Phenotype Variation in Niphargus (Amphipoda: Niphargidae): Possible Explanations and Open Challenges
Abstract
1. Introduction
2. Terminological Note
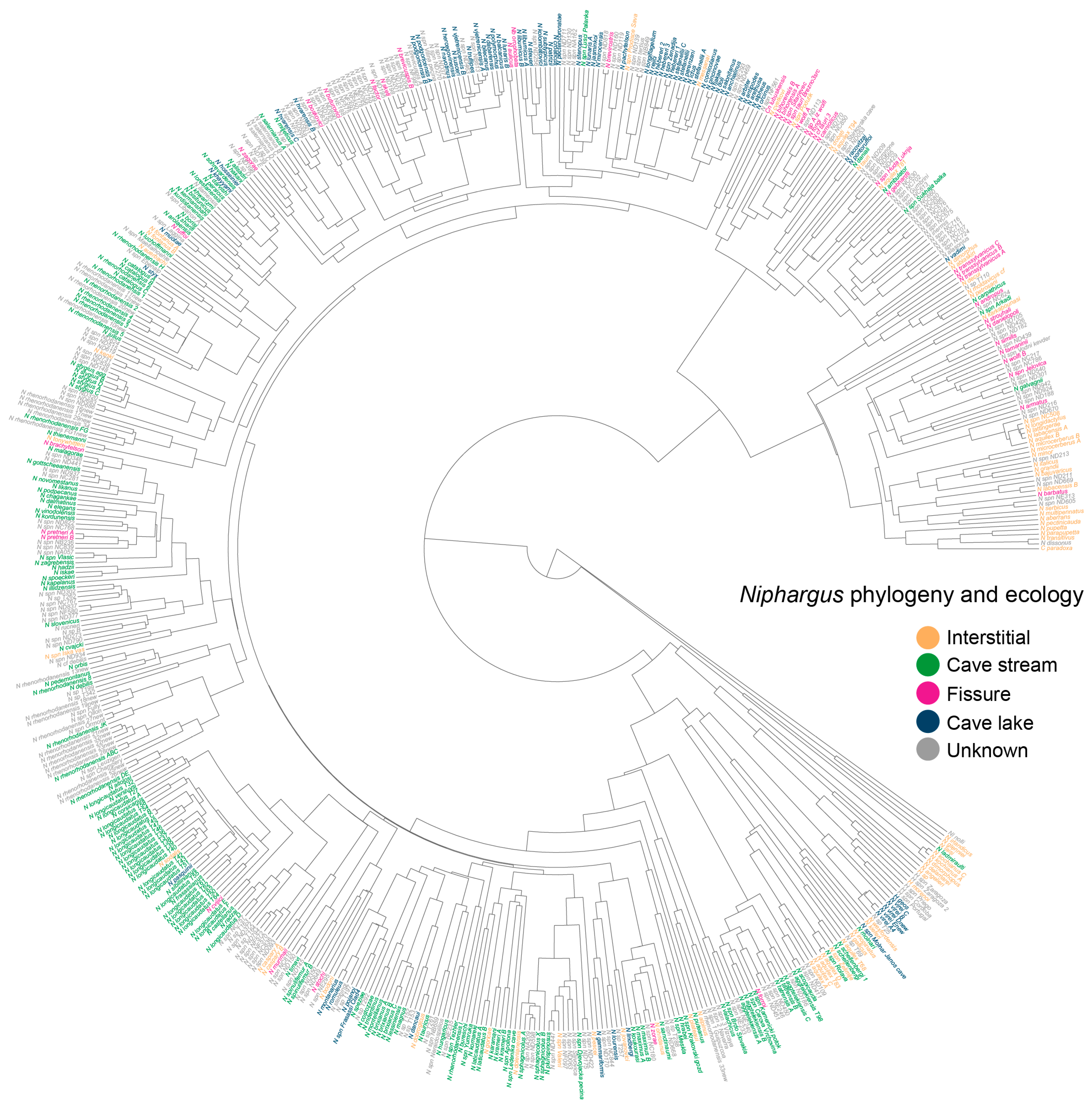
3. Phylogenetic Signal
| Trait | K | p Value (K) | λ | p Value (λ) |
|---|---|---|---|---|
| Gnathopod I carpus length | 0.14 | 0.240 | 0.67 | 0.000 |
| Antenna II length | 0.17 | 0.062 | 0.63 | 0.000 |
| Gnathopod II carpus length | 0.17 | 0.069 | 0.72 | 0.000 |
| Antenna I length | 0.20 | 0.040 | 0.66 | 0.000 |
| Pereopod VII basis width | 0.23 | 0.003 | 0.72 | 0.000 |
| Pereopod VII length | 0.24 | 0.016 | 0.79 | 0.000 |
| Gnathopod II perimeter | 0.25 | 0.015 | 0.84 | 0.000 |
| Pereopod V basis width | 0.25 | 0.010 | 0.71 | 0.000 |
| Pereopod VI basis width | 0.26 | 0.007 | 0.69 | 0.000 |
| Coxa II depth | 0.26 | 0.006 | 0.76 | 0.000 |
| Pereopod V length | 0.28 | 0.005 | 0.85 | 0.000 |
| Body length | 0.51 | 0.001 | 0.91 | 0.000 |
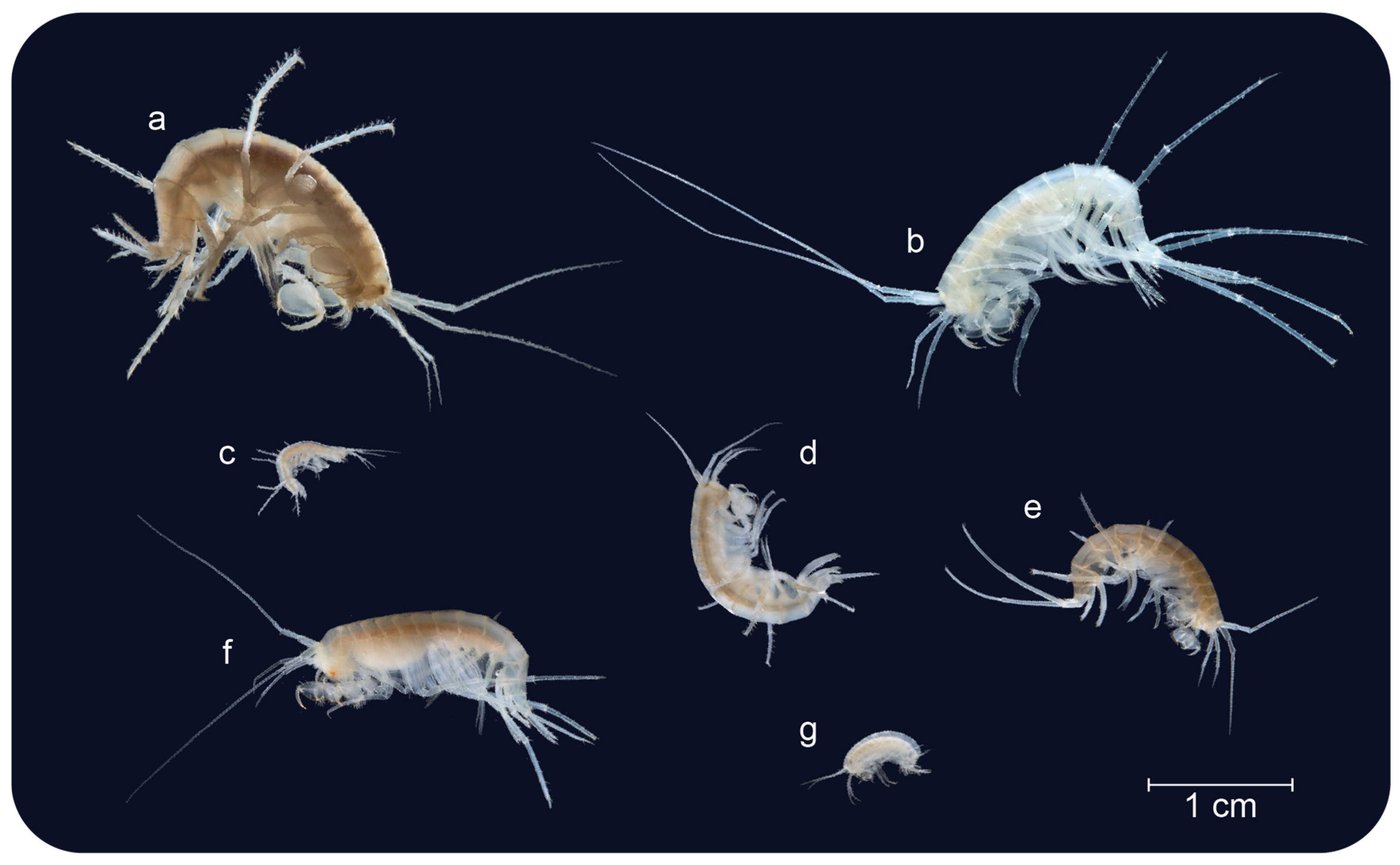
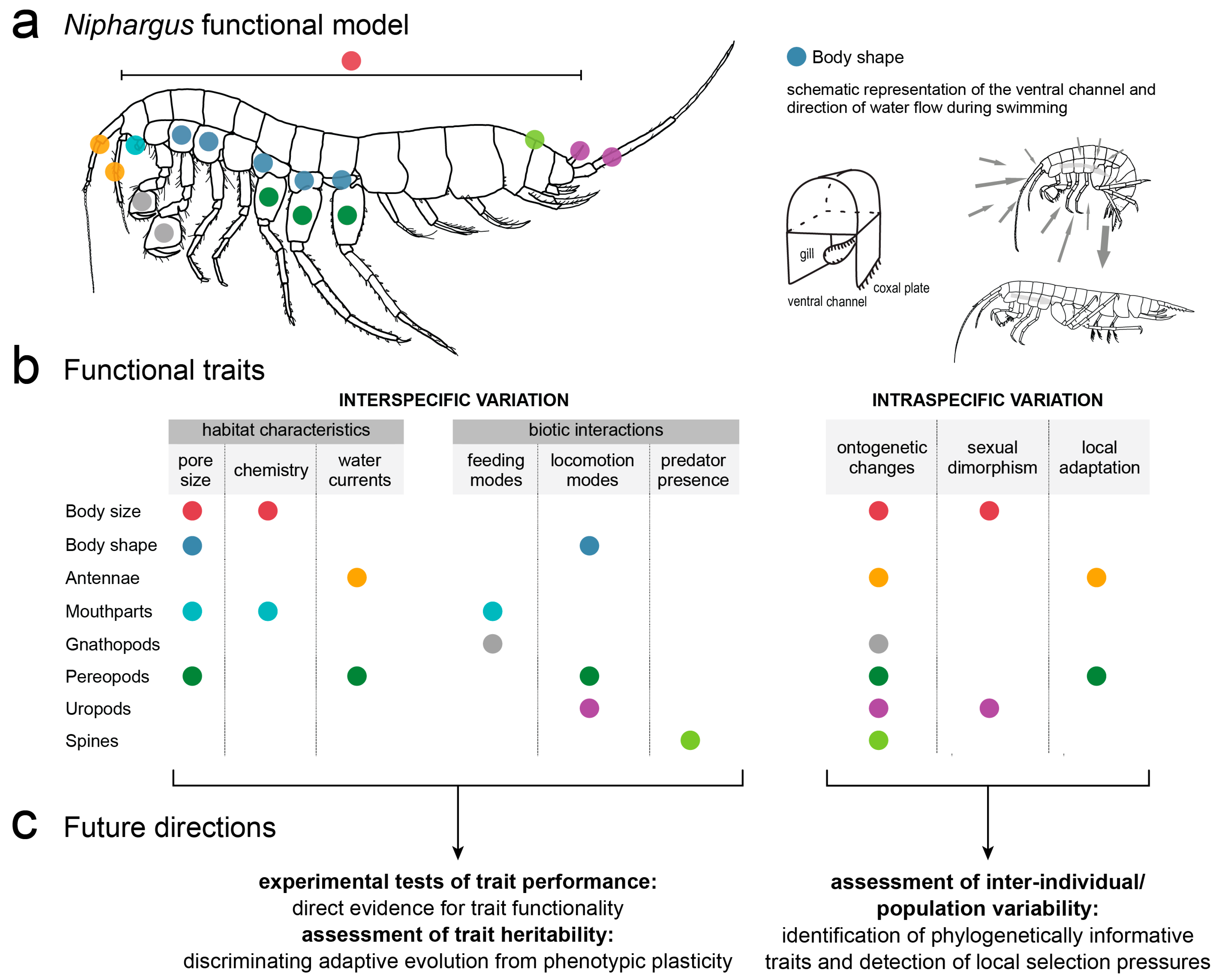
4. Functional Morphology
5. Intraspecific Variation
6. Discussion
6.1. Phylogenetic Contingency or Selection Acting on a Functional Trait or Both
6.2. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mayr, E. Systematics and the Origin of Species from the Viewpoint of Zoologist; Harvard University Press: Cambridge, MA, USA; London, UK, 1942; ISBN 0-674-88250-3. [Google Scholar]
- Aristotle Historia Animalium (Ton Peri ta Zoia Historion). Available online: https://archive.org/details/history_of_animals_2202_librivox (accessed on 1 June 2024).
- Gould, S.J. Ontogeny and Phylogeny; Belknap Press: Cambridge, MA, USA, 1985; ISBN 9780674639416. [Google Scholar]
- Darwin, C. On the Origin of the Species; John Murray: London, UK, 1859; Volume 5. [Google Scholar]
- Futuyma, D.J. Evolution, 2nd ed.; Sinauer Associates Inc.: Sunderland, MA, USA, 2009. [Google Scholar]
- Losos, J.B. Convergence, Adaptation, and Constraint. Evolution 2011, 65, 1827–1840. [Google Scholar] [CrossRef] [PubMed]
- Pfennig, D.W. Key Questions about Phenotypic Plasticity. In Phenotypic Plasticity & Evolution: Causes, Consequences, Controversies; Pfennig, D.W., Ed.; Taylor & Francis Group: Abingdon, UK, 2021; pp. 25–54. ISBN 9780429343001. [Google Scholar]
- Kitching, I.J.; Forey, P.L.; Humphries, C.J.; Williams, D.M. Cladistics: The Theory and Practice of Parsimony Analysis, 2nd ed.; Oxford University Press: Oxford, NY, USA, 1998; ISBN 0198501390. [Google Scholar]
- Hennig, W. Grundzüge Einer Theorie der Phylogenetischen Systematik; Deutscher Zentralverlag: Berlin, Germany, 1950. [Google Scholar]
- Christiansen, K. Convergence and Parallelism in Cave Entomobryinae. Evolution 1961, 15, 288–301. [Google Scholar] [CrossRef]
- Wiens, J.J. Character analysis in morphological phylogenetics: Problems and Solutions. Syst. Biol. 2001, 50, 689–699. [Google Scholar] [CrossRef]
- Gould, S.J.; Lewontin, R.C. The Spandrels of San Marco and the Panglossian Paradigm: A Critique of the Adaptationist Programme. Proc. R. Soc. London Ser. B 1979, 205, 581–598. [Google Scholar] [CrossRef]
- Herrel, A.; Huyghe, K.; Vanhooydonck, B.; Backeljau, T.; Breugelmans, K.; Grbac, I.; Van Damme, R.; Irschick, D.J. Rapid large-scale evolutionary divergence in morphology and performance associated with exploitation of a different dietary resource. Proc. Natl. Acad. Sci. USA 2008, 105, 4792–4795. [Google Scholar] [CrossRef]
- Schluter, D. Ecological Character Displacement in Adaptive Radiation. Am. Nat. 2000, 156, S4–S16. [Google Scholar] [CrossRef]
- Grant, B.R.; Grant, P.R. Songs of Darwin’s finches diverge when a new species enters the community. Proc. Natl. Acad. Sci. USA 2010, 107, 20156–20163. [Google Scholar] [CrossRef]
- Stuart, Y.E.; Losos, J.B. Ecological character displacement: Glass half full or half empty? Trends Ecol. Evol. 2013, 28, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Pfennig, K.S.; Pfennig, D.W. Character Displacement: Ecological and Reproductive Responses to A Common Evolutionary Problem. Q. Rev. Biol. 2009, 84, 253–276. [Google Scholar] [CrossRef]
- Wiens, J.J.; Chippindael, P.T.; Hillis, D.M. When Are Phylogenetic Analyses Misled by Convergence? A Case Study in Texas Cave Salamanders. Syst. Biol. 2003, 52, 501–514. [Google Scholar] [CrossRef]
- Felsenstein, J. Phylogenies and the Comparative Method. Am. Nat. 1985, 125, 1–15. [Google Scholar] [CrossRef]
- Horton, T.; Lowry, J.K.; De Broyer, C.; Bellan-Santini, D.; Coleman, C.O.; Daneliya, M.E.; Dauvin, J.C.; Fišer, C.; Gasca, R.; Grabowski, M.; et al. World Amphipoda Database. Available online: http://www.marinespecies.org/amphipoda (accessed on 19 April 2021).
- Horton, T.; De Broyer, C.; Bellan-Santini, D.; Coleman, C.O.; Copilaș-Ciocianu, D.; Corbari, L.; Daneliya, M.E.; Dauvin, J.-C.; Decock, W.; Fanini, L.; et al. The World Amphipoda Database: History and Progress. Rec. Aust. Mus. 2023, 75, 329–342. [Google Scholar] [CrossRef]
- Väinölä, R.; Witt, J.D.S.; Grabowski, M.; Bradbury, J.H.; Jazdzewski, K.; Sket, B. Global diversity of amphipods (Amphipoda; Crustacea) in freshwater. Hydrobiologia 2008, 595, 241–255. [Google Scholar] [CrossRef]
- Fišer, Ž.; Altermatt, F.; Zakšek, V.; Knapič, T.; Fišer, C. Morphologically cryptic Amphipod species sre “ecological clones” at regional but not at local scale: A case study of four Niphargus species. PLoS ONE 2015, 10, e0134384. [Google Scholar] [CrossRef] [PubMed]
- Fišer, C.; Sket, B.; Stoch, F. Distribution of four narrowly endemic Niphargus species (Crustacea: Amphipoda) in the western Dinaric region with description of a new species. Zool. Anz. 2006, 245, 77–94. [Google Scholar] [CrossRef]
- Sket, B. Distribution, ecological character and phylogenetic importance of Niphargus valachicus (Amphipoda, Gammaridae s. L.). Biološki Vestn. 1981, 29, 87–103. [Google Scholar]
- Copilaş-Ciocianu, D.; Fišer, C.; Borza, P.; Petrusek, A. Is subterranean lifestyle reversible? Independent and recent large-scale dispersal into surface waters by two species of the groundwater amphipod genus Niphargus. Mol. Phylogenet. Evol. 2018, 119, 37–49. [Google Scholar] [CrossRef]
- Sket, B. High biodiversity in hypogean waters and its endangerment—The situation in Slovenia, the Dinaric Karst, and Europe. Crustaceana 1999, 72, 767–779. [Google Scholar] [CrossRef]
- Trontelj, P.; Blejec, A.; Fišer, C. Ecomorphological Convergence of Cave Communities. Evolution 2012, 66, 3852–3865. [Google Scholar] [CrossRef] [PubMed]
- Borko, Š.; Trontelj, P.; Seehausen, O.; Moškrič, A.; Fišer, C. A subterranean adaptive radiation of amphipods in Europe. Nat. Commun. 2021, 12, 3688. [Google Scholar] [CrossRef]
- Karaman, G.S.; Sarbu, S.M. A new species of the genus Pontoniphargus Dancau, 1970 (Amphipoda Gammaridea, family Niphargidae) from Romania, P. ruffoi, n. sp. Bolletino Mus. Civ. Stor. Nat. Verona 1996, 20, 569–582. [Google Scholar]
- Brad, T.; Fišer, C.; Flot, J.-F.; Sarbu, S.M. Niphargus dancaui sp. nov. (Amphipoda, Niphargidae)—A new species thriving in sulfidic groundwaters in southeastern Romania. Eur. J. Taxon. 2015, 164, 1–28. [Google Scholar] [CrossRef][Green Version]
- Fišer, C.; Luštrik, R.; Sarbu, S.M.; Flot, J.-F.; Trontelj, P. Morphological Evolution of Coexisting Amphipod Species Pairs from Sulfidic Caves Suggests Competitive Interactions and Character Displacement, but No Environmental Filtering and Convergence. PLoS ONE 2015, 10, e0123535. [Google Scholar] [CrossRef]
- Flot, J.-F.; Bauermeister, J.; Brad, T.; Hillebrand-Voiculescu, A.; Sarbu, S.M.; Dattagupta, S. Niphargus—Thiothrix associations may be widespread in sulphidic groundwater ecosystems: Evidence from southeastern Romania. Mol. Ecol. 2014, 23, 1405–1417. [Google Scholar] [CrossRef]
- Sket, B. Niphargus im Brackwasser. Crustac. Suppl. 1977, 4, 188–191. [Google Scholar]
- Sket, B.; Velkovrh, F. Podzemeljske tivali v termalnih vodah (Subterranean animals in thermal waters). Biološki Vestn. 1981, 29, 91–120. [Google Scholar]
- Perez-Moreno, J.L.; Balazs, G.; Bracken-Grissom, H.D. Transcriptomic Insights into the Loss of Vision in Molnar Janos Cave’s Crustaceans. Integr. Comp. Biol. 2018, 58, 452–464. [Google Scholar] [CrossRef]
- Premate, E.; Borko, Š.; Delić, T.; Malard, F.; Simon, L.; Fišer, C. Cave amphipods reveal co-variation between morphology and trophic niche in a low-productivity environment. Freshw. Biol. 2021, 66, 1876–1888. [Google Scholar] [CrossRef]
- Fišer, C.; Kovacec, Ž.; Pustovrh, M.; Trontelj, P. The role of predation in the diet of Niphargus (Amphipoda: Niphargidae). Speleobiol. Notes 2010, 2, 4–6. [Google Scholar]
- Copilaș-Ciocianu, D.; Boros, B.V.; Šidagytė-Copilas, E. Morphology mirrors trophic niche in a freshwater amphipod community. Freshw. Biol. 2021, 66, 1968–1979. [Google Scholar] [CrossRef]
- Luštrik, R.; Turjak, M.; Kralj-Fišer, S.; Fišer, C. Coexistence of surface and cave amphipods in an ecotone environment. Contrib. Zool. 2011, 80, 133–141. [Google Scholar] [CrossRef]
- Copilaș-Ciocianu, D.; Boroş, B.V. Contrasting life history strategies in a phylogenetically diverse community of freshwater amphipods (Crustacea: Malacostraca). Zoology 2016, 11, 21–29. [Google Scholar] [CrossRef]
- Delić, T.; Trontelj, P.; Zakšek, V.; Fišer, C. Biotic and abiotic determinants of appendage length evolution in a cave amphipod. J. Zool. 2016, 299, 42–50. [Google Scholar] [CrossRef]
- McInerney, C.E.; Maurice, L.; Robertson, A.L.; Knight, L.R.F.D.; Arnscheidt, J.J.; Venditti, C.; Dooley, J.S.G.; Mathers, T.; Matthijs, S.; Eriksson, K.; et al. The ancient Britons: Groundwater fauna survived extreme climate change over tens of millions of years across NW Europe. Mol. Ecol. 2014, 23, 1153–1166. [Google Scholar] [CrossRef]
- Premate, E.; Zagmajster, M.; Fišer, C. Evaluating the overlap of surface protected areas with different facets of groundwater biodiversity: Glass half empty of half full? Biol. Conserv. 2024, 289, 110392. [Google Scholar] [CrossRef]
- Borko, Š.; Altermatt, F.; Zagmajster, M.; Fišer, C. A hotspot of groundwater amphipod diversity on a crossroad of evolutionary radiations. Divers. Distrib. 2022, 28, 2765–2777. [Google Scholar] [CrossRef]
- Eme, D.; Zagmajster, M.; Delić, T.; Fišer, C.; Flot, J.-F.; Konecny-Dupre, L.; Palsson, S.; Stoch, F.; Zakšek, V.; Douady, C.J.; et al. Do cryptic species matter in macroecology? Sequencing European groundwater crustaceans yields smaller ranges but does not challenge biodiversity determinants. Ecography 2017, 40, 424–436. [Google Scholar] [CrossRef]
- Delić, T.; Trontelj, P.; Rendoš, M.; Fišer, C. The importance of naming cryptic species and the conservation of endemic subterranean amphipods. Sci. Rep. 2017, 7, 3391. [Google Scholar] [CrossRef]
- Meleg, I.N.; Zakšek, V.; Fišer, C.; Kelemen, B.S.; Moldovan, O.T. Can Environment Predict Cryptic Diversity? The Case of Niphargus Inhabiting Western Carpathian Groundwater. PLoS ONE 2013, 8, e76760. [Google Scholar] [CrossRef]
- Weber, D.; Brad, T.; Weigand, A.; Flot, J. Water diviners multiplied: Cryptic diversity in the Niphargus aquilex species complex in Northern Europe. bioRxiv 2023, 1–7. [Google Scholar] [CrossRef]
- Stoch, F.; Knüsel, M.; Zakšek, V.; Alther, R.; Salussolia, A.; Altermatt, F.; Fišer, C.; Flot, J.F. Integrative taxonomy of the groundwater amphipod Niphargus bihorensis Schellenberg, 1940 reveals a species-rich clade. Contrib. Zool. 2024, in press. [Google Scholar] [CrossRef]
- Straškraba, M. Les groupements des espèces du genre Niphargus (senso lato). In Actes du Ier Colloque International sur le Genre Niphargus (1969); Ruffo, S., Ed.; Museo Civico di Storia Naturale di Verona: Verona, Italy, 1972; pp. 85–90. [Google Scholar]
- Karaman, G.S.; Ruffo, S. Amphipoda: Niphargus-group (Niphargidae sensu Bousfield, 1982). In Stygofauna Mundi: A Faunistic, Distributional, and Ecological Synthesis of the World Fauna Inhabiting Subterranean Waters (Including Marine Intersti); Botosaneanu, L., Ed.; Brill Academic Pub: Leiden, The Netherlands, 1986; pp. 514–534. [Google Scholar]
- Sket, B. Niphargobates orophobata n.g., n.sp. (Amphipoda, Gammaridae s.l.) from cave waters in Slovenia. Biološki Vestn. 1981, 29, 105–118. [Google Scholar]
- Karaman, G.S.; Sket, B. New genus and species of the family Niphargidae (Crustacea: Amphipoda: Senticaudata), Chaetoniphargus lubuskensis gen. nov., sp. nov. from Croatia. Zootaxa 2019, 4545, 249–263. [Google Scholar] [CrossRef] [PubMed]
- Jurado-Rivera, J.A.; Álvarez, G.; Caro, J.A.; Juan, C.; Pons, J.; Jaume, D. Molecular systematics of Haploginglymus, a genus of subterranean amphipods endemic to the Iberian Peninsula (Amphipoda: Niphargidae). Contrib. Zool. 2017, 86, 239–260. [Google Scholar] [CrossRef]
- Blomberg, S.P.; Garland, T.J.; Ives, A.R. Testing for phylogenetic signal in comparative data: Behavioral traits are more labile. Evolution 2003, 57, 717–745. [Google Scholar] [CrossRef] [PubMed]
- Pagel, M. Inferring the historical patterns of biological evolution. Nature 1999, 401, 877–884. [Google Scholar] [CrossRef] [PubMed]
- Kuntner, M.; Agnarsson, I. Are the Linnean and phylogenetic nomenclatural systems combinable? Recommendations for biological nomenclature. Syst. Biol. 2006, 55, 774–784. [Google Scholar] [CrossRef] [PubMed]
- Jażdżewski, K.; Kupryjanowicz, J. One More Fossil Niphargid (Malacostraca: Amphipoda) from Baltic Amber. J. Crustac. Biol. 2010, 30, 413–416. [Google Scholar] [CrossRef][Green Version]
- Coleman, C.O.; Ruffo, S. Another discovery of a niphargid amphipod (Crustaca) in Baltic amber. Mitteilungen Geol. Inst. Univ. Hambg. 2002, 86, 239–244. [Google Scholar]
- Coleman, C.O.; Myers, A.A. New Amphipoda from Baltic Amber. Pol. Arch. Hydrobiol. 2000, 47, 457–464. [Google Scholar]
- Sket, B. Vier Neue Aberrante Niphargus—Arten (Amphipoda, Gammaridae) und Einige Bemerkungen zur Taxonomie der Niphargus—Ähnlichen Gruppen. Diss. Acad. Sci. Artium Slov.-Cl. IV Hist. Nat. Med. 1971, 14, 1–25. [Google Scholar]
- Sket, B.; Notenboom, J. Phylogeny and biogeography of the Niphargus transitivus group of species (Crustacea, Amphipoda). Bijdr. tot Dierkd. 1993, 63, 149–161. [Google Scholar] [CrossRef]
- Karaman, S. Podrod Stygoniphargus u Sloveniji i Hrvatskoj. Prirodosl. Istraživanja 1952, 25, 5–38. [Google Scholar]
- Karaman, S.L. Podrod Orniphargus u Jugoslaviji II. O nekim amfipodima—Izopodima Balkana i o njihovoj sistematici. [O. nekim amfipodima—Izopodima Balkana i o njihovoj sistematici]. Poseb. Izd. Srp. Akad. Nauk. 1950, 163, 119–151. [Google Scholar]
- Karaman, S. Niphargus ilidžensis Schäferna i njegovi srodnici u Jugoslaviji. [O. nekim amfipodima—Izopodima Balkana i o njihovoj sistematici]. Poseb. Izd. Srp. Akad. Nauk. 1950, 163, 51–86. [Google Scholar]
- Karaman, S. Weitere Beitraege zur Kenntnis des jugoslavischen Niphargiden. Glas. Prir. Muzeja U Beogradu B 1960, 15, 75–90. [Google Scholar]
- Copilaș-Ciocianu, D.; Fišer, C.; Borza, P.; Balazs, G.; Angyal, D.; Petrusek, A. Low intraspecific genetic divergence and weak niche differentiation despite wide ranges and extensive sympatry in two epigean Niphargus species (Crustacea: Amphipoda). Zool. J. Linn. Soc. 2017, 10, 485–499. [Google Scholar] [CrossRef]
- Delić, T.; Borko, Š.; Premate, E.; Rexhepi, B.; Alther, R.; Knüsel, M.; Malard, F.; Weber, D.; Stoch, F.; Flot, J.-F.; et al. Evolutionary origin of morphologically cryptic species imprints co-occurrence and sympatry patterns. bioRxiv 2023. [Google Scholar] [CrossRef]
- Fišer, C.; Trontelj, P.; Sket, B. Phylogenetic analysis of the Niphargus orcinus species–aggregate (Crustacea: Amphipoda: Niphargidae) with description of new taxa. J. Nat. Hist. 2006, 40, 2265–2315. [Google Scholar] [CrossRef]
- Fišer, C.; Sket, B.; Trontelj, P. A phylogenetic perspective on 160 years of troubled taxonomy of Niphargus (Crustacea: Amphipoda). Zool. Scr. 2008, 37, 665–680. [Google Scholar] [CrossRef]
- Premate, E.; Fišer, C. Functional trait dataset of European groundwater Amphipoda: Niphargidae and Typhlogammaridae. Sci. Data 2024, 11, 188. [Google Scholar] [CrossRef]
- Revell, L.J. phytools: An R package for phylogenetic comparative biology (and other things). Methods Ecol. Evol. 2012, 3, 217–223. [Google Scholar] [CrossRef]
- Dahl, E. The amphipod functional model and its bearing upon systematics and phylogeny. Zool. Scr. 1977, 6, 221–228. [Google Scholar] [CrossRef]
- Premate, E.; Kepic, T.; Fišer, C. Is the relationship between body length and body mass consistent across habitats? A case study on Niphargus (Crustacea: Amphipoda). Zoology 2023, 161, 126120. [Google Scholar] [CrossRef]
- Kralj-Fišer, S.; Premate, E.; Copilaş-Ciocianu, D.; Volk, T.; Fišer, Ž.; Balázs, G.; Herczeg, G.; Delić, T.; Fišer, C. The interplay between habitat use, morphology and locomotion in subterranean crustaceans of the genus Niphargus. Zoology 2020, 139. [Google Scholar] [CrossRef]
- Premate, E.; Fišer, Ž.; Biro, A.; Copilaș-Ciocianu, D.; Fromhage, L.; Jennions, M.D.; Borko, Š.; Herczeg, G.; Balazs, G.; Kralj-Fišer, S.; et al. Sexual dimorphism in subterranean amphipod crustaceans covaries with subterranean habitat type. J. Evol. Biol. 2024, in press. [Google Scholar] [CrossRef]
- Fišer, C.; Zagmajster, M.; Zakšek, V. Coevolution of life history traits and morphology in female subterranean amphipods. Oikos 2013, 122, 770–778. [Google Scholar] [CrossRef]
- Fišer, C.; Delić, T.; Luštrik, R.; Zagmajster, M.; Altermatt, F. Niches within a niche: Ecological differentiation of subterranean amphipods across Europe’s interstitial waters. Ecography 2019, 42, 1212–1223. [Google Scholar] [CrossRef]
- Borko, Š.; Collette, M.; Brad, T.; Zakšek, V.; Flot, J.-F.; Vaxevanopoulos, M.; Sarbu, S.M.; Fišer, C. Amphipods in a Greek cave with sulphidic and non-sulphidic water: Phylogenetically clustered and ecologically divergent. Syst. Biodivers. 2019, 17, 558–572. [Google Scholar] [CrossRef]
- Flot, J.-F.; Wörheide, G.; Dattagupta, S. Unsuspected diversity of Niphargus amphipods in the chemoautotrophic cave ecosystem of Frasassi, central Italy. BMC Evol. Biol. 2010, 10, 171. [Google Scholar] [CrossRef]
- Karaman, S.L. Die Niphargiden des slovenischen Karstes, Istriens sowie des benachb. Ital. Acta Musei Maced. Sci. Nat. 1954, 2, 159–180. [Google Scholar]
- Karaman, G.S. Revizija Niphargus orcinus grupe I dio (fam. Niphargidae) (Contribution to the Knowledge of the Amphipoda 130). Montenegrin Acad. Sci. Arts Glas. Sect. Nat. Sci. 1984, 4, 7–79. [Google Scholar]
- Ramm, T.; Scholtz, G. No sight, no smell?—Brain anatomy of two amphipod crustaceans with different lifestyles. Arthropod Struct. Dev. 2017, 46, 537–551. [Google Scholar] [CrossRef]
- Mayer, G.; Maier, G.; Maas, A.; Waloszek, D. Mouthpart Morphology of Gammarus roeselii Compared to a Successful Invader, Dikerogammarus villosus (Amphipoda). J. Crustac. Biol. 2009, 29, 161–174. [Google Scholar] [CrossRef]
- Mayer, G.; Haug, J.T.; Maas, A.; Waloszek, D. Functional aspects of the gammaridean mandibles with special reference to the lacinia mobilis (Crustacea, Amphipoda). Zool. Anz. 2013, 252, 536–547. [Google Scholar] [CrossRef]
- Hutchins, B.T.; Schwartz, B.F.; Nowlin, W.H. Morphological and trophic specialization in a subterranean amphipod assemblage. Freshw. Biol. 2014, 59, 2447–2461. [Google Scholar] [CrossRef]
- Fišer, C.; Konec, M.; Alther, R.; Švara, V.; Altermatt, F. Taxonomic, phylogenetic and ecological diversity of Niphargus (Amphipoda: Crustacea) in the Hölloch cave system (Switzerland). Syst. Biodivers. 2017, 15, 218–237. [Google Scholar] [CrossRef]
- Karaman, G.S. Contribution to the knowledge of the Amphipoda 125. First discovery of genus Niphargopsis Chevr. 1922 in Yugoslavia with revision of the genus (Fam. Gammaridae). Poljopr. Sumar. 1982, 28, 87–103. [Google Scholar]
- Ginet, R. Compartement sexuel de Niphargus virei (Crustacé hypogé). Comparaison avec les autres Amphipodes. Rev. Comport. Anim. 1967, 4, 56. [Google Scholar]
- Marin, I.; Palatov, D. An occasional record of the amplexus in epigean Niphargus (Amphipoda: Niphargidae) from the Russian Western Caucasus. Zootaxa 2019, 4701, 97–100. [Google Scholar] [CrossRef]
- Sket, B. Die Niphargus jovanovici—Gruppe (Amphipoda, Gammaridae) in Jugoslawien und NO- Italien, Taxonomisch, Zoogeographisch und Phylogenetisch Betrachtet. Diss. Acad. Sci. Artium Slov.-Cl. IV Hist. Nat. Med. 1972, 15, 99–140. [Google Scholar]
- Belanger, J. Appendage diversity and mode of locomotion: Walking. In The Natural History of Crustacea: Functional Morphology & Diversity (Volume 1); Watling, L., Thiel, M., Eds.; Oxford University Press: New York, NY, USA, 2013; pp. 261–275. [Google Scholar]
- Delić, T.; Švara, V.; Coleman, C.O.; Trontelj, P.; Fišer, C. The giant cryptic amphipod species of the subterranean genus Niphargus (Crustacea, Amphipoda). Zool. Scr. 2017, 46, 740–752. [Google Scholar] [CrossRef]
- Premate, E.; Zagmajster, M.; Fišer, C. Inferring predator–prey interaction in the subterranean environment: A case study from Dinaric caves. Sci. Rep. 2021, 11, 21682. [Google Scholar] [CrossRef]
- Premate, E.; Borko, Š.; Altermatt, F.; Fišer, C. Context-dependent evolution of high trophic position drives functional disparity in subterranean crustaceans. Funct. Ecol. 2023, 37, 2523–2534. [Google Scholar] [CrossRef]
- Sket, B. Niphargus stygius (Schiödte) (Amphipoda, Gammaridae)—Die Neubeschreibung des Generotypus, Variabilität, Verbreitung und Biologie der Art, I. Biološki Vestn. 1974, 22, 91–103. [Google Scholar]
- Fišer, C.; Coleman, C.O.; Zagmajster, M.; Zwittnig, B.; Gerecke, R.; Sket, B. Old museum samples and recent taxonomy: A taxonomic, biogeographic and conservation perspective of the Niphargus tatrensis species complex (Crustacea: Amphipoda). Org. Divers. Evol. 2010, 10, 5–22. [Google Scholar] [CrossRef]
- Ginet, R. Ecologie, éthologie et biologie de Niphargus (Amphipodes Gammaridés hypogés). Ann. Spéléologie 1960, 15, 127–382. [Google Scholar]
- D’Ancona, U. I Niphargus Italiani Tentativo di valutazione critica delle minori unita sistematiche. Mem. Ist. Ital. Speleol. 1942, 4, 1–125. [Google Scholar]
- Fišer, C.; Bininda-Emonds, O.R.P.; Blejec, A.; Sket, B. Can heterochrony help explain the high morphological diversity within the genus Niphargus (Crustacea: Amphipoda)? Org. Divers. Evol. 2008, 8, 146–162. [Google Scholar] [CrossRef][Green Version]
- Premate, E.; Borko, Š.; Kralj-Fišer, S.; Jennions, M.; Fišer, Ž.; Balázs, G.; Bíró, A.; Bračko, G.; Copilaş-Ciocianu, D.; Hrga, N.; et al. No room for males in caves: Female-biased sex ratio in subterranean amphipods of the genus Niphargus. J. Evol. Biol. 2021, 34, 1653–1661. [Google Scholar] [CrossRef] [PubMed]
- Stock, J.H.; Gledhill, T. The Niphargus kochianus-group in North-Western Europe. Crustac. Suppl. 1977, 4, 212–243. [Google Scholar]
- Butler, M.A.; Sawyer, S.A.; Losos, J.B. Sexual dimorphism and adaptive radiation in Anolis lizards. Nature 2007, 447, 202–205. [Google Scholar] [CrossRef] [PubMed]
- Zakšek, V.; Delić, T.; Fišer, C.; Jalžić, B.; Trontelj, P. Emergence of sympatry in a radiation of subterranean amphipods. J. Biogeogr. 2019, 49, 657–669. [Google Scholar] [CrossRef]
- Schluter, D. The Ecology of Adaptive Radiation; Oxford University Press: Oxford, UK, 2000. [Google Scholar]
- Seehausen, O. Process and pattern in cichlid radiations—Inferences for understanding unusually high rates of evolutionary diversification. New Phytol. 2015, 207, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Losos, J.B. Lizards in an Evolutionary Tree. Ecology and Adaptive Radiation of Anoles; University of California Press: Berkeley, CA, USA; Los Angeles, CA, USA, 2009. [Google Scholar]
- Fišer, C.; Trontelj, P.; Luštrik, R.; Sket, B. Toward a unified taxonomy of Niphargus (Crustacea: Amphipoda): A review of morphological variability. Zootaxa 2009, 2061, 1–22. [Google Scholar] [CrossRef]
- Winchell, K.M.; Maayan, I.; Fredette, J.R.; Revell, L.J. Linking Locomotor Performance to Morphological Shifts in Urban Lizards. Proc. R. Soc. B 2018, 285. [Google Scholar] [CrossRef] [PubMed]
- Wainwright, P.C. Functional versus morphological diversity in macroevolution. Annu. Rev. Ecol. Evol. Syst. 2007, 38, 381–401. [Google Scholar] [CrossRef]
- Alfaro, M.E.; Bolnick, D.I.; Wainwright, P.C. Evolutionary consequences of many-to-one mapping of jaw morphology to mechanics in labrid fishes. Am. Nat. 2005, 165, E140–E154. [Google Scholar] [CrossRef]
- Mammola, S.; Lunghi, E.; Bilandžija, H.; Cardoso, P.; Grimm, V.; Schmidt, S.I.; Hesselberg, T.; Martinez, A. Collecting eco-evolutionary data in the dark: Impediments to subterranean research and how to overcome them. Ecol. Evol. 2021, 11, 5911–5926. [Google Scholar] [CrossRef]
- Bilandžija, H.; Hollifield, B.; Steck, M.; Meng, G.; Ng, M.; Koch, A.D.; Gračan, R.; Ćetković, H.; Porter, M.L.; Renner, K.J.; et al. Phenotypic plasticity as a mechanism of cave colonization and adaptation. eLife 2020, 9, e51830. [Google Scholar] [CrossRef]
- Rütz, N.K.; Marxsen, J.; Wolters, V. Long—Term cultivation of the groundwater amphipod Niphargus aquilex (Crustacea). Hydrobiologia 2022, 26, 269–281. [Google Scholar] [CrossRef]
- Alther, R.; Krähenbühl, A.; Bucher, P.; Altermatt, F. Optimizing laboratory cultures of Gammarus fossarum (Crustacea: Amphipoda) as a study organism in environmental sciences and ecotoxicology. Sci. Total Environ. 2023, 855. [Google Scholar] [CrossRef] [PubMed]
- Fong, D.W. Morphological Evolution of the Amphipod Gammarus minus in Caves: Quantitative Genetic Analysis. Am. Midl. Nat. 1989, 121, 361–378. [Google Scholar] [CrossRef]
- Formenti, G.; Theissinger, K.; Fernandes, C.; Bista, I.; Bombarely, A.; Bleidorn, C.; Ciofi, C.; Crottini, A.; Godoy, J.A.; Höglund, J.; et al. The era of reference genomes in conservation genomics. Trends Ecol. Evol. 2022, 37, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Pineda-ALarcon, L.; Zuluaga, M.; Ruíz, S.; Fernandez McCann, D.; Vélez, F.; Aguirre, N.; Puerta, Y.; Canon, J. Automated Software for Counting and Measuring Hyalella Genus Using Artificial Intelligence. Environ. Sci. Pollut. Res. 2023, 30, 123603–123615. [Google Scholar] [CrossRef]
- Visscher, P.M.; Brown, M.A.; McCarthy, M.I.; Yang, J. Five years of GWAS discovery. Am. J. Hum. Genet. 2012, 90, 7–24. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fišer, C.; Premate, E. Phenotype Variation in Niphargus (Amphipoda: Niphargidae): Possible Explanations and Open Challenges. Diversity 2024, 16, 375. https://doi.org/10.3390/d16070375
Fišer C, Premate E. Phenotype Variation in Niphargus (Amphipoda: Niphargidae): Possible Explanations and Open Challenges. Diversity. 2024; 16(7):375. https://doi.org/10.3390/d16070375
Chicago/Turabian StyleFišer, Cene, and Ester Premate. 2024. "Phenotype Variation in Niphargus (Amphipoda: Niphargidae): Possible Explanations and Open Challenges" Diversity 16, no. 7: 375. https://doi.org/10.3390/d16070375
APA StyleFišer, C., & Premate, E. (2024). Phenotype Variation in Niphargus (Amphipoda: Niphargidae): Possible Explanations and Open Challenges. Diversity, 16(7), 375. https://doi.org/10.3390/d16070375






