Abstract
Southern African grasslands with a rich flora, shaped by fire, grazing, climate and geology, as well as playing a role in carbon sequestration, are becoming more important in conservation. Fire is often used as a management tool to improve vegetation and to protect property against uncontrolled fire. We therefore attempt to determine the effect consecutive burning has on vegetation. Paired plots along firebreaks were used to collect vegetation data using the Braun-Blanquet cover abundance scale. Soil samples were also collected to determine the impact of fire on below-ground nitrogen (N) and carbon (C) stocks and ratios. The results indicate that there is no difference between the plant communities of the firebreaks and the adjacent grassland; however, there are certain species that are favoured by firebreaks and others by the adjacent grassland. There is also no difference in diversity between the firebreaks and adjacent grassland areas. Carbon and nitrogen stocks as well as C:N ratios did not differ significantly between the firebreaks and the adjacent grassland plots although trends indicate a decline in both C and N with repeated burning.
1. Introduction
Globally, the grassland biome is known for its high biodiversity [1,2] and various ecosystem services [2]. This biome is impacted by habitat loss, fragmentation and estimated future threats which include climate change and anthropogenic activities [3]. Other threats also include soil disturbance and the extirpation of plant species [4,5]. In South Africa, the grassland biome is the second largest and covers almost one-third of the land surface of the country [6]. This biome is species-rich with high diversity within communities and contains global centres of plant endemism [7]. It covers large parts of the Free State, but has largely been modified through cultivated agriculture, mostly maize and wheat [8], and less than 3% is formally protected [5]. This biome also contributes to the bioeconomy of South Africa in terms of its carbon sequestration and pollination [9], as well as acting as carbon and methane sinks and regional climate regulators [10]. Natural grasslands play an important role in the global carbon cycle. It is estimated that the soil carbon stocks of grasslands are between 10% and 30% of the total global carbon pools [11]. Scurlock and Hall (1998) [11] further indicated that below-ground storage, seasonal burning and regrowth are major players in the carbon cycle of grasslands. There is an intrinsic relationship between biodiversity, ecosystem stability and ecosystem processes [12]. This relationship will be destabilised in the future by management problems and pressures of growing agricultural demand, climate change and sea-level rise which will add additional pressure on the natural grassland ecosystems which provide humankind with multiple ecosystem services [2].
Dominated by the Poaceae (grasses), grasslands are structurally simple with a canopy cover that is moisture dependent. The canopy cover is also dependent on grazing, fire regimes [13] and soil fertility [6,14]. Nerlekar and Veldman (2020) [15] indicated that fire and herbivores shaped grasslands millions of years before the existence of humans. Grassland ecosystems are fire-prone, and therefore fire is vital in the maintenance of structural and textural patterns [6,16,17] as well as the climatic system due to carbon release into the atmosphere [18].
The intensity of fires in grasslands varies depending on fuel load (accumulation of biomass), fuel condition (compaction, moisture content), relative humidity, wind speed and topography. Furthermore, the implementation of incorrect fire management strategies can reduce heterogeneity and lower species diversity [4]. Although fires can occur at any time during the year, it is mostly beneficial at the beginning of the growing season when the grasses are still dormant [19].
Fire is widely used as a management tool in grasslands to maintain the vigour and palatability of the vegetation [13,20,21,22]. Le Maitre and Midgley (1992) [23] indicated that fire in grasslands typically occur every 1–4 years in late winter from July to September. Various studies have investigated the effects of fire on grasslands in South Africa; however, studies on the effects of firebreaks are limited [22,24]. These firebreaks are fixed on a property and normally the same area is subjected to annual burns. Firebreaks can comprise up to 10% of the surface area of a property [22,24], which comprises a substantial surface area of land and therefore needs more intense research as their ecological effects have been ignored [22]. Differences of opinion exist regarding the influence of firebreaks on the diversity of plants in southern Africa. Some studies indicate the effect as marginal [22,24,25,26], while others state that prescribed burning can potentially change the nutrient status and species composition of an ecosystem [27].
The negative effects on biodiversity, human health and the economy of runaway veld fires have resulted in the legal requirement for annual burning of firebreaks in the grassland and savanna biomes of South Africa [18]. According to the National Veld and Forest Fire Act 101 of 1998, South Africa, it is compulsory that landowners implement firebreaks along the boundaries of their properties. As a state-owned enterprise, the Golden Gate Highlands National Park (hereafter Golden Gate) needs to adhere to this law and therefore has prepared firebreaks along the boundaries of the park and any adjoining land [28]. The use of firebreaks has been implemented since 2006.
Firebreaks in Golden Gate are prepared from May by cutting vegetation by hand, brush cutters or using “bossie kappers” in combination with fuel reduction burns. The firebreaks can vary from seven to fifty meters wide and occur along certain roads and all infrastructure. These firebreaks are made by rangers and the Working on Fire team located in the park. Back fires are mostly used for the burning of the firebreaks. Fuel load is determined visually, to see if firebreaks are needed; however, good summer rainfall usually results in an increased growth of grasses that during winter produce a high fuel load which poses a high fire danger in the park. Therefore, these firebreaks are burned and maintained on an annual basis. The areas of the firebreaks are therefore devoid of vegetation until the onset of the rainy season which in the case of the Golden Gate is early November.
The aim of this study was to investigate the effect of annual burning of firebreaks on vegetation composition, species diversity, veld condition as well as soil carbon and nitrogen stocks in Golden Gate.
2. Materials and Methods
2.1. Study Site
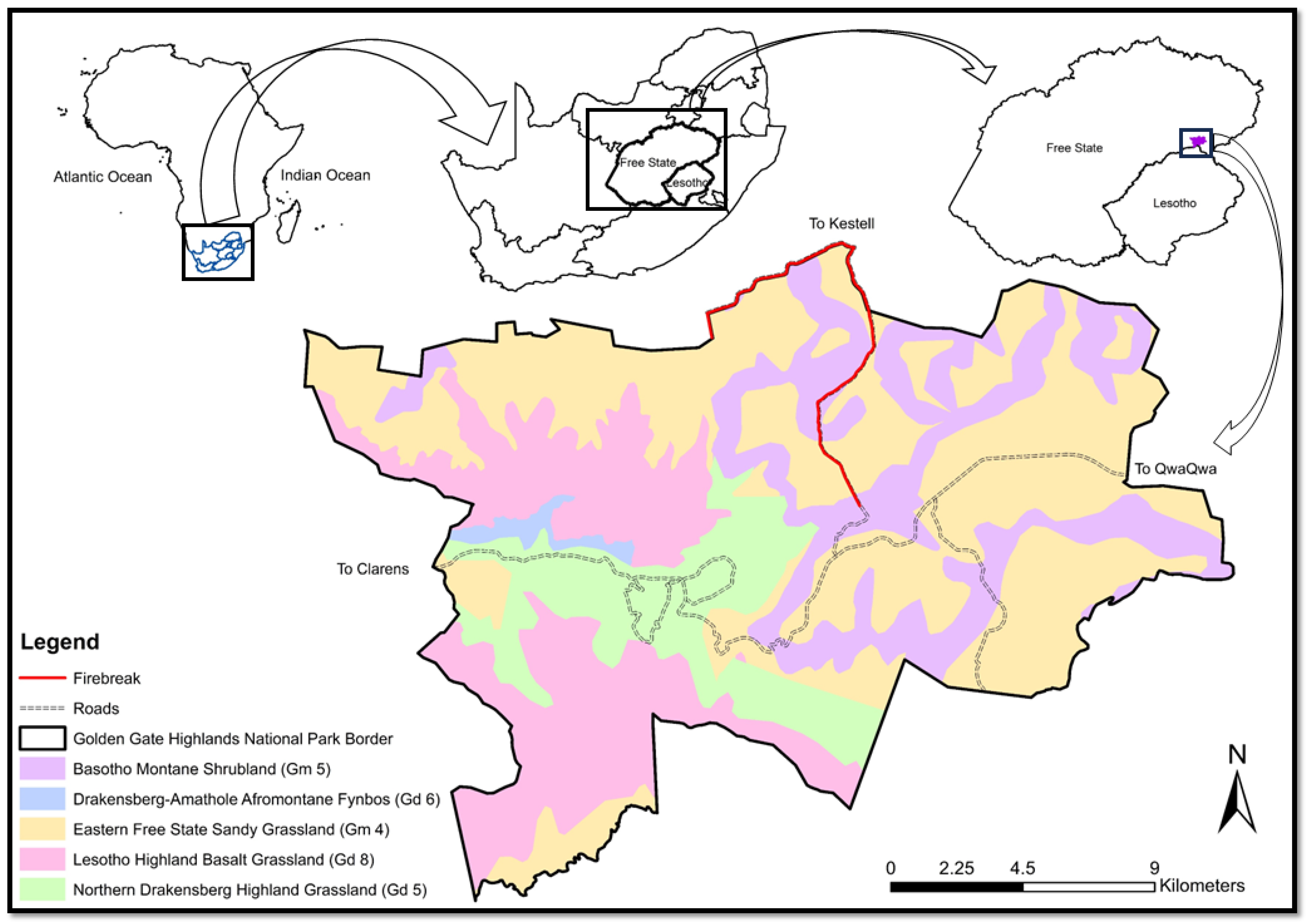
This study was conducted in Golden Gate, located in the eastern parts of the Free State Province, approximately 50 km southeast of Bethlehem and on the border of Lesotho [29] (Figure 1). The park covers 32,690 ha of the grassland biome and stretches across the Rooiberg Mountain Range (1892–2837 m.a.m.s.l), forming part of the greater Maloti-Drakensberg system [30].
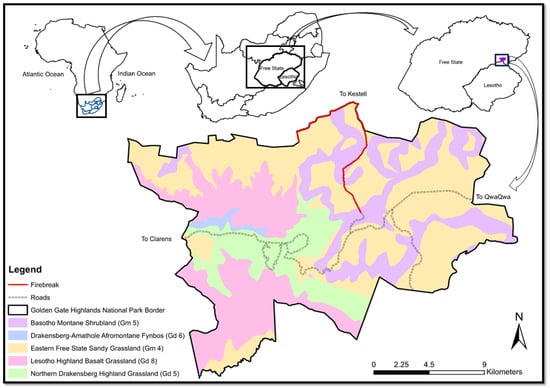
Figure 1.
Map of the Golden Gate Highlands National Park with the different vegetation types [6].
The reserve falls within the summer-rainfall region with mild to warm summers and cold winters [30]. Rainfall is mostly restricted to the months of November to April [30,31,32] and occurs in the form of thunderstorms [6,32,33]. Late afternoon thunderstorms are mostly accompanied by lightning and sometimes hail [32,33]. Rainfall average is around 764 mm per year, varying considerably across the region. During the cold winters, frost is a frequent phenomenon [32] because of the continental climate [6] and snow might fall on the higher peaks in the park [30].
The vegetation in the park is composed of the Basotho Montane Shrubland (Gm 5), Drakensberg Amathole Afromontane Fynbos (Gd 6), Eastern Free State Sandy Grassland (Gm 4), Lesotho Highveld Basalt Grassland (Gd 8) and the Northern Drakensberg Highveld Grassland (Gd 5) [6]. The firebreaks were situated in the middle of the park along the gravel road to Kestell and the northern boundary of the park (Figure 1). The vegetation in this area is the Eastern Free State Sandy Grassland (Gm 4) and plots were restricted to this vegetation type. This is seen as a closed grassland dominated by species such as Eragrostis curvula, Tristachya leucothrix and Themeda triandra. These are, however, not the only grasses, as Eragrostis capensis, E. racemose, E. plana, Elionirus mitucus, Cymbopogon pospischilii and Aristida junciformis can also be dominant. Numerous herbs, mostly from the Asteraceae family, increase the diversity [6].
2.2. Sampling Techniques
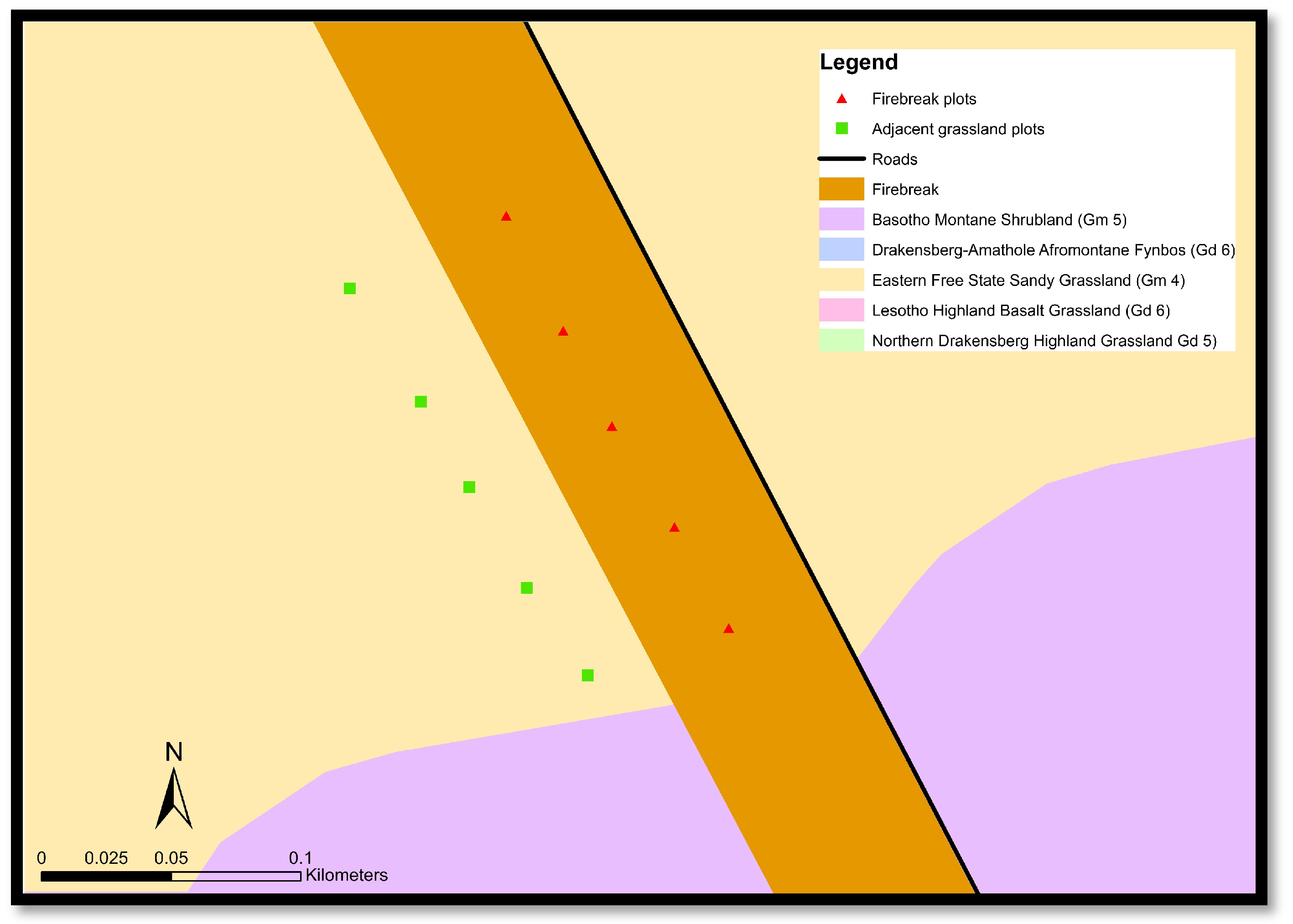
Twenty-four paired plots, consisting of a firebreak (treatment) and adjacent grassland (control) plots (Figure 2), were placed in areas experiencing similar environmental conditions, which include slope and topography, along the firebreaks in Golden Gate during January 2023. The unburned plots were located roughly 20 m from the firebreak boundary. The firebreaks in the Golden Gate are burned annually during the winter months of May/June.
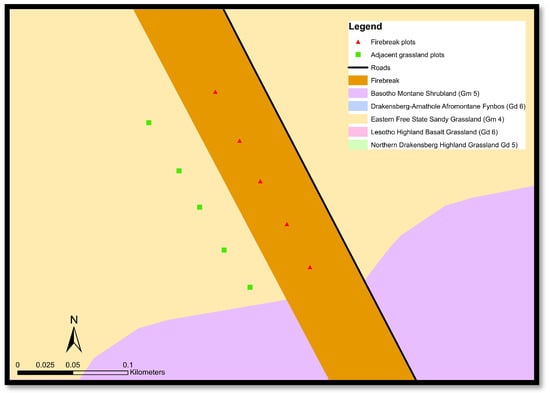
Figure 2.
Example of some paired data plots along the firebreaks and the adjacent grassland in Golden Gate.
The sample plot sizes for data collection were fixed at 16 m2 which is the size recommended by Brown et al. (2013) [34] for dry and moist grassland communities. Within each community, cover abundance values were assigned to each species using the Braun-Blanquet cover abundance scale [35,36,37]. This method is widely used in international and national vegetation classification studies [34,36,38]. The data collected were captured using VegCap; a macro-enabled Excel spreadsheet developed by N. Collins.
One soil sample was collected within each plot to a depth of 10 cm. The soil was analysed for carbon (C) and nitrogen (N) content. A core sample was also collected to determine the bulk density in order to calculate the carbon and nitrogen stocks of the topsoil.
2.3. Data Analysis
The following parameters were calculated and analyses were performed:
In order to compare species composition of firebreak sites with adjacent grasslands, vegetation classification was carried out using JUICE [39]. A Modified TWINSPAN classification [40] analysis was performed. Thereafter, Braun-Blanquet procedures [34] were used to refine the table in both JUICE and Excel for the final classification. Naming of communities and sub-communities were carried out according to the guidelines of Brown et al. (2013) [34]. The diagnostic, constant and dominant species were calculated using the Analysis of Columns of a Synoptic Table in JUICE. Frequency thresholds were set at 75, 60 and 50 for lower threshold and 80, 70 and 60 for upper threshold values for the respective diagnostic, constant and dominant species [34]. Species that have values higher than the threshold are indicated in bold.
We also compared local-scale (α-diversity) of firebreaks and adjacent grasslands. We used three measures, namely species richness (the number of species per site, S), the Shannon–Wiener index (H′) and the Simpson index (D), such that:
and
where pi are proportional contributes of the ith species in the set. H′ and D therefore differ from S in that they account for variation in the relative abundances and evenness of each species. These values were obtained by transforming Braun-Blanquet values to a numerical scale (r = 0.5; + = 1; 1 = 2; 2a = 8.5; 2b = 17.5; 3 = 35; 4 = 70; 5 = 140) [37,41]. All three α-diversity indices were estimated per site, and for each growth form (annual herb, geophyte, graminoid, parasite, perennial herb, sedge and shrub) separately, and compared using linear mixed models where the treatment (firebreak or adjacent grasslands) and growth form (nested within treatment) were fixed effects, and the plot pair was a random effect to account for the fact that parameter estimates may be biased due to the non-independence of paired sampling. For pairwise comparisons, we compared the 95% confidence intervals of the marginal means of each group. These analyses were conducted in R v. 4.3.1 [42], and the lmerTest package was used to fit mixed models [43]. Residuals were often non-normally distributed and/or heteroscedastic, and no transformation could be found that universally led to satisfying assumptions of the parametric tests. Therefore, we report on results of non-parametric approaches, i.e., using ranked series of S, H′ and D, respectively, and descriptive statistics are presented as medians and percentiles. Nevertheless, it is worth noting that results of parametric tests were qualitatively similar to the non-parametric counterparts, and therefore the ranking procedure did not appear to incur any significant loss of power.
The soils collected using the core method were used for the determination of bulk density [44]. Bulk density is seen as the mass per unit volume of soil in g cm−3. A 4.8 cm (diameter) metal core ring was pushed into the soil (5 cm deep) and a volume of 90.48 cm−3 undisturbed soil was collected. The sample was then placed in an oven at 105 °C for 48 h to dry and weighed afterwards to determine the dry mass [45]. The bulk density was then calculated using the following equation:
where ρb = bulk density (g cm−3), Ms = dry mass (g) and Vt = volume of core (cm3).
ρb = Ms /Vt
An auger-collected soil sample was used for the carbon and nitrogen analysis. A weighted sample of 0.2 g soil was combusted in a furnace up to 980 °C (dry combustion method) [46] using a Leco TruSpec CNS analyser. Since the soils do not contain inorganic C, the Leco derived C was assumed to be equal to the organic carbon. Soil organic carbon (SOC) and nitrogen stocks (NS) were determined using the following equation:
where, SOC and NS are the stocks in t ha−1; H is soil depth (10 cm); BD, bulk density (g cm−3); OC, soil organic carbon concentration (%). We only looked at the first 10 cm as fire will most likely impact this layer of the soil.
SOC or NS stock = H × BD × OC × 10
A single factor ANOVA analysis was carried out to determine whether there is a difference between the carbon and nitrogen stock as well as C:N ratios between the treatment (firebreaks) and control (adjacent grassland). Significant correlations were assumed if p < 0.05.
3. Results and Discussion
3.1. Phytosociological Classification
Table A1 (Appendix A) presents the phytosociological classification of the vegetation found in the firebreaks and adjacent grassland areas in Golden Gate, South Africa. Three plant communities, seven sub-communities and two variants were found during the analysis. The relevés indicated in orange are from the firebreaks (treatment). Species indicated with an * are alien invasive species.
- Tristachya leucothrix–Commelina africana Community
- 1.1.
- Tristachya leucothrix–Commelina africana–Pycreus nigricans Sub-community
- 1.2.
- Tristachya leucothrix–Commelina africana–Heteropogon contortus Sub-community
- Hyparrhenia tamba–Eragrostis curvula Community
- 2.1.
- Hyparrhenia tamba–Eragrostis curvula–Cyperus esculentus Sub-community
- 2.2.
- Hyparrhenia tamba–Eragrostis curvula–Conyza podocephala Sub-community
- 2.3.
- Hyparrhenia tamba–Eragrostis curvula–Helichrysum rugulosum Sub-community
- 2.3.1.
- Aristea woodii Variant
- 2.3.2.
- Pelargonium luridum Variant
- Sporobolus fimbriatus–Trifolium burchellianum Community
- 3.1.
- Sporobolus fimbriatus–Trifolium burchellianum–Cyperus longus Sub-community
- 3.2.
- Sporobolus fimbriatus–Trifolium burchellianum–Ipomoea oblongata Sub-community
3.2. Description of the Firebreak (Treatment) and Adjacent Grassland (Control) Communities
- 1
- Tristachya leucothrix–Commelina africana Community
- Diagnostic species: None
- Constant species: Eragrostis capensis 67
- Dominant species: Eragrostis chloromelas 8, Feurena pubescens 17, Helichrysium aureonitens 17, Heteropogon contortus 17, Kyllinga erecta 25
Community 1 is defined by the grass Tristachya leucothrix and perennial herb Commelina africana from Species Group A (Table A1; Appendix A). Species from Species Group A that define this community also occur in other communities, however, with low cover abundance values. O’Connor et al. (2004) [22] indicated that the grass Tristachya leucothrix is characteristic of conservation grasslands in general. Although there is no distinction between firebreak and adjacent grassland communities in this study, it is prominent that Tristachya leucothrix mostly occurs in the adjacent grassland and not in firebreak plots. This is confirmed by the sum of numerical values found in Table A2 (Appendix B), where Tristachya leucothrix has a value of 35.5 in the adjacent grassland and only 2.5 in the firebreak. This agrees with the research by Bachinger et al. (2016) [24] that indicated Tristachya leucothrix’s association with unburnt veld (adjacent grassland vegetation). Research by O’Connor et al. (2004) [22] also indicated that Commelina africana can withstand fire on account of their storage organs underground from which they resprout. This seems to correspond with the presence of this species mostly in the firebreak plots with the sum of numerical values being 15.5 compared to 7 for the adjacent grassland.
- 1.1
- Tristachya leucothrix–Commelina africana–Pycreus nigricans Sub-community
- Diagnostic species: Pycreus nigricans 100.0, Kyllinga erecta 79.5, Scirpoides burkei 79.5
- Constant species: Commelina africana 67, Pycreus nigricans 100, Eragrostis capensis 83, Fuirena pubescens 83, Helichrysium aureonitens 83, Kyllinga erecta 67, Monopsis decipiens 67, Scirpoides burkei 67
- Dominant species: Fuirena pubescens 33, Helichrysium aureonitens 33, Kyllinga erecta 50
Sub-community 1.1 is distinguished by the presence of species from Species Group B (Table A1; Appendix A). The sedge Pycreus nigricans is more prominent in the firebreaks (78.5) than in the adjacent grassland (39) (Table A2; Appendix B). Kyllinga erecta is more prominent in the adjacent grassland areas (140) where Fuerena pubescens is more prominent in the firebreaks (214) (Table A2; Appendix B). These species are mostly sedges, which might be indicative of wetness found within this sub-community.
- 1.2
- Tristachya leucothrix–Commelina africana–Heteropogon contortus Sub-community
- Diagnostic species: Brachiaria serrata 79.5, Lasiosiphon caffer 79.5, Indigofera tristoides 79.5
- Constant species: Brachiaria serrata 67, Eragrostis nindensis 67, Lasiosiphon caffer 67, Lasiosiphon kraussiana 67, Harpochloa falx 67, Heteropogon contortus 83, Hypoxis angustifolia 67, Indigofera tristoides 67, Senecio othonniflorus 67, Themeda triandra 83
- Dominant species: Eragrostis chloromelas 17, Heteropogon contortus 33
Sub-community 1.2 is distinguished by the presence of species from Species Group C (Table A1; Appendix A); these species are absent or occur with low cover abundances in other sub-communities. Research by Bachinger et al. (2016) [24] indicates that Heteropogon contortus and Themeda triandra that occur with high cover abundance values in this sub-community are associated with firebreaks on abandoned croplands. However, their findings contradict what this study found. When looking at Table A2 (Appendix B), it is seen that Heteropogon contortus (318.5) prefers the adjacent grassland, while Themeda triandra (Species Group O) (193) is more prominent in the firebreak compared to the adjacent grassland (134.5). However, both species occur in the firebreak plots and the adjacent grassland plots. O’Connor et al. (2004) [22] indicated that Themeda triandra shows no obvious environmental affiliation. Harpochloa falx, a species found in this community, occurs in conservation grasslands [22], which is also true for this study in Golden Gate, a conservation area. When looking at the sum of the numerical values, there is a tendency for Harpochloa falx to favour the adjacent grassland (5) and not the firebreaks (3) (Table A2; Appendix B).
- 2
- Hyparrhenia tamba–Eragrostis curvula Community
- Diagnostic species: None
- Constant species: Hyparrhenia tamba 68
- Dominant species: Eragrostis capensis 5, Eragrostis curvula 5, Helichrysium aureonitens 5, Helichrysum callicomum 5, Helichrysum nodifolium 5, Hyparrhenia tamba 36, Kyllinga pulchella 5, Nidorella anomala 5, Themeda triandra 9
Community 2 is dominated by the presence of Hyparrhenia tamba and Eragrostis curvula from Species Group D (Table A1; Appendix A). This community is again a combination of firebreak and adjacent grassland plots. Eragrostis curvula, one of the dominant species in this community, was found to be associated with grazing and disturbance as well as occurring (O’Connor et al., 2004) [22] on abandoned cropland areas (Bachinger et al., 2016) [24]. The findings of this study again contradict the findings of both O’Connor et al., 2004 [22] and Bachinger et al. (2016) [24] as the grass occur in firebreaks and adjacent grassland plots which are disturbed and undisturbed sites. From Table A2 (Appendix B), it is clear, however, that both Hyparrhenia tamba (963.5) and Eragrostis curvula (190) are more prominent in the firebreaks than the adjacent grassland. Furthermore, Bachinger et al. (2016) [24] indicated that Eragrosits curvula (Species Group D), E. chloromelas (Species Group E) and Hyparrhenia hirta (Species group H) are associated with unburnt plots. This is contradictory to our findings that indicate that the grass species (E. curvula, E. chloromelas and H. hirta) are mostly associated with firebreaks (Table A2; Appendix B). The difference here is that the areas studied were not croplands but rather natural grasslands. Furthermore, although there is a preference for firebreaks, the grasses also occur in the adjacent grassland.
- 2.1
- Hyparrhenia tamba–Eragrostis curvula–Cyperus esculentus Sub-community
- Diagnostic species: Cyperus esculentus 88.0
- Constant species: Cyperus esculentus 80, Hyparrhenia tamba 100
- Dominant species: Eragrostis curvula 20, Hyparrhenia tamba 60, Kyllinga pulchella 20, Nidorella anomala 20
This sub-community is distinguished by species from Species Group E (Cyperus esculentus, Eragrostis chloromelas and Kyllinga pulchella) (Table A1; Appendix A), which are either absent or occur with low cover abundance values in other sub-communities. Cyperus esculentus, which distinguishes this sub-community, is more prominent in firebreaks. Bachinger et al. (2016) [24] indicated that Eragrostis chloromelas was found in unburnt plots in abandoned croplands. However, in this study, E. chloromelas occurs in both firebreak (133; Table A2; Appendix B) and adjacent grassland (8.5) plots but is more prominent in firebreak (burnt) areas, which does not support the findings of Bachinger et al. (2016) [24]. One must however keep in mind that this research was undertaken in natural grasslands and not in abandoned croplands like that of Bachinger et al. (2016) [24].
- 2.2
- Hyparrhenia tamba–Eragrostis curvula–Conyza podocephala Sub-community
- Diagnostic species: None
- Constant species: Eragrostis curvula 100, Hyparrhenia tamba 100, Conyza podocephala 100
- Dominant species: Hyparrhenia tamba 100
The annual herb Conyza podocephala from Species Group F (Table A1; Appendix A) distinguishes this sub-community from all the other sub-communities. This sub-community is the only one that is composed of plots that were restricted to the firebreak. This sub-community also contains the lowest number of species (17) (Table A1; Appendix A) of all the sub-communities. When looking at Table A2 (Appendix B), it is also clear that Conyza podocephala prefers to occur in the firebreak plots (102) compared to the adjacent grassland (12.5) plots.
- 2.3
- Hyparrhenia tamba–Eragrostis curvula–Helichrysum rugulosum Sub-community
- Diagnostic species: None
- Constant species: Felicia muricata 62, Helichrysium aureonitens 69, Helichrysum nodifolium 77, Helichrysum rugulosum 92, Helictotrigon turgidulum 77, Themeda triandra 77
- Dominant species: Eragrostis capensis 8, Helichrysium aureonitens 8, Helichrysum callicomum 8, Helichrysum nodifolium 8, Hyparrhenia tamba 8, Themeda triandra 15
The presence of species from Species Group G (Table A1; Appendix A), which includes Helichrysum rugulosum, Helictotrigon turgidulum, Felicia muricata, Eragrostis plana, Helichrusum aureonitens and Ajuga ophrydis, distinguishes this sub-community from the other sub-communities. These species are absent or occur with low cover abundance values in other sub-communities. The dominating species are mostly perennial herbs. Table A2 (Appendix B) shows that Helichrusum rugulosum (121.5) is the only species that strongly occurs in the firebreaks, while Helictotrichon turgidulum (180.5) and Helichrysum aureonitens (348) strongly prefer the adjacent grasslands. The grass Eragrostis plana is, according to O’Connor et al. (2004) [22], commonly associated with grazing and disturbance, however, in this community it was more prominent in the adjacent grassland (25) compared to the firebreak (4). This might be an indication that the adjacent grassland is grazed by either game or domestic livestock. Another possible reason could be that the firebreaks are in the road reserve, outside the fence of the park, while the adjacent grassland is located inside the park. This is only the case where firebreaks occur on the border of the park.
- 2.3.1
- Aristea woodii Variant
- Diagnostic species: Aristea woodii 85.1
- Constant species: Aristea woodii 75, Centella asiatica 62, Conyza bonariensis 62, Dicoma anamala 62, Eragrostis capensis 62, Eragrostis curvula 75, Eragrostis plana 62, Felicia muricata 62, Gazania krebsiana 75, Helichrysium aureonitens 100, Helichrysum nodifolium 62, Helichrysum rugulosum 88, Helictotrigon turgidulum 88, Pollicha campestris 62, Themeda triandra 62
- Dominant species: Eragrostis capensis 12, Helichrysium aureonitens 12, Helichrysum callicomum 12
Variant 2.3.1 is distinguished from other variants by the presence of species from Species Group H (Table A1; Appendix A), mostly perennial herbs and grasses. This variant also contains some alien invasive species such as *Verbena bonariensis, *Hypochaeris radicata and *Richardia brasiliensis. The alien invasive *Richardia brasiliensis (78.5) and native grass Hyparrhenia hirta (56) are mostly associated with the firebreaks (Table A2; Appendix B).
- 2.3.2
- Pelargonium luridum Variant
- Diagnostic species: None
- Constant species: Helichrysum nodifolium 100, Helichrysum rugulosum 100, Oxalis obliquifolia 80, Pelargonium luridum 80, Themeda triandra 100
- Dominant species: Helichrysum nodifolium 20, Hyparrhenia tamba 20, Themeda triandra 40
This variant is dominated by species from Species Group I (Table A1; Appendix A), mostly composed of geophytes such as Pelargonium luridum, Oxalis obliquifolia and Gladiolus crassifolius. Gladiolus crassifolius (12.5), Pelargonium luridum (2.5) and Acalypha punctata (2) (Table A2; Appendix B) are more prominent in the adjacent grassland compared to Oxalis obliquifolia (11), which is more prominent in the firebreaks.
- 3
- Sporobolus fimbriatus–Trifolium burchellianum Community
- Diagnostic species: Sporobolus fimbriatus 93.5
- Constant species: Centella asiatica 71, Eragrostis capensis 86, Helichrysum nodifolium 64, Pseudognaphalium luteo album 64, Sporobolus fimbriatus 100, Themeda triandra 79, Trifolium burchellianum 64
- Dominant species: Helichrysum callicomum 7, Helictotrigon turgidulum 7, Ipomoea oblangata 7, Sporobolus fimbriatus 43, Themeda triandra 7, Trifolium burchellianum 7
Community 3 is dominated by Sporobolus fimbriatus, Trifolium burchellianum, Gnaphalium *polycaulon and Plantago lanceolata (Species Group J; Table A1; Appendix A), which are absent or occur with very low cover abundance values in other communities. Sporobolus fimbriatus is a climax species which is indicative of good growing conditions such as high rainfall, less grazing pressure and an intact local seed bank [47]. Although Sporobolus fimbriatus also occurs in the firebreaks (246 see Table A2; Appendix B), it is much more prominent in the adjacent grassland (464.5 see Table A2; Appendix B). Trifolium burchellianum (78.5) and Plantago lanceolata (13), on the other hand, are more prominent in the firebreaks. This community can be divided into two sub-communities.
- 3.1
- Sporobolus fimbriatus–Trifolium burchellianum–Cyperus longus Sub-community
- Diagnostic species: None
- Constant species: Centella asiatica 67, Cyperus longus 67, Eragrostis capensis 100, Helichrysum callicomum 67, Ledebouria ovatifolia 67, Lobelia flaccida 83, Pseudognaphalium luteo album 83, Sporobolus fimbriatus 100, Trifolium burchellianum 67
- Dominant species: Helichrysum callicomum 17, Helictotrigon turgidulum 17, Sporobolus fimbriatus 17, Themeda triandra 17
This sub-community is distinguished by the presence of the species form Species Group K (Table A1; Appendix A). No plots from the firebreaks occur in this sub-community. This might be due to the presence of moisture indicated by the high cover abundance of Cyperus longus. Table A2 (Appendix B) shows that, all the species distinguishing this sub-community occur with higher values in the adjacent grassland when compared to the firebreaks. Although fire was absent in this sub-community, it does not have the highest number of species present in it (34; Table A1; Appendix A).
- 3.2
- Sporobolus fimbriatus–Trifolium burchellianum–Ipomoea oblongata Sub-community
- Diagnostic species: Berkheya pinnatifida 84.9, Cymbopogon caesius 84.9, Ipomoea oblangata 92.6
- Constant species: Berkheya pinnatifida 75, Centella asiatica 75, Cymbopogon caesius 75, Dyschoriste setigera 75, Eragrostis capensis 75, Helichrysum nodifolium 75, Hermanmia depressa 88, Ipomoea oblangata 88, Nidorella podocephala 75, Plantago lanceolata 75, Pollicha campestris 88, Sporobolus fimbriatus 100, Themeda triandra 100, Trifolium burchellianum 62
- Dominant species: Ipomoea oblangata 12, Sporobolus fimbriatus 62, Trifolium burchellianum 12
Sub-community 3.2 is distinguished by the presence of species from Species Group L (Table A1; Appendix A) that occur with low cover abundance values or are absent from the other sub-communities. This sub-community is again a mix of firebreaks and adjacent grassland plots. All the species that distinguish this sub-community (Species Group L) are dominant in the firebreaks and not in the adjacent grassland (Table A2; Appendix B) plots.
The classification of plant communities did not show a difference between communities that occur in firebreaks and communities that occur in adjacent grassland areas. There is, however, one sub-community, the Hyparrhenia tamba–Eragrostis curvula–Conyza podocephala Sub-community, that only occurs in firebreak areas and one sub-community, the Sporobolus fimbriatus–Trifolium burchellianum–Cyperus longus Sub-community, that only occurs in the adjacent grasslands. These findings contradict that of Bachinger et al. (2016) [24] who found clear separations between the species assemblages of firebreaks and unburnt plots. A possible explanation for this difference could be that the study of Bachinger et al. (2016) [24] was conducted in grasslands on abandoned croplands. Although alien invasive species were limited, it is only *Richardia brasiliensis that is highly associated with the presence of fire and is mostly found in firebreaks (Table A2; Appendix B).
When looking at the grasses specifically, it is noticeable that most of the species are either sub-climax or climax grasses (Table A2; Appendix B); only Aristida congesta and Michracloa caffra (Table A2; Appendix B) are pioneer species. One can therefore not say that there are differences in successional stages between the firebreaks and the adjacent grassland. Bachinger et al. (2016) [24] indicated that Tristachya leucothrix and Brachiaria serrata are mostly associated with unburnt (adjacent grassland) plots, which corresponds to the findings of this study. There is, however, controversy about Themeda triandra. Bachinger et al. (2016) [24] indicate that T. triandra is mostly associated with firebreaks, similar to the findings of this study (Table A2; Appendix B). However, O’Connor et al. (2004) [22] indicate that T. triandra has no association with fire. Research has shown that grasslands in Mpumalanga that are dominated by Hyparrhenia species seldom progress to a more ecologically diverse stage [48,49]. In this study, the firebreaks were often dominated by either Hyparrhenia tamba (963.5 Table A2; Appendix B) or Hyparrhenia hirta (56 Table A2; Appendix B) often with limited other species in the plots.
3.3. Diversity
Diversity is a measure of the number of species, and their relative abundances [38]. The diversity indices used here, i.e., H′ and D, are thus interpreted alongside raw species counts (S) to compare the levels of diversity of firebreaks and the adjacent grassland, as well as how this varied across growth forms.
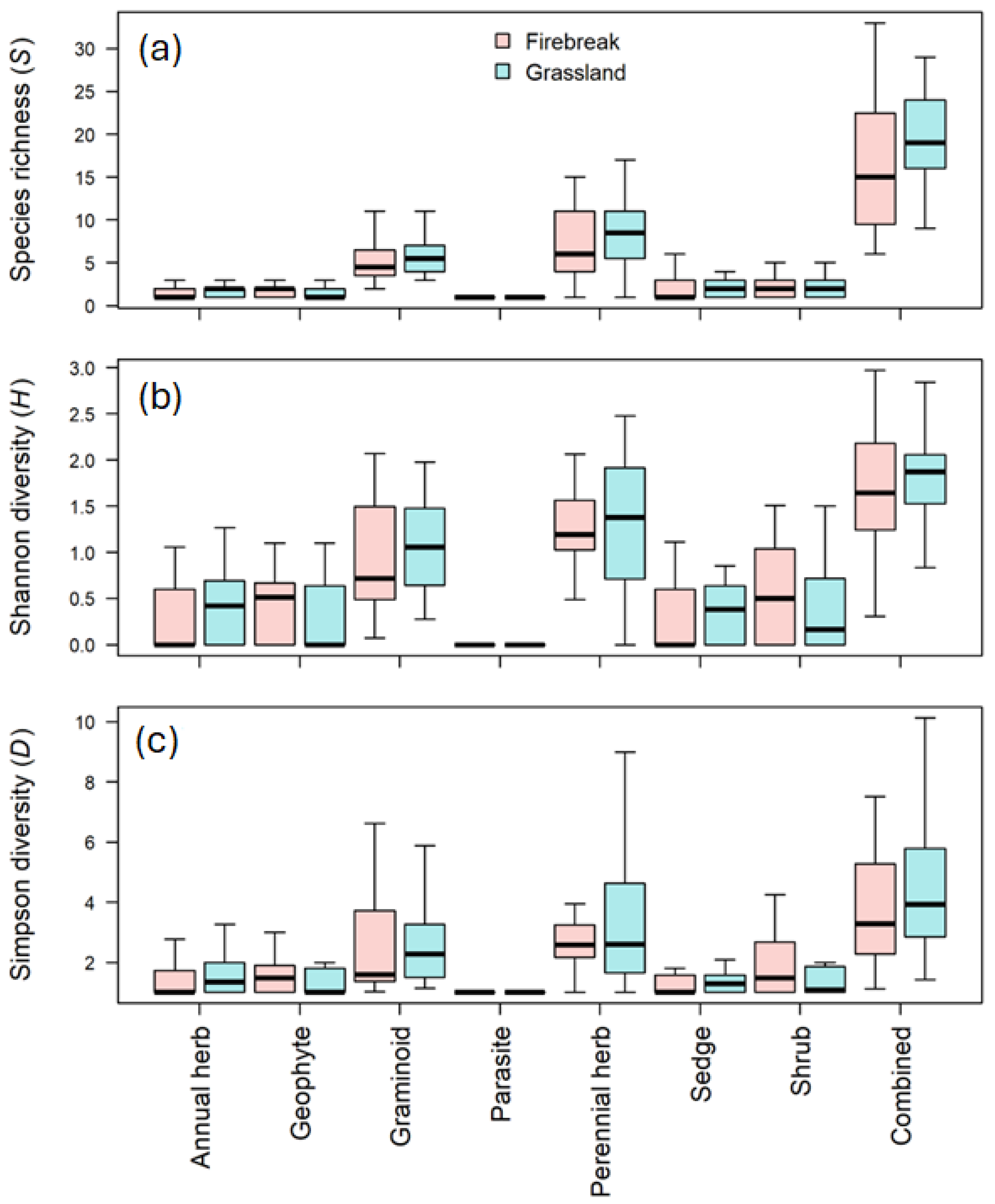
As was the case with the above evaluation of variation in species composition, we found no effect of treatment on α-diversity. In the mixed models fitted to the data, there were no significant differences between species richness (S) (p = 0.4912; Figure 3a), the Shannon–Wiener diversity (H′) (p = 0.6015; Figure 3b) or the Simpson index (D) (p = 0.5739; Figure 3c) of firebreaks and grasslands. Moreover, although the effect of growth form was significant in all three cases (p < 0.0001) (Table 1), this was only because certain growth forms (i.e., graminoids and perennial herbs) had higher diversity indices than other forms. None of the growth forms varied significantly in α-diversity levels between the two treatment levels, i.e., firebreaks and grassland (all had strongly overlapping 95% confidence intervals of their marginal means).
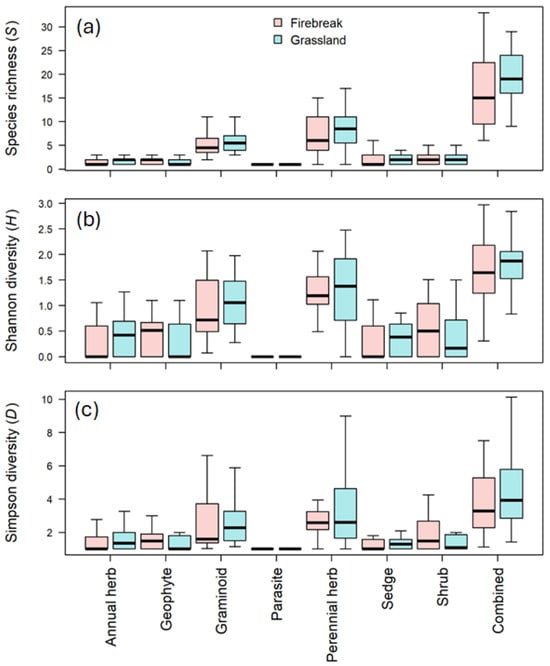
Figure 3.
Graph of the (a) species richness (S), (b) Shannon diversity (H’) and (c) Simpson diversity (D) for the firebreaks and adjacent grassland (grassland) in the Golden Gate Highlands National Park.

Table 1.
α-diversity analysis of the firebreaks and adjacent grassland as well as the growth forms nested within the firebreaks and adjacent grassland.
The research by O’Connor et al. (2004) [22] found a slightly greater graminoid density and a tendency for a greater total species richness on fire breaks, when compared to the adjacent grassland, which also does not correspond to this study. No difference between graminoids or species richness were found during this study.
The results in this study do not correspond to the findings of Bachinger et al. 2016 [24] who found a higher species richness in the fire-break plots than the unburnt plots (adjacent grassland) for both the grassland and abandoned cropland. The research by Bachinger et al. (2016) [24] further indicated that the firebreaks accumulate more species than the unburnt areas which again is in contrast to this study since there were no significant differences between the diversity of the firebreaks or the adjacent grassland.
Fire was in the past mostly used to improve grass quality for livestock production and thus diversity was not seen as important, as diversity lies in the forb richness [25]. In contrast to the findings of O’Connor et al. (2004) [22], there is no difference in species composition between the firebreaks and the adjacent grassland. From this research, it is evident that there are certain species that are more likely to occur in firebreaks than in the adjacent grassland and vice versa. In terms of biodiversity in the firebreaks and adjacent grassland, there is a no difference in diversity (Table 1), which contrasts with the findings of O’Conner et al. (2004) [22] that indicated only a slight effect on diversity.
3.4. Nitrogen and Carbon Stock
The Grassland biome can act as a carbon sink [10].
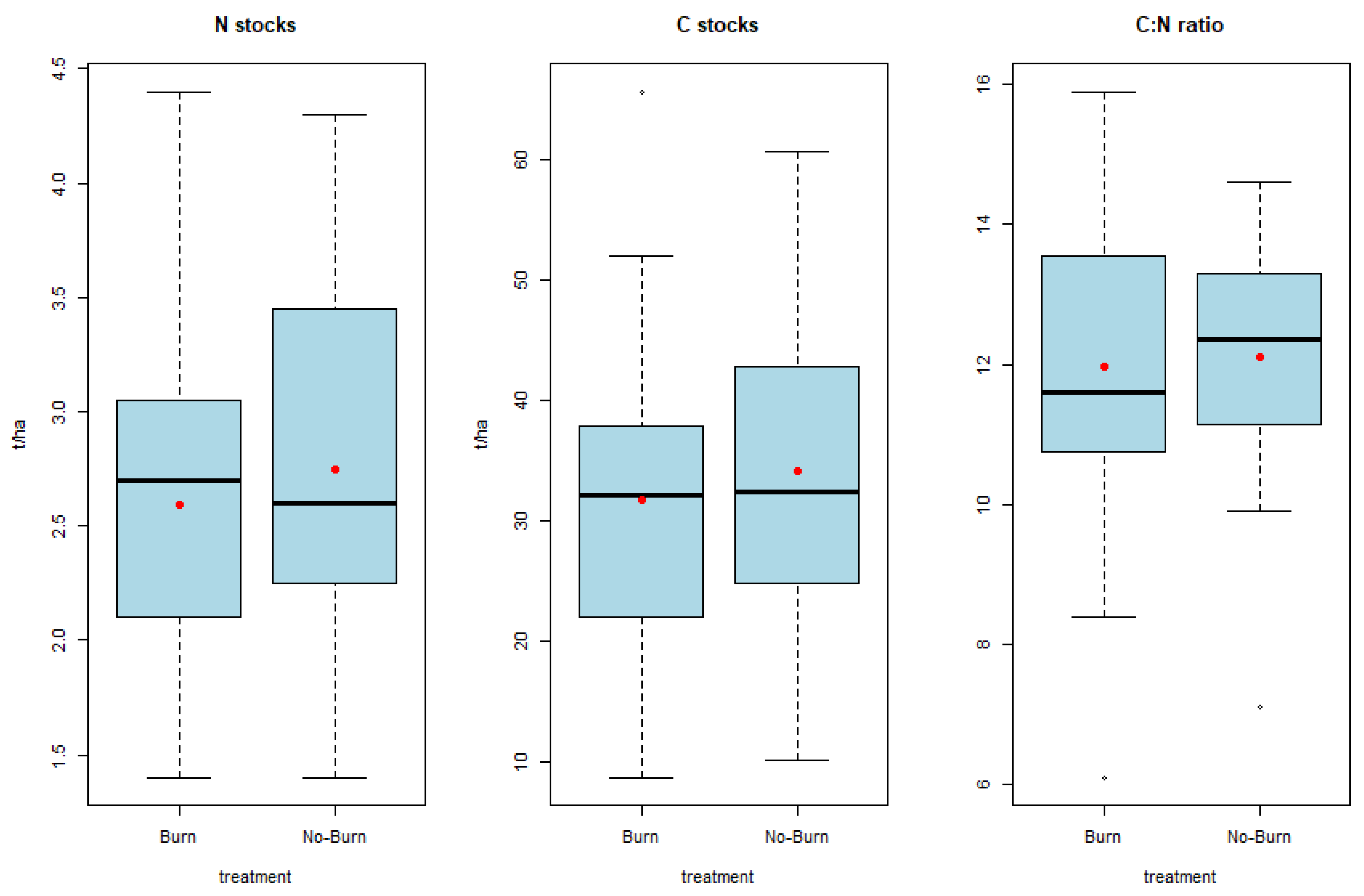
There were no significant differences observed in nitrogen (p = 0.435) or carbon stocks (p = 0.522) between the firebreaks and adjacent grassland (Figure 4). Additionally, no significant differences were noted in the C:N ratio (p = 0.808) (Figure 4). While most of the literature reports an increase in C and N stocks with fire suppression, some studies contradict this trend [50]. Local studies on firebreaks also indicate an increase in carbon and nitrogen concentrations outside the firebreaks [22,24]. A long-term study in a similar Afromontane environment also demonstrated that frequent fires do not lead to a decrease in topsoil carbon contents [51]. Conflicting findings are attributed to variations in soil type, fire behaviour, vegetation and seasonal fluctuations [52]. General trends in long-term studies indicate a decline in soil C due to burning [53]. Although significant differences were not found, the trend suggests an increase in C stocks in the grasslands compared to the firebreaks.
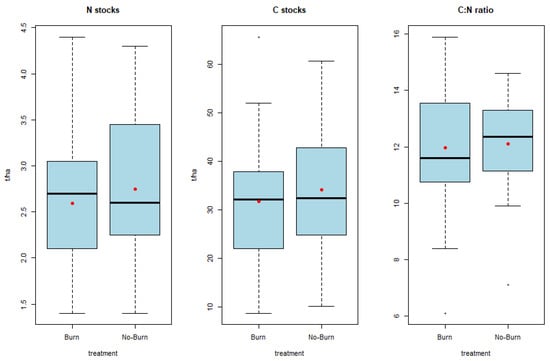
Figure 4.
Nitrogen and carbon stocks as well as C:N ratio of the firebreaks (burn) and adjacent grassland (no-burn) in the Golden Gate Highlands National Park. Means reported as red dots.
Despite localized increases in soil total N in certain long-term studies [54,55], a global analysis suggests an overall decline in N [53].
Ideal C:N ratios create a conducive environment for microbial organisms to cycle nutrients [56] and make them available for plant uptake. Optimal C:N ratios for mineralization typically fall between 20:1 and 30:1. Although the C:N ratios in our study were not significantly different (p = 0.808), the trend indicates more favourable ratios in the grassland compared to the firebreaks (Figure 4).
In summary, it appears that frequent fires do not alter the C and N dynamics in this grassland environment. Whether significant changes will occur in the long term remains to be seen. However, fire exclusion is certainly not recommended as a management practice to increase carbon stocks for carbon credits in this environment.
4. Conclusions
This research aims to contribute to the small body of knowledge on the effects of firebreaks on vegetation composition, diversity and soil properties. Firebreaks are burned annually as recommended by the National Veld and Forest Fire Act 101 of 1998 in South Africa to protect properties from runaway fires. However, there is limited research studying the effect of these firebreaks on plants and soils.
The research findings of this paper contradict or do not correspond to the research undertaken by other researchers in finding that firebreaks influenced the diversity of species. Our findings indicate that there is no difference in species richness or diversity. Instead, we found that firebreaks do not affect the community composition, nor the soil C and N stocks or ratios. There are also no differences between the successional stages of the grasses found in this research. We therefore suggest that the vegetation of the Eastern Free State Sandy Grassland (Gm 4) vegetation type in the eastern parts of the Free State is relatively resilient against annual fires, based on the data of one season as presented here. The limited research and the substantial surface area occupied by firebreaks underlines the urgent need for more research related to the effects of firebreaks on vegetation composition, diversity and soil properties, including the collection of data over more growing seasons (long-term studies), and the effect of fire on geophytes, parasites and shrubs.
The limitations of this study include:
- Only one vegetation type in the park was studied;
- Only areas with similar topography and slope were studied;
- Only carbon and nitrogen were studied in terms of soil properties;
- Only one growing season was studied.
Author Contributions
Conceptualization, A.C.v.A. and J.J.v.T.; methodology, A.C.v.A. and J.J.v.T.; software, A.C.v.A. and J.J.v.T.; formal analysis, A.C.v.A., J.C.L.d.J. and J.J.v.T.; investigation, A.C.v.A. and J.C.L.d.J.; resources, A.C.v.A.; data curation, A.C.v.A. and J.J.v.T.; writing—original draft preparation, A.C.v.A.; writing—review and editing, J.J.v.T. and J.C.L.d.J.; visualization, A.C.v.A. and J.J.v.T.; project administration, A.C.v.A.; funding acquisition, A.C.v.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Central Research Fund at the University of the Free State.
Institutional Review Board Statement
This study was conducted in accordance with the Environment and Biosafety Research Ethics Committee of The University of the Free State (UFS-ESD2021/0283.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available from the first author upon request.
Acknowledgments
We would like to than SANParks who allowed us to conduct research within the Golden Gate Highlands National Park, Louis Scott and Ettienne Theron for their assistance with the editing of the manuscript and Daryl Codron for his assistance with the statistical analysis.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Appendix A
This appendix contains the phytosociological classification of the firebreaks and adjacent grassland. All the communities, sub-communities and variants identified during the study are depicted here.

Table A1.
Caption Phytosociological classification of the vegetation associated with firebreaks and adjacent grassland plots in the Golden Gate Highlands National Park, Free State Province, South Africa. * Indicates alien invasive species.
Table A1.
Caption Phytosociological classification of the vegetation associated with firebreaks and adjacent grassland plots in the Golden Gate Highlands National Park, Free State Province, South Africa. * Indicates alien invasive species.
| Community | 1 | 2 | 3 | ||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sub-Community | 1.1 | 1.2 | 2.1 | 2.2 | 2.3 | 3.1 | 3.2 | ||||||||||||||||||||||||||||||||||||||||||
| Variants | 2.3.1 | 2.3.2 | |||||||||||||||||||||||||||||||||||||||||||||||
| Number of Species | 27 | 40 | 18 | 17 | 49 | 28 | 34 | 39 | |||||||||||||||||||||||||||||||||||||||||
| Number of Releves | 6 | 6 | 5 | 4 | 8 | 5 | 6 | 8 | |||||||||||||||||||||||||||||||||||||||||
| Releve Number | 25 | 22 | 23 | 24 | 26 | 21 | 19 | 40 | 39 | 18 | 17 | 20 | 51 | 50 | 37 | 49 | 52 | 7 | 8 | 6 | 5 | 42 | 46 | 41 | 45 | 34 | 43 | 44 | 35 | 54 | 48 | 53 | 38 | 47 | 33 | 3 | 4 | 36 | 2 | 1 | 9 | 13 | 11 | 15 | 10 | 16 | 12 | 14 | |
| Species Group A | Growth form | ||||||||||||||||||||||||||||||||||||||||||||||||
| Tristachya leucothrix | Grass | . | 1 | 1 | . | r | . | 1 | . | 1 | 1 | . | . | . | . | . | . | . | . | . | . | . | + | . | 2a | . | . | 2b | . | . | . | . | . | r | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . |
| Commelina africana | Perennial herb | . | 1 | . | 2a | 1 | 1 | . | + | 1 | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | 1 | . | 1 | . | . | r | . | r | . | . | . | . | . | . | . | . |
| Hypoxis angustifolia | Geophyte | . | + | r | . | + | . | 1 | . | . | r | r | r | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | + | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . |
| Aristida canescence | Grass | . | 1 | 1 | 1 | . | . | + | . | . | . | r | + | . | . | . | . | . | . | . | . | . | + | . | . | . | . | . | 2a | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . |
| Ischaemum fasciculatum | Grass | . | . | 1 | . | r | . | 1 | . | . | . | 1 | r | . | . | . | . | 1 | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . |
| Species Group B | |||||||||||||||||||||||||||||||||||||||||||||||||
| Pycreus nigricans | Sedge | 3 | 1 | 1 | 3 | 3 | 2a | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . |
| Kyllinga erecta | Sedge | 4 | . | 4 | . | 4 | 1 | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . |
| Fuirena pubescens | Sedge | 3 | 4 | . | 5 | 2a | 3 | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | 1 | . | . | . | 1 | + | . | . | . | . | 1 | r | . | + | . | . | . |
| Scirpoides burkei | Sedge | + | 2a | . | 1 | . | r | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . |
| Monopsis decipiens | Perennial herb | 2a | . | . | 2a | 1 | 2a | . | . | . | . | . | . | . | . | r | + | . | . | . | . | . | . | . | . | . | r | . | . | 1 | . | . | . | . | . | . | . | 1 | 1 | . | . | + | . | . | . | . | . | . | . |
| Eragrostis patentipilosa | Grass | 1 | . | . | 1 | . | 2a | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . |
| Hypericum lalandii | Perennial herb | r | r | . | . | . | . | . | . | . | . | . | . | . | . | r | . | . | . | . | . | . | . | . | . | . | r | . | . | . | . | . | r | . | + | r | . | . | r | . | . | . | . | . | . | . | . | . | . |
| Species Group C | |||||||||||||||||||||||||||||||||||||||||||||||||
| Heteropogon contortus | Grass | . | 2a | . | . | . | . | 5 | 1 | . | 5 | 3 | 2a | . | . | . | . | . | 1 | . | . | . | 1 | 1 | . | . | . | . | 1 | . | . | . | 1 | . | 2b | 2a | . | . | . | . | . | . | . | 1 | . | . | . | . | . |
| Eragrostis nindensis | Grass | . | 1 | . | . | . | . | 1 | . | . | 2a | 2a | 1 | . | . | . | . | . | . | . | . | . | 1 | . | 1 | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | 1 | . | . | . | . | . |
| Brachiaria serrata | Grass | . | . | . | . | . | . | 2a | . | . | r. | + | 1 | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . |
| Lasiosiphon caffer | Shrub | . | . | . | . | . | . | r | . | . | + | r | r | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . |
| Lasiosiphon kraussiana | Shrub | . | . | . | . | . | . | 1 | . | . | 1 | + | + | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | 1 | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . |
| Juncus dregeanus | Sedge | . | . | . | . | . | . | + | . | . | + | . | + | . | . | . | . | . | . | . | . | . | . | . | . | . | + | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . |
| Pentanisia prunelloides | Perennial herb | . | . | . | . | . | . | r | . | . | . | + | 1 | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . |
| Polygala hottentotta | Dwarf shrub | . | . | . | . | . | . | + | . | . | . | + | + | . | . | . | . | . | . | . | . | . | . | r | . | + | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . |
| Indigofera tristoides | Shrub | . | . | . | . | . | . | 1 | 2a | 2a | 1 | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . |
| Ficinia nigrescens | Sedge | . | . | 1 | . | . | . | + | . | . | . | 3 | 1 | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . |
| Andropagon appendiculatus | Grass | . | . | . | . | . | . | . | 2a | 2b | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . |
| Harpochloa falx | Grass | . | . | . | . | r | . | r | r | 1 | . | r | . | . | . | . | . | . | . | . | . | . | 1 | . | 1 | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . |
| Senecio othonniflorus | Perennial herb | 1 | . | . | . | . | . | 1 | r | . | r | . | r | . | . | . | . | . | . | . | . | . | . | 1 | 1 | . | . | . | . | 1 | . | . | . | . | . | 1 | . | . | 1 | . | . | . | . | . | . | . | . | . | . |
| Species Group D | |||||||||||||||||||||||||||||||||||||||||||||||||
| Hyparrhenia tamba | Grass | . | . | . | . | . | . | . | 1 | 2a | . | . | . | 4 | 3 | 5 | 3 | 5 | 4 | 5 | 5 | 4 | . | 2a | . | 2a | . | . | 2b | 1 | . | . | 5 | 2a | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . |
| Eragrostis curvula | Grass | . | . | . | . | . | . | . | . | . | . | . | . | 4 | . | . | 2a | 1 | 2b | 1 | 3 | 2a | 2a | 2a | . | 3 | . | 1 | 2a | 2a | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | + | 1 | 1 |
| Species Group E | |||||||||||||||||||||||||||||||||||||||||||||||||
| Cyperus esculentus | Sedge | . | . | . | . | . | . | . | . | . | . | . | . | 2b | 2a | . | 3 | 3 | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . |
| Eragrostis chloromelas | Grass | . | . | . | . | . | . | . | 4 | 2b | . | . | . | . | 2a | 2a | . | 3 | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | 1 | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . |
| Kyllinga pulchella | Sedge | . | . | . | . | . | . | . | . | . | . | . | . | r | 1 | . | 5 | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | r | . | . | . | . | . | . | . | . | . | . | . |
| Species Group F | |||||||||||||||||||||||||||||||||||||||||||||||||
| Conyza podocephala | Annual herb | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | 2a | 1 | 3 | 2a | . | r | . | . | . | . | . | . | . | . | . | . | . | . | + | . | . | + | . | . | 3 | . | 2a | 1 | 1 | 2a | 1 |
| Species Group G | |||||||||||||||||||||||||||||||||||||||||||||||||
| Helichrysum rugulosum | Perennial herb | . | . | . | . | . | . | 1 | . | . | . | 1 | . | . | . | . | . | . | . | . | + | . | 1 | 2a | 1 | 3 | . | 1 | 2a | 3 | 2a | 6 | 1 | 2a | 2a | . | . | . | 3 | . | . | . | 1 | . | 3 | . | 2a | . | 1 |
| Helictotrichon turgidulum | Grass | . | . | 1 | . | 1 | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | 2a | 2a | . | 1 | 3 | 2a | 2b | 3 | 2a | . | . | 1 | 1 | 2b | . | . | 4 | . | . | . | . | . | . | . | . | . | . |
| Felicia muricata | Shrub | . | . | . | . | . | . | . | . | . | . | 1 | 1 | . | . | . | . | . | . | . | . | . | 2a | 1 | 1 | . | r | . | + | . | 1 | 1 | . | . | 1 | . | . | . | . | . | . | . | . | . | . | . | . | . | . |
| Eragrostis plana | Grass | . | . | . | . | . | . | . | . | . | . | . | . | . | 2a | . | . | . | . | . | . | . | 1 | . | 1 | 1 | . | 1 | 1 | . | . | 2a | . | . | 1 | . | . | . | . | . | . | . | . | . | . | . | . | . | . |
| Helichrysum aureonitens | Perennial herb | . | 2b | 4 | 2b | 5 | 1 | . | 1 | 3 | . | . | . | . | + | 1 | . | . | . | . | . | . | 1 | 1 | 1 | 1 | 2b | 4 | + | 2b | . | . | . | 1 | . | 2a | . | . | 1 | . | . | . | . | . | 1 | . | 1 | . | . |
| Ajuga ophrydis | Perennial herb | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | + | . | r | r | . | . | + | 1 | . | r | . | . | . | . | . | . | . | . | . | . | . | . | . | . |
| Species Group H | |||||||||||||||||||||||||||||||||||||||||||||||||
| Aristea woodii | Perennial herb | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | + | r | 1 | r | . | 1 | r | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . |
| Erigeron bonariensis | Annual herb | . | . | . | . | + | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | r | + | r | . | . | r | + | . | . | . | . | . | . | . | . | . | . | . | . | . | . | r | . | . | . | 1 | . |
| Gazania krebsiana | Perennial herb | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | 1 | 1 | + | 1 | r | . | + | . | . | . | . | . | . | . | . | . | . | . | . | . | . | + | . | . | . | . | + |
| * Verbena bonarinesis | Annual herb | + | . | . | . | . | . | . | . | . | . | . | . | . | + | . | . | 1 | . | . | . | . | . | r | r | . | r | . | + | . | . | . | . | . | . | . | . | . | . | r | . | r | . | . | . | . | . | + | . |
| Dicoma anomala | Perennial herb | . | . | . | . | . | . | . | . | . | . | r | + | . | . | . | . | . | . | . | . | . | . | 1 | 1 | + | . | r | + | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . |
| Aristida congesta | Grass | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | 2b | 1 | 1 | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . |
| Melinis repens | Grass | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | 1 | . | 1 | . | . | 1 | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . |
| * Hypochaeris radicata | Perennial herb | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | 1 | . | . | + | . | r | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . |
| * Richardia brasiliensis | Perennial herb | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | 3 | . | 3 | . | . | 2a | . | . | . | . | . | . | . | . | + | . | 6 | . | . | . | . | . | . | . | . | . |
| Seriphium plumosum | Shrub | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | 1 | 1 | . | . | 2b | . | 1 | . | . | . | . | . | . | . | . | . | . | . | . | 1 | . | . | . | . | . | . | . |
| Felicia filifolia | Shrub | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | 1 | . | . | 1 | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | r | . | . | . |
| Lotononis eriantha | Perennial herb | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | 1 | . | . | . | . | 2a | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . |
| Euphorbia striata | Dwarf shrub | . | . | . | . | . | . | . | . | . | r | . | . | . | . | r | . | . | . | . | . | . | + | . | . | + | . | . | 1 | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . |
| Hyparrhenia hirta | Grass | . | . | . | . | r | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | 1 | . | 3 | . | 2a | . | . | 2a | . | . | . | . | . | . | . | . | . | . | . | . | . | 1 | . | . | . | . | . | . |
| Species Group I | |||||||||||||||||||||||||||||||||||||||||||||||||
| Pelargonium luridum | Geophyte | . | . | . | . | . | . | . | . | . | . | . | r | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | r | 1 | r | r | . | . | . | r | . | . | . | . | . | . | . | . | . | . | . |
| Oxalis obliquifolia | Geophyte | . | . | . | . | . | . | . | r | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | + | . | . | . | 1 | 1 | 2a | . | + | . | r | + | . | . | . | r | . | . | . | . | . | . | . |
| Gladiolus crassifolius | Geophyte | . | . | . | . | . | . | . | r | + | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | 1 | . | . | . | . | 1 | . | 1 | 2a | . | . | . | . | . | . | . | . | . | . | . | . | . | . |
| Acalypha punctata | Dwarf shrub | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | r | . | 1 | . | . | . | . | . | . | . | . | . | . | . | . | . | . |
| Crabbea acaulis | Perennial herb | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | r | . | . | . | r | . | . | . | . | . | . | . | 1 | . | 1 | . | . | . | . | . | . | . | 1 | . | . | . | . | . | . |
| Michracloa caffra | Grass | . | . | . | . | . | . | . | . | . | . | 1 | 1 | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | r | + | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . |
| Species Group J | |||||||||||||||||||||||||||||||||||||||||||||||||
| Sporobolus fimbriatus | Grass | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | r | . | . | . | . | . | . | + | . | 3 | 4 | 3 | r | 2a | 3 | 5 | 3 | 4 | 4 | 3 | 4 | 3 | 4 |
| Trifolium burchellianum | Perennial herb | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | + | . | 2a | 2a | . | 1 | 1 | . | . | 1 | 4 | 2a | 2a | 2a | . |
| Gnaphalium * polycaulon | Annual herb | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | 1 | . | . | . | . | . | . | . | . | . | . | . | . | . | . | 1 | . | . | 1 | 1 | . | . | + | . | + | . | 2a | . |
| Plantago lanceolata | Perennial herb | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | r | . | 1 | . | + | r | . | 1 | . | 1 | + | 2a |
| Species Group K | |||||||||||||||||||||||||||||||||||||||||||||||||
| Cyperus longus | Sedge | . | . | . | . | . | . | . | . | + | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | 3 | 3 | . | 2a | 3 | . | . | . | . | . | . | . | . |
| Ledebouria ovatifolia | Geophyte | . | . | . | . | r | . | . | . | . | 1 | r | . | . | . | . | . | . | . | . | . | r | . | . | . | . | . | . | . | . | . | . | . | . | . | r | r | . | . | r | 1 | . | . | . | . | . | . | . | 1 |
| * Erigeron sumatrensis | Annual herb | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | r | . | + | . | . | . | . | . | . | . | . | . | . |
| Lobelia flaccida | Annual herb | . | + | . | . | . | . | . | . | . | . | . | . | . | r | . | r | . | . | . | . | . | . | . | . | . | 1 | . | . | 2a | . | . | . | r | . | . | r | + | 2a | + | + | . | . | . | . | . | . | . | . |
| Helichrysum callicomum | Perennial herb | . | . | . | . | . | . | . | . | 2b | . | . | . | . | . | . | . | . | . | r | . | . | . | . | . | . | 2a | . | . | 4 | . | . | . | 2b | . | 4 | . | 1 | 2a | r | . | + | . | . | . | . | . | . | . |
| Species Group L | |||||||||||||||||||||||||||||||||||||||||||||||||
| Ipomoea oblongata | Perennial herb | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | r | 1 | . | + | + | 4 | 2b | 3 |
| Dyschoriste setigera | Dwarf shrub | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | 1 | . | 2a | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | 2a | 1 | 1 | . | . | 1 | r | 1 |
| Cymbopogon caesius | Grass | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | 1 | r | . | 1 | r | 1 | . | + |
| Berkheya pinnatifida | Perennial herb | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | + | 1 | + | . | . | 1 | 1 | 1 |
| Schistostephium crataegifolium | Perennial herb | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | + | 2a | . | 1 | . | . | . | 1 |
| Plantago major | Perennial herb | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | 1 | . | . | . | . | 1 | + | 1 |
| * Oenothera tretraptra | Perennial herb | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | 1 | r | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | + | + | + | . |
| Aristida bipartita | Grass | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | 1 | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | + | 1 |
| Gladiolus papilo | Geophyte | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | + | . | . | . | . | . | . | + | . | . | . | + | . | + |
| Rhynchosia totta | Perennial herb | . | . | . | . | . | . | + | . | . | + | . | . | . | . | . | . | . | r | . | . | . | . | . | r | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | + | . | + | . | 2a | . | . |
| Species Group M | |||||||||||||||||||||||||||||||||||||||||||||||||
| Centella asiatica | Perennial herb | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | 1 | 1 | 1 | 1 | . | . | + | . | . | . | . | . | . | . | + | + | . | r | r | + | + | . | 1 | r | 1 | . | + |
| Selago densiflora | Perennial herb | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | + | 1 | . | . | . | . | 1 | 2b | r | r | . | . | 1 | . | r | . | 1 | + | . | + | 1 | 1 | . | . | . | 1 | . |
| Species Group N | |||||||||||||||||||||||||||||||||||||||||||||||||
| Hermannia depressa | Perennial herb | . | . | . | . | . | . | . | . | . | . | . | 1 | . | . | . | . | . | . | . | . | 1 | . | . | + | + | . | . | + | . | . | 1 | 1 | . | 1 | . | . | . | . | r | . | 1 | 2a | 1 | 1 | 1 | . | 1 | 2a |
| Pseudognaphalium luteo-album | Annual herb | . | . | . | . | . | . | . | . | . | . | . | . | + | . | r | r | . | . | . | + | r | . | + | . | . | + | . | r | + | . | . | . | . | . | 2a | + | + | . | 1 | + | 1 | . | 1 | . | 2a | . | r | . |
| Pollicha campestris | Perennial herb | . | . | . | . | . | . | . | . | . | + | + | r | . | 1 | . | . | . | . | . | . | . | 1 | . | 1 | 1 | . | 1 | + | . | . | . | . | . | . | . | . | . | + | . | . | + | 1 | r | 1 | r | . | + | + |
| Elionuris miticus | Grass | . | . | . | . | . | . | 1 | . | 1 | . | . | . | . | . | . | . | . | . | . | . | . | . | . | 2b | 2a | . | 3 | 2a | . | 1 | . | . | . | 1 | . | . | . | . | . | . | + | 2a | 1 | 1 | . | . | . | . |
| Helichrysum nudifolium | Perennial herb | . | . | . | . | . | . | . | 2a | 1 | . | 1 | 1 | . | . | 1 | . | . | . | . | . | . | r | . | r | 1 | 3 | r | r | 2a | 2b | 4 | 2b | 1 | 3 | 1 | . | 1 | 1 | . | . | 3 | 1 | . | 2b | r | . | 1 | 2a |
| Species Group O | |||||||||||||||||||||||||||||||||||||||||||||||||
| Eragrostis lehmanniana | Grass | 1 | . | . | . | 1 | . | . | . | . | . | 1 | + | . | + | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | 1 | . | . | 2a | . | 3 | . | . | 2a | 2a | 1 | 1 | . | . | 1 | . | 1 | . |
| Themeda triandra | Grass | . | . | . | . | 1 | . | + | 1 | 2b | 1 | 1 | . | . | . | . | . | . | . | . | 1 | 2a | 1 | r | . | 2a | . | 2a | 1 | . | 4 | 3 | 1 | 4 | 1 | 4 | . | . | . | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Eragrostis capensis | Grass | 1 | 1 | . | 1 | 1 | 1 | r | . | . | . | + | + | . | . | 1 | . | . | . | . | . | . | 2a | . | 1 | . | 4 | r | + | . | . | 2a | . | . | 1 | 1 | r | 1 | 3 | 2a | + | 1 | . | 2 | 1 | 1 | . | 2a | 2a |
Appendix B
Appendix B contains the sum of all the numerical values for the species occurring in the firebreaks and adjacent grassland. This provides an idea of the preference each species has in terms of firebreaks vs. adjacent grassland environments.

Table A2.
Sum of the numerical values per species to compare adjacent grasslands with firebreaks. * Indicates alien invasive species.
Table A2.
Sum of the numerical values per species to compare adjacent grasslands with firebreaks. * Indicates alien invasive species.
| Adjacent Grassland | Fire-Breaks | |
|---|---|---|
| Species Group A | ||
| Tristachya leucothrix | 35.5 | 2.5 |
| Commelina africana | 7 | 15.5 |
| Hypoxis angustifolia | 5 | 2 |
| Aristida canescence | 6 | 12 |
| Ischaemum fasciculatum | 4.5 | 4.5 |
| Species Group B | ||
| Pycreus nigricans | 39 | 78.5 |
| Kyllinga erecta | 140 | 72 |
| Fuirena pubescens | 83 | 214 |
| Scirpoides burkei | 8.5 | 3.5 |
| Monopsis decipiens | 10.5 | 26 |
| Eragrostis patentipilosa | 0 | 12.5 |
| Hypericum lalandii | 3 | 1.5 |
| Species Group C | ||
| Heteropogon contortus | 318.5 | 40.5 |
| Eragrostis nindensis | 18.5 | 10.5 |
| Brachiaria serrata | 9 | 3 |
| Lasiosiphon caffer | 1.5 | 1 |
| Lasiosiphon kraussiana | 4 | 4 |
| Juncus dregeanus | 3 | 1 |
| Pentanisia prunelloides | 0.5 | 3 |
| Polygala hottentotta | 1 | 3.5 |
| Indigofera tristoides | 4 | 17 |
| Ficinia nigrescens | 3 | 37 |
| Andropogon appendiculatus | 0 | 26 |
| Harpochloa falx | 5 | 3 |
| Senecio othonniflorus | 10.5 | 5 |
| Species Group D | ||
| Hyparrhenia tamba | 72 | 963.5 |
| Eragrostis curvula | 29.5 | 190 |
| Species Group E | ||
| Cyperus esculentus | 43.5 | 52.5 |
| Eragrostis chloromelas | 8.5 | 133 |
| Kyllinga pulchella | 142.5 | 0.5 |
| Species Group F | ||
| Conyza podocephala | 12.5 | 102 |
| Species Group G | ||
| Helichrysum rugulosum | 92.5 | 121.5 |
| Helictotrichon turgidulum | 180.5 | 38.5 |
| Felicia muricata | 15 | 9 |
| Eragrostis plana | 25 | 4 |
| Helichrysum aureonitens | 348 | 69.5 |
| Ajuga ophrydis | 2 | 3.5 |
| Species Group H | ||
| Aristea woodii | 5 | 1.5 |
| Erigeron bonariensis | 5 | 2 |
| Gazania krebsiana | 4.5 | 6 |
| * Verbana bonariensis | 4 | 4.5 |
| Dicoma anamala | 2.5 | 5.5 |
| Aristida congesta | 2 | 19.5 |
| Melinis repens | 0 | 6 |
| * Hypochaeris radicata | 2.5 | 1 |
| * Richardia brasiliensis | 7 | 78.5 |
| Seriphium plumosum | 21.5 | 4 |
| Felicia filifolia | 0.5 | 4 |
| Lotononis eriantha | 10.5 | 0 |
| Euphorbia striata | 1.5 | 3.5 |
| Hyparrhenia hirta | 0.5 | 56 |
| Species Group I | ||
| Pelargonium luridum | 2.5 | 2 |
| Oxalis obliquifolia | 6 | 11 |
| Gladiolus crassifolius | 12.5 | 3.5 |
| Acalypha punctata | 2 | 0.5 |
| Crabbea acaulis | 2.5 | 4.5 |
| Michracloa caffra | 1 | 4.5 |
| Species Group J | ||
| Sporobolus fimbriatus | 464.5 | 246 |
| Trifolium burchellianum | 41 | 78.5 |
| Gnaphalium *polycaulon | 16.5 | 2 |
| Plantago lanceolata | 4.5 | 13 |
| Species Group K | ||
| Cyperus longus | 113.5 | 1 |
| Ledebouria ovatifolia | 6 | 3 |
| * Erigeron sumatrensis | 1.5 | 0 |
| Lobelia flaccida | 24.5 | 0.5 |
| Helichrysum callicomum | 160.5 | 35.5 |
| Species Group L | ||
| Ipomoea oblangata | 19 | 108 |
| Dyschoriste setigera | 11 | 16.5 |
| Cymbopogon caesius | 2.5 | 5.5 |
| Berkheya pinnatifida | 4 | 6 |
| Schistostephium crataegifolium | 1 | 12.5 |
| Plantago major | 3 | 4 |
| * Oenothera tretraptra | 2 | 3.5 |
| Aristida bipartita | 1 | 4 |
| Gladiolus papilo | 1 | 3 |
| Rhynchosia totta | 2.5 | 11 |
| Species Group M | ||
| Centella asiatica | 8.5 | 11 |
| Selago densiflora | 29.5 | 6.5 |
| Species Group N | ||
| Hermannia depressa | 13.5 | 27 |
| Pseudognaphalium luteo-album | 29 | 4.5 |
| Pollicha campestris | 13 | 9.5 |
| Elionuris miticus | 59.5 | 31.5 |
| Helichrysum nudifolium | 193.5 | 82 |
| Species Group O | ||
| Eragrostis lehmanniana | 71.5 | 7 |
| Themeda triandra | 134.5 | 193 |
| Eragrostis capensis | 157.5 | 21.5 |
References
- O’Mara, F.P. The role of grasslands in food security and climate change. Ann. Bot. 2012, 110, 1263–1270. [Google Scholar] [CrossRef]
- Bengtsson, J.; Bullock, J.M.; Egoh, B.; Everson, C.; Everson, T.; O’Connor, T.; O’Farrell, P.K.; Smith, H.G.; Lindborg, R. Grasslands—More important for ecosystem services than you might think. Ecosphere 2019, 10, e02582. [Google Scholar] [CrossRef]
- Neke, K.S.; Du Plessis, M.A. The Threat of Transformation: Qualitifying the Vulnerability of Grasslands in South Africa. Conserv. Biol. 2004, 18, 466–477. [Google Scholar] [CrossRef]
- Brown, L.R.; Bezuidenhout, H. Grassland vegetation of southern Africa. In Encyclopaedia of the Worlds Biomes; Dipaolo, D., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; Volume 3, pp. 814–826. ISBN 978-0-12-816097-8. [Google Scholar]
- Muller, M.; Siebert, S.J.; Ntloko, B.R.; Siebert, F. A floristic assessment of grassland diversity loss in South Africa. Bothalia 2021, 51, a11. [Google Scholar] [CrossRef]
- Mucina, L.; Rutherford, M.C. The Vegetation of South Africa, Lesotho and Swaziland. Strelitzia 19; South African National Biodiversity Institute: Pretoria, South Africa, 2006. [Google Scholar]
- O’Connor, T.G.; Kuyler, P. Impact of land use on the biodiversity integrity of the moist sub-biome of the grassland biome, South Africa. J. Environ. Manag. 2009, 90, 384–395. [Google Scholar] [CrossRef] [PubMed]
- Hensley, M.; le Roux, P.; du Preez, C.; van Huyssteen, C.; Kotze, E.; van Rensburg, L. Soils: The Free State’s Agricultural Base. S. Afr. Geogr. J. 2006, 88, 11–21. [Google Scholar] [CrossRef]
- De Wit, M. The Value of Biodiversity to the South African Economy: A Preliminary Study; Technical Report; Cooperative of Independent Consultants (CIC): Jeddah, Saudi Arabia, 2006. [Google Scholar] [CrossRef]
- Retallack, G.J. Cenozoic Expansion of Grasslands and Climate Cooling. J. Geol. 2001, 109, 407–426. [Google Scholar] [CrossRef]
- Scurlock, J.M.O.; Hall, D.O. The global carbon sink: A grassland perspective. Glob. Change Biol. 1998, 4, 229–233. [Google Scholar] [CrossRef]
- Watkinson, A.R.; Ormerod, S.T. Grasslands, Grazing and Biodiversity: Editors’ Introduction. J. Appl. Ecol. 2001, 38, 233–237. [Google Scholar]
- Bond, W.J. Fire. In Vegetation of Southern Africa; Cowling, R.M., Richardson, D.M., Pierce, S.M., Eds.; Cambridge University Press: Cambridge, UK, 1997; pp. 421–446. [Google Scholar]
- Blair, J.; Nippert, J.; Briggs, J. Grassland Ecology. In Ecology and the Environment, The Plant Sciences 8; Monson, R.K., Ed.; Springer Science and Business Media: New York, NY, USA, 2014; pp. 389–422. [Google Scholar]
- Nerlekar, A.N.; Veldman, J.W. High plant diversity and slow assembly of old-growth grasslands. Proc. Natl. Acad. Sci. USA 2020, 117, 18550–18556. [Google Scholar] [CrossRef]
- O’Connor, T.G.; Bredenkamp, G.J. Grassland. In Vegetation of Southern Africa; Cowling, R.M., Richardson, D.M., Pierce, S.M., Eds.; Cambridge University Press: Cambridge, UK, 1997; pp. 215–257. [Google Scholar]
- Archibald, S.; Roy, D.P.; van Wilgen, B.; Scholes, R.J. What limits fire? An examination of drivers of burnt area in Southern Africa. Glob. Change Biol. 2009, 15, 613–630. [Google Scholar] [CrossRef]
- Bowman, D.M.J.S.; Balch, J.K.; Artaxo, P.; Bond, W.J.; Carlson, J.M.; Cochrane, M.A.; D’Antonio, C.M.D.; DeFries, R.S.; Doyle, J.C.; Harrison, S.P.; et al. Fire in the Earth System. Science 2009, 324, 481–484. [Google Scholar] [CrossRef]
- Tainton, N. Veld Management in South Africa; University of Natal Press: Pietermaritzburg, South Africa, 1999; pp. 1–472. [Google Scholar]
- Gill, A.M. Fire Ecology in South Africa: A Visitor’s Impressions. S. Afr. J. Sci. 1981, 77, 62–64. [Google Scholar]
- Trollope, A.S.W.; Trollope, L.A.; Hartnett, D.C. Fire behaviour a key factor in the fire ecology of African grasslands and savannas. For. Fire Res. Wildland Fire Saf. 2002, 1–14. [Google Scholar]
- O’Connor, T.G.; Uys, R.G.; Mills, A.J. Ecological effects of firebreaks in the montane grasslands of the southern Drakensberg, South Africa. Afr. J. Range Forage Sci. 2004, 21, 1–9. [Google Scholar] [CrossRef]
- Le Maitre, D.C.; Midgley, J.J. Plant reproductive ecology. In The Ecology of Fynbos. Nutrients, Fire and Diversity; Cowling, R.M., Ed.; Oxford University Press: Cape Town, South Africa, 1992; pp. 35–174. [Google Scholar]
- Bachinger, L.M.; Brown, L.R.; Van Rooyen, M.W. The effects of fire-breaks on plant diversity and species composition in the grasslands of the Loskop Dam Nature Reserve, South Africa. Afr. J. Range Forage Sci. 2016, 33, 21–32. [Google Scholar] [CrossRef]
- Uys, R.G.; Bond, W.J.; Everson, T.M. The effect of different fire regimes on plant diversity in southern African grasslands. Biol. Conserv. 2004, 118, 489–499. [Google Scholar] [CrossRef]
- O’Connor, T.G.; Kuyler, P.; Kirkman, K.P.; Corcoran, B. Which grazing management practices are most appropriate for maintaining biodiversity in South African grasslands? Afr. J. Range Forage Sci. 2010, 27, 67–76. [Google Scholar] [CrossRef]
- Snyman, H.A. Fire and the dynamics of a semi-arid grassland: Influence on soil characteristics. Afr. J. Range Forage Sci. 2002, 19, 137–145. [Google Scholar] [CrossRef]
- Daemane, E.; Bezuidenhout, H.; Bissett, C.; Strydom, T.; Nariandeas, D.; Tshabalala, M.; Sikhosana, T. Proposed Changes to the Fire Management Plan of Golden Gate Highland National Park; Internal Report 08/2018; Scientific Services, South African National Parks: Knysna, South Africa, 2017. [Google Scholar]
- Kay, C.; Bredenkamp, G.J.; Theron, G.K. The plant communities of the Golden Gate Highlands National Park in the eastern Orange Free State. S. Afr. J. Bot. 1993, 59, 442–449. [Google Scholar] [CrossRef]
- Grab, S.W.; Goudie, A.S.; Viles, H.A.; Webb, N. Sandstone geomorphology of the Golden Gate Highlands National Park, South Africa, in a global context. Koedoe 2011, 53, 1–14. [Google Scholar] [CrossRef]
- Groenewald, G.H. Geology of the Golden Gate Highlands National Park. Koedoe 1986, 29, 165–181. [Google Scholar] [CrossRef]
- Moffett, R. A Field Guide to the Clarens Village Conservancy; Sun Press: Cape Town, South Africa, 2018. [Google Scholar]
- Van Zyl, D. South African Weather and Atmospheric Phenomena; Briza Publications: Pretoria, South Africa, 2003. [Google Scholar]
- Brown, L.R.; du Preez, P.J.; Bezuidenhout, H.; Bredenkamp, G.J.; Mostert, T.H.C.; Collins, N.B. Guidelines for phytosociological classifications and descriptions of vegetation in southern Africa. Koedoe 2013, 55, 1103. [Google Scholar] [CrossRef]
- Werger, M.J.A. On concepts and techniques applied in the Zürich-Montpellier method of vegetation survey. Bothalia 1974, 11, 309–323. [Google Scholar] [CrossRef]
- Westhoff, V.; van der Maarel, E. The Braun-Blanquet Approach. In Classification of Plant Communities; Whittaker, R.H., Ed.; Dr W. Junk Publishers: The Hague, The Netherlands; Boston, MA, USA, 1980; pp. 289–399. [Google Scholar]
- Van der Maarel, E. Transformation of cover-abundance values for appropriate numerical treatment—Alternatives to the proposals by Podani. J. Veg. Sci. 2007, 18, 767–770. [Google Scholar]
- Kent, M. Vegetation Description and Data Analysis: A Practical Approach, 2nd ed.; Wiley-Blackwell Publishers: West Sussex, UK, 2012. [Google Scholar]
- Tichy, L.; Holt, J. JUICE: A Program for Management, Analysis and Classification of Ecological Data; Vegetation Science Group, Masaryk University: Brno, Czech Republic, 2006. [Google Scholar]
- Roleček, J.; Lubomír, T.; David, Z.; Chytry, M. Modified TWINSPAN classification in which the hierarchy respects cluster heterogeneity. J. Veg. Sci. 2009, 20, 596–602. [Google Scholar] [CrossRef]
- Brown, L.R.; Bezuidenhout, H. Ecosystem description and diversity of the Jurisdam-Seekoegat sections of the Mountain Zebra National Park, South Africa. S. Afr. J. Bot. 2018, 118, 166–178. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. 2021. Available online: https://www.R-project.org/ (accessed on 20 April 2024).
- Kuznetsova, A.; Brockhoff, P.B.; Christensen RH, B. lmerTest Package: Tests in linear mixed effects models. J. Stat. Softw. 2017, 82, 1–26. [Google Scholar] [CrossRef]
- Okalebo, J.R.; Gathua, K.W.; Woomer, P.L. Laboratory Methods of Soil and Plant Analysis: A Working Manual, 2nd ed.; TSBF-CIAT and SACRED Africa: Nairobi, Kenya, 2002. [Google Scholar]
- Razakamanarivo, R.H.; Grinand, C.; Razafindrakoto, M.A.; Bernoux, M.; Albrecht, A. Mapping organic carbon stocks in eucalyptus plantations of the central highlands of Madagascar: A multiple regression approach. Geoderma 2011, 162, 335–346. [Google Scholar] [CrossRef]
- Kowalenko, C.G. Assessment of LECO CNS-2000 analyzer for simultaneously measuring total carbon, nitrogen and sulphur in soil. Commun. Soil Sci. Plant Anal. 2001, 32, 2065–2078. [Google Scholar] [CrossRef]
- Van Oudtshoorn, F. Guide to the Grasses of Southern Africa; Briza Publications: Pretoria, South Africa, 2018; pp. 1–287. [Google Scholar]
- Roux, E.R.; Warren, M. Plant Succession on Abandoned Fields in Central Oklahoma and in the Transvaal Highveld. Ecology 1963, 44, 576–579. [Google Scholar] [CrossRef]
- Van Rheede van Oudtshoorn, F.P. The Evaluation of Various Reseeding Methods for Restoring Old Croplands in the Highveld Region of South Africa. Master’s Thesis, University of South Africa, Johannesburg, South Africa, 2007. [Google Scholar]
- Wang, Y.; Xu, Z.; Zhou, Q. Impact of fire on soil gross nitrogen transformations in forest ecosystems. J. Soils Sediments 2014, 14, 1030–1040. [Google Scholar] [CrossRef]
- Findlay, N.; Manson, A.; Cromsigt, J.P.; Gordijn, P.; Nixon, C.; Rietkerk, M.; Thibaud, G.; Wassen, M.J.; Beest, M.T. Long-term frequent fires do not decrease topsoil carbon and nitrogen in an Afromontane grassland. Afr. J. Range Forage Sci. 2022, 39, 44–55. [Google Scholar] [CrossRef]
- Alcañiz, M.; Outeiro, L.; Francos, M.; Úbeda, X. Effects of prescribed fires on soil properties: A review. Sci. Total Environ. 2018, 613, 944–957. [Google Scholar] [CrossRef]
- Pellegrini, A.F.A.; Ahlström, A.; Hobbie, S.E.; Reich, P.B.; Nieradzik, L.P.; Staver, A.C.; Scharenbroch, B.C.; Jumpponen, A.; Anderegg, W.R.L.; Randerson, J.T.; et al. Fire frequency drives decadal changes in soil carbon and nitrogen and 3492 ecosystem productivity. Nature 2018, 553, 194–198. [Google Scholar] [CrossRef]
- Aranibar, J.N.; Macko, S.A.; Anderson, I.C.; Potgieter, A.L.F.; Sowry, R.; Shugart, H.H. Nutrient cycling responses to fire frequency in the Kruger National Park (South 2942 Africa) as indicated by Stable Isotope analysis. Isot. Environ. Health Stud. 2003, 39, 141–158. [Google Scholar] [CrossRef]
- Brye, K.R. Soil physiochemical changes following 12 years of annual burning in a humid–subtropical tallgrass prairie: A hypothesis. Acta Oecologica 2006, 30, 407–413. [Google Scholar] [CrossRef]
- Wan, X.; Huang, Z.; He, Z.; Yu, Z.; Wang, M.; Davis, M.R.; Yang, Y. Soil C: N ratio is the major determinant of soil microbial community structure in subtropical coniferous and broadleaf forest plantations. Plant Soil 2015, 387, 103–116. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).