Genetic Diversity and Relationships among Indian Jujube (Ziziphus mauritiana Lamk.) Cultivars Using Morphometric Characteristics, matK Barcoding, and ISSR Markers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Site and Plant Materials
2.2. Morphometric Characteristics
2.3. Molecular Marker Studies
2.4. Statistical Analysis
3. Results
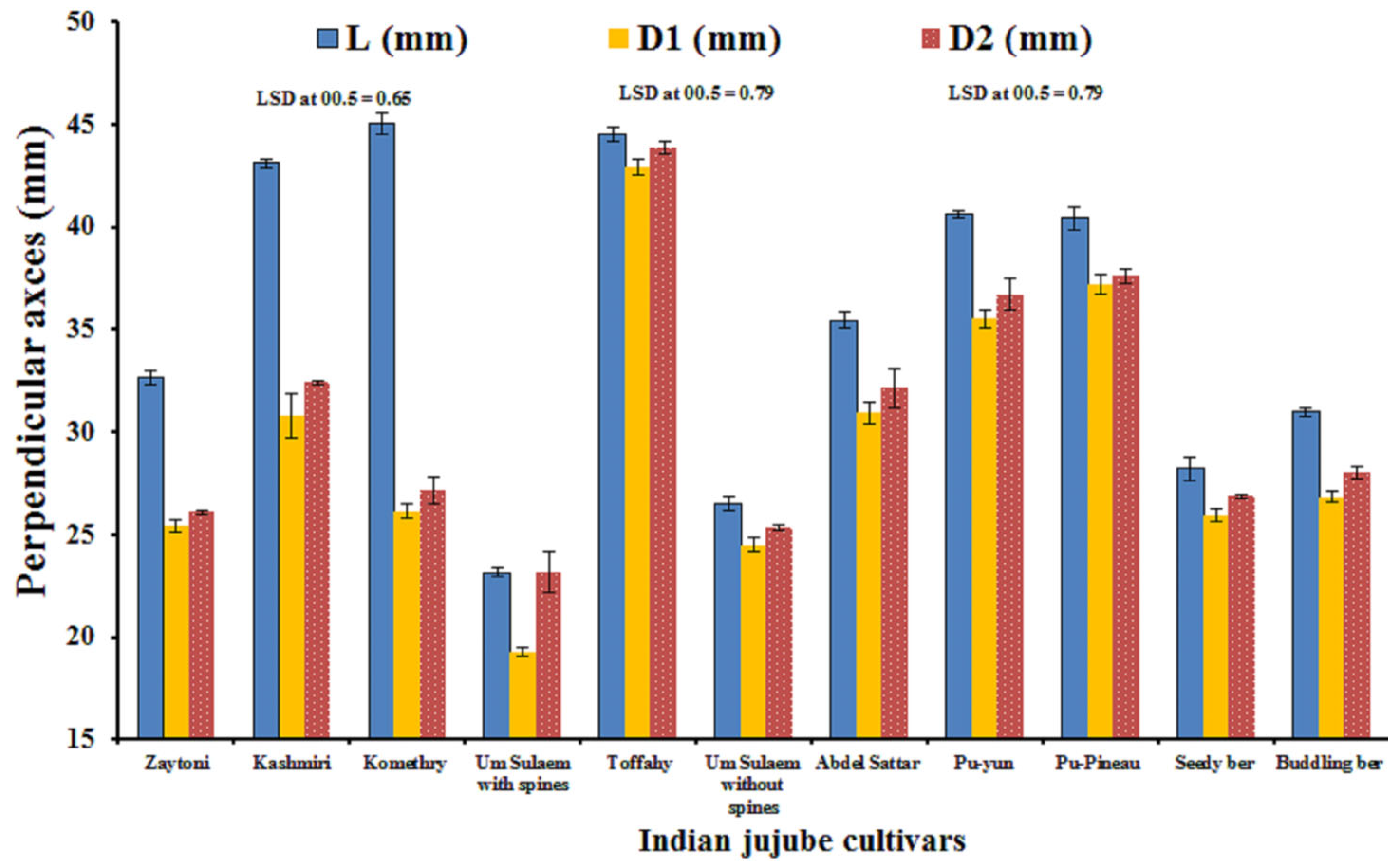
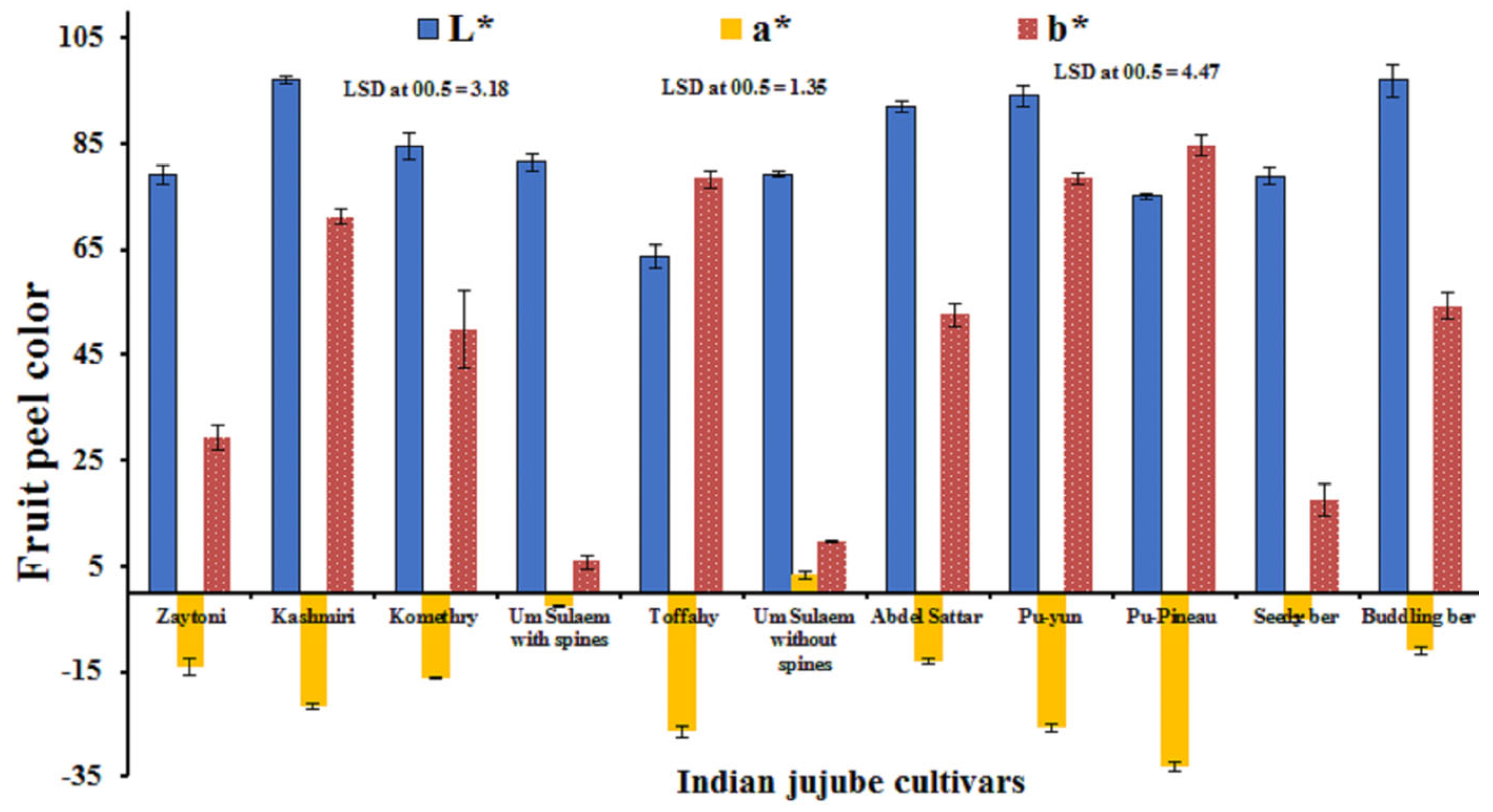
3.1. Fruit Morphometric Attributes
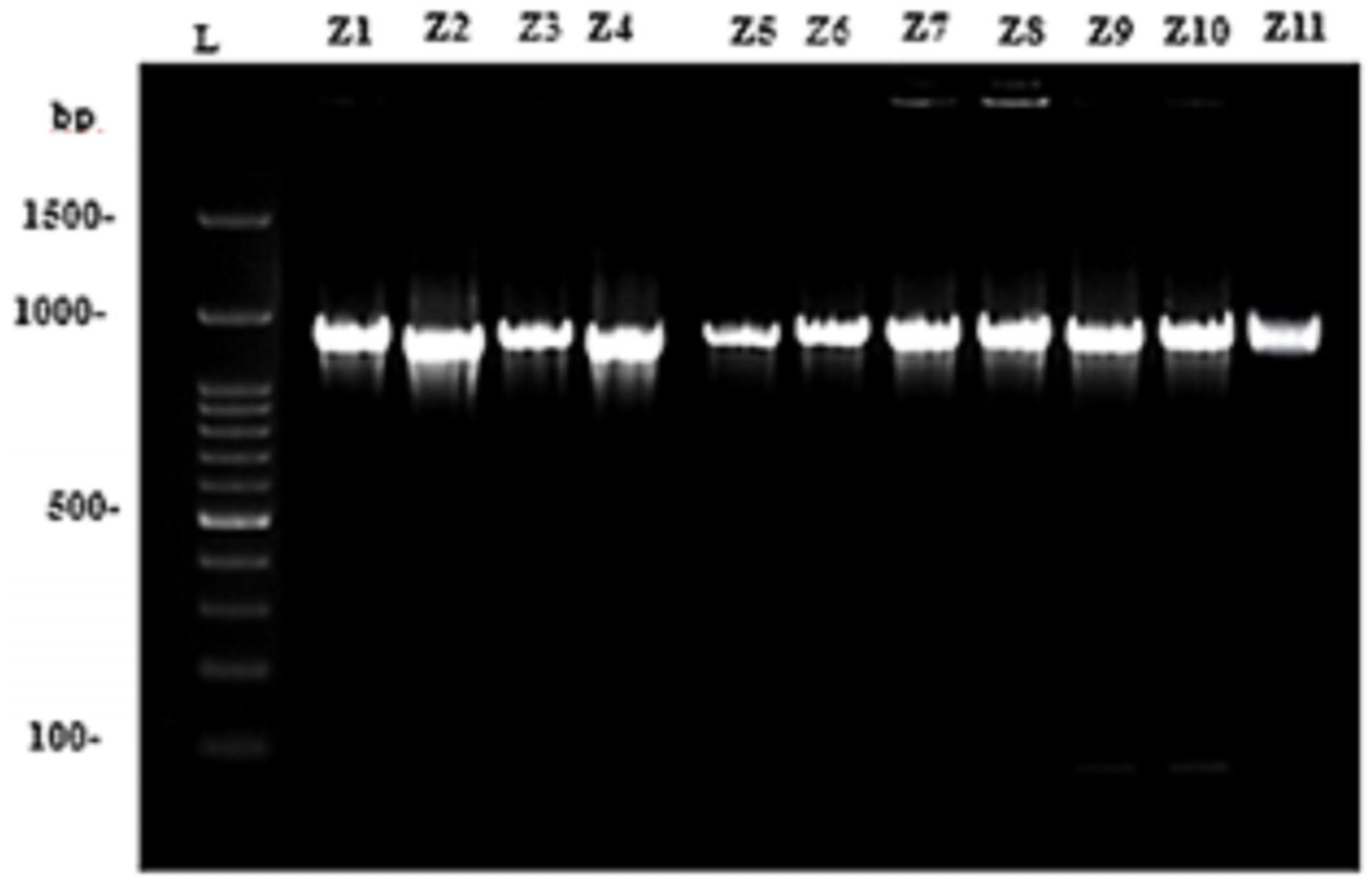
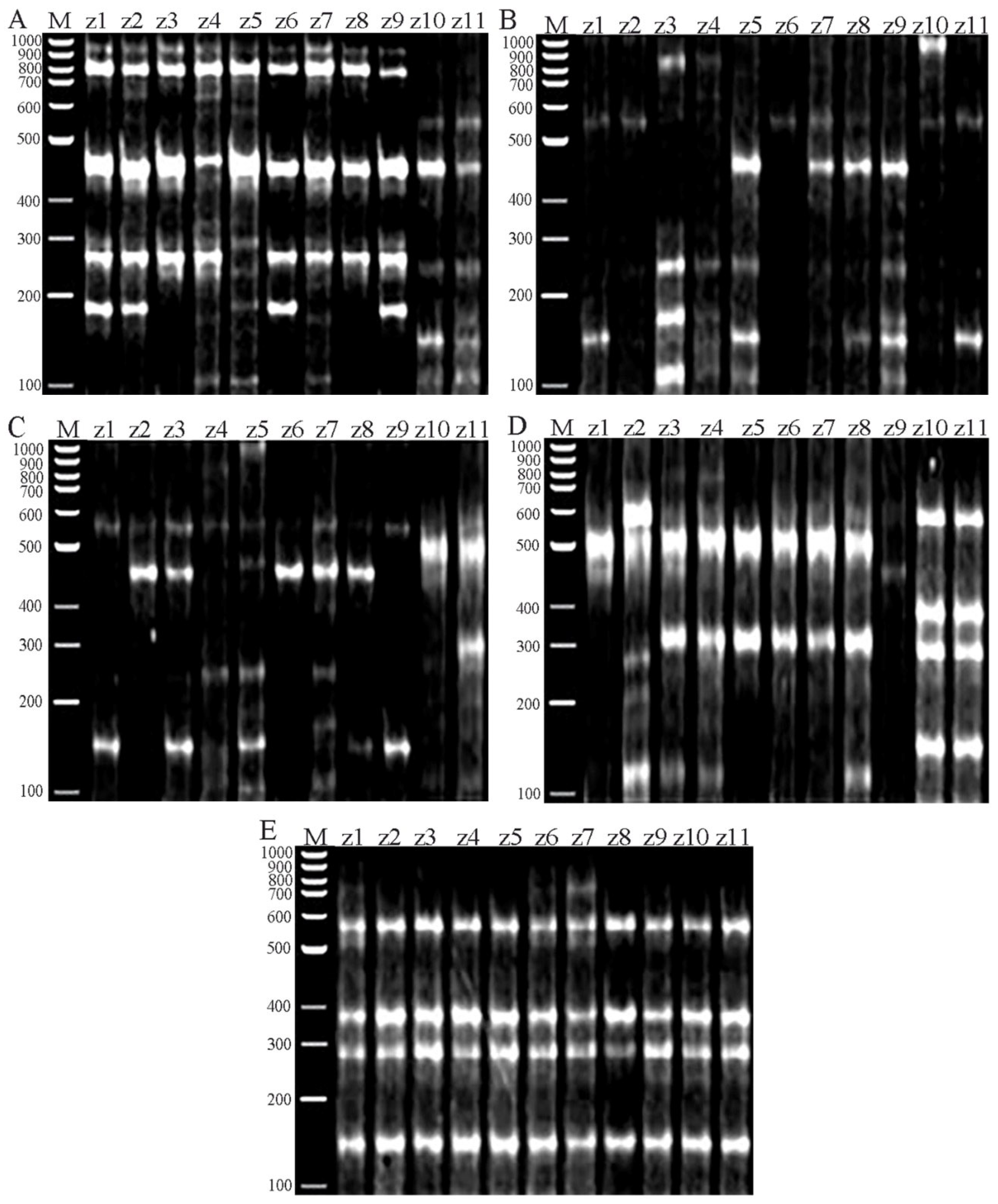
3.2. Molecular Marker Studies
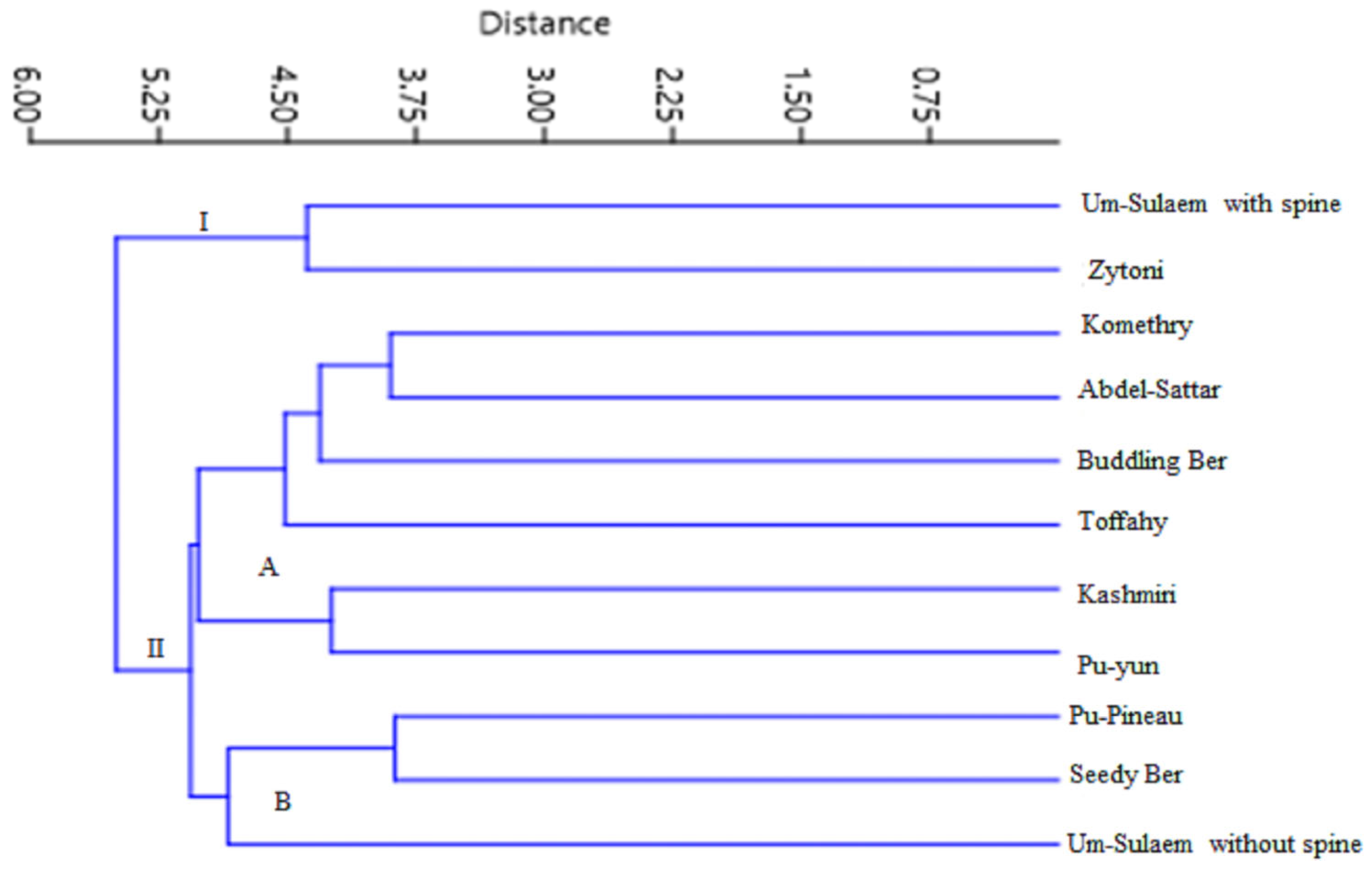
3.3. Relationships among Indian Jujube Cultivars
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, S.K.; Midha, A.; Kumar, U.; Singh, S.K. Phytochemical study of aerial part of different species of Ziziphus genus. Asian J. Pharm. Res. Dev. 2017, 5, 1–5. [Google Scholar]
- Naveed, F.; Nawaz, A.; Ali, S.; Ejaz, S. Xanthan gum coating delays ripening and softening of jujube fruit by reducing oxidative stress and suppressing cell wall polysaccharides disassembly. Postharvest Biol. Technol. 2024, 209, 112689. [Google Scholar] [CrossRef]
- Meighani, H.; Sadat-Hosseini, M. Assessment of Quality and Biochemical Changes in Indian Jujube Fruits (Ziziphus mauritiana Lamk.): Effect of Chitosan Coating and Putrescine Treatments. Int. J. Hort. Sci. Technol. 2024, 11, 481–490. [Google Scholar]
- Pareek, O.P. Fruits for the Future 2: Ber; International Centre for Underutilized Crops: Southampton, UK, 2001; 290p. [Google Scholar]
- Kumari, S.; Bhat, D.J.; Wali, V.K.; Bakshi, P.; Jasrotia, A. Physico-chemical studies of different ber (Zizyphus mauritiana Lamk.) germplasm under rainfed conditions of Jammu. The Bioscan 2015, 10, 1427–1430. [Google Scholar]
- Kumar, P.; Tripathi, V.K. Correlation Studies in Ber (Ziziphus mauriatiana Lamk.) in Eastern Region of Uttar Pradesh, India. Int. J. Plant Soil Sci. 2024, 36, 135–141. [Google Scholar] [CrossRef]
- Anjum, M.A.; Rauf, A.; Bashi, M.A.; Ahmad, R. The evaluation of biodiversity in some indigenous Indian jujube (Zizyphus mauritiana) germplasm the physico–chemical analysis. Acta Sci. Pol. Hortorum Cultus 2018, 17, 39–52. [Google Scholar] [CrossRef]
- Abdel-Sattar, M.; Almutairi, K.F.; Al-Saif, A.M.; Ahmed, K.A. Fruit properties during the harvest period of eleven Indian jujube (Ziziphus mauritiana Lamk.) cultivars. Saudi J. Biol. Sci. 2021, 28, 3424–3432. [Google Scholar] [CrossRef] [PubMed]
- Al-Saif, A.M.; Abdel-Sattar, M.; Aboukarima, A.M.; Eshra, D.H. Identification of Indian jujube cultivars cultivated in Saudi Arabia using an artificial neural network. Saudi J. Biol. Sci. 2021, 28, 5765–5772. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Tripathi, V.K. Phenotypic and Quantative Characterization of Ber (Ziziphus mauritiana Lamk.) Germplasm under Eastern Region of Uttar Pradesh, India. Int. J. Environ. Clim. Chang. 2024, 14, 264–272. [Google Scholar] [CrossRef]
- Bâ, M.; Danthu, P.; Duponnois, R.; Soleviev, P. Domestication of jujube fruit trees (Zizyphus mauritiana Lam.). In Crop Management and Postharvest Handling of Horticultural Crops; Science Publishers, Inc.: Enfield, NH, USA, 2003; Volume 3, Chapter 9; pp. 255–279. [Google Scholar]
- Owolarafe, T.A.; Salawu, K.; Ihegboro, G.O.; Ononamadu, C.J.; Alhassan, A.J.; Wudil, A.M. Investigation of cytotoxicity potential of different extracts of Ziziphus mauritiana (Lam.) leaf Allium cepa model. Toxicol. Rep. 2020, 7, 816–821. [Google Scholar] [CrossRef]
- Muhammad, G. Date fruits classification using texture descriptors and shape-size features. Eng. Appl. Artif. Intel. 2015, 37, 361–367. [Google Scholar] [CrossRef]
- Bahri, M.H.; Rashid, M.; Farahmandfar, R. Kiwi fruit shape classification based on geometrical attributes analysis. Agric. Eng. Res. J. 2017, 7, 1–5. [Google Scholar] [CrossRef]
- Çetin, N.; Yaman, M.; Karaman, K.; Bünyamin, D. Determination of some physicomechanical and biochemical parameters of hazelnut (Corylus avellana L.) cultivars. Turki. J. Agric. For. 2020, 44, 1–12. [Google Scholar] [CrossRef]
- Basile, B.; Mataffo, A.; Forlani, M.; Corrado, G. Diversity in Morphometric, Pomological, and Fruit-Quality Traits of Apricot (Prunus armeniaca) Traditional Varieties: Implications for Landrace Differentiation at Regional Scale. Diversity 2022, 14, 608. [Google Scholar] [CrossRef]
- Barthet, M. Expression and Function of the Chloroplast-Encoded Gene Matk. Ph.D. Thesis, Virginia Polytechnic Institute and State University, Blacksburg, VA, USA, 2006. [Google Scholar]
- Ali, A.N.A.; Al-Sokari, S.S.; Gushash, A.; Anwar, S.; Al-Karani, K.; Al-Khulaidi, A. Ethnopharmacological survey of medicinal plants in Albaha region, Saudi Arabia. Pharmacogn. Res. 2017, 9, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Chester, K.; Zahiruddin, S.; Ahmad, A.; Khan, W.; Paliwa, S.; Ahmad, S. Bioautography-based identification of antioxidant metabolites of Solanum nigrum L. and exploration its hepatoprotective potential against D-galactosamine-induced hepatic fibrosis in rats. Pharmacogn. Mag. 2017, 13, 179–188. [Google Scholar] [CrossRef]
- Parmentier, I.; Duminil, J.; Kuzmina, M.; Philippe, M.; Thomas, D.W.; Kenfack, D.; Chuyong, G.B.; Cruaud, C.; Hardy, O.J. How Effective Are DNA Barcodes in the Identification of African Rainforest Trees? PLoS ONE 2013, 8, e54921. [Google Scholar] [CrossRef]
- Parson, W.; Pegoraro, K.; Niederstätter, H.; Föger, M.; Steinlechner, M. Species identification by means of the cytochrome b gene. Int. J. Legal Med. 2000, 114, 23–28. [Google Scholar] [CrossRef]
- Hollingsworth, P.M.; Graham, S.W.; Little, D.P. Choosing and using a plant DNA barcode. PLoS ONE 2011, 6, e19254. [Google Scholar] [CrossRef]
- Coissac, E.; Hollingsworth, P.M.; Lavergne, S.; Taberlet, P. From barcodes to genomes: Extending the concept of DNA barcoding. Mol. Ecol. 2016, 25, 1423–1428. [Google Scholar] [CrossRef]
- Alasmari, A. DNA-Barcoding of Some Medicinal Plant Species in Saudi Arabia Using rbcL and matK Genes. Phyton 2020, 89, 1059–1081. [Google Scholar] [CrossRef]
- CBOL Plant Working Group; Hollingsworth, P.M.; Forrest, L.L.; Spouge, J.L.; Hajibabaei, M.; Ratnasingham, S.; van der Bank, M.; Chase, M.W.; Cowan, R.S.; Erickson, D.L.; et al. A DNA barcode for land plants. Proc. Natl. Acad. Sci. USA. 2009, 106, 12794–12797. [Google Scholar] [CrossRef] [PubMed]
- Cutarelli, G.G.; Capuano, F.C.F. Species identification by means of mitochondrial cytochrome b DNA sequencing in processed anchovy, sardine and tuna products. Food Nutr. Sci. 2018, 9, 369–375. [Google Scholar] [CrossRef]
- Lucas, C.; Thangaradjou, T.; Papenbrock, J. Development of a DNA barcoding system for seagrasses: Successful but not simple. PLoS ONE 2012, 7, e29987. [Google Scholar] [CrossRef] [PubMed]
- Zietkiewicz, E.; Rafalski, A.; Labuda, D. Genome fingerprinting by simple sequence repeat (SSR)-anchored polymerase chain reaction amplification. Genomics 1994, 20, 176–193. [Google Scholar] [CrossRef] [PubMed]
- Younis, A.; Ramzan, F.; Ramzan, Y.; Zulfiqar, F.; Ahsan, M.; Lim, K.B. Molecular Markers Improve Abiotic Stress Tolerance in Crops: A Review. Plants 2020, 9, 1374. [Google Scholar] [CrossRef]
- Shaban, A.S.; Arab, S.A.; Basuoni, M.M.; Abozahra, M.S.; Abdelkawy, A.M.; Mohamed, M.M. SCoT, ISSR, and SDS-PAGE investigation of genetic diversity in several Egyptian wheat genotypes under normal and drought conditions. Int. J. Agron. 2022, 14, 7024028. [Google Scholar] [CrossRef]
- Vaishnav, K.; Tiwari, V.; Durgapal, A.; Meena, B.; Rana, T.S. Estimation of genetic diversity and population genetic structure in Gymnema sylvestre (Retz.) R. Br. ex Schult. populations using DAMD and ISSR markers. J. Genet. Eng. Biotechnol. 2023, 21, 42. [Google Scholar] [CrossRef]
- Wen, S.; Zhao, H.; Zhang, M.; Qiao, G.; Shen, X. IRAPs in Combination with Highly Informative ISSRs Confer Effective Potentials for Genetic Diversity and Fidelity Assessment in Rhododendron. Int. J. Mol. Sci. 2023, 24, 6902. [Google Scholar] [CrossRef] [PubMed]
- Khuntia, S.; Kar, B.; Sahoo, S. Molecular Marker Based Genetic Diversity Study of Curcuma Caesia Roxb.—A Mini Review. J. Microbiol. Biotechnol. 2023, 8, 2. [Google Scholar] [CrossRef]
- Itle, R.A.; Kabelka, E.A. Correlation between L*a*b* color space values and carotenoid content in pumpkins and squash (Cucurbita spp.). HortScience 2009, 44, 633–637. [Google Scholar] [CrossRef]
- Vivek, K.; Mishra, S.; Pradhan, R.C. Physicochemical characterization and mass modelling of Sohiong (Prunus nepalensis L.) fruit. J. Food Meas. Charact. 2018, 12, 923–936. [Google Scholar] [CrossRef]
- Sunmonu, M.O.; Iyanda, M.O.; Odewole, M.M.; Moshood, A.N. Determination of some mechanical properties of almond seed related to design of food processing machines. Niger. J. Technol. Dev. 2015, 12, 22–26. [Google Scholar] [CrossRef]
- Altuntas, E.; Saracoglu, O.; Polatci, H. Physico-mechanical, colour and chemical properties of selected cherry laurel genotypes of Turkey. Adv. Agric. Sci. 2018, 6, 61–76. [Google Scholar]
- Yu, J.; Xue, J.H.; Zhou, S.L. New universal matK primers for DNA barcoding angiosperms. J. Syst. Evol. 2011, 49, 176–181. [Google Scholar] [CrossRef]
- Alansi, S.; Tarroum, M.; Al-Qurainy, F.; Khan, S.; Nadeem, M. Use of ISSR markers to assess the genetic diversity in wild medicinal Ziziphus spina-christi (L.) Willd. collected from different regions of Saudi Arabia. Biotechnol. Biotechnol. Equip. 2016, 30, 942–947. [Google Scholar] [CrossRef]
- Snedecor, G.W.; Cochran, W.G. Statistical Methods, 7th ed.; The Iowa State University Press: Ames, IA, USA, 1990; p. 593. [Google Scholar]
- Gomez, K.A.; Gomez, A.A. Statistical Procedures for Agricultural Research; John Wiley & Sons: New York, NY, USA, 1984. [Google Scholar]
- XLSTAT Statistical Package Software. The XLSTAT System for Windows; Version 2019.1; Excel Add-Ins Soft SARL: New York, NY, USA, 2019. [Google Scholar]
- Rohlf, F.J. NTSYS-pc: Numerical Taxonomy and Multivariate Analysis System; Exeter Publishing: Exeter, UK, 1988. [Google Scholar]
- Metsalu, T.; Vilo, J. ClustVis: A web tool for visualizing clustering of multivariate data using Principal Component Analysis and heatmap. Nucleic Acids Res. 2015, 43, 566–570. [Google Scholar] [CrossRef] [PubMed]
- Obeed, R.S.; Harhash, M.M.; Abdel-Mawgood, A.L. Fruit properties and genetic diversity of five ber (Ziziphus mauritiana Lamk.) cultivars. Pak. J. Biol. Sci. 2008, 11, 888–893. [Google Scholar] [CrossRef] [PubMed]
- Mencarelli, F.; Bellincontro, A.; Forniti, R.; Vizovitis, K.; Botondi, R.; Valentini, M.; Sequi, P.; DiNatale, C.; Basile, B.; Romano, R. Factors affecting the apricot quality for the consumer with special attention to the use of 1-MCP and of NDT for detection of bruising. Acta Hortcult. 2006, 717, 315–320. [Google Scholar] [CrossRef]
- Ghosh, S.N.; Mathew, B. Performance of nine ber cultivars (Zizyphus mauritiana Lamk.) on top working in semi-arid region of West Bengal. J. Appl. Hortic. 2002, 4, 49–51. [Google Scholar] [CrossRef]
- Razi, M.F.; Anwar, R.; Basra, S.M.A.; Khan, M.M.; Khan, I.A. Morphological characterization of leaves and fruit jujube (Ziziphus mauritiana Lamk.) Germplasm in Faisalabad, Pakistan. Pak. J. Agric. Sci. 2013, 50, 211–216. [Google Scholar]
- Cardenas-Perez, S.; Chanona-Perez, J.; Mendez-Mendez, J.V.; Calderon-Dominguez, G.; Lopez-Santiago, R.; Perea-Flores, M.J.; Arzate-Vazquez, I. Evaluation of the ripening stages of apple (Golden Delicious) by means of computer vision system. Biosyst. Eng. 2017, 159, 46–58. [Google Scholar] [CrossRef]
- Mahajan, B.V.C.; Dhillon, S.B. Evaluation of different cultivars of litchi (Litchi chinensis Sonn.) under sub-mountainous regions of Punjab. Haryana J. Hort. Sci. 2000, 29, 184. [Google Scholar]
- Kumar, H.; Katiyar, P.N.; Singh, A.K.; Rajkumar, B.V. Effect of different pruning severity on physico-chemical properties of ber (Zizyphus mauritiana Lamk.) cv. Banarasi Karaka. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 935–940. [Google Scholar]
- Bhargava, R.; Shukla, A.K.; Chauhan, N.; Vashishtha, B.B.; Dhandar, D.G. Impact of hybridity on flavonoid spectrum of ber (Ziziphus mauritiana Lamk.). Environ. Exp. Bot. 2005, 53, 135–138. [Google Scholar] [CrossRef]
- Shukla, A.K.; Awasthi, O.P.; Shukla, A.K.; Vashishtha, B.B.; Bhargava, R. Evaluation of ber (Ziziphus mauritiana L.) cultivars under hot arid ecosystem of Rajasthan. Progress. Hortic. 2007, 39, 22–27. [Google Scholar]
- Singh, A.; Singh, R.K.; Kumar, A.; Ashwani, K.; Kumar, R.; Kumar, N.; Sheoran, P.; Yadav, R.K.; Sharma, D.K. Adaptation to social-ecological stressors: A case study with Indian jujube (Ziziphus mauritiana Lam.) growers of north-western India. Environ. Dev. Sustain. 2021, 23, 3265–3288. [Google Scholar] [CrossRef]
- Corrado, G.; Forlani, M.; Rao, R.; Basile, B. Diversity and Relationships among Neglected Apricot (Prunus armeniaca L.) Landraces Using Morphological Traits and SSR Markers: Implications for Agro-Biodiversity Conservation. Plants 2021, 10, 1341. [Google Scholar] [CrossRef]
- Alkaraki, A.K.; Aldmoor, M.A.; Lahham, J.N.; Nusair, S.D. DNA Barcoding of Selected Medicinal Plant Species from Jordan Using matK, rbcL, and rpoC1 Genes. Int. J. Biol. Biomed. Eng. 2021, 15, 376–411. [Google Scholar] [CrossRef]
- Moudi, M.; Kouhi, S.M.M.; Mood, S.G.; Ayoobi, A. DNA barcoding of Ziziphus jujuba Mill. in Iran using chloroplast genes (rbcL and matK). J. Plant Prod. Resch. 2021, 28, 115–130. [Google Scholar] [CrossRef]
- El-Haggar, M.A.; Mahgoub, Y.A.; Aly, H.M.; Ghazy, N.M.; El-Fiky, F.K.; El-Hawiet, A.M. DNA barcodes, ISSR, RAPD and SCAR markers as potential quality control tools for molecular authentication of black and white mulberry. Food Control 2023, 152, 109821. [Google Scholar] [CrossRef]
- Singh, A.K.; Devanshi; Singh, R.; Singh, B.; Koundal, K.R.; Singh, N.K. Assessment of genetic diversity in Ziziphus mauritiana using inter-simple sequence repeat markers. J. Plant Biochem. Biotechnol. 2007, 16, 35–40. [Google Scholar] [CrossRef]
- Sareen, A.; Sharma, V.; Gupta, R.C. Assessment of genetic diversity and population structure in wild Ziziphus species from northwest India using SSR marker technique. J. Genet. Eng. Biotechnol. 2023, 21, 4. [Google Scholar] [CrossRef] [PubMed]
- Botstein, D.; White, R.L.; Skalnick, M.H.; Davies, R.W. Construction of a genetic linkage map in man using restriction fragment length polymorphism. Am. J. Hum. Gen. 1980, 32, 314–331. [Google Scholar]
- Khodaee, L.; Azizinezhad, R.; Etminan, A.R.; Khosroshahi, M. Assessment of genetic diversity among Iranian aegilops triuncialis accessions using ISSR, SCoT, and CBDP markers. J. Genet. Eng. Biotech. 2021, 19, 5. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Lateif, K.S.; Hewedy, O. Genetic diversity among Egyptian wheat cultivars using SCoT and ISSR markers. J. Breed. Genet. 2018, 50, 36–45. [Google Scholar]
- Cornea-Cipcigan, M.; Pamfil, D.; Sisea; Rodica Margaoan, C.R. Characterization of Cyclamen genotypes using morphological descriptors and DNA molecular markers in a multivariate analysis. Front. Plant Sci. 2023, 14, 1100099. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Meinhardt, L.W.; Bailley, B.; Zhan, D. Genetic improvement of Chinese gugube for disease resistances: Status, knowledge gaps and research needs. Crop Breed. Genet. Genom. 2019, 1, e190015. [Google Scholar] [CrossRef]
- Cabrera-Toledo, D.; Vargas-Ponce, O.; Ascencio-Ramírez, S.; Valadez-Sandoval, L.M.; Pérez-Alquicira, J.; Morales-Saavedra, J.; Huerta-Galván, O.F. Morphological and Genetic Variation in Monocultures, Forestry Systems and Wild Populations of Agave maximiliana of Western Mexico: Implications for Its Conservation. Front. Plant Sci. 2020, 11, 817. [Google Scholar] [CrossRef]
- Qiao, Q.; Ye, M.; Wu, C.; Wang, J.; Liu, Q.; Tao, J.; Zhang, L.; Feng, Z. Analysis of leaf morphology variation and genetic diversity via SRAP markers for near-threatened plant Acer truncatum. Glob. Ecol. Conserv. 2022, 33, e01980. [Google Scholar] [CrossRef]
- Song, Y.; Fan, L.; Chen, H.; Zhang, M.; Ma, Q.; Zhang, S.; Wu, J. Identifying genetic diversity and a preliminary core collection of Pyrus pyrifolia cultivars by a genome-wide set of SSR markers. Sci. Hortic. 2014, 167, 5–16. [Google Scholar] [CrossRef]
- Nizar, M.; Mulani, R. Genetic diversity in indigenous and exotic linseed germplasm (Linum usitatissimum L.). Elect. J. Plant Breed 2015, 6, 848–854. [Google Scholar]
- Pissard, A.; Arbizu, C.; Ghislain, M.; Faux, A.; Paulet, S.; Bertin, B. Congruence between morphological and molecular markers inferred from the analysis of the intra-morphotype genetic diversity and the spatial structure of Oxalis tuberosa Mol. Genetica 2007, 132, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Normohamadi, S.; Solouki, M.; Heidari, F. Diversity in Cucumber Genotypes Based on Morphological Traits and SSR Molecular Markers. Biosci. Biotechnol. Res. Asia 2017, 14, 775–782. [Google Scholar] [CrossRef]





| Primer Name | Sequences | Reference |
|---|---|---|
| ISSR1 | AGAGAGAGAGAGAGAGYA | Alansi, et al. [39] |
| ISSR2 | TGTGTGTGTGTGTGTGRT | |
| ISSR3 | GAGAGAGAGAGAGAGAYG | |
| ISSR4 | AGAGAGAGAGAGAGAGGT | |
| ISSR5 | ACACACACACACACACTG |
| Cultivars | Fruit Volume (cm3) | Pulp/Stone Ratio | Fruit Density (g/cm3) | Sphericity Index (%) |
|---|---|---|---|---|
| Zaytoni | 12.37 g | 12.81 de | 0.97 bc | 85.3 e |
| Kashmiri | 24.43 d | 24.01 b | 0.96 bc | 81.25 f |
| Komethry | 17.6 f | 13.33 d | 0.98 b | 70.49 g |
| Um-Sulaem with spines | 4.8 j | 44.62 a | 0.99 b | 94.06 c |
| Toffahy | 44 a | 17.85 c | 0.92 cd | 98.34 a |
| Um-Sulaem without spines | 7.13 i | 46.68 a | 0.98 b | 95.94 b |
| Abdel-Sattar | 19.03 e | 15.03 cd | 0.99 b | 92.47 d |
| Pu-Yun | 30.67 c | 16.38 c | 0.94 bc | 92.4 d |
| Pu-Pineau | 33.1 b | 23.55 b | 0.98 b | 94.97 bc |
| Seedy Ber | 8.83 h | 9.81 e | 0.87 d | 95.67 b |
| Buddling Ber | 12.6 g | 12.47 de | 1.06 a | 92.17 d |
| LSD00.5 | 0.7356 | 3.0033 | 0.0555 | 1.4692 |
| Primer Name | Monomorphic Band | Polymorphic Band | Unique Band | % of Polymorphic Band | Marker Indices | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| H 1 | PIC 2 | E 3 | H.av 4 | MI 5 | D 6 | |||||
| ISSR1 | 1 | 17 | 1 | 94.4 | 0.565 | 0.478 | 1.000 | 0.565 | 0.565 | 0.519 |
| ISSR2 | 0 | 15 | 1 | 100 | 0.546 | 0.475 | 1.000 | 0.546 | 0.546 | 0.473 |
| ISSR3 | 0 | 13 | 3 | 100 | 0.474 | 0.424 | 1.000 | 0.474 | 0.474 | 0.436 |
| ISSR4 | 0 | 14 | 2 | 100 | 0.525 | 0.462 | 1.000 | 0.525 | 0.525 | 0.484 |
| ISSR5 | 2 | 8 | 1 | 80 | 0.576 | 0.495 | 1.000 | 0.576 | 0.576 | 0.586 |
| Total | 3 | 67 | 8 | 474.4 | 2.69 | 2.33 | 5.00 | 2.69 | 2.69 | 2.50 |
| Average | 0.6 | 13.4 | 1.6 | 94.88 | 0.54 | 0.47 | 1.00 | 0.54 | 0.54 | 0.50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdel-Sattar, M.; Al-Obeed, R.S.; Rihan, H.Z.; El-Badan, G.E. Genetic Diversity and Relationships among Indian Jujube (Ziziphus mauritiana Lamk.) Cultivars Using Morphometric Characteristics, matK Barcoding, and ISSR Markers. Diversity 2024, 16, 313. https://doi.org/10.3390/d16060313
Abdel-Sattar M, Al-Obeed RS, Rihan HZ, El-Badan GE. Genetic Diversity and Relationships among Indian Jujube (Ziziphus mauritiana Lamk.) Cultivars Using Morphometric Characteristics, matK Barcoding, and ISSR Markers. Diversity. 2024; 16(6):313. https://doi.org/10.3390/d16060313
Chicago/Turabian StyleAbdel-Sattar, Mahmoud, Rashid S. Al-Obeed, Hail Z. Rihan, and Ghada E. El-Badan. 2024. "Genetic Diversity and Relationships among Indian Jujube (Ziziphus mauritiana Lamk.) Cultivars Using Morphometric Characteristics, matK Barcoding, and ISSR Markers" Diversity 16, no. 6: 313. https://doi.org/10.3390/d16060313






