Maxent Predictive Species Distribution Models and Model Accuracy Assessment for Two Species of Psilochalcis Kieffer (Hymenoptera: Chalcididae) Occurring in the Eastern Great Basin of Utah, USA
Abstract
1. Introduction
2. Materials and Methods
2.1. Species Occurrence Data
2.2. Environmental Variables
2.3. Species Distribution Model Construction
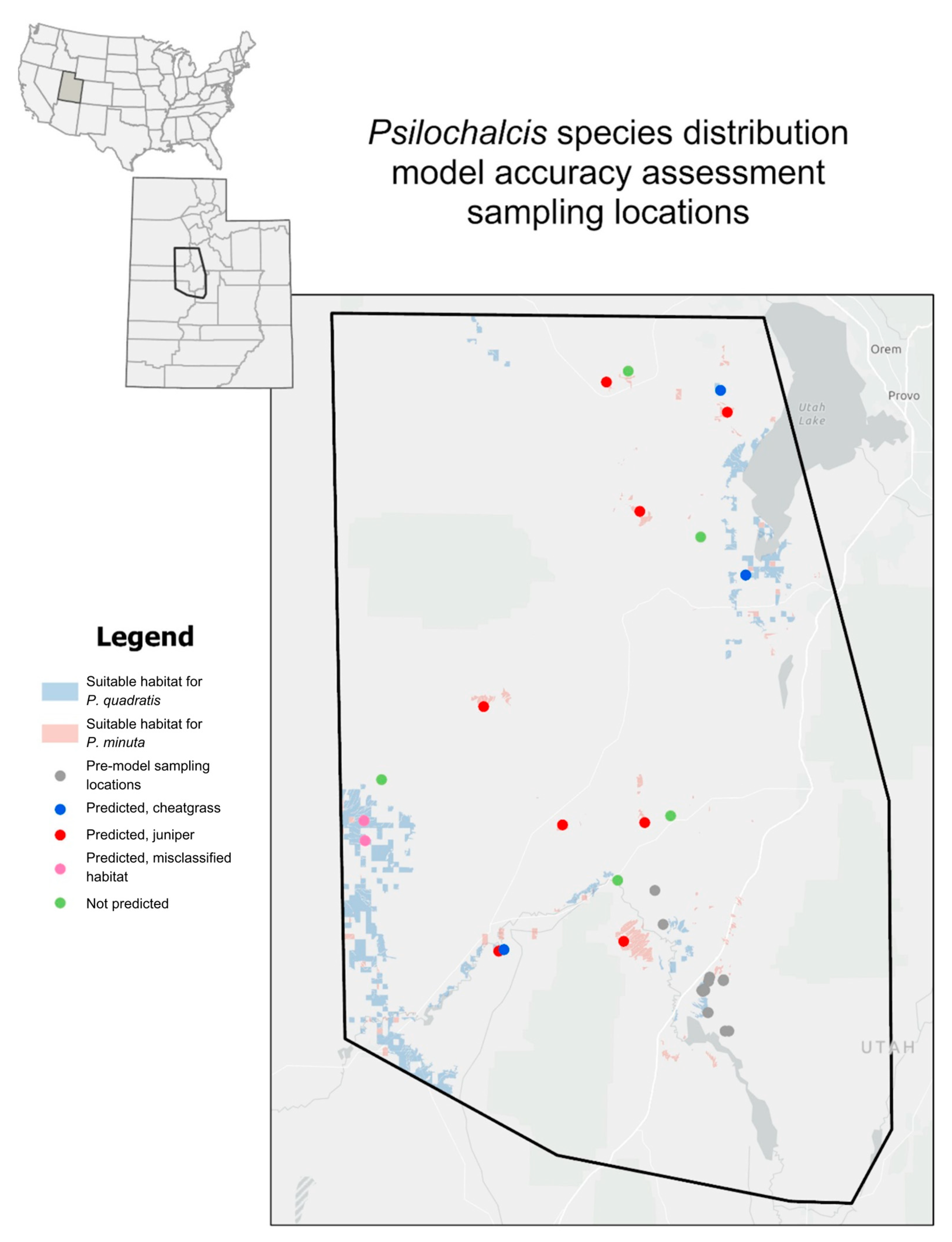
2.4. Sampling Location Selection for Model Accuracy Assessment
3. Results
3.1. Model Accuracy Assessment Sampling Summary
3.2. Maxent Modeling Results
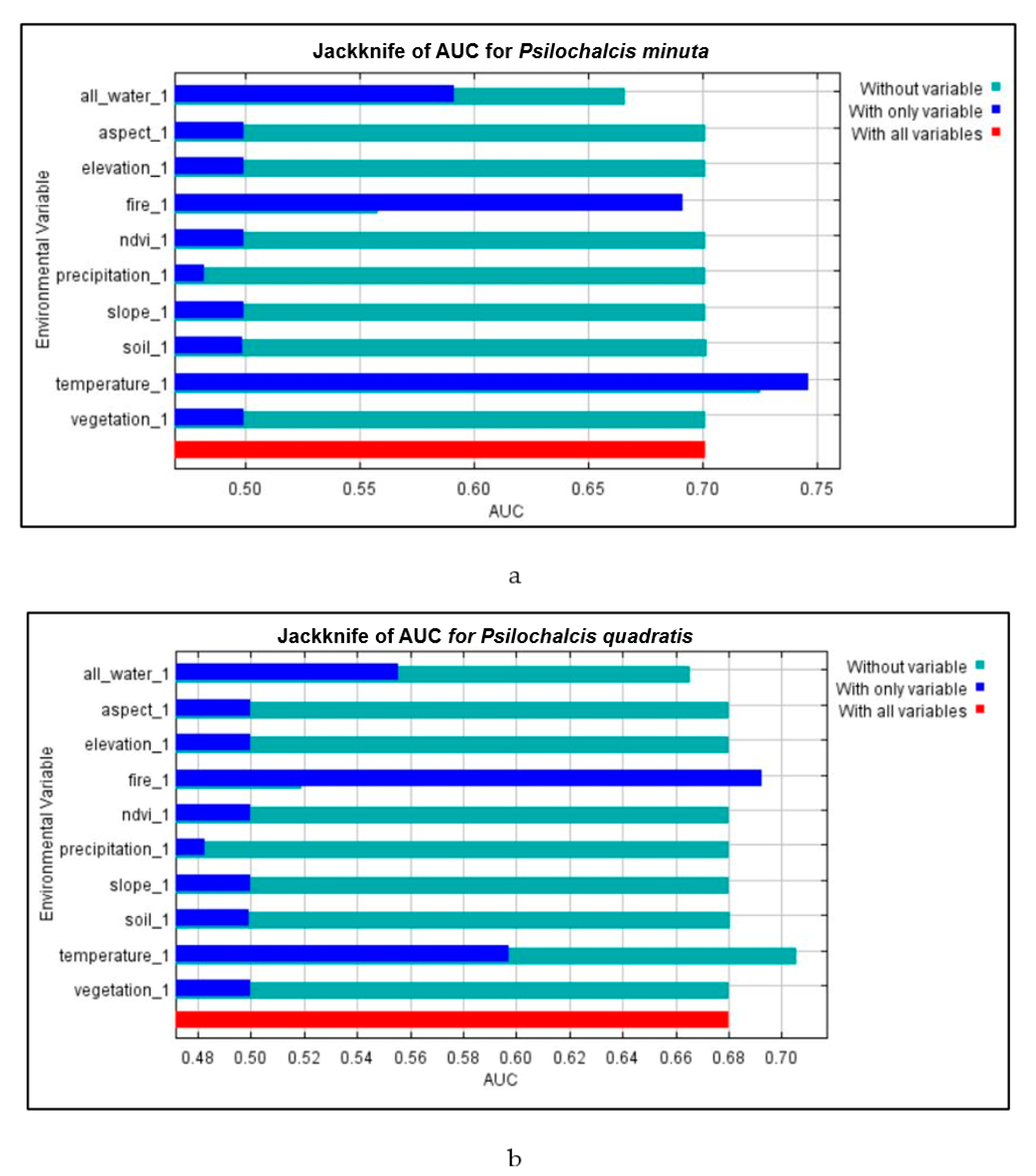
3.3. Environmental Variable Analyses
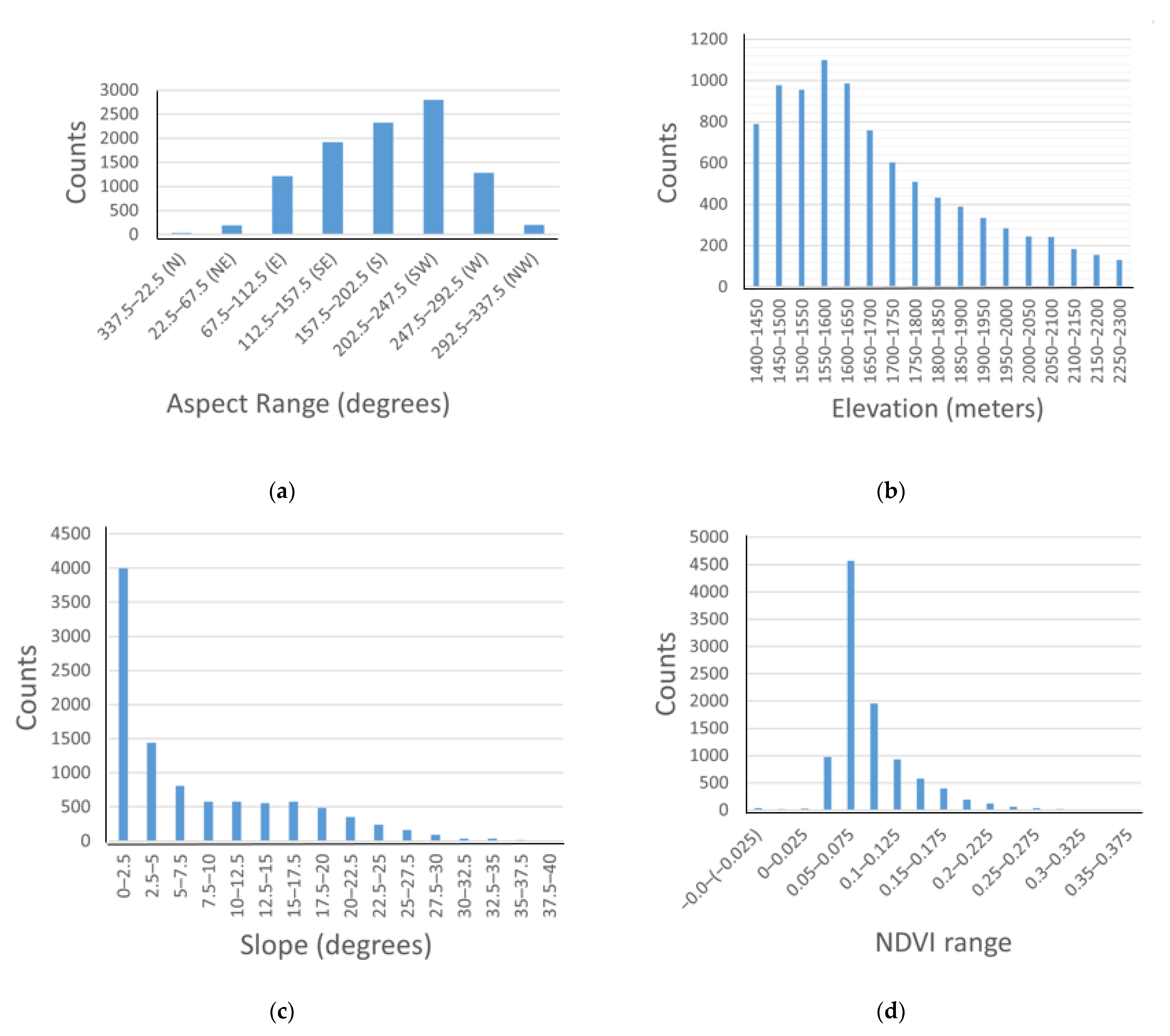
3.4. Variable Response Graphs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Johnson, R.L.; Anderson, V.J.; Clark, S.M. Chapter 1 Impact of Habitat Alterations to Bee Diversity in Sagebrush and Pinyon/Juniper Communities of the Eastern Great Basin. Unpublished Dissertation, Department of Plant and Wildlife Sciences, Brigham Young University, Provo, UT, USA, 2008. [Google Scholar]
- Petersen, M.J.; Johnson, R.L.; Anderson, V.J. A review of Psilochalcis Kieffer (Hymenoptera: Chalcidoidea: Chalcididae) from the western United States with descriptions of three new species from Utah and surrounding states. West. N. Am. Nat. 2022, 82, 704–718. [Google Scholar] [CrossRef]
- Petersen, M.J.; Anderson, V.J.; Johnson, R.L.; Eggett, D.L. Seasonal Trap Abundance of Two Species of Psilochalcis Kieffer (Hymenoptera: Chalcididae) in Rangelands of the Eastern Great Basin of Utah, USA. Land 2023, 12, 54. [Google Scholar] [CrossRef]
- Franklin, J. Mapping Species Distributions: Spatial Inference and Prediction; Cambridge University Press: Cambridge, UK, 2010. [Google Scholar]
- Wiersma, Y.F.; Huettmann, F.; Drew, C.A. Introduction. landscape modeling of species and their habitats: History, uncertainty, and complexity. In Predictive Species and Habitat Modeling in Landscape Ecology; Springer: New York, NY, USA, 2011; pp. 1–6. [Google Scholar]
- Davies, A.J.; Wisshak, M.; Orr, J.C.; Roberts, J.M. Predicting suitable habitat for the cold-water coral Lophelia pertusa (Scleractinia). Deep Sea Res. Part I Oceanogr. Res. Pap. 2008, 55, 1048–1062. [Google Scholar] [CrossRef]
- Ulrichs, C.; Hopper, K.R. Predicting insect distributions from climate and habitat data. BioControl 2008, 53, 881–894. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Yoon, S.; Lee, W.H. Methodological analysis of bioclimatic variable selection in species distribution modeling with application to agricultural pests (Metcalfa pruinosa and Spodoptera litura). Comput. Electron. Agric. 2021, 190, 106430. [Google Scholar] [CrossRef]
- Byeon, D.H.; Jung, S.; Lee, W.H. Review of CLIMEX and MaxEnt for studying species distribution in South Korea. J. Asia-Pac. Biodivers. 2018, 11, 325–333. [Google Scholar] [CrossRef]
- Sunil, K.; Stohlgren, T.J. MaxEnt modeling for predicting suitable habitat for threatened and endangered tree Canacomyrica monticola in New Caledonia. J. Ecol. Nat. Environ. 2009, 1, 094–098. [Google Scholar]
- Elith, J.; Graham, C.H.; Anderson, R.P.; Dudík, M.; Ferrier, S.; Guisan, A.; Hijmans, R.J.; Huettmann, F.; Leathwick, J.R.; Lehmann, A.; et al. Novel methods improve prediction of species’ distributions from occurrence data. Ecography 2006, 29, 129–151. [Google Scholar] [CrossRef]
- Senula, S.F.; Scavetta, J.T.; Banta, J.A.; Mueller, U.G.; Seal, J.N.; Kellner, K. Potential distribution of six north American higher-attine fungus-farming ant (Hymenoptera: Formicidae) species. J. Insect Sci. 2019, 19, 24. [Google Scholar] [CrossRef]
- van Proosdij, A.S.; Sosef, M.S.; Wieringa, J.J.; Raes, N. Minimum required number of specimen records to develop accurate species distribution models. Ecography 2016, 39, 542–552. [Google Scholar] [CrossRef]
- Hernandez, P.A.; Graham, C.H.; Master, L.L.; Albert, D.L. The effect of sample size and species characteristics on performance of different species distribution modeling methods. Ecography 2006, 29, 773–785. [Google Scholar] [CrossRef]
- Mafuwe, K.; Broadley, S.; Moyo, S. Use of maximum entropy (Maxent) niche modelling to predict the occurrence of threatened freshwater species in a biodiversity hotspot of Zimbabwe. Afr. J. Ecol. 2022, 60, 557–565. [Google Scholar] [CrossRef]
- Walters, A.D.; Ford, D.; Chong, E.T.; Williams, M.G.; Ford, N.B.; Williams, L.R.; Banta, J.A. High-resolution ecological niche modelling of threatened freshwater mussels in east Texas, USA. Aquat. Conserv. Mar. Freshw. Ecosyst. 2017, 27, 1251–1260. [Google Scholar] [CrossRef]
- Pearson, R.G.; Raxworthy, C.J.; Nakamura, M.; Townsend Peterson, A. Predicting species distributions from small numbers of occurrence records: A test case using cryptic geckos in Madagascar. J. Biogeogr. 2007, 34, 102–117. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, D.; Wang, L.; Fang, J.; Sun, W.; Ren, B. Effects of altered precipitation on insect community composition and structure in a meadow steppe. Ecol. Entomol. 2014, 39, 453–461. [Google Scholar] [CrossRef]
- Rohde, A.T.; Pilliod, D.S.; Novak, S.J. Insect communities in big sagebrush habitat are altered by wildfire and post-fire restoration seeding. Insect Conserv. Divers. 2019, 12, 216–230. [Google Scholar] [CrossRef]
- Wenninger, E.J.; Inouye, R.S. Insect community response to plant diversity and productivity in a sagebrush–steppe ecosystem. J. Arid. Environ. 2008, 72, 24–33. [Google Scholar] [CrossRef]
- Bradley, B.A.; Curtis, C.A.; Fusco, E.J.; Abatzoglou, J.T.; Balch, J.K.; Dadashi, S.; Tuanmu, M.N. Cheatgrass (Bromus tectorum) distribution in the intermountain Western United States and its relationship to fire frequency, seasonality, and ignitions. Biol. Invasions 2018, 20, 1493–1506. [Google Scholar] [CrossRef]
- U.S. Geological Survey, Earth Resources Observation and Science Center. U.S. Landsat Analysis Ready Data (ARD). Available online: https://dwtkns.com/srtm30m/ (accessed on 9 May 2022).
- LANDFIRE (LF), LF 2016 Remap, U.S. Department n of the Interior, Geological Survey, and U.S Department of Agriculture. Available online: https://www.landfire.gov/viewer/ (accessed on 8 January 2023).
- PRISM Climate Group, Oregon State University. (Data Created 20 September 2020). Available online: http://prism.oregonstate.edu (accessed on 9 May 2022).
- State Geographic Information Database. NHD Lakes (Data Layer), NHD Streams (Data Layer), NHD Springs (Point Layer). Utah Geospatial Resource Center. Available online: https://gis.utah.gov/data/water/lakes-river-dams/ (accessed on 7 January 2023).
- Soil Survey Staff, Natural Resources Conservation Service, United States Department of Agriculture. Web Soil Survey. Available online: https://websoilsurvey.sc.egov.usda.gov/ (accessed on 7 January 2023).
- Warren, D.L.; Glor, R.E.; Turelli, M. ENMTools: A toolbox for comparative studies of environmental niche models. Ecography 2010, 33, 607–611. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.R-pfoject.org/ (accessed on 23 July 2023).
- Phillips, S.J.; Dudik, M.; Schapire, R.E. Maxent Software for Modeling Species Niches and Distributions (Version 3.4.3). Available online: https://biodiversityinformatics.amnh.org/open_source/maxent/ (accessed on 6 April 2022).
- Warren, D.L.; Seifert, S.N. Ecological niche modeling in Maxent: The importance of model complexity and the performance of model selection criteria. Ecol. Appl. 2011, 21, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.P.; Gonzalez, I., Jr. Species-specific tuning increases robustness to sampling bias in models of species distributions: An implementation with Maxent. Ecol. Model. 2011, 222, 2796–2811. [Google Scholar] [CrossRef]
- Muscarella, R.; Galante, P.J.; Soley-Guardia, M.; Boria, R.A.; Kass, J.M.; Uriarte, M.; Anderson, R.P. ENM eval: An R package for conducting spatially independent evaluations and estimating optimal model complexity for Maxent ecological niche models. Methods Ecol. Evol. 2014, 5, 1198–1205. [Google Scholar] [CrossRef]
- Warren, D.L.; Wright, A.N.; Seifert, S.N.; Shaffer, H.B. Incorporating model complexity and spatial sampling bias into ecological niche models of climate change risks faced by 90 California vertebrate species of concern. Divers. Distrib. 2014, 20, 334–343. [Google Scholar] [CrossRef]
- Merow, C.; Smith, M.J.; Silander, J.A., Jr. A practical guide to MaxEnt for modeling species’ distributions: What it does, and why inputs and settings matter. Ecography 2013, 36, 1058–1069. [Google Scholar] [CrossRef]
- Stolar, J.; Nielsen, S.E. Accounting for spatially biased sampling effort in presence-only species distribution modelling. Divers. Distrib. 2015, 21, 595–608. [Google Scholar] [CrossRef]
- Syfert, M.M.; Smith, M.J.; Coomes, D.A. The effects of sampling bias and model complexity on the predictive performance of MaxEnt species distribution models. PLoS ONE 2013, 8, e55158. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudík, M.; Elith, J.; Graham, C.H.; Lehmann, A.; Leathwick, J.; Ferrier, S. Sample selection bias and presence-only distribution models: Implications for background and pseudo-absence data. Ecol. Appl. 2009, 19, 181–197. [Google Scholar] [CrossRef] [PubMed]
- Pearce, J.; Ferrier, S. Evaluating the predictive performance of habitat models developed using logistic regression. Ecol. Model. 2000, 133, 225–245. [Google Scholar] [CrossRef]
- Liao, Y.; Lei, Y.; Ren, Z.; Chen, H.; Li, D. Predicting the potential risk area of illegal vaccine trade in China. Sci. Rep. 2017, 7, 3883. [Google Scholar] [CrossRef]
- Elith, J.; Phillips, S.J.; Hastie, T.; Dudík, M.; Chee, Y.E.; Yates, C.J. A statistical explanation of MaxEnt for ecologists. Divers. Distrib. 2011, 17, 43–57. [Google Scholar] [CrossRef]
- Bradley, B.A.; Fleishman, E. Relationships between expanding pinyon–juniper cover and topography in the central Great Basin, Nevada. J. Biogeogr. 2008, 35, 951–964. [Google Scholar] [CrossRef]
- Merrill, K.R.; Meyer, S.E.; Coleman, C.E. Population genetic analysis of Bromus tectorum (Poaceae) indicates recent range expansion may be facilitated by specialist genotypes. Am. J. Bot. 2012, 99, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Balch, J.K.; Bradley, B.A.; D’Antonio, C.M.; Gómez-Dans, J. Introduced annual grass increases regional fire activity across the arid western USA (1980–2009). Glob. Chang. Biol. 2013, 19, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Whisenant, S.G. Changing fire frequencies on Idaho’s Snake River Plains: Ecological and management implications. In Proceedings: Symposium on Cheatgrass Invasion, Shrub Die-Off, and Other Aspects of Shrub Biology and Management; General Technical Report INT-276; McArthur, E.D., Romney, E.M., Smith, S.D., Tueller, P.T., Eds.; USDA Forest Service Intermountain Research Station: Ogden, UT, USA, 1990; pp. 4–10. [Google Scholar]
- Meyer, S.E.; Leger, E.A. Inbreeding, genetic variation, and invasiveness: The strange case of Bromus tectorum. Rangelands 2010, 32, 6–11. [Google Scholar] [CrossRef]
- Paysen, T.E.; Ansley, R.J.; Brown, J.K.; Gottfried, G.J.; Haase, S.M.; Harrington, M.G.; Narog, M.G.; Sackett, S.S.; Wilson, R.C. Fire in western shrubland, woodland, and grassland ecosystems. Wildland Fire Ecosyst. Eff. Fire Flora 2000, 2, 121–159. [Google Scholar]
- Ardestani, E.G.; Tarkesh, M.; Bassiri, M.; Vahabi, M.R. Potential habitat modeling for reintroduction of three native plant species in central Iran. J. Arid. Land 2015, 7, 381–390. [Google Scholar] [CrossRef]
- Westerband, A.; Dovčiak, M.; La Quay-Velázquez, G.; Medeiros, J.S. Aspect reduces soil moisture and tree cover, but not nitrogen mineralization or grass cover, in semiarid pinyon-juniper woodlands of the Southwestern United States. Southwest. Nat. 2015, 60, 21–29. [Google Scholar] [CrossRef]
- Petersen, S.L.; Stringham, T.K. Infiltration, runoff, and sediment yield in response to western juniper encroachment in southeast Oregon. Rangel. Ecol. Manag. 2008, 61, 74–81. [Google Scholar] [CrossRef]
- Pinder, J.E.; Kroh, G.C.; White, J.D.; Basham May, A.M. The relationships between vegetation type and topography in Lassen Volcanic National Park. Plant Ecol. 1997, 131, 17–29. [Google Scholar] [CrossRef]
- Boŭcek, Z.; Halstead, J.A. Chalcididae. In Annotated Keys to the Genera of Nearctic Chalcidoidea (Hymenoptera); Gibson, G.A., Huber, J.T., Woolley, J.B., Eds.; NRC Research Press: Ottawa, ON, Canada, 1997; pp. 151–164. [Google Scholar]
- Wall, R.; Berberet, R.C. Parasitoids associated with lepidopterous pests on peanuts; Oklahoma fauna. Environ. Entomol. 1975, 4, 877–882. [Google Scholar] [CrossRef]
- Veldtman, R.; Daly, D.; Bekker, G.F.V.G. Spatio–environmental analysis of Vespula germanica nest records explains slow invasion in South Africa. Insects 2021, 12, 732. [Google Scholar] [CrossRef] [PubMed]
- Howse, M.W.; Haywood, J.; Lester, P.J. Bioclimatic modelling identifies suitable habitat for the establishment of the invasive European paper wasp (Hymenoptera: Vespidae) across the southern hemisphere. Insects 2020, 11, 784. [Google Scholar] [CrossRef] [PubMed]

| Variable | Data Source |
|---|---|
| Elevation | EROS, SRTM |
| Aspect | Generated in ArcGIS |
| Slope | Generated in ArcGIS |
| Historic fire disturbance | LANDFIRE |
| Existing vegetation type | LANDFIRE |
| Precipitation | PRISM Climate Group |
| Temperature | PRISM Climate Group |
| Distance to closest water source | UGRC calculated in ArcGIS |
| NDVI | USGS/EROS |
| Soil composition | WSS/NRCS |
| Sampling Location Latitude Longitude | Predicted Habitat | Observed Habitat | Number of P. minuta Retrieved | Number of P. quadratis Retrieved |
|---|---|---|---|---|
| 40.21209, −111.97083 | Pinyon/juniper | Pinyon/juniper | 2 | 0 |
| 39.65849, −112.24926 | Pinyon/juniper | Pinyon/juniper | 21 | 5 |
| 39.48876, −112.35678 | Pinyon/juniper | Pinyon/juniper | 10 | 0 |
| 40.25033, −112.18350 | Pinyon/juniper | Pinyon/juniper | 21 | 0 |
| 40.07829, −112.12203 | Pinyon/juniper | Pinyon/juniper | 5 | 0 |
| 39.66354, −112.10690 | Pinyon/juniper | Pinyon/juniper | 51 | 2 |
| 39.50412, −112.14085 | Pinyon/juniper | Pinyon/juniper | 165 | 2 |
| 39.81450, −112.38919 | Pinyon/juniper | Pinyon/juniper | 9 | 0 |
| 39.66003, −112.59415 | Pinyon/juniper | * Greasewood | 0 | 0 |
| 40.24126, −111.98340 | Cheatgrass | Cheatgrass | 0 | 1 |
| 39.49077, −112.34794 | Cheatgrass | Cheatgrass | 0 | 0 |
| 39.59726, −111.56220 | Cheatgrass | Cheatgrass | 0 | 0 |
| 39.63317, −112.59161 | Cheatgrass | * Greasewood | 0 | 2 |
| 40.26538, −112.14526 | No prediction | Pinyon/juniper | 21 | 3 |
| 40.04515, −112.01527 | No prediction | Pinyon/juniper and sagebrush | 7 | 1 |
| 39.67244, −112.06180 | No prediction | Mixed shrub and grasses | 0 | 0 |
| 39.58563, −112.15250 | No prediction | Greasewood | 5 | 4 |
| 39.71542, −112.56461 | No prediction | Barren | 0 | 1 |
| Model | p-Value | AUC | Standard Deviation | Linear/Quadratic Product | Categorical | Threshold | Hinge |
|---|---|---|---|---|---|---|---|
| P. minuta | 0.02 | 0.701 | 0.119 | 1.000 | 0.530 | 1.920 | 0.500 |
| P. quadratis | 0.02 | 0.680 | 0.058 | 1.000 | 0.530 | 1.920 | 0.500 |
| Model | Aspect | Elevation | Slope | Soil | Fire | Water | Vegetation | NDVI | Temperature | Precipitation |
|---|---|---|---|---|---|---|---|---|---|---|
| P. minuta | 40.8 | 13.4 | 5.3 | 10.1 | 14.3 | 0.3 | 4.1 | 7.0 | 4.5 | 0.3 |
| P. quadratis | 40.3 | 12.6 | 6.8 | 8.8 | 14.3 | 0.2 | 4.9 | 7.9 | 4.0 | 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petersen, M.J.; Ortiz Cano, H.G.; Gomez, T.; Johnson, R.L.; Anderson, V.J.; Petersen, S.L. Maxent Predictive Species Distribution Models and Model Accuracy Assessment for Two Species of Psilochalcis Kieffer (Hymenoptera: Chalcididae) Occurring in the Eastern Great Basin of Utah, USA. Diversity 2024, 16, 348. https://doi.org/10.3390/d16060348
Petersen MJ, Ortiz Cano HG, Gomez T, Johnson RL, Anderson VJ, Petersen SL. Maxent Predictive Species Distribution Models and Model Accuracy Assessment for Two Species of Psilochalcis Kieffer (Hymenoptera: Chalcididae) Occurring in the Eastern Great Basin of Utah, USA. Diversity. 2024; 16(6):348. https://doi.org/10.3390/d16060348
Chicago/Turabian StylePetersen, Mark J., Hector G. Ortiz Cano, Teresa Gomez, Robert L. Johnson, Val Jo Anderson, and Steven L. Petersen. 2024. "Maxent Predictive Species Distribution Models and Model Accuracy Assessment for Two Species of Psilochalcis Kieffer (Hymenoptera: Chalcididae) Occurring in the Eastern Great Basin of Utah, USA" Diversity 16, no. 6: 348. https://doi.org/10.3390/d16060348
APA StylePetersen, M. J., Ortiz Cano, H. G., Gomez, T., Johnson, R. L., Anderson, V. J., & Petersen, S. L. (2024). Maxent Predictive Species Distribution Models and Model Accuracy Assessment for Two Species of Psilochalcis Kieffer (Hymenoptera: Chalcididae) Occurring in the Eastern Great Basin of Utah, USA. Diversity, 16(6), 348. https://doi.org/10.3390/d16060348






