Migratory Ecology of Pseudoplatystoma fasciatum in the Amazon Basin Revealed by Otolith Microchemistry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Area of Study and Sampling
2.2. Analytical Design
2.2.1. Laboratory Analysis
2.2.2. Migration in Different Waters
2.2.3. Distance Migrated
2.2.4. What Is the Directionality of the Migrations of P. fasciatum?
2.2.5. Is the Migration of P. fasciatum Related to Age?
3. Results
3.1. Migration of P. fasciatum between Waters with Different Sr and Chemical Compositions
3.2. Distance Migrated
3.3. Migration at Age
3.4. Directionality of Migration
4. Discussion
Implications for Management and Conservation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baras, E.; Lucas, M.C. Impacts of man’s modifications of river hydrology on the migration of freshwater fishes: A mechanistic perspective. Int. J. Ecohydrol. Hydrobiol. 2001, 1, 291–304. [Google Scholar]
- Brönmark, C.; Hulthén, K.; Nilsson, P.A.; Skov, C.; Hansson, L.A.; Brodersen, J.; Chapman, B.B. There and back again: Migration in freshwater fishes. Can. J. Zool. 2014, 92, 467–479. [Google Scholar] [CrossRef]
- Reis-Santos, P.; Gillanders, B.M.; Sturrock, A.M.; Izzo, C.; Oxman, D.S.; Lueders-Dumont, J.A.; Hüssy, K.; Tanner, S.E.; Rogers, T.; Doubleday, Z.A.; et al. Reading the biomineralized book of life: Expanding otolith biogeochemical research and applications for fisheries and ecosystem-based management. Rev. Fish Biol. Fish. 2023, 33, 411–449. [Google Scholar] [CrossRef]
- Duponchelle, F.; Isaac, V.J.; Doria, C.; Van Damme, P.A.; Herrera-R, G.A.; Anderson, E.P.; Cruz, R.E.A.; Hauser, M.; Hermann, T.W.; Agudelo, E.; et al. Conservation of migratory fishes in the Amazon basin. Aquat. Conserv. Mar. Freshw. Ecosyst. 2021, 31, 1087–1105. [Google Scholar] [CrossRef]
- Winemiller, K.O.; Poff, N.L.; Brown, C.M.; Grantham, T.E.; Matthews, J.H.; Palmer, M.A.; Spence, C.M.; Wilby, R.L.; Haasnoot, M.; Mendoza, G.F.; et al. Sustainable water management under future uncertainty with eco-engineering decision scaling. Nat. Clim. Change 2016, 6, 25–34. [Google Scholar] [CrossRef]
- Junk, W.J. General aspects of floodplain ecology with special reference to Amazonian floodplains. In The Central Amazon Floodplain: Ecology of a Pulsing System; Springer: Berlin/Heidelberg, Germany, 1989; pp. 3–20. [Google Scholar]
- Herrera, G.A.; Heilpern, S.A.; Couto, T.B.A.; Victoria-Lacy, L.; Duponchelle, F.; Correa, S.B.; Farah-Pérez, A.; López-Casas, S.; Cañas-Alva, C.M.; Doria, C.R.C.; et al. A synthesis of the diversity of freshwater fish migrations in the Amazon basin. Fish Fish. 2023, 25, 114–133. [Google Scholar] [CrossRef]
- Hermann, T.W.; Stewart, D.J.; Limburg, K.E.; Castello, L. Unravelling the life history of Amazonian fishes through otolith microchemistry. R. Soc. Open Sci. 2016, 3, 160206. [Google Scholar] [CrossRef]
- Hermann, T.W.; Duponchelle, F.; Castello, L.; Limburg, K.E.; Pereira, L.A.; Hauser, M. Harnessing the potential for otolith microchemistry to foster the conservation of Amazonian fishes. Aquat. Conserv. Mar. Freshw. Ecosyst. 2021, 31, 1206–1220. [Google Scholar] [CrossRef]
- Goulding, M.; Lowe-McConnell, R.H. The Fishes and the Forest; Goulding, M., Lowe-McConnell, R.H., Eds.; University of California Press: Berkeley, CA, USA, 1980. [Google Scholar]
- Mariac, C.; Renno, J.-F.; Garcia-Davila, C.; Vigouroux, Y.; Mejia, E.; Angulo, C.; Ruiz, D.C.; Estivals, G.; Nolorbe, C.; Vasquez, A.G.; et al. Species-level ichthyoplankton dynamics for 97 fishes in two major river basins of the Amazon using quantitative metabarcoding. Mol. Ecol. 2021, 31, 1627–1648. [Google Scholar] [CrossRef] [PubMed]
- Barthem, R.B.; Goulding, M.; Leite, R.G.; Cañas, C.; Forsberg, B.; Venticinque, E.; Petry, P.; De, M.L.; Ribeiro, B.; Chuctaya, J.; et al. Goliath catfish spawning in the far western Amazon confirmed by the distribution of mature adults, drifting larvae and migrating juveniles. Sci. Rep. 2017, 7, 41784. [Google Scholar] [CrossRef]
- Barthem, R.; Goulding, M. Os Bagres Balizadores: Ecologia, Migração e Conservação de Peixes Amazônicos; Sociedade Civil Mamirauá: Tefé, AM, Brazil, 1997; Available online: https://www.bdpa.cnptia.embrapa.br/consulta/busca?b=ad&id=575548&biblioteca=vazio&busca=(autoria:%22BARTHEM,%20R.%22)&qFacets=(autoria:%22BARTHEM,%20R.%22)&sort=&paginacao=t&paginaAtual=1 (accessed on 1 May 2023).
- Hauser, M.; Duponchelle, F.; Hermann, T.W.; Limburg, K.E.; Castello, L.; Stewart, D.J.; Torrente-Vilara, G.; García-Vásquez, A.; García-Davila, C.; Pouilly, M.; et al. Unmasking continental natal homing in goliath catfish from the upper Amazon. Freshw. Biol. 2020, 65, 325–336. [Google Scholar] [CrossRef]
- Osorio, D.; Terborgh, J.; Alvarez, A.; Ortega, H.; Quispe, R.; Chipollini, V.; Davenport, L.C. Lateral migration of fish between an oxbow lake and an Amazonian headwater river. Ecol. Freshw. Fish 2011, 20, 619–627. [Google Scholar] [CrossRef]
- Castello, L. Lateral migration of Arapaima gigas in floodplains of the Amazon. Ecol. Freshw. Fish 2008, 17, 38–46. [Google Scholar] [CrossRef]
- Duponchelle, F.; Pouilly, M.; Pécheyran, C.; Hauser, M.; Renno, J.F.; Panfili, J.; Darnaude, A.M.; García-Vasquez, A.; Carvajal-Vallejos, F.; García-Dávila, C.; et al. Trans-Amazonian natal homing in giant catfish. J. Appl. Ecol. 2016, 53, 1511–1520. [Google Scholar] [CrossRef]
- Miranda-Chumacero, G.; Álvarez, G.; Luna, V.; Wallace, R.B.; Painter, L. First observations on annual massive upstream migration of juvenile catfish Trichomycterus in an Amazonian River. Environ. Biol. Fish. 2015, 98, 1913–1926. [Google Scholar] [CrossRef]
- Flecker, A.S.; McIntyre, P.B.; Moore, J.W.; Anderson, J.T.; Taylor, B.W.; Hall, R.O. Migratory fishes as material and process subsidies in riverine ecosystems. Am. Fish. Soc. Symp. 2010, 73, 559–592. [Google Scholar]
- Heilpern, S.A.; Defries, R.; Fiorella, K.; Flecker, A.; Sethi, S.A.; Uriarte, M.; Naeem, S. Declining diversity of wild-caught species puts dietary nutrient supplies at risk. Sci. Adv. 2021, 7, 9967–9995. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L.A.; Castello, L.; Orth, D.J.; Duponchelle, F.; Hallerman, E.M. A synthesis of the ecology and conservation of Pseudoplatystoma catfishes in the Neotropics. Fishes 2023, 8, 306. [Google Scholar] [CrossRef]
- Loubens, G.; Panfili, J. Biologie de Pseudoplatystoma fasciatum et P. tigrinum (Teleostei: Pimelodidae) dans le basin du Mamoré (Amazonie Bolivienne). Ichthyol. Explor. Freshw. 2000, 11, 13–34. [Google Scholar]
- Diaz-Sarmiento, J.A.; Alvarez-Leon, R. Migratory Fishes of the Colombian Amazon. In Migratory Fishes of South America: Biology, Fisheries and Conservation Status; Carolsfeld, J., Harvey, B., Ross, C., Baer, A., Eds.; World Fisheries Trust: Victoria, BC, Canada, 2003; pp. 303–344. [Google Scholar]
- Hahn, L.; Martins, E.G.; Nunes, L.D.; da Câmara, L.F.; Machado, L.S.; Garrone-Neto, D. Biotelemetry reveals migratory behaviour of large catfish in the Xingu River, eastern Amazon. Sci. Rep. 2019, 9, 8464. [Google Scholar] [CrossRef]
- Agudelo, E.; Acosta-Santos, A.; Gómez, G.; Gil, B.D.; Ajiaco-Martínez, R.E.; Ramírez-Gil, H. Pseudoplatystoma punctifer (Siluriformes, Pimelodidae). In I. Catálogo de los Recursos Pesqueros Continentales de Colombia. Serie Editorial Recursos Hidro-biológicos y Pesqueros continentales de Colombia; Instituto de Investigación de Recursos Biológicos Alexander von Humboldt (IAvH): Bogotá, Colombia, 2011; Capitulo 7; pp. 509–512. Available online: https://scholar.google.com/scholar?hl=en&as_sdt=0%2C47&q=Agudelo++2011+Pseudoplatystoma&btnG= (accessed on 23 May 2024).
- Doria, C.R.C.; Duponchelle, F.; Lima, M.A.L.; Garcia, A.; Carvajal-Vallejos, F.M.; Méndez, C.C.; Catarino, M.F.; Freitas, C.E.d.C.; Vega, B.; Miranda-Chumacero, G.; et al. Review of fisheries resource use and status in the Madeira River basin (Brazil, Bolivia, and Peru) before hydroelectric dam completion. Rev. Fish. Sci. Aquac. 2018, 26, 494–514. [Google Scholar] [CrossRef]
- Isaac, V.; Ruffino, M.; McGrath, D. In search of a new approach to fisheries management in the middle Amazon region. In Fishery Stock Assessment Models; Alaska Sea Grant, University of Alaska Fairbanks: Fairbanks, AK, USA, 1998; pp. 889–902. [Google Scholar] [CrossRef]
- Poff, L.N.; Hart, D.D. How dams vary and why it matters for the emerging science of dam removal. BioScience 2002, 52, 659–668. [Google Scholar] [CrossRef]
- Marmulla, G. Dams, Fish and Fisheries Opportunities, Challenges and Conflict Resolution; FAO Fisheries Technical Paper 419; Food and Agriculture Organization of the United Nations: Rome, Italy, 2001. [Google Scholar]
- Campana, S.E. Otolith science entering the 21st century. Mar. Freshw. Res. 2005, 56, 485–495. [Google Scholar] [CrossRef]
- Kerr, L.A.; Campana, S.E. Chemical composition of fish hard parts as a natural marker of fish stocks. In Stock Identification Methods; Cadrin, S.X., Kerr, L.A., Mariani, S., Eds.; Academic Press: New York, NY, USA, 2014; pp. 205–225. [Google Scholar]
- Hauser, M.; Doria, C.R.C.; Santos, R.V.; García-Vasquez, A.; Pouilly, M.; Pécheyran, C.; Ponzevera, E.; Torrente-Vilara, G.; Bérail, S.; Panfili, J.; et al. Shedding light on the migratory patterns of the Amazonian goliath catfish, Brachyplatystoma platynemum, using otolith 87Sr/86Sr analyses. Aquat. Conserv. Mar. Freshw. Ecosyst. 2019, 29, 397–408. [Google Scholar] [CrossRef]
- Hegg, J.C.; Giarrizzo, T.; Kennedy, B.P. Diverse early life-history strategies in migratory Amazonian catfish: Implications for conservation and management. PLoS ONE 2015, 10, e0129697. [Google Scholar] [CrossRef]
- Garcez, R.C.S.; Humston, R.; Harbor, D.; Freitas, C.E.C. Otolith geochemistry in young-of-the-year peacock bass Cichla temensis for investigating natal dispersal in the Rio Negro (Amazon—Brazil) river system. Ecol. Freshw. Fish 2015, 24, 242–251. [Google Scholar] [CrossRef]
- Souza, R.G.C.; Humston, R.; Freitas, C.E.C. Movement patterns of adult peacock bass Cichla temensis between tributaries of the middle Negro River basin (Amazonas—Brazil): An otolith geochemical analysis. Fish. Manag. Ecol. 2016, 23, 76–87. [Google Scholar] [CrossRef]
- Santos, R.V.; Sondag, F.; Cochonneau, G.; Lagane, C.; Brunet, P.; Hattingh, K.; Chaves, J.G.S. Source area and seasonal 87Sr/86Sr variations in rivers of the Amazon basin. Hydrol. Process. 2015, 29, 187–197. [Google Scholar] [CrossRef]
- Hauser, M.; Doria, C.R.C.; Melo, L.R.C.; Santos, A.R.; Ayala, D.M.; Nogueira, L.D.; Amadio, S.; Fabré, N.; Torrente-Vilara, G.; García-Vásquez, Á.; et al. Age and growth of the Amazonian migratory catfish Brachyplatystoma rousseauxii in the Madeira River basin before the construction of dams. Neotrop. Ichthyol. 2018, 16, e170130. [Google Scholar] [CrossRef]
- Pereira, L.A.; Castello, L.; Hallerman, E.; Rodrigues, E.R.F.; Doria, C.R.C.; Duponchelle, F. Flood pulse effects on the growth of Pseudoplatystoma fasciatum in the Amazon basin. Fishes 2024, 9, 223. [Google Scholar] [CrossRef]
- Claverie, F.; Fernández, B.; Pécheyran, C.; Alexis, J.; Donard, O.F.X. Elemental fractionation effects in high repetition rate IR femtosecond laser ablation ICP-MS analysis of glasses. J. Anal. At. Spectrom. 2009, 24, 891–902. [Google Scholar] [CrossRef]
- Tabouret, H.; Bareille, G.; Claverie, F.; Pécheyran, C.; Prouzet, P.; Donard, O.F.X. Simultaneous use of strontium: Calcium and barium: Calcium ratios in otoliths as markers of habitat: Application to the European eel (Anguilla anguilla) in the Adour basin, South West France. Mar. Environ. Res. 2010, 70, 35–45. [Google Scholar] [CrossRef] [PubMed]
- El Meknassi, S.; Dera, G.; Cardone, T.; De Rafélis, M.; Brahmi, C.; Chavagnac, V. Sr isotope ratios of modern carbonate shells: Good and bad news for chemostratigraphy. Geology 2018, 46, 1003–1006. [Google Scholar] [CrossRef]
- Rosman, K.J.R.; Taylor, P.D.F. Isotopic compositions of the elements 1997. Pure Appl. Chem. 1998, 70, 217–235. [Google Scholar] [CrossRef]
- Yang, Y.-H.; Wu, F.-Y.; Yang, J.-H.; Chew, D.M.; Xie, L.-W.; Chu, Z.-Y.; Zhang, Y.-B.; Huang, C. Sr and Nd isotopic compositions of apatite reference materials used in U–Th–Pb geochronology. Chem. Geol. 2014, 385, 35–55. [Google Scholar] [CrossRef]
- Jochum, K.P.; Stoll, B.; Weis, U.; Kuzmin, D.V.; Sobolev, A.V. In situ Sr isotopic analysis of low Sr silicates using LA-ICP-MS. J. Anal. At. Spectrom. 2009, 24, 1237–1243. [Google Scholar] [CrossRef]
- Sioli, H. The Amazon and its main affluents: Hydrography, morphology of the river courses, and river types. In The Amazon: Limnology and Landscape Ecology of a Mighty Tropical River and Its Basin; Springer: Dordrecht, The Netherlands, 1984. [Google Scholar]
- Araújo-Lima, C.; Goulding, M. Os Frutos do Tambaqui: Ecologia, Conservação e Cultivo na Amazônia; Sociedade Civil de Mamirauá: Tefé, AM, Brazil, 1998; Available online: https://books.google.com.hk/books/about/Os_frutos_do_tambaqui.html?id=OOtXAAAACAAJ&redir_esc=y (accessed on 3 May 2023).
- Pavlov, S.; Nezdoliy, V.; Urteaga, A.; Sanches, O. Downstream migration of juvenile fishes in the rivers of Amazonian Peru. J. Ichthyol. /Vopr. Ikhtiologii 1995, 35, 227–248. [Google Scholar]
- Armas, M.; Ortega, H.; García-Vasquez, A.; García-Dávila, C.; Vargas, G.; Nuñez, J.; Renno, J.-F.; Duponchelle, F. Age validation and contrasted growth performances of Pseudoplatystoma punctifer (Siluriformes: Pimelodidae) in two river systems of the Western Amazon. Neotrop. Ichthyol. 2022, 20, e210099. [Google Scholar] [CrossRef]
- Castillo, O. Aspectos Biologicos y Pesqueros Sobre los Peces Comerciales del Bajo Llano con Enfasis en los Bagres (Orden Siluriformes). Ph.D. Dissertation, Universidad Central de Venezuela, Caracas, Venezuela, 1988. [Google Scholar]
- Mago-Leccia, F.; Nass, P.; Castillo, O. Larvas, Juveniles y Adultos de la Familia de los Pimelodidae (Teleostei, Siluriformes) de Venezuela; Proyecto S1-1500-CONICIT, Informe Final; Venezuela, 1986; 168p. [Google Scholar]
- Barbarino Duque, A.; Winemiller, K.O. Dietary segregation among large catfishes of the Apure and Arauca Rivers, Venezuela. J. Fish Biol. 2003, 63, 410–427. [Google Scholar] [CrossRef]
- Ganias, K. Determining the indeterminate: Evolving concepts and methods on the assessment of the fecundity pattern of fishes. Fish. Res. 2013, 138, 23–30. [Google Scholar] [CrossRef]
- Pereira, L.H.G.; Foresti, F.; Oliveira, C. Genetic structure of the migratory catfish Pseudoplatystoma corruscans (Siluriformes: Pimelodidae) suggests homing behaviour. Ecol. Freshw. Fish. 2009, 18, 215–225. [Google Scholar] [CrossRef]
- Comte, L.; Olden, J.D. Fish dispersal in flowing waters: A synthesis of movement-and genetic-based studies. Fish Fish. 2018, 19, 1063–1077. [Google Scholar] [CrossRef]
- Batista, J.; Alves-Gomes, J. Phylogeography of Brachyplatystoma rousseauxii (Siluriformes-Pimelodidae) in the Amazon Basin offers preliminary evidence for the first case of “homing” for an Amazonian migratory catfish. Genet. Mol. Res. 2006, 5, 723–740. [Google Scholar] [PubMed]
- Salmenkova, E.A. Mechanisms of homing in salmonids. Biol. Bull. Rev. 2017, 7, 287–298. [Google Scholar] [CrossRef]
- Benedito-Cecilio, E.; Araujo-Lima, C.A.R.M.; Forsberg, B.R.; Bittencourt, M.M.; Martinelli, L.C. Carbon sources of Amazonian fisheries. Fish. Manag. Ecol. 2000, 7, 305–315. [Google Scholar] [CrossRef]
- Forsberg, B.R.; Araujo-Lima, C.A.R.M.; Martinelli, L.A.; Victoria, R.L.; Bonassi, J.A. Autotrophic carbon sources for fish of the central Amazon. Ecology 1993, 74, 643–652. [Google Scholar] [CrossRef]
- Castello, L.; McGrath, D.G.; Beck, P.S.A. Resource sustainability in small-scale fisheries in the Lower Amazon floodplains. Fish. Res. 2011, 110, 356–364. [Google Scholar] [CrossRef]
- Ruffino, M.L.; Isaac, V.J. Dinamica populacional de Surubim-tigre. Pseudoplatystoma Acta Amazon. 1999, 29, 463–476. [Google Scholar] [CrossRef]

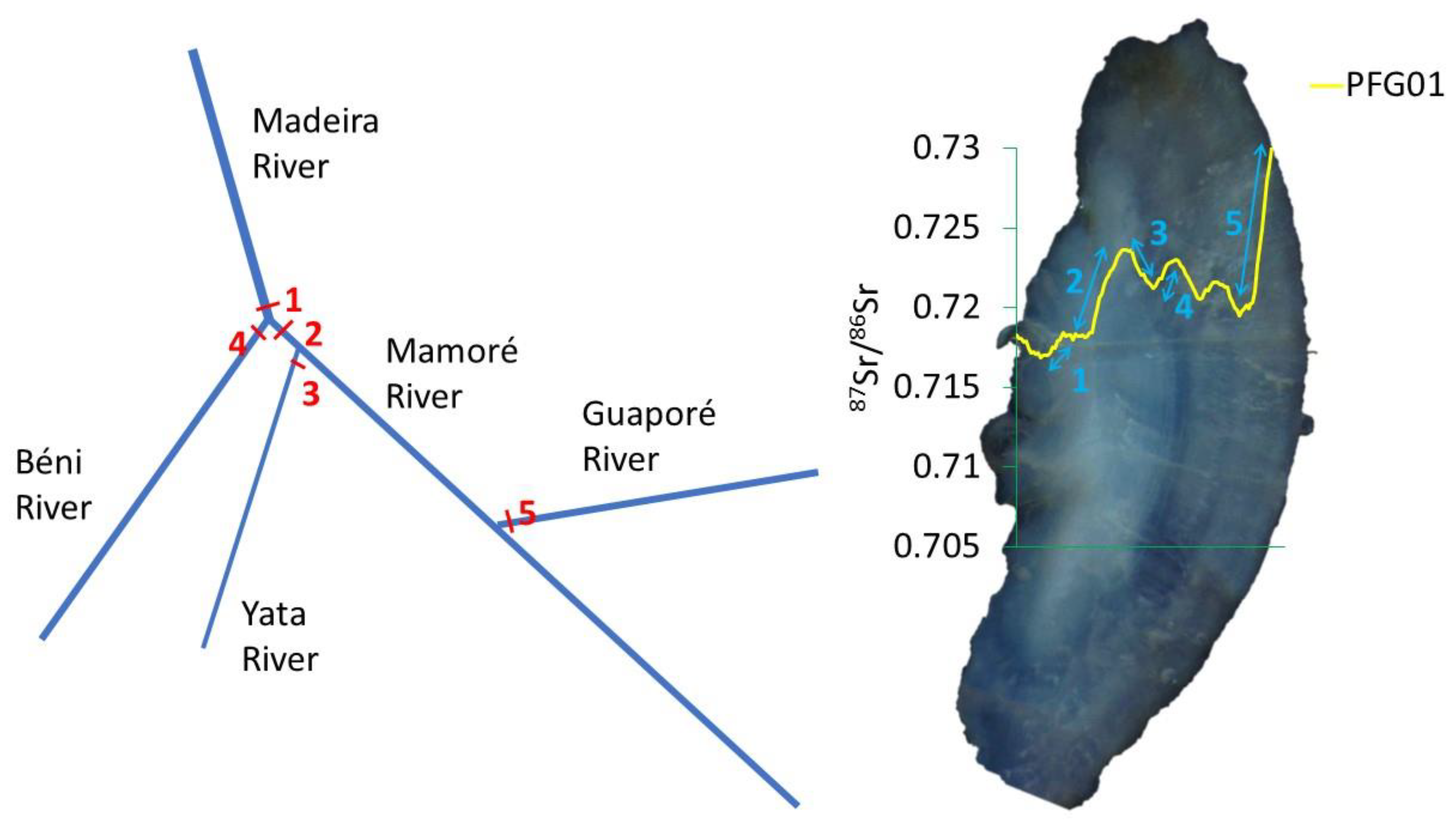
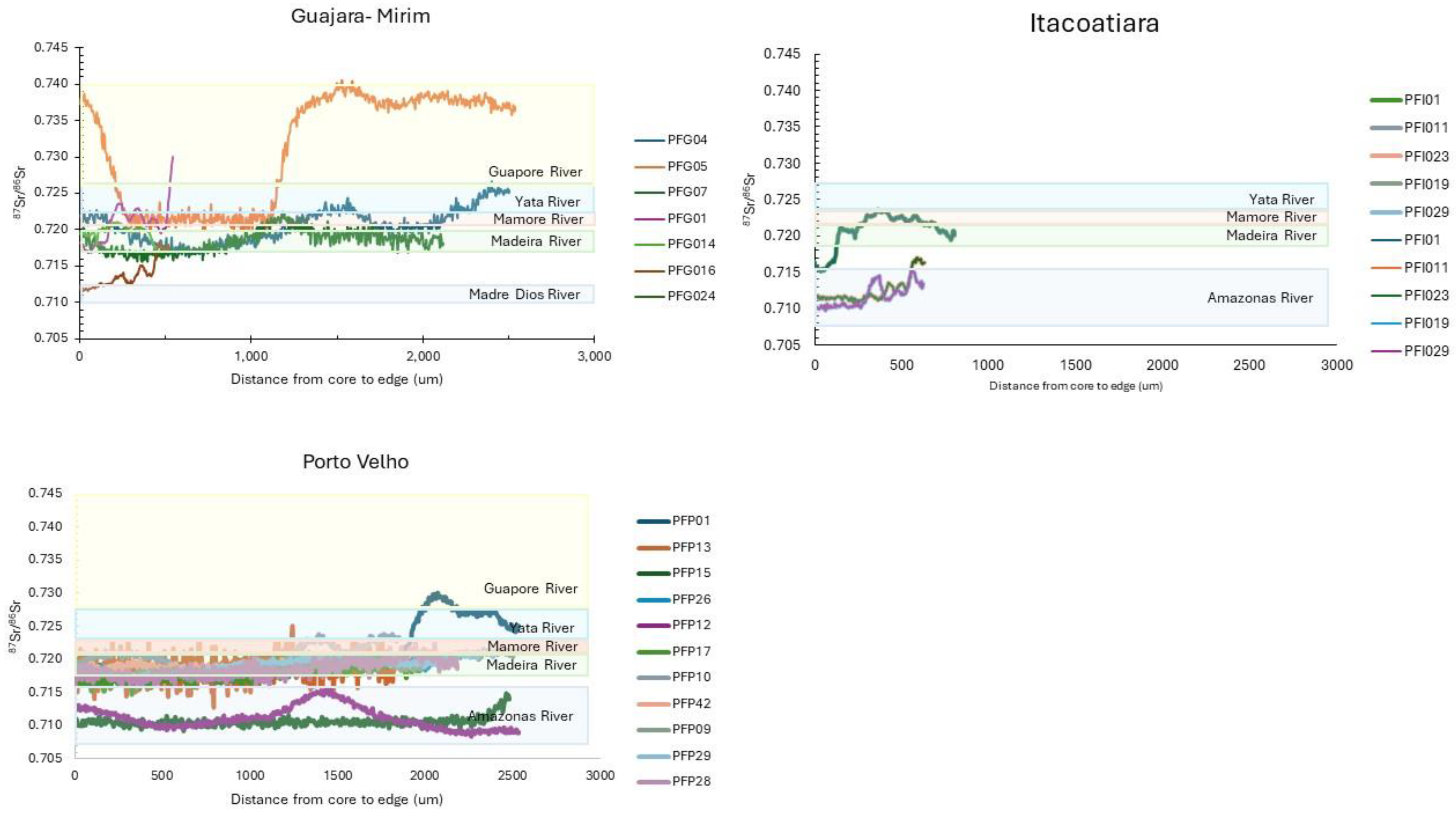

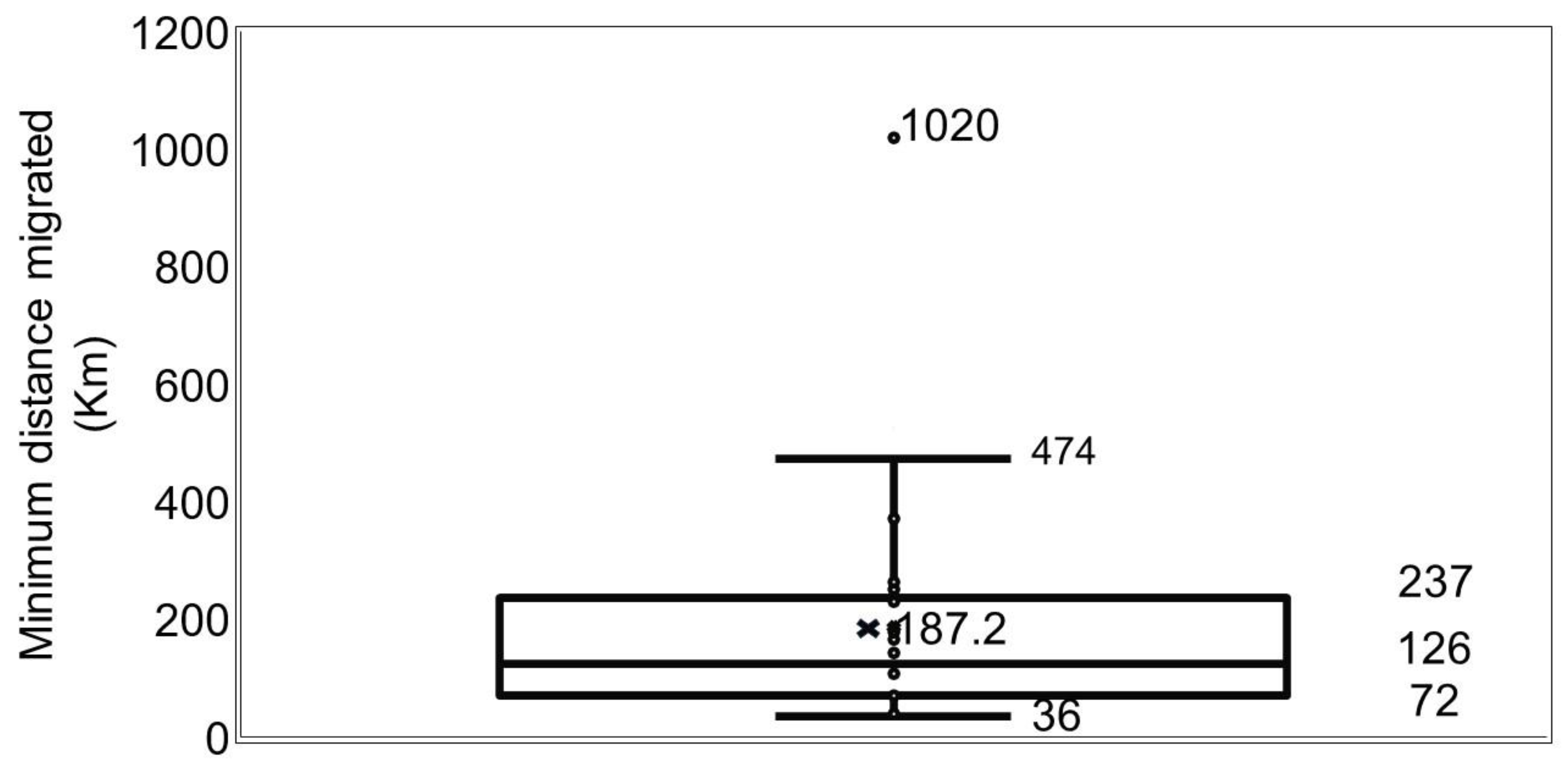
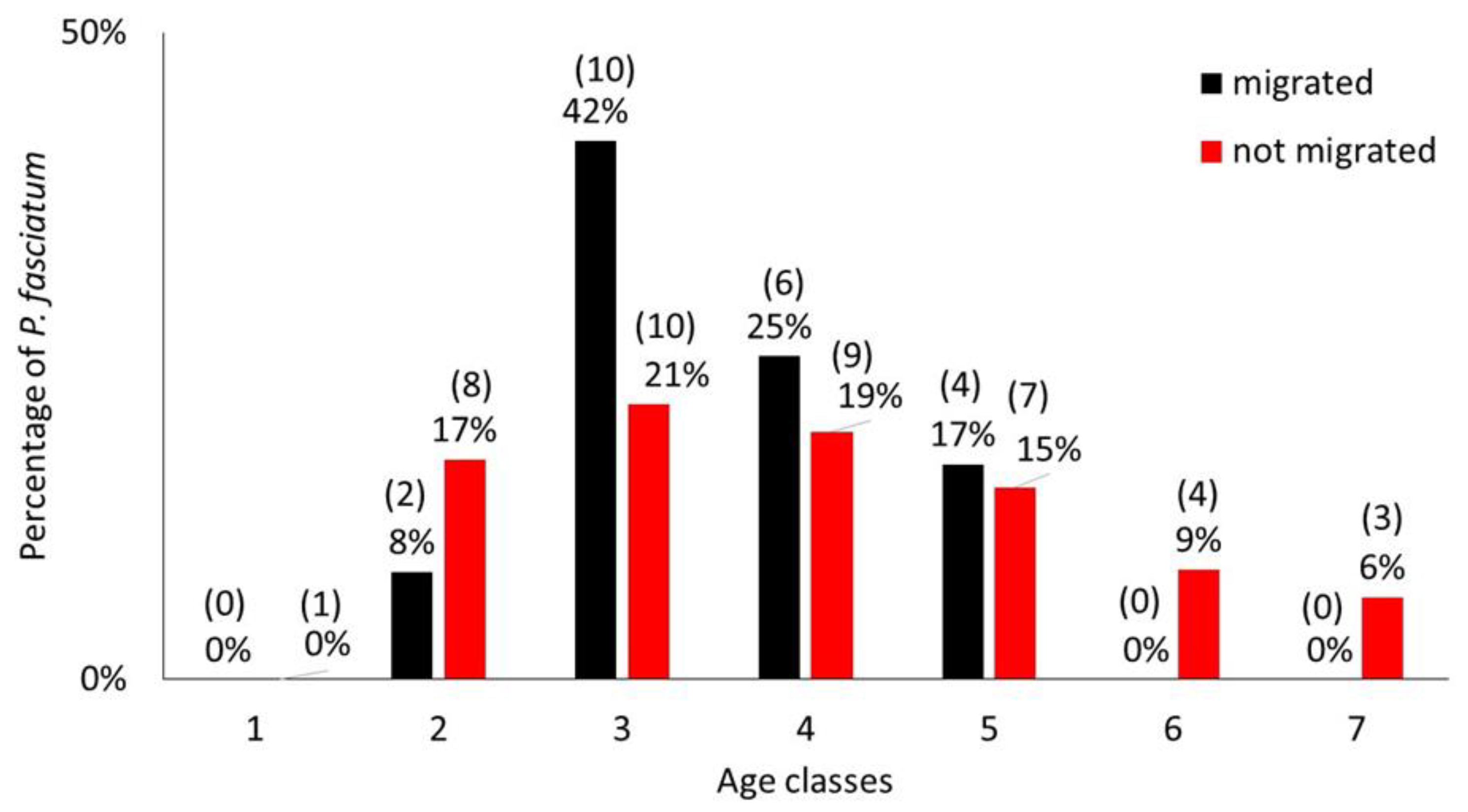

| Site | N | Length Range | Age Range |
|---|---|---|---|
| Guajara-Mirim | 10 | 50–70 cm | 2–5 years |
| Itacoatiara | 11 | 23–68 cm | 1–5 years |
| Manacapuru | 13 | 52–85 cm | 4–7 years |
| Porto Velho | 16 | 57–105 cm | 2–6 years |
| Santarem | 20 | 50–73 cm | 2–5 years |
| Total | 71 | 23–105 cm | 1–7 years |
| Fish | Locality | Age | Length TL (mm) | Moved between Rivers? | Moved between Water Types? | Minimum Estimated Distance Migrated | Direction | Homing? |
|---|---|---|---|---|---|---|---|---|
| PFG01 | GUA | 4.0 | 660 | yes | yes | 232 | Bi | no |
| PFG04 | GUA | 3.0 | 600 | yes | no | 372 | Bi | no |
| PFG05 | GUA | 4.7 | 800 | yes | yes | 474 | Bi | yes |
| PFG07 | GUA | 2.8 | 630 | yes | no | 72 | Bi | yes |
| PFG14 | GUA | 2.3 | 640 | yes | no | 180 | Bi | no |
| PFG15 | GUA | 2.3 | 570 | no | no | 166 | NA | no |
| PFG16 | GUA | 3.3 | 550 | yes | no | 252 | Uni | no |
| PFG19 | GUA | 3.4 | 590 | no | no | 36 | NA | no |
| PFG20 | GUA | 5.4 | 660 | yes | yes | 252 | Uni | no |
| PFG21 | GUA | 2.4 | 700 | no | no | 80 | NA | no |
| PFG24 | GUA | 3.5 | 700 | yes | yes | 36 | Uni | no |
| PFG27 | GUA | 3.5 | 610 | no | no | 1020 | NA | no |
| PFI01 | ITA | 3.2 | 680 | yes | no | 264 | Bi | yes |
| PFI09 | ITA | 3.2 | 570 | no | no | 252 | NA | no |
| PFI11 | ITA | 3.2 | 557 | yes | yes | 80 | Bi | yes |
| PFI14 | ITA | 1.2 | 460 | no | no | 172 | NA | no |
| PFI19 | ITA | 3.2 | 545 | yes | no | 40 | Uni | no |
| PFI23 | ITA | 5.2 | 230 | yes | no | 1020 | Uni | no |
| PFI28 | ITA | 3.2 | 620 | no | no | 40 | NA | no |
| PFI29 | ITA | 3.3 | 600 | yes | no | 40 | Uni | no |
| PFI33 | ITA | 2.3 | 505 | no | no | 72 | NA | no |
| PFI37 | ITA | 3.3 | 573 | no | no | 144 | NA | no |
| PFM02 | MANA | 3.7 | 600 | no | no | 108 | NA | no |
| PFM05 | MANA | 3.7 | 600 | no | no | 72 | NA | no |
| PFM07 | MANA | 3.7 | 740 | no | no | NA | NA | no |
| PFM12 | MANA | 4.7 | 850 | no | no | NA | NA | no |
| PFM18 | MANA | 4.7 | 780 | no | no | NA | NA | no |
| PFM20 | MANA | 4.7 | 580 | no | no | NA | NA | no |
| PFM27 | MANA | 5.7 | 520 | no | no | NA | NA | no |
| PFM29 | MANA | 5.7 | 570 | no | no | NA | NA | no |
| PFM30 | MANA | 5.7 | 560 | no | no | NA | NA | no |
| PFM34 | MANA | 5.7 | 550 | no | no | NA | NA | no |
| PFM37 | MANA | 6.7 | 700 | no | no | NA | NA | no |
| PFM38 | MANA | 6.7 | 690 | no | no | NA | NA | no |
| PFM41 | MANA | 6.7 | 600 | no | no | NA | NA | no |
| PFP01 | PV | 2.9 | 750 | yes | yes | 252 | Uni | no |
| PFP07 | PV | 4.0 | 600 | no | no | NA | NA | no |
| PFP09 | PV | 5.0 | 690 | yes | no | 108 | Bi | no |
| PFP10 | PV | 4.0 | 660 | yes | yes | 172 | Bi | no |
| PFP12 | PV | 3.4 | 580 | yes | no | 80 | Uni | yes |
| PFP13 | PV | 3.4 | 570 | yes | no | 72 | Bi | yes |
| PFP15 | PV | 3.7 | 570 | yes | no | 40 | Uni | no |
| PFP17 | PV | 4.9 | 620 | yes | no | 144 | Bi | yes |
| PFP20 | PV | 5.9 | 105 | no | no | NA | NA | no |
| PFP23 | PV | 5.9 | 770 | no | no | NA | NA | no |
| PFP26 | PV | 3.5 | 570 | yes | no | 72 | Bi | yes |
| PFP28 | PV | 5.8 | 870 | yes | no | 144 | Bi | yes |
| PFP29 | PV | 4.8 | 810 | yes | no | 108 | Bi | no |
| PFP35 | PV | 5.9 | 840 | no | no | NA | NA | no |
| PFP42 | PV | 4.0 | 660 | yes | no | 72 | Bi | yes |
| PFP43 | PV | 5.0 | 590 | no | no | NA | NA | no |
| PFS05 | SANTA | 4.8 | 635 | no | no | NA | NA | no |
| PFS06 | SANTA | 4.8 | 610 | no | no | NA | NA | no |
| PFS09 | SANTA | 2.8 | 535 | no | no | NA | NA | no |
| PFS14 | SANTA | 4.8 | 542 | no | no | NA | NA | no |
| PFS15 | SANTA | 5.7 | 572 | no | no | NA | NA | no |
| PFS18 | SANTA | 3.6 | 526 | no | no | NA | NA | no |
| PFS19 | SANTA | 3.1 | 510 | no | no | NA | NA | no |
| PFS22 | SANTA | 3.5 | 560 | no | no | NA | NA | no |
| PFS23 | SANTA | 3.1 | 510 | no | no | NA | NA | no |
| PFS25 | SANTA | 2.0 | 520 | no | no | NA | NA | no |
| PFS31 | SANTA | 3.1 | 510 | no | no | NA | NA | no |
| PFS32 | SANTA | 2.1 | 520 | no | no | NA | NA | no |
| PFS33 | SANTA | 3.1 | 540 | no | no | NA | NA | no |
| PFS34 | SANTA | 3.1 | 500 | no | no | NA | NA | no |
| PFS35 | SANTA | 4.1 | 500 | no | no | NA | NA | no |
| PFS36 | SANTA | 2.1 | 500 | no | no | NA | NA | no |
| PFS38 | SANTA | 2.1 | 520 | no | no | NA | NA | no |
| PFS44 | SANTA | 3.5 | 520 | no | no | NA | NA | no |
| PFS46 | SANTA | 3.5 | 550 | no | no | NA | NA | no |
| PFS48 | SANTA | 3.5 | 730 | no | no | NA | NA | no |
| Sites | Size Range of Length (cm) | Percentage of Fish That Did Not Move between Rivers with Different Sr Signatures | Percentage of Fish That Did Move between Rivers with Different Sr Signatures | Percentage of Fish That Moved between Different Types of Water | Percentage of Fish That Did Not Moved between Different Types of Water |
|---|---|---|---|---|---|
| Guajara-Mirim | 550–800 | 0.7 | 0.6 | 0.6 | 0.2 |
| Itacoatiara | 230–680 | 0.5 | 0.5 | 0.3 | 0.8 |
| Manacapuru | 520–850 | 0.0 | 1.0 | NA | NA |
| Porto Velho | 570–1050 | 0.7 | 0.3 | 0.3 | 0.8 |
| Santarem | 500–730 | 0.0 | 1.0 | NA | NA |
| All | 230–1050 | 0.7 | 0.3 | 0.1 | 0.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, L.A.; Castello, L.; Hallerman, E.; Orth, D.; Duponchelle, F. Migratory Ecology of Pseudoplatystoma fasciatum in the Amazon Basin Revealed by Otolith Microchemistry. Diversity 2024, 16, 378. https://doi.org/10.3390/d16070378
Pereira LA, Castello L, Hallerman E, Orth D, Duponchelle F. Migratory Ecology of Pseudoplatystoma fasciatum in the Amazon Basin Revealed by Otolith Microchemistry. Diversity. 2024; 16(7):378. https://doi.org/10.3390/d16070378
Chicago/Turabian StylePereira, Luciana A., Leandro Castello, Eric Hallerman, Donald Orth, and Fabrice Duponchelle. 2024. "Migratory Ecology of Pseudoplatystoma fasciatum in the Amazon Basin Revealed by Otolith Microchemistry" Diversity 16, no. 7: 378. https://doi.org/10.3390/d16070378







