Abstract
Reptiles are threatened by anthropogenic landscape transformation, largely due to agriculture. However, ecosystems nowadays constitute a matrix of fragmented landscapes. We analyzed a heterogeneous landscape’s reptile taxonomic and functional diversity patterns across ten land cover/use types in Jalisco state, in west-central Mexico. At the alpha diversity level, we assessed the taxonomic diversity using q-order indices, and functional diversity with multidimensional indices (FRic, FDiv, and FEve) by land cover/use. We evaluated the differences in species composition among land cover/use types (beta diversity). We utilized multidimensional distance-based analyses (dbRDA) to evaluate the association between reptile diversity, habitat structure, and environmental variables. Species richness did not correlate with functional richness across land cover types overall, except for riparian habitat surrounded by crops (RH-C), which exhibited higher species richness and functional diversity. Secondary vegetation surrounded by temperate forest (SV-TF), riparian habitat surrounded by tropical dry forest (RH-TDF), and RH-C were the land cover/use types with the most functional groups. Herbaceous cover is crucial for preserving both reptile diversity facets in this landscape. These findings suggest that the availability of resources (e.g., riparian habitat and herbaceous cover) regardless of perturbation level could be more relevant for reptile diversity than the condition (tropical vs. temperate) due to the high plasticity and adaptation of the group. It is essential to recognize the ecological value of these habitats by adopting a holistic approach that values the intrinsic and ecological importance of reptile diversity.
Keywords:
crops; alpha diversity; beta diversity; medium scale; diversity facets; snakes; lizards; turtles 1. Introduction
The reptile class comprises turtles (Testudines), crocodiles (Crocodylia), squamates (Squamata: lizards, snakes and amphisbaenians), and tuatara (Rhynchocephalia). Reptiles are a paraphyletic group with a wide range of body shapes, habitat associations, and functional roles within their ecosystems. These adaptations allow reptiles to colonize and even dominate all ecosystems except the Poles [1]. They contribute to the energy flow of ecosystems in many ways. Ectotherms efficiently convert ingested energy into biomass, making it available to higher trophic levels [2,3]. On the other hand, they considerably influence food webs by controlling prey population sizes. Moreover, they participate in seed dispersal, bioturbation [2], and even pollination [4].
Reptiles are one of the most speciose vertebrate groups in the world (~11,940 species) [5]. The same pattern is shown in Mexico, where reptiles, following birds, are the second most diverse group of terrestrial vertebrates [6]. This country ranks second place in reptile richness around the world [6,7] with 975 species, of which 60.1% are endemic (586 species) [8]. The highest endemism is found in central and south Mexico (Guerrero, Puebla, Veracruz, and Oaxaca states) [8], while western central Mexico shelters a medium species richness but high endemism of reptiles [9,10].
Despite the high diversity in Mexico, several species are disappearing because of human activities, mainly habitat fragmentation [11,12]. Currently, out of the 10,000 reptile species assessed by the IUCN, 21% are at risk of extinction [13]. Reptiles are mainly threatened by anthropogenic landscape transformation such as agriculture, logging, urban development [14,15] as well as roadkill [15], invasive species, and by possible climate change [14]. Therefore, most ecosystems nowadays constitute a matrix of fragmented landscapes [16]. The Valles Region is situated in the state of Jalisco, in west-central Mexico. It is characterized by a complex geography with valleys surrounded by mountains. It shelters diverse ecosystems, from pine-oak forest to dry tropical forest as well as corn and sugar cane where there used to be natural vegetation [17].
The dimensions of taxonomic and functional diversity are crucial in illustrating functional patterns by considering species richness within a specific area. Both facets are crucial to understanding the response of biological assemblages to environmental changes resulting from habitat transformation [18]. However, these metrics are not necessarily correlated [19,20] because they describe complementary patterns when considering different functional attributes, particularly in the case of multi-trait functional diversity indices [21]. In various studies, researchers have contrasted the taxonomic and functional diversity of reptiles in a local area [20]. For example, in reptile assemblages exposed to fire regimes where species richness is maintained, the dominant species and functional traits change [22]. Tsianou et al. [23] showed that climate and human intervention (e.g., agricultural land, urban land, population density) are the key factors influencing the functional diversity and species richness of reptiles on a continental spatial scale. Other studies have focused on assessing the effect of heterogeneous cropped landscapes in shaping reptile taxonomic diversity [24,25]. It has been found that reptile assemblages are more influenced by climatic factors than by anthropogenic factors in altitudinal gradients [26], altered habitats in dry forests [27], and in sites around roads with different degrees of buffering [15]. However, not many studies have evaluated both facets of diversity across human-modified landscapes. Studying the facets of taxonomic and functional diversities is essential to understanding the community dynamics and ecosystem functioning, leading to well-informed conservation strategies in regions where biodiversity is declining [28].
On the other hand, the landscape approach has gained prominence in searching for solutions to reconcile biological conservation and development tradeoffs [29]. Understanding the intermediate landscape-scale patterns and processes could be the way to connect the knowledge about species ecology between local habitat studies and larger regions (e.g., biogeographical) [30]. In this sense, the Valles region in Jalisco state, west-central Mexico is a suitable area to evaluate patterns of functional diversity due to its intermediate scale and the fact that it includes both conserved areas and areas subject to anthropogenic pressures. This work aimed to analyze the taxonomic and functional diversity across land cover/use types (diverse landscape) and to determine the relationship between the taxonomic and functional diversity with the habitat characteristics in Jalisco state, a west-central region of Mexico. We hypothesized that both biodiversity facets would have similar trends according to a perturbation level and condition (tropical vs. temperate): the greatest taxonomic and functional diversity is anticipated to exist in tropical native vegetation cover, especially in riparian habitats surrounded by tropical dry forest due to the more complex vegetation structure of tropical land covers.
2. Materials and Methods
2.1. Study Area
The research area is located in the Valles region of the Jalisco state (20°33′50″–20° 47′40″ N, 103° 47′30″–103° 51′20″ W) in western central Mexico. It has an elevational gradient from 1247 to 2080 m asl (Figure 1). The weather is temperate-subhumid to semicalid-subhumid. The annual average temperature is from 18 °C at higher altitudes to 21.2 °C at lower ones. The average yearly precipitation is 900 mm at higher altitudes and 948 mm at lower ones [31]. There are two main seasons: the dry season from late October to mid-June, and the wet season from late June to early October.
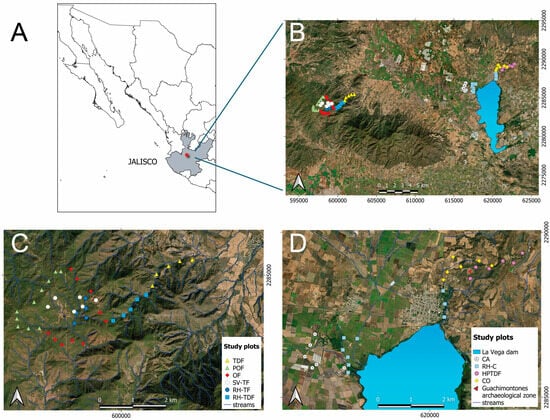
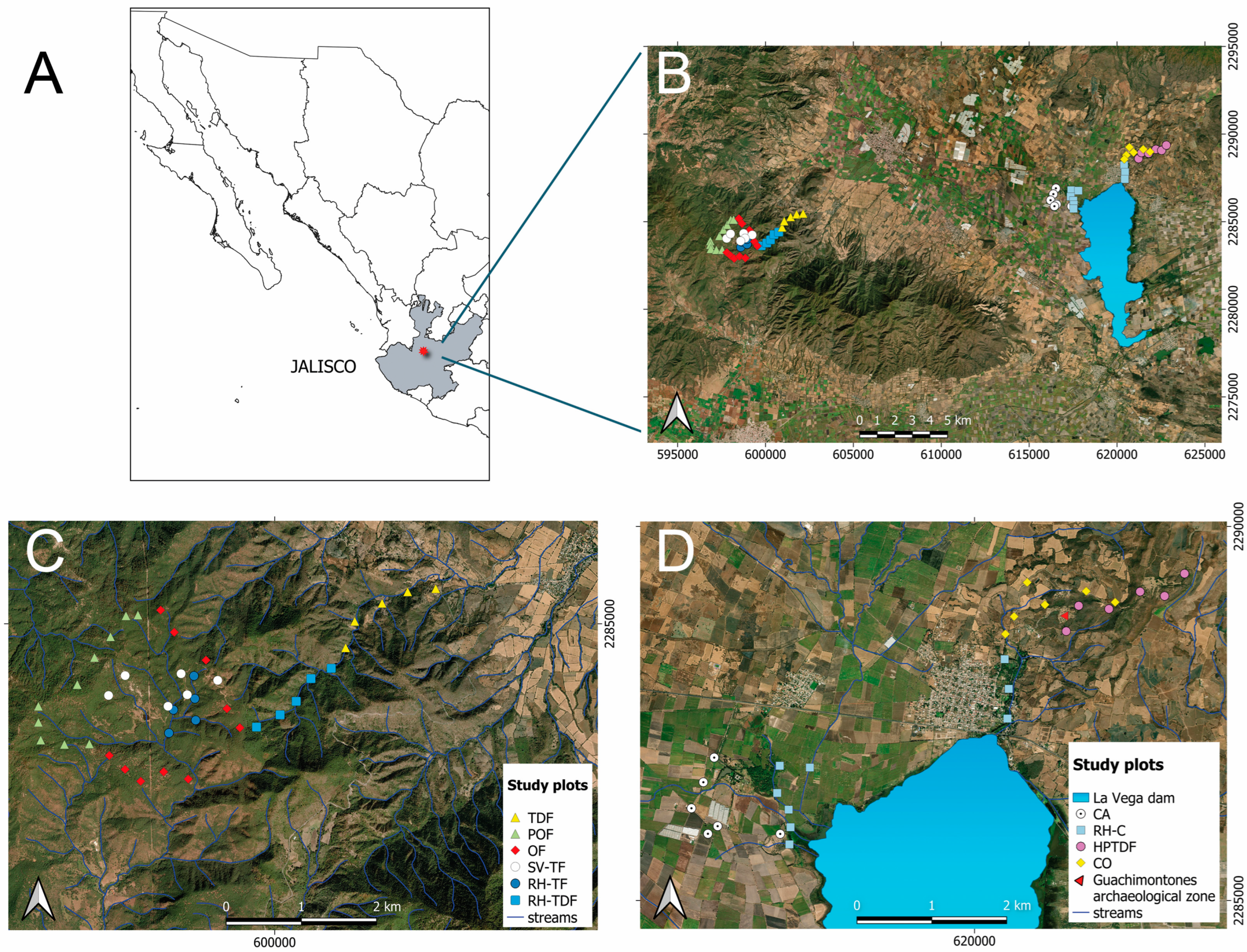
Figure 1.
Map showing the research area in Jalisco state, located in west-central Mexico (A). Sites for surveying in the Valles region (B). Sampling plots (C,D). Land cover and land use type abbreviations can be found in the Supplementary Materials, Table S3.
The land cover/use types were determined by analyzing the vegetation cover cartography, altitude, and current land use, while closely considering the plant species composition and physiognomic characteristics [32]. Field sampling was conducted throughout a heterogeneous landscape [33,34,35]. We chose eight land cover types: riparian habitat surrounded by crops (RH-C), highly perturbed tropical dry forest (HPTDF), TDF, RH-TDF, riparian habitat surrounded temperate forest (RH-TF), secondary vegetation surrounded by temperate forest (SV-TF), oak forest (OF), and pine-oak forest (POF). Additionally, we selected two land use types: sugar cane field (CA) and cornfield (CO). Please refer to García-Martínez and Rodríguez [33], and Rendón and Cedano [35] for comprehensive information on the native vegetation land cover types including elevation, dominant plant species, and level of disturbance.
2.2. Data Collection
We fixed circular daylight plots (A = 500 m2 each one) in each land cover/use type. The diurnal plots were separated by at least a 400 m distance from each one. At each plot, an intensive unrestricted visual search was carried out on the microhabitats preferred by these reptile species. The searches were performed by the same three people. We recorded all individuals observed, and when possible, measured and photographed them. They were later released at the capture site; no animals were collected. We conducted nine, monthly, samplings of reptile communities from July 2011 to August 2012 in the TDF, RH-TDF, RH-TF, SV-TF, OF, and POF. Additionally, we recorded reptiles for eight months from September 2012 to September 2013 in CA, RH-C, CO, and HPTDF. Once a month during the day, we surveyed 12,500 m2 in TDF, RH-TDF, and RH-TF; 15,000 m2 in CA, CO, and HPTDF; 17,500 m2 in SV-TF; 25,000 m2 in OF and POF; and 22,500 m2 in RH-C.
The vegetation structure was also characterized by each diurnal circular parcel for reptiles. In each plot, we recorded the arboreal, shrub, and herbaceous coverage as well as the dominant species in each layer. Additionally, we recorded different habitat variables for each survey site using a qualitative scale: rocks (structures > 1 m in diameter), stones (<1 m in diameter), leaf litter, fire, and cow foraging (Supplementary Materials, Table S1).
2.3. Data Analysis
We evaluated the taxonomic and functional diversity with a two-way experimental design with crossed factors. The land cover/use type was a fixed factor with ten levels (CA, RH-C, CO, HPTDF, TDF, RH-TDF, RH-TF, SV-TF, OF, and POF), and the season was a fixed factor with two levels (dry and wet). The months per land cover/use type were considered replicates.
2.3.1. Alpha Taxonomic and Functional Diversity
We evaluated the sampling effort of the reptile inventories in each land-use type using sample-based rarefactions based on the non-parametric estimators Chao1, Jackknife1, and Jackknife2 built from 10,000 randomizations without replacement. This analysis was performed using the program EstimateS 9.1 [36].
Reptile taxonomic diversity was measured by the land cover/use type. The diversity of q orders (qD) represents the effective number of species and the reptile total abundance (N). In this assessment, q = 0 (0D) signifies species richness; q = 1 (1D) Shannon diversity (i.e., the abundant species); when q = 2 (2D) Simpson diversity (i.e., very abundant species) [37]; and 21D = 2D/1D Hill evenness. Several permutational analyses of variance (ANOVA) were used to determine differences per site, season, and the interaction regarding 0D, 1D, 2D, 21D, N, and 21D. By each index, a resemblance matrix was generated with Euclidean distance [38].
For the analysis of functional diversity, we collected data on six functional traits related to the reproduction, habitat, and morphology of reptiles (Supplementary Materials, Table S2). These findings were primarily derived from field records, individual measurements taken during this study, and others in neighboring areas of Jalisco state, west-central Mexico as well as from the relevant literature [2,27,39]. Afterward, we quantified three multidimensional diversity indices: (a) functional richness (FRic), which indicates the distribution of functional traits in a multidimensional space as well as the amount of functional space occupied by the species within a community; (b) functional evenness (FEve), which reflects the homogeneity in the distribution of the abundances of the species of a community in a functional space; and (c) functional divergence (FDiv) as a measure of functional similarity between the dominant species of a community [40]. These functional metrics were estimated using the “FD” package in R-project [41,42].
Permutational ANOVAs determined differences per site and season regarding FRic, FEve, and FDiv. In each case, a resemblance matrix was generated with Euclidean distance. The statistical significance of permutational ANOVAs was tested with 10,000 permutations under a reduced model and a type III sum of squares. Pairwise comparisons were used to assess the significant difference between habitat types.
The cluster analysis identified reptile functional groups. This analysis was based on a Gower similarity index, which we constructed from the matrix of functional traits. It was applied with a complete linkage method and we set the threshold for cutting the dendrogram at 60% according to the knowledge of the group.
2.3.2. Beta Taxonomic and Functional Diversity
Permutational multidimensional analysis of variance (PERMANOVA) was performed following the same experimental design to determine differences in the reptile composition and abundance among sampling sites across climatic seasons. The PERMANOVA model uses a square root transformation as data pre-treatment and Bray–Curtis similarity. The statistical significance in PERMANOVA was also tested with 10,000 permutations under a reduced model and a type III sum of squares. Pairwise comparisons were applied in cases of significant differences in the interaction or any factor. The PCO ordination, based on the previously mentioned resemblance coefficient and the same data pre-treatment, provided a visualization of the reptile composition and abundance changes. All species were represented as vectors produced by multiple correlations. Furthermore, considering the reptile abundance of each species with respect to the total abundance of species in all habitats, a shade plot diagram was created to envisage the species’ contribution to land cover/use types. For this purpose, we used the reptiles’ functional groups generated by the previous cluster analysis and the Whittaker’s coefficient of association.
2.3.3. Taxonomic and Functional Diversity Facing Environmental Variables
We assessed the connection between reptile taxonomic diversity and ecological variables by employing distance-based linear models (DistLM) and distance-based redundancy analysis (dbRDA) [38]. Previously conducting these analyses, we reduced the multicollinearity by using Pearson correlations to exclude predictable variables (r ≥ 0.9). Nine out of ten environmental variables were contemplated in the analyses (Supplementary Materials, Table S1). To analyze taxonomic diversity, DistLM and dbRDA analyses were performed at the habitat level using a Bray–Curtis similarity matrix from reptile abundances with a fourth root transformation. In DistLM, predictive variables were selected using a forward selection procedure and the adjusted coefficient of determination (R2adj). The statistical significance of the marginal and sequential tests was assessed using 10,000 permutations. The dbRDA ordination was constrained by the best-fit global model explanatory environmental variables. All analyses (sample-based rarefactions, permutational ANOVA, PERMANOVA, cluster analysis, PCO, shade diagram, DistLM, and dbRDA were conducted in PRIMER v7 and PERMANOVA+ software from PRIMER-e, Clarke & Gorley, Albany, New Zealand [43].
We included a table containing abbreviations for land cover/use types, recorded the reptile species, taxonomic and functional diversity measured parameters, and statistical analyses (Supplementary Materials, Table S3).
3. Results
We registered 39 reptile species from 15 families and 28 genera in the study area (Table 1). The families with the highest number of species were Colubridae (9), Phrynosomatidae (7), and Dipsadidae (5). Additionally, over 50% of the species were endemic to Mexico (24). Most of these species are native, except for Indotyphlops braminus (Eastern Hemisphere). According to NOM-059-SEMARNAT-2010 [44], 15 species with conservation problems, of which 11 were in the category “Subject to special protection”, and four were in the category “Threatened”. On the other hand, two species were in some category of protection according to the IUCN [45], of which one was “Near Threatened” and one was “Vulnerable”.

Table 1.
Reptile checklist distribution over a land-use landscape of Jalisco state, west-central Mexico. Abbreviations: endemic to Mexico (E), native to Mexico (N), exotic (EX), protected (Pr), threatened (A), least concern (LC), vulnerable (VU), and near threatened (NT), number of registered individuals (Indiv). Land cover/use types codes are in the Supplementary Materials, Table S3.
The sampling effort of the land cover/use types varied from 56.7 of representativeness for CO to 100% for CA from the expected species richness of reptiles in the Valles region of Jalisco state, western central Mexico (Supplementary Materials, Table S4).
3.1. Alpha Taxonomic and Functional Diversity
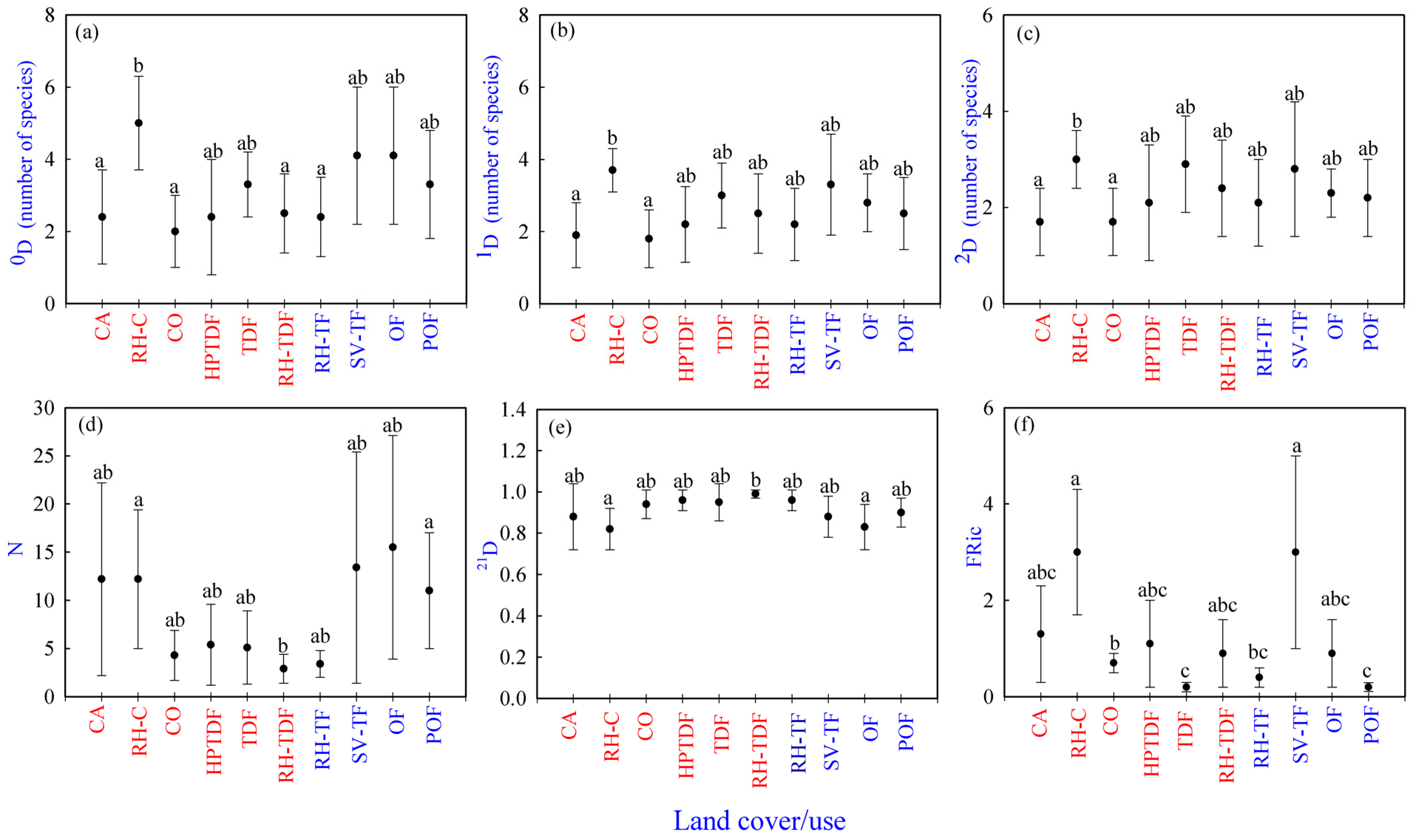
Regarding 0D, 1D, 2D, N, and 21D, there were significant differences only among sites but not seasonally or in the interaction. The results for species richness (0D) showed two significantly different groups. CO, CA, RH-TDF, and RH-TF had the lowest species richness (2.0–2.5); however, RH-C showed the highest (5). Additionally, HPTDF, TDF, POF, SV-TF, and OF were a share group with medium richness (2.44–4.13) (Supplementary Materials, Table S5) (Figure 2a).
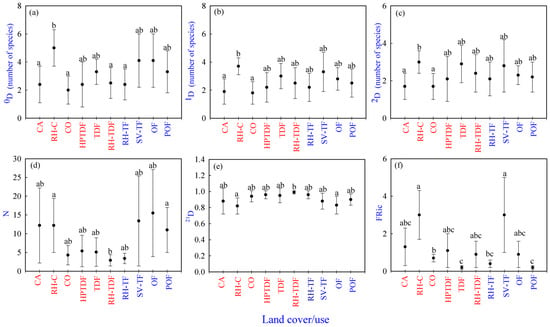
Figure 2.
Reptile taxonomic and functional diversity per land cover/use type in Jalisco state, west-central Mexico. (a–c) Averages values of taxonomic diversity (0D, 1D, and 2D), (d) abundances (N), (e) 21D evenness, (f) functional richness [FRic]. Tropical habitats are represented in red, and temperate habitats are shown in blue. Different lowercase letters indicate significant differences (p ≤ 0.05) among cover/use types. Equal lowercase letters indicate no statistical differences among cover/use types. A land cover/use type that shares two or three letters with other land cover/use types indicates that it is not significantly different from the types that share those letters. Land cover/use types as well as the taxonomic and functional diversity indices codes are available in the Supplementary Materials, Table S3.
The habitats with the lowest values of abundant species (1D) were CO and CA (1.79 and 1.86, respectively) and the one with the highest value was RH-C (3.70). All the other land cover/use types formed a shared group with medium values (2.24–3.25) of abundant species (Supplementary Materials, Table S5) (Figure 2b).
Concerning the very abundant species (2D), CO and CA had the lowest values of very abundant species (1.66 and 1.69, respectively). In contrast, RH-C showed the highest value (3.03). Moreover, POF, OF, RH-TF, HPTDF, RH-TDF, SV-TF, and TDF (2.10–2.87) were shared between the two groups. They had medium values of abundant species (Supplementary Materials, Table S5) (Figure 2c).
Habitats with lower values of N were CO, RH-TDF, RH-TF, HPTDF, and TDF (2.88–5.23). Instead, CA, RH-C, OF, POF, SV-TF (11–15.5) had higher abundances (Supplementary Materials, Table S5) (Figure 2d). Concerning 21D, RH-C, OF, SV-TF, CA, and POF showed lower values (0.82–0.90), and CO, TDF, HPTDF, RH-TF, and RH-TDF were higher (0.94–0.9) (Supplementary Materials, Table S5) (Figure 2e).
Regarding the functional richness (FRic), there was a significant difference among land cover/use types but not seasonally or in the interaction. FEve and FDiv showed no differences in the factors or the interaction. In the FRic, the land cover/use types with the lowest values were POF and TDF (0.20–0.24, respectively), followed by CO (0.68) and another group with the highest values in RH-C and SV-TF (3.03, respectively). All the other land cover/use types with medium values were shared with the two groups just mentioned (Figure 2f).
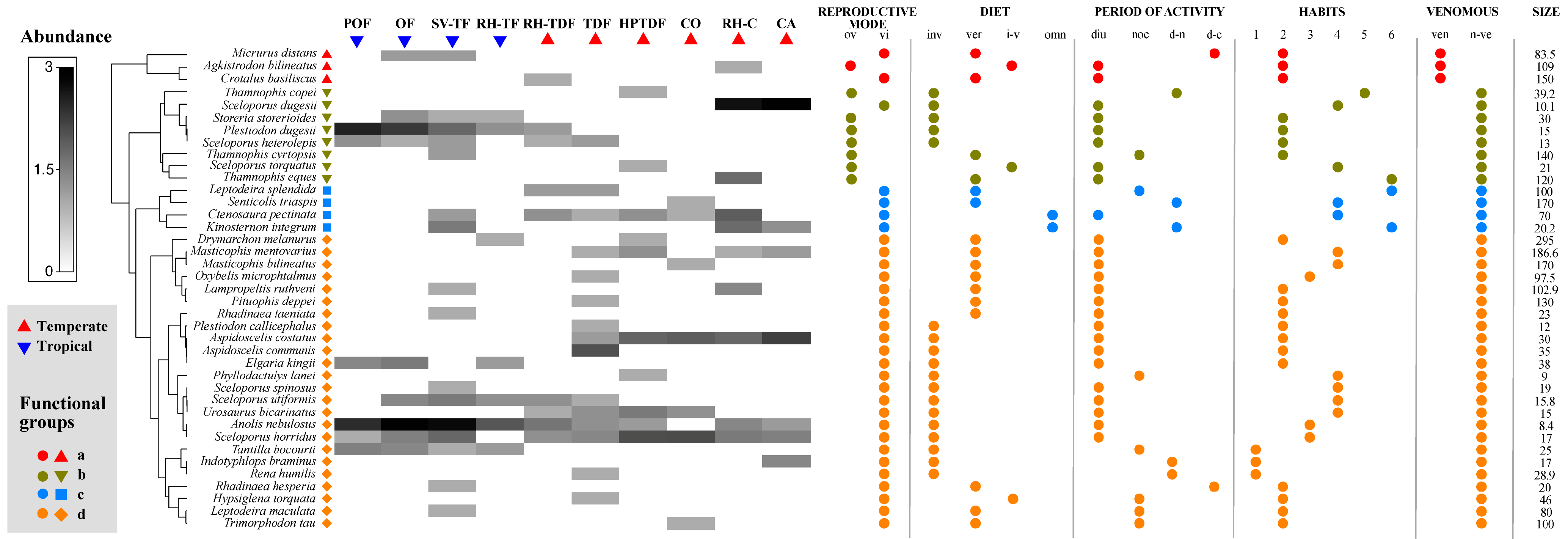
The cluster analysis showed four different functional groups among the reptile species. Group “a” was associated with the snakes Micrurus distans, Agkistrodon bilineatus, and Crotalus basiliscus. This group was featured by being venomous, medium to large size, and terrestrial habitat; group “b” was formed by no venomous and viviparous lizards (Plestiodon dugesi, Sceloporus dugesi, Sceloporus heterolepis, Sceloporus torquatus) and snakes (Storeira storerioides, Thamnophis copei, Thamnophis cyrtopsis, and Thamnophis eques). In contrast, group “c” was integrated by snakes (Leptodeira splendida and Senticolis triaspis), a turtle (Kinosternon integrum), and a lizard (Ctenosaura pectinata), and was featured by species of medium and large size, not venomous, omnivorous, or carnivorous, terrestrial-arboreal or terrestrial-aquatic, and oviparous. Finally, group “d” corresponded to all other species of lizards and snakes. This group was variable in size, not venomous, fossorial, terrestrial, arboreal, or terrestrial-arboreal and oviparous (Figure 3).
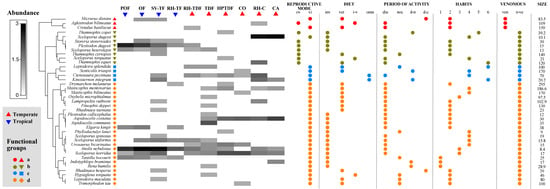
Figure 3.
Reptile species composition, functional groups, and functional traits across different land cover/use types. The intensity of color in the matrix signifies the relative abundance of the species. A cluster analysis determined the amphibian functional groups: Group “a” was characterized by being venomous and terrestrial habitat; group “b” by non-venomous and viviparous; group “c” by medium or big size, non-venomous, omnivorous, or carnivorous (vertebrates), terrestrial-arboreal or terrestrial-aquatic, and oviparous; group “d” by the variable size, not venomous, fossorial, terrestrial, arboreal or terrestrial-arboreal, and oviparous. Abbreviations: oviparous (ov), viviparous (vi), invertebrates (inv), vertebrates (ver), invertebrates-vertebrates (i-v), omnivorous (on), diurnal (diu), nocturnal (noc), diurnal-nocturnal (d-n), diurnal-crepuscular (d-c), terrestrial (1), (2), (3), (4), (5), (6), venomous (ve), non-venomous (n-ve), and size measured in cm. Land cover/use types abbreviations are available in the Supplementary Materials, Table S3.
3.2. Beta Taxonomic and Functional Diversity
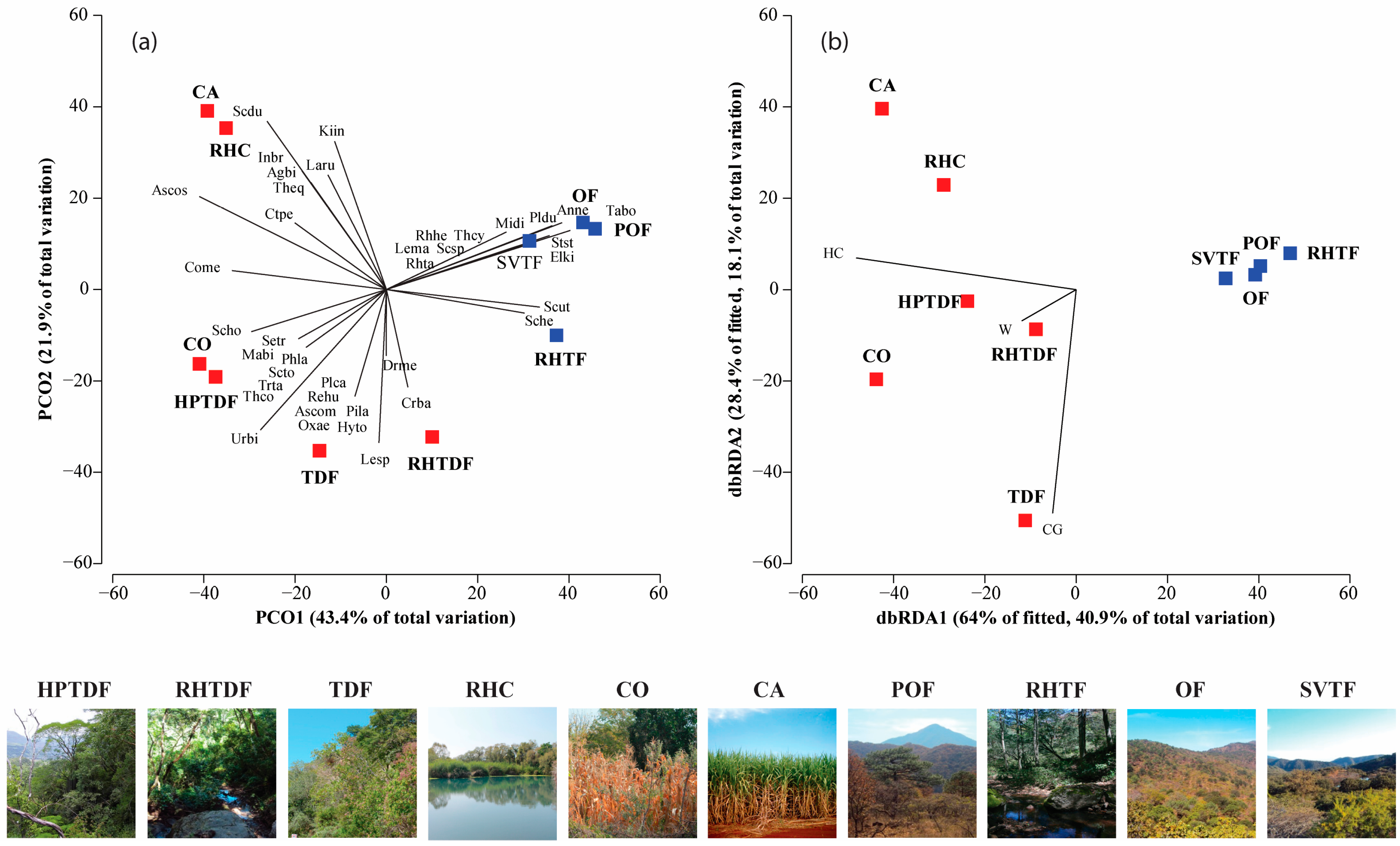
The PERMANOVA outcomes displayed differences in species composition and the abundance differed significantly only among the land cover/used types (Supplementary Materials, Table S5). The PCO ordination of species composition and abundance explained 65.3% of the total variation on the first two axes. There were significant differences in species similarity concerning the land cover/use types. There were four land cover/use type groups: the first one formed by POF, OF, SVTF, and RH-TF; the second by CO and HPTDF; the third by TDF and RH-TDF; and the last by CA and RH-C (Figure 4a).
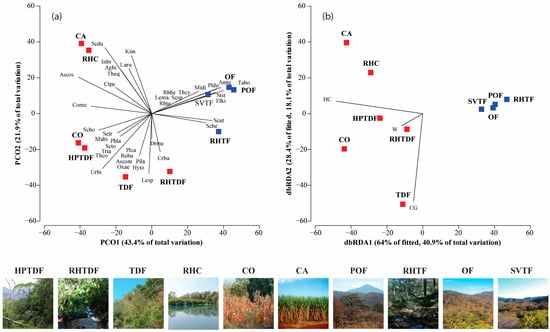
Figure 4.
PCO and dbRDA ordination. (a) The correlation between the types of land cover/use and the composition of reptile species; (b) relationship between environmental variables that best explained the reptile community variation across land cover/use types. Colors: Red for tropical habitats; blue for temperate habitats. Land cover/use types and reptile scientific name abbreviations can be found in the Supplementary Materials, Table S3.
None of the land cover or use types included all four functional groups. Only SV-TF, RH-TDF, and RH-C had four functional groups. All other sites had three functional groups, with the exception of POF, RH-TF, and CO, which had two. Group “d” with 24 species had the highest number of species, indicating greater functional redundancy compared to groups “a”, “b”, and “c”, which were represented by three, eight, and four species, respectively. The functional group “d” was present in all land cover/use types, while groups “a”, “b”, and “c” were present in four, nine, and seven types, respectively. “Furthermore, there were variations in the dominant functional groups associated with each land cover/use type”. In POF, OF, RH-C, RH-TF, and CA, the most abundant functional groups (total number of individuals) were “b” and “d”, and in SV-TF and TDF, it was “d”; however, in RH-TDF, all functional groups were present but not very abundant, while in HPTDF, it was “b”, “c”, and “d”, and in CO, it was “c” and “d” (Figure 3).
The shade plot showed that the lizards A. nebulosus and P. dugesi were the most abundant species in POF, OF, and SV-TF. In contrast, S. dugesi was in RH-C and CA. No species dominated the other habitats, but the species with intermedium abundance were A. costata and S. horridus in HPTDF and CO, and A. communis in TDF (Figure 4). Concerning the rarity of the reptile species, TDF and SV-TF had the highest number of unique species (10 and 9). In contrast, RH-C, RH-TF, OF, POF, and CA (2, 2, 1, 1, and 0, respectively) had the lowest number. Regarding the duplicate species, CA and SV-TF had the highest number (4, respectively), and the rest of the land cover/use types had two or fewer. SV-TF and TD displayed the highest number of singletons (7 and 8, respectively) and doubletons (3 and 4, respectively), and all the other sites showed four or less for doubletons and singletons (Figure 3).
3.3. Reptile Diversity Facing Environmental Variables
The DistLM showed that the variation in reptile composition and abundance was primarily attributed to herbaceous cover and cow grazing (R2adj = 0.45, p = 0.004) among the land cover/use types (Supplementary Materials, Table S6). The dbRDA ordination accounted for 59% of the total variation, indicating that herbaceous cover was the most significant predictor of tropical land cover/use types (Figure 4b). Sceloporus horridus, Phyllodactylus lanei, Oxybelis microphthalmus, S. triaspis, Rhadinaea hesperia, and T. copei were more abundant in CO and HPTDF. The PCO ordination explained 65.3% of the total variation and showed that C. basiliscus, Drymarchon melanurus, and L. splendida were associated with RH-TDF. Moreover, S. dugesii, Kinosternum integrum, I. braminus, Lampropeltis ruthveni, T. eques, A. bilineatus, and Masticophis bilineatus were linked with CA and RH-C (Figure 4a). However, TDF was the sole type of tropical land cover that was linked to cow foraging and a high contribution of Sceloporus spinosus, Urosauros bicarinatus, Plestiodon callicephalus, Aspidocelis communis, Sceloporus torquatus, Pituophis deppei, Trimorphodon tau, Hypsiglena torquata, and Rena humilis (Figure 4a). In contrast, the temperate areas were characterized by lower values of herbaceous cover (Figure 4b). Storeria storerioides, Elgaria kingii, M. distans, Anolis nebulosus, P. dugesi, Tantilla bocourti, Leptodeira maculata, Rhadinaea taeniata, and T. cyrtopsis were correlated with POF, OF, and SV-TF, and Sceloporus utiformis and Sceloporus heterolepis with RH-TF (Figure 4a).
4. Discussion
Facets of taxonomic and functional diversity are sensitivity measures of diversity patterns that consider the breadth of the ecological functions of species and their differentiation across landscapes. In contrast to our hypothesis of the highest levels of diversity in native (perturbation level) and tropical (condition) land/use cover types, we found the highest values in perturbed tropical (RH-C) and secondary vegetation enclosed by temperate forest (SV-TF) covers. Additionally, we found no matching between facets of diversity (taxonomic and functional). Our results show that RH-C and SV-TF had the highest values of species richness (q0) and functional richness (FRic), but at the same time showed a great proportion of dominant species (q2). This may suggest that in heterogeneous environments, the disturbance degree may influence spatial patterns benefiting some dominant reptile species more than condition (temperate vs. tropical) due to their exceptional plasticity and adaptation in environments experiencing transformative processes.
This study examined the taxonomic and functional diversity of the reptile species in Mexico’s ten land cover/use types. In this study, we discovered that riparian habitats, CO and CA, had the lowest species richness compared to all of the other land cover types with medium, except RH-C, with the highest. Human activities including land-use practices and changes significantly influence species richness and abundance through habitat fragmentation [46,47,48]. Moreover, these activities negatively impact specialist species by decreasing their abundance [47]. In the study area, the habitats with native vegetation exhibited a more complex vegetation structure with a higher number of strata, greater plant richness, and coverage, along with less anthropogenic impact compared to transformed habitats. Based on this, this research hypothesized that the native habitats, in particular the TDF and the RH-TDF, fostered higher taxonomic and functional reptile diversity than land use types. In contrast to our assumption, our results revealed that from tropical native habitats, only the RH-C benefited from these characteristics. Given the more complex vegetation structure, we anticipated that tropical land covers (HPTDF, TDF, RH-TDF, and RH-C) would exhibit higher reptile diversity compared to temperate ones (POF, OF, RH-TF, and SV-TF). However, our findings showed that RH-TF and RH-TDF exhibited similarly low species richness compared to the other land cover types, which support the idea regarding the influence of disturbance degree in spatial patterns more than condition [49].
Regarding the functional richness, RH-C and SV-TF had high values, and the rest of the land cover types had low or medium values regardless of whether they were tropical or temperate. The high richness of the RH-C could be attributed to the accessibility of sites to rest, hide, and reproduce, but more importantly, with prey within the site and in the land cover/use types surrounding it. RH-C is next to the CA and CO, which mainly shelter mammals and reptile prey. In the case of SV-TF, even it had intermedium species richness but high functional richness due to a higher shrub and herbaceous cover and the existence of a temporary big pond (15 m × 20 m), which escalated the prey presence compared with the other temperate land cover types. Environmental heterogeneity promotes species richness by providing food resources, space, and a variety of microhabitats [46].
In contrast to our hypothesis, no correlation existed between taxonomic and functional diversity in any land cover or land use types, except for RH-C. This research displayed that the RH-C had higher values of species richness (0D), abundant (1D) and very abundant species (2D), and functional richness (FRic), but with no difference in functional evenness (FEve) or functional divergence (FDiv) with other land cover/use types. These findings suggest that dominant species in the RH-C exhibit varying functional traits, face reduced competition, and adequately utilize resources through niche complementarity [50]. For instance, Suazo-Ortuño et al. [49] discovered a higher abundance and diversity of lizards and snakes near-stream, even though they were in the TDF and disturbed TDF.
The lowest values of 0D were recorded in both crops (CA and CO) and riparian habitats (RH-TF and RH-TDF), but the lowest functional richness was in CO, RH-TF, TDF, and POF. The low number of species and functional indices in these land cover/use types might indicate reduced competition among species and available ecological niches. Instead, this fact could show the presence of ecological niches resulting from environmental stress and limited resources [50] such as a lack of prey availability. Our findings partially align with those of Berriozabal-Islas et al. [27], who reported lower richness and functional richness in disturbed TDF for lizard species than the TDF. We found that HPTDF and TDF had medium species richness values, and TDF had a lower functional richness. Easily accessible ecological niches can greatly increase the potential for exotic or translocated species to access and fill vacant niches [50,51].
Different reptile species are associated with various habitats based on their physiological, morphological, and reproductive attributes. For example, venomous snakes in functional group “a” were recorded in different land cover/use types, where C. basiliscus was in the RH-TDF, M. distans in temperate land cover types, and A. bilineatus in RH-C. These three snakes are poisonous, have a medium to large size, and terrestrial habitat. They are opportunistic predators and tolerate habitat perturbation. Crotalus basiliscus is widely distributed in temperate and tropical habitats [52]. In other studies in the Jalisco state, we have observed that C. basiliscus and A. bilineatus can inhabit sugar cane and corn crops.
Group “b” was formed by non-venomous and viviparous lizards (P. dugesi, S. dugesi, S. heterolepis, S. torquatus) and snakes (S. storerioides, T. copei, T. cyrtopsis, and T. eques). For example, S. dugesii was abundant in CA, and P. dugesii in POF and OF. Viviparity is an adaptation because pregnant female reptiles sustain more constant temperatures than are accessible in nests [53,54]. Moreover, it enhances fitness by increasing the female’s ability to anticipate the post-hatching conditions of her offspring [55]. This functional group occurred in temperate and tropical land cover types and CA.
In contrast, group “c”, comprising snakes (L. splendida and S. triaspis), a turtle (K. integrum), and a lizard (C. pectinata), was characterized by medium to large sizes, non-venomous nature, and diverse diets ranging from omnivorous to carnivorous (specifically vertebrates). Their habitats varied between terrestrial-arboreal and terrestrial-aquatic environments, and they reproduce by laying eggs (oviparous). This functional group was associated with tropical sites except for the SV-TF, where K. integrum was registered in the existing pond and juveniles of C. pectinata. Some adult reptiles including chelonian and squamate reptiles are known to be herbivorous [56]. For instance, C. pectinata adults primarily consume the leaves and flowers from various plant species, but not the juveniles, which feed mainly on insects. Juvenile and immature reptiles must maintain a diverse diet through opportunistic feeding to survive the high predation risk during the growth period [57]. Conversely, K. integrum is a generalist that feeds opportunistically on available prey items (plant material and invertebrates) [58].
Finally, group “d” corresponded to all other species of lizards and snakes. This group was variable in size, non-venomous, fossorial, terrestrial, arboreal, or terrestrial-arboreal and oviparous. As ectotherms, different attributes of the reptiles’ physiology and behavior are modulated by ambient temperatures [3]. Moreover, in oviparous species, there is a sensitivity of embryogenesis to thermal variance. The offspring’s physical traits are altered by the daily distribution of incubation temperatures and the average environmental temperature [54].
Areas with reduced indigenous vegetation cover, reduced landscape connectivity, altered native vegetation, and intensive land use are at high risk and are highly susceptible to experiencing species extinction. Species with critical functional roles or within key functional groups are the most heavily impacted [16]. Habitat shift alters environmental conditions, vegetation composition, and structure, resulting in changes to habitat features [59]. For example, lizard species richness (15 species), species diversity, functional richness, functional dispersion, and the number of functional groups are lower in disturbed sites than in undisturbed tropical dry forest sites in western central Mexico [27]. In contrast, Suazo-Ortuño et al. [48] found a high resilience for lizards and snakes, which did not show a significant difference in species abundance, richness, or diversity among most of the different vegetation successional stages in an anthropogenically modified tropical dry forest landscape in western Mexico. Monocultures significantly impact reptile and amphibian populations based on the agricultural practices and the specific crops grown [60,61]. For instance, applying herbicides, fertilizers, fungicides, and controlled burns in corn farming and coffee plantations impacts the vegetation structure and crop growth, whether this is annual or perennial. Conversely, the richness of herpetofauna species in avocado orchards is similar to that of undisturbed forests [62]. The composition of species on the site is directly determined by the adjacent vegetation [60]. In fragmented tropical landscapes, Suazo-Ortuño et al. [48] showed that the composition and quality of the surrounding matrix influence the conservation of amphibians and reptiles.
The region of west-central Mexico consists of a diverse combination of land cover and land use types. In this study, RH-C sheltered the highest taxonomic diversity and TDF, HPTDF, SV-TF, OF, and POF intermedium, while RH-C and SV-TF had the highest functional diversity and CO intermedium. In contrast, Peña-Joya et al. [63] mentioned that POF and pine forest showed high levels of functional diversity. Additionally, the gallery forest and riparian habitat play a crucial role in connecting different types of land cover [49]. Cornfields and sugar cane plots are common agricultural landscapes in Mexico. These habitats are limiting for reptiles due to the absence of plant cover upon establishment, exposing the animals to predators. Notably, in the case of sugar cane, the culture is set on fire every 5–6 years to harvest the plants and allow new growth. When this happens, several animals die, and others have to move to other land cover/use types because shifts in photoperiod, humidity, and temperature can impact the survival of organisms living in the disturbed environment, leading to a decrease in their richness and abundance [64]. For example, Leyte-Manrique et al. [65] found that TDF presented higher values of taxonomic diversity than areas with corn fields.
Reptiles are imperiled by habitat destruction and degradation (fragmentation and patch isolation) [66,67], pollution, use for food and pet commerce, exotic species, diseases, global climate change [66], and urbanization (e.g., roadkill) [66]. For example, Vega-Agavo et al. [47] suggested that POF conversion to avocado orchards is a driver favoring the persistence and success of generalist but not specialist herpetofauna species. Suazo-Ortuño et al. [60] observed that some tropical habitats changed by humans contained higher reptile species richness and diversity than undisturbed environments. Original vegetation fragments provide shelter and food, and certain species exhibit remarkable plasticity and adaptation in habitats undergoing transformation processes.
Reptile species react differently to perturbation. This study demonstrated varying reptile taxonomic and functional diversity patterns across different land cover/use types. At different spatial scales, a number of factors have an impact on these facets. At the local level, these factors include the vegetation structure, water availability, temperature, and human use [63]. In contrast, at the landscape level, these factors are associated with native vegetation patches and spatial connectivity [63]. Finally, at a broad spatial scale, climate factors such as temperature, precipitation, temperature seasonality, and precipitation seasonality [23] as well as land cover diversity [63] have a significant impact on functional diversity and the number of species. Furthermore, population density and urban sprawl may have adverse effects on reptile functional diversity and species richness [23]. This research was carried out on a landscape scale, and we found that individuals of different species from bordering habitats are important in sustaining the number of reptile species and abundance in the corn and sugar cane land-use types. In landscapes impacted by agricultural and livestock activities, reptiles may be favored due to their endurance for temporal variations in temperature, precipitation, and humidity. This results in a greater utilization of accessible microhabitats and food [68,69]. Given the diverse land cover found in most landscapes, it is imperative to assess the taxonomic and functional diversity changes to implement management policies to conserve biodiversity.
This study’s findings underscore the critical role of diverse land cover/use types such as pine-oak forests, oak forests, and secondary vegetation, in maintaining reptile biodiversity in west-central Mexico. These habitats not only support a variety of reptile species including those that are endemic, but also contribute to species with critical conservation status [70]. Understanding these habitats is crucial to integrating conservation strategies into local and regional planning. This includes promoting sustainable land-use practices that minimize habitat disruption, enhancing legal protections for crucial biodiversity areas, and fostering community engagement in conservation efforts [71]. Particularly in Mexico, an alternative to reduce habitat fragmentation is the inclusion of forest remnants in a program of remuneration for environmental services [72,73]. On the other hand, restoring structural elements of habitat and its interaction with grazing and agricultural management can be important for ameliorating conservation actions for reptiles in heterogenous landscapes [74]. Thus, recognizing the ecological value of these land cover/use types in environmental education and policy making can bolster support for conservation initiatives.
Conservation strategies should prioritize the restoration of degraded habitats and the creation of ecological corridors to facilitate wildlife movement and genetic exchange [75]. Furthermore, considering climate change and its impact on habitat suitability, adaptive management practices are essential to guarantee the enduring survival of reptile species in this region. By adopting a holistic approach that values the intrinsic and ecological importance of reptile diversity, stakeholders can safeguard these species and their habitats for future generations [74]. Collaborative efforts involving government agencies, non-governmental organizations, researchers, and local communities will be vital to achieving sustainable conservation outcomes [71].
Supplementary Materials
Information available for download: Table S1: Structural habitat variables measured on the reptile’s land cover/use plots.; Table S2: Functional traits of reptiles and their ecological meaning.; Table S3: Abbreviations used in the manuscript.; Table S4: Sampled-based rarefaction results of expected reptile species richness from central-western Mexico. Observed and expected species for land cover/use type by non-parametric procedures: Chao1, Jackknife 1, Jackknife 2.; Table S5: Permutational ANOVA for 0D (species richness), 1D (abundant species), 2D (very abundant species), N (abundance), 21D (evenness), Fric (functional richness), FEve (functional evenness), FDiv functional divergence), and PERMANOVA for the reptile’s composition and abundance. Table S6: Results of the DistLM marginal and sequential tests to obtain the best predictable model between the reptile species composition and environmental variables across land cover/use types.
Author Contributions
Conceptualization and methodology, V.C.R.-E., F.A.R.-Z., K.E.P.-J. and A.L.S.-P.; Formal analysis and investigation, V.C.R.-E., E.Á.-G., F.A.R.-Z., K.E.P.-J. and A.A.G.-G.; Resources, V.C.R.-E. and A.L.S.-P.; Writing original draft preparation, V.C.R.-E., F.A.R.-Z., K.E.P.-J. and F.M.H.-M.; Writing-review and editing, V.C.R.-E., F.A.R.-Z., K.E.P.-J., A.L.S.-P., E.Á.-G., A.A.G.-G. and F.M.H.-M.; Project administration and funding acquisition, V.C.R.-E., F.A.R.-Z. and A.L.S.-P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data are contained within the article and Supplementary Material.
Acknowledgments
We thank U. O. García Vázquez and M. Dominguez Laso for their support in the digital deposit of the photographs at the MZFZ (UNAM). We thank H. Ayuntamiento de Ahualulco del Mercado, Ejido de Santa Cruz de Bárcenas, and the H. Ayuntamiento de Teuchitlán (HAT). We thank L. Padilla Miranda and E. S. Blanco Morales (Centro Interpretativo Guachimontones) for the logistical and economic support, M. Ureña Díaz (HAT) for the permits, R. Orozco Wences (Restarurant Soky), and E. Lucke Gracián (Hacienda Labor de Rivera) for their financial support as well as J. Arreola Aguirre, Á. Urzúa Sánchez, H. Franz-Chávez, J. G. Navarro Flores, and R. Vázquez Arias for their field support.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Pincheira-Donoso, D.; Bauer, A.M.; Meiri, S.; Uetz, P. Global Taxonomic Diversity of Living Reptiles. PLoS ONE 2013, 8, e59741. [Google Scholar] [CrossRef]
- Valencia-Aguilar, A.; Cortés-Gómez, A.M.; Ruiz-Agudelo, C.A. Ecosystem services provided by amphibians and reptiles in Neotropical ecosystems. Int. J. Biodivers. Sci. Ecosyst. Serv. Manag. 2013, 9, 257–272. [Google Scholar] [CrossRef]
- Vitt, L.J.; Caldwell, J.P. Herpetology, Introductory Biology of Amphibians and Reptiles, 4th ed.; Elsevier: Amsterdam, The Netherlands; Academic Press: San Diego, CA, USA, 2014; p. 776. [Google Scholar]
- Nelson, E.C. Lizards on Aeonium lancerottense in Lanzarote, Canary Islands: A new example of pollination by reptiles? Bradleya 2010, 28, 15–18. [Google Scholar] [CrossRef]
- Uetz, P.; Freed, P.; Aguilar, R.; Reyes, F.; Hošek, J. (Eds.) The Reptile Database. Available online: https://www.reptile-database.org (accessed on 5 May 2024).
- Comisión Nacional Para el Conocimiento y Uso de la Biodiversidad (CONABIO). Biodiversidad Mexicana. Available online: http://www.biodiversidad.gob.mx (accessed on 5 May 2024).
- Flores-Villela, O.; García-Vázquez, U. Biodiversidad de reptiles en México. Rev. Mex. Biodivers. 2014, 85, 467–475. [Google Scholar] [CrossRef]
- Ramírez-Bautista, A.; Torres-Hernández, L.A.; Cruz-Elizalde, R.; Berriozabal-Islas, C.; Hernández-Salinas, U.; Wilson, L.D.; Johnson, J.D.; Porras, L.W.; Balderas-Valdivia, C.J.; González-Hernández, A.J.X.; et al. An updated list of the Mexican herpetofauna: With a summary of historical and contemporary studies. Zookeys 2023, 1166, 287–306. [Google Scholar] [CrossRef] [PubMed]
- García, A. Using ecological niche modeling to identify diversity hotspots for the herpetofauna of Pacific lowlands and adjacent interior valleys of Mexico. Biol. Conserv. 2006, 130, 25–46. [Google Scholar] [CrossRef]
- Ochoa-Ochoa, L.M.; Flores-Villela, O.A. Áreas de Diversidad y Endemismo de la Herpetofauna Mexicana; Universidad Nacional Autónoma de México-CONABIO: Mexico City, México, 2006; p. 218. [Google Scholar]
- Baena, M.L.; Halfter, G. Extinción de especies. In Capital Natural de México, Volume I: Conocimiento Actual de la Biodiversidad; Sarukhán, J., Ed.; CONABIO: Mexico City, México, 2008; pp. 264–275. [Google Scholar]
- Ceballos, G.; Ehrlich, P.R.; Barnosky, A.D.; García, A.; Pringle, R.M.; Palmer, T.M. Accelerated modern human-induced species losses: Entering the sixth mass extinction. Sci. Adv. 2015, 1, e1400253. [Google Scholar] [CrossRef] [PubMed]
- IUCN. World’s Reptiles Comprehensively Assessed-IUCN Red List. 2022. Available online: https://www.iucn.org/news/species/202204/worlds-reptiles-comprehensively-assessed-iucn-red-list (accessed on 10 June 2024).
- Cox, N.; Young, B.E.; Bowles, P.; Fernadez, M.; Marin, J.; Rapacciuolo, G.; Böhm, M.; Brooks, T.M.; Hedges, S.B.; Hilton-Taylor, C.; et al. A global reptile assessment highlights shared conservation needs of tetrapods. Nature 2022, 605, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Rincón-Aranguri, M.; Toro-Cardona, F.A.; Galeano, S.P.; Roa-Fuentes, L.; Urbina-Cardona, N. Functional diversity of snakes is explained by the landscape composition at multiple areas of influence. Ecol. Evol. 2023, 13, e10352. [Google Scholar] [CrossRef]
- Fisher, J.; Lindenmayer, D.B. Landscape modification and habitat fragmentation: A synthesis. Glob. Ecol. Biogeogr. 2007, 16, 265–280. [Google Scholar] [CrossRef]
- Instituto de Información Estadística y Geografía del Estado de Jalisco (IIEG). Valles Diagnóstico de la Región. Available online: https://iieg.gob.mx/ns/wp-content/uploads/2023/08/Valles.pdf (accessed on 5 May 2024).
- Petchey, O.L.; Gaston, K.J. Functional diversity: Back to basics and looking forward. Ecol. Lett. 2006, 9, 741–758. [Google Scholar] [CrossRef] [PubMed]
- Galindo-Uribe, D.M.; Hoyos-Hoyos, J.M.; Isaacs-Cubides, P.; Corral-Gómez, N.; Urbina-Cardona, N. Classification and sensitivity of taxonomic and functional diversity indices of anurans in the Andean coffee cultural landscape. Ecol. Indic. 2022, 135, 108650. [Google Scholar] [CrossRef]
- Hernández-Salinas, U.; Cruz-Elizalde, R.; Ramírez-Bautista, A.; Wilson, L.D.; Berriozabal-Islas, C.; Johnson, J.D.; Mata-Silva, V. Taxonomic and functional diversity of the amphibian and reptile community of the state of Durango, Mexico. Community Ecol. 2023, 24, 229–242. [Google Scholar] [CrossRef]
- Villeger, S.; Ramos-Miranda, J.; Flores-Hernández, D.; Mouillot, D. Contrasting changes in taxonomic vs. functional diversity of tropical fish communities after habitat degradation. Ecol. Appl. 2010, 20, 1512–1522. [Google Scholar] [CrossRef] [PubMed]
- Santos, X.; Cheylan, M. Taxonomic and functional response of a Mediterranean reptile assemblage to a repeated fire regime. Biol. Conserv. 2013, 168, 90–98. [Google Scholar] [CrossRef]
- Tsianou, M.A.; Lazarina, M.; Andrikou-Charitidou, A.; Michailidou, D.; Kallimanis, A.S. The Effect of Climate and Human Pressures on Functional Diversity and Species Richness Patterns of Amphibians, Reptiles and Mammals in Europe. Diversity 2021, 13, 275. [Google Scholar] [CrossRef]
- Berriozabal-Islas, C.; Ramírez-Bautista, A.; Cruz-Elizalde, R.; Hernández-Salinas, U. Modification of landscape as promoter of change in structure and taxonomic diversity of reptile’s communities: An example in tropical landscape in the central region of Mexico. Nat. Conserv. 2018, 28, 33–49. [Google Scholar] [CrossRef]
- López-Bedoya, P.A.; Cardona-Galvis, E.A.; Urbina-Cardona, J.N.; Edwards, F.A.; Edwards, D.P. Impacts of pastures and forestry plantations on herpetofauna: A global meta-analysis. J. Appl. Ecol. 2022, 59, 3038–3048. [Google Scholar] [CrossRef]
- Barnagaud, J.; Geniez, P.; Cheylan, M.; Crochet, P.A. Climate overrides the effects of land use on the functional composition and diversity of Mediterranean reptile assemblages. Divers. Distrib. 2021, 27, 50–64. [Google Scholar] [CrossRef]
- Berriozabal-Islas, C.; Badillo-Saldaña, L.M.; Ramírez-Bautista, A.; Moreno, C.M. Effects of hábitat disturbance on lizard functional diversity in a tropical dry forest of the Pacific Coast of Mexico. Trop. Conserv. Sci. 2017, 10, 1–11. [Google Scholar] [CrossRef]
- Pollock, L.J.; O’Connor, L.M.J.; Mokany, K.; Rosauer, D.F.; Talluto, M.V.; Thuiller, W. Protecting biodiversity (in all its complexity): New models and methods. Trends Ecol. Evol. 2020, 35, 1119–1128. [Google Scholar] [CrossRef] [PubMed]
- Sayer, J.; Sunderland, T.; Ghazoul, J.; Pfund, J.L.; Sheil, D.; Meijaard, E.; Venter, M.; Boedhihartono, A.K.; Day, M.; Garcia, C.; et al. Ten principles for a landscape approach to reconciling agriculture, conservation, and other competing land uses. Proc. Natl. Acad. Sci. USA 2013, 110, 8349–8356. [Google Scholar] [CrossRef] [PubMed]
- Freemark, K.E.; Probst, J.R.; Dunning, J.B.; Hejl, S.J. Status and management of neotropical migratory birds: 21–25 September 1992, Estes Park, Colorado. In General Technical Reports RM-229; Finch, D.M., Stangel, P.W., Eds.; Department of Agriculture, Forest Service: Washington, DC, USA, 1993; pp. 346–352. [Google Scholar]
- Instituto Nacional de Estadística y Geografía (INEGI). Conjunto de Datos Vectoriales Climatología, Escala 1:250,000, Serie VI. Available online: https://www.inegi.org.mx/temas/climatologia/ (accessed on 5 May 2024).
- Rzedowski, J. Vegetación de México; Comisión Nacional Para el Conocimiento y Uso de la Biodiversidad: México City, México, 2006; p. 504. Available online: https://www.biodiversidad.gob.mx/publicaciones/librosDig/pdf/VegetacionMxPort.pdf (accessed on 5 May 2024).
- García-Martínez, M.A.; Rodríguez, A. Vegetación y flora fanerogámica del área natural protegida Piedras Bola, Jalisco, México. Polibotánica 2018, 46, 71–90. [Google Scholar]
- Instituto Nacional de Estadística y Geografía (INEGI). Conjunto de Datos Vectoriales de Uso Del Suelo y Vegetación, Escala 1:250,000, Serie VII. Available online: https://www.inegi.org.mx/temas/usosuelo/default.html#Descargas/ (accessed on 5 May 2024).
- Rendón, S.F.J.; Cedano, M.M. Guía de excursión botánica: Los Guachimontones y humedales de Teuchitlán. In Guía de las Excursiones Botánicas en Jalisco. XVIII Congreso Mexicano; Ramírez, D.R., Reynoso, D.J.J., Rodríguez, C.A., Eds.; Universidad de Guadalajara: Guadalajara, México, 2010; pp. 88–106. [Google Scholar]
- Colwell, R.K. EstimateS: Statistical Estimation of Species Richness and Shared Species from Samples. Version 7.5. Available online: https://www.robertkcolwell.org/pages/1407 (accessed on 5 May 2024).
- Jost, L. Entropy and diversity. Oikos 2006, 113, 363–375. [Google Scholar] [CrossRef]
- Anderson, M.J.; Gorley, R.N.; Clarke, K.R. PERMANOVA+ for PRIMER: Guide to Software and Statistical Methods; PRIMER-E: Plymouth, UK, 2008. [Google Scholar]
- Cortés-Gómez, A.M.; Ruiz-Agudelo, C.A.; Valencia-Aguilar, A.; Ladle, R.J. Ecological functions of neotropical amphibians and reptiles: A review. Univ. Sci. 2015, 2, 229–245. [Google Scholar] [CrossRef]
- Laliberte, E.; Legendre, P. A distance-based framework for measuring functional diversity from multiple traits. Ecology 2010, 9, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Laliberte, E.; Legendre, P.; Shipley, B. FD Measuring Functional Diversity from Multiple Traits, and Other Tools for Functional Ecology. R Package Version 1.0–12, 2014. Available online: https://cran.r-project.org/web/packages/FD/index.html (accessed on 9 January 2022).
- RStudio Team. RStudio: Integrated Development for R. Computer Software v0.98.1074. Available online: www.rstudio.com/ (accessed on 5 May 2024).
- Clarke, K.R.; Gorley, R.N.; Somerfield, P.J.; Warwick, R.M. Change in Marine Communities: An Approach to Statistical Analysis and Interpretation, 3rd ed.; PRIMER-E Ltd.: Plymouth, UK, 2014. [Google Scholar]
- Diario Oficial de la Federación. Secretaría de Medio Ambiente y Recursos Naturales NOM–059–SEMARNAT–2010. Protección Ambiental. Especies Nativas de México de Flora y Fauna Silvestres-Categorías de Riesgo y Especificaciones Para su Inclusión, Exclusión o Cambio. Lista de Especies en Riesgo. Modificación del Anexo Normativo III, Publicada en el Diario Oficial de la Federación el 30 de Diciembre del 2010, Págs.: 1–78. Available online: https://www.dof.gob.mx/nota_detalle.php?codigo=5578808&fecha=14/11/2019#gsc.tab=0 (accessed on 5 May 2024).
- IUCN. The IUCN Red List of Threatened Species. 2021. Available online: https://www.iucnredlist.org/ (accessed on 5 May 2024).
- Luja, V.H.; López, J.A.; Cruz-Elizalde, R.; Ramírez-Bautista, A. Herpetofauna inside and outside from a natural protected area: The case of Reserva Estatal de la Biósfera Sierra San Juan, Nayarit, Mexico. Nat. Conserv. 2017, 21, 15–38. [Google Scholar] [CrossRef]
- Vega-Agavo, M.I.; Suazo-Ortuño, E.; Lopez-Toledo, L.; Gómez-Tagle, A.; Sillero, N.; Pineda-López, R.; Alvarado-Díaz, J. Influence of avocado orchard landscapes on amphibians and reptiles in the tans-Mexican volcanic belt. Biotropica 2021, 53, 1631–1645. [Google Scholar] [CrossRef]
- Suazo-Ortuño, I.; Alvarado-Díaz, J.; Mendoza, E.; López-Toledo, L.; Lara-Uribe, N.; Márquez-Camargo, C.; Paz-Gutiérrez, J.G.; Rangel-Orozco, J.D. High resilience of herpetofaunal communities in a human-modified tropical dry forest landscape in western Mexico. Trop. Conserv. Sci. 2015, 8, 396–423. [Google Scholar] [CrossRef]
- Suazo-Ortuño, I.; Alvarado-Díaz, J.; Martínez-Ramos, M. Riparian Areas and Conservation of Herpetofauna in a Tropical Dry Forest in Western Mexico. Biotropica 2011, 43, 237–245. [Google Scholar] [CrossRef]
- Mason, N.W.H.; Mouillot, D.; Lee, W.G.; Wilson, J.B. Functional richness, functional evenness, and functional divergence: The primary components of functional diversity. Oikos 2005, 111, 112–118. [Google Scholar] [CrossRef]
- Mouchet, M.A.; Villéger, S.; Mason, N.W.H.; Mouillot, D. Functional diversity measures: An overview of their redundancy and their ability to discriminate community assembly rules. Funct. Ecol. 2010, 24, 867–876. [Google Scholar] [CrossRef]
- McCranie, J.R. Crotalus basiliscus (Cope) Mexican west coast rattlesnake. Cat. Am. Amphib. Rept. 1981, 283, 1–2. Available online: https://repositories.lib.utexas.edu/browse/subject?scope=3ee72c9e-726f-45fd-8c72-4e7c583b3d74&value=Crotalus%20basiliscus&bbm.return=1 (accessed on 5 May 2024).
- Shine, R. The evolution of viviparity in reptiles: An ecological analysis. Biol. Reptil. 1985, 15, 605–694. [Google Scholar]
- Webb, J.K.; Shine, R.; Christian, K.A. The adaptive significance of reptilian viviparity in the tropics: Testing the maternal manipulation hypothesis. Evolution 2006, 60, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Tinkle, D.W.; Gibbons, J.W. The distribution and evolution of viviparity in reptiles. Misc. Publ. Mus. Zool. Univ. Mich. 1977, 154, 1–55. [Google Scholar]
- Bjorndal, K.A. Fermentation in reptiles and amphibians. In Gastrointestinal Microbiology; Mackie, R.I., White, B.A., Eds.; Springer Science+ Business Media: New York, NY, USA, 1997; Volume 1, pp. 199–230. [Google Scholar]
- Durtsche, D. Ontogenetic plasticity of food habits in the Mexican spiny-tailed iguana, Ctenosaura pectinata. Oecologia 2000, 124, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Macip-Ríos, R.; Sustaita-Rodriguez, V.H.; Barrios-Quiroz, G.; Casas-Andreu, G. Alimentary Habits of the Mexican Mud Turtle (Kinosternon integrum) in Tonatico, Estado de México. Chelonian Conserv. Biol. 2010, 9, 90–97. [Google Scholar] [CrossRef]
- Tuff, K.T.; Tuff, T.; Davies, K.F. A framework for integrating thermal biology into fragmentation research. Ecol. Lett. 2016, 19, 361–374. [Google Scholar] [CrossRef]
- Suazo-Ortuño, I.; Alvarado-Díaz, J.; Martínez-Ramos, M. Effects of conversion of dry tropical forest to agricultural mosaic on herpetofaunal assemblage. Conserv. Biol. 2008, 22, 362–374. [Google Scholar] [CrossRef]
- Rosas-Espinoza, V.C.; Peña-Joya, K.E.; Álvarez-Grzybowska, E.; Godoy-González, A.A.; Santiago-Pérez, A.L.; Rodríguez-Zaragoza, F.A. Amphibian Taxonomic and Functional Diversity in a Heterogeneous Landscape of West-Central Mexico. Diversity 2022, 14, 738. [Google Scholar] [CrossRef]
- Marroquín-Páramo, J.A.; Suazo-Ortuño, I.; Mendoza, E.; Alvarado-Díaz, J.; Siliceo-Cantero, H.H. Herpetofaunal diversity in avocado orchards and in conserved habitats in Michoacán, Mexico. Rev. Mex. Biodivers. 2017, 88, 234–240. [Google Scholar] [CrossRef]
- Peña-Joya, K.E.; Cupul-Magaña, F.G.; Rodríguez-Zaragoza, F.A.; Moreno, C.E.; Téllez-López, J. Spatio-temporal discrepancies in lizard species and functional diversity. Community Ecol. 2020, 21, 1–12. [Google Scholar] [CrossRef]
- Cruz-Elizalde, R.; Berriozabal-Islas, C.; Hernández-Salinas, U.; Martínez-Morales, M.A.; Ramírez-Bautista, A. Amphibian species richness and diversity in a modified tropical environment of central Mexico. Trop. Ecol. 2016, 57, 407–417. [Google Scholar]
- Leyte-Manrique, A.; Buelna-Chontal, A.A.; Torres-Diaz, M.A.; Berriozabal-Islas, C.; Maciel-Mata, C.A.A. Comparison of Amphibian and Reptile Diversity Between Disturbed and Undisturbed Environments of Salvatierra, Guanajuato, Mexico. Trop. Conserv. Sci. 2019, 12, 1–12. [Google Scholar] [CrossRef]
- Gibbons, J.W.; Scott, D.E.; Ryan, T.J.; Buhlmann, K.A.; Tuberville, T.D.; Metts, B.S.; Greene, J.L.; Mills, T.; Leiden, Y.; Poppy, S.; et al. The global decline of reptiles, déjà vu amphibians: Reptile species are declining on a global scale. Six significant threats to reptile populations are habitat loss and degradation, introduced invasive species, environmental pollution, disease, unsustainable use, and global climate change. Bioscience 2000, 50, 653–666. [Google Scholar]
- Keinath, D.A.; Doak, D.F.; Hodges, K.E.; Prugh, L.R.; Fagan, W.; Sekercioglu, C.H.; Buchart, S.H.M.; Kauffman, M. A global analysis of traits predicting species sensitivity to habitat fragmentation. Glob. Ecol. Biogeogr. 2017, 26, 115–127. [Google Scholar] [CrossRef]
- Macip-Ríos, R.; Casas-Andreu, G. Los cafetales en México y su importancia para la conservacion de los anfibios y reptiles. Acta Zool. Mex. 2008, 24, 143–159. [Google Scholar] [CrossRef]
- Macip-Ríos, R.; Muñoz-Alonso, A. Diversidad de lagartijas en cafetales y bosque primario en el Soconusco Chiapaneco. Rev. Mex. Biodiv. 2008, 79, 185–195. [Google Scholar] [CrossRef]
- Wilson, L.D.; Mata-Silva, V.; Johnson, J.D. A conservation reassessment of the reptiles of Mexico based on the EVS measure. Amphib. Reptile Conserv. 2013, 7, 97–127. [Google Scholar]
- Brandon, K.; Gorenflo, L.J.; Rodrigues, A.S.; Waller, R.W. Reconciling biodiversity conservation, people, protected areas, and agricultural suitability in Mexico. World Dev. 2005, 33, 1403–1418. [Google Scholar] [CrossRef]
- Ramirez-Reyes, C.; Sims, K.; Potapov, P.; Radeloff, V.C. Payments for ecosystem services in Mexico reduce forest fragmentation. Ecol. Appl. 2018, 28, 1982–1997. [Google Scholar] [CrossRef] [PubMed]
- Costedoat, S.; Corbera, E.; Ezzine-de-Blas, D.; Honey-Rosés, J.; Baylis, K.; Castillo-Santiago, M.A. How effective are biodiversity conservation payments in Mexico? PLoS ONE 2015, 10, e0119881. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, J.L.; Foster, C.N.; Blanchard, W.; Florance, D.; Michael, D.R.; Lindenmayer, D.B. Reversing habitat loss: An experimental test of the interactive effects of grazing exclusion and surface rock restoration on reptile conservation. J. Appl. Ecol. 2023, 60, 1778–1789. [Google Scholar] [CrossRef]
- Donald, P.F.; Evans, A.D. Habitat connectivity and matrix restoration: The wider implications of agri-environment schemes. J. Appl. Ecol. 2006, 43, 209–218. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).