Habitat Fragmentation Enhances the Difference between Natural and Artificial Reefs in an Urban Marine Coastal Tract
Abstract
:1. Introduction
2. Materials and Methods
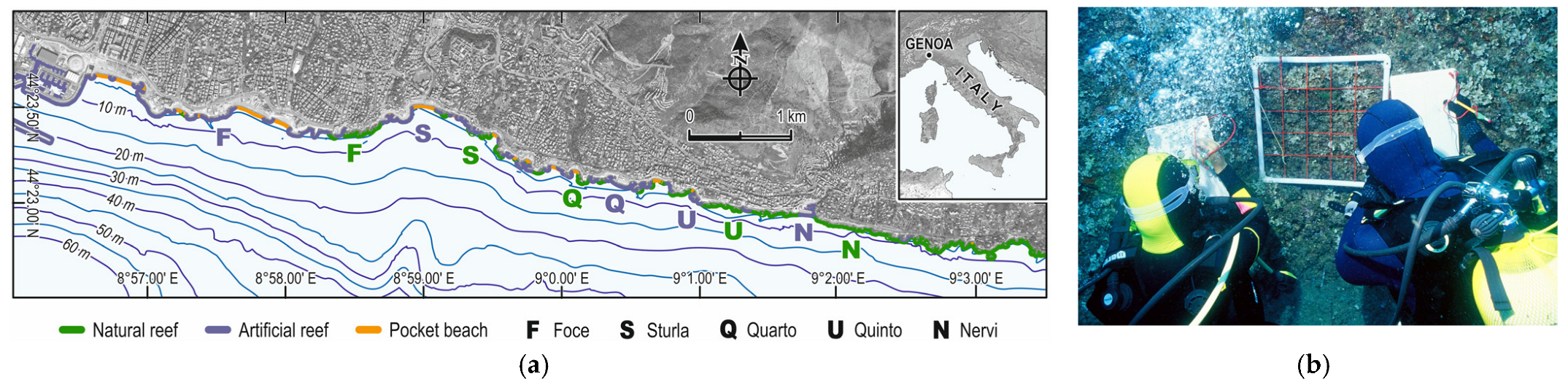
2.1. Study Area and Field Activities
2.2. Data Management
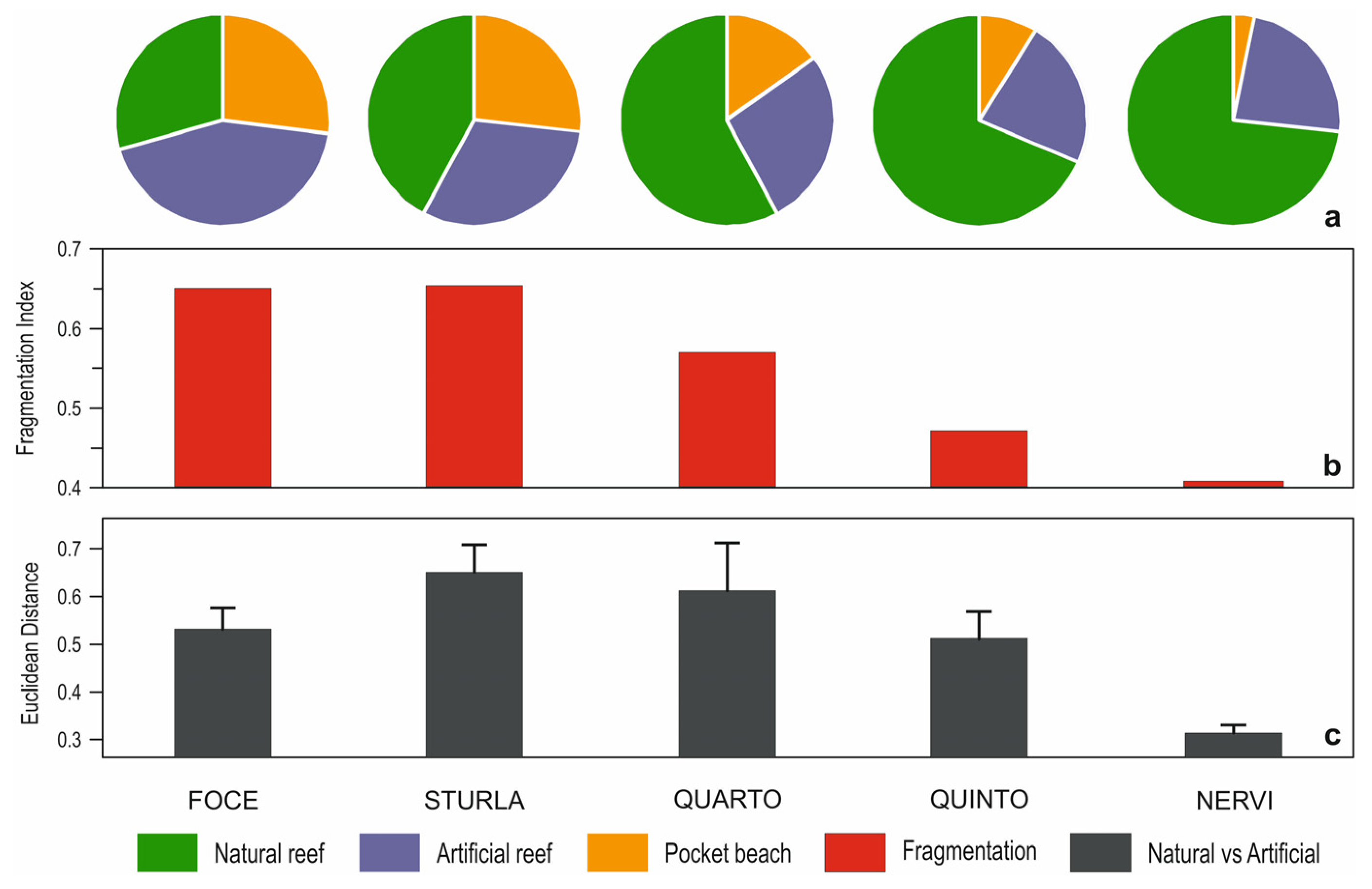
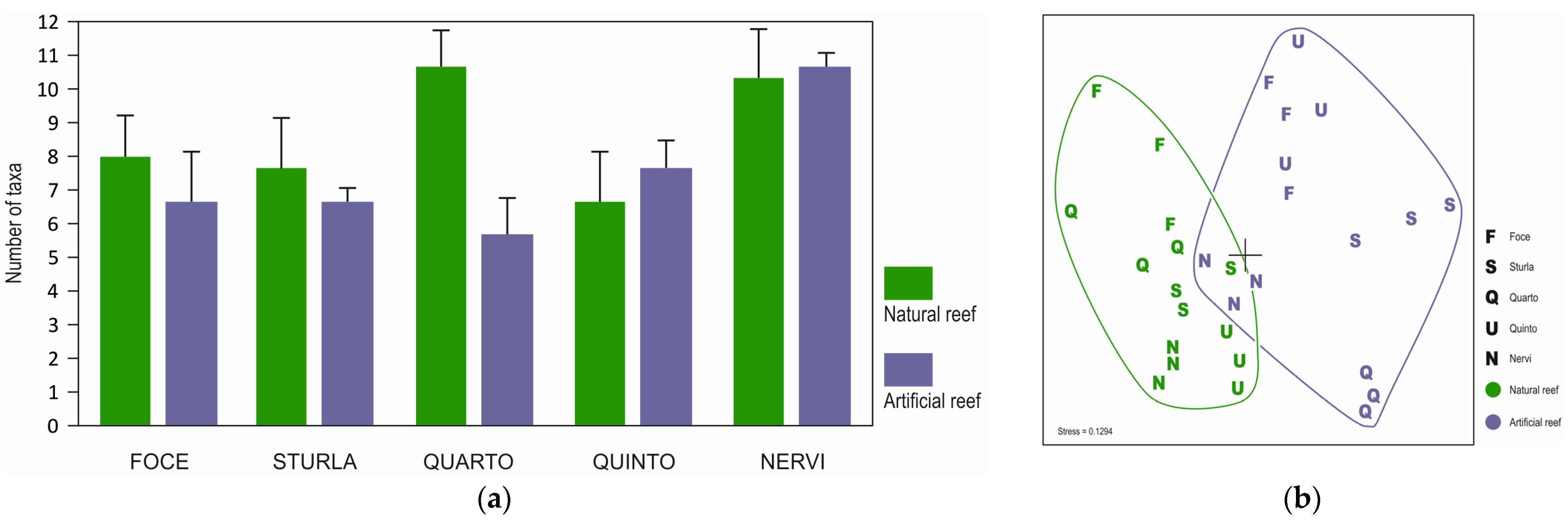
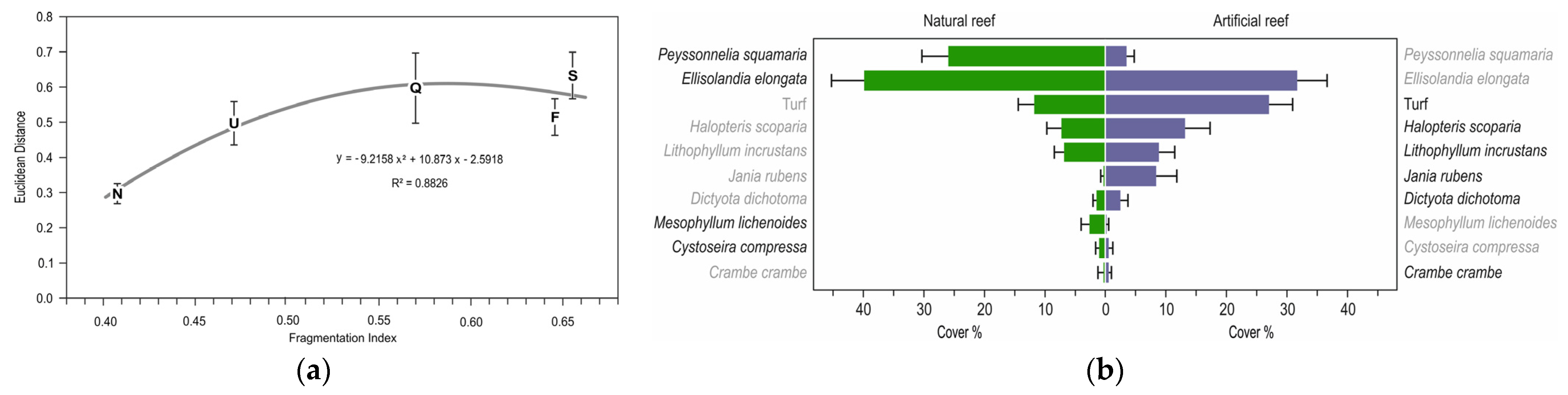
3. Results
4. Discussion
| NAT | ART | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Dissim | Contrib % | m | se | m | se | n | t | P | ||
| 1 | Peyssonnelia squamaria | 13.41 | 25.41 | 26.0 | 4.27 | 3.6 | 1.23 | 15 | 5.041 | 0.000 *** |
| 2 | Ellisolandia elongata | 11.52 | 21.84 | 39.9 | 5.32 | 32.5 | 4.79 | 15 | 1.034 | 0.310 ns |
| 3 | Turf | 9.63 | 18.25 | 11.8 | 2.40 | 27.7 | 3.75 | 15 | −3.571 | 0.001 ** |
| 4 | Halopteris scoparia | 6.71 | 12.71 | 7.3 | 2.18 | 13.5 | 4.05 | 15 | −1.348 | 0.188 ns |
| 5 | Lithophyllum incrustans | 4.26 | 8.08 | 6.9 | 1.51 | 9.1 | 2.54 | 15 | −0.745 | 0.463 ns |
| 6 | Jania rubens | 4.18 | 7.92 | 0.4 | 0.28 | 8.6 | 3.40 | 15 | −2.404 | 0.023 * |
| 7 | Dictyota dichotoma | 1.05 | 2.00 | 1.5 | 0.36 | 2.6 | 1.17 | 15 | −0.899 | 0.377 ns |
| 8 | Mesophyllum lichenoides | 1.03 | 1.94 | 2.7 | 1.14 | 0.3 | 0.21 | 15 | 2.036 | 0.048 * |
| 9 | Cystoseira compressa | 0.64 | 1.21 | 1.0 | 0.48 | 0.6 | 0.55 | 15 | 0.548 | 0.588 ns |
| 10 | Crambe crambe | 0.34 | 0.65 | 0.4 | 0.70 | 0.6 | 0.32 | 15 | −0.260 | 0.797 ns |
| 11 | Protula tubularia | 0 | 0 | 0.5 | 0.20 | 0.2 | 0.12 | 15 | ||
| 12 | Ircinia oros | 0 | 0 | 0.4 | 0.16 | 0.2 | 0.15 | 15 | ||
| 13 | Chondrosia reniformis | 0 | 0 | 0.4 | 0.22 | 0.1 | 0.07 | 15 | ||
| 14 | Sphaerococcus coronopifolius | 0 | 0 | 0.4 | 0.24 | 0.0 | 0.00 | 15 | ||
| 15 | Amphiroa rigida | 0 | 0 | 0.2 | 0.21 | 0.0 | 0.00 | 15 | ||
| 16 | Hydrozoa | 0 | 0 | 0.1 | 0.06 | 0.1 | 0.07 | 15 | ||
| 17 | Padina pavonica | 0 | 0 | 0.1 | 0.10 | 0.0 | 0.00 | 15 | ||
| 18 | Ircinia variabilis | 0 | 0 | 0.1 | 0.07 | 0.1 | 0.05 | 15 | ||
| 19 | Asparagopsis armata | 0 | 0 | 0.0 | 0.00 | 0.1 | 0.07 | 15 | ||
| 20 | Serpulidae | 0 | 0 | 0.0 | 0.00 | 0.1 | 0.10 | 15 | ||
| 21 | Aiptasia mutabilis | 0 | 0 | 0.1 | 0.07 | 0.0 | 0.00 | 15 | ||
| 22 | Cliona celata | 0 | 0 | 0.0 | 0.00 | 0.1 | 0.07 | 15 | ||
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Timmerman, P.; White, R. Megahydropolis: Coastal cities in the context of global environmental change. Global Environ. Chang. 1997, 7, 205–234. [Google Scholar] [CrossRef]
- Bulleri, F.; Chapman, M.G. The introduction of coastal infrastructure as a driver of change in marine environments. J. Appl. Ecol. 2010, 47, 26–35. [Google Scholar] [CrossRef]
- Crossett, K.M.; Culliton, T.J.; Wiley, P.C.; Goodspeed, T.R. Population Trends along the Coastal United States: 1980–2008; NOAA: Silver Spring, MD, USA, 2004; pp. 1–54. [Google Scholar]
- Hugo, G. Future demographic change and its interactions with migration and climate change. Glob. Environ. Chang. 2011, 21, S21–S33. [Google Scholar] [CrossRef]
- Barragán, J.M.; De Andrés, M. Analysis and trends of the world’s coastal cities and agglomerations. Ocean Coast. Manag. 2015, 114, 11–20. [Google Scholar] [CrossRef]
- McGranahan, G.; Balk, D.; Aderson, B. The rising tide: Assessing the risks of climate change and human settlements in low elevation coastal zones. Environ. Urban. 2007, 19, 17–37. [Google Scholar] [CrossRef]
- Espinosa, F.; Bazairi, H. Impacts, evolution, and changes of pressure on marine ecosystems in recent times. Toward new emerging and unforeseen impacts within a changing world. In Coastal Habitat Conservation. New Perspectives and Sustainable Development of Biodiversity in the Anthropocene; Espinosa, F., Ed.; Elsevier: Amsterdam, The Netherland, 2023; pp. 1–16. [Google Scholar]
- Glasby, T.M.; Gibson, P.T.; Cruz-Motta, J.J. Differences in rocky reef habitats related to human disturbances across a latitudinal gradient. Mar. Environ. Res. 2017, 129, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Firth, L.B.; Airoldi, L.; Bulleri, F.; Challinor, S.; Chee, S.; Evans, A.J.; Hanley, M.E.; Knights, A.M.; O’Shaughnessy, K.; Thompson, R.C.; et al. Greening of grey infrastructure should not be used as a Trojan horse to facilitate coastal development. J. Appl. Ecol. 2020, 57, 1762–1768. [Google Scholar] [CrossRef]
- Dafforn, K.A.; Glasby, T.M.; Airoldi, L.; Rivero, N.K.; Mayer-Pinto, M.; Johnston, E.L. Marine urbanization: An ecological framework for designing multifunctional artificial structures. Front. Ecol. Environ. 2015, 13, 82–90. [Google Scholar] [CrossRef]
- Fahrig, L. Effects of habitat fragmentation on biodiversity. Annu. Rev. Ecol. Evol. Syst. 2003, 34, 487–515. [Google Scholar] [CrossRef]
- Marrone, A.; Mangano, M.C.; Deidun, A.; Berlino, M.; Sarà, G. Effects of habitat fragmentation of a Mediterranean marine reef on the associated fish community: Insights from biological traits analysis. J. Mar. Sci. Eng. 2023, 11, 1957. [Google Scholar] [CrossRef]
- Ostalé-Valriberas, E.; Martín-Zorrilla, A.; Sempere-Valverde, J.; García-Gómez, J.C.; Espinosa, F. Ecological succession within microhabitats (tidepools) created in riprap structures hosting climax communities: An economical strategy for mitigating the negative effects of coastal defence structure on marine biodiversity. Ecol. Eng. 2024, 200, 107187. [Google Scholar] [CrossRef]
- Bevilacqua, S.; Airoldi, L.; Ballesteros, E.; Benedetti-Cecchi, L.; Boero, F.; Bulleri, F.; Cebrian, E.; Cerrano, C.; Claudet, J.; Colloca, F.; et al. Mediterranean rocky reefs in the Anthropocene: Present status and future concerns. Adv. Mar. Bio. 2021, 89, 1–51. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Rodríguez, A.; Aburto-Oropeza, O.; Erisman, B.; Jiménez-Esquivel, V.M.; Hinojosa-Arango, G. Rocky reefs: Preserving biodiversity for the benefit of the communities in the aquarium of the world. In Ethnobiology of Corals and Coral Reefs; Narchi, N., Leimar Price, L., Eds.; Springer International: Cham, Switzerland, 2015; pp. 177–208. [Google Scholar] [CrossRef]
- Giglio, V.J.; Aued, A.W.; Cordeiro, C.A.; Eggertsen, L.; Ferrari, D.S.; Gonçalves, L.R.; Hanazaki, N.; Luiz, O.J.; Luza, A.L.; Mendes, T.C.; et al. A Global Systematic Literature Review of Ecosystem Services in Reef Environments. Environ. Manag. 2023, 73, 634–645. [Google Scholar] [CrossRef] [PubMed]
- Rilov, G.; Benayahu, Y. Vertical artificial structures as an alternative habitat for coral reef fishes in disturbed environments. Mar. Environ. Res. 1998, 45, 431–451. [Google Scholar] [CrossRef]
- Sempere-Valverde, J.; Guerra-García, J.M.; García-Gómez, J.C.; Espinosa, F. Coastal urbanization, an issue for marine conservation. In Coastal Habitat Conservation. New Perspectives and Sustainable Development of Biodiversity in the Anthropocene; Espinosa, F., Ed.; Elsevier: Amsterdam, The Netherland, 2023; pp. 41–79. [Google Scholar]
- Ostalé-Valriberas, E.; Sempere-Valverde, J.; Coppa, S.; García-Gómez, J.C.; Espinosa, F. Creation of microhabitats (tidepools) in ripraps with climax communities as a way to mitigate negative effects of artificial substrate on marine biodiversity. Ecol. Eng. 2018, 120, 522–531. [Google Scholar] [CrossRef]
- López, I.; Tinoco, H.; Aragonés, L.; Garcia-Barba, J. The multifunctional artificial reef and its role in the defence of the Mediterranean coast. Sci. Total Environ. 2016, 550, 910–923. [Google Scholar] [CrossRef] [PubMed]
- Bombace, G. Artificial reefs in the Mediterranean Sea. Bull. Mar. Sci. 1989, 44, 1023–1032. [Google Scholar]
- Grossman, G.D.; Jones, G.P.; Seaman, W.J., Jr. Do artificial reefs increase regional fish production? A review of existing data. Fisheries 1997, 22, 17–23. [Google Scholar] [CrossRef]
- Lima, J.S.; Sanchez-Jerez, P.; dos Santos, L.N.; Zalmon, I.R. Could artificial reefs increase access to estuarine fishery resources? Insights from a long-term assessment. Estuar. Coast. Shelf Sci. 2020, 242, 106858. [Google Scholar] [CrossRef]
- Han, C.; Liu, K.; Kinoshita, T.; Guo, B.; Zhao, Y.; Ye, Y.; Liu, Y.; Yamashita, O.; Zheng, D.; Wang, W.; et al. Assessing the attractive effects of floating artificial reefs and combination reefs on six local marine species. Fishes 2023, 8, 248. [Google Scholar] [CrossRef]
- Folpp, H.; Lowry, M.; Gregson, M.; Suthers, I.M. Fish assemblages on estuarine artificial reefs: Natural rocky-reef mimics or discrete assemblages? PLoS ONE 2013, 8, e63505. [Google Scholar] [CrossRef] [PubMed]
- Bracho-Villavicencio, C.; Matthews-Cascon, H.; Rossi, S. Artificial reefs around the world: A review of the state of the art and a meta-analysis of its effectiveness for the restoration of marine ecosystems. Environments 2023, 10, 121. [Google Scholar] [CrossRef]
- Carvalho, S.; Moura, A.; Cúrdia, J.; da Fonseca, L.C.; Santos, M.N. How complementary are epibenthic assemblages in artificial and nearby natural rocky reefs? Mar. Environ. Res. 2013, 92, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, M.A.; Broitman, B.R.; Thiel, M. Spatial variability in community composition on a granite breakwater versus natural rocky shores: Lack of microhabitats suppresses intertidal biodiversity. Mar. Pollut. Bull. 2014, 87, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Schroeter, S.C.; Reed, D.C.; Raimondi, P.T. Effects of reef physical structure on development of benthic reef community: A large-scale artificial reef experiment. Mar. Ecol. Prog. Ser. 2015, 540, 43–55. [Google Scholar] [CrossRef]
- Fabi, G.; Spagnolo, A. Artificial Reefs in the Management of Mediterranean Sea Fisheries; CRC Press: Boca Raton, FL, USA, 2011; pp. 167–181. [Google Scholar]
- Airoldi, L.; Beck, W.M. Loss, status and trends for coastal marine habitats of Europe. Oceanogr. Mar. Biol. Annu. Rev. 2007, 45, 345–405. [Google Scholar]
- Furlani, S.; Pappalardo, M.; Gómez-Pujol, L.; Chelli, A. The rock coast of the Mediterranean and Black seas. Geol. Soc. Lond. Mem. 2014, 40, 89–123. [Google Scholar] [CrossRef]
- Meinesz, A.; Lefèvre, J.R. Destruction de l’étage infralittoral des Alpes-Maritimes (France) et de Monaco par les restructurations du rivage. Bull. Ecol. 1978, 9, 259–276. [Google Scholar]
- Meinesz, A.; Astier, J.M.; Lefèvre, J.R. Impact de l’aménagement du domaine maritime sur l’étage infralittoral du Var, France (Méditerranée occidentale). Ann. Inst. Océanogr. 1981, 57, 65–77. [Google Scholar]
- Mineur, F.; Cook, E.J.; Minchin, D.; Bohn, K.; MacLeod, A.; Maggs, C.A. Changing coasts: Marine aliens and artificial structures. Oceanogr. Mar. Biol. Annu. Rev. 2012, 50, 189–234. [Google Scholar] [CrossRef]
- Sedano, F.; Florido, M.; Rallis, I.; Espinosa, F.; Gerovasileiou, V. Comparing sessile benthos on shallow artificial versus natural hard substrates in the Eastern Mediterranean Sea. Mediterr. Mar. Sci. 2019, 20, 688–702. [Google Scholar] [CrossRef]
- Cattaneo Vietti, R.; Albertelli, G.; Aliani, S.; Bava, S.; Bavestrello, G.; Benedetti Cecchi, L.; Bianchi, C.N.; Bozzo, E.; Capello, M.; Castellano, M.; et al. The Ligurian Sea: Present status, problems and perspectives. Chem. Ecol. 2010, 26, 319–340. [Google Scholar] [CrossRef]
- Burgos, E.; Montefalcone, M.; Ferrari, M.; Paoli, C.; Vassallo, P.; Morri, C.; Bianchi, C.N. Ecosystem functions and economic wealth: Trajectories of change in seagrass meadows. J. Clean. Prod. 2017, 168, 1108–1119. [Google Scholar] [CrossRef]
- Mangialajo, L.; Ruggieri, N.; Asnaghi, V.; Chiantore, M.; Povero, P.; Cattaneo-Vietti, R. Ecological status in the Ligurian Sea: The effect of coastline urbanisation and the importance of proper reference sites. Mar. Pollut. Bull. 2007, 55, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Albertelli, G.; Balduzzi, A.; Cattaneo, R. Analisi strutturale su alcuni popolamenti bentonici lungo il litorale genovese. Atti Assoc. Ital. Oceanogr. Limnol. 1985, 6, 187–193. [Google Scholar]
- Montefalcone, M.; Albertelli, G.; Morri, C.; Bianchi, C.N. Urban seagrass: Status of Posidonia oceanica facing the Genoa city waterfront (Italy) and implications for management. Mar. Pollut. Bull. 2007, 54, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, C.N.; Pronzato, R.; Cattaneo-Vietti, R.; Benedetti Cecchi, L.; Morri, C.; Pansini, M.; Chemello, R.; Milazzo, M.; Fraschetti, S.; Terlizzi, A.; et al. Hard bottoms. Biol. Mar. Med. 2004, 11, 185–215. [Google Scholar]
- Weinberg, S. The minimal area problem in invertebrate communities of Mediterranean rocky substrata. Mar. Biol. 1978, 49, 33–40. [Google Scholar] [CrossRef]
- Bellan-Santini, D. Contribution à l’étude des peuplements infralittoraux sur substrat rocheux (étude qualitative et quantitative de la frange supérieure). Rec. Trav. St. Mar. Endoume 1969, 47, 1–294. [Google Scholar]
- Boudouresque, C.F.; Belsher, T. Une méthode de determination de l’aire minimale qualitative. Rapp. Comm. Int. Mer Médit. 1979, 25/26, 273–275. [Google Scholar]
- Bianchi, C.N.; Azzola, A.; Cocito, S.; Morri, C.; Oprandi, A.; Peirano, A.; Sgorbini, S.; Montefalcone, M. Biodiversity monitoring in Mediterranean marine protected areas: Scientific and methodological challenges. Diversity 2022, 14, 43. [Google Scholar] [CrossRef]
- Bianchi, C.N.; Castelli, A.; Abbiati, M.; Giangrande, A.; Lardicci, C.; Morri, C. Étude bionomique comparatif de la zonation verticale des Polychètes le long d’une falaise littorale en Méditerranée nord-occidentale. Rapp. Comm. Int. Mer Médit. 1988, 31, 18. [Google Scholar]
- Morri, C.; Bellan-Santini, D.; Giaccone, G.; Bianchi, C.N. Principles of bionomy: Definition of assemblages and use of taxonomic descriptors (macrobenthos). Biol. Mar. Medit 2004, 11 (Suppl. 1), 573–600. [Google Scholar]
- Lymperaki, M.M.; Hill, C.E.; Hoeksema, B.W. The effects of wave exposure and host cover on coral-associated fauna of a centuries-old artificial reef in the Caribbean. Ecol. Eng. 2022, 176, 106536. [Google Scholar] [CrossRef]
- Fraschetti, S.; Bianchi, C.N.; Terlizzi, A.; Fanelli, G.; Morri, C.; Boero, F. Spatial variability and human disturbance in shallow subtidal hard substrate assemblages: A regional approach. Mar. Ecol. Prog. Ser. 2001, 212, 1–12. [Google Scholar] [CrossRef]
- Cattaneo-Vietti, R.; Albertelli, G.; Bavestrello, G.; Bianchi, C.N.; Cerrano, C.; Chiantore, M.C.; Gaggero, L.; Morri, C. Can rock composition affect sublittoral epibenthic communities? PSZN Mar. Ecol. 2002, 23 (Suppl. 1), 65–77. [Google Scholar] [CrossRef]
- Guidetti, P.; Bianchi, C.N.; Chiantore, M.C.; Schiaparelli, S.; Morri, C.; Cattaneo-Vietti, R. Living on the rocks: Substrate mineralogy and the structure of subtidal rocky substrate communities in the Mediterranean Sea. Mar. Ecol. Progr. Ser. 2004, 274, 57–68. [Google Scholar] [CrossRef]
- Longobardi, L.; Bavestrello, G.; Betti, F.; Cattaneo-Vietti, R. Long-term changes in a Ligurian infralittoral community (Mediterranean Sea): A warning signal? Reg. Stud. Mar. Sci. 2017, 14, 15–26. [Google Scholar] [CrossRef]
- NOAA Physical Sciences Laboratory. Available online: http://www.esrl.noaa.gov/psd/cgi-bin/data/timeseries/timeseries1.pl (accessed on 5 January 2024).
- Google Earth. Available online: https://earth.google.com (accessed on 17 January 2024).
- Magurran, A.E. Measuring Biological Diversity; Wiley-Blackwell: Hoboken, NJ, USA, 2013; pp. 1–272. [Google Scholar]
- Legendre, P.; Legendre, L. Numerical Ecology, 3rd ed.; Elsevier: Amsterdam, The Netherland, 2012; pp. 1–1006. [Google Scholar]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PaSt: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 4. [Google Scholar]
- Augier, H. Inventory and classification of the marine benthic biocoenoses of the Mediterranean. Council of Europe, Strasbourg, Nat. Envir. Ser. 1982, 25, 1–57. [Google Scholar]
- Pérès, J.M. The Mediterranean benthos. Oceanogr. Mar. Biol. Ann. Rev. 1967, 5, 449–533. [Google Scholar]
- Ardizzone, G.D.; Belluscio, A.; Gravina, M.F.; Somaschini, A. Colonization and disappearance of Mytilus galloprovincialis Lam. on an artificial habitat in the Mediterranean Sea. Estuar. Coast. Shelf Sci. 1996, 43, 665–676. [Google Scholar] [CrossRef]
- Bracchetti, L.; Capriotti, M.; Fazzini, M.; Cocci, P.; Palermo, F.A. Mass mortality event of Mediterranean mussels (Mytilus galloprovincialis) in the Middle Adriatic: Potential implications of the climate crisis for marine ecosystems. Diversity 2024, 16, 130. [Google Scholar] [CrossRef]
- Veiga, P.; Ramos-Oliveira, C.; Sampaio, L.; Rubal, M. The role of urbanisation in affecting Mytilus galloprovincialis. PLoS ONE 2020, 15, e0232797. [Google Scholar] [CrossRef] [PubMed]
- Cocito, S.; Bianchi, C.N.; Morri, C.; Peirano, A. First survey of sessile communities on subtidal rocks in an area with hydrothermal vents: Milos Island, Aegean Sea. Hydrobiologia 2000, 426, 113–121. [Google Scholar] [CrossRef]
- Pizzuto, F. On the structure, typology and periodism of a Cystoseira brachycarpa J. Agardh emend. Giaccone community and of a Cystoseira crinita Duby community from the eastern coast of Sicily (Mediterranean Sea). Plant Biosyst. 1999, 133, 15–35. [Google Scholar] [CrossRef]
- Muguerza, N.; Bustamante, M.; Díez, I.; Quintano, E.; Tajadura, F.J.; Saiz-Salinas, J.I.; Gorostiaga, J.M. Long-term surveys reveal abrupt canopy loss with immediate changes in diversity and functional traits. Mar. Biol. 2020, 167, 61. [Google Scholar] [CrossRef]
- Connell, S.D.; Foster, M.S.; Airoldi, L. What are algal turfs? Towards a better description of turfs. Mar. Ecol. Prog. Ser. 2014, 495, 299–307. [Google Scholar] [CrossRef]
- Rodríguez-Prieto, C.; Ballesteros, E.; Boisset, F.; Afonso-Carrillo, J. Guía de las Macroalgas y Fanerógamas Marinas del Mediterráneo Occidental; Omega: Barcelona, Spain, 2013; pp. 1–656. [Google Scholar]
- Porzio, L.; Buia, M.C.; Lorenti, M.; Vitale, E.; Amitrano, C.; Arena, C. Ecophysiological response of Jania rubens (Corallinaceae) to ocean acidification. Rendi. Lincei. Sci. Fis. Nat. 2018, 29, 543–546. [Google Scholar] [CrossRef]
- Sempere-Valverde, J.; Ostalé-Valriberas, E.; Farfán, G.M.; Espinosa, F. Substratum type affects recruitment and development of marine assemblages over artificial substrata: A case study in the Alboran Sea. Estuar. Coast. Shelf. Sci. 2018, 204, 56–65. [Google Scholar] [CrossRef]
- Hill, C.E.L.; Lymperaki, M.M.; Hoeksema, B.W. A centuries-old manmade reef in the Caribbean does not substitute natural reefs in terms of species assemblages and interspecific competition. Mar. Pollut. Bull. 2021, 169, 112576. [Google Scholar] [CrossRef]
- Pisano, E.; Bianchi, C.N.; Matricardi, G.; Relini, G. Accumulo della biomassa su substrati artificiali immersi lungo la falesia di Portofino (Mar Ligure). In Atti del Convegno delle Unità Operative Afferenti ai Sottoprogetti Risorse Biologiche ed Inquinamento Marino; CNR: Rome, Italy, 1982; pp. 93–105. [Google Scholar]
- Huvé, M.P. Recherches sur la Genèse de Quelques Peuplements Algaux Marins de la Roche Littorale dans la Région de Marseille. Ph.D. Thesis, University of Paris, Paris, France, 1970. [Google Scholar]
- Bianchi, C.N. Ecologia dei Serpuloidea (Annelida, Polychaeta) del piano infralitorale presso Portofino (Genova). Boll. Mus. Ist. Biol. Univ. Genova 1979, 47, 101–115. [Google Scholar]
- Pinn, E.H.; Mitchel, K.; Corkill, J. The assemblages of groynes in relation to substratum age, aspect and microhabitat. Estuar. Coast. Shelf Sci. 2005, 62, 271–282. [Google Scholar] [CrossRef]
- Gacia, E.; Satta, M.P.; Martin, D. Low crested coastal defence structures on the Catalan coast of the Mediterranean Sea: How they compare with natural rocky shores. Sci. Mar. 2007, 71, 259–267. [Google Scholar] [CrossRef]
- Sempere-Valverde, J.; Chebaane, S.; Bernal-Ibáñez, A.; Silva, R.; Cacabelos, E.; Ramalhosa, P.; Jiménez, J.; Gama Monteiro, J.; Espinosa, F.; Navarro-Barranco, C.; et al. Surface integrity could limit the potential of concrete as a bio-enhanced material in the marine environment. Mar. Pollut. Bull. 2024, 200, 116096. [Google Scholar] [CrossRef]
- Firth, L.B.; Knights, A.M.; Bridger, D.; Evans, A.J.; Mieszkowska, N.; Moore, P.J.; O’Connor, N.E.; Sheehan, E.V.; Thompson, R.C.; Hawkins, S.J. Ocean sprawl: Challenges and opportunities for biodiversity management in a changing world. Oceanogr. Mar. Biol. Annu. Rev. 2016, 54, 193–269. [Google Scholar]
- Rallis, I.; Chatzigeorgiou, G.; Florido, M.; Sedano, F.; Procopiou, A.; Chertz-Bynichaki, M.; Vernadou, E.; Plaiti, W.; Koulouri, P.; Dounas, C.; et al. Early succession patterns of benthic assemblages on artificial reefs in the oligotrophic eastern Mediterranean Basin. J. Mar. Sci. Eng. 2022, 10, 620. [Google Scholar] [CrossRef]
- Bavestrello, G.; Bianchi, C.N.; Calcinai, B.; Cattaneo-Vietti, R.; Cerrano, C.; Morri, C.; Puce, S.; Sarà, M. Bio-mineralogy as a structuring factor for marine epibenthic communities. Mar. Ecol. Progr. Ser. 2000, 193, 241–249. [Google Scholar] [CrossRef]
- Green, D.S.; Chapman, M.G.; Blockley, D.J. Ecological consequences of the type of rock used in the construction of artificial boulder-fields. Ecol. Eng. 2012, 46, 1–10. [Google Scholar] [CrossRef]
- Corsi, B.; Elter, F.M.; Giammarino, S. Structural fabric of the Antola Unit (Riviera di Levante, Italy) and implications for its alpine versus Apennine origin. Ofioliti 2001, 26, 1–8. [Google Scholar]
- Cortesogno, L.; Palenzona, A. Le Nostre Rocce. Le Rocce della Liguria: Riconoscerle e Capirne la Storia; Sagep: Genoa, Italy, 1986; pp. 1–176. [Google Scholar]
- Bulleri, F.; Benedetti-Cecchi, L.; Acunto, S.; Cinelli, F.; Hawkins, S.J. The influence of canopy algae on vertical patterns of distribution of low-shore assemblages on rocky coasts in the northwest Mediterranean. J. Exp. Mar. Biol. Ecol. 2002, 267, 89–106. [Google Scholar] [CrossRef]
- Asnaghi, V.; Chiantore, M.; Bertolotto, R.M.; Parravicini, V.; Cattaneo-Vietti, R.; Gaino, F.; Moretto, P.; Privitera, D.; Mangialajo, L. Implementation of the European Water Framework Directive: Natural variability associated with the CARLIT method on the rocky shores of the Ligurian Sea (Italy). Mar. Ecol. 2009, 30, 505–513. [Google Scholar] [CrossRef]
- Blanfuné, A.; Boudouresque, C.F.; Verlaque, M.; Thibaut, T. The ups and downs of a canopy-forming seaweed over a span of more than one century. Sci. Rep. 2019, 9, 5250. [Google Scholar] [CrossRef]
- Mangialajo, L.; Chiantore, M.; Cattaneo-Vietti, R. Loss of fucoid algae along a gradient of urbanisation, and structure of benthic assemblages. Mar. Ecol. Prog. Series 2008, 358, 63–74. [Google Scholar] [CrossRef]
- Goodsell, P.J.; Chapman, M.G.; Underwood, A.J. Differences between biota in anthropogenically fragmented habitats and in naturally patchy habitats. Mar. Ecol. Prog. Ser. 2007, 351, 15–23. [Google Scholar] [CrossRef]
- Sanabria-Fernandez, J.A.; Lazzari, N.; Riera, R.; Becerro, M.A. Building up marine biodiversity loss: Artificial substrates hold lower number and abundance of low occupancy benthic and sessile species. Mar. Environ. Res. 2018, 140, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Tyrrell, M.C.; Byers, J.E. Do artificial substrates favor nonindigenous fouling species over native species? J. Exp. Mar. Biol. Ecol. 2007, 342, 54–60. [Google Scholar] [CrossRef]
- Sheehy, D.J.; Vik, S.F. The role of constructed reefs in non-indigenous species introductions and range expansions. Ecol. Eng. 2010, 36, 1–11. [Google Scholar] [CrossRef]
- Siguan, M.A.R. Review of non-native marine plants in the Mediterranean Sea. In Invasive Aquatic Species of Europe. Distribution, Impacts and Management; Leppäkoski, E., Gollasch, S., Olenin, S., Eds.; Kluwer: Dordrecht, The Netherlands, 2002; pp. 291–310. [Google Scholar] [CrossRef]
- Mancini, I.; Bianchi, C.N.; Morri, C.; Azzola, A.; Oprandi, A.; Robello, C.; Montefalcone, M. A marine invasion story: Caulerpa cylindracea (Chlorophyta, Ulvophyceae) in the marine protected area of Portofino (Ligurian Sea). Biol. Mar. Medit. 2024, in press.
- Piazzi, L.; Ceccherelli, G.; Balata, D.; Cinelli, F. Early patterns of Caulerpa racemosa recovery in the Mediterranean Sea: The influence of algal turfs. J. Mar. Bio. Assoc. UK 2003, 83, 27–29. [Google Scholar] [CrossRef]
- Bulleri, F.; Airoldi, L. Artificial marine structures facilitate the spread of a non-indigenous green alga, Codium fragile ssp. tomentosoides, in the north Adriatic Sea. J. Appl. Ecol. 2005, 42, 1063–1072. [Google Scholar] [CrossRef]
- Dong, Y.; Huang, X.; Wang, W.; Li, Y.; Wang, J. The marine ‘great wall’ of China: Local- and broad-scale ecological impacts of coastal infrastructure on intertidal macrobenthic communities. Divers. Distrib. 2016, 22, 731–744. [Google Scholar] [CrossRef]
- Bonnici, L.; Borg, J.A.; Evans, J.; Lanfranco, S.; Schembri, P.J. Of rocks and hard places: Comparing biotic assemblages on concrete jetties versus natural rock along a microtidal Mediterranean shore. J. Coast. Res. 2018, 34, 1136–1148. [Google Scholar] [CrossRef]
- Bae, S.; Ubagan, M.D.; Shin, S.; Kim, D.G. Comparison of recruitment patterns of sessile marine invertebrates according to substrate characteristics. Int. J. Environ. Res. Public Health 2022, 19, 1083. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Caballero, C.A.; Borges-Souza, J.M.; Chavez-Hidalgo, A.; Abelson, A. Assessing benthic reef assemblages: A comparison between no-take artificial reefs and partially protected natural reefs. Estuar. Coast. Shelf Sci. 2023, 287, 108347. [Google Scholar] [CrossRef]
- Airoldi, L.; Beck, M.W.; Firth, L.B.; Bugnot, A.B.; Steinberg, P.D.; Dafforn, K.A. Emerging solutions to return nature to the urban ocean. Ann. Rev. Mar. Sci. 2021, 13, 445–477. [Google Scholar] [CrossRef]

| Ochrophyta |
| Cystoseira compressa (Esper) Gerloff and Nizamuddin, 1975 |
| Dictyota dichotoma (Hudson) J.V.Lamouroux, 1809 |
| Halopteris scoparia (Linnaeus) Sauvageau, 1904 |
| Padina pavonica (Linnaeus) Thivy, 1960 |
| Rhodophyta |
| Amphiroa rigida J.V.Lamouroux, 1816 |
| Ellisolandia elongata (J.Ellis and Solander) K.R.Hind and G.W.Saunders, 2013 |
| Asparagopsis armata Harvey, 1855 (Falkenbergia rufolanosa stadium) |
| Jania rubens (Linnaeus) J.V.Lamouroux, 1816 |
| Lithophyllum incrustans Philippi, 1837 |
| Mesophyllum lichenoides (J.Ellis) Me.Lemoine, 1928 |
| Peyssonnelia squamaria (S.G.Gmelin) Decaisne ex J.Agardh, 1842 |
| Sphaerococcus coronopifolius Stackhouse, 1797 |
| Turf |
| Filamentous algae indet. |
| Porifera |
| Chondrosia reniformis Nardo, 1847 |
| Cliona celata Grant, 1826 |
| Crambe crambe (Schmidt, 1862) |
| Ircinia oros (Schmidt, 1864) |
| Ircinia variabilis (Schmidt, 1862) |
| Cnidaria |
| Aiptasia mutabilis (Gravenhorst, 1831) |
| Hydrozoa indet. |
| Annelida |
| Protula tubularia (Montagu, 1803) |
| Serpulidae indet. |
| Source | SS | Df | R2 | F | P |
|---|---|---|---|---|---|
| Reef type | 10.8 | 1 | 10.8 | 4 | 0.05927 ns |
| Locale | 48.5333 | 4 | 12.1333 | 4.494 | 0.009412 ** |
| Interaction | 32.5333 | 4 | 8.13333 | 3.012 | 0.04263 * |
| Within | 54 | 20 | 2.7 | ||
| Total | 145.867 | 29 |
| Source | SS | Df | R2 | F | P |
|---|---|---|---|---|---|
| Reef type | 0.734667 | 1 | 0.73467 | 8.7115 | 0.0001 *** |
| Locale | 0.64 | 4 | 0.16 | 1.8972 | 0.0312 * |
| Interaction | 0.692 | 4 | 0.173 | 2.0514 | 0.0199 * |
| Residual | 1.68667 | 20 | 0.084333 | ||
| Total | 3.7533 | 29 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mancini, I.; Azzola, A.; Bianchi, C.N.; Capello, M.; Cutroneo, L.; Morri, C.; Oprandi, A.; Montefalcone, M. Habitat Fragmentation Enhances the Difference between Natural and Artificial Reefs in an Urban Marine Coastal Tract. Diversity 2024, 16, 316. https://doi.org/10.3390/d16060316
Mancini I, Azzola A, Bianchi CN, Capello M, Cutroneo L, Morri C, Oprandi A, Montefalcone M. Habitat Fragmentation Enhances the Difference between Natural and Artificial Reefs in an Urban Marine Coastal Tract. Diversity. 2024; 16(6):316. https://doi.org/10.3390/d16060316
Chicago/Turabian StyleMancini, Ilaria, Annalisa Azzola, Carlo Nike Bianchi, Marco Capello, Laura Cutroneo, Carla Morri, Alice Oprandi, and Monica Montefalcone. 2024. "Habitat Fragmentation Enhances the Difference between Natural and Artificial Reefs in an Urban Marine Coastal Tract" Diversity 16, no. 6: 316. https://doi.org/10.3390/d16060316
APA StyleMancini, I., Azzola, A., Bianchi, C. N., Capello, M., Cutroneo, L., Morri, C., Oprandi, A., & Montefalcone, M. (2024). Habitat Fragmentation Enhances the Difference between Natural and Artificial Reefs in an Urban Marine Coastal Tract. Diversity, 16(6), 316. https://doi.org/10.3390/d16060316









