Reptile Biodiversity and Vulnerability in Bolivia’s Beni Department: Informing Conservation Priorities in a Neglected Frontier
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- INE. Boletín Estadístico Producto Interno Bruto al Segundo Trimestre. 2020. Available online: https://www.ine.gob.bo/index.php/publicaciones/boletin-estadistico-producto-interno-bruto-alsegundo-trimestre-2020/ (accessed on 15 December 2023).
- Embert, D. Distribution, Diversity, and Conservation Status of Bolivian Reptiles. Ph.D. Dissertation, Rheinischen Friedrichs-Wilhelms-Universitat Bonn, Bonn, Germany, 2007; p. 429. [Google Scholar]
- Aliaga-Rossel, E. Distribution and abundance of the river dolphin (Inia geoffrensis) in the Tijamuchi River, Beni, Bolivia. Aquat. Mamm. 2002, 28, 312–323. [Google Scholar]
- Larrea-Alcázar, D.M.; Embert, D.; Aguirre, L.F.; Ríos-Uzeda, B.; Quintanilla, M.; Vargas, A. Spatial patterns of biological diversity in a neotropical lowland savanna of northeastern Bolivia. Biodivers. Conserv. 2011, 20, 1167–1182. [Google Scholar]
- Erickson, C.L. The domesticated landscapes of the Bolivian Amazon. In Time and Complexity in Historical Ecology: Studies in the Neotropical Lowlands; Balée, W.L., Erickson, C.L., Eds.; Columbia University Press: New York, NY, USA, 2006; pp. 235–278. [Google Scholar]
- Walker, J.H. Pre-Columbian ring ditches along the Yacuma and Rapulo Rivers, Beni, Bolivia: A preliminary review. J. Field Archaeol. 2008, 33, 413–427. [Google Scholar] [CrossRef]
- Erickson, C.L. The transformation of environment into landscape: The historical ecology of monumental earthwork construction in the Bolivian Amazon. Diversity 2010, 2, 618–652. [Google Scholar] [CrossRef]
- Beck, H.E.; Zimmermann, N.E.; McVicar, T.R.; Vergopolan, N.; Berg, A.; Wood, E.F. Present and future Köppen-Geiger climate classification maps at 1-km resolution. Sci. Data 2018, 5, 180214. [Google Scholar] [CrossRef] [PubMed]
- Navarro, G. Vegetación y Unidades Biogeográficas. In Geografía Ecológica de Beni. Vegetación y Ambientes Acuáticos; Navarro, G., Maldonado, M., Eds.; Editorial Centro de Ecología Difusión Simón I. Patiño: Cochabamba, Bolivia, 2002; pp. 1–500. [Google Scholar]
- Navarro, G. Clasificación de la Vegetación de Bolivia; Centro de Ecología Difusión Simón I. Patiño: Santa Cruz, Bolivia, 2011. [Google Scholar]
- Navarro, G.; Ferreira, W. Biogeografía de Bolivia. In Libro Rojo de Parientes Silvestres de Cultivos de Bolivia; Moraes, M., Mostacedo, B., Altamirano, S., Eds.; Viceministerio de Medio Ambiente, Biodiversidad y Cambio Climático (VMABCC), Plural Editores: La Paz, Bolivia, 2009; pp. 23–39. [Google Scholar]
- Myster, R.W. The Physical Structure of Forests in the Amazon Basin: A Review. Bot. Rev. 2016, 82, 407–427. [Google Scholar] [CrossRef]
- Bredin, Y.K.; Hawes, J.E.; Peres, C.A.; Haugaasen, T. Structure and composition of Terra Firme and Seasonally Flooded Várzea Forests in the Western Brazilian Amazon. Forests 2020, 11, 1361. [Google Scholar] [CrossRef]
- Moraes, R. Flora de Palmeras de Bolivia, 2nd ed.; Universidad Mayor de San Andrés: La Paz, Bolivia, 2020. [Google Scholar]
- Ibisch, P.L.; Beck, S.G.; Gerkmann, B.; Carretero, A. Ecoregiones y ecosistemas. In Biodiversidad: La Riqueza de Bolivia; Ibisch, P.L., Me, G., Eds.; Editorial FAN: Santa Cruz de la Sierra, Bolivia, 2003; pp. 47–88. [Google Scholar]
- Langstroth, R.P. Forest Islands in an Amazonian Savanna of Northeastern Bolivia. Ph.D. Dissertation, University of Wisconsin, Madison, WI, USA, 1996; p. 452. [Google Scholar]
- Langstroth, R.P. Biogeography of the Llanos de Moxos: Natural and anthropogenic determinants. Geogr. Helv. 2011, 66, 183–192. [Google Scholar] [CrossRef]
- Haase, R.; Beck, G. Structure and composition of savanna vegetation in northern Bolivia: A preliminary report. Brittonia 1989, 41, 80–100. [Google Scholar] [CrossRef]
- Eversole, C.B.; Powell, R.L.; Lizarro, D.; Moreno, F.; Vaca, G.C.; Aparicio, J.; Crocker, A.V. Introduction of a novel natural history collection: A model for global scientific collaboration and enhancement of biodiversity infrastructure with a focus on developing countries. Biodivers. Conserv. 2019, 28, 1921–1931. [Google Scholar] [CrossRef]
- Steinberg, P. Environmental Leadership in Developing Countries: Transnational Relations of Biodiversity Policy in Costa Rica and Bolivia; MIT Press: Cambridge, MA, USA, 2001. [Google Scholar]
- Moravec, J.; Aparicio, J. Notes on the herpetofauna of Nacebe (Provincia Abuna, Departamento Pando, Bolivia). Časopis Národního Muz. Řada Přírodovědná 2004, 173, 13–28. [Google Scholar]
- Eversole, C.B.; Powell, R.L.; Lizarro, D.; Crocker, A.V.; Vaca, G.C.; Quintana, P.L. Herpetofauna of the Reserva de la Biósfera Estación Biológica del Beni and the Chimane Reserve Indigenous Territory, Bolivia. Neotrop. Biodivers. 2021, 7, 146–154. [Google Scholar] [CrossRef]
- Wilson, L.D.; Mata-Silva, V.; Johnson, J.D. A conservation reassessment of the reptiles of Mexico based on the EVS measure. Amphib. Reptile Conserv. 2013, 7, 1–47. [Google Scholar]
- Gibbons, J.W.; Scott, D.E.; Ryan, T.J.; Buhlmann, K.A.; Tuberville, T.D.; Metts, B.S.; Greene, J.L.; Mills, T.; Leiden, Y.; Poppy, S.; et al. The global decline of reptiles, déja vu amphibians. BioScience 2000, 50, 653–666. [Google Scholar] [CrossRef]
- Uetz, P.; Freed, P.; Aguilar, R.; Reyes, F.; Kudera, J.; Hošek, J. The Reptile Database. 2023. Available online: http://www.reptile-database.org/ (accessed on 1 June 2024).
- Lemos-Espinal, J.A.; Smith, G.R.; Rorabaugh, J. A conservation checklist of the amphibians and reptiles of Sonora, Mexico, with updated species lists. ZooKeys 2019, 829, 131–160. [Google Scholar] [CrossRef] [PubMed]
- International Union for the Conservation of Nature (IUCN). 2022. IUCN Red List of Threatened Species. 2019. Available online: http://www.iucnredlist.org (accessed on 15 December 2023).
- Wilson, L.D.; McCranie, J.R. The conservation status of the herpetofauna of Honduras. Amphib. Reptile Conserv. 2004, 3, 6–33. [Google Scholar] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 15 December 2023).
- Ogle, D.H.; Doll, J.C.; Wheeler, P.; Dinno, A. FSA: Fisheries Stock Analysis. R Package Version 0.9.1. 2021. Available online: https://github.com/droglenc/FSA (accessed on 15 December 2023).
- Gardner, T.A.; Barlow, J.; Chazdon, R.; Ewers, R.M.; Harvey, C.A.; Peres, C.A.; Sodhi, N.S. Prospects for tropical forest biodiversity in a human-modified world. Ecol. Lett. 2009, 12, 561–582. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.S.L.; Andelman, S.J.; Bakarr, M.I.; Boitani, L.; Brooks, T.M.; Cowling, R.M.; Fishpool, L.D.C.; da Fonseca, G.A.B.; Gaston, K.J.; Hoffmann, M.; et al. Effectiveness of the global protected area network in representing species diversity. Nature 2006, 440, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Mace, G.M.; Collar, N.J.; Gaston, K.J.; Hilton-Taylor, C.; Akçakaya, H.R.; Leader-Williams, N.; Stuart, S.N. Quantification of extinction risk: IUCN’s system for classifying threatened species. Conserv. Biol. 2008, 22, 1424–1442. [Google Scholar] [CrossRef]
- Beng, K.C.; Corlett, R.T. Applications of environmental DNA (eDNA) in ecology and conservation: Opportunities, challenges, and prospects. Biodivers. Conserv. 2020, 29, 2089–2121. [Google Scholar] [CrossRef]
- Tan, W.C.; Herrel, A.; Rödder, D. A global analysis of habitat fragmentation research in reptiles and amphibians: What have we done so far? Biodivers. Conserv. 2023, 32, 439–468. [Google Scholar] [CrossRef]
- Foster, R.B.; Parker, T.A., III; Gentry, A.H.; Emmons, L.H.; Chicchon, A.; Schulenberg, T.; Rodriguez, L.; Lamas, G.; Ortega, H.; Icochea, J.; et al. The Tambopata-Candamo Reserved Zone of Southeastern Peru: A Biological Assessment; Rapid Assessment Program Working Paper 6; Conservation International: Washington, DC, USA, 1994; p. 184. [Google Scholar]
- Gentry, A.H.; León, B. Tambopata region, Peru. In Centres of Plant Diversity: A Guide and Strategy for Their Conservation; Davis, S.D., Heywood, V.H., Herrera-Macbryde, O., Villa-Lobos, J., Hamilton, A.C., Eds.; WWF and IUCN: New York, NY, USA, 1997; pp. 355–359. [Google Scholar]
- Laurencio, D.; Malone, J.H. The amphibians and reptiles of Parque Nacional Carara, a transitional herpetofaunal assemblage in Costa Rica. Herpetol. Conserv. Biol. 2009, 4, 120–131. [Google Scholar]
- Köhler, J.; Price, A.H. New species of Eleutherodactylus (Anura: Leptodactylidae) from cloud forest of Bolivia. Copeia 2000, 2000, 516–520. [Google Scholar] [CrossRef]
- Reichle, S.; Embert, D. New species of Clelia (Colubridae) from the inter-Andean dry valleys of Bolivia. J. Herpetol. 2005, 39, 379–383. [Google Scholar] [CrossRef]
- Crandall, K.A.; Bininda-Emonds, O.R.P.; Mace, G.M.; Wayne, R.K. Considering evolutionary processes in conservation biology. Trends Ecol. Evol. 2000, 15, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Campos-Silva, J.V.; Hawes, J.E.; Andrade, P.C.M.; Peres, C.A. Unintended multispecies co-benefits of an Amazonian community-based conservation program. Nat. Sustain. 2018, 1, 650–656. [Google Scholar] [CrossRef]
- Onyishi, I.E.; Nwonyi, S.K.; Pazda, A.; Prokop, P. Attitudes and behaviour toward snakes on the part of Igbo people in southeastern Nigeria. Sci. Total Environ. 2021, 763, 143045. [Google Scholar] [CrossRef] [PubMed]
- Fahrig, L. Effects of habitat fragmentation on biodiversity. Annu. Rev. Ecol. Evol. Syst. 2003, 34, 487–515. [Google Scholar] [CrossRef]
- Newbold, T.; Hudson, L.N.; Hill, S.L.L.; Contu, S.; Lysenko, I.; Senior, R.A.; Börger, L.; Bennett, D.J.; Choimes, A.; Collen, B.; et al. Global effects of land use on local terrestrial biodiversity. Nature 2015, 520, 45–50. [Google Scholar] [CrossRef]
- Böhm, M.; Collen, B.; Baillie, J.E.M.; Bowles, P.; Chanson, J.; Cox, N.; Hammerson, G.; Hoffmann, M.; Livingstone, S.R.; Ram, M.; et al. The conservation status of the world’s reptiles. Biol. Conserv. 2013, 157, 372–385. [Google Scholar] [CrossRef]
- Urbina-Cardona, J.N.; Loyola, R.D. Applying niche-based models to predict endangered-hylid potential distributions: Are neotropical protected areas effective enough? Trop. Conserv. Sci. 2008, 1, 417–445. [Google Scholar] [CrossRef]
- Le Saout, S.; Hoffmann, M.; Shi, Y.; Hughes, A.; Bernard, C.; Brooks, T.M.; Bertzky, B.; Butchart, S.H.M.; Stuart, S.N.; Badman, T.; et al. Protected areas and effective biodiversity conservation. Science 2013, 342, 803–805. [Google Scholar] [CrossRef]
- Cazalis, V.; Princé, K.; Mihoub, J.B.; Kelly, J.; Butchart, S.H.M.; Rodrigues, A.S.L. Effectiveness of protected areas in conserving tropical forest birds. Nat. Commun. 2020, 11, 4461. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, P.J.; Russell, A.P. Systematics and biogeography of the widespread Neotropical gekkonid genus Thecadactylus (Squamata), with the new description of a new cryptic species. Zool. J. Linn. Soc. 2007, 149, 339–370. [Google Scholar] [CrossRef]
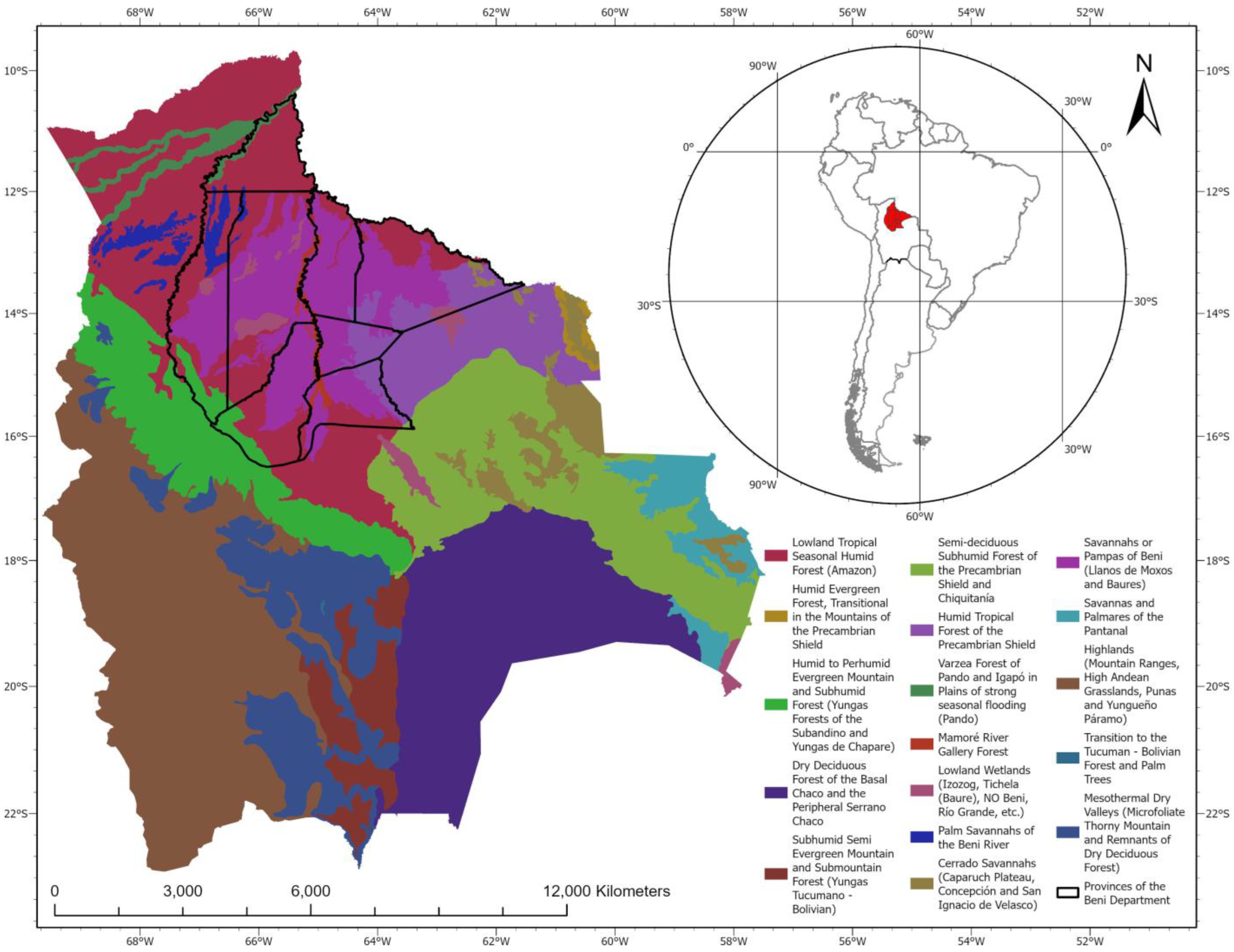
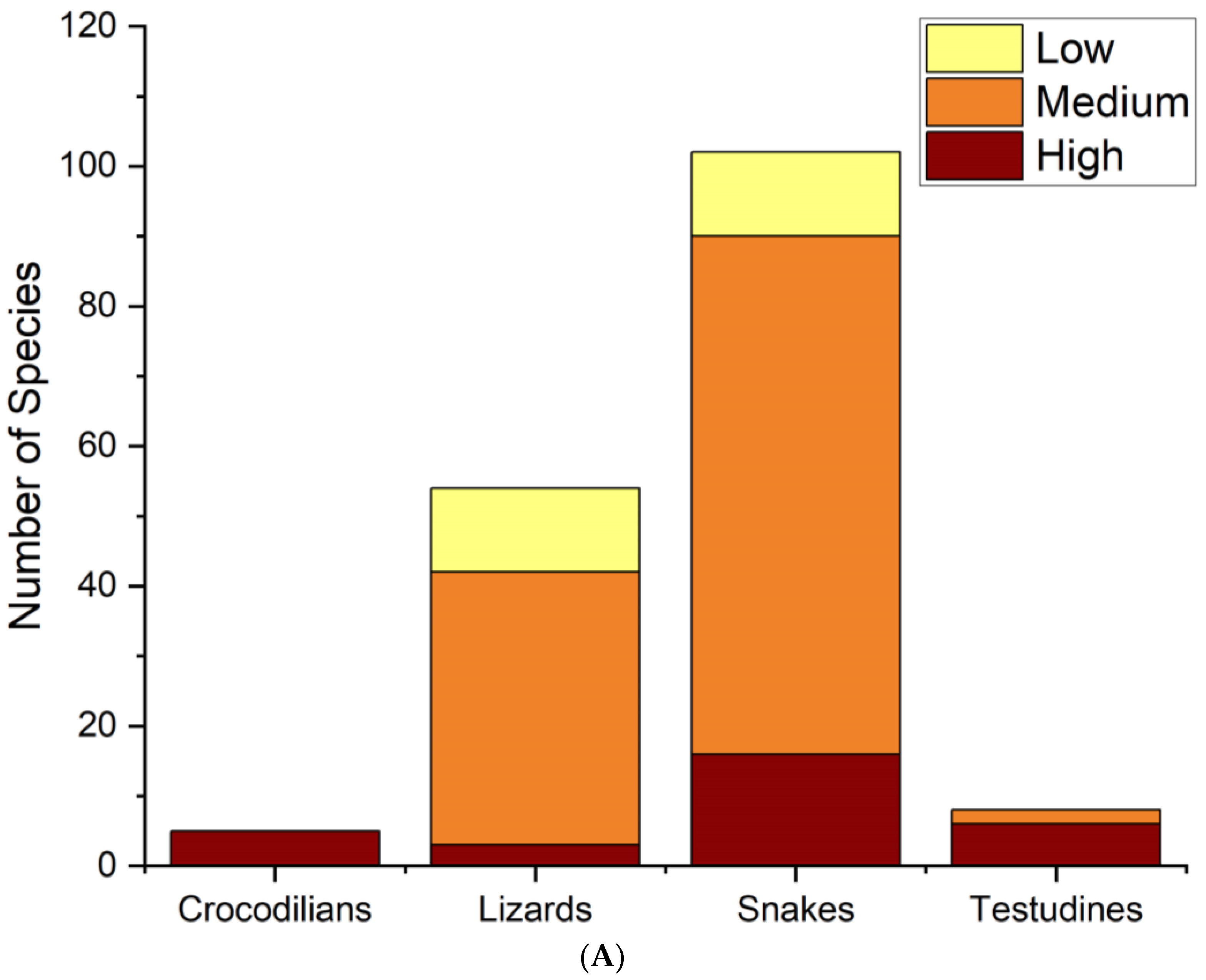
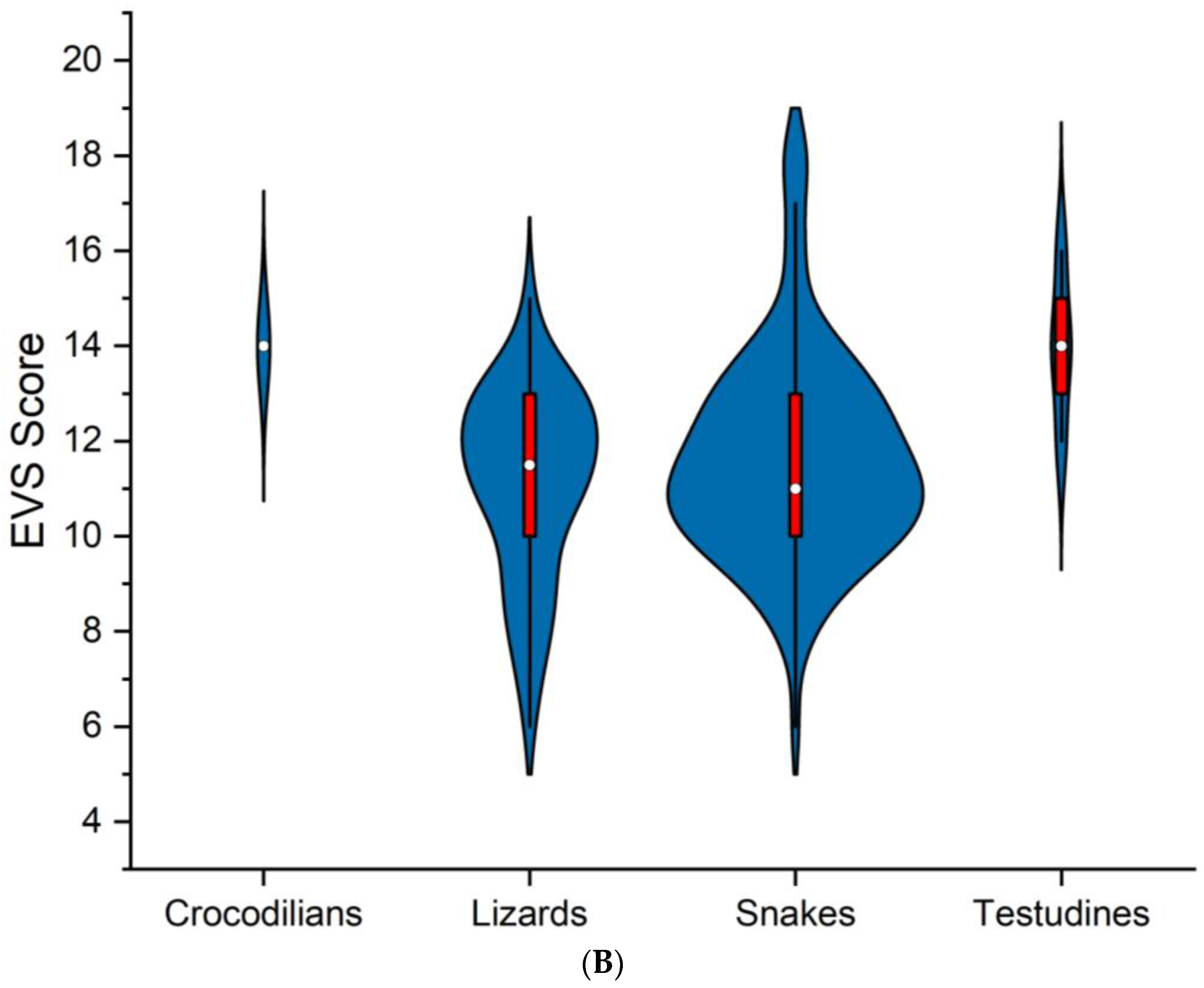
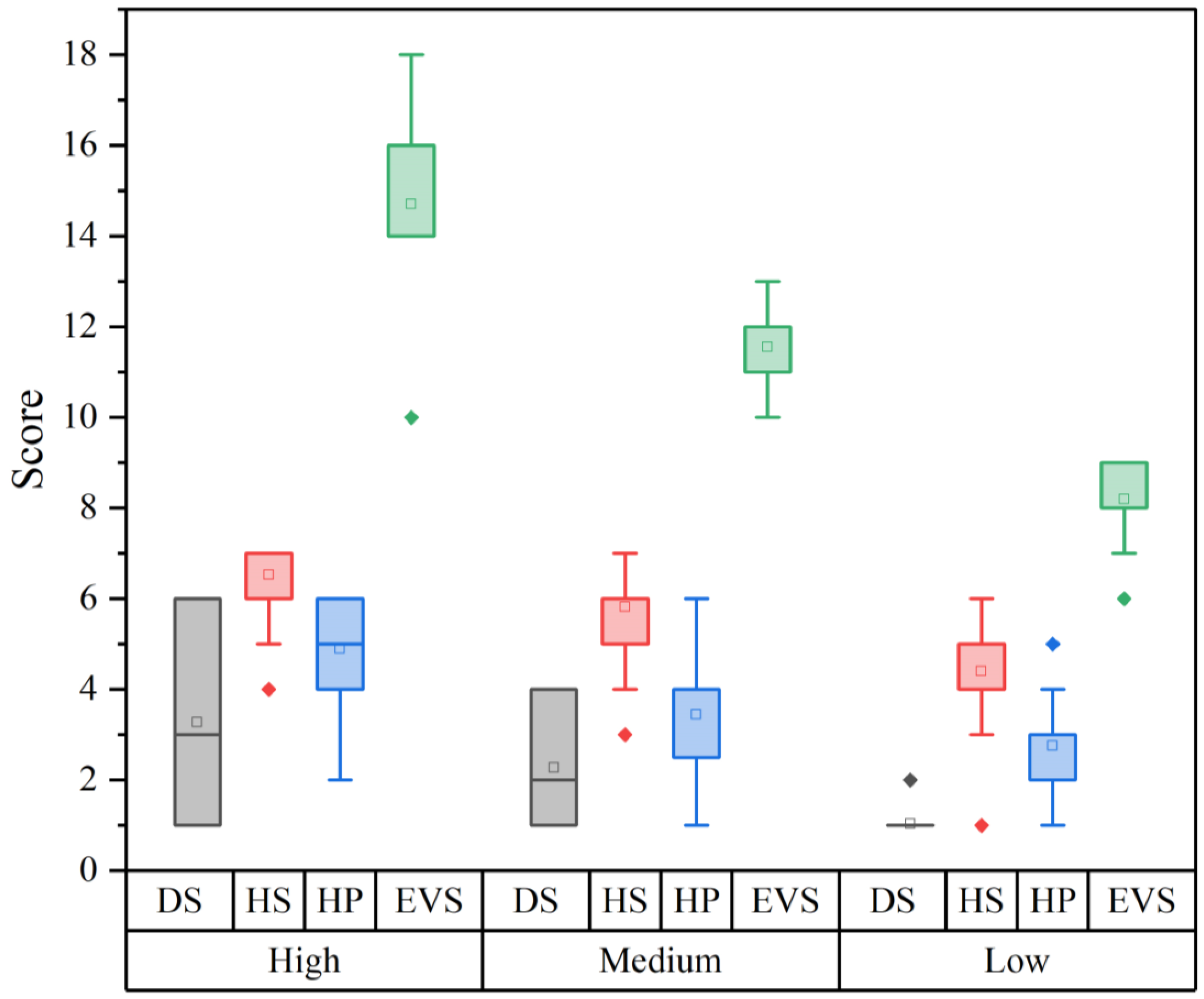
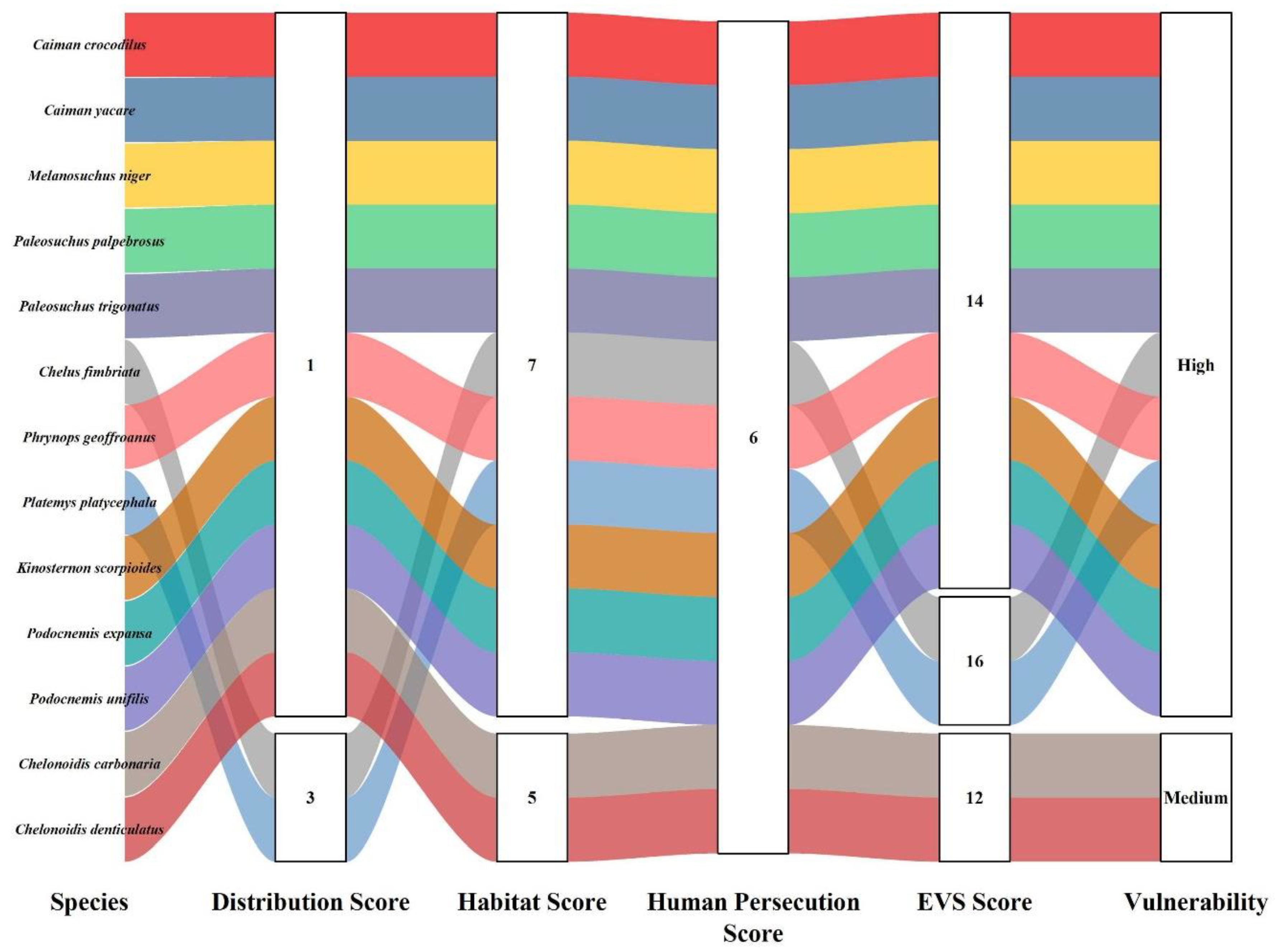
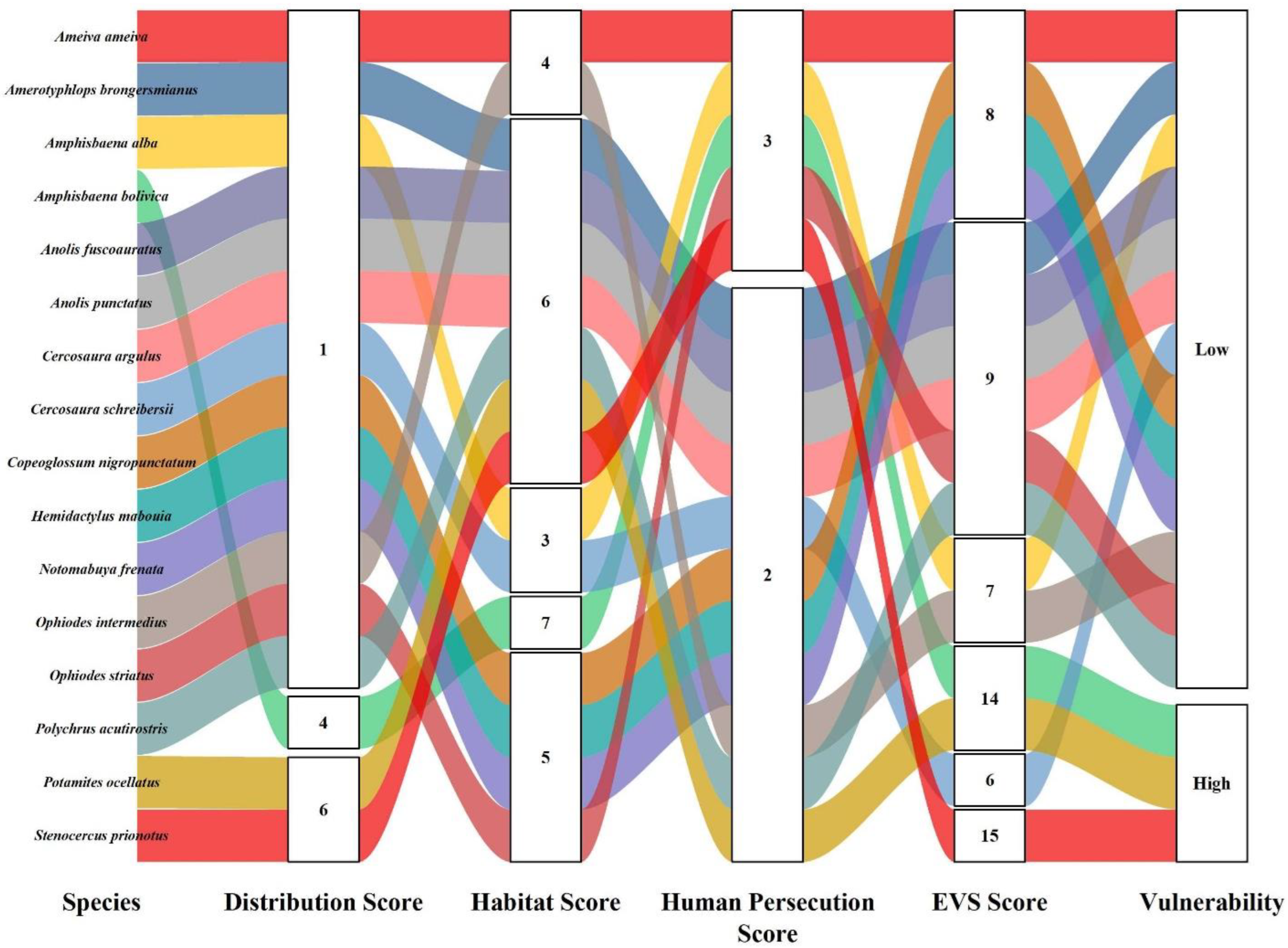
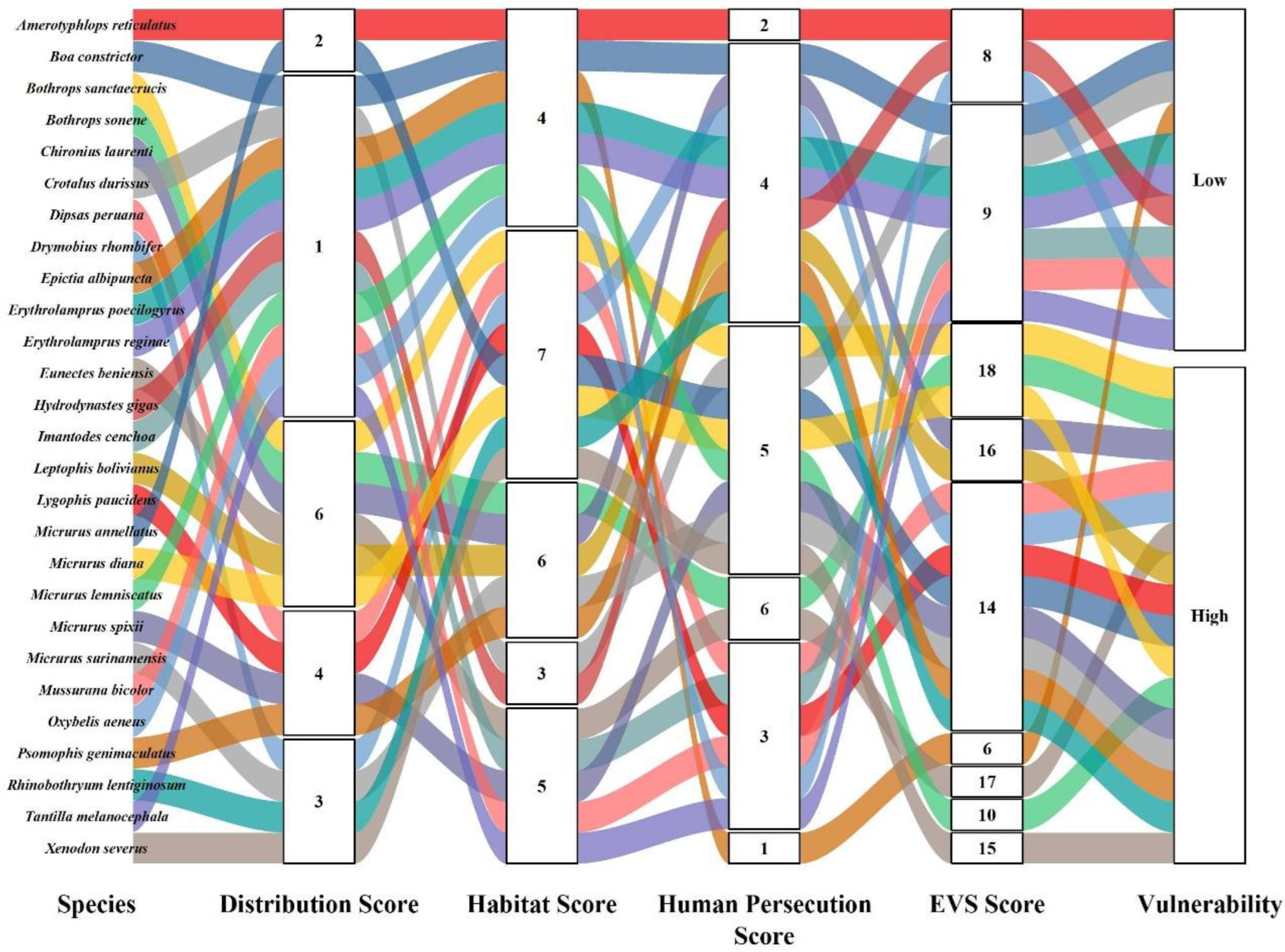
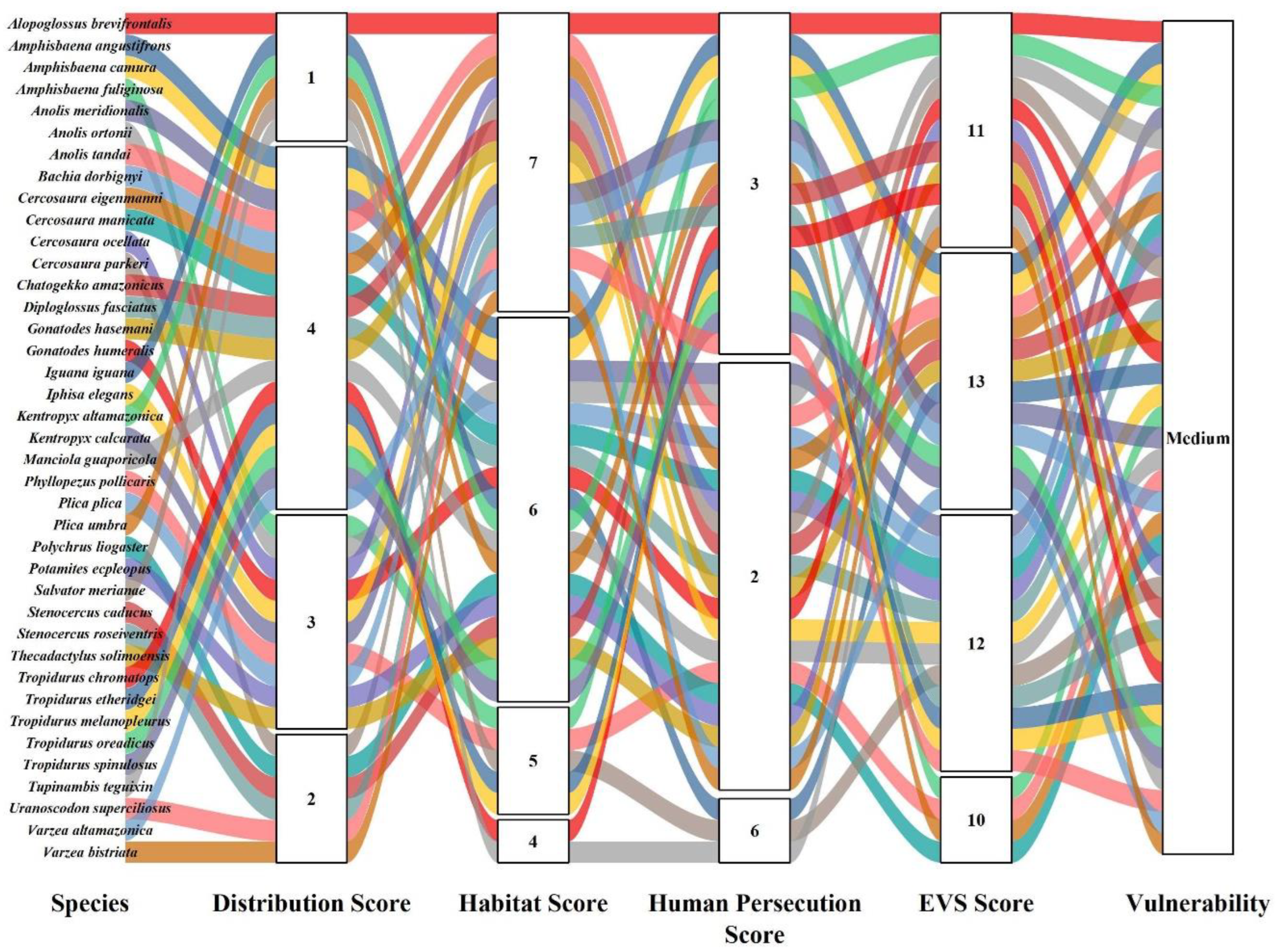
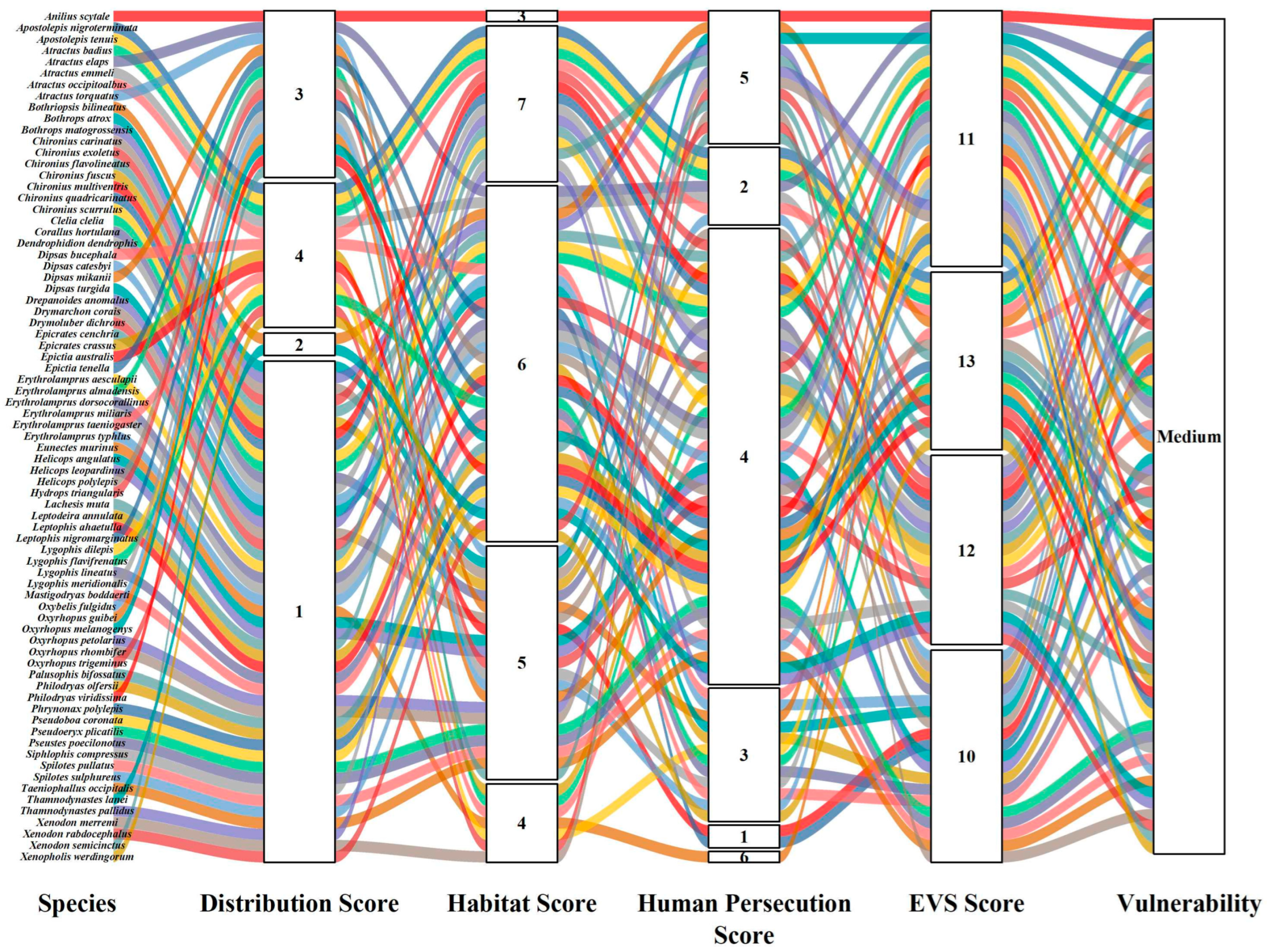
| Order | Family | Species | Authority | DS | HS | HP | EVS | Vulnerability | IUCN | Pop. Status |
|---|---|---|---|---|---|---|---|---|---|---|
| Crocodilia | Alligatoridae | Caiman crocodilus | (Linnaeus, 1758) | 1 | 7 | 6 | 14 | High | LC | Stable |
| Caiman yacare | (Daudin, 1801) | 1 | 7 | 6 | 14 | High | LC | Stable | ||
| Melanosuchus niger | (Spix, 1825) | 1 | 7 | 6 | 14 | High | LC | Unk | ||
| Paleosuchus palpebrosus | (Cuvier, 1807) | 1 | 7 | 6 | 14 | High | LC | Stable | ||
| Paleosuchus trigonatus | (Schneider, 1801) | 1 | 7 | 6 | 14 | High | LC | Stable | ||
| Squamata | Alopoglossidae | Alopoglossus brevifrontalis | (Boulenger, 1912) | 1 | 7 | 3 | 11 | Medium | LC | Unk |
| Amphisbaenidae | Amphisbaena alba | (Linnaeus, 1758) | 1 | 3 | 3 | 7 | Low | LC | Stable | |
| Amphisbaena angustifrons | Cope, 1861 | 4 | 6 | 3 | 13 | Medium | LC | Unk | ||
| Amphisbaena bolivica | (Mertens, 1929) | 4 | 7 | 3 | 14 | High | LC | Stable | ||
| Amphisbaena camura | Cope, 1862 | 4 | 6 | 3 | 13 | Medium | LC | Stable | ||
| Amphisbaena fuliginosa | (Linnaeus, 1758) | 3 | 5 | 3 | 11 | Medium | LC | Stable | ||
| Aniliidae | Anilius scytale | (Linnaeus, 1758) | 3 | 3 | 5 | 11 | Medium | LC | Stable | |
| Boidae | Boa constrictor | (Linnaeus, 1758) | 1 | 4 | 4 | 9 | Low | LC | Decreasing | |
| Corallus hortulana | (Linnaeus, 1758) | 1 | 5 | 4 | 10 | Medium | LC | Stable | ||
| Epicrates cenchria | (Linnaeus, 1758) | 1 | 7 | 4 | 12 | Medium | LC | Stable | ||
| Epicrates crassus | Cope, 1862 | 4 | 4 | 4 | 12 | Medium | LC | Unk | ||
| Eunectes beniensis | (Dirksen, 2002) | 6 | 5 | 6 | 17 | High | LC | Unk | ||
| Eunectes murinus | (Linnaeus, 1758) | 1 | 4 | 6 | 11 | Medium | LC | Unk | ||
| Colubridae | Adelphostigma occipitalis | (Jan, 1863) | 1 | 5 | 4 | 10 | Medium | LC | Stable | |
| Apostolepis nigroterminata | Boulenger, 1896 | 4 | 7 | 2 | 13 | Medium | LC | Unk | ||
| Apostolepis tenuis | Ruthven, 1927 | 4 | 7 | 2 | 13 | Medium | DD | Unk | ||
| Atractus elaps | (Günther, 1858) | 3 | 6 | 2 | 11 | Medium | LC | Unk | ||
| Atractus emmeli | (Boettger, 1888) | 4 | 6 | 2 | 12 | Medium | LC | Unk | ||
| Atractus occipitoalbus | (Jan, 1862) | 4 | 7 | 2 | 13 | Medium | NT | Unk | ||
| Atractus torquatus | (Duméril, Bibron and Duméril, 1854) | 3 | 5 | 2 | 10 | Medium | LC | Stable | ||
| Chironius carinatus | (Linnaeus, 1758) | 1 | 5 | 4 | 10 | Medium | LC | Stable | ||
| Chironius exoletus | (Linnaeus, 1758) | 1 | 7 | 4 | 12 | Medium | LC | Stable | ||
| Chironius flavolineatus | (Jan, 1863) | 1 | 6 | 4 | 11 | Medium | LC | Unk | ||
| Chironius fuscus | (Linnaeus, 1758) | 1 | 5 | 4 | 10 | Medium | LC | Stable | ||
| Chironius laurenti | (Dixon, Wiest and Cei, 1993) | 6 | 6 | 4 | 16 | High | LC | Unk | ||
| Chironius multiventris | Schmidt and Walker, 1943 | 1 | 7 | 4 | 12 | Medium | LC | Stable | ||
| Chironius quadricarinatus | (Boie, 1827) | 1 | 7 | 4 | 12 | Medium | LC | Unk | ||
| Chironius scurrulus | (Wagler, 1824) | 1 | 6 | 4 | 11 | Medium | LC | Stable | ||
| Clelia clelia | (Daudin, 1803) | 1 | 6 | 4 | 11 | Medium | LC | Stable | ||
| Dendrophidion dendrophis | (Schlegel, 1837) | 1 | 7 | 4 | 12 | Medium | LC | Stable | ||
| Dipsas bucephala | (Shaw, 1802) | 4 | 6 | 3 | 13 | Medium | LC | Unk | ||
| Dipsas catesbyi | (Sentzen, 1796) | 1 | 6 | 3 | 10 | Medium | LC | Unk | ||
| Dipsas mikanii | (Schlegel, 1837) | 3 | 5 | 3 | 11 | Medium | LC | Stable | ||
| Dipsas peruana | (Boettger, 1898) | 4 | 7 | 3 | 14 | High | LC | Stable | ||
| Dipsas turgida | (Cope, 1868) | 1 | 6 | 3 | 10 | Medium | LC | Stable | ||
| Drepanoides anomalus | (Jan, 1863) | 1 | 7 | 4 | 12 | Medium | LC | Stable | ||
| Drymarchon corais | (Boie, 1827) | 1 | 5 | 4 | 10 | Medium | LC | Stable | ||
| Drymobius rhombifer | (Günther, 1860) | 3 | 7 | 4 | 14 | High | LC | Stable | ||
| Drymoluber dichrous | (Peters, 1863) | 1 | 6 | 4 | 11 | Medium | LC | Stable | ||
| Erythrolamprus aesculapii | (Linnaeus, 1758) | 1 | 7 | 4 | 12 | Medium | LC | Stable | ||
| Erythrolamprus almadensis | (Wagler, 1824) | 3 | 4 | 4 | 11 | Medium | LC | Stable | ||
| Erythrolamprus dorsocorallinus | (Esqueda, Natera, La Marca and Ilija-Fistar, 2007) | 1 | 6 | 4 | 11 | Medium | LC | Unk | ||
| Erythrolamprus miliaris | (Linnaeus, 1758) | 1 | 6 | 4 | 11 | Medium | LC | Stable | ||
| Erythrolamprus poecilogyrus | (Wied-Neuwied, 1824) | 1 | 4 | 4 | 9 | Low | LC | Increasing | ||
| Erythrolamprus reginae | (Linnaeus, 1758) | 1 | 4 | 4 | 9 | Low | LC | Stable | ||
| Erythrolamprus taeniogaster | (Linnaeus, 1758) | 4 | 4 | 4 | 12 | Medium | LC | Stable | ||
| Erythrolamprus typhlus | (Linnaeus, 1758) | 1 | 6 | 4 | 11 | Medium | LC | Stable | ||
| Helicops angulatus | (Linnaeus, 1758) | 1 | 5 | 4 | 10 | Medium | LC | Stable | ||
| Helicops leopardinus | (Schlegel, 1837) | 1 | 5 | 4 | 10 | Medium | LC | Stable | ||
| Helicops polylepis | (Günther, 1861) | 3 | 6 | 4 | 13 | Medium | LC | Stable | ||
| Hydrodynastes gigas | (Duméril, Bibron and Duméril, 1854) | 1 | 3 | 4 | 8 | Low | LC | Stable | ||
| Hydrops triangularis | (Wagler, 1824) | 3 | 5 | 4 | 12 | Medium | LC | Stable | ||
| Imantodes cenchoa | (Linnaeus, 1758) | 1 | 5 | 3 | 9 | Low | LC | Stable | ||
| Leptodeira annulata | (Linnaeus, 1758) | 1 | 6 | 3 | 10 | Medium | LC | Stable | ||
| Leptophis ahaetulla | (Linnaeus, 1758) | 1 | 6 | 4 | 11 | Medium | LC | Stable | ||
| Leptophis bolivianus | Oliver, 1942 | 6 | 6 | 4 | 16 | High | LC | Stable | ||
| Leptophis nigromarginatus | (Gunther, 1866) | 3 | 6 | 4 | 13 | Medium | LC | Stable | ||
| Lygophis dilepis | Cope, 1862 | 4 | 4 | 3 | 11 | Medium | LC | Stable | ||
| Lygophis flavifrenatus | Cope, 1862 | 4 | 6 | 3 | 13 | Medium | LC | Stable | ||
| Lygophis lineatus | (Linnaeus, 1758) | 1 | 6 | 3 | 10 | Medium | LC | Stable | ||
| Lygophis meridionalis | (Schenkel, 1901) | 3 | 5 | 3 | 11 | Medium | LC | Unk | ||
| Lygophis paucidens * | Hoge, 1953 | 4 | 7 | 3 | 14 | High | LC | Unk | ||
| Mastigodryas boddaerti | (Sentzen, 1796) | 1 | 6 | 3 | 10 | Medium | LC | Stable | ||
| Mussurana bicolor | (Peracca, 1904) | 1 | 5 | 3 | 9 | Low | LC | Stable | ||
| Oxybelis aeneus | (Wagler, 1824) | 1 | 4 | 3 | 8 | Low | LC | Stable | ||
| Oxybelis fulgidus | (Daudin, 1803) | 3 | 5 | 3 | 11 | Medium | LC | Stable | ||
| Oxyrhopus guibei | Hoge and Romano 1977 | 3 | 5 | 4 | 13 | Medium | LC | Stable | ||
| Oxyrhopus melanogenys | (Tschudi, 1845) | 3 | 6 | 4 | 13 | Medium | LC | Stable | ||
| Oxyrhopus petolarius | (Linnaeus, 1758) | 1 | 5 | 5 | 11 | Medium | LC | Unk | ||
| Oxyrhopus rhombifer | Duméril, Bibron and Duméril, 1854 | 1 | 5 | 5 | 11 | Medium | LC | Stable | ||
| Oxyrhopus trigeminus | Duméril, Bibron and Duméril, 1854 | 4 | 4 | 5 | 13 | Medium | LC | Stable | ||
| Palusophis bifossatus | (Raddi, 1820) | 1 | 6 | 5 | 12 | Medium | LC | Unk | ||
| Philodryas olfersii | (Lichtenstein, 1823) | 1 | 6 | 4 | 11 | Medium | LC | Stable | ||
| Philodryas viridissima | (Linnaeus, 1758) | 3 | 6 | 4 | 13 | Medium | LC | Stable | ||
| Phrynonax polylepis | (Peters, 1867) | 1 | 6 | 4 | 11 | Medium | LC | Stable | ||
| Pseudoboa coronata | Schneider, 1801 | 1 | 6 | 4 | 11 | Medium | LC | Stable | ||
| Pseudoeryx plicatilis | (Linnaeus, 1758) | 1 | 5 | 4 | 10 | Medium | LC | Stable | ||
| Pseustes poecilonotus | (Günther, 1858) | 1 | 5 | 4 | 10 | Medium | LC | Stable | ||
| Psomophis genimaculatus | (Boettger, 1885) | 4 | 6 | 4 | 14 | High | LC | Stable | ||
| Rhinobothryum lentiginosum | (Scopoli, 1785) | 3 | 7 | 4 | 14 | High | LC | Stable | ||
| Siphlophis compressus | (Daudin, 1803) | 1 | 7 | 4 | 12 | Medium | LC | Stable | ||
| Spilotes pullatus | (Linnaeus, 1758) | 1 | 5 | 4 | 10 | Medium | LC | Stable | ||
| Spilotes sulphureus | (Wagler, 1824) | 1 | 6 | 4 | 11 | Medium | LC | Stable | ||
| Tantilla melanocephala | (Linnaeus, 1758) | 1 | 5 | 3 | 9 | Low | LC | Stable | ||
| Thamnodynastes lanei | Bailey, Thomas and Da Silva, 2005 | 2 | 6 | 4 | 12 | Medium | LC | Unk | ||
| Thamnodynastes pallidus | (Linnaeus, 1758) | 1 | 7 | 4 | 12 | Medium | LC | Stable | ||
| Xenodon merremi | (Wagler, 1824) | 1 | 4 | 5 | 10 | Medium | LC | Unk | ||
| Xenodon rabdocephalus | (Wied-Neuwied, 1824) | 1 | 6 | 5 | 12 | Medium | LC | Stable | ||
| Xenodon semicinctus * | (Duméril, Bibron and Duméril, 1854) | 3 | 5 | 5 | 13 | Medium | LC | Stable | ||
| Xenodon severus | (Linnaeus, 1758) | 3 | 7 | 5 | 15 | High | LC | Stable | ||
| Xenopholis werdingorum | Jansen, Álvarez and Köhler, 2009 | 4 | 6 | 3 | 13 | Medium | LC | Stable | ||
| Dactyloidae | Anolis fuscoauratus | D’Orbigny, 1837 | 1 | 6 | 2 | 9 | Low | LC | Stable | |
| Anolis meridionalis | Boettger, 1885 | 4 | 6 | 2 | 12 | Medium | LC | Stable | ||
| Anolis ortonii | Cope, 1868 | 3 | 6 | 2 | 11 | Medium | LC | Stable | ||
| Anolis punctatus | Daudin, 1802 | 1 | 6 | 2 | 9 | Low | LC | Unk | ||
| Anolis tandai * | Avila-Pires, 1995 | 4 | 7 | 2 | 13 | Medium | LC | Unk | ||
| Diploglossidae | Diploglossus fasciatus | (Gray,1831) | 4 | 6 | 2 | 12 | Medium | LC | Unk | |
| Ophiodes intermedius * | Boulenger, 1894 | 1 | 4 | 2 | 7 | Low | LC | Stable | ||
| Ophiodes striatus | Spix (1824) | 1 | 5 | 3 | 9 | Low | LC | Unk | ||
| Elapidae | Micrurus annellatus | Peters, 1871 | 2 | 7 | 5 | 14 | High | LC | Unk | |
| Micrurus diana | Roze, 1983 | 6 | 7 | 5 | 18 | High | LC | Unk | ||
| Micrurus lemniscatus | (Linnaeus, 1758) | 1 | 4 | 5 | 10 | High | LC | Decreasing | ||
| Micrurus spixii | Wagler, 1824 | 4 | 5 | 5 | 14 | High | LC | Stable | ||
| Micrurus surinamensis | (Cuvier, 1816) | 3 | 6 | 5 | 14 | High | LC | Stable | ||
| Gekkonidae | Hemidactylus mabouia | (Moreau de Jonnès, 1818) | 1 | 5 | 2 | 8 | Low | LC | Stable | |
| Gymnophthalmidae | Bachia dorbignyi | (Duméril and Bibron, 1839) | 4 | 6 | 2 | 12 | Medium | LC | Unk | |
| Cercosaura argulus | Peters, 1862 | 1 | 6 | 2 | 9 | Low | LC | Unk | ||
| Cercosaura eigenmanni * | (Griffin, 1917) | 4 | 7 | 2 | 13 | Medium | LC | Stable | ||
| Cercosaura manicata | O’Shaughnessy, 1881 | 4 | 6 | 2 | 12 | Medium | LC | Unk | ||
| Cercosaura ocellata | Wagler, 1830 | 3 | 7 | 2 | 12 | Medium | LC | Stable | ||
| Cercosaura parkeri | (Ruibal, 1952) | 2 | 7 | 2 | 11 | Medium | LC | Stable | ||
| Cercosaura schreibersii | Wiegmannnnnn, 1834 | 1 | 3 | 2 | 6 | Low | LC | Stable | ||
| Potamites ocellatus | (Sinitsin, 1930) | 6 | 6 | 2 | 14 | High | VU | Decreasing | ||
| Iphisa elegans | Gray, 1851 | 3 | 7 | 2 | 12 | Medium | LC | Stable | ||
| Potamites ecpleopus | (Cope, 1875) | 3 | 6 | 2 | 11 | Medium | LC | Unk | ||
| Iguanidae | Iguana iguana | (Linnaeus, 1758) | 1 | 6 | 6 | 13 | Medium | LC | Unk | |
| Leptotyphlopidae | Epictia albipuncta | (Burmeister, 1861) | 1 | 4 | 1 | 6 | Low | LC | Stable | |
| Epictia australis | (Freiberg and Orejas-Miranda, 1968) | 4 | 5 | 1 | 10 | Medium | LC | Stable | ||
| Epictia tenella | (Klauber, 1939) | 3 | 6 | 1 | 10 | Medium | LC | Stable | ||
| Phyllodactylidae | Phyllopezus pollicaris | (Spix, 1825) | 3 | 5 | 2 | 10 | Medium | LC | Unk | |
| Thecadactylus solimoensis | Bergmann and Russell, 2007 | 3 | 6 | 2 | 11 | Medium | LC | Stable | ||
| Polychrotidae | Polychrus acutirostris | (Spix, 1825) | 1 | 6 | 2 | 9 | Low | LC | Stable | |
| Polychrus liogaster | Boulenger, 1908 | 2 | 6 | 2 | 10 | Medium | LC | Unk | ||
| Scincidae | Copeoglossum nigropunctatum | (Spix, 1825) | 1 | 5 | 2 | 8 | Low | LC | Decreasing | |
| Manciola guaporicola | (Dunn, 1935) | 4 | 6 | 2 | 12 | Medium | LC | Decreasing | ||
| Notomabuya frenata | (Cope, 1862) | 1 | 5 | 2 | 8 | Low | LC | Stable | ||
| Varzea altamazonica | Miralles, Barrio-Amoros, Rivas, Chaparro-Auza, 2006 | 4 | 7 | 2 | 13 | Medium | LC | Unk | ||
| Varzea bistriata | (Spix, 1825) | 2 | 7 | 2 | 11 | Medium | LC | Unk | ||
| Sphaerodactylidae | Chatogekko amazonicus * | (Andersson, 1918) | 4 | 7 | 2 | 13 | Medium | LC | Stable | |
| Gonatodes hasemani | (Griffin, 1917) | 4 | 7 | 2 | 13 | Medium | LC | Stable | ||
| Gonatodes humeralis | (Guichenot, 1855) | 3 | 6 | 2 | 11 | Medium | LC | Stable | ||
| Teiidae | Ameiva ameiva | (Linnaeus, 1758) | 1 | 4 | 3 | 8 | Low | LC | Stable | |
| Kentropyx altamazonica | (Cope, 1875) | 1 | 6 | 3 | 10 | Medium | LC | Stable | ||
| Kentropyx calcarata | Spix, 1825 | 3 | 7 | 3 | 13 | Medium | LC | Stable | ||
| Salvator merianae | Duméril and Bibron, 1839 | 1 | 5 | 6 | 12 | Medium | LC | Stable | ||
| Tupinambis teguixin | (Linnaeus, 1758) | 1 | 4 | 6 | 11 | Medium | LC | Stable | ||
| Tropiduridae | Plica plica | (Linnaeus, 1758) | 3 | 7 | 3 | 13 | Medium | LC | Stable | |
| Plica umbra | (Linnaeus, 1758) | 1 | 6 | 3 | 10 | Medium | LC | Stable | ||
| Stenocercus caducus | (Copes, 1862) | 2 | 6 | 3 | 11 | Medium | LC | Stable | ||
| Stenocercus prionotus | Cadle, 2001 | 6 | 6 | 3 | 15 | High | LC | Unk | ||
| Stenocercus roseiventris | D’Orbigny in Duméril and Bibron, 1837 | 2 | 7 | 3 | 12 | Medium | LC | Stable | ||
| Tropidurus chromatops | Harvey and Gutberlet, 1998 | 4 | 4 | 3 | 11 | Medium | LC | Decreasing | ||
| Tropidurus Etheridgei * | Cei, 1982 | 4 | 5 | 3 | 12 | Medium | LC | Unk | ||
| Tropidurus melanopleurus | Boulenger, 1902 | 4 | 5 | 3 | 12 | Medium | LC | Stable | ||
| Tropidurus oreadicus * | Rodrigues, 1987 | 4 | 6 | 3 | 13 | Medium | LC | Unk | ||
| Tropidurus spinulosus | (Cope, 1862) | 4 | 6 | 3 | 13 | Medium | LC | Unk | ||
| Uranoscodon superciliosus | (Linnaeus, 1758) | 2 | 7 | 3 | 12 | Medium | LC | Stable | ||
| Typhlopidae | Amerotyphlops brongersmianus | (Vanzolini, 1976) | 1 | 6 | 2 | 9 | Low | LC | Stable | |
| Amerotyphlops reticulatus | (Linnaeus, 1758) | 2 | 4 | 2 | 8 | Low | LC | Stable | ||
| Viperidae | Bothriopsis bilineatus | (Wied-Neuwied, 1821) | 2 | 6 | 5 | 13 | Medium | LC | Stable | |
| Bothrops atrox | (Linnaeus, 1758) | 1 | 5 | 5 | 11 | Medium | LC | Stable | ||
| Bothrops matogrossensis | (Amaral, 1925) | 1 | 6 | 5 | 12 | Medium | LC | Unk | ||
| Bothrops sanctaecrucis | Hoge, 1966 | 6 | 7 | 5 | 18 | High | LC | Decreasing | ||
| Bothrops sonene | Carrasco et al. 2019 | 6 | 6 | 6 | 18 | High | NE | Unk | ||
| Crotalus durissus | (Linnaeus, 1758) | 1 | 3 | 5 | 9 | Low | LC | Unk | ||
| Lachesis muta | (Linnaeus, 1766) | 1 | 7 | 5 | 13 | Medium | LC | Unk | ||
| Testudines | Chelidae | Chelus fimbriata | (Schneider, 1783) | 3 | 7 | 6 | 16 | High | LC | Unk |
| Phrynops geoffroanus | (Schweigger, 1812) | 1 | 7 | 6 | 14 | High | LC | Unk | ||
| Platemys platycephala | (Schneider, 1792) | 3 | 7 | 6 | 16 | High | LC | Unk | ||
| Kinosternidae | Kinosternon scorpioides | (Linnaeus, 1766) | 1 | 7 | 6 | 14 | High | LC | Stable | |
| Podocnemididae | Podocnemis expansa | (Schweigger, 1812) | 1 | 7 | 6 | 14 | High | LC | Unk | |
| Podocnemis unifilis | (Troschel, 1848) | 1 | 7 | 6 | 14 | High | VU | Unk | ||
| Testudinidae | Chelonoidis carbonaria | (Spix, 1824) | 1 | 5 | 6 | 12 | Medium | NE | Unk | |
| Chelonoidis denticulatus | (Linnaeus, 1766) | 1 | 5 | 6 | 12 | Medium | VU | Unk |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eversole, C.B.; Powell, R.L.; Rivas, L.R.; Lizarro, D.E. Reptile Biodiversity and Vulnerability in Bolivia’s Beni Department: Informing Conservation Priorities in a Neglected Frontier. Diversity 2024, 16, 335. https://doi.org/10.3390/d16060335
Eversole CB, Powell RL, Rivas LR, Lizarro DE. Reptile Biodiversity and Vulnerability in Bolivia’s Beni Department: Informing Conservation Priorities in a Neglected Frontier. Diversity. 2024; 16(6):335. https://doi.org/10.3390/d16060335
Chicago/Turabian StyleEversole, Cord B., Randy L. Powell, Luis R. Rivas, and Dennis E. Lizarro. 2024. "Reptile Biodiversity and Vulnerability in Bolivia’s Beni Department: Informing Conservation Priorities in a Neglected Frontier" Diversity 16, no. 6: 335. https://doi.org/10.3390/d16060335
APA StyleEversole, C. B., Powell, R. L., Rivas, L. R., & Lizarro, D. E. (2024). Reptile Biodiversity and Vulnerability in Bolivia’s Beni Department: Informing Conservation Priorities in a Neglected Frontier. Diversity, 16(6), 335. https://doi.org/10.3390/d16060335







