Abstract
The Kasatura Bay region is one of Türkiye’s Important Plant Areas and plant biodiversity hotspots. In this study, the diversity and gradient of the sand-dune vegetation in Kasatura Bay were studied. Vegetation sampling was carried out by using the Braun-Blanquet method. The vegetation dataset was recorded in the TURBOVEG database management program. For classification, Beta-Flexible clustering (β = −0.25) and the correlation similarity index were used in the PC-ORD program. Diagnostic species of the communities were determined in the JUICE program using the φ-coefficient (higher than 0.30). Detrended Canonical Correspondence analysis was applied to data in the CANOCO program to understand the effect of ecological factors on vegetation diversity. Ellenberg ecological indicator values were used as the ecological variables. As a result, seven different plant communities were identified at the Kasatura Bay sand dunes. A new association of Sileno thymifoliae–Cionuretum erectae ass. nova was identified under the alliance Sileno thymifoliae–Jurineion kilaeae. The sand-dune vegetation represents high diversity, also including endemic plants, some of which are globally threatened. Due to all this diversity, settled on sensitive conditions, conservation strategies need to be developed to protect and ensure the continuity of Kasatura Bay sand-dune vegetation in the face of intense human pressure.
Keywords:
classification; Kasatura; nature conservation; ordination; phytosociology; sand dune; vegetation 1. Introduction
Sand-dune ecosystems are important components of habitat diversity, with unique ecological conditions. Although they are distributed in a very narrow area in the world, they form a transition between marine and terrestrial ecosystems and provide many ecosystem services [1]: they protect the inner regions against storms and strong waves, have unique flora and fauna, provide an economic contribution by reducing the damages to the infrastructures from strong storms, and offer recreational opportunities [2].
Despite the importance of dune ecosystems in terms of both nature protection and ecosystem services, their distribution areas are shrinking day by day. Tourism activities, urbanization, and highway construction are the main reasons for this degradation. As a result of these effects, dune ecosystems in Europe have largely disappeared [3]. There are sand-dune ecosystems covering very large areas in Türkiye consisting of two peninsulas: Thrace and Anatolia. However, most of these ecosystems have been destroyed, mainly due to tourism and urbanization on the Mediterranean coast and highway construction and urbanization on the Black Sea coast.
Ecologists draw attention to this decline and state that importance should be given to the protection of dune ecosystems due to their biodiversity and that damaged areas should be rehabilitated [4,5,6,7]. Understanding the structure of dune ecosystems is important for rehabilitating them. At this point, it is particularly evident that we do not sufficiently know about the natural vegetation structures of the dunes.
Kasatura Bay is located in the southeastern part of Europe on the Black Sea coast and situated in the north of European Türkiye. The dune ecosystem in Kasatura Bay has a special place with its biodiversity and relatively preserved structure [8]. The Kasatura Bay region, one of Türkiye’s Important Plant Areas (IPAs) [9], is considered one of the hotspots in Türkiye due to its rich plant diversity [10]. Although some flora studies and vegetation samplings have been carried out in the area in the past, a comprehensive plant community analysis has not been conducted in the area. This study is important in terms of both understanding the diversity of the area and contributing to the Black Sea dune vegetation, most of which has disappeared.
Cynanchica littoralis, Centaurea kilaea, Isatis arenaria, Linum tauricum subsp. bosphori, Erysimum sorgerae, and Silene sangaria have been determined as endemic taxa in Kasatura sand dunes [11]. However, it has been determined that Silene sangaria is synonymous with the Silene thymifolia species [12], which are known to be widespread in Türkiye [13]. Additionally, many rare species like Jurinea kilaea, Lepidotrichum uechtritzianum, and Matthiola fruticulosa are distributed in the sand dunes. Gèhu and Uslu [14], in their large-scale phytosociological assessment of the halophilous and psammaphilous vegetation of the western Black Sea, Marmara Sea, and northern Aegean coasts of Türkiye, included some vegetation sampling from Kasatura Bay (only 8 relevés). Based on this limited sampling, they described some communities from the area. For a better understanding of the vegetation diversity of the sand dunes, there was still a need for detailed research. In this context, we aimed to determine the plant communities of the Kasatura Bay sand-dune vegetation, make a syntaxonomical scheme of the communities, and understand the main environmental variables causing the floristic differentiation of communities.
2. Materials and Methods
2.1. Study Site
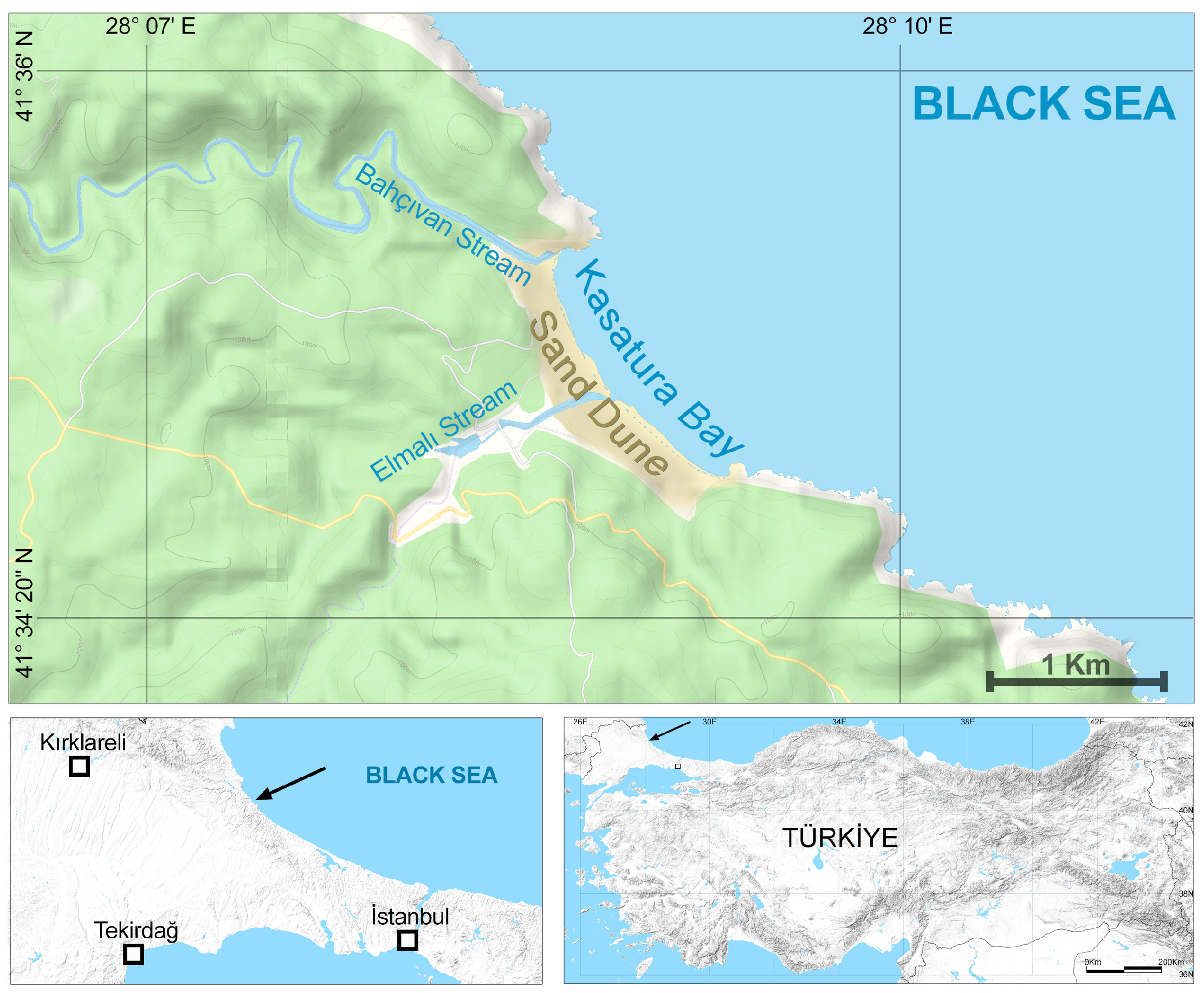
The study was conducted in Kasatura Bay. It is located in the northwestern part of Türkiye, at the northwest end of Istanbul, at the conjunction of the cities of Tekirdağ, Kırklareli, and Istanbul (Figure 1). Kasatura Bay covers a wide sand-dune area with a length of 1800 m along the coast (Figure 2). One hundred meters of the sand dunes are located in Kırklareli, eight hundred meters are in Tekirdağ, and nine hundred meters are in Istanbul. There are limestone rocks at both ends of the dunes. The Bahçıvan stream, to the north of the dunes, borders the province of Kırklareli–Tekirdağ; the Elmalı stream, which flows into the sea from the middle of the dunes, forms the provincial border of Tekirdağ–Istanbul.
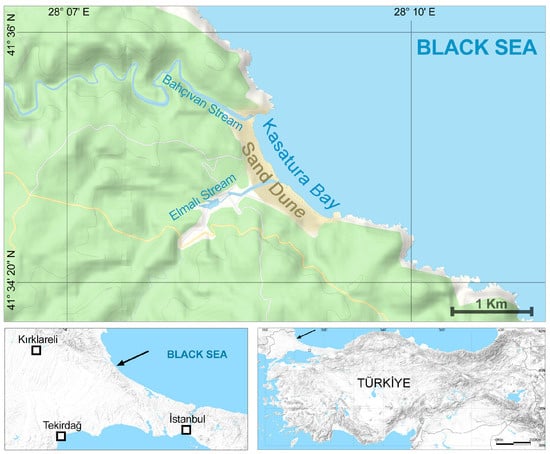
Figure 1.
The geographical location of Kasatura Bay. Arrows show the location of the study site.

Figure 2.
General view of the study site.
The Bahçıvan stream is in the north, while the Elmalı stream in the middle reaches the sea and divides the Kasatura sand-dunes into three parts. Sultanbahçe Dam and Elmalı Stream Dam are located on these streams, and their waters are used for the water needs of Istanbul. The surrounding area of the bay is formed by floodplain forests of Fraxinus angustifolia and Alnus glutinosa, deciduous forests of oak (Quercus petraea, Q. frainetto, Q. cerris), Carpinus betulus and C. orientalis, and relict Pinus nigra forests.
According to the Thornthwaite [15] classification system based on the Istanbul Kumköy Meteorology Station data, the research area has a semi-humid, mesothermal, oceanic climate with a moderate water deficit in summer [16]. The average temperature is 13.8 °C, and the amount of annual precipitation is about 800 mm. February is the coldest month and August is the hottest month on the site.
2.2. Vegetation Sampling and Data Analysis
Kasatura sand-dunes are moving sand-dunes, with the feature of being displaced by the wind. Vegetation sampling on the sand-dune area was carried out in the direction from the sea coast to the inner part of the land. Due to the patchy vegetation structure, the study site was divided into subgroups according to plant population structures with stratified random sampling. The sizes of the sampling plots (relevés) were determined separately for each plant population according to the minimal area method [17]. The sampling points were taken from locations on the sand dunes that were homogeneous in size and quality to represent different plant communities, which were observed to be very variable. The total number of vegetation plots was 74.
Vegetation sampling studies on the sand-dune area were carried out between June and July of 2005. In each sampling area, all vascular plants were recorded with their aerial cover in both the shrub and herb layers. Cover-abundance values for all plant taxa were determined according to the Braun-Blanquet scales [18,19].
To identify the plant species, information from several sources [20,21,22,23,24,25,26,27,28,29,30,31,32] and plant samples from the ISTO and ISTE Herbariums were used. In some controversial and necessary cases, experts of the genus were consulted. The plant names were checked according to Güner et al. [33] and Powo [34]. Abbreviations belonging to threat categories of rare and endemic plants [35] are presented in parentheses next to the plants’ names where it is necessary in the paper.
The vegetation plots were recorded in the TURBOVEG database management program [36]. This dataset was transferred into the JUICE program [37] for further analysis. Data classification studies were performed in the PC-ORD 4 program [38]. For classification, Beta-Flexible clustering (β = −0.25) and the correlation similarity index as a resemblance measure were used. Diagnostic species of each vegetation type were defined by calculating the fidelity of each species to each vegetation type [39] using the φ-coefficient as a fidelity measure. Species with a φ-value higher than 0.30 were considered diagnostic species (frequency value at least 50%), and the dominant species (plants with at least a 10% frequency value and at least 10% coverage) representing each plant group were calculated in the JUICE program.
To determine the effect of ecological factors on floristic differentiation, Detrended Canonical Correspondence analysis (DCA), which is an indirect orientation technique, was applied due to the heterogeneous structure of the data. The analysis was carried out using the CANOCO program [40]. Ellenberg ecological indicator values, Raunkiaer’s life forms [41], and the distribution to phytogeographic regions were shown passively on the ordination. The correlation between the DCA vegetation plot scores and explanatory variables was calculated using the non-parametric Kendall coefficient in STATISTICA [42]. Box–whisker diagrams for the Ellenberg ecological values, life forms, phytogeographic regions, vegetative biodiversity, and each plant group in the vegetation layers were presented. These graphs were established using the JUICE-R function in the JUICE program.
3. Results
3.1. Classification
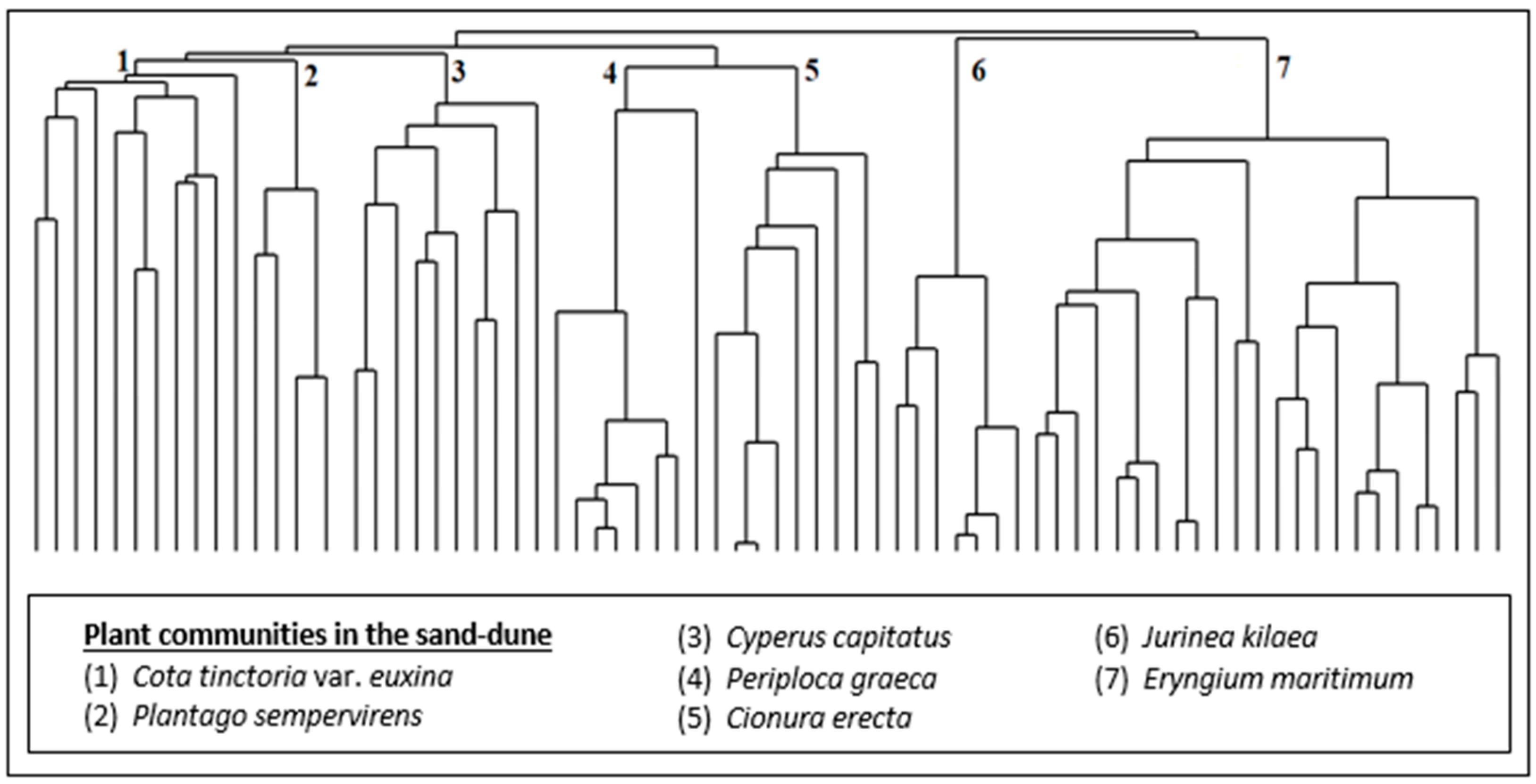
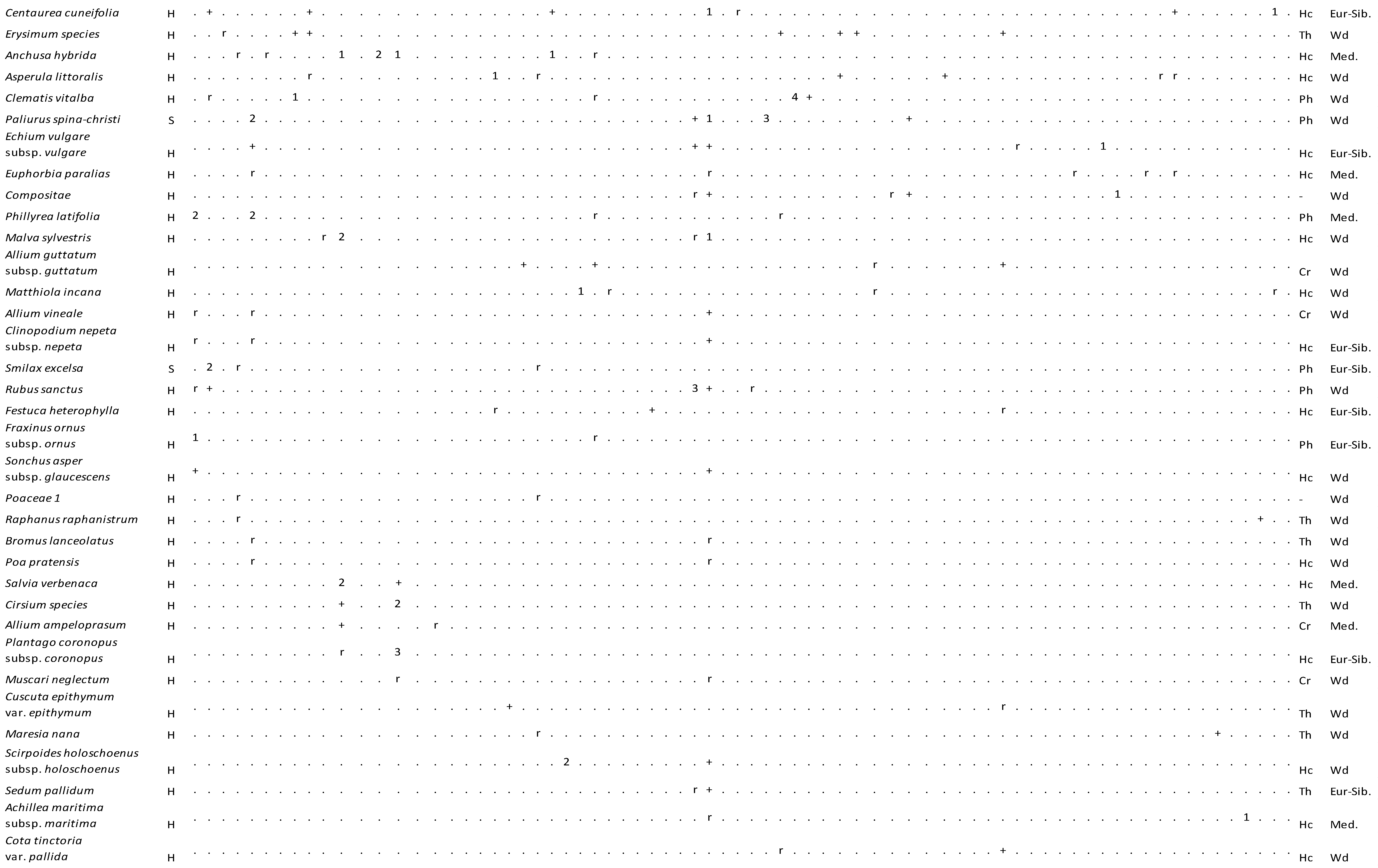
As a result of the numerical classification analysis, seven (7) different plant communities (Figure 3 and Figure 4) were defined in the sand-dune vegetation of Kasatura Bay. The clusters in Figure 3 are characterized by the Cota tinctoria var. euxina, Plantago sempervirens, Cyperus capitatus, Periploca graeca, Cionura erecta, Jurinea kilaea, and Eryngium maritimum communities, respectively. These communities and their diagnostic, constant, and dominant species, along with their cluster numbers (Figure 3), are as follows:
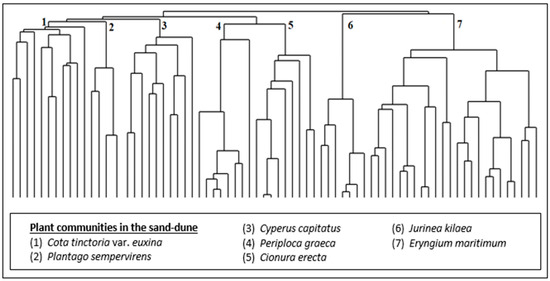
Figure 3.
Hierarchical dendrogram of the vegetation plots in the sand-dune vegetation of Kasatura Bay.
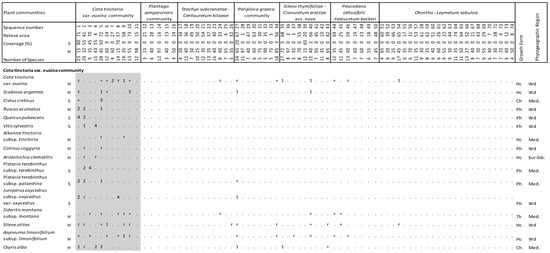
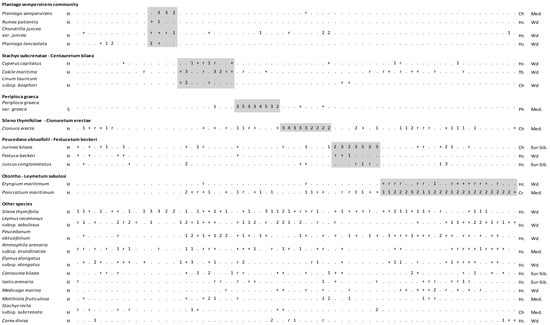
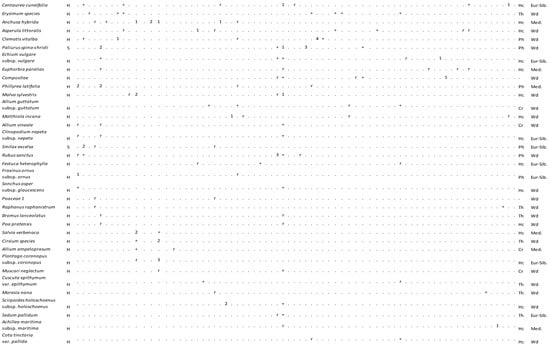

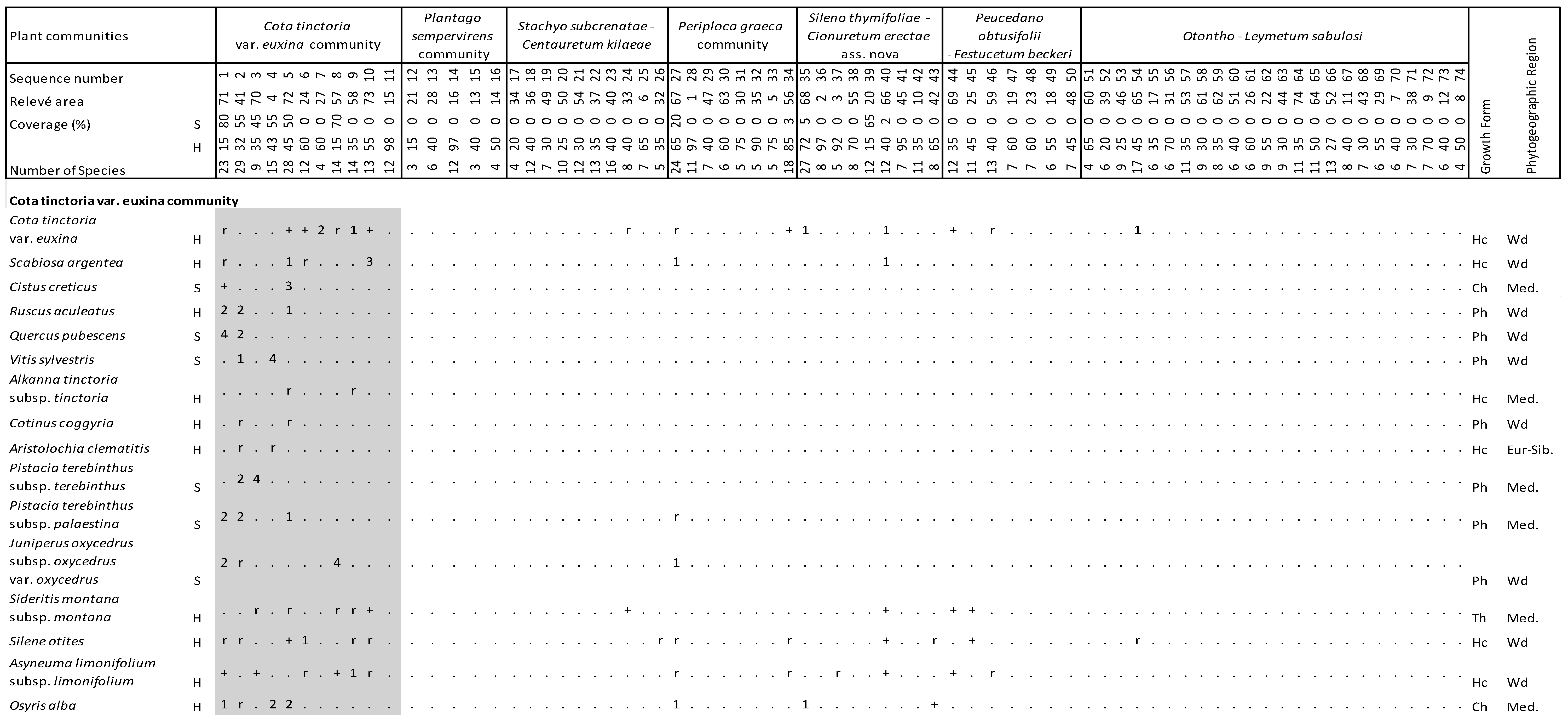
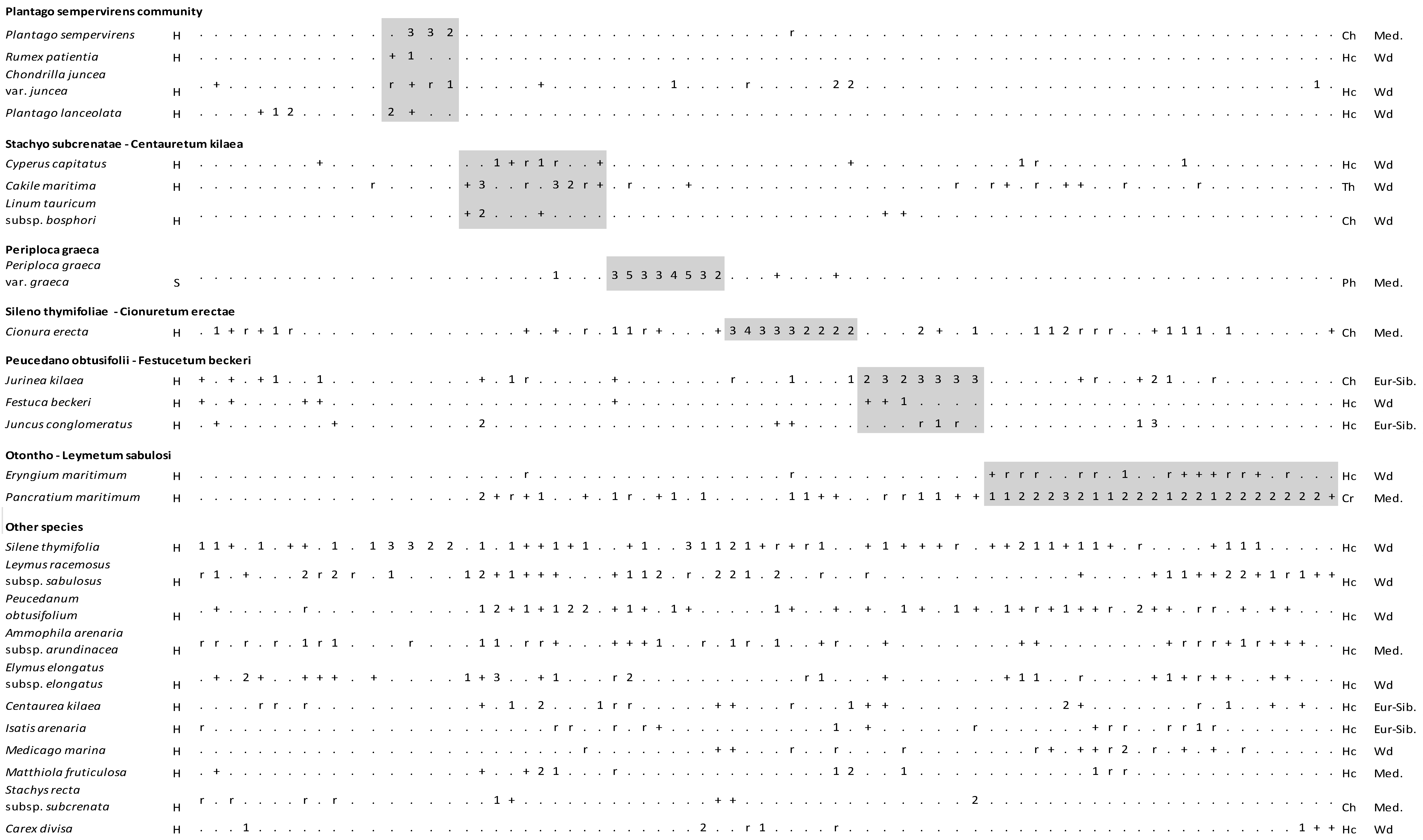
Figure 4.
Vegetation table of Kasatura Bay sand-dunes. Diagnostic species of each community are shown separately with gray shading. They are listed in decreasing order of fidelity. The threshold of φ-coefficient as a fidelity measure is 0.30. Other species are listed in decreasing order of presence in the total dataset. S and H represent shrub and herb layers, respectively. Cover-abundance values (r, +, 1, 2, 3, 4, 5) represent the Braun-Blanquet scales. Growth forms: Ph—Phanerophyte, Ch—Chamaephyte, Hc—Hemicryptophyte, Cr—Cryptophyte, Th—Therophyte. Phytogeographical regions: Med.—Mediterranean, Eur-Sib.—Euro-Siberian, Wd—wide distribution.
3.1.1. Eryngium maritimum Community (Cluster 7)
The diagnostic species of this community are Eryngium maritimum and Pancratium maritimum. The constant species are Leymus racemosus subsp. sabulosus, Peucedanum obtusifolium, and Silene thymifolia, whereas the dominant species is Pancratium maritimum.
The approximately 25 m wide belt on the seaside and the sand-dune area to the south of the Elmalı stream had a lack of vegetation. Just behind this area, hills with a height of 1 to 5 m had formed. An Eryngium maritimum community was growing in front of these sand-dune hills. This community, which was about 5 m wide, continued towards the south, interrupted in places in the Kasatura dunes. Centaurea kilaea and Isatis arenaria are endemic plants, and it was observed that their densities were greater in this community.
3.1.2. Cyperus capitatus Community (Cluster 3)
The diagnostic species of this community are Cakile maritima and Cyperus capitatus. The constant taxa are Leymus racemosus subsp. sabulosus, Pancratium maritimum, Peucedanum obtusifolium, and Silene thymifolia. The dominant taxa are Cakile maritima, Centaurea kilaea, Elymus elongatus subsp. elongatus, Juncus conglomeratus, Leymus racemosus subsp. sabulosus, Linum tauricum subsp. bosphori, Matthiola fruticulosa, Pancratium maritimum, Peucedanum obtusifolium, Scirpoides holoschoenus subsp. holoschoenus, and Teucrium polium subsp. polium. The Cyperus capitatus community was found closer to the sea in the north of the dunes, and it finds cover behind the Cionura erecta community as it stretches to the southeast.
Linum tauricum subsp. bosphori, Centaurea kilaea, and Cynanchica littoralis are endemic plants, and it was observed that their densities were greater in this community. In particular, Linum tauricum subsp. bosphori is located in areas between the streams of Bahçıvan and Elmalı, away from the coast and close to the roadside.
3.1.3. Jurinea kilaea Community (Cluster 6)
This community is also represented by only one diagnostic species, Jurinea kilaea. Pancratium maritimum, Peucedanum obtusifolium, and Silene thymifolia are placed in the floristic composition of the community as constant species. The dominant taxa of the community are Cionura erecta, Jurinea kilaea, and Stachys recta subsp. subcrenata. The Jurinea kilaea community is distributed close to the Cyperus capitatus community, and in the south direction, its distribution moves away from the sea and even extends into the innermost part of the dunes by the Elmalı stream.
3.1.4. Cionura erecta Community (Cluster 5)
Cionura erecta is the only diagnostic species of the community. The constant taxa are Ammophila arenaria subsp. arundinacea and Silene thymifolia, while the dominant taxa are Paliurus spina-christi, Chondrilla juncea var. juncea, Cionura erecta, Clematis vitalba, Leymus racemosus subsp. sabulosus, Matthiola fruticulosa, and Silene thymifolia.
The Cionura erecta community is also located behind the Eryngium maritimum community and together with the Periploca graeca community in the middle belt. This community, located on the sand-dune hills formed on the coast, grew narrowly in these places and widely in locations along the coastline.
3.1.5. Periploca graeca Community (Cluster 4)
The diagnostic species of this community is Periploca graeca var. graeca. The constant taxa are composed of Ammophila arenaria subsp. arundinacea, Cionura erecta, Leymus racemosus subsp. sabulosus, Pancratium maritimum, Peucedanum obtusifolium, and Silene thymifolia. The dominant taxa are Periploca graeca var. graeca, Alnus glutinosa subsp. glutinosa, Carex divisa, Elymus elongatus subsp. elongatus, Leymus racemosus subsp. sabulosus, Rubus sanctus, and Silene thymifolia.
This plant community was located behind the Eryngium maritimum community along the coastline. While it was located closer to the seashore in the north, it moved more and more inland towards the south. In the south, there was a transition zone from a sand-dune area to a forest area near the Elmalı stream.
3.1.6. Plantago sempervirens Community (Cluster 2)
The diagnostic species of this community are Chondrilla juncea var. juncea, Plantago lanceolata, Plantago sempervirens, and Rumex patientia. Silene thymifolia is the only constant species in this community. The dominant taxa are Anchusa hybrida, Cirsium sp., Plantago coronopus subsp. coronopus, Plantago lanceolata, Plantago sempervirens, and Silene thymifolia. This community was located only in the north of the sand-dune area and in the back most flat area, where the dune movement stopped and became stable.
3.1.7. Cota tinctoria var. euxina Community (Cluster 1)
The diagnostic species of this community are Cistus creticus, Pistacia terebinthus subsp. terebinthus, Quercus pubescens, Vitis sylvestris, Alkanna tinctoria subsp. tinctoria, Cota tinctoria var. euxina, Aristolochia clematitis, Cotinus coggygria, Ruscus aculeatus, and Scabiosa argentea. While Ammophila arenaria subsp. arundinacea, Asyneuma limonifolium subsp. limonifolium, Cionura erecta, Elymus elongatus subsp. elongatus, Leymus racemosus subsp. sabulosus, Silene otites, and Silene thymifolia appear as constant species in this community, the dominant taxa are Juniperus oxycedrus subsp. oxycedrus var. oxycedrus, Pistacia terebinthus subsp. palaestina, Pistacia terebinthus subsp. terebinthus, Quercus pubescens, Leymus racemosus subsp. sabulosus, Osyris alba, and Phillyrea latifolia. Allium longispathum shows very limited distribution in Türkiye, and it also has been found in this community, with a small number of individuals only in the north of the sand-dune area.
Although this community is enriched by herb species, scrub species also join the floristic composition, even as dominant species in some locations. In the transition area to the forest, where the dune movement became stable in the northwest of the area, the Cota tinctoria var. euxina community was distributed closer to the Plantago sempervirens community. In the south of the area, behind the Jurinea kilaea and Cyperus capitatus communities, it moved to the innermost part of the dunes on the side of the Elmalı stream.
3.2. Ordination
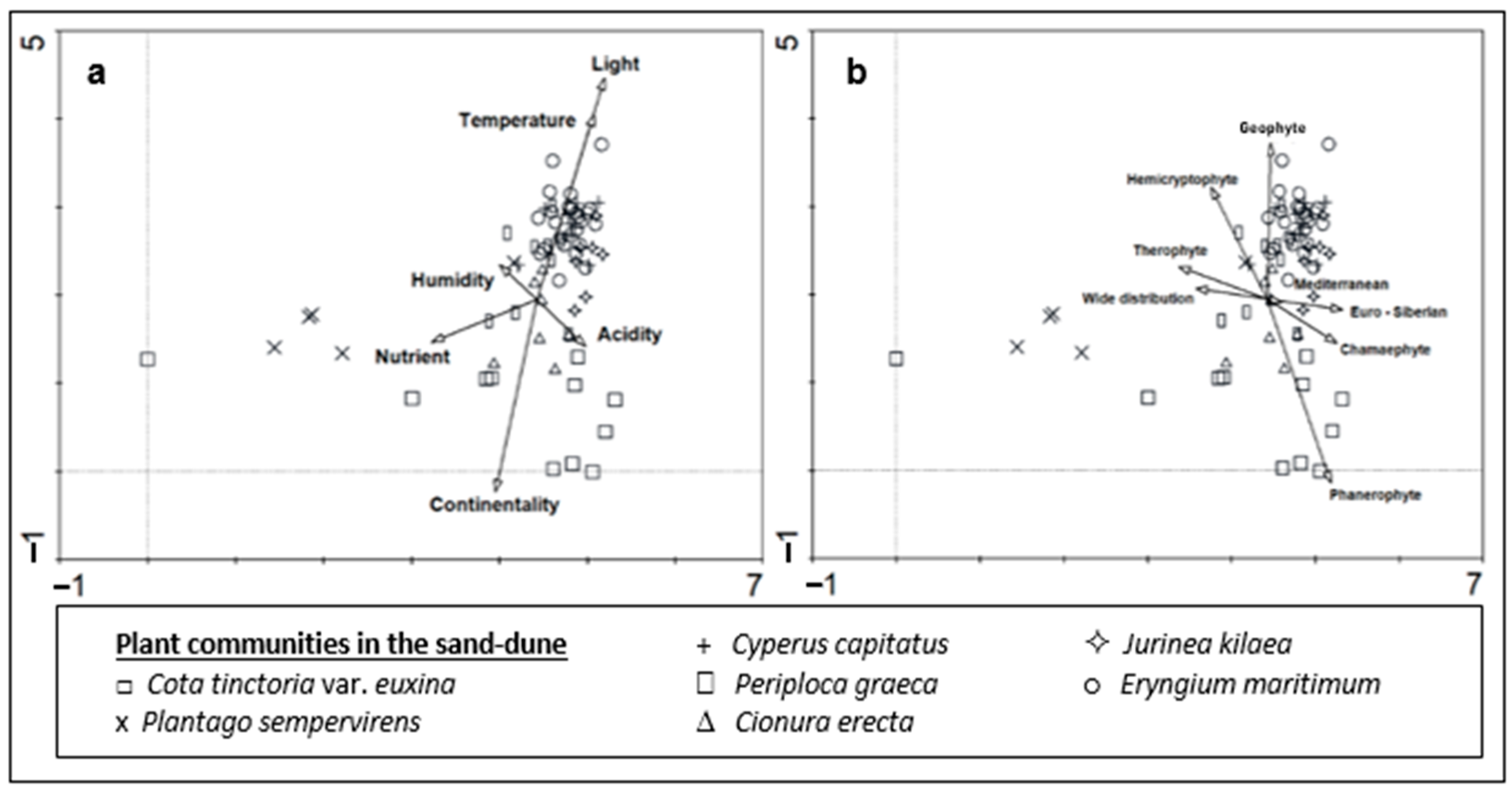
DCA showed that there is a clear ecological difference between the plant communities in the sand-dune vegetation of Kasatura Bay (Figure 5a). Gradients along both axes of the ordination plane were evident. Some ecological variables were also significantly correlated with the DCA values of the vegetation plots (Figure 5a and Table 1), which shows their effects on plant community differentiation in the sand dunes.
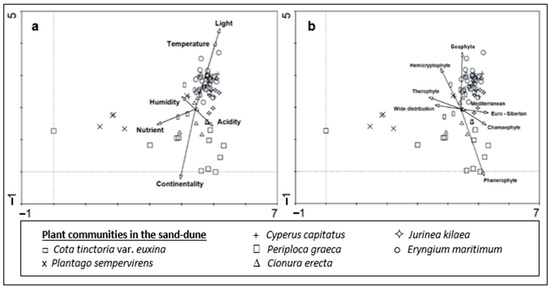
Figure 5.
DCA ordination of the plant groups in the sand-dune vegetation of Kasatura Bay: (a) Ellenberg ecological indicator values, (b) growth forms and phytogeographical regions are passively projected.

Table 1.
Kendall correlation coefficients between DCA ordination values and ecological indicator values, growth forms, and phytogeographical regions and their significance levels.
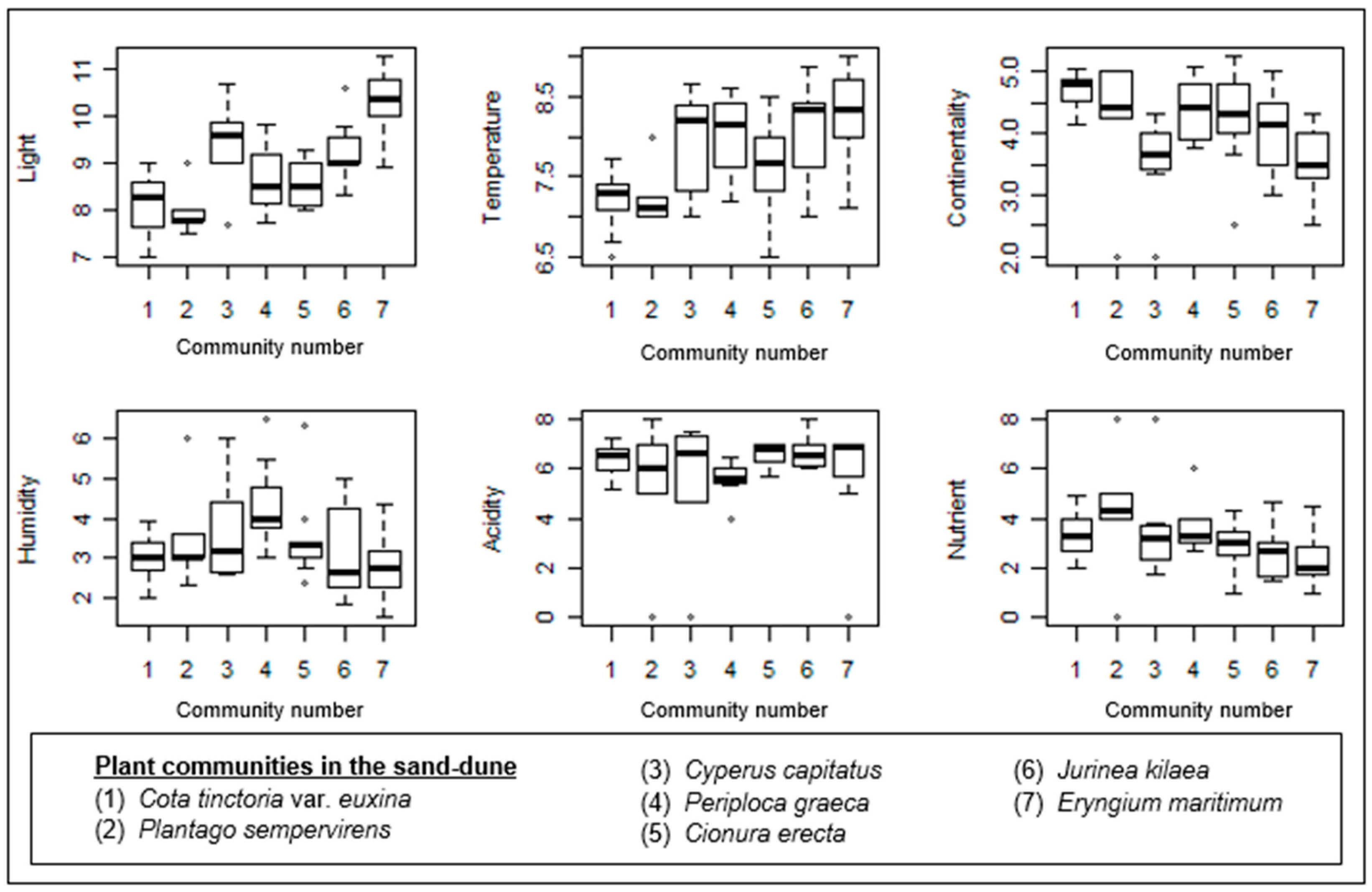
The gradient along Axis 1 mainly results from the Plantago sempervirens-dominated community, which is mostly situated in the left part of the ordination. Similarly, two plots from Cota tinctoria var. euxina dominated the sand dunes accompanied by this community on the left side of the ordination. Nutrients showed a significant correlation with Axis 1 (Table 1). Therefore, the Plantago sempervirens-dominated community represents the most nutrient-rich site in the sand dunes.
The gradient along Axis 2 is very diverse, and the correlations of ecological variables like light, temperature, and continentality along this axis are also very significant (Figure 5a). The lower part of Axis 2 is formed by the Cota tinctoria var. euxina-dominated community. In this direction, continentality also shows a significant increase. This shows the continentality of this community in comparison with the others (Figure 6). Along the upper part of Axis 2, Cionura erecta-, Jurinea kilaea-, Periploca greaca-, Cyperus capitatus-, and Eryngium maritimum-dominated communities appear, respectively. The effects of light and temperature on floristic differentiation were very much evident in this direction of the axis. The Eryngium maritimum- and Jurinea kilaea-dominated communities are characterized by the highest light and temperature. The Periploca greaca-dominated community represents the most moisture-rich sites in the sand dunes. The acidity is also lowest in this community. The Cyperus capitatus- and Cionura erecta-dominated communities are placed in relatively moderate sites in terms of the ecological conditions (Figure 6).
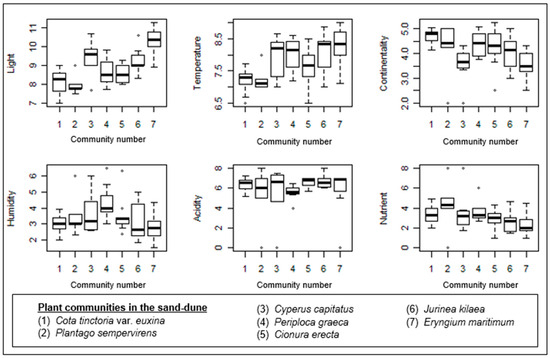
Figure 6.
Box–whisker diagrams of Ellenberg ecological indicator values. -: Median, ▯: 25–75%, I Non-outlier range, ◦: outliers.
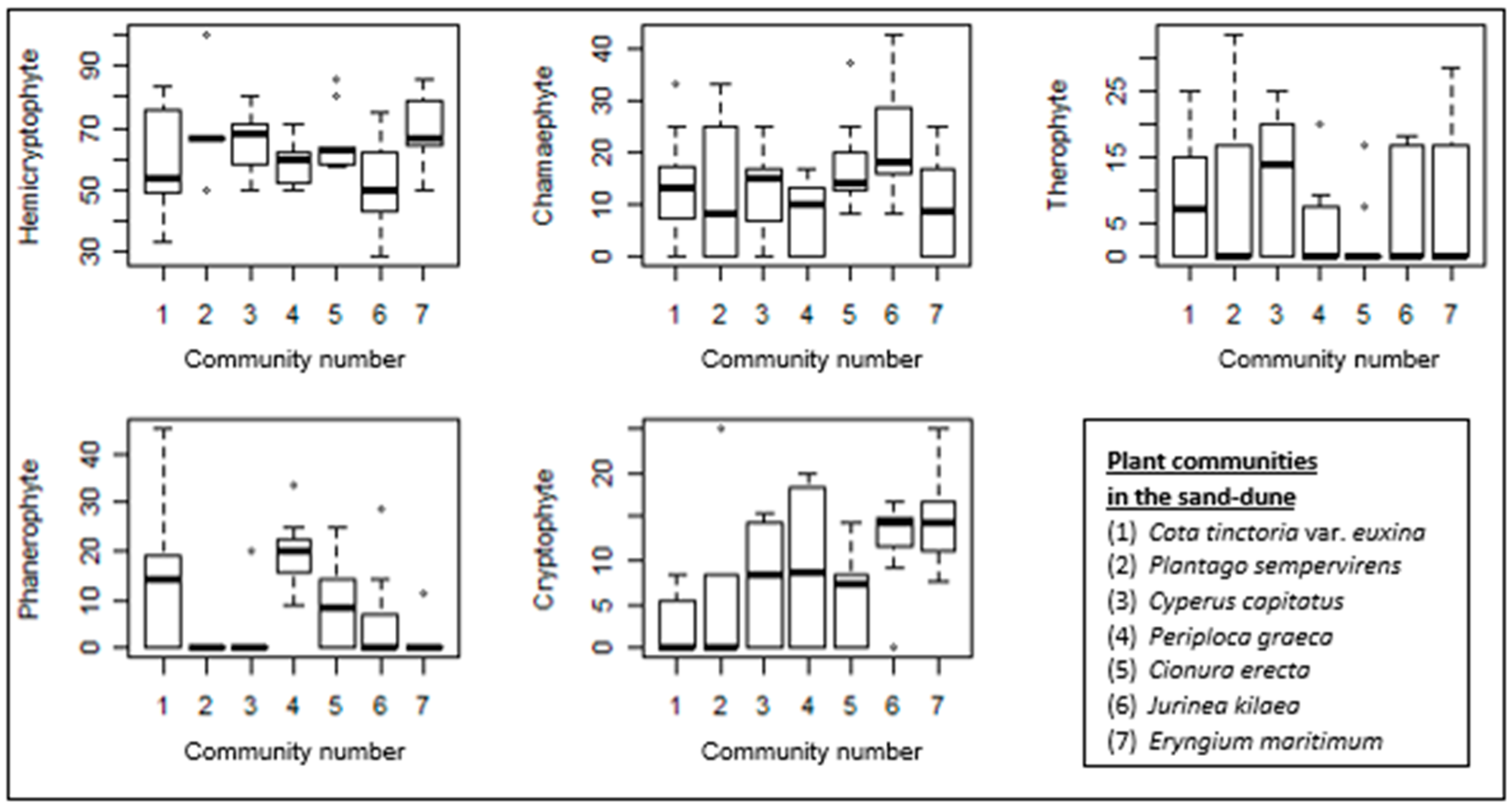
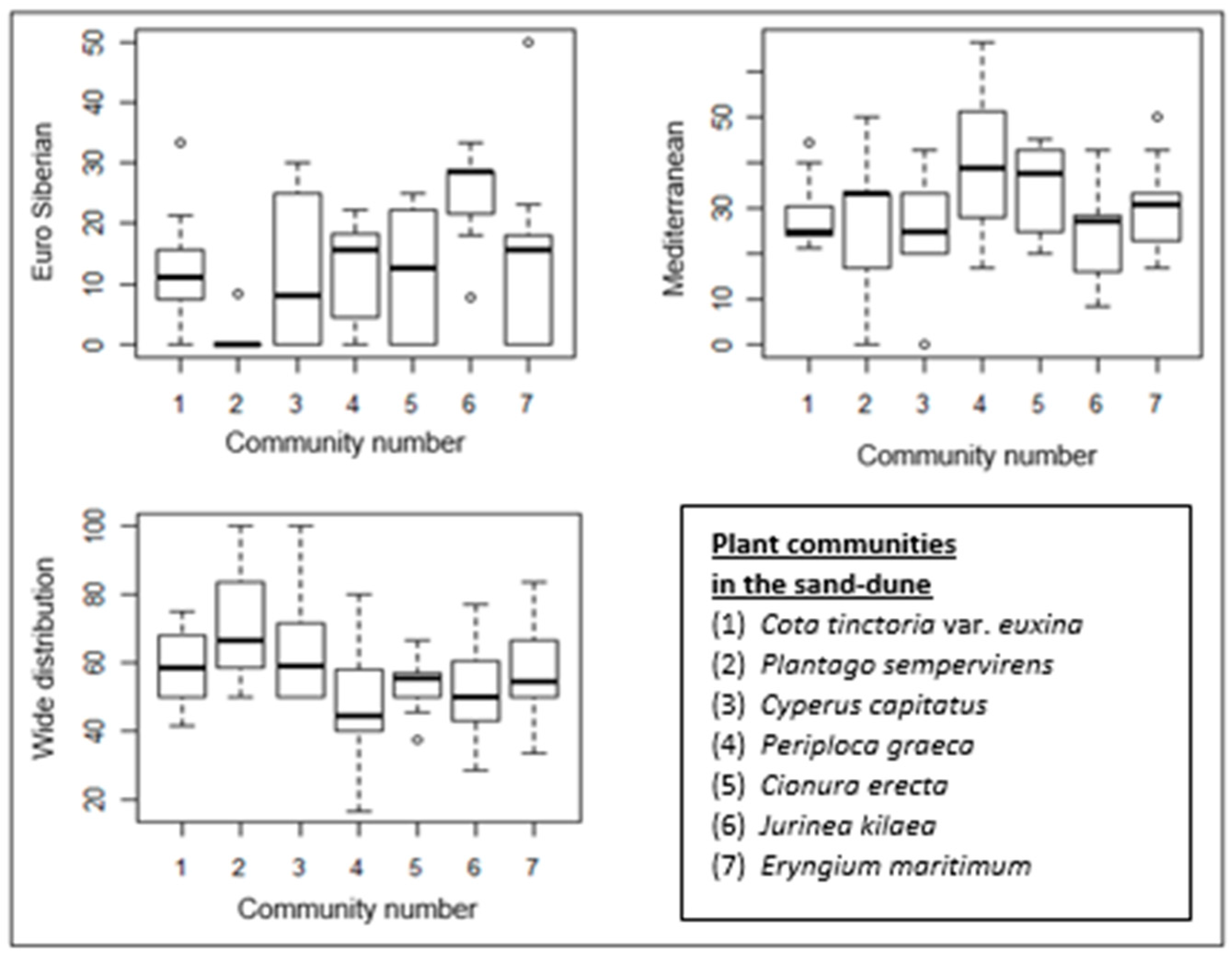
The plant community also shows significant differences in terms of its growth forms and phytogeographical region spectrums (Figure 4, Figure 5b and Figure 7). Phanerophyte and cryptophyte are the growth forms, in which the plant communities show significant differences. In terms of the phytogeographical regions, the plant communities are differentiated only by the proportion of Euro-Siberian plants. The number of plant taxa in the 7 plant communities identified from dune area, the phytogeographic regions they belong to, and the representation of these different flora region elements in numbers and percentages for each community are presented in Table S1.
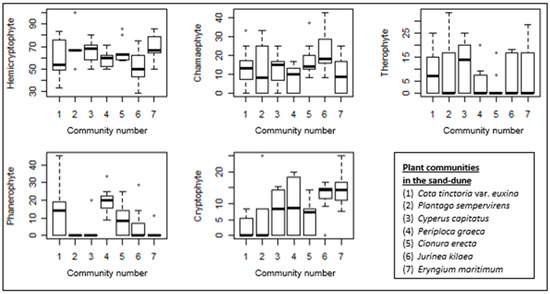
Figure 7.
Box–whisker diagrams of growth forms. -: Median, ▯: 25–75%, I Non-outlier range, ◦: outliers.
The proportion of hemicryptophytes is the highest in all the sand-dune communities. Although sand-dune vegetation is generally formed by low-growing plants, the high proportion of phanerophytes in the Periploca graeca-dominated community is clear. This community is also rich in cryptophytes. The Jurinea kilaea-dominated community is also rich in cryptophytes. A high proportion of chamaephytes is also evident in this community. Despite the generally low proportion of therophytes in these communities, they are well represented in the Cyperus capitatus community and relatively well represented in the Cota tinctoria var. euxina-dominated community.
The proportion of Mediterranean elements is higher in all almost the communities than that of Euro-Siberian plants, except for the Jurinea kilaea-dominated community. The proportion of Mediterranean elements in the Periploca graeca and Cionura erecta communities is higher than in the other communities. Interestingly, Euro-Siberian plants are almost absent in the floristic composition of the Plantago sempervirens-dominated community, which is represented by the highest proportion of wide-distribution plants (Figure 5b and Figure 8).
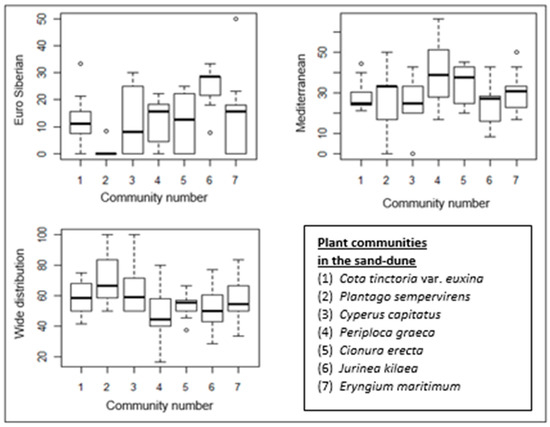
Figure 8.
Box–whisker diagrams of phytogeographical regions. -: Median, ▯: 25–75%, I Non-outlier range, ◦: outliers.
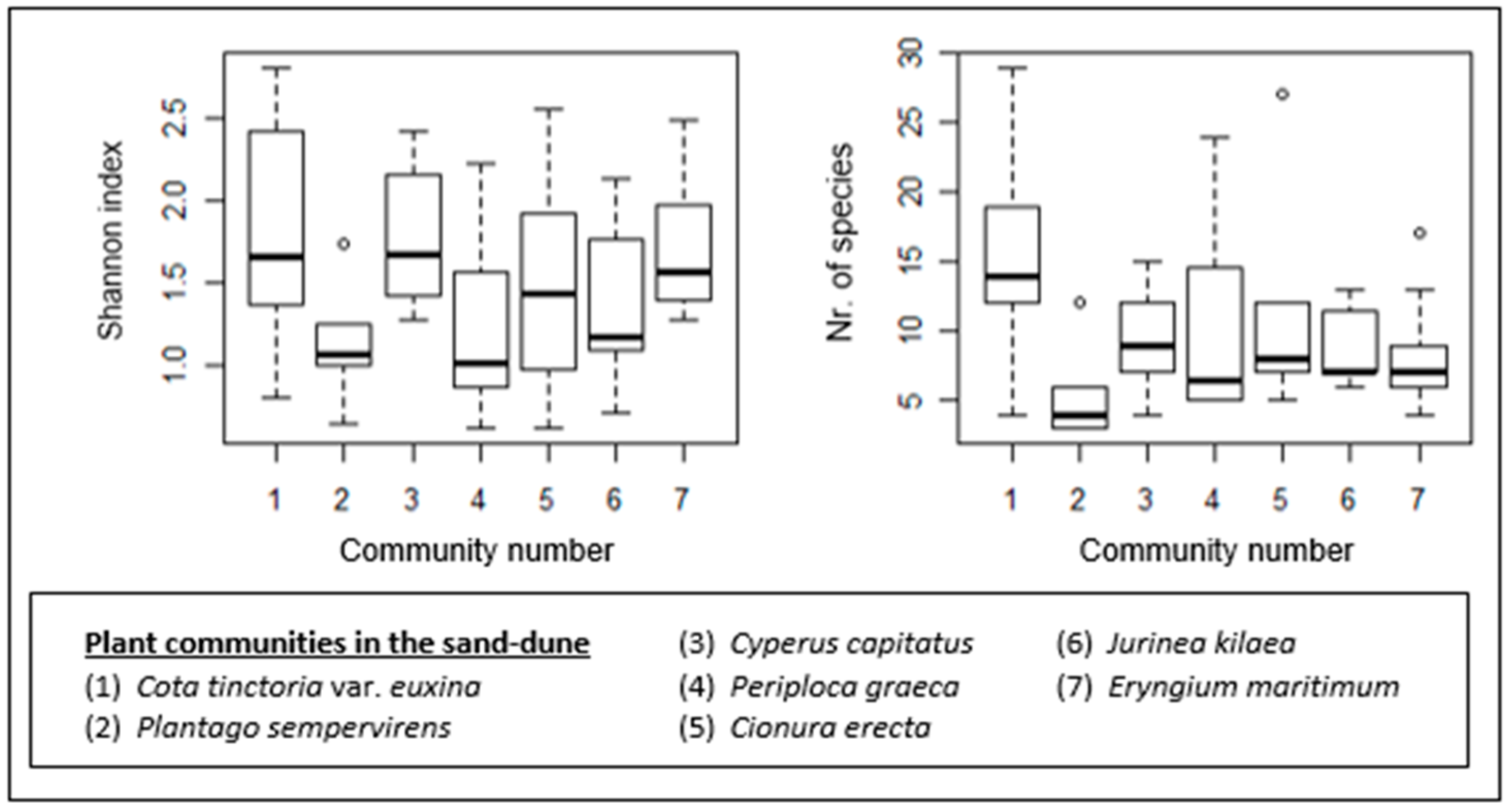
The sand-dune plant communities also show differences in terms of their species richness and diversity (Figure 9). The Cota tinctoria var. euxina (1)-dominated community is the most species-rich. It also shows a higher species diversity, together with the Cyperus capitatus (3)- and Eryngium maritimum (7)-dominated communities. The poorest community in terms of species number is the Plantago sempervirens (2)-dominated community. The species diversity of this community is low as well. Other communities with low species diversity are the Periploca graeca (4)- and Jurinea kilaea (6)-dominated communities.
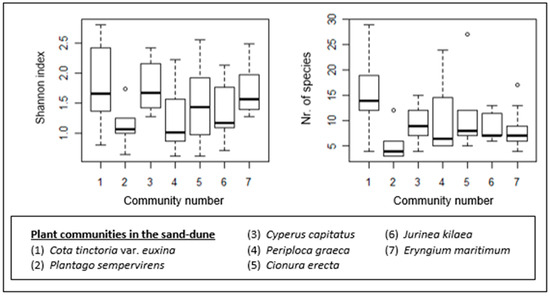
Figure 9.
Box–whisker diagrams for species richness (number of species) and species diversity. - Median, ▯: 25–75%, I Non-outlier range, ◦: outliers.
4. Discussion
4.1. Syntaxonomical Assessment
Gèhu and Uslu [14], in their large-scale phytosociological assessment, classified four different associations in Kasatura Bay’s sand-dune vegetation, despite the limited number of sampling plots used. These associations were Otontho–Leymetum sabulosi (2 relevés), Stachyo subcrenatae–Centauretum kilaeae (1 relevé), Peucedano obtusifolii–Festucetum beckeri (3 relevés), and Marsdenio erectae–Juniperetum macrocarpae (2 relevés). Although the sampling size from Kasatura Bay was narrow in this study, the association identification carried out by Géhu and Uslu [14] is directive of our numerical classification.
Although Turkish Thracian Black Sea coastal vegetation shows floristic similarity to Bulgarian and Greek dune ecosystems [4,43], they have different vegetation composition in terms of their phytosociological structures, which was clearly shown by Géhu and Uslu [14] and Kavgacı [16]. Shifting dunes are characterized by the Otantho–Leymetum sabulosi association on the Turkish Black Sea coast, and the embryonic dune community of Eryngium maritimum can be classified under this association of Elymion gigantei (Ammophiletalia, Ammophiletea).
The Cyperus capitatus and Jurinea kilaea communities are distributed under highly similar ecological conditions in Kasatura Bay. Despite this, due to the floristic differences that appeared as a result of numerical classification, they represent different associations. One of the diagnostic species of the Jurinea kilaea community is Festuca beckeri, which was also mentioned as the diagnostic species of the Peucedano obtusifolii–Festucetum beckeri association by Géhu and Uslu [14] in Kasatura Bay. Another diagnostic species, Linum tauricum subsp. bosphori, which was mentioned by Géhu and Uslu [14] is also placed in the floristic composition of the Jurinea kilaea community. Therefore, it seems suitable to identify this community under this association of the Silene thymifoliae–Jurinion kilaeae alliance, under the order Medicago–Seselietalia tenderiensis and the class Ammophiletea [44].
Another community described by Gèhu and Uslu [14] from Kasatura Bay is Stachyo subcrenatae–Centauretum kilaeae, with only one relevé (relevé 4 in Figure 4). Cyperus capitatus and Cakile maritima, both of which are diagnostic species of the Cyperus capitatus community in our study, appear in the floristic composition of this association. However, the Cyperus capitatus community includes Centaurea kilaea and Stachys recta subsp. subcrenata in its floristic composition, both of which were accepted as the diagnostic species of the association by Gèhu and Uslu [14] as well. Therefore, we decide to classify this community under this association (Stachyo subcrenatae–Centauretum kilaeae) of the alliance Silene thymifoliae–Jurinion kilaeae too.
The fixed part of Kasatura Bay is well represented by the Cionura erecta community. Similar vegetation dominated by Cionura erecta in İğneada province, located in Northwest Thrace in Türkiye, was classified as a Medicago rigidula–Cionura erecta basal community under the alliance Maresion nanae [16]. Although Cionura erecta-dominated communities are indicative of the stabilized coastal grey and hind dunes in Northern Thrace and have a large distributional range, no association has been attributed to this kind of vegetation yet. Therefore, we propose a new association of Silene thymifoliae–Cionuretum erectae ass. nova (Holotypus: Figure 4, relevé number 68) under the alliance Sileno thymifoliae–Jurineion kilaeae, which is the characteristic alliance of dwarf scrub vegetation found in the stabilized hind dunes on the southwestern coast of the Black Sea [14].
The Plantago sempervirens community located at the back of the sand dunes represents a mixture of ruderal species of trampled soils. This community could be characterized by the destruction of the littoral, with a complex mixture of ruderal species like Anchusa hybrida, Chondrilla juncea var. juncea, Plantago sempervirens, P. lanceolata, P. coronopus subsp. coronopus, and Rumex patienta. A similar structure of sand-dune vegetation was also noted in İğneada sand-dune vegetation [16], and this kind of community was referred to as a meadow behind sand dunes. Also, in our case, it seems suitable not to make any syntaxonomic attribution for this community since we believe that a wider syntaxonomic assessment of ruderal communities is required.
Another association described by Gèhu and Uslu [14] in Kasatura Bay is Marsdenio–Juniperetum macrocarpae, with only two relevés. Since this community is narrowly distributed in the bay, we also could not sample it with a sufficient number of relevés to describe it as a different cluster in the analyses. For this reason, the relevés of this association are placed under the cluster of the Cota tinctoria var. euxina community, which is a transitional one, consisting of sand-dune plants and also zonal shrubland and forest vegetation. As seen in the floristic composition of this community, herb-dominated relevés characterizing the sand dunes are grouped with the shrub- and tree-species-dominated ones, representing shrubland and deciduous forests. Due to the transitional character of this group, it is not classified at the association level and is called a community in the syntaxonomic list.
The Periploca graeca community is another transitional community in Kasatura Bay. Periploca graeca is a characteristic species of floodplain forests [45]. Just behind the sand dunes in the study area and along the rivers, floodplain forests dominated by Alnus glutinosa subsp. glutinosa and Fraxinus angustifolia subsp. angustifolia have grown. Therefore, in the floristic composition of the Periploca graeca community, in addition to the characteristic species of sand dunes, species of floodplain forests are seen, like Alnus glutinosa. Owing to this floristic composition and the transitional character of the Periploca graeca community, it was also described at the community level in the syntaxonomic list.
Following the discussion above, a syntaxonomic list of Kasatura Bay’s sand-dune vegetation was formed as follows:
| Ammophiletea Br.-Bl. and Tx. ex Westhoff et al. 1946 |
| Ammophiletalia Br.-Bl. and Tx. ex Westhoff et al. 1946 |
| Elymion gigantei Morariu 1957 |
| Otontho–Leymetum sabulosi Gèhu et Uslu 1989 |
| Medicago–Seselietalia tenderiensis Umanets et V. Solomakha 1999 |
| Sileno thymifoliae–Jurineion kilaeae Gèhu et Uslu ex Mucina 2016 |
| Stachyo subcrenatae–Centauretum kilaeae Gèhu et Uslu 1989 |
| Peucedano obtusifolii–Festucetum beckeri Gèhu et Uslu 1989 |
| Sileno thymifoliae–Cionuretum erectae ass. nova (Holotypus: Figure 4, relevé number 68) |
| Quercetea ilicis Br.-Bl. ex A.Bolòs et O. de Bolòs in A.Bolòs y Vayreda 1950 |
| Pistacio lentisci-Rhamnetalia alaterni Rivas-Mart 1975 |
| Asparago orientalis–Juniperion macrocarpae (Diez Garretas et Asensi 2014) |
| Mucina in Mucina et al. 2016 |
| Marsdenio–Juniperetum macrocarpae Gèhu et Uslu 1989 |
| Cota tinctoria var. euxina community |
| Plantago sempervirens community |
| Periploca graeca community |
4.2. Ecological Interpretation
Whether sand-dunes have fixed or shifting character is decisive on the ecological conditions and therefore their vegetation structure [4,16]. Oberdorfer [46] divided sand-dune ecosystems into three groups: the first was the place closest to the sea with a lack of vegetation due to the impact of waves from the sea, the second was behind the first parts with an unstable morphological structure and with high numbers of graminoids, and the last was the farthest parts from the sea, consisting of dwarf shrubs with stable conditions. Similarly, Tzonev et al. [4] also stated that the dune ecosystems in the Black Sea could be grouped into two main groups: unstable dunes and fixed dunes behind the unstable dunes.
This is also clear for Kasatura Bay’s sand-dune vegetation, and seven different plant communities appear as a result of ecological differences. Not only the morphological structure of the sand dunes but also the zonal vegetation behind the sand-dunes and the two streams reaching the sea that divide the sand-dunes effect the floristic composition of sand-dunes plant communities in the study area. This zonal vegetation is formed of pseudomaquis and deciduous-oak-dominated forests. The Bahçıvan stream in the north of the bay and Elmalı in the south create humid conditions and therefore have formed floodplain forests and swamps.
The Eryngium maritimum community represents the embryonic dunes in Kasatura Bay. This is followed by shifting vegetation, formed by Cyperus capitatus, Jurinea kilaea, and Cionura erecta communities. The back of the sand dunes is composed of Cota tinctoria var. euxina, Plantago sempervirens, and Periploca graeca communities, indicating fixed dune characteristics. The low moisture and poor nutrient content here have determined vegetation structures suitable for areas close to the sea. Through the inland and streams, with an increase in the moisture and nutrient conditions, the vegetation composition changes, with the types of plants indicating these kinds of sites. Therefore, the plant communities in the area are indicators of different site conditions in the sand dunes.
In addition to these floristic differences in the communities, the morphological changes in and the interactions of different ecosystems in the sand dunes also affect the distributional patterns of communities [16]. The vegetation composition and pattern change within a short distance in the sand dunes from the sea coast to the interior land. This is not only a phenomenon observed by the distance to the coast but also along the sea coast, from north to south.
The presence of the Bahçıvan stream in the north and the Elmalı stream in the south has affected the floristic composition in the areas where these streams meet the sea. The location where the sand dunes extend inland from the sea coast is near the Elmalı stream. Especially in this area, there were riparian vegetation plants in groups. In the north, the dunes have become narrower, and the transition to the forest is faster. Contrary to the north, the transition to the forestland is slower in the south after wide dunes. This situation has caused the communities in the north to be closer to each other and even to overlap with each other. In other words, the Eryngium maritimum, Cionura erecta, Periploca graeca, Jurinea kilaea, and Cyperus capitatus communities were very close to each other in the north part of the plant population distribution area. It is easier to see these communities, in order more or less as indicated, as you go south of the area. In the transition area from the dunes to the forestland, there are Plantago sempervirens and Cota tinctoria var. euxina communities in the north, while there is only a Cota tinctoria var. euxina community in the south.
In this study, we saw that the prevailing wind affects the structure of the sand-dune area and the presence of vegetation growing in the area. In particular, the vegetation density in the northern part of the Kasatura dunes and the presence of forest vegetation behind the dunes indicate that the effect of wind on dune movement was relatively low here. The southeastern part of the dune area following the Elmalı stream had a lack of vegetation, as it receives a lot of wind and does not have good growing conditions.
There was a dormant dune on the flat land behind the moving dunes. The floristic composition in this area, which is at the transition to the forest, was under intensive human pressure and on the side of the road. In the plant transition community behind this dune, the number of shrub groups and stream vegetation plants increased further toward the south.
4.3. Nature Conservation
Seven different sand-dune plant communities in Kasatura Bay indicate the plant biodiversity richness of the area and therefore its vulnerability in terms of nature conservation. Not only the plant community differentiation but also the endemic and rare species occurring in the sand dunes increase the importance of the bay. There are five endemic taxa in Kasatura Bay’s sand-dune vegetation, and four of them (Cynanchica littoralis (Sm.) P.Caputo and Del Guacchio (Syn. Asperula littoralis) (VU) [47], Centaurea kilaea (EN), Isatis arenaria (EN), and Linum tauricum subsp. bosphori (CR)), are globally endangered [11]. It was estimated that one of the three largest known colonies of Isatis arenaria and Linum tauricum subsp. bosphori from these plants was located in this dune [48]. The latter endemic species, Erysimum sorgerae, grew in the dune–forest transition, with a small number of individuals. Linum tauricum subsp. bosphori was concentrated in groups only in the transition from the dune area to the forest. Peucedanum obtusifolium (VU) was very common in the dune area and mostly occurred together with Pancratium maritimum.
In addition to these plants, the sand dunes of Kasatura were the habitat of many rare plants, such as Jurinea kilaea, Lepidotrichum uechtritzianum (Syn. Aurinia uechtritziana), and Matthiola fruticulosa. Lepidotrichum uechtritzianum (VU) was a rare species in the transition from the dunes to the forest. It was observed that Jurinea kilaea also showed distribution not only within its community but also close to the transitional community zone behind the sand dunes away from the sea. Matthiola fruticulosa was observed in the inner parts of the dunes away from the sea and in small numbers. Additionally, Allium longispathum (Syn. A.dentiferum), which is widespread in the Mediterranean basin, has a very limited distribution in Türkiye, and its distribution in Kasatura Bay was newly recorded [49], so it has been suggested to assign it to the Endangered (EN) category since its population size in the area is too small. Allium longispathum has a limited distribution north of the sand-dune area within the Cota tinctoria var. euxina community.
Erysimum sorgerae is on the rare plants list in Türkiye [50]. The other endemic plants in danger at a global scale are [50] Cynanchica littoralis, Vulnerable (VU); Centaurea kilaea, and Isatis arenaria, Endangered (EN); and Linum tauricum subsp. bosphori, Critically Endangered (CR), according to the threatened plant categories [35].
As seen in Figure 4, 108 plant taxa were identified in the vegetation sampling plots. Among these, 24 out of 108 taxa were from the Mediterranean flora region, and 19 were members of the European–Siberian flora region. Centaurea kilaea, Isatis arenaria, and Erysimum sorgerae of these endemic plants are members of the European–Siberian flora region. The results showed that about 20% of the total taxa identified in the vegetation sampling belong to the Mediterranean flora region. That means the area is under the influence of a Mediterranean climate even though the area is located on the Black Sea coast. These climatical conditions have created rich plant diversity in the area.
The Kasatura Bay sand-dune area is intensively used by people for recreation activities, especially for camping in the summer, and the effect of this intensive use has resulted in degradation, as seen in the noticeable presence of a trampled soil surface. The part used as a camping area is the forest area behind the sand-dunes to the south of the Bahçıvan stream. Human pressure is seen in the dormant flat area on the roadside behind the moving front sand-dunes and in the form of trampling effects on the plants on the side of the road leading to the sea in the north. Also, people in the camping area collect sand lily flowers for ornamental purposes. Therefore, in the management plans of the region, the nature conservation value of the bay has to be taken into account, and it is of great importance to implement measures to limit recreation activities and human access to the sand-dune area.
5. Conclusions
Kasatura Bay’s sand-dune vegetation represents a unique floristic and ecological structure within the scale of the entire Black Sea coast. Not only the plant communities with high diversity but also the presence of many globally endangered species in a narrow sand-dune area make the site important and sensitive in terms of its management. The association Sileno thymifoliae–Cionuretum erectae ass. nova, which was first described in the area, increases its vulnerability. The region is normally under the effect of a temperate climate. But one-fifth of the plant taxa in the sand-dune area are members of the Mediterranean flora region. This can be connected with the effects of the Mediterranean climate along the Black Sea coast. However, on a larger scale, this may also be evaluated in terms of the future distributional patterns of species and communities in the context of climate change.
The nomenclature type of the association Sileno thymifoliae-Cionuretum erectae ass. nov. holotypus:
Authors, D.Oral, A.Kavgacı, A.Efe (1 July 2005). Plot size: 50 m2, Altitude: 12 m, southwest aspect, in the sand dune of Kasatura Bay, shrub layer: 5%, herb layer: 72%.
Shrub layer: Paliurus spina-christi: 1.
Herb layer: Cionura erecta: 3, Silene thymifolia: 2, Leymus racemosus subsp. sabulosus: 2, Ammophila arenaria subsp. arundinacea: 1, Centaurea cuneifolia: 1, Cota tinctoria var. euxina: 1, Malva sylvestris: 1, Osyris alba: 1, Allium vineale: +, Centaurea kilaea: +, Clinopodium nepeta subsp. nepeta: +, Echium vulgare subsp. vulgare: +, Euphorbia paralias: +, Medicago marina: +, Rubus sanctus: +, Scirpoides holoschoenus subsp. holoschoenus: +, Sedum pallidum: +, Sonchus asper subsp. glaucescens: +, Stachys recta subsp. subcrenata: +, Achillea maritima subsp. maritima: r, Bromus lanceolatus: r, Jurinea kilaea: r, Melica ciliata: r, Muscari neglectum: r, Poa pratensis: r.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/d16060318/s1, Table S1: Presence of phytogeographic region element in plant communities.
Author Contributions
D.O. carried out the vegetation sampling, the identification of the plant species, the creation of the raw data table, and project management and additionally prepared the manuscript, together with A.K. On the other hand, A.K. analyzed the data, classified the plant communities, and took part in the manuscript preparation. Although A.E. has passed away, we have included her name in the manuscript because she was D.O.’s supervisor during D.O.’s PhD research project. Since A.E. has passed away, we could not obtain her agreement to the publication of the manuscript. D.O. and A.K. have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Scientific Research Projects Unit of Istanbul University with project number T-960/06102006. The funder had no role in the design of this study, the interpretation of its data, the decision to publish the results or the writing of the manuscript.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, and upon request, further inquiries can be directed to the authors.
Acknowledgments
We thank Ferhat Gökbulak for his comments on the content of the manuscript and Ferdi Akarsu for the preparation of the map and graphical abstract. We also thank Andraž Čarni for his help with the computer programs used for the statistical analyses. This publication was based on Demir Oral’s PhD dissertation [49], supervised by Asuman Efe, who passed away in 2010.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sigren, J.M.; Figlus, J.; Armitage, A.R. Coastal sand-dunes and dune vegetation: Restoration, erosion, and storm protection. Shore Beach 2014, 82, 5–12. [Google Scholar]
- Calvão, T.; Pessoa, M.F.; Lidon, F.C. Impact of human activities on coastal vegetation -A review. Emir. J. Food Agric. 2013, 25, 926–944. [Google Scholar] [CrossRef]
- Brown, A.C.; McLachlan, A. Sandy shore ecosystems and the threats facing them: Some predictions for the year 2025. Environ Conserv. 2002, 29, 62–77. [Google Scholar] [CrossRef]
- Tzonev, R.; Dimitrov, M.; Roussakova, V. Dune vegetation of the Bulgarian Black Sea Coast. Hacquetia 2005, 4, 7–32. Available online: https://ojs.zrc-sazu.si/hacquetia/article/view/2974 (accessed on 12 March 2024).
- Çakan, H.; Yılmaz, K.T.; Alphan, H.; Ünlükaplan, Y. The classification and assessment of vegetation for monitoring coastal sand-dune succession: The case of Tuzla in Adana, Turkey. Turk. J. Bot. 2011, 35, 697–711. [Google Scholar] [CrossRef]
- Šilc, U.; Mullaj, A.; Alegro, A.; Ibraliu, A.; Stevanović, Z.D.; Luković, M.; Stešević, D. Sand-dune vegetation along the eastern Adriatic coast. Phytocoenologia 2016, 46, 339–355. [Google Scholar] [CrossRef]
- Stešević, D.; Küzmič, F.; Milanović, D.; Stanišić-Vujačić, M.; Šilc, U. Coastal sand-dune vegetation of Velika plaža (Montenegro). Acta Bot. Croat. 2019, 79, 43–54. [Google Scholar] [CrossRef]
- Akalın-Uruşak, E.; Özhatay, F.N.; Güler, N.; Ersoy, H.; Başak, N.; Yeşil, Y.; Oral, D.; Demirci, S. The flora of Yıldız Mountains (Kırklareli) Biosphere Project area. Turk. J. Bot. 2013, 37, 225–269. [Google Scholar] [CrossRef]
- Özhatay, E.; Çırpıcı, A.; Byfield, A. IPA 6-Terkos–Kasatura Coasts. In Important Plant Areas in Turkey; Özhatay, N., Byfield, A., Atay, S., Eds.; WWF: Istanbul, Türkiye, 2005; pp. 13–16. (In Turkish) [Google Scholar]
- Özhatay, N.; Akalın, E.; Güler, N.; Ersoy, H.; Yeşil, Y.; Demirci, S. Floristic richness and conservation priority sites in the northwest of European Turkey: Mt Yıldız-Kırklareli. Phytol. Balc. 2013, 19, 77–88. [Google Scholar]
- Oral, D.; Efe, A. New Registered Plants from Trakya-Çamlikoy. Proceedings book. In Proceedings of the International Scientific And Vocational Studies Congress (Bilmes 2018), Nevşehir, Türkiye, 28 June–8 July 2018; pp. 599–603. [Google Scholar]
- Yıldız, K.; Ay, G.; Kuh, M.; Fırat, M.; Tan, S. Ecological Characteristics of Türkiye’s Silene Genus and Revision of its 17 Sections. In Manisa Celal Bayar University Scientific Research Projects Coordination (2015–2017); Project No: 2014-125; Manisa Celal Bayar University: Manisa, Türkiye, 2017. [Google Scholar]
- Yıldız, K. Silene. In Türkiye Plant List (Vascular Plants); Güner, A., Aslan, S., Ekim, T., Vural, M., Babaç, M.T., Eds.; Nezahat Gökyiğit Botanical Garden and Flora Research Association Publications: İstanbul, Türkiye, 2012; pp. 354–365. [Google Scholar]
- Géhu, J.M.; Uslu, T. Données sur la végetation littorale de la Turquie du Nord–Ouest. Phytocoenologia 1989, 17, 449–505. [Google Scholar] [CrossRef]
- Thornthwaite, C.W. An approach toward a rational classification of climate. Geogr. Rev. 1948, 38, 55–94. [Google Scholar] [CrossRef]
- Kavgacı, A. Sand-Dune Vegetation of Iğneada Coast in the Thracian Part of Turkey. Hacquetia 2007, 6, 171–182. [Google Scholar] [CrossRef]
- Gökbulak, F. Vegetation Analysis in Pastures; İst. Üniv. Publ. Number: 5151, Forest Faculty Publ. number: 503; İstanbul University Press: İstanbul, Türkiye, 2013; p. 17. [Google Scholar]
- Braun-Blanquet, J. Pflanzensoziologie. Grundzüge der Vegetationskunde; Springer: Wien, NY, USA, 1964; 865p. [Google Scholar]
- Westhoff, V.; Van Der Maarel, E. The Braun-Blanquet approach. In Handbook of Vegetation Science; Tüxen, R., Ed.; Part V, Ordination and classification of communities, Whittaker, R.H. Ed.; Junk: The Hague, The Netherlands, 1973; pp. 617–626. [Google Scholar]
- Davis, P.H. Flora of Turkey and The East Aegean Islands, Vol. I–IX; The University Press: Edinburgh, UK, 1985. [Google Scholar]
- Davis, P.H.; Mill, R.R.; Tan, K. Flora of Turkey And The East Aegean Islands. Vol. X; The University Press: Edinburgh, UK, 1988. [Google Scholar]
- Güner, A.; Özhatay, N.; Ekim, T.; Başer, K.H.C. Flora of Turkey and the East Aegean Islands. Vol. XI; The University Press: Edinburgh, UK, 2000. [Google Scholar]
- Bonnier, B. Flore Complete Illustree En Couleurs de France; Tome 1–7; Suisse et Belgique: Paris, France, 1886. [Google Scholar]
- Fiori, A. Flora Italiana Illustrata; Proprieta Letteraria Riservata: Firenze, Italy, 1974; 549p. [Google Scholar]
- Tutin, T.G.; Heywood, V.H.; Burges, N.A.; Moore, D.M.; Valentine, D.H.; Walters, S.M.; Webb, D.A. Flora Europaea; Cambridge University Press/CUP: Cambridge, UK, 1980; Volume 1–5. [Google Scholar]
- Boissier, E. Flora Orientalis; Lugduni Batavorum: Genéve, Switzerland, 1888; Volume 1–5. [Google Scholar]
- Yaltırık, F. Türkiye Oaks Diagnostic Guide; General Directorate of Forestry Publications: Istanbul, Türkiye, 1984; 84p. (In Turkish) [Google Scholar]
- Yaltırık, F. Dendrology Textbook II, Angiosperms; No. 35096/309; IU. Forestry Faculty Publications: Istanbul, Türkiye, 1988; 256p. (In Turkish) [Google Scholar]
- Yaltırık, F.; Efe, A. Herbaceous Plants Systematics; Istanbul University Publication No: 3568, I.S.T. Publication No: 3; Institute of Science and Technology Publications: Istanbul, Türkiye, 1989; 518p. (In Turkish) [Google Scholar]
- 30. Kılınç ,M.; Özkanca, R. Flora of Coastal Dunes of the Central Black Sea Region. Turk. J. Bot 1991, 15, 314–327. (In Turkish)
- Kılınç, M.; Özkanca, R. Vegetation of Central Black Sea Region Coastal Dunes. Turk. J. Bot 1991, 15, 328–348. (In Turkish) [Google Scholar]
- Pils, G. Flowers of Turkey (A Photo Guide); Gerhard Pils (Privately Published): Linz, Austria, 2006; 448p. [Google Scholar]
- Güner, A.; Aslan, S.; Ekim, T.; Vural, M.; Babaç, M.T. Türkiye Plant List (Vascular Plants); Nezahat Gökyiğit Botanical Garden and Flora Research Association Publications: İstanbul, Türkiye, 2012. [Google Scholar]
- Powo. Plants of the World Online. Facilitated by the Royal Botanic Gardens, Kew. 2024. Available online: http://www.plantsoftheworldonline.org/ (accessed on 22 March 2024).
- Ekim, T.; Koyuncu, M.; Vural, M.; Duman, H.; Aytaç, Z.; Adıgüzel, N. Red Book of Plants of Türkiye; Ferns and Seed Plants; Türkiye Nature Conservation Association, and Van Centennial University: Ankara, Türkiye, 2000; pp. 1–96. [Google Scholar]
- Hennekens, S.M.; Schaminée, J.H.J. TURBOVEG, A comprehensive data base management system for vegetation data. J. Veg. Sci. 2001, 12, 589–591. [Google Scholar] [CrossRef]
- Tichý, L. JUICE, software for vegetation classification. J. Veg. Sci. 2002, 13, 45–453. [Google Scholar] [CrossRef]
- McCune, B.; Mefford, M.J. PC-ORD. Multivariate Analysis of Ecological Data, Version 4; MjM Sofware Design: Gleneden Beach, OR, USA, 2001; 237p.
- Chytrý, M.; Tichý, L.; Kolt, J.; Botta-Duká, Z. Determination of diagnostic species with statistical fidelity measures. J. Veg. Sci. 2001, 13, 79–90. [Google Scholar] [CrossRef]
- Šmilauer, P.; Lepš, J. Multivariate Analysis of Ecological Data Using CANOCO 5; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Raunkiaer, C. The Life Forms of Plants and Statistical Plant Geography; Oxford University Press: Oxford, UK, 1934; 632p. [Google Scholar]
- Anon. STATISTICA (Data Analysis Software System), Version 8.0; StatSoft, Inc.: Tulsa, OK, USA, 2007. Available online: https://statistica.software.informer.com/8.0/ (accessed on 12 October 2023)Available online:.
- Sykora, K.V.; Babalonas, D.; Papastergiadou, E.D. Strandline and sand-dune vegetation of coast of Greece and some other Aegean countries. Phytocoenologia 2003, 33, 409–446. [Google Scholar] [CrossRef]
- Mucina, L.; Bültmann, H.; Dierßen, K.; Theurillat, J.P.; Raus, T.; Čarni, A.; Šumberová, K.; Willner, W.; Dengler, J.; García, R.G.; et al. Vegetation of Europe: Hierarchical floristic classification system of vascular plant, bryophyte, lichen, and algal communities. Appl. Veg. Sci. 2016, 19, 3–264. [Google Scholar] [CrossRef]
- Kavgacı, A.; Yalçın, E.; Korkmaz, H. Numerical classification and ordination of the floodplain forests in the Euxine region of Turkey. Turk. J. Bot. 2016, 40, 164–175. [Google Scholar] [CrossRef]
- Oberdorfer, E. Beitrag zur Kenntnis der nordagaischen Küstenvegetation. Vegetatio 1952, 3, 320–349. [Google Scholar] [CrossRef]
- Guacchio, E.D.; Caputo, P. Splitting Asperula (Rubiaceae): A proposal for consistency purposes within sections Cynanchicae, Thliphthisa and Hexaphylla. Plant Biosyst Int. J. Deal. All Asp. Plant Biol. 2020, 154, 766–782. [Google Scholar] [CrossRef]
- Byfield, A.; Özhatay, N. Report on the Protection of Türkiye’s Northern Sand Dunes; Revised and translated: S. Atay and N.Özhatay; WWF: İstanbul, Türkiye, 1996. [Google Scholar]
- Demir Oral, D. Flora and Vegetation of Kasatura Gulf and Its Environment (Kırklareli-Tekirdağ-Istanbul). Ph.D. Thesis, Istanbul University, Institute of Science and Technology, Inst. Sci., İst. Univ., Istanbul, Türkiye, 2010; 328p. Available online: https://tez.yok.gov.tr/UlusalTezMerkezi/tezSorguSonucYeni.jsp (accessed on 12 March 2024). (In Turkish).
- Özhatay, N.; Byfield, A.; Atay, S. Important Plant Areas in Türkiye; WWF: İstanbul, Türkiye, 2005. (In Turkish) [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).