Soil Studies for Fungal Diversity to Enable the Conservation Translocation of Green-Winged Orchid
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil Collecting
2.2. Soil Processing and Chemical Analyses
2.3. Soil DNA Extraction
2.4. Metabarcoding
2.5. Statistical Analysis
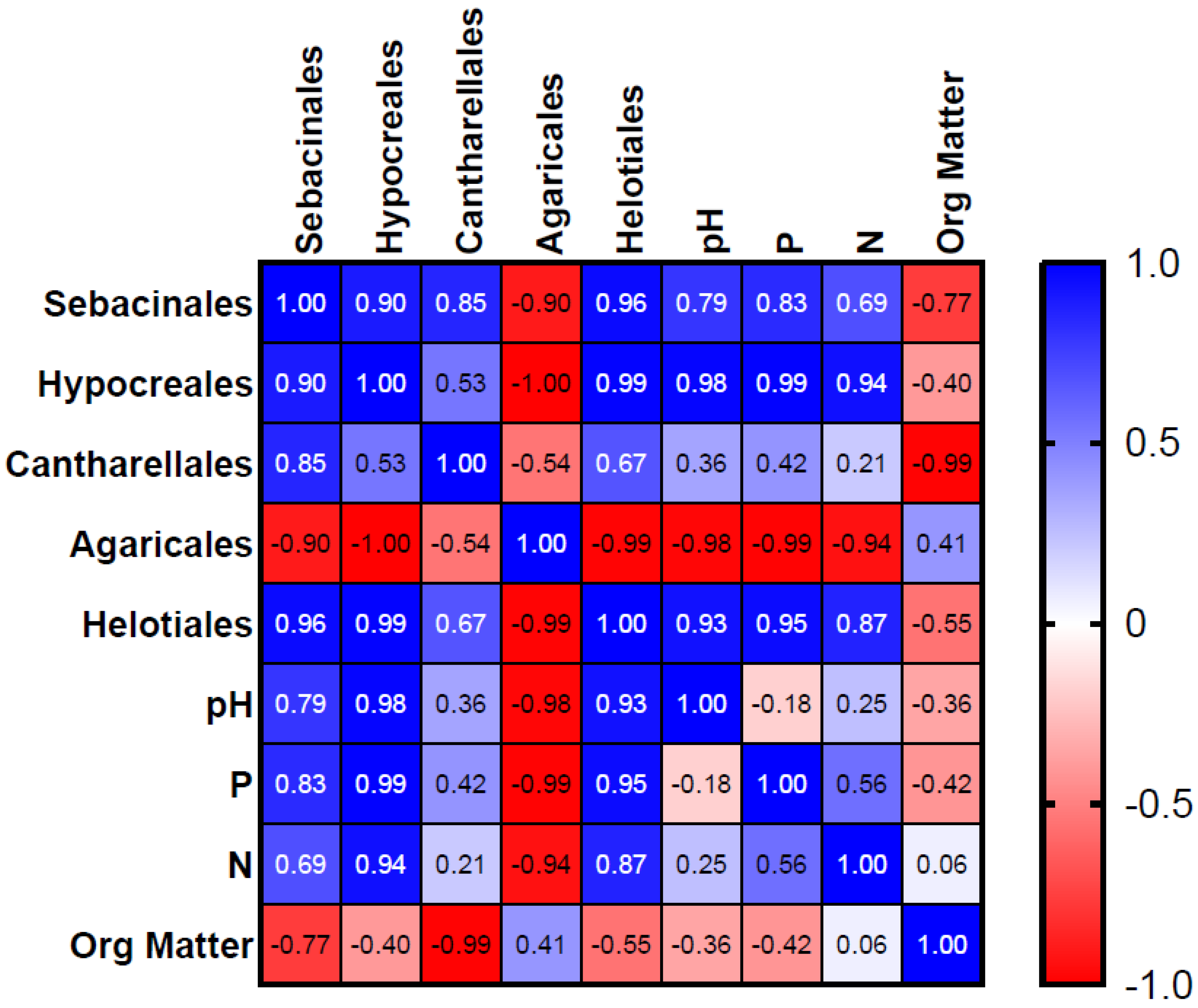
3. Results
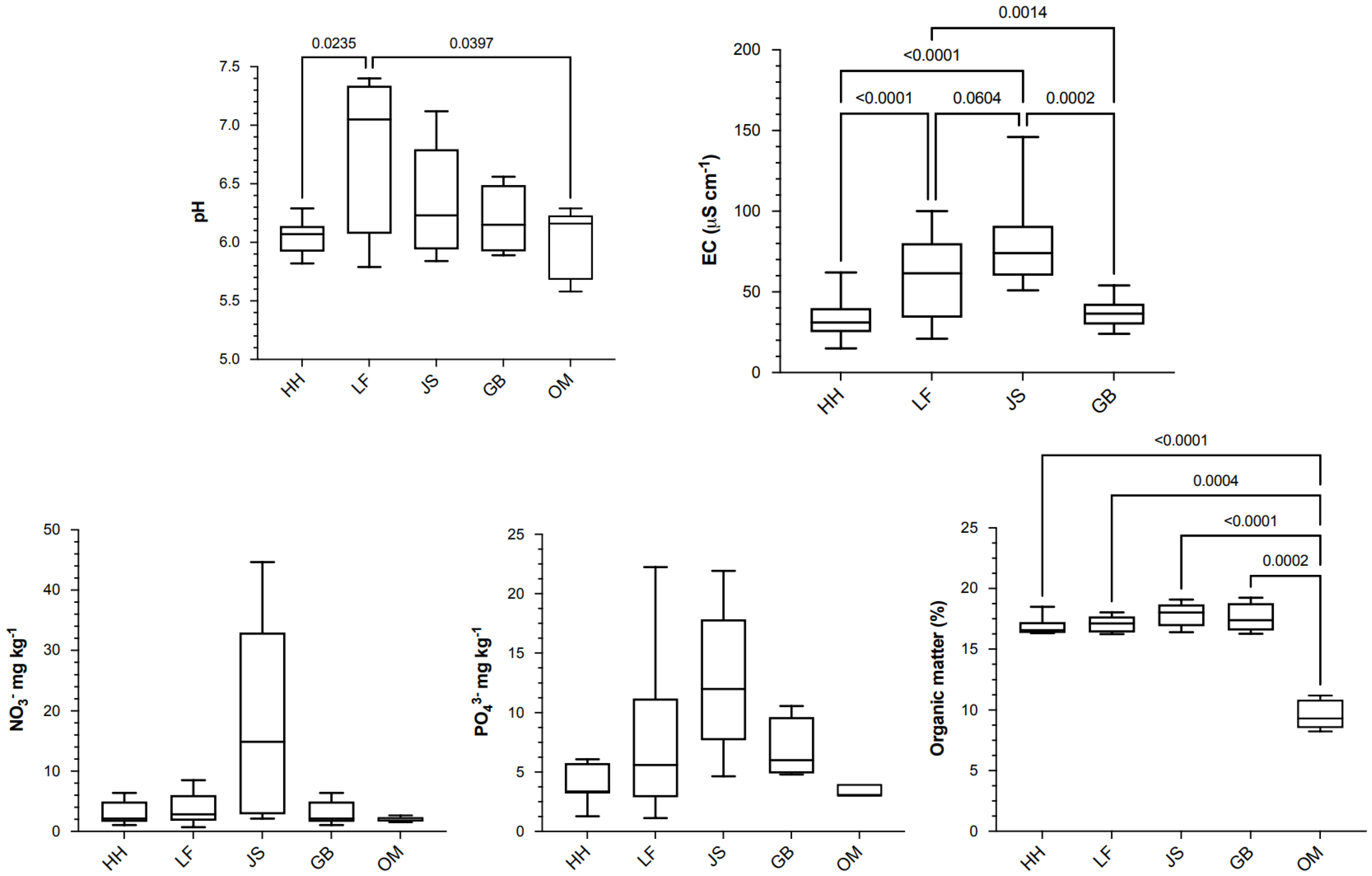
3.1. Soil Analysis
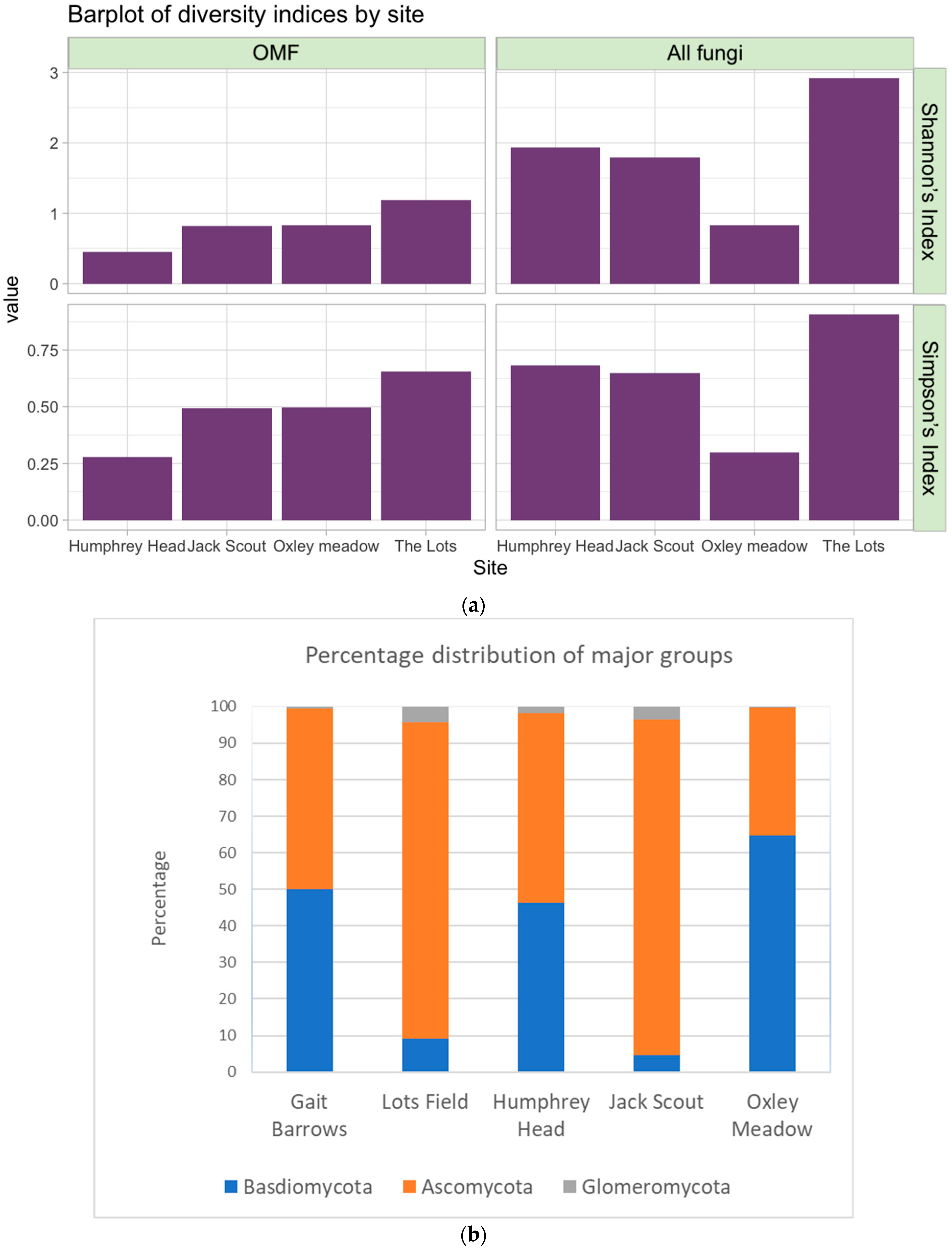
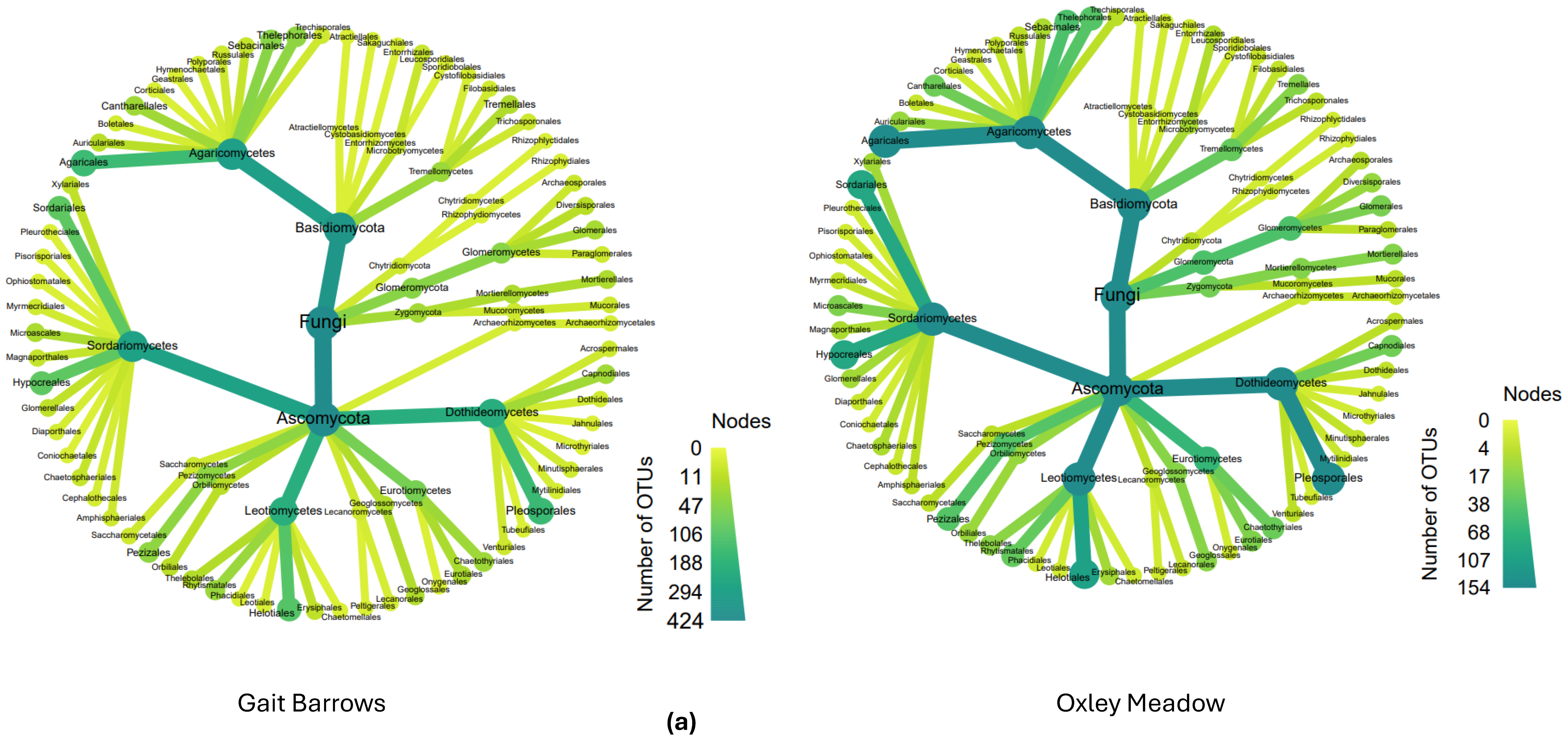
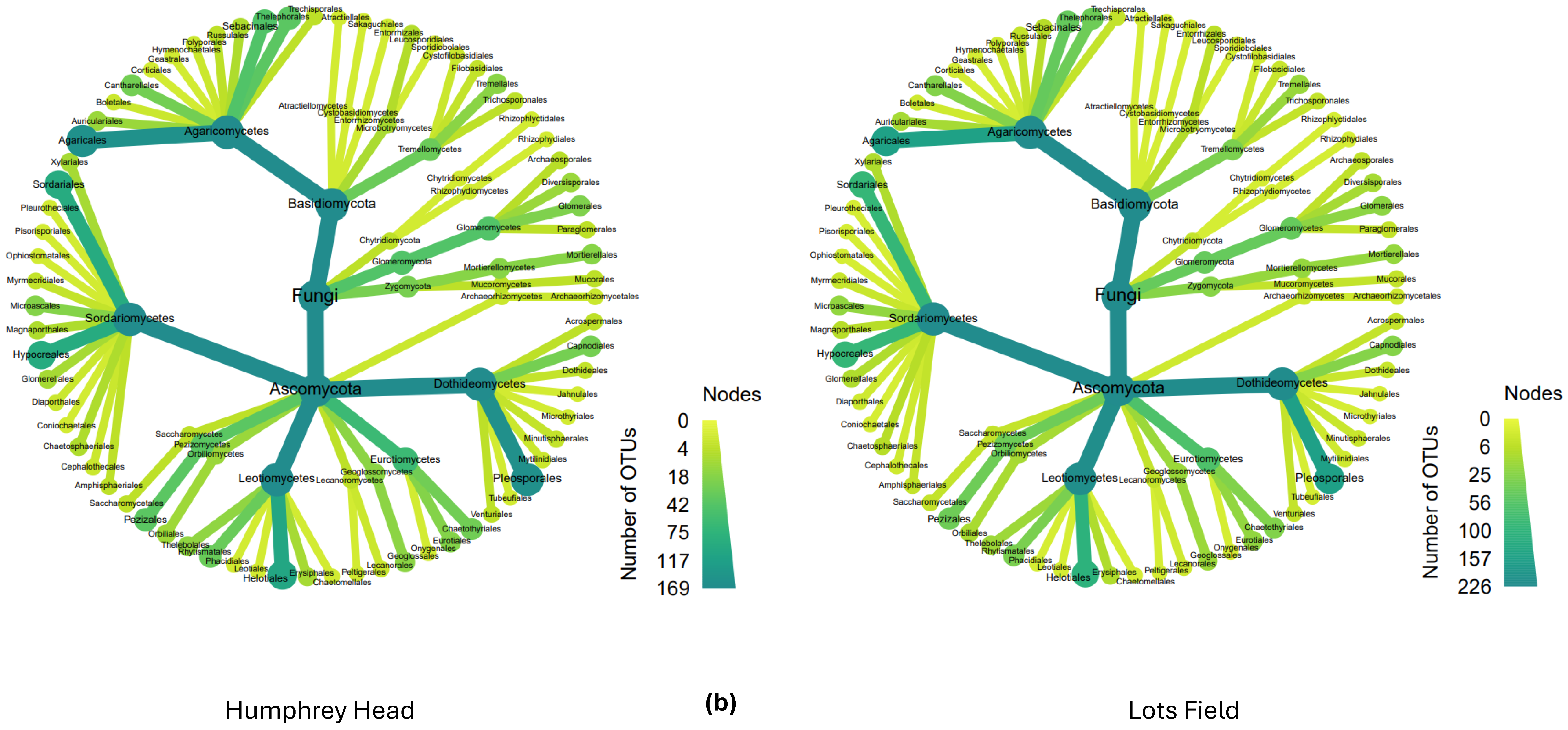
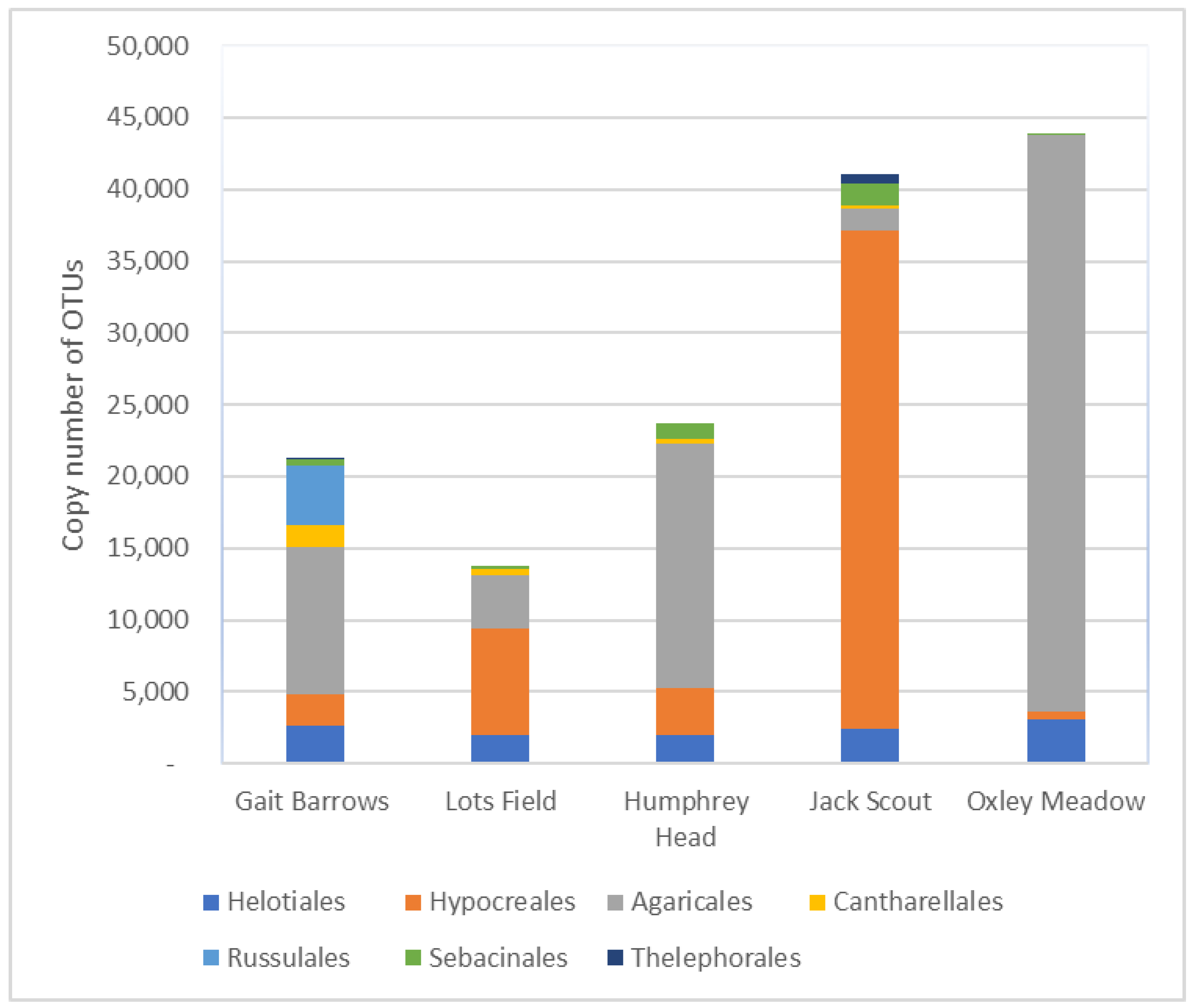
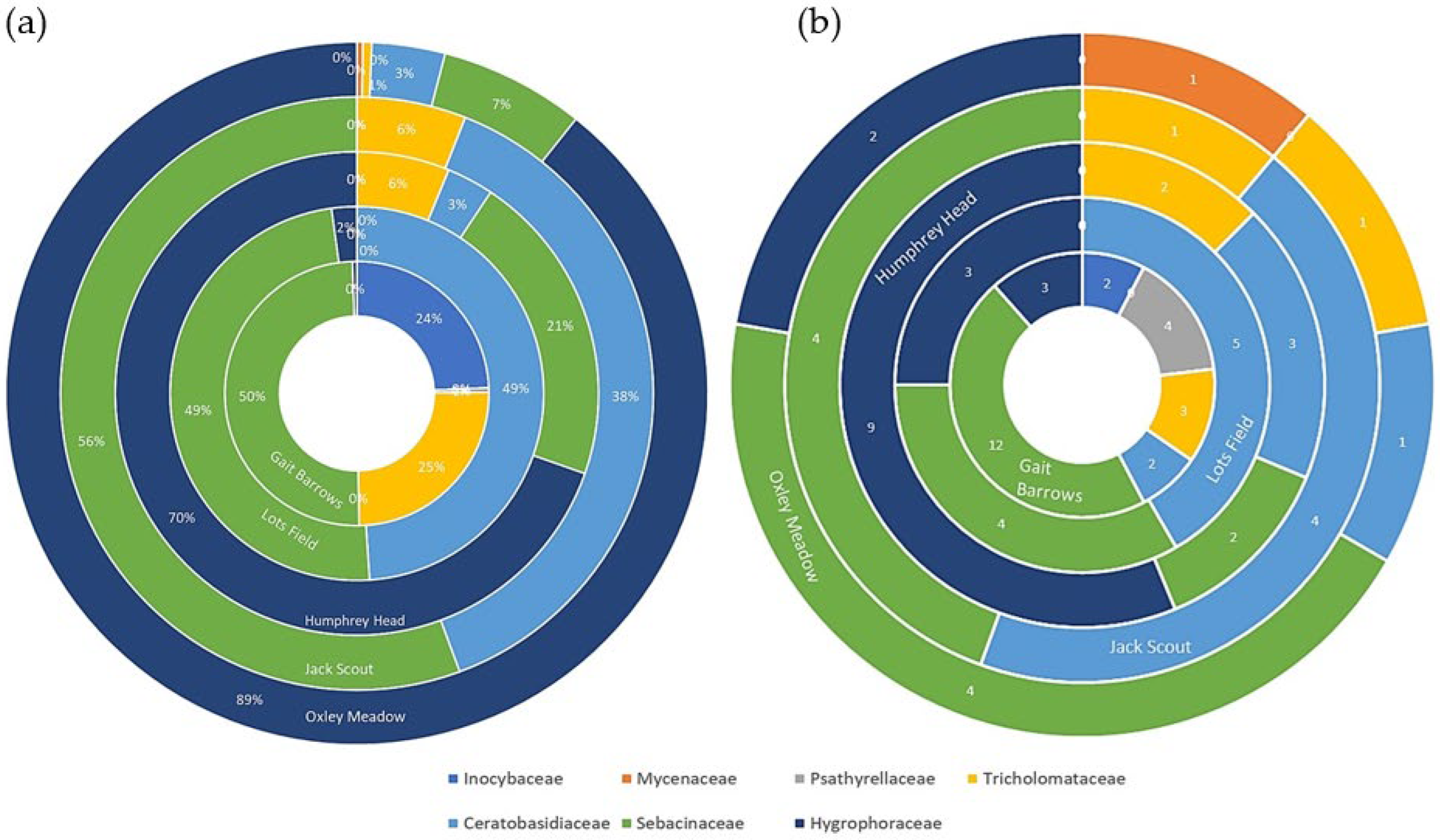
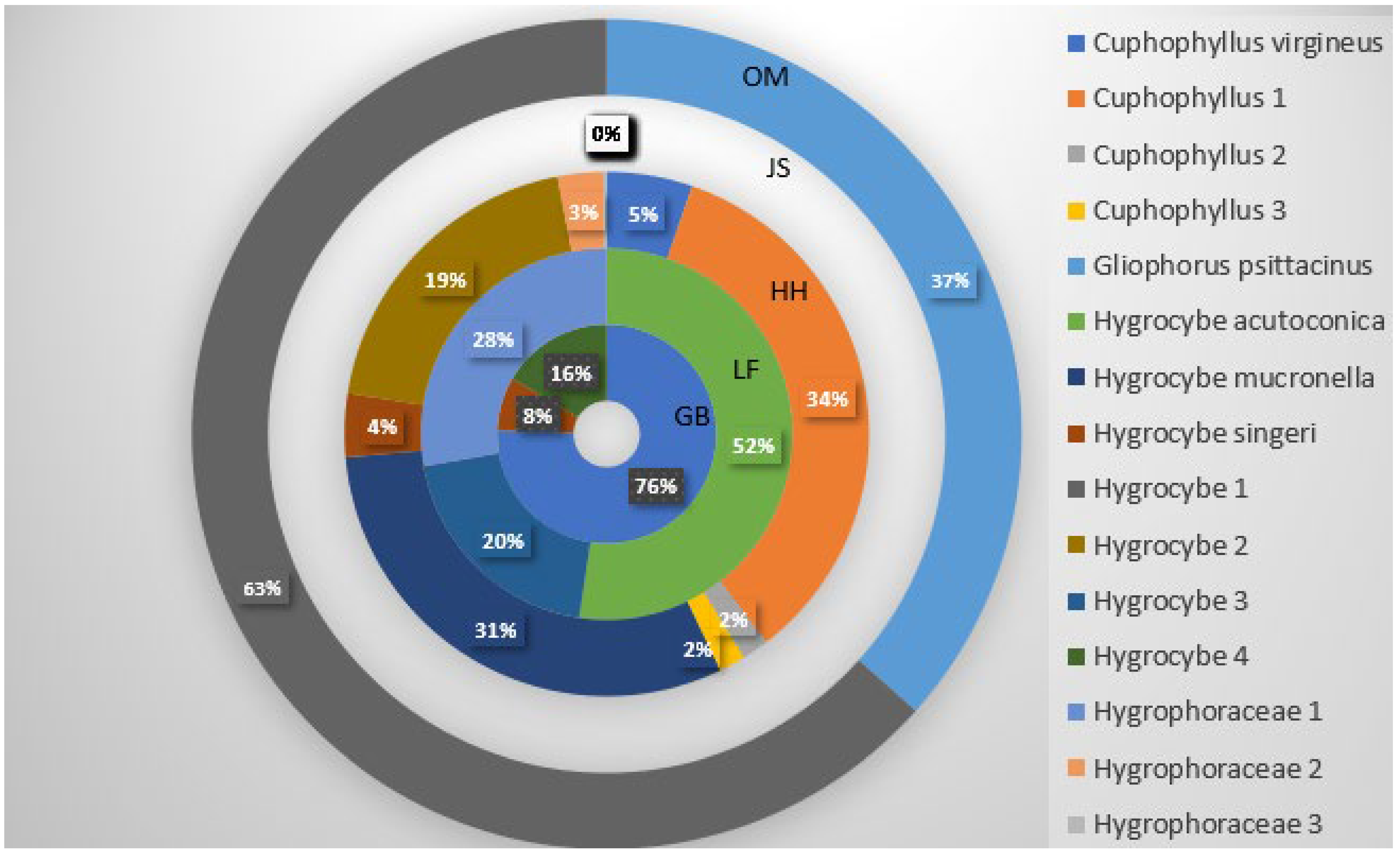
3.2. Fungal Distribution and Abundance
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Christenhusz, M.J.M.; Byng, J.W. The Number of Known Plants Species in the World and Its Annual Increase. Phytotaxa 2016, 261, 201–207. [Google Scholar] [CrossRef]
- Stroh, P.A. Long-Term Monitoring of Green-Winged Orchid (Anacamptis morio) at Upwood Meadows NNR, Huntingdonshire. Br. Ir. Bot. 2019, 1, 107–116. [Google Scholar] [CrossRef]
- Silvertown, J.; Wells, D.A.; Gillman, M.; Dodd, M.E.; Robertson, H.; Lakhani, K.H. Short-Term Effects and Long-Term after-Effects of Fertilizer Application on the Flowering Population of Green-Winged Orchid Orchis Morio. Biol. Conserv. 1994, 69, 191–197. [Google Scholar] [CrossRef]
- Kull, T.; Hutchings, M.J. A Comparative Analysis of Decline in the Distribution Ranges of Orchid Species in Estonia and the United Kingdom. Biol. Conserv. 2006, 129, 31–39. [Google Scholar] [CrossRef]
- Barman, D.; Devadas, R. Climate Change on Orchid Population and Conservation Strategies: A Review. J. Crop Weed 2013, 9, 1–12. [Google Scholar]
- Liu, H.; Tropi, F. Home, Home Outside the Range? Science 2010, 329, 1592–1594. [Google Scholar]
- Melillo, J.M.; McGuire, A.D.; Kicklighter, D.W.; Moore, B.; Vorosmarty, C.J.; Schloss, A.L. Global Climate Change and Terrestrial Net Primary Production. Nature 1993, 363, 234–240. [Google Scholar] [CrossRef]
- Thomas, C.D. Translocation of Species, Climate Change, and the End of Trying to Recreate Past Ecological Communities. Trends Ecol. Evol. 2011, 26, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Simmons, C.L.; Auld, T.D.; Hutton, I.; Baker, W.J.; Shapcott, A. Will Climate Change, Genetic and Demographic Variation or Rat Predation Pose the Greatest Risk for Persistence of an Altitudinally Distributed Island Endemic? Biology 2012, 1, 736–765. [Google Scholar] [CrossRef]
- Hornemann, G.; Michalski, S.G.; Durka, W. Short-Term Fitness and Long-Term Population Trends in the Orchid Anacamptis Morio. Plant Ecol. 2012, 213, 1583–1595. [Google Scholar] [CrossRef]
- Bidartondo, M.I.; Read, D.J. Fungal Specificity Bottlenecks during Orchid Germination and Development. Mol. Ecol. 2008, 17, 3707–3716. [Google Scholar] [CrossRef]
- Rasmussen, H.N.; Rasmussen, F.N. Seedling Mycorrhiza: A Discussion of Origin and Evolution in Orchidaceae. Bot. J. Linn. Soc. 2014, 175, 313–327. [Google Scholar] [CrossRef]
- McCormick, M.K.; Jacquemyn, H. What Constrains the Distribution of Orchid Populations? New Phytol. 2014, 202, 392–400. [Google Scholar] [CrossRef]
- McCormick, M.K.; Taylor, D.L.; Whigham, D.F.; Burnett, R.K. Germination Patterns in Three Terrestrial Orchids Relate to Abundance of Mycorrhizal Fungi. J. Ecol. 2016, 104, 744–754. [Google Scholar] [CrossRef]
- Dove, A.; Charters, M.D.; Campbell, M.J.; Blake, H.; Menon, M.; Sarasan, V. Fungal Community Composition at the Last Remaining Wild Site of Yellow Early Marsh Orchid (Dactylorhiza incarnata ssp. ochroleuca). Microorganisms 2023, 11, 2124. [Google Scholar] [CrossRef] [PubMed]
- Sarasan, V.; Pankhurst, T.; Yokoya, K.; Sriskandarajah, S.; McDiarmid, F. Preventing Extinction of a Critically Endangered Dactylorhiza incarnata Subsp. ochroleua in Britain Using Symbiotic Seedlings for Reintroduction. Microorganisms 2021, 9, 1421. [Google Scholar] [CrossRef]
- Yokoya, K.; Zettler, L.W.; Bell, J.; Kendon, J.P.; Jacob, A.S.; Schofield, E.; Rajaovelona, L.; Sarasan, V. The Diverse Assemblage of Fungal Endophytes from Orchids in Madagascar Linked to Abiotic Factors and Seasonality. Diversity 2021, 13, 96. [Google Scholar] [CrossRef]
- Verbruggen, E.; Rillig, M.C.; Wehner, J.; Hegglin, D.; Wittwer, R.; van der Heijden, M.G.A. Sebacinales, but Not Total Root Associated Fungal Communities, Are Affected by Land-Use Intensity. New Phytol. 2014, 203, 1036–1040. [Google Scholar] [CrossRef]
- Jurišić, M.; Radočaj, D.; Plaščak, I.; Rapčan, I. A UAS and Machine Learning Classification Approach to Suitability Prediction of Expanding Natural Habitats for Endangered Flora Species. Remote Sens. 2022, 14, 3054. [Google Scholar] [CrossRef]
- McCormick, M.K.; Whigham, D.F.; Canchani-Viruet, A. Mycorrhizal fungi affect orchid distribution and population dynamics. New Phytol. 2018, 219, 1207–1215. [Google Scholar] [CrossRef]
- Downing, J.L.; Liu, H.; McCormick, M.K.; Arce, J.; Alonso, D.; Lopez-Perez, J. Generalized Mycorrhizal Interactions and Fungal Enemy Release Drive Range Expansion of Orchids in Southern Florida. Ecosphere 2020, 11, e03228. [Google Scholar] [CrossRef]
- Rominger, K.; Meyer, S. Application of UAV-Based Methodology for Census of an Endangered Plant Species in a Fragile Habitat. Remote Sens. 2019, 11, 719. [Google Scholar] [CrossRef]
- Gröschler, K.-C.; Oppelt, N. Using Drones to Monitor Broad-Leaved Orchids (Dactylorhiza majalis) in High-Nature-Value Grassland. Drones 2022, 6, 174. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.D.; Lee, S.B.; Taylor, J.W. Amplification and Direct Sequencing of Fungal Ribosomal RNA Genes for Phylogenetics. In PCR Protocols: A Guide to Methods and Applications, 315th–322th ed.; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: Cambridge, MA, USA, 1990. [Google Scholar]
- Bengtsson-Palme, J.; Ryberg, M.; Hartmann, M.; Branco, S.; Wang, Z.; Godhe, A.; De Wit, P.; Sanchez-Garcia, M.; Ebersberger, I.; de Sousa, F.; et al. Improved Software Detection and Extraction of ITS1 and ITS 2 from Ribosomal ITS Sequences of Fungi and Other Eukaryotes for Analysis of Environmental Sequencing Data. Methods Evol. Ecol. 2013, 4, 914–919. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly Accurate OTU Sequences from Microbial Amplicon Reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic Local Alignment Search Tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: Architecture and Applications. BMC Bioinform. 2009, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Diez, J.M.; Pulliam, H.R. Hierarchical analysis of species distributions and abundance across environmental gradients. Ecology 2007, 88, 3144–3152. [Google Scholar] [CrossRef]
- Tsiftsis, S.; Tsiripidis, I.; Papaioannou, A. Ecology of the Orchid Goodyera repens in Its Southern Distribution Limits. Plant Biosyst. 2012, 146, 857–866. [Google Scholar] [CrossRef]
- Djordjević, V.; Tsiftsis, S. Patterns of Orchid Species Richness and Composition in Relation to Geological Substrates. Wulfenia 2019, 26, 1–21. [Google Scholar]
- Li, T.; Wu, S.; Yang, W.; Selosse, M.-A.; Gao, J. How Mycorrhizal Associations Influence Orchid Distribution and Population Dynamics. Front. Plant Sci. 2021, 12, 647114. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Zhao, C.; Garland, G.; Edlinger, A.; García-Palacios, P.; Romdhane, S.; Degrune, F.; Pescador, D.S.; Herzog, C.; Camuy-Velez, L.A.; et al. Biotic Homogenization, Lower Soil Fungal Diversity and Fewer Rare Taxa in Arable Soils across Europe. Nat. Commun. 2024, 15, 327. [Google Scholar] [CrossRef] [PubMed]
- Carron, A.I.; Garibaldi, L.A.; Marquez, S.; Fontenla, S. The Soil Fungal Community of Native Woodland in Andean Patagonian Forest: A Case Study Considering Experimental Forest Management and Seasonal Effects. Ecol. Manag. 2020, 461, 117955. [Google Scholar] [CrossRef]
- Lambers, H.; Brundrett, M.C.; Raven, J.A.; Hopper, S.D. Plant Mineral Nutrition in Ancient Landscapes: High Plant Species Diversity on Infertile Soils Is Linked to Functional Diversity for Nutritional Strategies. Plant Soil 2011, 348, 7–27. [Google Scholar] [CrossRef]
- Arnolds, E. The Changing Macromycete Flora in the Netherlands. Trans. Br. Mycol. Soc. 1988, 90, 391–406. [Google Scholar] [CrossRef]
- Lodge, D.J.; Padamsee, M.; Matheny, P.B.; Aime, M.C.; Cantrell, S.A.; Boertmann, D.; Kovalenko, A.; Vizzini, A.; Dentinger, B.T.M.; Kirk, P.M.; et al. Molecular Phylogeny, Morphology, Pigment Chemistry and Ecology in Hygrophoraceae (Agaricales). Fungal Divers. 2014, 64, 1–99. [Google Scholar] [CrossRef]
- Rasmussen, H.N. Terrestrial Orchids; Cambridge University Press: Cambridge, UK, 1995; ISBN 9780521451659. [Google Scholar]
- Selosse, M.A. The Latest News from Biological Interactions in Orchids: In Love, Head to Toe. New Phytol. 2014, 202, 337–340. [Google Scholar] [CrossRef]
- Oja, J.; Kohout, P.; Tedersoo, L.; Kull, T.; Kõljalg, U. Temporal Patterns of Orchid Mycorrhizal Fungi in Meadows and Forests as Revealed by 454 Pyrosequencing. New Phytol. 2015, 205, 1608–1618. [Google Scholar] [CrossRef]
- Voyron, S.; Ercole, E.; Ghignone, S.; Perotto, S.; Girlanda, M. Fine-Scale Spatial Distribution of Orchid Mycorrhizal Fungi in the Soil of Host-Rich Grasslands. New Phytol. 2017, 213, 1428–1439. [Google Scholar] [CrossRef]
- Seitzman, B.H.; Ouimette, A.; Mixon, R.L.; Hobbie, E.A.; Hibbett, D.S. Conservation of Biotrophy in Hygrophoraceae Inferred from Combined Stable Isotope and Phylogenetic Analyses. Mycologia 2011, 103, 280–290. [Google Scholar] [CrossRef]
- McHugh, R.; Mitchel, D.; Wright, M.; Anderson, R. The Fungi of Irish Grasslands and Their Value for Nature Conservation. Biol. Environ. 2001, 101, 225–243. [Google Scholar]
- Ainsworth, A.; Cannon, P.; Dentinger, B. DNA Barcoding and Morphological Studies Reveal Two New Species of Waxcap Mushrooms (Hygrophoraceae) in Britain. MycoKeys 2013, 7, 45–62. [Google Scholar] [CrossRef]
- Arnolds, E. De Oecologie en Sociologie van Wasplaten (Hygrophorus Subgenus Hygrocybe Sensu Lato). Natura 1980, 77, 17–44. [Google Scholar]


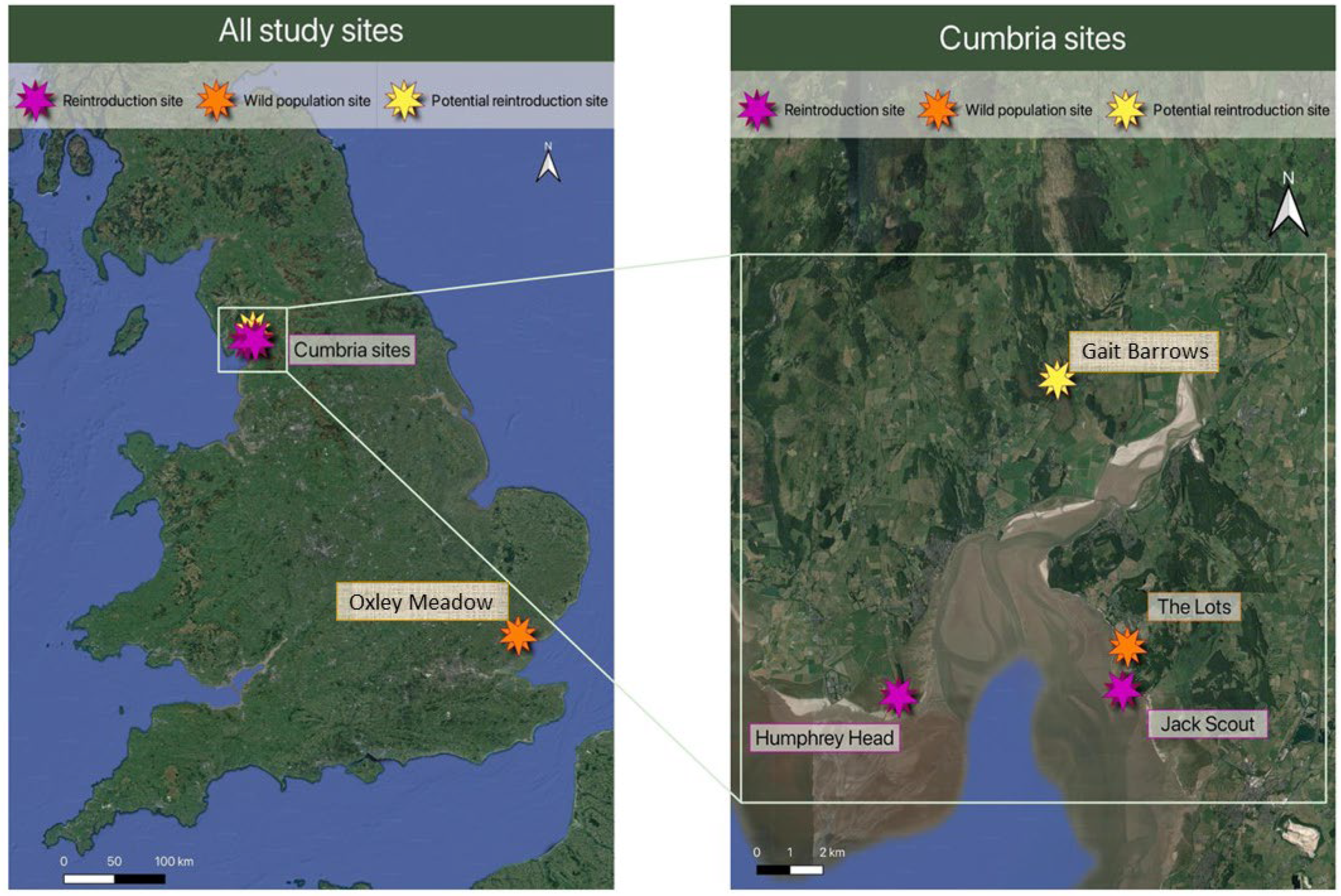


| Site Name | County (England) | Site Type |
|---|---|---|
| Lots Field (LF) | Cumbria | Wild site |
| Jack Scout (JS) | Cumbria | Potential receiver site |
| Humphrey Head (HH) | Cumbria | Potential receiver site |
| Gait Barrows (GB) | Cumbria | Potential receiver site |
| Oxley Meadow (OM) | Essex | Wild site |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Newmarch, M.B.; Velde, M.; Menon, M.; Sarasan, V. Soil Studies for Fungal Diversity to Enable the Conservation Translocation of Green-Winged Orchid. Diversity 2024, 16, 327. https://doi.org/10.3390/d16060327
Newmarch MB, Velde M, Menon M, Sarasan V. Soil Studies for Fungal Diversity to Enable the Conservation Translocation of Green-Winged Orchid. Diversity. 2024; 16(6):327. https://doi.org/10.3390/d16060327
Chicago/Turabian StyleNewmarch, Millie Brigitte, Mélusine Velde, Manoj Menon, and Viswambharan Sarasan. 2024. "Soil Studies for Fungal Diversity to Enable the Conservation Translocation of Green-Winged Orchid" Diversity 16, no. 6: 327. https://doi.org/10.3390/d16060327






