The City as an Evolutionary Hothouse—The Search for Rapid Evolution in Urban Settings
Abstract
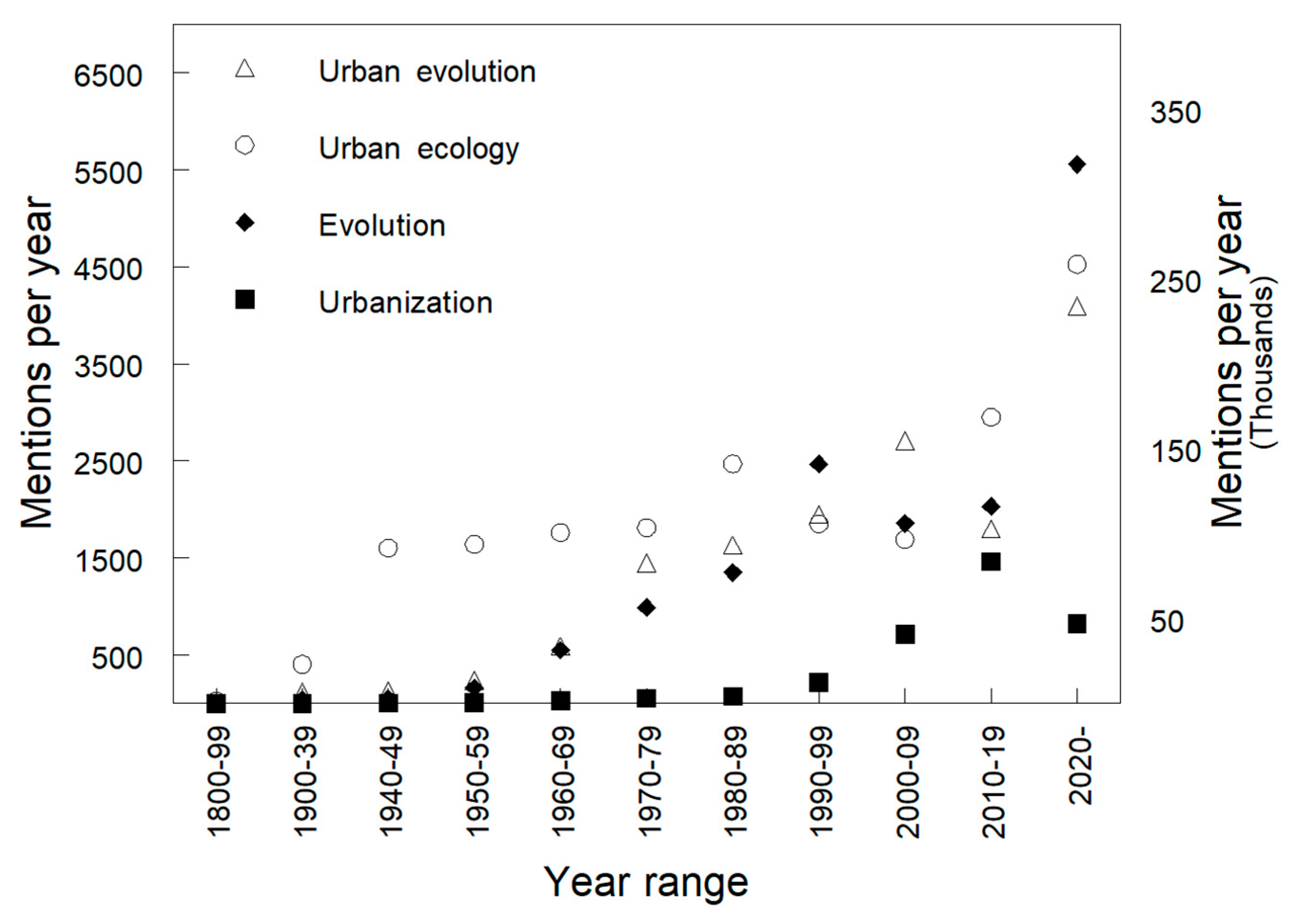
:1. Introduction
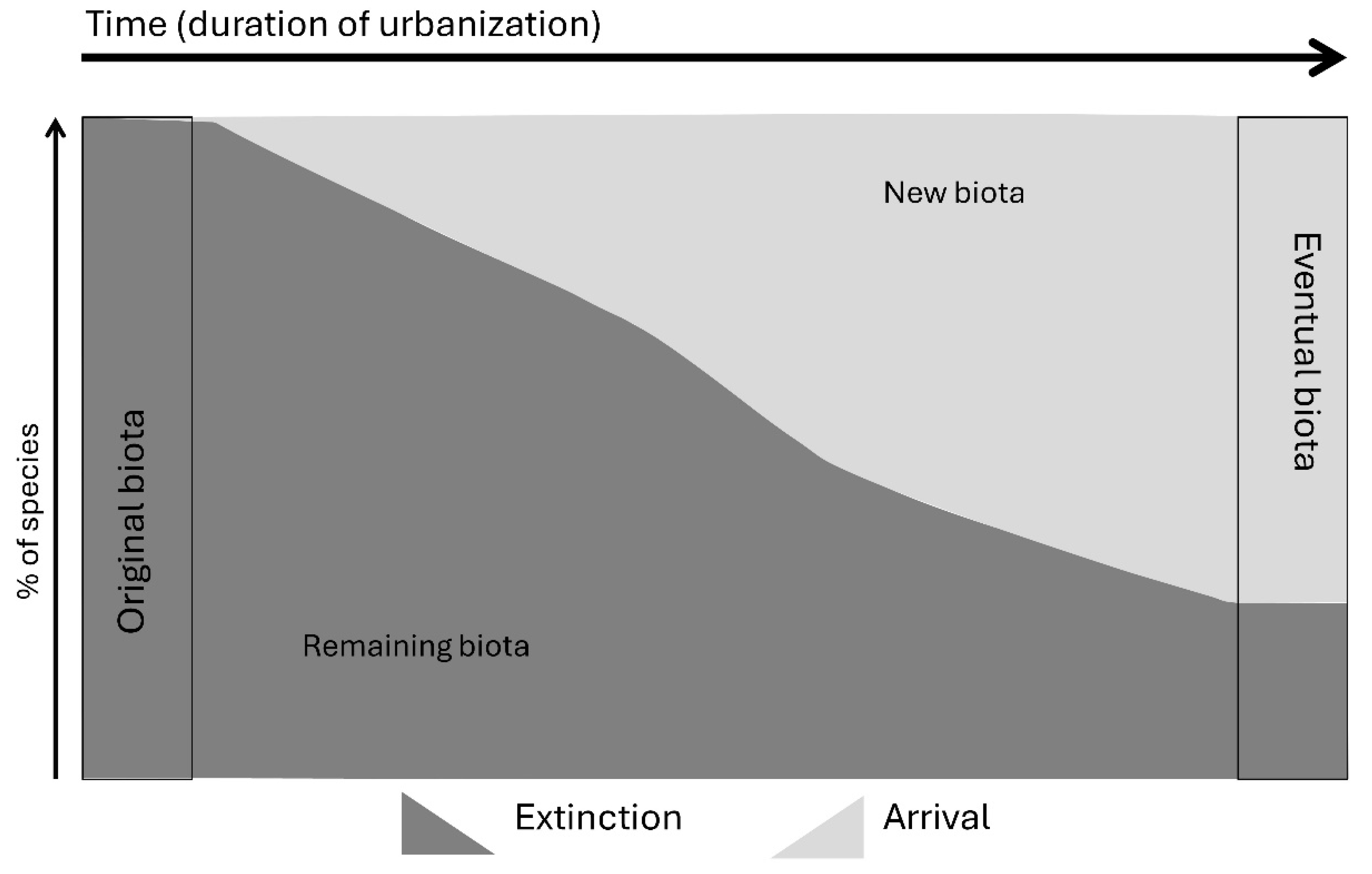
2. Urbanization as an Ecological Phenomenon and a Conservation Concern
3. Urban Generalists, Specialists, and Invaders: Different Settings for Evolutionary Processes?
4. Urbanization as a Driver of Evolution
4.1. Mutation
4.2. Genetic Drift
4.3. Gene Flow
4.4. Selection and Adaptation
5. Urbanization and Pathogen Evolution
6. Emergence of New Forms under Urbanization
7. Epigenetics and Urbanization
8. Research Prospects
8.1. Where Should We Look?
8.2. Which Species Should We Study?
8.3. Convergent or Parallel Evolution?
8.4. Impact of Biotic Environment
8.5. Importance of Epigenetic
8.6. Urban Human Evolution
8.7. Relevance for Urban Conservation
9. Conclusions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hartl, D.L. A Primer of Population Genetics, 3rd ed.; Sinauer Associates, Inc.: Sunderland, MA, USA, 2000; 221p. [Google Scholar]
- Wang, M.S.; Murray, G.G.R.; Mann, D.; Groves, P.; Vershinina, A.O.; Supple, M.A.; Kapp, J.D.; Corbett-Detig, R.; Crump, S.E.; Stirling, I.; et al. A polar bear paleogenome reveals extensive ancient gene flow from polar bears into brown bears. Nat. Ecol. Evol. 2022, 6, 936–944. [Google Scholar] [CrossRef]
- Dufresnes, C.; Brelsford, A.; Jeffries, D.L.; Mazepa, G.; Suchan, T.; Canestrelli, D.; Nicieza, A.; Fumagalli, L.; Dubey, S.; Martínez-Solano, I.; et al. Mass of genes rather than master genes underlie the genomic architecture of amphibian speciation. Proc. Natl. Acad. Sci. USA 2021, 118, e2103963118. [Google Scholar] [CrossRef]
- Holliday, T.W. Neanderthals and modern humans: An example of a mammalian syngameon? In Neanderthals Revisited: New Approaches and Perspectives, 1st ed.; Hublin, J.-J., Harvati, K., Harrison, T., Eds.; Springer: Dordrecht, The Netherlands, 2006; pp. 281–297. [Google Scholar]
- Semaw, S. The world’s oldest stone artefacts from Gona, Ethiopia: Their implications for understanding stone technology and patterns of human evolution between 2·6–1·5 million years ago. J. Archaeol. Sci. 2000, 27, 1197–1214. [Google Scholar] [CrossRef]
- Galway-Witham, J.; Cole, J.; Stringer, C. Aspects of human physical and behavioural evolution during the last 1 million years. J. Quat. Sci. 2019, 34, 355–378. [Google Scholar] [CrossRef]
- Grant, P.R.; Grant, B.R. Evolution of character displacement in Darwin’s finches. Science 2006, 313, 224–226. [Google Scholar] [CrossRef]
- Darwin, C.R. Variation of Animals and Plants under Domestication; John Murray: London, UK, 1868. [Google Scholar]
- Göttert, T.; Perry, G. Going wild in the city—Animal feralization and its impacts on biodiversity in urban environments. Animals 2023, 13, 747. [Google Scholar] [CrossRef]
- Zeller, U.; Göttert, T. The relations between evolution and domestication reconsidered-implications for systematics, ecology, and nature conservation. Glob. Ecol. Conserv. 2019, 20, e00756. [Google Scholar] [CrossRef]
- Howe, N.S.; Hale, M.C.; Waters, C.D.; Schaal, S.M.; Shedd, K.R.; Larson, W.A. Genomic evidence for domestication selection in three hatchery populations of Chinook salmon, Oncorhynchus tshawytscha. Evol. Appl. 2024, 17, e13656. [Google Scholar] [CrossRef]
- Davis, K.; Golden, H.H. Urbanization and the development of pre-industrial areas. Econ. Dev. Cult. Chang. 1954, 3, 6–26. Available online: https://www.jstor.org/stable/1151656 (accessed on 1 May 2024). [CrossRef]
- Chandler, T.; Fox, G. 3000 Years of Urban Growth; Academic Press: New York, NY, USA, 1974. [Google Scholar]
- Diamond, J. Natural selection: Rapid evolution of urban birds. Nature 1986, 324, 107–108. [Google Scholar] [CrossRef]
- Schilthuizen, M. Darwin Comes to Town: How the Urban Jungle Drives Evolution; Picador: London, UK, 2019. [Google Scholar]
- McDonald, R.I.; Mansur, A.V.; Ascensão, F.; Colbert, M.L.; Crossman, K.; Elmqvist, T.; Gonzalez, A.; Güneralp, B.; Haase, D.; Hamann, M.; et al. Research gaps in knowledge of the impact of urban growth on biodiversity. Nat. Sustain. 2020, 3, 16–24. [Google Scholar] [CrossRef]
- Johnson, M.T.; Munshi-South, J. Evolution of life in urban environments. Science 2017, 358, eaam8327. [Google Scholar] [CrossRef] [PubMed]
- Rivkin, L.R.; Santangelo, J.S.; Alberti, M.; Aronson, M.F.; de Keyzer, C.W.; Diamond, S.E.; Fortin, M.J.; Frazee, L.J.; Gorton, A.J.; Hendry, A.P.; et al. A roadmap for urban evolutionary ecology. Evol. Appl. 2019, 12, 384–398. [Google Scholar] [CrossRef] [PubMed]
- Hahs, A.K.; Fournier, B.; Aronson, M.F.; Nilon, C.H.; Herrera-Montes, A.; Salisbury, A.B.; Threlfall, C.G.; Rega-Brodsky, C.C.; Lepczyk, C.A.; La Sorte, F.A.; et al. Urbanisation generates multiple trait syndromes for terrestrial animal taxa worldwide. Nat. Commun. 2023, 14, 4751. [Google Scholar] [CrossRef] [PubMed]
- Roque, D.V.; Göttert, T.; Zeller, U.; Macandza, V.A. Modeling the drivers of large herbivore distribution in human-dominated southern African savannas. Ecosphere 2024, 15, e4770. [Google Scholar] [CrossRef]
- Thompson, K.A.; Rieseberg, L.H.; Schluter, D. Speciation and the city. Trends Ecol. Evol. 2018, 33, 815–826. [Google Scholar] [CrossRef] [PubMed]
- Ravinet, M.; Elgvin, T.O.; Trier, C.; Aliabadian, M.; Gavrilov, A.; Sætre, G.P. Signatures of human-commensalism in the house sparrow genome. Proc. R. Soc. Lond. B 2018, 285, 20181246. [Google Scholar] [CrossRef] [PubMed]
- Patten, M.A.; Burger, J.C. Reserves as double-edged sword: Avoidance behavior in an urban-adjacent wildland. Biol. Conserv. 2018, 218, 233–239. [Google Scholar] [CrossRef]
- Ditchkoff, S.S.; Saalfeld, S.T.; Gibson, C.J. Animal behavior in urban ecosystems: Modifications due to human-induced stress. Urban Ecosyst. 2006, 9, 5–12. [Google Scholar] [CrossRef]
- Caro, T. Behavior and conservation, conservation and behavior. Curr. Opin. Behav. Sci. 2016, 12, 97–102. [Google Scholar] [CrossRef]
- Geslin, B.; Gauzens, B.; Thébault, E.; Dajoz, I. Plant pollinator networks along a gradient of urbanization. PLoS ONE 2013, 8, e63421. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Li, Z.; Geisen, S.; Qiao, Z.; Breed, M.F.; Sun, X. Degree of urbanization and vegetation type shape soil biodiversity in city parks. Sci. Total Environ. 2023, 899, 166437. [Google Scholar] [CrossRef] [PubMed]
- Schlaeppi, K.; Bulgarelli, D. The plant microbiome at work. Mol. Plant Microbe Interact. 2015, 28, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Dornelas, M.; Gotelli, N.J.; Shimadzu, H.; Moyes, F.; Magurran, A.E.; McGill, B.J. A balance of winners and losers in the Anthropocene. Ecol. Lett. 2019, 22, 847–854. [Google Scholar] [CrossRef]
- de Andrade, A.C. Metropolitan lizards? Urbanization gradient and the density of lagartixas (Tropidurus hispidus) in a tropical city. Ecol. Evol. 2020, 10, 1740–1750. [Google Scholar] [CrossRef]
- Beckerman, A.P.; Boots, M.; Gaston, K.J. Urban bird declines and the fear of cats. Anim. Conserv. 2007, 10, 320–325. [Google Scholar] [CrossRef]
- Slabbekoorn, H.; Ripmeester, E.A.P. Birdsong and anthropogenic noise: Implications and applications for conservation. Mol. Ecol. 2008, 17, 72–83. [Google Scholar] [CrossRef]
- Cooke, S.J.; Piczak, M.L.; Singh, N.J.; Åkesson, S.; Ford, A.T.; Chowdhury, S.; Mitchell, G.W.; Norris, D.R.; Hardesty-Moore, M.; McCauley, D.; et al. Animal migration in the Anthropocene: Threats and mitigation options. Biol. Rev. 2024; early view. [Google Scholar] [CrossRef]
- Perry, G.; Cox, R.D. Opportunities for biodiversity conservation via urban ecosystem regeneration. Diversity 2024, 16, 131. [Google Scholar] [CrossRef]
- Pabijan, M.; Palomar, G.; Antunes, B.; Antoł, W.; Zieliński, P.; Babik, W. Evolutionary principles guiding amphibian conservation. Evol. Appl. 2020, 13, 857–878. [Google Scholar] [CrossRef] [PubMed]
- Padayachee, A.L.; Irlich, U.M.; Faulkner, K.T.; Gaertner, M.; Procheş, Ş.; Wilson, J.R.; Rouget, M. How do invasive species travel to and through urban environments? Biol. Invasions 2017, 19, 3557–3570. [Google Scholar] [CrossRef]
- Zeller, U.; Perry, G.; Göttert, T. Biodiversity and the Urban-Rural Interface: Conflicts vs. opportunities-Proceedings of an International Workshop in Linde, Germany; Humboldt-Universität zu Berlin: Berlin, Germany, 2019. [Google Scholar] [CrossRef]
- Von Lührte, A.; Krauss, M. Die Ausbreitung des Fischotters in Berlin. Beitr. J. Wildf. 2023, 48, 249–257. [Google Scholar]
- Mayer, M.; Sunde, P. Colonization and habitat selection of a declining farmland species in urban areas. Urban Ecosyst. 2020, 23, 543–554. [Google Scholar] [CrossRef]
- Pagaldai, N.; Arizaga, J.; Jiménez-Franco, M.V.; Zuberogoitia, I. Colonization of urban habitats: Tawny owl abundance is conditioned by urbanization structure. Animals 2021, 11, 2954. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, B. Diversity of birds in Bharatpur metropolitan city, Chitwan, Nepal. Vibek Multi-Discip. Peer-Rev. J. 2018, 34, 36–49. [Google Scholar]
- Rycken, S.J.; Warren, K.S.; Yeap, L.; Donaldson, R.; Mawson, P.; Dawson, R.; Shephard, J.M. Forest specialist species in the urban landscape: Do different levels of urbanization affect the movements of forest red-tailed black cockatoos (Calyptorhynchus banksii naso)? Avian Conserv. Ecol. 2022, 17, 11. [Google Scholar] [CrossRef]
- Isaksson, C. Urbanization, oxidative stress and inflammation: A question of evolving, acclimatizing or coping with urban environmental stress. Funct. Ecol. 2015, 29, 913–923. [Google Scholar] [CrossRef]
- van Velzen, E.; Etienne, R.S. The evolution and coexistence of generalist and specialist herbivores under between-plant competition. Theor. Ecol. 2013, 6, 87–98. [Google Scholar] [CrossRef]
- Fraebel, D.T.; Gowda, K.; Mani, M.; Kuehn, S. Evolution of generalists by phenotypic plasticity. Iscience 2020, 23, 101678. [Google Scholar] [CrossRef] [PubMed]
- Clark, P.J.; Reed, J.M.; Chew, F.S. Effects of urbanization on butterfly species richness, guild structure, and rarity. Urban Ecosyst. 2007, 10, 321–337. [Google Scholar] [CrossRef]
- Clavel, J.; Julliard, R.; Devictor, V. Worldwide decline of specialist species: Toward a global functional homogenization? Front. Ecol. Environ. 2011, 9, 222–228. [Google Scholar] [CrossRef]
- Winchell, K.M.; Aviles-Rodriguez, K.J.; Carlen, E.J.; Miles, L.S.; Charmantier, A.; De León, L.F.; Gotanda, K.M.; Rivkin, L.R.; Szulkin, M.; Verrelli, B.C. Moving past the challenges and misconceptions in urban adaptation research. Ecol. Evol. 2022, 12, e9552. [Google Scholar] [CrossRef]
- Kassen, R. The experimental evolution of specialists, generalists, and the maintenance of diversity. J. Evol. Biol. 2002, 15, 173–190. [Google Scholar] [CrossRef]
- Gilchrist, G.W. Specialists and generalists in changing environments. I. Fitness landscapes of thermal sensitivity. Am. Nat. 1995, 146, 252–270. [Google Scholar] [CrossRef]
- Schlaepfer, M.A.; Guinaudeau, B.P.; Martin, P.; Wyler, N. Quantifying the contributions of native and non-native trees to a city’s biodiversity and ecosystem services. Urban For. Urban Green. 2020, 56, 126861. [Google Scholar] [CrossRef]
- Epp Schmidt, D.J.; Pouyat, R.; Szlavecz, K.; Setälä, H.; Kotze, D.J.; Yesilonis, I.; Cilliers, S.; Hornung, E.; Dombos, M.; Yarwood, S.A. Urbanization erodes ectomycorrhizal fungal diversity and may cause microbial communities to converge. Nat. Ecol. Evol. 2017, 1, 0123. [Google Scholar] [CrossRef]
- Borden, J.B.; Flory, S.L. Urban evolution of invasive species. Front. Ecol. Environ. 2021, 19, 184–191. [Google Scholar] [CrossRef]
- Francis, R.A.; Chadwick, M.A. Urban invasions: Non-native and invasive species in cities. Geography 2015, 100, 144–151. [Google Scholar] [CrossRef]
- Santana Marques, P.; Resende Manna, L.; Clara Frauendorf, T.; Zandonà, E.; Mazzoni, R.; El-Sabaawi, R. Urbanization can increase the invasive potential of alien species. J. Anim. Ecol. 2020, 89, 2345–2355. [Google Scholar] [CrossRef]
- Ducatez, S.; Baguette, M.; Trochet, A.; Chaput-Bardy, A.; Legrand, D.; Stevens, V.; Fréville, H. Flight endurance and heating rate vary with both latitude and habitat connectivity in a butterfly species. Oikos 2013, 122, 601–611. [Google Scholar] [CrossRef]
- Schoville, S.D.; Widmer, I.; Deschamps-Cottin, M.; Manel, S. Morphological clines and weak drift along an urbanization gradient in the butterfly, Pieris rapae. PLoS ONE 2013, 8, e83095. [Google Scholar] [CrossRef] [PubMed]
- Rocabert, C.; Fenet, S.; Kaufmann, B.; Gippet, J.M. Accounting for the topology of road networks to better explain human-mediated dispersal in terrestrial landscapes. Ecography 2023, 2024, e07068. [Google Scholar] [CrossRef]
- Kozakiewicz, C.P.; Burridge, C.P.; Funk, W.C.; Salerno, P.E.; Trumbo, D.R.; Gagne, R.B.; Boydston, E.E.; Fisher, R.N.; Lyren, L.M.; Jennings, M.K.; et al. Urbanization reduces genetic connectivity in bobcats (Lynx rufus) at both intra–and interpopulation spatial scales. Mol. Ecol. 2019, 28, 5068–5085. [Google Scholar] [CrossRef] [PubMed]
- Vellend, M.; Harmon, L.J.; Lockwood, J.L.; Mayfield, M.M.; Hughes, A.R.; Wares, J.P.; Sax, D.F. Effects of exotic species on evolutionary diversification. Trends Ecol. Evol. 2007, 22, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Tranquillo, C.; Bisi, F.; Wauters, L.A.; Preatoni, D.; Martinoli, A.; Santicchia, F. The impact of urbanisation on chipmunks, arboreal and flying squirrels: A global systematic review. Mammal Rev. 2023, 54, 150–177. [Google Scholar] [CrossRef]
- Schell, C.J.; Dyson, K.; Fuentes, T.L.; Des Roches, S.; Harris, N.C.; Miller, D.S.; Woelfle-Erskine, C.A.; Lambert, M.R. The ecological and evolutionary consequences of systemic racism in urban environments. Science 2020, 369, eaay4497. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.L.; Nizamani, M.M.; Guo, L.Y.; Cui, J.; Padullés Cubino, J.; Hughes, A.C.; Wang, H.F. Interplay of socio-economic and environmental factors in shaping urban plant biodiversity: A comprehensive analysis. Front. Ecol. Evol. 2024, 12, 1344343. [Google Scholar] [CrossRef]
- Marzluff, J.M.; Shulenberger, E.; Endlicher, W.; Alberti, M.; Bradley, G.; Ryan, C.; ZumBrunnen, C.; Simon, U. An International Perspective on the Interaction between Humans and Nature; Springer Science and Business Media: New York, NY, USA, 2008. [Google Scholar]
- Gaston, K.J. Urban Ecology; Oxford University Press: New York, NY, USA, 2010. [Google Scholar]
- McCleery, R.A.; Moorman, C.E.; Peterson, M.N. Urban Wildlife Conservation: Theory and Practice; Springer: New York, NY, USA, 2014. [Google Scholar]
- Alberti, M. Advances in Urban Ecology: Integrating Humans and Ecological Processes in Urban Ecosystems; Springer: New York, NY, USA, 2008; 366p. [Google Scholar]
- Hedblom, M.; Murgui, E. Urban bird research in a global perspective. In Ecology and Conservation of Birds in Urban Environments; Hedblom, M., Murgui, E., Eds.; Springer: Cham, Switzerland, 2017; pp. 3–10. [Google Scholar]
- Fidino, M.; Magle, S.B. Trends in long-term bird research. In Ecology and Conservation of Birds in Urban Environments; Hedblom, M., Murgui, E., Eds.; Springer: Cham, Switzerland, 2017; pp. 161–184. [Google Scholar]
- Yeh, P.J.; Price, T.D. Adaptive phenotypic plasticity and the successful colonization of a novel environment. Am. Nat. 2004, 164, 531–542. [Google Scholar] [CrossRef]
- Shochat, E.; Warren, P.S.; Faeth, S.H.; McIntyre, N.E.; Hope, D. From patterns to emerging processes in mechanistic urban ecology. Trends Ecol. Evol. 2006, 21, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Alberti, M. Eco-evolutionary dynamics in an urbanizing planet. Trends Ecol. Evol. 2015, 30, 114–126. [Google Scholar] [CrossRef]
- Partecke, J. Mechanisms of phenotypic responses following colonization of urban areas: From plastic to genetic adaptation. In Avian Urban Ecology: Behavioural and Physiological Adaptations; Gil, D., Brumm, H., Eds.; Oxford University Press: New York, NY, USA, 2014; pp. 131–142. [Google Scholar]
- Alberti, M. Forward. In Urban Evolutionary Biology; Szulkin, M., Munshi-South, J., Charmantier, A., Eds.; Oxford University Press: New York, NY, USA, 2020; pp. vii–x. [Google Scholar]
- Verrelli, B.C.; Alberti, M.; Des Roches, S.; Harris, N.C.; Hendry, A.P.; Johnson, M.T.; Savage, A.M.; Charmantier, A.; Gotanda, K.M.; Govaert, L.; et al. A global horizon scan for urban evolutionary ecology. Trends Ecol. Evol. 2022, 37, 1006–1019. [Google Scholar] [CrossRef]
- Nachman, M.W.; Crowell, S.L. Estimate of the mutation rate per nucleotide in humans. Genetics 2000, 156, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Whitlock, M.C.; Bürger, R. Fixation of New Mutations in Small Populations; IIASA Interim Report; IIASA: Laxenburg, Austria, 2004; IR-04-064; Available online: https://pure.iiasa.ac.at/id/eprint/7385/1/IR-04-064.pdf (accessed on 1 May 2024).
- Møller, A.P.; Mousseau, T.A. Conservation consequences of Chernobyl and other nuclear accidents. Biol. Conserv. 2011, 144, 2787–2798. [Google Scholar] [CrossRef]
- Mousseau, T.A. The biology of Chernobyl. Annu. Rev. Ecol. Evol. Syst. 2021, 52, 87–109. [Google Scholar] [CrossRef]
- Dubrova, Y.E.; Nesterov, V.N.; Krouchinsky, N.G.; Ostapenko, V.A.; Neumann, R.; Neil, D.L.; Jeffreys, A.J. Human minisatellite mutation rate after the Chernobyl accident. Nature 1996, 380, 683–686. [Google Scholar] [CrossRef] [PubMed]
- Yauk, C.L.; Quinn, J.S. Multilocus DNA fingerprinting reveals high rate of heritable genetic mutation in herring gulls nesting in an industrialized urban site. Proc. Natl. Acad. Sci. USA 1996, 93, 12137–12141. [Google Scholar] [CrossRef]
- Yauk, C.L.; Fox, G.A.; McCarry, B.E.; Quinn, J.S. 2000 Induced minisatellite germline mutations in herring gulls (Larus argentatus) living near steel mills. Mutat. Res. 2000, 452, 211–218. [Google Scholar] [CrossRef]
- Johnson, M.T.; Arif, I.; Marchetti, F.; Munshi-South, J.; Ness, R.W.; Szulkin, M.; Verrelli, B.C.; Yauk, C.L.; Anstett, D.N.; Booth, W.; et al. Effects of urban-induced mutations on ecology, evolution and health. Nat. Ecol. Evol. 2024, 1–13. [Google Scholar] [CrossRef]
- White, P.A.; Claxton, L.D. Mutagens in contaminated soil: A review. Mutat. Res. 2004, 567, 227–345. [Google Scholar] [CrossRef]
- Claxton, L.D.; Woodall, G.M., Jr. A review of the mutagenicity and rodent carcinogenicity of ambient air. Mutat. Res. 2007, 636, 36–94. [Google Scholar] [CrossRef]
- Sepp, T.; Ujvari, B.; Ewald, P.W.; Thomas, F.; Giraudeau, M. Urban environment and cancer in wildlife: Available evidence and future research avenues. Proc. R. Soc. Lond. B 2019, 286, 20182434. [Google Scholar] [CrossRef]
- Lynch, M.; Gabriel, W. Mutation load and the survival of small populations. Evolution 1990, 44, 1725–1737. [Google Scholar] [CrossRef]
- Soulé, M.E. Land use planning and wildlife maintenance: Guidelines for conserving wildlife in an urban landscape. J. Am. Plann. Assoc. 1991, 57, 313–323. [Google Scholar] [CrossRef]
- Willi, Y.; Van Buskirk, J.; Hoffmann, A.A. Limits to the adaptive potential of small populations. Annu. Rev. Ecol. Evol. Syst. 2006, 37, 433–458. [Google Scholar] [CrossRef]
- Miles, L.S.; Rivkin, L.R.; Johnson, M.T.; Munshi-South, J.; Verrelli, B.C. Gene flow and genetic drift in urban environments. Mol. Ecol. 2019, 28, 4138–4151. [Google Scholar] [CrossRef]
- Wood, D.A.; Rose, J.P.; Halstead, B.J.; Stoelting, R.E.; Swaim, K.E.; Vandergast, A.G. Combining genetic and demographic monitoring better informs conservation of an endangered urban snake. PLoS ONE 2020, 15, e0231744. [Google Scholar] [CrossRef]
- Konorov, E.A.; Yurchenko, V.; Patraman, I.; Lukashev, A.; Oyun, N. The effects of genetic drift and genomic selection on differentiation and local adaptation of the introduced populations of Aedes albopictus in southern Russia. PeerJ 2021, 9, e11776. [Google Scholar] [CrossRef]
- Schmidt, C.; Garroway, C.J. The population genetics of urban and rural amphibians in North America. Mol. Ecol. 2021, 30, 3918–3929. [Google Scholar] [CrossRef]
- Johnson, M.T.; Prashad, C.M.; Lavoignat, M.; Saini, H.S. Contrasting the effects of natural selection, genetic drift and gene flow on urban evolution in white clover (Trifolium repens). Proc. R. Soc. Lond. B 2018, 285, 20181019. [Google Scholar] [CrossRef]
- Richardson, J.L.; Michaelides, S.; Combs, M.; Djan, M.; Bisch, L.; Barrett, K.; Silveira, G.; Butler, J.; Aye, T.T.; Munshi-South, J.; et al. Dispersal ability predicts spatial genetic structure in native mammals persisting across an urbanization gradient. Evol. Appl. 2021, 14, 163–177. [Google Scholar] [CrossRef]
- Munshi-South, J.; Zolnik, C.P.; Harris, S.E. Population genomics of the Anthropocene: Urbanization is negatively associated with genome-wide variation in white-footed mouse populations. Evol. Appl. 2016, 9, 546–564. [Google Scholar] [CrossRef]
- Schmidt, C.; Domaratzki, M.; Kinnunen, R.P.; Bowman, J.; Garroway, C.J. Continent-wide effects of urbanization on bird and mammal genetic diversity. Proc. R. Soc. Lond. B 2020, 287, 20192497. [Google Scholar] [CrossRef]
- Miles, L.S.; Carlen, E.J.; Winchell, K.M.; Johnson, M.T. Urban evolution comes into its own: Emerging themes and future directions of a burgeoning field. Evol. Appl. 2021, 14, 3–11. [Google Scholar] [CrossRef]
- Thaweepworadej, P.; Evans, K.L. Squirrel and tree-shrew responses along an urbanisation gradient in a tropical mega-city–reduced biodiversity, increased hybridisation of Callosciurus squirrels, and effects of habitat quality. Anim. Conserv. 2023, 26, 46–60. [Google Scholar] [CrossRef]
- Salmón, P.; Jacobs, A.; Ahrén, D.; Biard, C.; Dingemanse, N.J.; Dominoni, D.M.; Helm, B.; Lundberg, M.; Senar, J.C.; Sprau, P.; et al. Continent-wide genomic signatures of adaptation to urbanisation in a songbird across Europe. Nat. Commun. 2021, 12, 2983. [Google Scholar] [CrossRef]
- Perry, G.; Powell, R.; Watson, H. Keeping invasive species off Guana Island, British Virgin Islands. Iguana 2006, 13, 272–277. [Google Scholar]
- Zapfe, L.; Freeland, J.R. Heterosis in invasive F1 cattail hybrids (Typha × glauca). Aquat. Bot. 2015, 125, 44–47. [Google Scholar] [CrossRef]
- Pieper, S.; Dorken, M.; Freeland, J. Genetic structure in hybrids and progenitors provides insight into processes underlying an invasive cattail (Typha × glauca) hybrid zone. Heredity 2020, 124, 714–725. [Google Scholar] [CrossRef]
- Geddes, P.; Murphy, L.; Astudillo-Scalia, Y.; Blasini, D.; Nugent, S.; Ríos, M.J.; Schirmer, A.E.; Olfelt, J.P. Microsatellite markers reveal unprecedented high frequencies of hybridization among Typha species in the Midwestern US. Wetlands 2021, 41, 24. [Google Scholar] [CrossRef]
- Abbott, R.J.; Lowe, A.J. Origins, establishment and evolution of new polyploid species: Senecio cambrensis and S. eboracensis in the British Isles. Biol. J. Linn. Soc. 2004, 82, 467–474. [Google Scholar] [CrossRef]
- Environmental Pollution Panel; United States President’s Science Advisory Committee; President’s Science Advisory Committee. Restoring the Quality of our Environment. The White House. 1965. Available online: https://www.documentcloud.org/documents/3227654-PSAC-1965-Restoring-the-Quality-of-Our-Environment (accessed on 1 May 2024).
- IPCC. IPCC Sixth Assessment Report. 2021. Available online: https://www.ipcc.ch/assessment-report/ar6/ (accessed on 1 May 2024).
- Martin, R.A.; Chick, L.D.; Yilmaz, A.R.; Diamond, S.E. Evolution, not transgenerational plasticity, explains the adaptive divergence of acorn ant thermal tolerance across an urban–rural temperature cline. Evol. Appl. 2019, 12, 1678–1687. [Google Scholar] [CrossRef]
- Janas, K.; Gudowska, A.; Drobniak, S.M. Avian colouration in a polluted world: A meta-analysis. Biol. Rev. 2024; early view. [Google Scholar] [CrossRef]
- Sepp, T.; McGraw, K.J.; Giraudeau, M. Urban sexual selection. In Urban Evolutionary Biology; Szulkin, M., MunshiSuh, J., Charmantier, A., Eds.; Oxford University Press: New York, NY, USA, 2020; pp. 234–252. [Google Scholar]
- Brans, K.I.; Jansen, M.; Vanoverbeke, J.; Tüzün, N.; Stoks, R.; De Meester, L. The heat is on: Genetic adaptation to urbanization mediated by thermal tolerance and body size. Glob. Chang. Biol. 2017, 23, 5218–5227. [Google Scholar] [CrossRef]
- Brans, K.I.; Stoks, R.; De Meester, L. Urbanization drives genetic differentiation in physiology and structures the evolution of pace-of-life syndromes in the water flea Daphnia magna. Proc. R. Soc. Lond. B 2018, 285, 20180169. [Google Scholar] [CrossRef]
- Brans, K.I.; Almeida, R.A.; Fajgenblat, M. Genetic differentiation in pesticide resistance between urban and rural populations of a nontarget freshwater keystone interactor, Daphnia magna. Evol. Appl. 2021, 14, 2541–2552. [Google Scholar] [CrossRef]
- Perry, G.; Buchanan, B.W.; Fisher, R.N.; Salmon, N.; Wise, S.E. Effects of artificial night lighting on reptiles and amphibians in urban environments. In Urban Herpetology; Jung, R.E., Mitchell, J.C., Eds.; Herpetological Conservation, Society for the Study of Amphibians and Reptiles: Salt Lake, UT, USA, 2008; Volume 3, pp. 239–256. [Google Scholar]
- Sordello, R.; Ratel, O.; Flamerie De Lachapelle, F.; Leger, C.; Dambry, A.; Vanpeene, S. Evidence of the impact of noise pollution on biodiversity: A systematic map. Environ. Evid. 2020, 9, 1–27. [Google Scholar] [CrossRef]
- Zipf, L.; Primack, R.B.; Rothendler, M. Citizen scientists and university students monitor noise pollution in cities and protected areas with smartphones. PLoS ONE 2020, 15, e0236785. [Google Scholar] [CrossRef]
- Stroud, J.T.; Losos, J.B. Ecological opportunity and adaptive radiation. Annu. Rev. Ecol. Evol. S. 2016, 47, 507–532. [Google Scholar] [CrossRef]
- Lambert, M.R.; Donihue, C.M. Urban biodiversity management using evolutionary tools. Nat. Ecol. Evol. 2020, 4, 903–910. [Google Scholar] [CrossRef]
- Prokop, P. Urban environment decreases pollinator availability, fertility, and prolongs anthesis in the field bindweed (Convolvulus arvensis Linnaeus, 1753). Plant Signal. Behav. 2024, 19, 2325225. [Google Scholar] [CrossRef]
- Badyaev, A.V.; Young, R.L.; Oh, K.P.; Addison, C. Evolution on a local scale: Developmental, functional, and genetic bases of divergence in bill form and associated changes in song structure between adjacent habitats. Evolution 2008, 62, 1951–1964. [Google Scholar] [CrossRef]
- Campbell-Staton, S.C.; Winchell, K.M.; Rochette, N.C.; Fredette, J.; Maayan, I.; Schweizer, R.M.; Catchen, J. Parallel selection on thermal physiology facilitates repeated adaptation of city lizards to urban heat islands. Nat. Ecol. Evol. 2020, 4, 652–658. [Google Scholar] [CrossRef]
- Winchell, K.M.; Campbell-Staton, S.C.; Losos, J.B.; Revell, L.J.; Verrelli, B.C.; Geneva, A.J. Genome-wide parallelism underlies contemporary adaptation in urban lizards. Proc. Natl. Acad. Sci. USA 2023, 120, e2216789120. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.N. Health and the Rise of Civilization; Yale University Press: New Haven, CT, USA, 1989. [Google Scholar]
- Perry, G.; Stone, L.A.; Obaid, O. Adapting U.S. foreign assistance for a rapidly urbanizing world. Sci. Dipl. 2021, 10, 1–16. Available online: https://www.sciencediplomacy.org/article/2021/adapting-us-foreign-assistance-for-rapidly-urbanizing-world (accessed on 1 May 2024).
- Ben Maamar, S.; Hu, J.; Hartmann, E.M. Implications of indoor microbial ecology and evolution on antibiotic resistance. J. Expo. Sci. Environ. Epidemiol. 2020, 30, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, R.A.; Polk, J.S.; Datta, T.; Parekh, R.R.; Agga, G.E. Occurrence of antibiotic resistant bacteria in urban karst groundwater systems. Water 2022, 14, 960. [Google Scholar] [CrossRef]
- Almakki, A.; Jumas-Bilak, E.; Marchandin, H.; Licznar-Fajardo, P. Antibiotic resistance in urban runoff. Sci. Total Environ. 2019, 667, 64–76. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.N.; McEachran, A.D.; Wooten, K.J.; Blackwell, B.R. A preliminary evaluation of veterinary antibiotics, estrogens, in vitro estrogenic activity and microbial communities in airborne particulate matter collected near dairy production facilities. Aerobiologia 2019, 35, 315–326. [Google Scholar] [CrossRef]
- Neiderud, C.J. How urbanization affects the epidemiology of emerging infectious diseases. Infect. Ecol. Epidemiol. 2015, 5, 27060. [Google Scholar] [CrossRef] [PubMed]
- Combs, M.A.; Kache, P.A.; VanAcker, M.C.; Gregory, N.; Plimpton, L.D.; Tufts, D.M.; Fernandez, M.P.; Diuk-Wasser, M.A. Socio-ecological drivers of multiple zoonotic hazards in highly urbanized cities. Glob. Chang. Biol. 2022, 28, 1705–1724. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Z.; Wang, J.; Li, M.; Wang, S.; He, X.; Zhou, C. Evolution and control of the COVID-19 pandemic: A global perspective. Cities 2022, 130, 103907. [Google Scholar] [CrossRef]
- Yu, D.; Li, X.; Yu, J.; Shi, X.; Liu, P.; Tian, P. Whether urbanization has intensified the spread of infectious diseases—Renewed question by the COVID-19 pandemic. Front. Public Health 2021, 9, 699710. [Google Scholar] [CrossRef]
- Abdelwhab, E.M.; Mettenleiter, T.C. Zoonotic animal influenza virus and potential mixing vessel hosts. Viruses 2023, 15, 980. [Google Scholar] [CrossRef]
- Albery, G.F.; Carlson, C.J.; Cohen, L.E.; Eskew, E.A.; Gibb, R.; Ryan, S.J.; Sweeny, A.R.; Becker, D.J. Urban-adapted mammal species have more known pathogens. Nat. Ecol. Evol. 2022, 6, 794–801. [Google Scholar] [CrossRef]
- Hassell, J.M.; Muloi, D.M.; VanderWaal, K.L.; Ward, M.J.; Bettridge, J.; Gitahi, N.; Ouko, T.; Imboma, T.; Akoko, J.; Karani, M.; et al. Epidemiological connectivity between humans and animals across an urban landscape. Proc. Natl. Acad. Sci. USA 2023, 120, e2218860120. [Google Scholar] [CrossRef]
- Mackenstedt, U.; Jenkins, D.; Romig, T. The role of wildlife in the transmission of parasitic zoonoses in peri-urban and urban areas. Int. J. Parasitol. Parasites. Wildl. 2015, 4, 71–79. [Google Scholar] [CrossRef]
- Tan, C.C.; van Dorp, L.; Balloux, F. The evolutionary drivers and correlates of viral host jumps. Nat. Ecol. Evol. 2024, 8, 960–971. [Google Scholar] [CrossRef]
- Kürschner, T.; Scherer, C.; Radchuk, V.; Blaum, N.; Kramer-Schadt, S. Resource asynchrony and landscape homogenization as drivers of virulence evolution: The case of a directly transmitted disease in a social host. Ecol. Evol. 2024, 14, e11065. [Google Scholar] [CrossRef] [PubMed]
- McKinney, M.L. Urbanization as a major cause of biotic homogenization. Biol. Conserv. 2006, 127, 247–260. [Google Scholar] [CrossRef]
- Bradley, C.A.; Altizer, S. Urbanization and the ecology of wildlife diseases. Trends Ecol. Evol. 2007, 22, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Heisler, G.M.; Brazel, A.J. The urban physical environment: Temperature and urban heat islands. Urban Ecosyst. Ecol. 2010, 55, 29–56. [Google Scholar]
- Gusa, A.; Yadav, V.; Roth, C.; Williams, J.D.; Shouse, E.M.; Magwene, P.; Heitmann, J.; Jinks-Robertson, S. Genome-wide analysis of heat stress-stimulated transposon mobility in the human fungal pathogen Cryptococcus deneoformans. Proc. Natl. Acad. Sci. USA 2023, 120, e2209831120. [Google Scholar] [CrossRef]
- Heidecke, J.; Lavarello Schettini, A.; Rocklöv, J. West Nile virus eco-epidemiology and climate change. PLoS Clim. 2023, 2, e0000129. [Google Scholar] [CrossRef]
- Murray, M.H.; Lankau, E.W.; Kidd, A.D.; Welch, C.N.; Ellison, T.; Adams, H.C.; Lipp, E.K.; Hernandez, S.M. Gut microbiome shifts with urbanization and potentially facilitates a zoonotic pathogen in a wading bird. PLoS ONE 2020, 15, e0220926. [Google Scholar] [CrossRef] [PubMed]
- Barnes, I.; Duda, A.; Pybus, O.G.; Thomas, M.G. Ancient urbanization predicts genetic resistance to tuberculosis. Evolution 2011, 65, 842–848. [Google Scholar] [CrossRef] [PubMed]
- Starik, N.; Göttert, T. Bats adjust echolocation and social call design as a response to urban environments. Front. Ecol. Evol. 2022, 10, 939408. [Google Scholar] [CrossRef]
- Miranda, A.C.; Schielzeth, H.; Sonntag, T.; Partecke, J. Urbanization and its effects on personality traits: A result of microevolution or phenotypic plasticity? Glob. Chang. Biol. 2013, 19, 2634–2644. [Google Scholar] [CrossRef] [PubMed]
- Partecke, J.; Schwabl, I.; Gwinner, E. Stress and the city: Urbanization and its effects on the stress physiology in European blackbirds. Ecology 2006, 87, 1945–1952. [Google Scholar] [CrossRef] [PubMed]
- Morelli, F.; Benedetti, Y.; Ibáñez-Álamo, J.D.; Jokimäki, J.; Mänd, R.; Tryjanowski, P.; Møller, A.P. Evidence of evolutionary homogenization of bird communities in urban environments across Europe. Glob. Ecol. Biogeogr. 2016, 25, 1284–1293. [Google Scholar] [CrossRef]
- Dennis, R. Making the Underground underground. Lond. J. 2013, 38, 203–225. [Google Scholar] [CrossRef]
- Byrne, K.; Nichols, R.A. Culex pipiens in London Underground tunnels: Differentiation between surface and subterranean populations. Heredity 1999, 82, 7–15. [Google Scholar] [CrossRef]
- Haba, Y.; McBride, L. Origin and status of Culex pipiens mosquito ecotypes. Cur. Biol. 2022, 32, R237–R246. [Google Scholar] [CrossRef]
- Dudley, W.R. The Cayuga Flora: Part I: A Catalogue of the Phaenogamia Growing Without Cultivation in the Cayuga Lake Basin; Andrus & Church: Ithaca, NY, USA, 1886; Volume 2. [Google Scholar]
- Liker, A. Biologia Futura: Adaptive changes in urban populations. Biol. Futur. 2020, 71, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Perrier, C.; Caizerugues, A.; Charmantier, A. Adaptation genomics in urban environments. In Urban Evolutionary Biology; Szulkin, M., MunshiSuh, J., Charmantier, A., Eds.; Oxford University Press: New York, NY, USA, 2020; pp. 74–90. [Google Scholar]
- Caizergues, A.E.; Le Luyer, J.; Grégoire, A.; Szulkin, M.; Senar, J.C.; Charmantier, A.; Perrier, C. Epigenetics and the city: Non-parallel DNA methylation modifications across pairs of urban-forest great tit populations. Evol. Appl. 2022, 15, 149–165. [Google Scholar] [CrossRef] [PubMed]
- Galea, S.; Uddin, M.; Koenen, K. The urban environment and mental disorders: Epigenetic links. Epigenetics 2011, 6, 400–404. [Google Scholar] [CrossRef] [PubMed]
- McNew, S.M.; Beck, D.; Sadler-Riggleman, I.; Knutie, S.A.; Koop, J.A.; Clayton, D.H.; Skinner, M.K. Epigenetic variation between urban and rural populations of Darwin’s finches. BMC Evol. Biol. 2017, 17, 183. [Google Scholar] [CrossRef] [PubMed]
- Watson, H.; Powell, D.; Salmón, P.; Jacobs, A.; Isaksson, C. Urbanization is associated with modifications in DNA methylation in a small Passerine bird. Evol. Appl. 2021, 14, 85–98. [Google Scholar] [CrossRef]
- Thorson, J.L.; Smithson, M.; Sadler-Riggleman, I.; Beck, D.; Dybdahl, M.; Skinner, M.K. Regional epigenetic variation in asexual snail populations among urban and rural lakes. Environ. Epigenet. 2019, 5, dvz020. [Google Scholar] [CrossRef] [PubMed]
- Donelan, S.C.; Hellmann, J.K.; Bell, A.M.; Luttbeg, B.; Orrock, J.L.; Sheriff, M.J.; Sih, A. Transgenerational plasticity in human-altered environments. Trends Ecol. Evol. 2020, 35, 115–124. [Google Scholar] [CrossRef]
- Diamond, S.E.; Martin, R.A. Evolution in cities. Annu. Rev. Ecol. Evol. Syst. 2021, 52, 519–540. [Google Scholar] [CrossRef]
- Hamnett, C. Is Chinese urbanization unique? Urban Stud. 2020, 57, 690–700. [Google Scholar] [CrossRef]
- Andermann, T.; Faurby, S.; Turvey, S.T.; Antonelli, A.; Silvestro, D. The past and future human impact on mammalian diversity. Sci. Adv. 2020, 6, eabb2313. [Google Scholar] [CrossRef]
- Zeller, U.; Starik, N.; Göttert, T. Biodiversity, land use and ecosystem services—An organismic and comparative approach to different geographical regions. Glob. Ecol. Conserv. 2017, 10, 114–125. [Google Scholar] [CrossRef]
- Zeller, U.; Göttert, T. Humans, megafauna and landscape structure–Rock engravings from Namibia encourage a comparative approach to central Europe and southern Africa. Vertebr. Zool. 2021, 71, 631–643. [Google Scholar] [CrossRef]
- Güneralp, B.; Lwasa, S.; Masundire, H.; Parnell, S.; Seto, K.C. Urbanization in Africa: Challenges and opportunities for conservation. Environ. Res. Lett. 2017, 13, 015002. [Google Scholar] [CrossRef]
- Cilliers, S.S.; Bouwman, H.E.N.K.; Drewes, E. Comparative urban ecological research in developing countries. In Ecology of Cities and Towns: A Comparative Approach; McDonnell, M.J., Hahs, A.K., Breuste, J.H., Eds.; Cambridge University Press: Cambridge, UK, 2009; pp. 90–111. [Google Scholar]
- UN-Department of Economic and Social Affairs (UN-DESA). World Urbanization Prospects. The 2018 Revision. The UN-Department of Economic and Social Affairs. 2018. Available online: https://www.un.org/en/desa/2018-revision-world-urbanization-prospects (accessed on 1 April 2024).
- Hardoy, J.E.; Satterthwaite, D. Why small and intermediate urban centres? In Small and Intermediate Urban Centres: Their Role in Regional and National Development in the Third World; Hardoy, J.E., Satterthwaite, D., Eds.; Routledge: New York, NY, USA, 2019; pp. 1–17. [Google Scholar]
- Keeling, D.J. Transport and the world city paradigm. In World Cities in a World System; Knox, P.L., Taylor, P.L., Eds.; Cambridge University Press: Cambridge, UK, 1995; pp. 115–131. [Google Scholar]
- Valjarević, A.; Milić, M.; Valjarević, D.; Stanojević-Ristić, Z.; Petrović, L.; Milanović, M.; Filipović, D.; Ristanović, B.; Basarin, B.; Lukić, T. Modelling and mapping of the COVID-19 trajectory and pandemic paths at global scale: A geographer’s perspective. Open Geosci. 2020, 12, 1603–1616. [Google Scholar] [CrossRef]
- Cheptu, P.O.; Lambrecht, S.C. Sidewalk plants as a model for studying adaptation to urban environments. In Urban Evolutionary Biology; Szulkin, M., MunshiSuh, J., Charmantier, A., Eds.; Oxford University Press: New York, NY, USA, 2020; pp. 130–141. [Google Scholar]
- Dholakia, S.; Buckler, J.; Jeans, J.P.; Pillai, A.; Eagles, N.; Dholakia, S. Pubic lice: An endangered species? Sex. Transm. Dis. 2014, 41, 388–391. [Google Scholar] [CrossRef] [PubMed]
- Cox, G.W. Alien Species and Evolution: The Evolutionary Ecology of Exotic Plants, Animals, Microbes, and Interacting Native Species; Island Press: Washington, DC, USA, 2004. [Google Scholar]
- Calzada Preston, C.E.; Pruett-Jones, S. The number and distribution of introduced and naturalized parrots. Diversity 2021, 13, 412. [Google Scholar] [CrossRef]
- Francis, R.A.; Chadwick, M.A. What makes a species synurbic? Appl. Geogr. 2012, 32, 514–521. [Google Scholar] [CrossRef]
- Langerhans, R.B.; Kern, E.M.A. Urbanization and evolution in aquatic environments. In Urban Evolutionary Biology; Szulkin, M., MunshiSuh, J., Charmantier, A., Eds.; Oxford University Press: New York, NY, USA, 2020; pp. 157–174. [Google Scholar]
- Pianka, E.R.; Pianka, H.D. The ecology of Moloch horridus (Lacertilia: Agamidae) in western Australia. Copeia 1970, 1970, 90–103. [Google Scholar]
- Stern, D.L. The genetic causes of convergent evolution. Nat. Rev. Genet. 2013, 14, 751–764. [Google Scholar] [CrossRef] [PubMed]
- Herring, C.D.; Raghunathan, A.; Honisch, C.; Patel, T.; Applebee, M.K.; Joyce, A.R.; Albert, T.J.; Blattner, F.R.; Van den Boom, D.; Cantor, C.R.; et al. Comparative genome sequencing of Escherichia coli allows observation of bacterial evolution on a laboratory timescale. Nat. Genet. 2006, 38, 1406–1412. [Google Scholar] [CrossRef]
- Santangelo, J.S.; Miles, L.S.; Breitbart, S.T.; Murray-Stoker, D.; Rivkin, L.R.; Johnson, M.T.; Ness, R.W.; Szulkin, M.; Munshi-South, J.; Charmantier, A. Urban environments as a framework to study parallel evolution. In Urban Evolutionary Biology; Szulkin, M., MunshiSuh, J., Charmantier, A., Eds.; Oxford University Press: New York, NY, USA, 2020; pp. 36–53. [Google Scholar]
- Barraclough, T.G. How do species interactions affect evolutionary dynamics across whole communities? Ann. Rev. Ecol. Evol. Syst. 2015, 46, 25–48. [Google Scholar] [CrossRef]
- Brans, K.I.; Govaert, L.; De Meester, L. Evolutionary dynamics of metacommunities in urbanized landscapes. In Urban Evolutionary Biology; Szulkin, M., MunshiSuh, J., Charmantier, A., Eds.; Oxford University Press: New York, NY, USA, 2020; pp. 175–196. [Google Scholar]
- Leppäkoski, E.; Olenin, S. Non-native species and rates of spread-lessons from the brackish Baltic Sea. In Proceedings of the VII International Congress of Ecology, Florence, Italy, 19–25 July 1998. [Google Scholar]
- Cronjé, H.T.; Elliott, H.R.; Nienaber-Rousseau, C.; Pieters, M. Leveraging the urban–rural divide for epigenetic research. Epigenomics 2020, 12, 1071–1081. [Google Scholar] [CrossRef]
- Lederbogen, F.; Kirsch, P.; Haddad, L.; Streit, F.; Tost, H.; Schuch, P.; Wüst, S.; Pruessner, J.C.; Rietschel, M.; Deuschle, M.; et al. City living and urban upbringing affect neural social stress processing in humans. Nature 2011, 474, 498–501. [Google Scholar] [CrossRef]
- Sampson, L.; Ettman, C.K.; Galea, S. Urbanization, urbanicity, and depression: A review of the recent global literature. Curr. Opin. Psychiatry. 2020, 33, 233–244. [Google Scholar] [CrossRef]
- Milot, E.; Stearns, S.C. Selection on humans in cities. In Urban Evolutionary Biology; Szulkin, M., MunshiSuh, J., Charmantier, A., Eds.; Oxford University Press: New York, NY, USA, 2020; pp. 268–288. [Google Scholar]
- Aitken, R.J. The changing tide of human fertility. Hum. Reprod. 2022, 37, 629–638. [Google Scholar] [CrossRef]
- Svechkina, A.; Portnov, B.A.; Trop, T. The impact of artificial light at night on human and ecosystem health: A systematic literature review. Landsc. Ecol. 2020, 35, 1725–1742. [Google Scholar] [CrossRef]
- Schell, C.J.; Stanton, L.A.; Young, J.K.; Angeloni, L.M.; Lambert, J.E.; Breck, S.W.; Murray, M.H. The evolutionary consequences of human–wildlife conflict in cities. Evol. Appl. 2021, 14, 178–197. [Google Scholar] [CrossRef]
- DeVos, T.B.; Bock, D.G.; Kolbe, J.J. Rapid introgression of non-native alleles following hybridization between a native Anolis lizard species and a cryptic invader across an urban landscape. Mol. Ecol. 2023, 32, 2930–2944. [Google Scholar] [CrossRef]
- Brauer, C.J.; Sandoval-Castillo, J.; Gates, K.; Hammer, M.P.; Unmack, P.J.; Bernatchez, L.; Beheregaray, L.B. Natural hybridization reduces vulnerability to climate change. Nat. Clim. Chang. 2023, 13, 282–289. [Google Scholar] [CrossRef]
- Sadanandan, K.R.; Low, G.W.; Sridharan, S.; Gwee, C.Y.; Ng, E.Y.; Yuda, P.; Prawiradilaga, D.M.; Lee, J.G.H.; Tritto, A.; Rheindt, F.E. The conservation value of admixed phenotypes in a critically endangered species complex. Sci. Rep. 2020, 10, 15549. [Google Scholar] [CrossRef]
- Jacobsen, F.; Nesje, M.; Bachmann, L.; Lifjeld, J.T. Significant genetic admixture after reintroduction of peregrine falcon (Falco peregrinus) in Southern Scandinavia. Conserv. Genet. 2008, 9, 581–591. [Google Scholar] [CrossRef]
- Rega-Brodsky, C.C.; Aronson, M.F.; Piana, M.R.; Carpenter, E.S.; Hahs, A.K.; Herrera-Montes, A.; Knapp, S.; Kotze, D.J.; Lepczyk, C.A.; Moretti, M.; et al. Urban biodiversity: State of the science and future directions. Urban Ecosyst. 2022, 25, 1083–1096. [Google Scholar] [CrossRef]
- Awoyemi, A.G.; Ibánez-Álamo, J.D. Status of urban ecology in Africa: A systematic review. Landsc. Urban Plan. 2023, 233, 104707. [Google Scholar] [CrossRef]
- Brum, P.H.R.; Gonçalves, S.R.A.; Strüssmann, C.; Teixido, A.L. A global assessment of research on urban ecology of reptiles: Patterns, gaps and future directions. Anim. Conserv. 2023, 26, 1–13. [Google Scholar] [CrossRef]
- Ibáñez-Álamo, J.D.; Rubio, E.; Bitrus Zira, K. The degree of urbanization of a species affects how intensively it is studied: A global perspective. Front. Ecol. Evol. 2017, 5, 41. [Google Scholar] [CrossRef]
- Shultz, A.J.; Adams, B.J.; Bell, K.C.; Ludt, W.B.; Pauly, G.B.; Vendetti, J.E. Natural history collections are critical resources for contemporary and future studies of urban evolution. Evol. Appl. 2021, 14, 233–247. [Google Scholar] [CrossRef]
- Kendal, D.; Egerer, M.; Byrne, J.A.; Jones, P.J.; Marsh, P.; Threlfall, C.G.; Allegretto, G.; Kaplan, H.; Nguyen, H.K.; Pearson, S.; et al. City-size bias in knowledge on the effects of urban nature on people and biodiversity. Environ. Res. Lett. 2020, 15, 124035. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perry, G.; Göttert, T. The City as an Evolutionary Hothouse—The Search for Rapid Evolution in Urban Settings. Diversity 2024, 16, 308. https://doi.org/10.3390/d16060308
Perry G, Göttert T. The City as an Evolutionary Hothouse—The Search for Rapid Evolution in Urban Settings. Diversity. 2024; 16(6):308. https://doi.org/10.3390/d16060308
Chicago/Turabian StylePerry, Gad, and Thomas Göttert. 2024. "The City as an Evolutionary Hothouse—The Search for Rapid Evolution in Urban Settings" Diversity 16, no. 6: 308. https://doi.org/10.3390/d16060308
APA StylePerry, G., & Göttert, T. (2024). The City as an Evolutionary Hothouse—The Search for Rapid Evolution in Urban Settings. Diversity, 16(6), 308. https://doi.org/10.3390/d16060308






