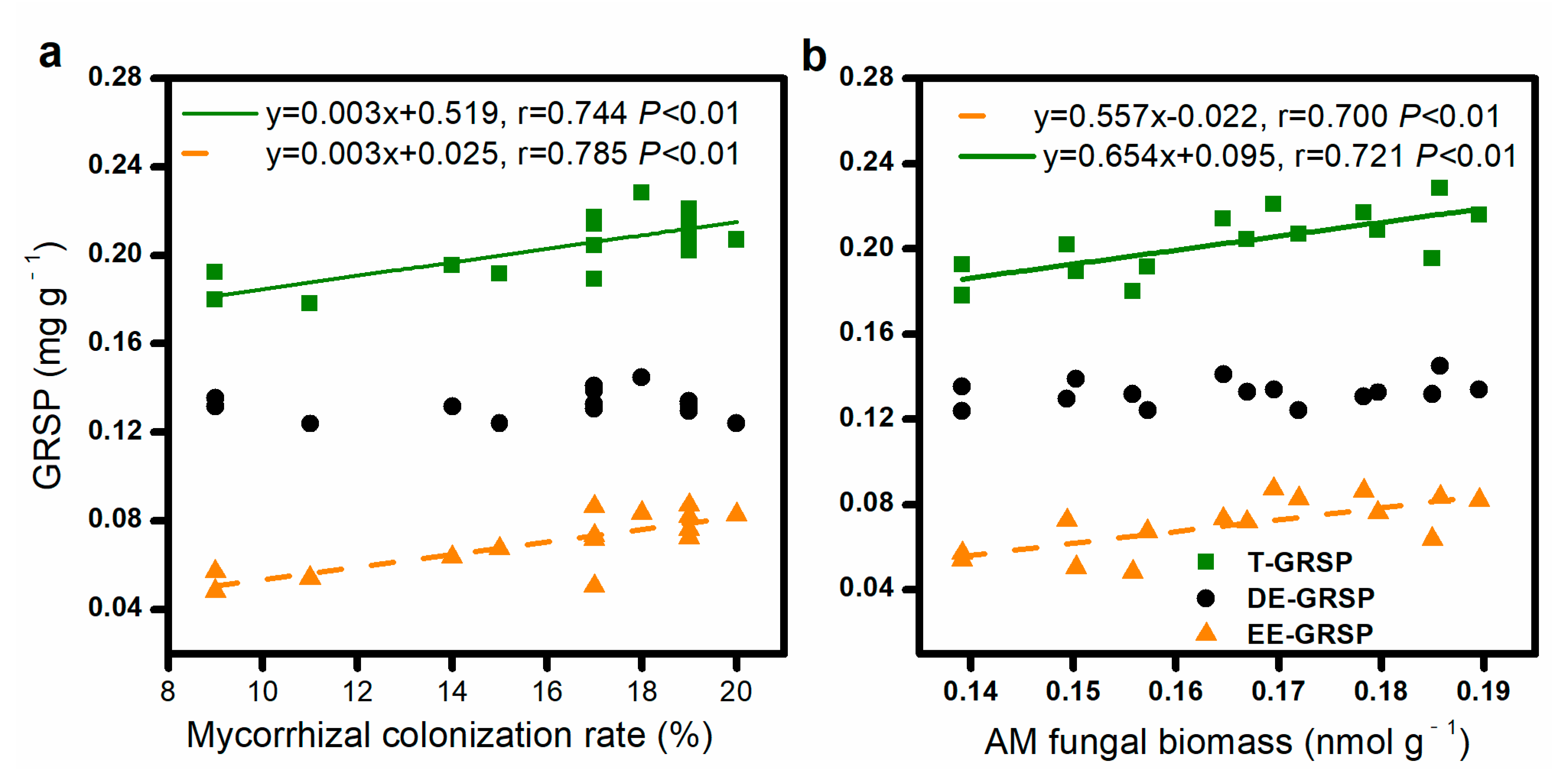
3.1. Mycorrhizal Colonization Rate, AM Fungal Biomass, and GRSP Content
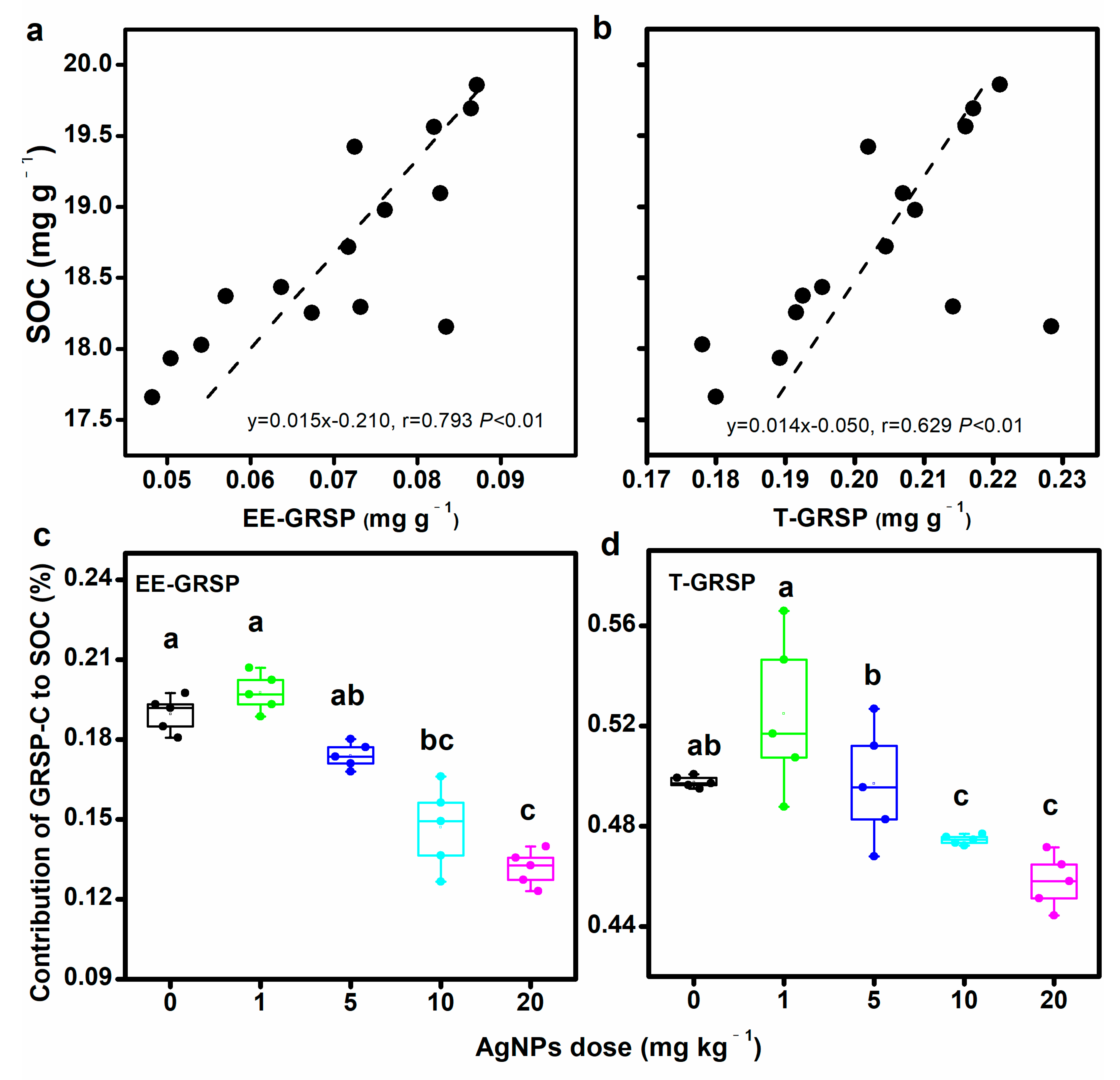
3.2. Contribution of GRSP to SOC
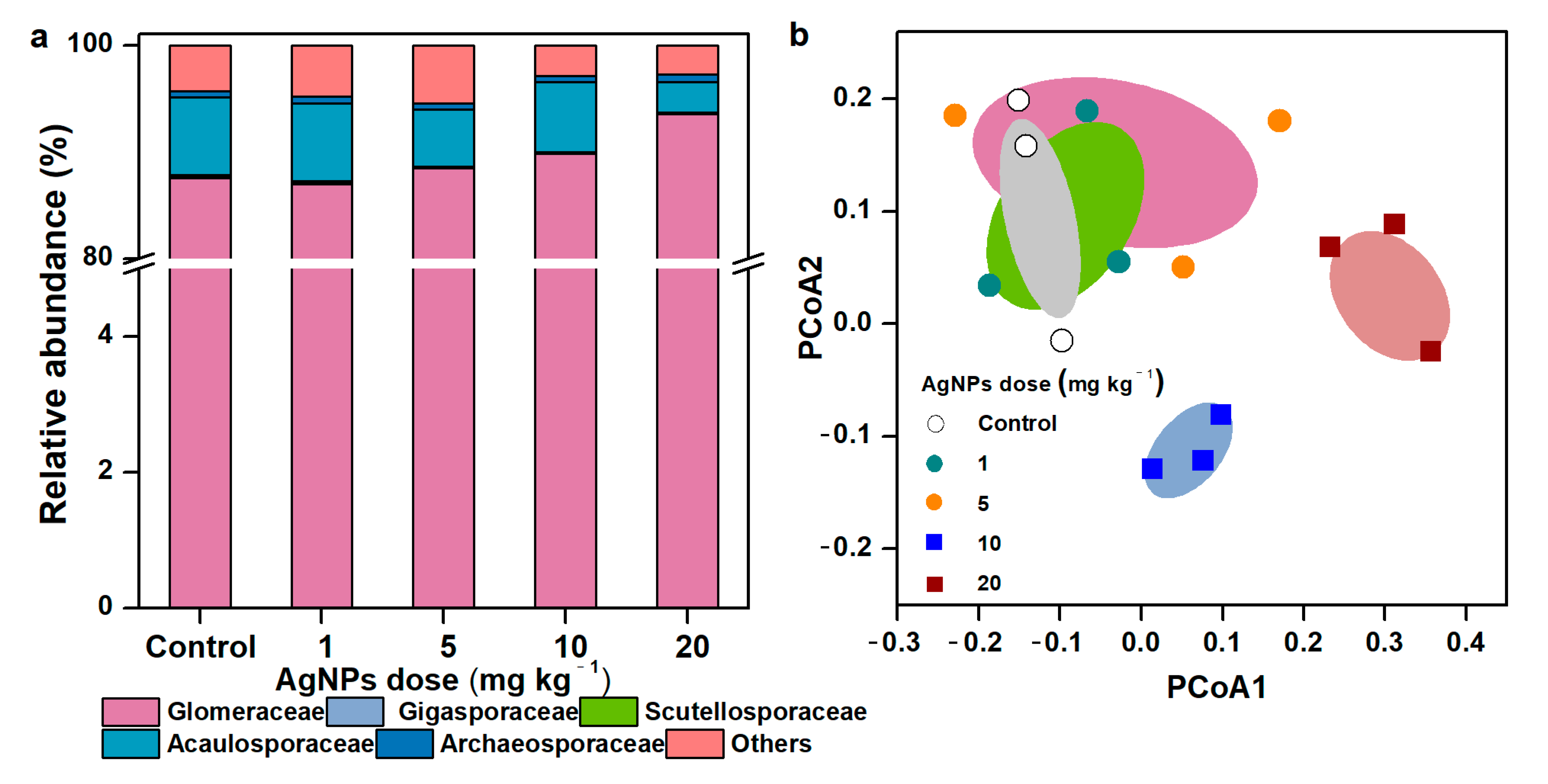
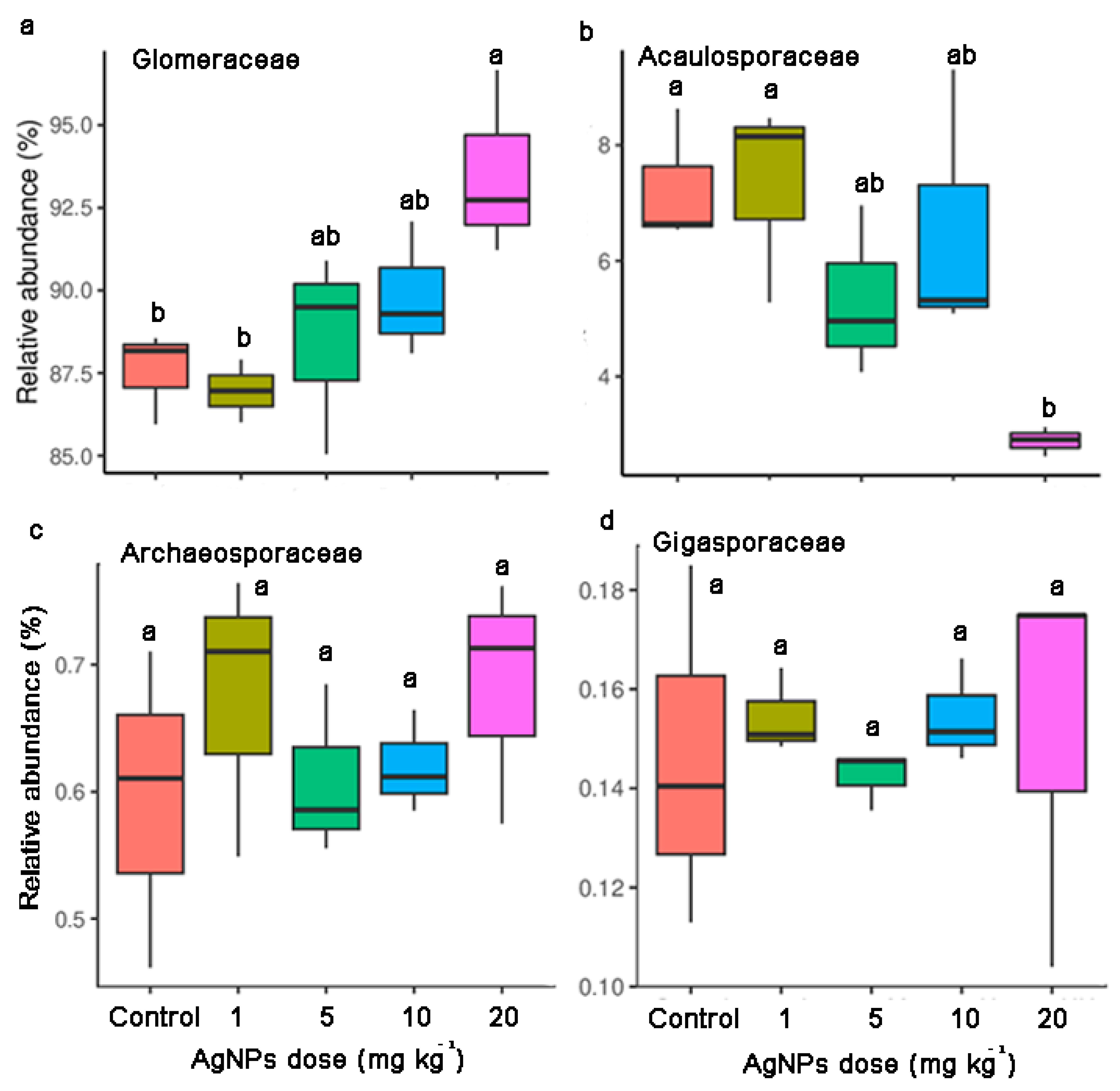
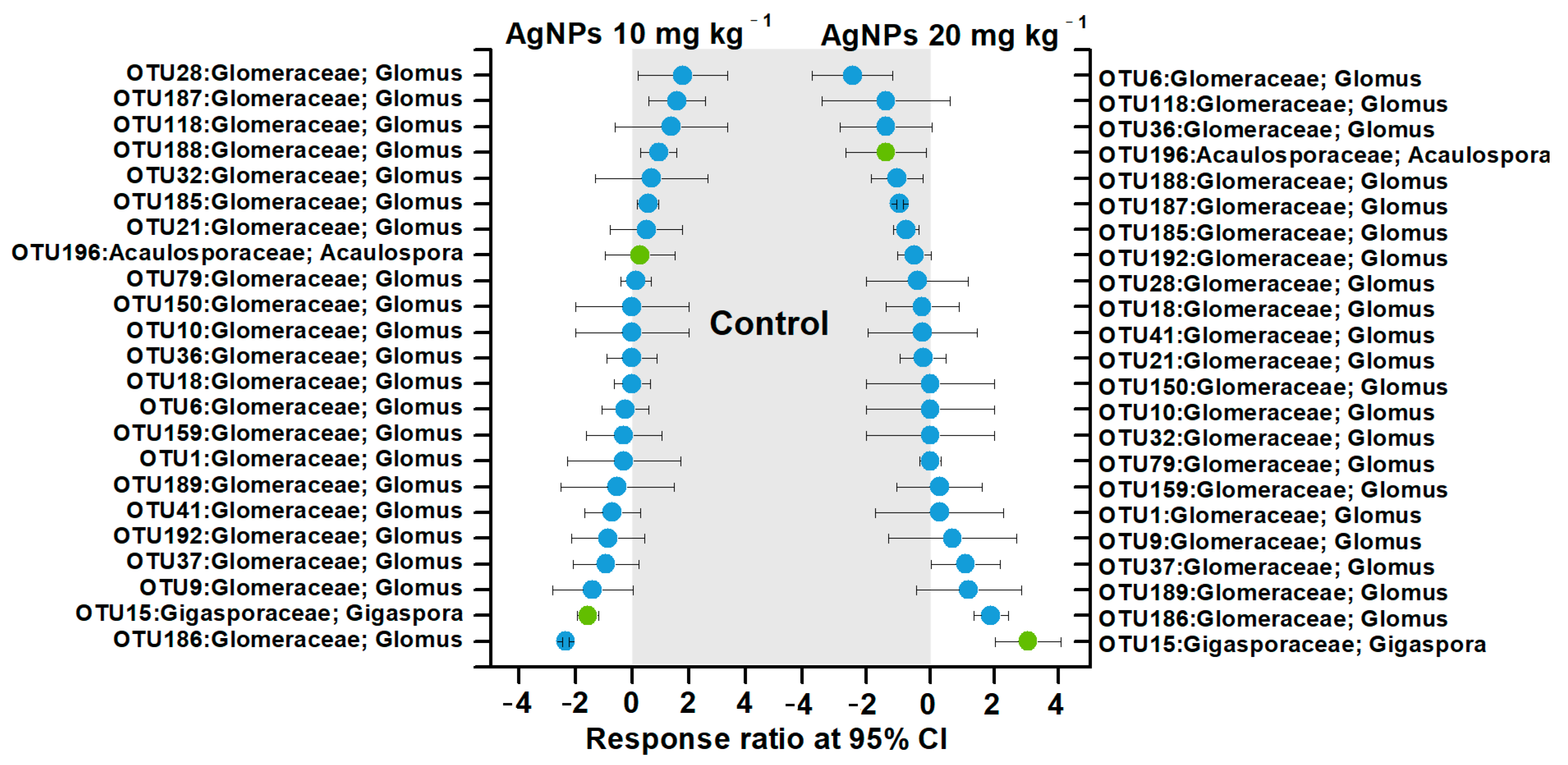
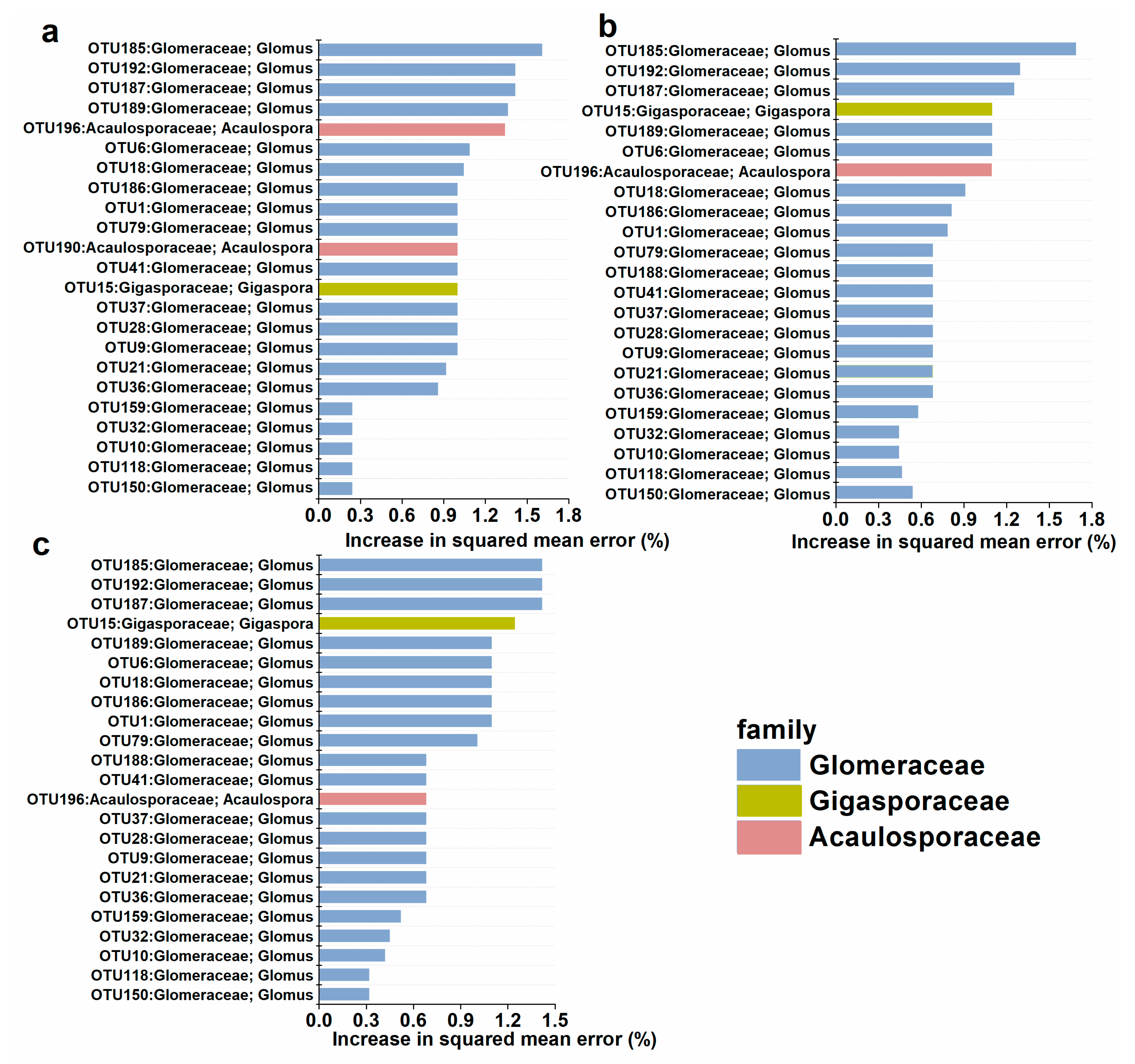
3.3. Taxonomic Distribution and Community Composition of AM Fungal Communities
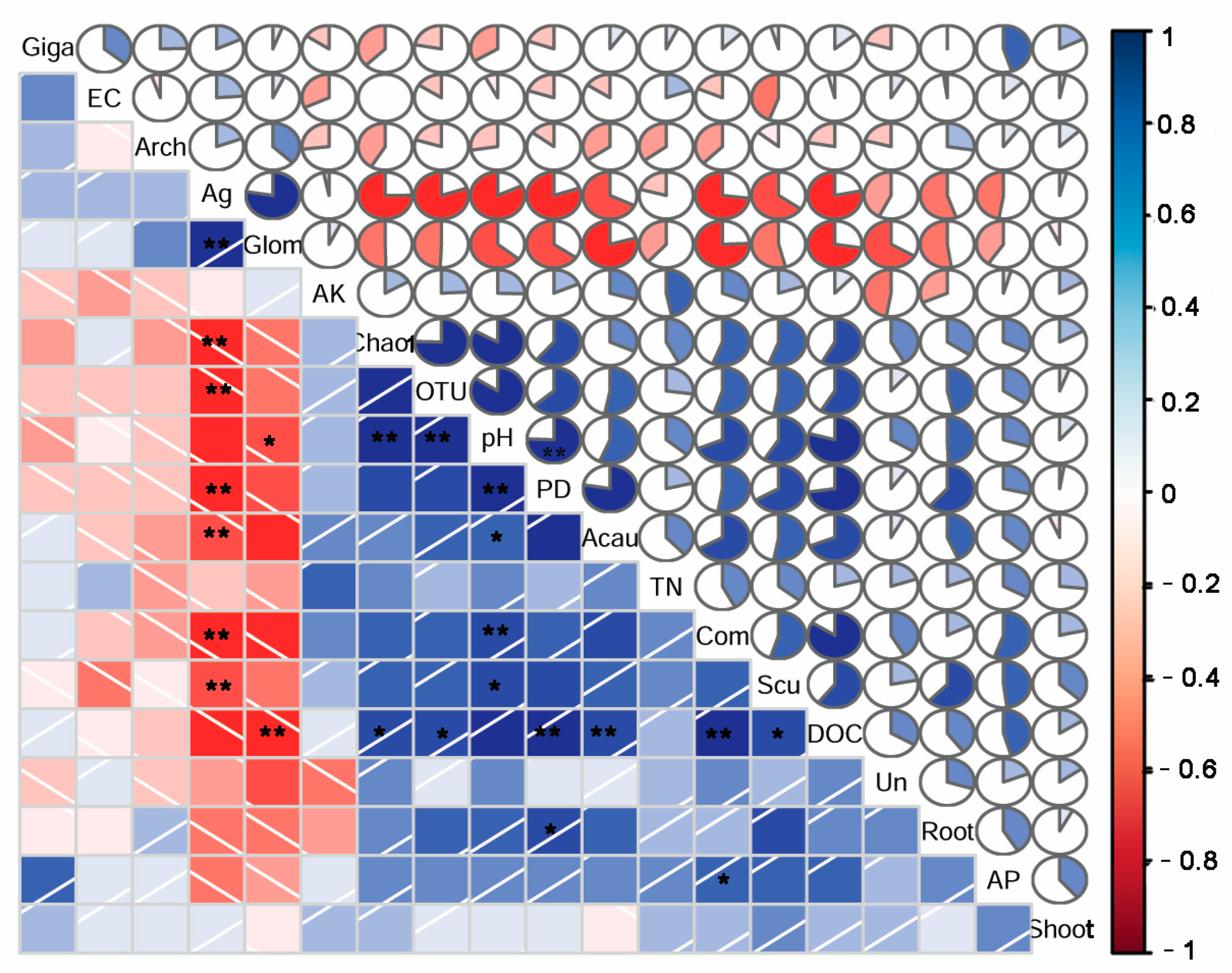
3.4. Correlations between AM Fungal Communities and Soil Properties and Plant Biomasses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Rillig, M.C.; Wright, S.F.; Nichols, K.A.; Schmidt, W.F.; Torn, M.S. Large contribution of arbuscular mycorrhizal fungi to soil carbon pools in tropical forest soils. Plant Soil. 2001, 233, 167–177. [Google Scholar] [CrossRef]
- Wright, S.F.; Upadhyaya, A. A survey of soils for aggregate stability and glomalin, a glycoprotein produced by hyphae of arbuscular mycorrhizal fungi. Plant Soil. 1998, 198, 97–107. [Google Scholar] [CrossRef]
- Gillespie, A.W.; Farrell, R.E.; Walley, F.L.; Ross, A.R.S.; Leinweber, P.; Eckhardt, K.U. Glomalin-related soil protein contains non-mycorrhizal-related heat-stable proteins, lipids and humic materials. Soil. Biol. Biochem. 2011, 43, 766–777. [Google Scholar] [CrossRef]
- Lovelock, C.E.; Wright, S.F.; Nichols, K.A. Using glomalin as an indicator for arbuscular mycorrhizal hyphal growth: An example from a tropical rain forest soil. Soil. Biol. Biochem. 2004, 36, 1009–1012. [Google Scholar] [CrossRef]
- Smith, S.E.; Read, D.J. Mycorrhizal symbiosis; Academic Press: New York, NY, USA, 2008. [Google Scholar]
- Wilson, G.W.T.; Rice, C.W.; Rillig, M.C.; Springer, A.; Hartnett, D.C. Soil aggregation and carbon sequestration are tightly correlated with the abundance of arbuscular mycorrhizal fungi: Results from long-term field experiments. Ecol. Lett. 2009, 12, 452. [Google Scholar] [CrossRef] [PubMed]
- Madanayake, N.H.; Perera, N.; Adassooriya, N.M. Engineered nanomaterials: Threats, releases, and concentrations in the environment. In Emerging Contaminants in the Environment; Sarma, H., Dominguez, D.C., Lee, W.Y., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 225–240. [Google Scholar]
- Vance, M.E.; Kuiken, T.; Vejerano, E.P.; McGinnis, S.P.; Hochella, M.F.J.; Rejeski, D.; Hull, M.S. Nanotechnology in the real world: Redeveloping the nanomaterial consumer products inventory. Beilstein J. Nanotechnol. 2015, 6, 1769–1780. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Pan, Z.; Chen, S. Rice exposure to silver nanoparticles in a life cycle study: Effect of dose responses on grain metabolomic profile, yield, and soil bacteria. Environ. Sci. Nano 2022, 9, 2195. [Google Scholar] [CrossRef]
- Kwak, J.I.; Nam, S.H.; An, Y.J. Assessing the risks of silver nanoparticle-concentrated matrix application in agricultural soil: Implications for plant and soil enzymes. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2023, 269, 109631. [Google Scholar] [CrossRef]
- Lee, W.M.; Kwak, J.I.; An, Y.J. Effect of silver nanoparticles in crop plants Phaseolus radiatus and Sorghum bicolor: Media effect on phytotoxicity. Chemosphere 2012, 86, 491–499. [Google Scholar] [CrossRef]
- Gupta, S.D.; Agarwal, A.; Pradhan, S. Phytostimulatory effect of silver nanoparticles (AgNPs) on rice seedling growth: An insight from antioxidative enzyme activities and gene expression patterns. Ecotoxicol. Environ. Saf. 2018, 161, 624–633. [Google Scholar] [CrossRef]
- Shen, H.Y.; Liu, Y.Y.; Liu, Y.N.; Duan, Z.M.; Wu, P.P.; Lin, Z.F.; Sun, H.Y. Hormetic dose-responses for silver antibacterial compounds, quorum sensing inhibitors, and their binary mixtures on bacterial resistance of Escherichia coli. Sci. Total Environ. 2021, 786, 147464. [Google Scholar] [CrossRef] [PubMed]
- Falco, W.F.; Scherer, M.D.; Oliveira, S.L.; Wender, H.; Colbeck, I.; Lawson, T.; Caires, A.R. Phytotoxicity of silver nanoparticles on Vicia faba: Evaluation of particle size effects on photosynthetic performance and leaf gas exchange. Sci. Total Environ. 2020, 701, 134816. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Xiao, Y. Silver nanoparticles and arbuscular mycorrhizal fungi influence Trifolium repen root-associated AMF community structure and its co-occurrence pattern. Sci. Hortic. 2023, 320, 112232. [Google Scholar] [CrossRef]
- Cao, J.L.; Feng, Y.Z.; He, S.Y.; Lin, X.G. Silver nanoparticles deteriorate the mutual interaction between maize (Zea mays L.) and arbuscular mycorrhizal fungi: A soil microcosm study. Applied Soil. Ecology 2017, 119, 307–316. [Google Scholar] [CrossRef]
- Yin, K.J.; Cao, L.B.; Xu, X.F.; Shi, Z.Y. Effects of molybdenum pollution on arbuscular mycorrhizal fungi and GRSP. Soil. Fertil. Sci. China 2022, 1, 180–187. [Google Scholar]
- Aalipour, H.; Nikbakht, A.; Etemadi, N. Physiological response of Arizona cypress to Cd-contaminated soil inoculated with arbuscular mycorrhizal fungi and plant growth promoting rhizobacteria. Rhizosphere 2021, 18, 100354. [Google Scholar] [CrossRef]
- Jia, X.; Zhao, Y.; He, Y. Glomalin-related soil protein in the rhizosphere of Robinia pseudoacacia L. seedlings under higher air temperature combined with Cd-contaminated soil. Eur. J. Soil. Sci. 2018, 69, 634–645. [Google Scholar] [CrossRef]
- Vodnik, D.; Grčman, H.; Maček, I.; van Elteren, J.T.; Kovačevič, M. The contribution of glomalin-related soil protein to Pb and Zn sequestration in polluted soil. Sci. Total Environ. 2008, 392, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Requejo, R.; Tena, M. Proteome analysis of maize roots reveals that oxidative stress a main contributing factor to plant arsenic toxicity. Phytochemistry 2005, 66, 1519–1528. [Google Scholar] [CrossRef]
- Cao, J.L.; Feng, Y.Z.; Lin, X.G.; Wang, J.H. A beneficial role of arbuscular mycorrhizal fungi in influencing the effects of silver nanoparticles on plant-microbe systems in a soil matrix. Environ. Sci. Pollut. Res. 2020, 27, 11782–11796. [Google Scholar] [CrossRef]
- Noori, A.; White, J.C.; Newman, L.A. Mycorrhizal fungi influence on silver uptake and membrane protein gene expression following silver nanoparticle exposure. J. Nanoparticle Res. 2017, 19, 66. [Google Scholar] [CrossRef]
- Giese, B.; Klaessig, F.; Park, B.; Kagegi, R.; Steinfeldt, M.; Wigger, H.; Gleich, A.V.; Gottschalk, F. Risks, Release and Concentrations of Engineered Nanomaterial in the Environment. Sci. Rep. 2018, 8, 1565. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.J.; Conrad, R. Bacteria rather than Archaea dominate microbial ammonia oxidation in an agricultural soil. Environ. Microbiol. 2009, 11, 1658–1671. [Google Scholar] [CrossRef] [PubMed]
- Philippot, L.; Raaijmakers, J.M.; Lemanceau, P.; van der Putten, W.H. Going back to the roots: The microbial ecology of the rhizosphere. Nat. Rev. Microbiol. 2013, 11, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Bray, R.H.; Kurtz, L.T. Determination of total, organic, and available forms of phosphorus in soils. Soil. Sci. 1945, 59, 39–45. [Google Scholar] [CrossRef]
- Lindsay, W.L.; Norvell, W.A. Development of a DTPA soil test for zinc, iron, manganese, and copper. Soil. Sci. Soc. Am. J. 1978, 42, 421–428. [Google Scholar] [CrossRef]
- Giovannetti, M.; Mosse, B. An evaluation of techniques for measuring vesicular-arbuscular mycorrhizal infection in roots. New Phytol. 1980, 84, 489–500. [Google Scholar] [CrossRef]
- Frostegård, Å.; Tunlid, A.; Bååth, E. Microbial biomass measured as total lipid phosphate in soils of different organic content. J. Microbiol. Methods 1991, 14, 151–163. [Google Scholar] [CrossRef]
- Olsson, P.A. Signature fatty acids provide tools for determination of the distribution and interactions of mycorrhizal fungi in soil. FEMS Microbiol. Ecol. 1999, 29, 303–310. [Google Scholar] [CrossRef]
- Olsson, P.A.; Bååth, E.; Jakobsen, I.; Söderström, B. The use of phospholipid and neutral lipid fatty acids to estimate biomass of arbuscular mycorrhizal fungi in soil. Mycol. Res. 1995, 99, 623–629. [Google Scholar] [CrossRef]
- Treseder, K.K.; Turner, K.M. Glomalin in ecosystems. Soil. Sci. Soc. Am. J. 2007, 71, 1257–1266. [Google Scholar] [CrossRef]
- Wright, S.F.; Upadhyaya, A. Extraction of an abundant and unusual protein from soil and comparison with hyphal protein of arbuscular mycorrhizal fungi. Soil. Sci. 1996, 161, 575–586. [Google Scholar] [CrossRef]
- Schindler, F.V.; Mercer, E.J.; Rice, J.A. Chemical characteristics of glomalinrelated soil protein (GRSP) extracted from soils of varying organic matter content. Soil. Biol. Biochem. 2007, 39, 320–329. [Google Scholar] [CrossRef]
- Zhang, J.; Tang, X.L.; He, X.H.; Liu, J.X. Glomalin-related soil protein responses to elevated CO2 and nitrogen addition in a subtropical forest: Potential consequences for soil carbon accumulation. Soil. Biol. Biochem. 2015, 83, 142–149. [Google Scholar] [CrossRef]
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon and organic matter. In Methods of Soil Analysis Part 2; American Society of Agronomy: Madison, WI, USA, 1982; pp. 539–580. [Google Scholar]
- Biddle, J.F.; Fitz-Gibbon, S.; Schuster, S.C.; Brenchley, J.E.; House, C.H. Metagenomic signatures of the Peru Margin subseafloor biosphere show a genetically distinct environment. Proc. Natl. Acad. Sci. USA 2008, 105, 10583–10588. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Pena, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of highthroughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Opik, M.; Vanatoa, A.; Vanatoa, E.; Moora, M.; Davison, J.; Kalwij, J.M.; Reier, U.; Zobel, M. The online database MaarjAM reveals global and ecosystemic distribution patterns in arbuscular mycorrhizal fungi (Glomeromycota). New Phytol. 2010, 188, 223–241. [Google Scholar] [CrossRef]
- Colwell, R.K. EstimateS: Statistical Estimation of Species Richness and Shared Species from Samples. 2013. Available online: https://purl.oclc.org/estimates (accessed on 1 April 2024).
- Paradis, E.; Claude, J.; Strimmer, K. APE: Analyses of phylogenetics and evolution in R language. Bioinformatics 2004, 20, 289–290. [Google Scholar] [CrossRef]
- Luo, Y.Q.; Hui, D.F.; Zhang, D.Q. Elevated CO2 stimulates net accumulations of carbon and nitrogen in land ecosystems: A meta-analysis. Ecology 2006, 87, 53–63. [Google Scholar] [CrossRef]
- Hauke, J.; Kossowski, T. Comparison of values of Pearson’s and Spearman’s correlation coefficients on the same sets of data. Quaest. Geogr. 2011, 30, 87–93. [Google Scholar] [CrossRef]
- Liaw, A.; Wiener, M. Classification and regression by randomForest. R News 2002, 2, 18–22. [Google Scholar]
- Das, P.; Barua, S.; Sarkar, S.; Chatterjee, S.K.; Mukherjee, S.; Goswami, L.; Das, S.; Bhattacharya, S.; Karak, N.; Bhattacharya, S.S. Mechanism of toxicity and transformation of silver nanoparticles: Inclusive assessment in earthworm-microbe-soil-plant system. Geoderma 2018, 314, 73–84. [Google Scholar] [CrossRef]
- Kim, J.S.; Kuk, E.; Yu, K.N.; Kim, J.H.; Park, S.J.; Lee, H.J.; Kim, S.H.; Park, Y.K.; Park, Y.H.; Hwang, C.Y.; et al. Antimicrobial effects of silver nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2007, 3, 95–101. [Google Scholar] [CrossRef]
- Parvin, S.; Geel, M.V.; Yeasmin, T.; Lievens, B.; Honnay, O. Variation in arbuscular mycorrhizal fungal communities associated with lowland rice (Oryza sativa) along a gradient of soil salinity and arsenic contamination in Bangladesh. Sci. Total Environ. 2019, 686, 546–554. [Google Scholar] [CrossRef]
- Zheng, J.Q.; Cui, M.M.; Wang, C.; Wang, J.; Wang, S.L.; Sun, Z.J.; Ren, F.R.; Wan, S.Q.; Han, S.J. Elevated CO2, warming, N addition, and increased precipitation affect different aspects of the arbuscular mycorrhizal fungal community. Sci. Total Environ. 2022, 806, 150522. [Google Scholar] [CrossRef]
- Chen, Y.L.; Xu, Z.W.; Xu, T.L.; Veresoglou, S.D.; Yang, G.W.; Chen, B.D. Nitrogen deposition and precipitation induced phylogenetic clustering of arbuscular mycorrhizal fungal communities. Soil. Biol. Biochem. 2017, 115, 233–242. [Google Scholar] [CrossRef]
- Hart, M.M.; Reader, R.J. Taxonomic basis for variation in the colonization strategy of arbuscular mycorrhizal fungi. New Phytol. 2002, 153, 335–344. [Google Scholar] [CrossRef]
- Daniell, T.J.; Husband, R.; Fitter, A.H.; Young, J.P.W. Molecular diversity of arbuscular mycorrhizal fungi colonising arable crops. Fems Microbiol. Ecol. 2001, 36, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Maček, I.; Clark, D.R.; Šibanc, N.; Moser, G.; Vodnik, D.; Müller, C.; Dumbrell, A.J. Impacts of long-term elevated atmospheric CO2 concentrations on communities of arbuscular mycorrhizal fungi. Mol. Ecol. 2019, 28, 3445–3458. [Google Scholar] [CrossRef]
- Cotton, T.A.; Fitter, A.H.; Miller, R.M.; Dumbrell, A.J.; Helgason, T. Fungi in the future: Interannual variation and effects of atmospheric change on arbuscular mycorrhizal fungal communities. New Phytol. 2015, 205, 1598–1607. [Google Scholar] [CrossRef]
- Johnson, D.; Leake, J.R.; Read, D.J. Transfer of recent photosynthate into mycorrhizal mycelium of an upland grassland: Short-term respiratory losses and accumulation of C-14. Soil. Biol. Biochem. 2002, 34, 1521–1524. [Google Scholar] [CrossRef]
- Angelard, C.; Tanner, C.J.; Fontanillas, P.; Niculita-Hirzel, H.; Masclaux, F.; Sanders, I.R. Rapid genotypic change and plasticity in arbuscular mycorrhizal fungi is caused by a host shift and enhanced by segregation. Isme J. 2014, 8, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Priester, J.H.; Van De Werfhorst, L.C.; Walker, S.L.; Nisbet, R.M.; An, Y.-J.; Schimel, J.P.; Gardea-Torresdey, J.L.; Holden, P.A. Soybean plants modify metal oxide nanoparticle effects on soil bacterial communities. Environ. Sci. Technol. 2014, 48, 13489–13496. [Google Scholar] [CrossRef] [PubMed]
- Dumbrell, A.J.; Nelson, M.; Helgason, T.; Dytham, C.; Fitter, A.H. Relative roles of niche and neutral processes in structuring a soil microbial community. ISME J. 2010, 4, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Xiu, Z.M.; Zhang, Q.B.; Puppala, H.L.; Colvin, V.L.; Alvarez, P.J.J. Negligible particle-specific antibacterial activity of silver nanoparticles. Nano Lett. 2012, 12, 4271–4275. [Google Scholar] [CrossRef] [PubMed]
- Bainard, L.D.; Bainard, J.D.; Hamel, C.; Gan, Y. Spatial and temporal structuring of arbuscular mycorrhizal communities is differentially influenced by abiotic factors and host crop in a semi-arid prairie agroecosystem. Fems Microbiol. Ecol. 2014, 88, 333–344. [Google Scholar] [CrossRef]
- Yang, Y.R.; Song, Y.Y.; Scheller, H.V.; Ghosh, A.; Ban, Y.H.; Chen, H.; Tang, M. Community structure of arbuscular mycorrhizal fungi associated with Robinia pseudoacacia in uncontaminated and heavy metal contaminated soils. Soil. Biol. Biochem. 2015, 86, 146–158. [Google Scholar] [CrossRef]
- Zheng, Z.; Ma, X.; Zhang, Y.; Liu, Y.J.; Zhang, S.H. Soil properties and plant community-level traits mediate arbuscular mycorrhizal fungal response to nitrogen enrichment and altered precipitation. Appl. Soil. Ecol. 2022, 169, 104245. [Google Scholar] [CrossRef]
- Dumbrell, A.J.; Ashton, P.D.; Aziz, N.; Feng, G.; Nelson, M.; Dytham, C.; Fitter, A.H.; Helgason, T. Distinct seasonal assemblages of arbuscular mycorrhizal fungi revealed by massively parallel pyrosequencing. New Phytol. 2011, 190, 794–804. [Google Scholar] [CrossRef]
- Qin, H.; Wu, Q.F.; Chen, J.H.; Li, Y.C.; Liang, C.F.; Xu, Q.F.; Fuhrmann, J.J.; Shen, Y. Bamboo forest expansion increases soil organic carbon through its effect on soil arbuscular mycorrhizal fungal community and abundance. Plant Soil. 2017, 420, 407–421. [Google Scholar] [CrossRef]
- Voets, L.; De La Providencia, I.; Declerck, S. Glomeraceae and Gigasporaceae differ in their ability to form hyphal networks. New Phytol. 2006, 172, 185–188. [Google Scholar] [CrossRef] [PubMed]
- Rillig, M.C.; Mummey, D.L. Mycorrhizas and soil structure. New Phytol. 2006, 171, 41–53. [Google Scholar] [CrossRef]
- Ren, A.T.; Mickan, B.S.; Li, J.Y.; Zhou, R.; Zhang, X.C.; Ma, M.S.; Wesly, K.; Xiong, Y.C. Soil labile organic carbon sequestration is tightly correlated with the abundance and diversity of arbuscular mycorrhizal fungi in semiarid maize fields. Land. Degrad. Dev. 2021, 32, 1224–1236. [Google Scholar] [CrossRef]
- Lee, J.E.; Lee, E.H.; Eom, A.H. Effects of arbuscular mycorrhizal fungal inoculation on the growth of red pepper and soil glomalin content. Korean J. Mycol. 2021, 4, 517–524. [Google Scholar]
- Chi, G.G.; Wu, Q.S. Effects of mycorrhizal fungi on plant and growth soil properties in trifoliate orange seedlings grown in a root–box. Philipp. Agric. Sci. 2017, 3, 271–277. [Google Scholar]
- Zhang, F.; Wang, P.; Zou, Y.N.; Wu, Q.S.; Kuča, K. Effects of mycorrhizal fungi on root-hair growth and hormone levels of taproot and lateral roots in trifoliate orange under drought stress. Arch. Agron. Soil. Sci. 2019, 65, 1316–1330. [Google Scholar] [CrossRef]
- Zhang, J.; Tang, X.L.; Zhong, S.Y.; Yin, G.C.; Gao, Y.F.; He, X.H. Recalcitrant carbon components in glomalin-related soil protein facilitate soil organic carbon preservation in tropical forests. Sci. Rep. 2017, 7, 2391. [Google Scholar] [CrossRef] [PubMed]
- He, J.D.; Chi, G.G.; Zou, Y.N.; Shu, B.; Wu, Q.S.; Strivastava, A.K.; Kuča, K. Contribution of glomalin-related soil proteins to soil organic carbon in trifoliate orange. Appl. Soil. Ecol. 2020, 154, 103592. [Google Scholar] [CrossRef]
- Wang, Q.; Li, L.W.; Chen, J.Y.; Hong, H.L.; Lu, H.L.; Liu, J.C.; Dong, Y.W.; Yan, C.L. Glomalin-related soil protein deposition and carbon sequestration in the old Yellow River delta. Sci. Total Environ. 2018, 625, 619–626. [Google Scholar] [CrossRef]
- Wu, Q.S.; Cao, M.Q.; Zou, Y.N.; He, X.H. Direct and indirect effects of glomalin, mycorrhizal hyphae, and roots on aggregate stability in rhizosphere of trifoliate orange. Sci. Rep. 2014, 4, 5823. [Google Scholar] [CrossRef]
- Treseder, K.K.; Turner, K.M.; Mack, M.C. Mycorrhizal responses to nitrogen fertilization in boreal ecosystems: Potential consequences for soil carbon storage. Glob. Chang. Biol. 2007, 13, 78–88. [Google Scholar] [CrossRef]

| AgNPs (mg kg−1) | EE-GRSP/SOC | DE-GRSP/SOC | T-GRSP/SOC |
|---|---|---|---|
| Control | 0.426 ± 0.022 a | 0.679 ± 0.018 a | 1.105 ± 0.007 ab |
| 1 | 0.438 ± 0.021 a | 0.711 ± 0.077 a | 1.145 ± 0.056 a |
| 5 | 0.386 ± 0.014 ab | 0.716 ± 0.052 a | 1.101 ± 0.066 ab |
| 10 | 0.332 ± 0.045 bc | 0.723 ± 0.047 a | 1.055 ± 0.005 ab |
| 20 | 0.295 ± 0.019 c | 0.723 ± 0.032 a | 1.018 ± 0.030 b |
| AgNPs (mg kg−1) | PD | Chao1 | Observed OTUs |
|---|---|---|---|
| Control | 2.23 ± 0.09 a | 47.92 ± 8.06 a | 35.60 ± 4.93 a |
| 1 | 2.30 ± 0.12 a | 44.83 ± 5.01 ab | 36.13 ± 7.03 a |
| 5 | 1.99 ± 0.22 ab | 46.45 ± 3.98 ab | 32.67 ± 5.76 ab |
| 10 | 2.03 ± 0.24 ab | 37.89 ± 3.24 ab | 29.03 ± 3.85 ab |
| 20 | 1.66 ± 0.19 b | 33.00 ± 3.61 b | 20.73 ± 3.31 b |
| Matrix | EE-GRSP | DE-GRSP | T-GRSP |
|---|---|---|---|
| Community composition a | 0.711 ** | −0.001 | 0.623 * |
| Diversity b | 0.772 ** | 0.042 | 0.695 ** |
| Glomeraceae | 0.623 * | 0.020 | 0.556 * |
| Acaulosporaceae | −0.694 * | 0.079 | −0.576 * |
| Archaeosporaceae | −0.139 | 0.203 | −0.039 |
| Gigasporaceae | −0.216 | 0.373 | −0.040 |
| Scutellosporaceae | 0.794 ** | 0.118 | 0.743 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, H.; Liu, Z.; Han, Y.; Cao, J. Impact of Silver Nanoparticles on Arbuscular Mycorrhizal Fungi and Glomalin-Related Soil Proteins in the Rhizosphere of Maize Seedlings. Diversity 2024, 16, 273. https://doi.org/10.3390/d16050273
Zhao H, Liu Z, Han Y, Cao J. Impact of Silver Nanoparticles on Arbuscular Mycorrhizal Fungi and Glomalin-Related Soil Proteins in the Rhizosphere of Maize Seedlings. Diversity. 2024; 16(5):273. https://doi.org/10.3390/d16050273
Chicago/Turabian StyleZhao, Haiying, Zhiyuan Liu, Yu Han, and Jiling Cao. 2024. "Impact of Silver Nanoparticles on Arbuscular Mycorrhizal Fungi and Glomalin-Related Soil Proteins in the Rhizosphere of Maize Seedlings" Diversity 16, no. 5: 273. https://doi.org/10.3390/d16050273
APA StyleZhao, H., Liu, Z., Han, Y., & Cao, J. (2024). Impact of Silver Nanoparticles on Arbuscular Mycorrhizal Fungi and Glomalin-Related Soil Proteins in the Rhizosphere of Maize Seedlings. Diversity, 16(5), 273. https://doi.org/10.3390/d16050273





