Abstract
Most of the attention given to intraspecific crop, livestock, and aquaculture diversity in agricultural production systems has been targeted at their roles in providing provisioning services, such as food and fibre or their cultural services, providing non-material benefits, such as places for recreation and inspiration. The additional role that intraspecific crop, livestock, and aquaculture diversity has in providing regulating and supporting ecosystem services for agricultural productivity and ecosystem resilience has been largely neglected. A literature review was carried out across sectors (crop, livestock, aquaculture), both on the counterfactual, i.e., the lack of intraspecific diversity in the production system and on the direct and indirect roles that intraspecific diversity plays in maintaining seven of the regulating and supporting ecosystem services: (i) regulating pest and diseases; (ii) maintaining and regulating water and soil quality; (iii) regulating and improving the flow of reproductive diversity; (iv) buffering excess or lack of water; (v) regulating soil erosion; (vi) nutrient cycling in water and soil; and (vii) supporting habitat maintenance. Benefits from the use of intraspecific diversity, diversity per se, and adaptive traits include a limited use of chemical inputs and unsustainable practices and their negative impact on livelihoods, ecosystem functioning, and productivity. All sectors (crop, livestock, and aquaculture) should be examined in the agricultural production system to understand the provision of the different ecosystem services by intraspecific diversity. Differences in structure, functioning, and temporal and spatial scales of these sectors should also be considered. Supporting and regulating ecosystem services often have relatively longer-term processes than food provisioning and rely not only on the current diversity but also on its presence over time. The presented regulating and supporting ecosystem services rely on the presence of the diversity from the farm to the landscape and to agroecological zone. Neglecting the additional role that intraspecific crop, livestock, and aquaculture diversity has in providing regulating and supporting ecosystem services is shown in this review to be detrimental to agricultural productivity and landscape resilience.
1. Introduction
A considerable amount of agricultural biodiversity continues to be maintained in the agroecosystems of small-scale farmers, pastoralists, and aquaculture systems in the form of traditional crop varieties, livestock breeds, and farmed-fish types [1,2,3,4,5,6,7,8]. These varieties, breeds and farmed types as well as their potential inner genetic variability are referred to as intraspecific agricultural diversity. This intraspecific diversity is an essential part of the livelihood strategies for small-scale farming populations [9,10,11,12]. These small-scale farmers and pastoralists are providing nearly 30 percent of our global food production [13,14,15].
Domestication and selection of modern crop varieties and livestock breeds have resulted in the following overall characteristics: widespread, highly specialized, and requiring high-external inputs. These varieties and breeds are for the most part more genetically uniform than their ancestors, ensuring a calibrated and high production under specific conditions and inputs. The history of crop and livestock domestication was initiated some 12,000 years ago (10,000 BC). Over the last 150 years, modern breeding with the use of scientific tools and for commercial purposes has produced major genetic changes for some major crops such as rice, wheat, maize, high-value fruit and vegetables, and oilseeds, leading to a great loss of genetic diversity [5,16,17,18]. Despite this early history, for the livestock sector, it was only about two centuries ago that the concept of breed, and with it a much stronger selection towards phenotypically homogenized breeds, was implemented. Only decades ago, heavy selection pressures were used to improve breeds productivity without enough consideration on the importance of preserving their overall genetic diversity [19]. Genetic improvement and domestication are much more limited for aquatic organisms with the exception of a few fish species [8,20]. The genetic diversity found in wild fish populations and the level of domestication of farmed fish remains much lower than that of livestock, leaving great potential for future domestication through genetic improvement and improved husbandry [8,21,22,23]. There is therefore a general concern over how to best conserve this intraspecific diversity.
To date, the majority of attention given to intraspecific crop, livestock, and aquaculture diversity in agricultural production systems has been targeted at their roles in providing provisioning services, such as food and fibre [24,25,26,27] and, to a lesser extent, to cultural services that provide non-material benefits, such as places for recreation and inspiration [3,25,28,29,30,31,32,33,34,35,36,37,38,39,40]. Moreover, most of these studies have focussed on the use of specific adaptive traits from traditional genetic resources for breeding programmes and for marketing crop, livestock, or aquatic resources [21,41,42,43,44]. Much less attention has been given to the potential role that this intraspecific diversity has in providing ecosystem services for regulation and maintenance [45] and increasing resilience across all three sectors: crop, livestock, and aquatic genetic resources. Moreover, the counterfactual is missing from these studies: the question of how the lack of intraspecific diversity can affect regulating and supporting services. It should nevertheless be noted that most of the publications explored in the literature review refer to a variety of terminologies and classifications.
We conducted a literature review on a selection of regulation and maintenance ecosystem services associated with intraspecific diversity in agriculture. The targeted services are (i) pest and disease control; (ii) water conditions and regulation of soil quality; (iii) lifecycle maintenance through the flow of reproductive materials; (iv) water flow and extreme-water-event regulation; (v) maintenance of soil and soil conditions; (vi) nutrient cycling in soil and water; and (vii) habitat maintenance and protection. The names of the ecosystem services have been chosen based on the names found in the literature review and a tentative harmonization with the Common International Classification of Ecosystem Services (CICES) [45,46]. In this review, we focussed on major crops, both annual and perennial (fruit trees), where significant intraspecific diversity has been documented. For livestock, we examine diversity among and within breeds, concentrating on major livestock species. For aquaculture, the review is a first exploration in an unexploited field, and articles were found for a limited number of target species or groups. The provision of or contribution to several of the ecosystem services, such as water flow and extreme-water-event regulation, maintenance of soil conditions, or nutrient cycling (in soil and water), depends on the very presence of animals in the agroecosystems. Local livestock breeds commonly display higher levels of adaptability to local conditions as they have often co-evolved within specific production systems, sometimes in harsh and extreme conditions, and with limited inputs [24,47,48]. For each studied ecosystem service, we examine both the effect of the lack and the presence, at various temporal and spatial scales, of intraspecific diversity.
2. Methodology and Literature Characteristics
Building on the work of Hajjar and colleagues [10], which focussed on intraspecific crop diversity, this paper looks at the potential for crop, livestock, and aquatic genetic diversity to enhance specific ecosystem functions through diversity per se and adapted traits [10,49]. Starting with the counterfactual, we examined all examples of the relation between low intraspecific diversity in the production system and the studied ecosystem services. This is followed by a review of evidence where intraspecific diversity for crop, livestock, and aquatic organisms has played a role in providing and maintaining the ecosystem services considered.
This review is based on 202 publications from 1960 to 2024 found through abstract searches on AGRIS, CABI, CORE, and AGRICOLA, as well as a search on Google Scholar, Scopus, ScienceDirect, and Web of Science research portals. Keywords used for the search included the names of the different ecosystem services targeted as well as the words variet*, breed, intraspecific, genetic diversity, and genetic variability, filtered for the three sectors, crop, livestock, and aquaculture. It is important to note that when searching and filtering using the above keywords, publications that mention the characteristics of breeds, varieties, or farming types that enable them to thrive in particular environments, but without mentioning ecosystem services, were not selected. Publications from FAO and various development projects were also searched. From the very large number of publications obtained via the keyword search, a sub-selection was made by checking their titles, then abstracts, and then finally the full publications themselves. Thirty-eight of the publications searched helped develop the counterfactual. When examining the role of intraspecific genetic resources, 60% of the references clearly mentioned the intraspecific level while we had to search for additional publications demonstrating the presence of the intraspecific diversity in the given contexts for the remaining 40%.
In addition to the 202 publications, 69 references helped structure this paper and define the concepts. Table 1 presents the number of publications per sector and ecosystem service.

Table 1.
Number of publications on the role of each sector per studied ecosystem service.
Slightly more than half of the publications gathered were not attached to a specific geographical location but either treated ecosystem services and functions in a general or worldwide manner or were based on experiments with controlled environments. Table 2 summarizes these results with publications referring to specific World Regions or general regions experiments in controlled environments, or the global level. Note that some publications are reported in more than one World Region.

Table 2.
Distribution of articles per World Region or in the category “general, experiment, global”.
Journal articles represent 80% of the publications of this review. Other publications searched are books, book sections, technical papers, proceedings, and project and thesis reports.
Looking at the temporal distribution of the publications by sector and of publications explicitly mentioning the intraspecific level, presented in Figure 1, we see that the focus for publications on ecosystem services was first directed to the crop sector then slightly later to the livestock sector and then even more recently to the aquaculture sector.
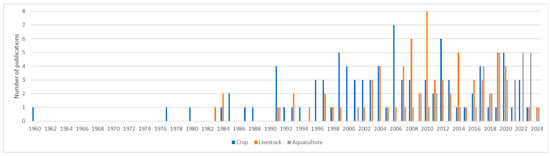
Figure 1.
Total number of publications per year per sector.
3. Intraspecific Genetic Resources of Crops, Livestock, and Aquaculture and Their Role in Supporting and Regulating Ecosystems
Table 3 presents the results of the literature review for the three sectors for each targeted ecosystem service. Counterfactual elements are also reported.

Table 3.
Intraspecific genetic resources of crop, livestock, and aquaculture and their role in supporting and regulating ecosystem services.
Based on the literature review conducted, we identified the main negative impacts of low intraspecific agricultural diversity and the potential roles of its presence in the production system. Positive effects of low diversity, such as the simplification of the work, are not reported and were not studied. Table 4 presents these results.

Table 4.
Effects in the production system of low intraspecific agricultural diversity and of its presence for the studied ecosystem services for regulation and maintenance.
From the literature review, we find that, when documented, low intraspecific diversity has a direct negative impact on ecosystem services, particularly when replaced with chemical agricultural inputs and unsustainable management practices. In turn, these chemical inputs and management practices have indirect negative effects on several ecosystem services such as pest and disease regulation or soil and water quality regulation. Moreover, because of the many interlinks between regulating and supporting ecosystem services, low diversity often has a negative domino effect.
Low intraspecific diversity affects the regulation of pest and disease in the agroecosystem: not only is the service lost or limited, but there is an increased use of agrochemicals; this in turn leads to the indirect effect of increased disease and pest resistance to chemicals, as well as a larger spread of epidemics and infestations because of genetic uniformity. For water and soil quality regulation, direct effects are similar with loss of service, but indirect effects take the form of soil and water pollution, the use of agrochemicals, and other unsustainable agriculture practices. Direct effects are similar for nutrient cycling with indirect effects being the disruption of nutrient cycles and their replacement by human interventions. In the case of pollination and seed dispersal, the direct effect of low intraspecific diversity is the loss of natural populations providing the ecosystem service and therefore of the service itself and the introduction of beehives or manual pollination practices as a replacement. Indirect effects are found in another type of ecosystem service: food provision. For buffering excess and lack of water, low diversity has mainly indirect impact with the degradation of the service in the form of more extreme water events or the establishment of water retention infrastructures such as dams and reservoirs. Similarly for soil erosion regulation, the indirect effects of low diversity are the degradation of the service with soil erosion and the increased use of heavy tillage and other non-conservation agriculture practices. Finally, the impact of low diversity on habitat maintenance is the cumulation of direct effects for the other regulating and supporting ecosystems services leading to poor habitat quality; indirect effects are habitat destruction or fragmentation because of poor soil management, extreme events, etc. This is coherent with the results gathered by Duval and colleagues [272] for the contribution of agrobiodiversity, from the ecosystem level to the intraspecific level, to the resilience of production systems.
This diversity also acts as an abatement factor for loss as an alternative to moderating or reducing the use of chemicals and medical treatments and, as such, has an impact on water and soil quality [119,273].
We also find that, while faunal ecosystem services—such as pollination, pest and disease control, and seed dispersal—are largely considered to be strictly provided by wildlife population, the potential of domesticated animals to contribute to these regulation services should be considered [50].
In aquaculture, genetic variability appears to be an asset sometimes resulting from an adaptation to environments [274]. Ecosystem-based approaches are key to the sustainable development of aquaculture with the inclusion of potentially regulating and supporting ecosystem services provided by these production systems [275].
Temporal and Spatial Scales
Spatial and temporal scales at which intraspecific diversity occurs and matters are closely linked to the characteristics of each sector and to the scales at which ecosystem services are produced.
The use of a single variety, breed, or farmed aquatic type as being deliberately chosen for adaptation to a specific environment is distinguished from the use of diversity per se as insurance to maintain regulating and supporting services under heterogeneous environments or changing environmental or social economic conditions. Adaptive traits in agricultural systems allow production for specific conditions at a given time. Diversity per se allows us to ensure productivity over time, have sustainable production, or have diversity to choose from in the future. Different farmers have different populations of the same crop variety [276]. Genetically diverse livestock populations provide the society with a greater range of options for meeting future challenges. They allow farmers and pastoralists to select stocks or develop new breeds in response to changing conditions, including climate change, new or resurgent disease threats, new knowledge of human nutritional requirements, and changing market conditions or changing societal needs. If they are lost, the options for future generations will be severely curtailed [24,277]. Many endangered breeds are found in harsh production systems and may possess unique genetic adaptations and disease-resistance characteristics [278]. Moreover, when focussing on adaptive traits, it is possible to have a limited number of breeds or varieties that have been selected for specific conditions, sometimes at a very small geographical scale in case of soil characteristics or uneven terrains used at small levels. Looking at larger spatial scales, landscape to agroecological zones, the diversity of all the selected breeds and varieties appears again as key for thriving in all the conditions included at these scales [279]. Another important element to note is that although overall genetic diversity might be high, functional diversity can be low if the various varieties and breeds all carry the same selected functional traits. A diversity of functional traits are important for farmers and livestock keepers to enable them to adapt to changing environmental and socio-economic conditions [5,280,281].
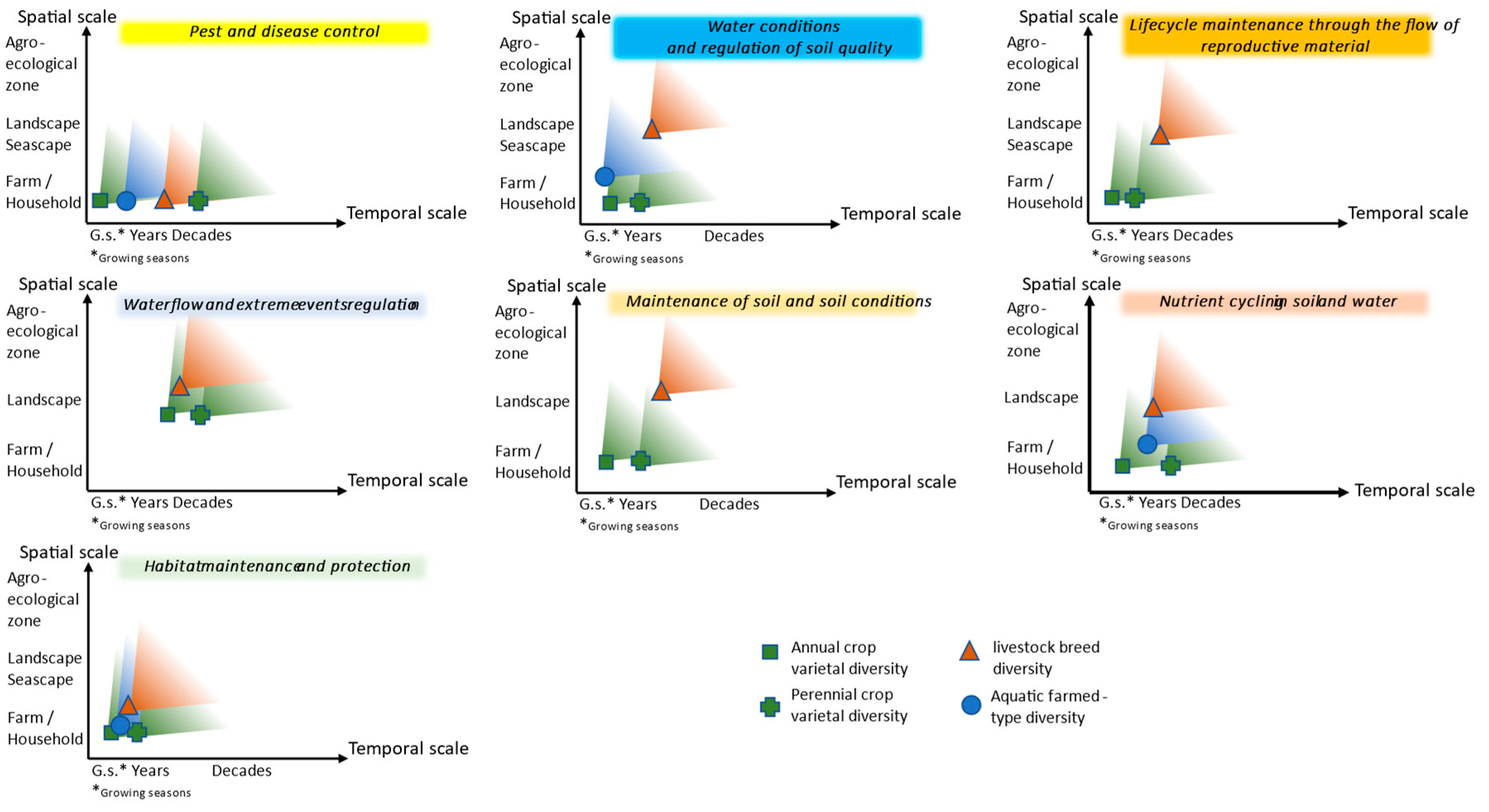
Based on the various publications collected during the literature review, Figure 2 presents a schematic view comparing the spatial and temporal “starting points” of crop, livestock, and aquaculture intraspecific diversity’s impact on provisioning and supporting ecosystem services. While the spatial aspects vary depending on the characteristics of the sector studied, the minimum temporal scales where diversity impacts these services are more closely linked to the operating timeframe of a given ecosystem service. Axes present the order of magnitude and do not pretend to propose precise values. Figure 2 is the result of the authors’ analysis of the many publications gathered.
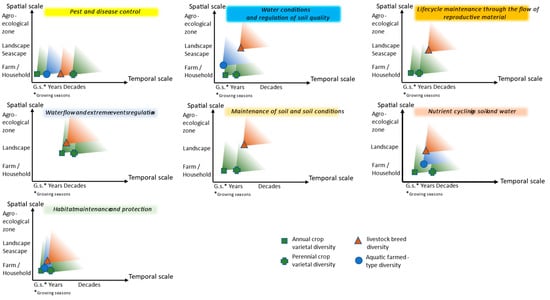
Figure 2.
Starting points from which diversity begins to have impact on the provision of ecosystem services.
For the spatial scale axis, we considered the farm or household level, the community and landscape or seascape, the agroecosystem level, and then wider with no reference. We refer to a local community as a social geographic group where people at a particular point in time have common interests and live in a defined geographical area, rural or urban, within a broader society [17]. For the temporal axis, we considered growing seasons, years, and decades as they are the timeframe for production and lifespan for the different sectors.
For pest and disease control, we found from the literature review that spatial scales where diversity matters are small, as for all sectors, intraspecific diversity at plot, pasture, or pond level means better tolerance or resistance to pests, diseases, and parasites. For livestock, the intraspecific diversity is found at small spatial scales in indigenous or locally adapted breeds kept by local communities. For these breeds, a wide array of traits and plasticity has been shown within and between breeds, indicating that livestock genetic diversity provides a range of options that are likely to be valuable in climate change adaptation, including tolerance of climatic extremes such as hot temperatures, adaptation to poor-quality diets or to feeding in harsh conditions, and resistance and tolerance to specific diseases [24]. Indigenous breeds thrive in harsh environments but generally do not have high productivity rates [282,283]. Temporal scales where intraspecific diversity is key for pest and disease control follow the temporal scales of the lifespan of the domesticated plants, animals, or aquatic organisms. Lifespan, reproductive maturity, and intervals between generations vary greatly for the different sectors and for species within a sector, from annual crops to perennial crops, from cattle to poultry, from bivalves to algae.
In the case of water conditions and regulation of soil quality, spatial scales are small for crops where multiple varieties can be adapted to a given plot and for aquaculture where healthy bivalves and algae are genetically diverse. Larger spatial scales are found for livestock where breeds are adapted to each specific condition where their controlled grazing contributes to the service. The diversity is “found” only when taking a step back. The relevant temporal scale is short for aquaculture with constant filtration and nutrient removal activities. It is also short for crops depending on growing seasons. The temporal scale depends on the lifespan of animals for livestock. For lifecycle maintenance through the flow of reproductive material, we found that for the crop sector, the plot scale and the growing season are relevant, and in the case of livestock, larger scales for dispersal and lifespan of animals are relevant. For the ecosystem service maintenance of soil and soil conditions, we found for the crop sector that the plot scale, where varieties will protect the soil, is the starting point and the temporal framework of annual and perennial crops. Larger spatial scales are relevant for livestock breeds with their grazing ability and, again, the lifespans of animals. In the case of nutrient cycling in soil and water, plot levels and growing seasons are essential for crops. Livestock breeds’ presence and adaptedness across landscapes is required, and the time scale follows the lifespan or production period. As for aquaculture, we have a small spatial scale and production period. Habitat maintenance and protection are dependent on all the other regulation services, and the smallest and shortest scales are key for this service.
Selection, in all sectors, to obtain higher productivity brings light on the trade-offs between provisioning ecosystem services and regulation services [51,284]. Indigenous or local varieties, breeds, or farmed types that thrive better than selected ones in many harsh and changing environments will not have high production rates [201,282,283]. Management of genetically diverse rather than uniform systems can require more decision choices, time, and adapted processing equipment, for farmers and livestock keepers in the short term. However, managing diversity has the potential to maximize both current and future productivity and reduce the potential to future loss due to unsustainable management practices [285].
Because farmers and pastoralists must deal with obstacles and risks all along the production or growing season and from one season to the other, the temporal scale at which diversity occurs and operates will determine the use they make of it in their strategy for risk management. The spatial and temporal scales at which regulating and supporting ecosystem services are operating require the presence over these scales of the various service providers among which intraspecific genetic diversity in each sector when this diversity is contributing to the provision or maintenance of these services. Annual and perennial crops, livestock, and aquatic intraspecific diversity present different characteristics that are reflected in the scales from which they are first detected or the “starting point” when they have an impact on the provision of regulating and supporting ecosystem services. If the impact is detected at a small spatial or temporal scale, this is the “starting point” where it will continue to have impact at larger spatial and temporal scales.
In the case of annual crops, the household level, as well as short temporal scales such as growing seasons, is often crucial. For perennial crops, the landscape level is more present and important temporal scales will be years and decades. Livestock breed diversity matters at landscape level and longer temporal scales because of its spatial distribution and the lifespans of animals. Less information was gathered for aquatic farmed diversity, and therefore, the scales identified are more difficult to analyse although we know there are high levels of functional diversity at small scales. Overall, the contribution to regulating pests and diseases starts at small spatial and short temporal scales, while supporting habitat maintenance starts at larger and longer scales. Other studied ecosystem services are spread between those two extremes.
The different levels of genetic diversity within farmed crop varieties, livestock breeds, and aquatic populations are related to the length of the different sectors’ history of domestication and the lifespan of the entity considered. This is directly related to levels of genetic diversity available in improved varieties, breeds, and aquatic farmed populations. This timeline has meant that modern breeding has caused much higher levels of genetic erosion in crops than in livestock than, in turn, in aquatic farmed populations. Plants are not mobile themselves but their genetic material is; breeds are usually less diverse at the farm level, but they are mobile and can move from pasture to pasture, sometimes over a wide distance as potentially in transhumant or nomadic systems. This is also reflected in Figure 2.
4. Conclusions
The detrimental effects of low intraspecific diversity in all sectors (crop, livestock, and aquaculture) on supporting and regulating ecosystem services, on agricultural productivity, and on the overall ecosystem health are visible in the counterfactual information provided. Intraspecific diversity provides both direct and indirect benefits to agricultural productivity and ecosystem health. Diversity allows avoiding resorting to large quantities of chemical inputs and to unsustainable agronomic or zootechnical practices. This, in turn, limits the negative impact of the use of these inputs and practices and their damaging side effects on livelihoods, ecosystem functioning, and in the long-term on productivity gains. Both the diversity, per se, within a species and a species’ adaptive traits are crucial to deliver these benefits today and in the future, particularly in the context of climate change. Reaping these benefits, however, requires access to diversity and knowledge, intellectual investment in planning and organisation, and appropriate agricultural tools and policies supporting the use of agricultural diversity.
Temporal and spatial scales differ for the provision of the different ecosystem services by intraspecific diversity. Provisional ecosystem services in the form of the production of food and non-food products tend to have relatively short time scales, while supporting and regulating ecosystem services often have longer-term processes. Supporting and regulating services rely not only on the availability of the current diversity, but also the continued presence of diversity over time. Temporal and spatial scales for the provision of ecosystem services also differ according to sector. At landscape level, genetic diversity is found both between and within crop varieties. In contrast, for a given livestock species, genetic diversity is more likely found within a breed or between a small number of breeds rather than a community of farmers or pastoralists keeping animals of numerous distinct breeds. Diversity measured by the number of varieties compared to the number of breeds is likely to be higher for crops than for livestock at the farm, community, and landscape scales. In contrast, the levels of functional genetic diversity, for some selected traits, can be similar for crops and livestock (such as disease tolerance traits).
Agricultural production systems should be seen as holistic, using a comprehensive lens that includes the role of intraspecific diversity of crop, livestock, and aquaculture species as an integral part of the regulating and provisioning services they provide. While this approach needs to be cross-sectoral and interdisciplinary, differences across sectors in structure, functionality, and temporal and spatial scales must be taken into account. Neglecting the additional role that intraspecific crop, livestock, and aquaculture diversity has in providing regulating and supporting ecosystem services is detrimental to agricultural productivity for sustainable agriculture with lower agricultural inputs and landscape resilience. In order to better understand and benefit from agricultural biodiversity, data should be collected more systematically, enabling any correlations to be identified between crop varieties, livestock breeds, aquatic farmed types, the management practices adopted by farmers, livestock keepers, and ecological parameters.
Author Contributions
Conceptualization, D.I.J. and L.C.; methodology, A.B.-F., D.I.J. and L.C.; validation, A.B.-F., D.I.J. and F.A.; formal analysis, A.B.-F., D.I.J., B.S., L.S., Y.Z., F.A. and L.C.; investigation, A.B.-F., D.I.J., B.S., L.S. and Y.Z.; writing—original draft preparation, A.B.-F., D.I.J., B.S., L.S. and Y.Z.; writing—review and editing, A.B.-F., D.I.J. and F.A.; visualization, A.B.-F. and D.I.J.; supervision, D.I.J., F.A. and L.C.; funding acquisition, D.I.J. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the United Nations Food and Agricultural Organization (FAO), the United Nations Environment Programme (UNEP), the Global Environmental Facility (GEF), and the Chinese Academy of Agricultural Sciences (CAAS)—CGIAR Research Fellowship program.
Acknowledgments
We are grateful for the discussions with scientists affiliated with the FAO Commission of Genetic Resources for Food and Agriculture, including Damiano Luchetti and Devin Bartley, with Toby Hodgkin, Platform for Agrobiodiversity Research, and with scientists from Bioversity International, including Adam Drucker, Paola De Santis, and Carlo Fadda that led to a more complete cross-sectoral review of the available literature. We thank Loredana Maria, Elisabetta Rossetti, and Nicole Demers for their administrative support.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Barker, J.S.F. Conservation and management of genetic diversity: A domestic animal perspective. Can. J. For. Res. 2001, 31, 588–595. [Google Scholar] [CrossRef]
- FAO. The Second Report on the State of the World’s Plant Genetic Resources for Food and Agriculture; FAO: Rome, Italy, 2010. [Google Scholar]
- FAO. The State of World Fisheries and Aquaculture; FAO: Rome, Italy, 2010. [Google Scholar]
- FAO. The State of the Word’s Animal Genetic Resources for Food and Agriculture; FAO: Rome, Italy, 2007. [Google Scholar]
- Jarvis, D.I.; Hodgkin, T.; Brown, A.H.D.; Tuxill, J.; Noriega, I.; Smale, M.; Sthapit, B. Crop Genetic Diversity in the Field and on the Farm: Principles and Applications in Research Practices; Yale University Press: New Haven, NY, USA; London, UK, 2016. [Google Scholar] [CrossRef]
- Köhler-Rollefson, I.; Mathias, E. Animating diversity: Supporting endogenous development of livestock keepers. Development 2010, 53, 425–428. [Google Scholar] [CrossRef]
- Köhler-Rollefson, I. Indigenous practices of animal genetic resource management and their relevance for the conservation of domestic animal diversity in developing countries. J. Anim. Breed. Genet. 1997, 114, 231–238. [Google Scholar] [CrossRef]
- Lind, C.; Ponzoni, R.; Nguyen, N.; Khaw, H. Selective Breeding in Fish and Conservation of Genetic Resources for Aquaculture. Reprod. Domest. Anim. 2012, 47, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, G.; van Duijvendijk, C.; Collette, L. Biodiversity for Food and Agriculture. Contributing to Food Security and Sustainability in a Changing World; PAR Platforml; FAO: Rome, Italy, 2011. [Google Scholar]
- Hajjar, R.; Jarvis, D.I.; Gemmill-Herren, B. The utility of crop genetic diversity in maintaining ecosystem services. Agric. Ecosyst. Environ. 2008, 123, 261–270. [Google Scholar] [CrossRef]
- Halwart, M.; Bartley, D.M. Aquatic Biodiversity in Rice-Based Ecosystems. In Managing Biodiversity in Agricultural Ecosystems; Jarvis, D., Padoch, C., Cooper, H.D., Eds.; Columbia University Press: New York, NY, USA; Chichester, UK; West Sussex, UK, 2013; pp. 181–199. [Google Scholar] [CrossRef]
- Lazard, J.; Baruthio, A.; Mathé, S.; Rey-Valette, H.; Chia, E.; Clément, O.; Aubin, J.; Morissens, P.; Mikolasek, O.; Legendre, M.; et al. Aquaculture system diversity and sustainable development: Fish farms and their representation. Aquat. Living Resour. 2010, 23, 187–198. [Google Scholar] [CrossRef]
- IAASTD. Agriculture at a Crossroads—Synthesis Report; IAASTD: Washington, DC, USA, 2009. [Google Scholar]
- Ricciardi, V.; Ramankutty, N.; Mehrabi, Z.; Jarvis, L.; Chookolingo, B. How much of the world’s food do smallholders produce? Glob. Food Sec. 2018, 17, 64–72. [Google Scholar] [CrossRef]
- Ritchie, H. Smallholders Produce One-Third of the World’s Food, Less Than Half of What Many Headlines Claim—Our World in Data [WWW Document]. 2021. Available online: https://ourworldindata.org/smallholder-food-production (accessed on 31 May 2022).
- Miller, A.J.; Gross, B.L. From forest to field: Perennial fruit crop domestication. Am. J. Bot. 2011, 98, 1389–1414. [Google Scholar] [CrossRef] [PubMed]
- FAO. Community-Based Management of Animal and Genetic Resources; FAO: Rome, Italy, 2003. [Google Scholar]
- Scherf, B. (Ed.) World Watch List for Domestic Animal Diversity, 3rd ed.; FAO: Rome, Italy, 2000. [Google Scholar] [CrossRef]
- Taberlet, P.; Valentini, A.; Rezaei, H.R.; Naderi, S.; Pompanon, F.; Negrini, R.; Ajmone-Marsan, P. Are cattle, sheep, and goats endangered species? Mol. Ecol. 2008, 17, 275–284. [Google Scholar] [CrossRef]
- Gjedrem, T.; Baranski, M. Domestication and the Application of Genetic Improvement in Aquaculture. In Selective Breeding in Aquaculture; Springer: Dordrecht, The Netherlands, 2009; pp. 5–11. [Google Scholar] [CrossRef]
- Houston, R.D.; Bean, T.P.; Macqueen, D.J.; Gundappa, M.K.; Jin, Y.H.; Jenkins, T.L.; Selly, S.L.C.; Martin, S.A.M.; Stevens, J.R.; Santos, E.M.; et al. Harnessing genomics to fast-track genetic improvement in aquaculture. Nat. Rev. Genet. 2020, 21, 389–409. [Google Scholar] [CrossRef]
- Li, C. Conservation of genetic resources for sustainable aquaculture. J. World Aquac. Soc. 2022, 53, 4–7. [Google Scholar] [CrossRef]
- Pullin, R.S.V.; Froese, R.; Pauly, D. Indicators for the Sustainability of Aquaculture. In Ecological and Genetic Implications of Aquaculture Activities. Methods and Technologies in Fish Biology and Fisheries; Bert, T.M., Ed.; Springer: Dordrecht, The Netherlands, 2007; pp. 53–72. [Google Scholar] [CrossRef]
- Hoffmann, I.; From, T.; Boerma, D. Ecosystem Services Provided by Livestock Species and Breeds, with Special Consideration to the Contributions of Small-Scale Livestock Keepers and Pastoralists; BSP CGRFA: Rome, Italy, 2014. [Google Scholar]
- MEA. Millennium Ecosystem Assessment—Ecosystems and Human Well-Being: Synthesis, Ecosystems; MEA: Washington, DC, USA, 2005. [Google Scholar] [CrossRef]
- Pérez-Soba, M.; Elbersen, B.; Kempen, M.; Braat, L.; Staristky, I.; van Wijngaart, R.; Kaphengst, T.; Andersen, E.; Germer, L.; Smith, L. Study on the role of agriculture as provisioning ecosystem service. In Interim Report to the Institute for Environment and Sustainability (JRC/IES); Alterra Wageningen UR: Wageningen, The Netherlands; Ecologic Institute: Berlin, Germany; University of Copenhagen: Copenhagen, Denmark; EuroCARE: Saint Ives, UK, 2012. [Google Scholar]
- Zhang, W.; Ricketts, T.H.; Kremen, C.; Carney, K.; Swinton, S.M. Ecosystem services and dis-services to agriculture. Ecol. Econ. 2007, 64, 253–260. [Google Scholar] [CrossRef]
- Bellon, M.R.; Pham, J.L.; Jackson, M.T. Genetic conservation: A role for rice farmers. In Plant Conservation: The In Situ Approach; Hawkes, J.G., Ed.; Chapman & Hall: London, UK, 1997. [Google Scholar]
- Brush, S.; Kesselli, R.; Ortega, R.; Cisneros, P.; Zimmerer, K.; Quiros, C. Potato diversity in the Andean center of crop domestication. Conserv. Biol. 1995, 9, 1189–1198. [Google Scholar] [CrossRef]
- Brush, S. Farmers’ Bounty: Locating Crop Diversity in the Contemporary World; Yale University Press: New Haven, NY, USA, 2004. [Google Scholar]
- Ficiciyan, A.; Loos, J.; Sievers-Glotzbach, S.; Tscharntke, T. More than Yield: Ecosystem Services of Traditional versus Modern Crop Varieties Revisited. Sustainability 2018, 10, 2834. [Google Scholar] [CrossRef]
- Jarvis, D.I.; Hodgkin, T.; Sthapit, B.R.; Fadda, C.; Lopez-Noriega, I. An Heuristic Framework for Identifying Multiple Ways of Supporting the Conservation and Use of Traditional Crop Varieties within the Agricultural Production System. Crit. Rev. Plant Sci. 2011, 30, 125–176. [Google Scholar] [CrossRef]
- Hall, S.J.G. Livestock biodiversity as interface between people, landscapes and nature. People Nat. 2019, 1, 284–290. [Google Scholar] [CrossRef]
- Marsoner, T.; Egarter Vigl, L.; Manck, F.; Jaritz, G.; Tappeiner, U.; Tasser, E. Indigenous livestock breeds as indicators for cultural ecosystem services: A spatial analysis within the Alpine Space. Ecol. Indic. 2018, 94, 55–63. [Google Scholar] [CrossRef]
- Nabban, G.P. Cultures of Habitat: On Nature, Culture, and Story; Counterpoint, Perseus Book Group: Washington, DC, USA, 1989. [Google Scholar]
- Pretty, J. Agricultural sustainability: Concepts, principles and evidence. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2008, 363, 447–465. [Google Scholar] [CrossRef]
- Pelicice, F.M.; Agostinho, A.A.; Azevedo-Santos, V.M.; Bessa, E.; Casatti, L.; Garrone-Neto, D.; Gomes, L.C.; Pavanelli, C.S.; Petry, A.C.; dos Santos Pompeu, P.; et al. Ecosystem services generated by Neotropical freshwater fishes. Hydrobiologia 2022, 850, 2903–2926. [Google Scholar] [CrossRef]
- Rana, R.B.; Garforth, C.; Sthapit, B.R. Farmers’ management of rice varietal diversity in the mid hills of Nepal: Implications for on-farm conservation and crop improvement. Plant Genet. Resour. Charact. Utiliz. 2008, 28, 1–14. [Google Scholar] [CrossRef]
- Tuxill, J.; Reyes, L.A.; Latournerie, L.; Cob, V.; Jarvis, D.I. All maize is not equal: Maize variety choices and mayan foodways in rural Yucatan, Mexico. In Pre-Columbian Foodways: Interdisciplinary Approaches to Food, Culture and Markets in Mesoamerica; Staller, J.E., Carrasco, M.D., Eds.; Springer: New York, NY, USA, 2009; pp. 467–486. [Google Scholar] [CrossRef]
- Velado-Alonso, E.; Gómez-Sal, A.; Bernués, A.; Martín-Collado, D. Disentangling the Multidimensional Relationship between Livestock Breeds and Ecosystem Services. Animals 2021, 11, 2548. [Google Scholar] [CrossRef] [PubMed]
- Brush, S. The issues of in situ conservation of crop genetic resources. In Genes in the Field; CRC Press, LLC: Boca Raton, FL, USA, 2000. [Google Scholar]
- Drucker, A.G.; Gomez, V.; Anderson, S. The economic valuation of farm animal genetic resources: A survey of available methods. Ecol. Econ. 2001, 36, 1–18. [Google Scholar] [CrossRef]
- Hoffmann, I. Climate change and the characterization, breeding and conservation of animal genetic resources. Anim. Genet. 2010, 41, 32–46. [Google Scholar] [CrossRef] [PubMed]
- Smale, M.; Bellon, M.R.; Jarvis, D.; Sthapit, B. Economic concepts for designing policies to conserve crop genetic resources on farms. Genet. Resour. Crop Evol. 2004, 51, 121–135. [Google Scholar] [CrossRef]
- Haines-Young, R.H.; Potschin, M.B. Common International Classification of Ecosystem Services (CICES) V5.1 and Guidance on the Application of the Revised Structure. Fabis Consulting Ltd., 2018. Available online: https://cices.eu/content/uploads/sites/8/2018/01/Guidance-V51-01012018.pdf (accessed on 24 February 2024). (In English).
- Czúcz, B.; Arany, I.; Potschin-Young, M.; Bereczki, K.; Kertész, M.; Kiss, M.; Aszalós, R.; Haines-Young, R. Where concepts meet the real world: A systematic review of ecosystem service indicators and their classification using CICES. Ecosyst. Serv. 2018, 29, 145–157. [Google Scholar] [CrossRef]
- Leroy, G.; Baumung, R.; Boettcher, P.; Besbes, B.; From, T.; Hoffmann, I. Animal genetic resources diversity and ecosystem services. Glob. Food Secur. 2018, 17, 84–91. [Google Scholar] [CrossRef]
- Naskar, S.; Gowane, G.R.; Chopra, A. Strategies to improve livestock genetic resources to counter climate change impact. In Climate Change Impact on Livestock: Adaptation and Mitigation; Springer: New Delhi, India, 2015; pp. 441–475. [Google Scholar] [CrossRef]
- Mulder, C.P.H.; Uliassi, D.D.; Doak, D.F. Physical stress and diversity-productivity relationships: The role of positive interactions. Proc. Natl. Acad. Sci. USA 2001, 98, 6704–6708. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Arellano, C.; Mulligan, M. A review of regulation ecosystem services and disservices from faunal populations and potential impacts of agriculturalisation on their provision, globally. Nat. Conserv. 2018, 30, 1–39. [Google Scholar] [CrossRef]
- Mengist, W.; Soromessa, T.; Feyisa, G.L. A global view of regulatory ecosystem services: Existed knowledge, trends, and research gaps. Ecol. Process. 2020, 9, 40. [Google Scholar] [CrossRef]
- Schowalter, T.D.; Noriega, J.A.; Tscharntke, T. Insect effects on ecosystem services—Introduction. Basic. Appl. Ecol. 2018, 26, 1–7. [Google Scholar] [CrossRef]
- Marshall, D.R. The advantages and hazards of genetic homogeneity. Ann. N. Y Acad. Sci. 1977, 287, 1–20. [Google Scholar] [CrossRef]
- Mitchell, C.E.; Tilman, D.; Groth, J.V. Effects of grassland species diversity, abundance, and composition on foliar fungal disease. Ecology 2002, 83, 1713–1726. [Google Scholar] [CrossRef]
- Schumann, G.L. Plant Diseases: Their Biology and Social Impact; APS Press, American Phytopathological Society: St. Paul, MN, USA, 1991. [Google Scholar]
- Allen, A.; da Silva, N.; Corubolo, E. Environmental Problems and Opportunities of the Peri-Urban Interface and Their Impact upon the Poor; Environ. Urban; UCL: London, UK, 1999. [Google Scholar]
- Calonnec, A.; Goyeau, H.; de Vallavieille-Pope, C. Effects of induced resistance on infection efficiency and sporulation of Puccinia striiformis on seedlings in varietal mixtures and on field epidemics in pure stands. Eur. J. Plant Pathol. 1996, 102, 733–741. [Google Scholar] [CrossRef]
- Cantelo, W.W.; Sanford, L.L. Insect population response to mixed and uniform planting of resistant and susceptible plant material. Environ. Entomol. 1984, 13, 1443–1445. [Google Scholar] [CrossRef]
- Dawson, J.; Goldringer, I. Breeding for genetically diverse populations. In Organic Crop Breeding; van Bueren, E.T.L., Myers, J.R., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2012; pp. 77–98. [Google Scholar] [CrossRef]
- Didelot, F.; Brun, L.; Parisi, L. Effects of cultivar mixtures on scab control in apple orchards. Plant Pathol. 2007, 56, 1014–1022. [Google Scholar] [CrossRef]
- Döring, T.F.; Knapp, S.; Kovacs, G.; Murphy, K.; Wolfe, M.S. Evolutionary plant breeding in cereals—Into a new era. Sustainability 2011, 3, 1944–1971. [Google Scholar] [CrossRef]
- Finckh, M.R.; Mundt, C.C. Plant competition and disease in genetically diverse wheat populations. Oecologia 1992, 91, 82–92. [Google Scholar] [CrossRef]
- Holt, J.; Chancellor, T.C.B. Modelling the spatio-temporal deployment of resistant varieties to reduce the incidence of rice tungro disease in a dynamic cropping system. Plant Pathol. 1999, 48, 453–461. [Google Scholar] [CrossRef]
- Lannou, C.; Mundt, C.C. Evolution of a pathogen population in host mixtures: Rate of emergence of complex races. Theor. Appl. Genet. 1997, 94, 991–999. [Google Scholar] [CrossRef]
- Matson, P.A.; Parton, W.J.; Power, A.G.; Swift, M.J. Agricultural Intensification and Ecosystem Properties. Science (1979) 1997, 277, 504–509. [Google Scholar] [CrossRef]
- Morand, S.; Guégan, J.-F. How the biodiversity sciences may aid biological tools and ecological engineering to assess the impact of climatic changes. Rev. Sci. Tech. 2008, 27, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Mulumba, J.W.; Nankya, R.; Adokorach, J.; Kiwuka, C.; Fadda, C.; De Santis, P.; Jarvis, D.I. A risk-minimizing argument for traditional crop varietal diversity use to reduce pest and disease damage in agricultural ecosystems of Uganda. Agric. Ecosyst. Environ. 2012, 157, 70–86. [Google Scholar] [CrossRef]
- Schläpfer, F.; Schmid, B. Ecosystem effects of biodiversity: A classification of hypotheses and exploration of empirical results. Ecol. Appl. 1999, 9, 893–912. [Google Scholar] [CrossRef]
- Teshome, A.; Brown, A.H.D.; Hodgkin, T. Diversity in Landraces of Cereal and Legume Crops. In Plant Breeding Reviews; John Wiley & Sons, Inc.: Oxford, UK, 2010; pp. 221–261. [Google Scholar] [CrossRef]
- Tooker, J.F.; Frank, S.D. Genotypically diverse cultivar mixtures for insect pest management and increased crop yields. J. Appl. Ecol. 2012, 49, 974–985. [Google Scholar] [CrossRef]
- Wolfe, M.S. The Current Status and Prospects of Multiline Cultivars and Variety Mixtures for Disease Resistance. Annu. Rev. Phytopathol. 1985, 23, 251–273. [Google Scholar] [CrossRef]
- Wolfe, M.S.; Finckh, M.R. Diversity of host resistance within the crop: Effects on host, pathogen and disease. In Plant Resistance to Fungal Diseases; Hartleb, H., Heitefuss, R., Hoppe, H.H., Eds.; G. Fischer Verlag: Jena, Germany, 1997; pp. 378–400. [Google Scholar]
- Zhu, Y.; Chen, H.; Fan, J.; Wang, Y.; Li, Y.; Chen, J.; Fan, J.; Yang, S.; Hu, L.; Leung, H.; et al. Genetic diversity and disease control in rice. Nature 2000, 406, 718–722. [Google Scholar] [CrossRef]
- Mourkas, E.; Taylor, A.J.; Méric, G.; Bayliss, S.C.; Pascoe, B.; Mageiros, L.; Calland, J.K.; Hitchings, M.D.; Ridley, A.; Vidal, A.; et al. Agricultural intensification and the evolution of host specialism in the enteric pathogen Campylobacter jejuni. Proc. Natl. Acad. Sci. USA 2020, 117, 11018–11028. [Google Scholar] [CrossRef] [PubMed]
- Springbett, A.J.; MacKenzie, K.; Woolliams, J.A.; Bishop, S.C. The Contribution of Genetic Diversity to the Spread of Infectious Diseases in Livestock Populations. Genetics 2003, 165, 1465–1474. [Google Scholar] [CrossRef]
- Baker, R.L. Genetic resistance to endoparasites in sheep and goats. A review of genetic resistance to gastrointestinal nematode parasites in sheep and goats in the tropics and evidence for resistance in some sheep and goat breeds in sub-humid coastal Kenya. Anim. Genet. Resour. Inf. 1998, 24, 13–30. [Google Scholar] [CrossRef]
- Bishop, S.C.; Chesnais, J.; Stear, M.J. Breeding for disease resistance: Issues and opportunities. In Proceedings of the 7th World Congress on Genetics Applied to Livestock Production, Montpellier, France, 19–23 August 2002. [Google Scholar]
- Bowles, D.; Carson, A.; Isaac, P. Genetic Distinctiveness of the Herdwick Sheep Breed and Two Other Locally Adapted Hill Breeds of the UK. PLoS ONE 2014, 9, e87823. [Google Scholar] [CrossRef]
- Claxton, J.; Leperre, P. Parasite burdens and host susceptibility of Zebu and N’Dama cattle in village herds in Gambia. Vet. Parasitol. 1991, 40, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Emery, D.L.; Stewart, D.J.; Clark, B.L. The comparative susceptibility of five breeds of sheep to foot-rot. Aust. Vet. J. 1984, 61, 85–88. [Google Scholar] [CrossRef]
- FAO. Animal genetic resources and resistance to disease. In State of the World Animal Genetic Resources for Food and Agriculture; FAO: Rome, Italy, 2007; pp. 101–112. [Google Scholar]
- FAO. The Second Report on the State of the World’s Animal Genetic Resources for Food and Agriculture—Key Findings; FAO: Rome, Italy, 2015. [Google Scholar] [CrossRef]
- Gauly, M.; Bollwein, H.; Breves, G.; Brügemann, K.; Dänicke, S.; Daş, G.; Demeler, J.; Hansen, H.; Isselstein, J.; König, S.; et al. Future consequences and challenges for dairy cow production systems arising from climate change in Central Europe—A review. Animal 2013, 7, 843–859. [Google Scholar] [CrossRef] [PubMed]
- Glass, E.J.; Preston, P.M.; Springbett, A.; Craigmile, S.; Kirvar, E.; Wilkie, G.; Brown, C.G.D. Bos taurus and Bos indicus (Sahiwal) calves respond differently to infection with Theileria annulata and produce markedly different levels of acute phase proteins. Int. J. Parasitol. 2005, 35, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.K.; Afify, M.A.; Aly, M.M. Genetic Resistance of Egyptian Chickens to Infectious Bursal Disease and Newcastle Disease. Trop. Anim. Health Prod. 2004, 36, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hogerwerf, L.; Wallace, R.G.; Ottaviani, D.; Slingenbergh, J.; Prosser, D.; Bergmann, L.; Gilbert, M. Persistence of Highly Pathogenic Avian Influenza H5N1 Virus Defined by Agro-Ecological Niche. Ecohealth 2010, 7, 213–225. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kim, S.J.; Ka, S.; Ha, J.W.; Kim, J.; Yoo, D.A.; Kim, K.; Lee, H.K.; Lim, D.; Cho, S.; Hanotte, O.; et al. Cattle genome-wide analysis reveals genetic signatures in trypanotolerant N’Dama. BMC Genom. 2017, 18, 371. [Google Scholar] [CrossRef] [PubMed]
- Lauvie, A.; Alexandre, G.; Angeon, V.; Couix, N.; Fontaine, O.; Gaillard, C.; Meuret, M.; Mougenot, C.; Moulin, C.H.; Naves, M.; et al. Is the ecosystem services concept relevant to capture the multiple benefits from farming systems using livestock biodiversity? A framework proposal. Genet. Resour. 2023, 4, 15–28. [Google Scholar] [CrossRef]
- Marcos-Carcavilla, A.; Calvo, J.H.; González, C.; Moazami-Goudarzi, K.; Laurent, P.; Bertaud, M.; Hayes, H.; Beattie, A.E.; Serrano, C.; Lyahyai, J.; et al. Structural and functional analysis of the HSP90AA1 gene: Distribution of polymorphisms among sheep with different responses to scrapie. Cell Stress Chaperones 2008, 13, 19–29. [Google Scholar] [CrossRef]
- Mattioli, R.C.; Bah, M.; Faye, J.; Kora, S.; Cassama, M. A comparison of field tick infestation on N’Dama, Zebu and N’Dama×Zebu crossbred cattle. Vet. Parasitol. 1993, 47, 139–148. [Google Scholar] [CrossRef]
- Mattioli, R.C.; Bah, M.; Kora, S.; Cassama, M.; Clifford, D.J. Susceptibility to different tick genera in Gambian N’Dama and Gobra zebu cattle exposed to naturally occurring tick infestations. Trop. Anim. Health Prod. 1995, 27, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Meyer, B. Egyptian Chicken Plan Hatches … 50 Years Later [WWW Document]. The Iowa Stater. 1997. Available online: https://web.archive.org/web/19971122083922/https://www.iastate.edu/IaStater/1997/may/chicken.html (accessed on 29 July 2021).
- Minga, U.M.; Msoffe, P.L.; Gwakisa, P.S. Biodiversity (Variation) in Disease Resistance and in Pathogens Within Rural Chicken Populations. In Proceedings of the International Health Network for Family Poultry (INFD), World Poultry Congress, Istanbul, Turkey, 8–13 June 2004. [Google Scholar]
- Murray, M.; Trail, J.C.M.; Davis, C.E.; Black, S.J. Genetic Resistance to African Trypanosomiasis. J. Infect. Dis. 1984, 149, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Ngowi, E.E.; Chenyambuga, S.W.; Gwakisa, P.S. Socio-economic values and traditional management practices of Tarime zebu cattle in Tanzania. Livest. Res. Rural. Dev. 2008, 20, 94. [Google Scholar]
- Paling, R.W.; Dwinger, R.H. Potential of trypanotolerance as a contribution to sustainable livestock production in tsetse affected Africa. Vet. Q. 1993, 15, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.A.; Estuningsih, E.; Widjayanti, S.; Wiedosari, E.; Partoutomo, S.; Spithill, T.W. Resistance of Indonesian thin tail sheep against Fasciola gigantica and F. hepatica. Vet. Parasitol. 1997, 68, 69–78. [Google Scholar] [CrossRef]
- Schilling, M.A.; Memari, S.; Cavanaugh, M.; Katani, R.; Deist, M.S.; Radzio-Basu, J.; Lamont, S.J.; Buza, J.J.; Kapur, V. Conserved, breed-dependent, and subline-dependent innate immune responses of Fayoumi and Leghorn chicken embryos to Newcastle disease virus infection. Sci. Rep. 2019, 9, 7209. [Google Scholar] [CrossRef]
- Sonaiya, E.B.; Branckaert, R.D.S.; Guèye, E.F. Research and development options for family poultry. Introductory paper. In Proceedings of the First International Network for Family Poultry Development/Food and Agriculture Organization of the United NationsElectronic Conference on Family Poultry, 7 December 1998–5 March 1999; Available online: https://www.fao.org/4/y5169e/y5169e0b.htm (accessed on 15 July 2024).
- Twomey, A.J.; Graham, D.A.; Doherty, M.L.; Blom, A.; Berry, D.P. Little genetic variability in resilience among cattle exists for a range of performance traits across herds in Ireland differing in Fasciola hepatica prevalence. J. Anim. Sci 2018, 96, 2099–2112. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, R.C.; Santos, M.; Bernardino, G.; Hrbek, T.; Farias, I.P. From river to farm: An evaluation of genetic diversity in wild and aquaculture stocks of Brycon amazonicus (Spix & Agassiz, 1829), Characidae, Bryconinae. Hydrobiologia 2018, 805, 75–88. [Google Scholar] [CrossRef]
- Grebe, G.S.; Byron, C.J.; Gelais, A.S.; Kotowicz, D.M.; Olson, T.K. An ecosystem approach to kelp aquaculture in the Americas and Europe. Aquac. Rep. 2019, 15, 100215. [Google Scholar] [CrossRef]
- Kennedy, D.A.; Kurath, G.; Brito, I.L.; Purcell, M.K.; Read, A.F.; Winton, J.R.; Wargo, A.R. Potential drivers of virulence evolution in aquaculture. Evol. Appl. 2016, 9, 344–354. [Google Scholar] [CrossRef]
- Kim, J.K.; Yarish, C.; Hwang, E.K.; Park, M.; Kim, Y. Seaweed aquaculture: Cultivation technologies, challenges and its ecosystem services. Algae 2017, 32, 1–13. [Google Scholar] [CrossRef]
- Agha, R.; Gross, A.; Rohrlack, T.; Wolinska, J. Adaptation of a chytrid parasite to its cyanobacterial host is hampered by host intraspecific diversity. Front. Microbiol. 2018, 9, 921. [Google Scholar] [CrossRef] [PubMed]
- Altermatt, F.; Ebert, D. Genetic diversity of Daphnia magna populations enhances resistance to parasites. Ecol. Lett. 2008, 11, 918–928. [Google Scholar] [CrossRef] [PubMed]
- Colsoul, B.; Boudry, P.; Pérez-Parall, L.; Bratos Cetini, A.; Hugh-Jones, T.; Arzul, I.; Mérou, N.; Wegner, K.M.; Peter, C.; Merk, V.; et al. Sustainable large-scale production of European flat oyster (Ostrea edulis) seed for ecological restoration and aquaculture: A review. Rev. Aquac. 2021, 13, 1423–1468. [Google Scholar] [CrossRef]
- Conejeros, P.A.; Calderón, C.; Gómez, D.; Nilo, L.; Marshall, S.H. High immune diversity in farmed Atlantic salmon (Salmo salar L.). Aquac. Int. 2011, 19, 999–1005. [Google Scholar] [CrossRef]
- Pogoda, B.; Brown, J.; Hancock, B.; Preston, J.; Pouvreau, S.; Kamermans, P.; Sanderson, W.; von Nordheim, H. The Native Oyster Restoration Alliance (NORA) and the Berlin Oyster Recommendation: Bringing back a key ecosystem engineer by developing and supporting best practice in Europe. Aquat. Living Resour. 2019, 32, 13. [Google Scholar] [CrossRef]
- Bünemann, E.K.; Bongiorno, G.; Bai, Z.; Creamer, R.E.; de Deyn, G.; de Goede, R.; Fleskens, L.; Geissen, V.; Kuyper, T.W.; Mäder, P.; et al. Soil quality—A critical review. Soil Biol. Biochem. 2018, 120, 105–125. [Google Scholar] [CrossRef]
- Carter, M.R.; Gregorich, E.G.; Anderson, D.W.; Doran, J.W.; Janzen, H.H.; Pierce, F.J. Chapter 1 Concepts of soil quality and their significance. Dev. Soil Sci. 1997, 25, 1–19. [Google Scholar] [CrossRef]
- Jenkins, M.B.; Endale, D.M.; Schomberg, H.H.; Sharpe, R.R. Fecal bacteria and sex hormones in soil and runoff from cropped watersheds amended with poultry litter. Sci. Total Environ. 2006, 358, 164–177. [Google Scholar] [CrossRef]
- Karlen, D.L.; Ditzler, C.A.; Andrews, S.S. Soil quality: Why and how? Geoderma 2003, 114, 145–156. [Google Scholar] [CrossRef]
- Karlen, D.L.; Mausbach, M.J.; Doran, J.W.; Cline, R.G.; Harris, R.F.; Schuman, G.E. Soil Quality: A Concept, Definition, and Framework for Evaluation (A Guest Editorial). Soil Sci. Soc. Am. J. 1997, 61, 4. [Google Scholar] [CrossRef]
- Parron, L.M.; Villanueva, A.J.; Glenk, K. Estimating the value of ecosystem services in agricultural landscapes amid intensification pressures: The Brazilian case. Ecosyst. Serv 2022, 57, 101476. [Google Scholar] [CrossRef]
- Bellon, M.R.; Taylor, J.E. “Folk” Soil Taxonomy and the Partial Adoption of New Seed Varieties. Econ. Dev. Cult. Chang. 1993, 41, 763. [Google Scholar] [CrossRef]
- Bhargava, A.; Carmona, F.F.; Bhargava, M.; Srivastava, S. Approaches for enhanced phytoextraction of heavy metals. J. Environ. Manag. 2012, 105, 103–120. [Google Scholar] [CrossRef]
- Döring, T.F.; Elsalahy, H. Quantifying compensation in crop mixtures and monocultures. Eur. J. Agron. 2022, 132, 126408. [Google Scholar] [CrossRef]
- Dudley, N.; Attwood, S.J.; Goulson, D.; Jarvis, D.; Bharucha, Z.P.; Pretty, J. How should conservationists respond to pesticides as a driver of biodiversity loss in agroecosystems? Biol. Conserv. 2017, 209, 449–453. [Google Scholar] [CrossRef]
- Hatt, S.; Döring, T.F. Designing pest suppressive agroecosystems: Principles for an integrative diversification science. J. Clean. Prod. 2023, 432, 139701. [Google Scholar] [CrossRef]
- Hue, N.T.N. In situ Project staff. On-farm conservation of rice genetic diversity under salinity stress: Case study in a lowland agrosystem of Vietnam. In Enhancing the Use of Crop Genetic Diversity to Manage Abiotic Stress in Agricultural Production Systems; Jarvis, D.I., Mar, I., Sears, L., Eds.; Bioversity International: Rome, Italy, 2006; pp. 49–54. [Google Scholar]
- Mundt, C.C. Use of multiline cultivars and cultivar mixtures for disease management. Annu. Rev. Phytopathol. 2002, 40, 381–410. [Google Scholar] [CrossRef]
- Ninkovic, V.; Al Abassi, S.; Ahmed, E.; Glinwood, R.; Pettersson, J. Effect of within-species plant genotype mixing on habitat preference of a polyphagous insect predator. Oecologia 2011, 166, 391–400. [Google Scholar] [CrossRef]
- Power, A.G. Leafhopper response to genetically diverse maize stands. Entomol. Exp. Appl. 1988, 49, 213–219. [Google Scholar] [CrossRef]
- Rhouma, A.; Nasr, N.; Zirari, A.; Belguedj, M. Indigenous knowledge in management of abiotic stress: Date palm genetic resources diversity in the oases of Maghreb region. In Enhancing the Use of Crop Genetic Diversity to Manage Abiotic Stress in Agricultural Production Systems. 23-27 May, Budapest; Jarvis, D., Mar, I., Sears, L., Eds.; Bioversity International: Rome, Italy, 2006; pp. 55–61. [Google Scholar]
- Black, S.H.; Hodges, N.; Vaughan, M.; Shepherd, M. Pollinators in Natural Areas: A Primer on Habitat Management, Invertebrate Conservation Factsheet; The Xerces Society for Invertebrate Conservation: Portland, OR, USA, 2007. [Google Scholar]
- Dumont, B.; Rook, A.J.; Coran, C.; Röver, K.-U. Effects of livestock breed and grazing intensity on biodiversity and production in grazing systems. 2. Diet selection. Grass Forage Sci. 2007, 62, 159–171. [Google Scholar] [CrossRef]
- Hatfield, P.; Goosey, H.; Lenssen, A.; Blodgett, S. Sheep Grazing to Manage Crop Residues, Insects and Weeds in Northern Plains Grain and Alfalfa Systems; SARE Agricultural Innovations Fact Sheet; Sustainable Agriculture Research & Education: College Park, MD, USA, 2011. [Google Scholar]
- Metera, E.; Sakowski, T.; Słoniewski, K.; Romanowicz, B. Grazing as a tool to maintain biodiversity of grassland—A review. Anim. Sci. Pap. Rep. 2010, 28, 315–334. [Google Scholar]
- Osoro, K.; García, U.; Jáuregui, B.M.; Ferreira, L.M.M.; Rook, A.J.; Celaya, R. Diet selection and live-weight changes of two breeds of goats grazing on heathlands. Animal 2007, 1, 449–457. [Google Scholar] [CrossRef]
- Pauler, C.M.; Isselstein, J.; Braunbeck, T.; Schneider, M.K. Influence of Highland and production-oriented cattle breeds on pasture vegetation: A pairwise assessment across broad environmental gradients. Agric. Ecosyst. Environ. 2019, 284, 106585. [Google Scholar] [CrossRef]
- Pauler, C.M.; Isselstein, J.; Berard, J.; Braunbeck, T.; Schneider, M.K. Grazing Allometry: Anatomy, Movement, and Foraging Behavior of Three Cattle Breeds of Different Productivity. Front. Vet. Sci. 2020, 7, 547387. [Google Scholar] [CrossRef]
- Pauler, C.M.; Isselstein, J.; Suter, M.; Berard, J.; Braunbeck, T.; Schneider, M.K. Choosy grazers: Influence of plant traits on forage selection by three cattle breeds. Funct. Ecol. 2020, 34, 980–992. [Google Scholar] [CrossRef]
- Petz, K.; Alkemade, R.; Bakkenes, M.; Schulp, C.J.E.; van der Velde, M.; Leemans, R. Mapping and modelling trade-offs and synergies between grazing intensity and ecosystem services in rangelands using global-scale datasets and models. Glob. Environ. Chang. 2014, 29, 223–234. [Google Scholar] [CrossRef]
- Rook, A.J.; Dumont, B.; Isselstein, J.; Osoro, K.; WallisDeVries, M.F.; Parente, G.; Mills, J. Matching type of livestock to desired biodiversity outcomes in pastures—A review. Biol. Conserv. 2004, 119, 137–150. [Google Scholar] [CrossRef]
- Rosa García, R.; Fraser, M.D.; Celaya, R.; Ferreira, L.M.M.; García, U.; Osoro, K. Grazing land management and biodiversity in the Atlantic European heathlands: A review. Agrofor. Syst. 2012, 87, 19–43. [Google Scholar] [CrossRef]
- Bell, G. The ecology and genetics of fitness in Chlamydomonas. IV. The properties of mixtures of genotypes of the same species. Evolution 1991, 45, 1036–1046. [Google Scholar] [CrossRef]
- Alleway, H.; Barrett, L.T.; Theuerkauf, S. Restorative Aquaculture Shows We Can Have More Habitat, and Eat It Too [WWW Document]. The Fish Site. 2021. Available online: https://thefishsite.com/articles/restorative-aquaculture-shows-we-can-have-more-habitat-and-eat-it-too (accessed on 19 July 2022).
- Barr, J.M.; Munroe, D.; Rose, J.M.; Calvo, L.; Cheng, K.M.; Bayer, S.; Kreeger, D. Seasonal Feeding Behavior of Aquaculture Eastern Oysters (Crassostrea virginica) in the Mid-Atlantic. Estuaries Coasts 2023, 1, 789–804. [Google Scholar] [CrossRef]
- Cottingham, A.; Bossie, A.; Valesini, F.; Tweedley, J.R.; Galimany, E. Quantifying the Potential Water Filtration Capacity of a Constructed Shellfish Reef in a Temperate Hypereutrophic Estuary. Diversity 2023, 15, 113. [Google Scholar] [CrossRef]
- Cubillo, A.M.; Lopes, A.S.; Ferreira, J.G.; Moore, H.; Service, M.; Bricker, S.B. Quantification and valuation of the potential of shellfish ecosystem services in mitigating coastal eutrophication. Estuar. Coast. Shelf Sci. 2023, 293, 108469. [Google Scholar] [CrossRef]
- Ferreira, J.; Sequeira, A.; Hawkins, A.; Newton, A.; Nickell, T.; Pastres, R.; Forte, J.; Bodoy, A.; Bricker, S. Analysis of coastal and offshore aquaculture: Application of the FARM model to multiple systems and shellfish species. Aquaculture 2009, 289, 32–41. [Google Scholar] [CrossRef]
- Galimany, E.; Wikfors, G.H.; Dixon, M.S.; Newell, C.R.; Meseck, S.L.; Henning, D.; Li, Y.; Rose, J.M. Cultivation of the Ribbed Mussel (Geukensia demissa) for Nutrient Bioextraction in an Urban Estuary. Environ. Sci. Technol. 2017, 51, 13311–13318. [Google Scholar] [CrossRef]
- Lai, Q.T.; Irwin, E.R.; Zhang, Y. Estimating nitrogen removal services of eastern oyster (Crassostrea virginica) in Mobile Bay, Alabama. Ecol. Indic. 2020, 117, 106541. [Google Scholar] [CrossRef]
- Nielsen, P.; Cranford, P.J.; Maar, M.; Petersen, J.K. Magnitude, spatial scale and optimization of ecosystem services from a nutrient extraction mussel farm in the eutrophic Skive Fjord, Denmark. Aquac. Environ. Interact. 2016, 8, 311–329. [Google Scholar] [CrossRef]
- Petersen, J.K.; Saurel, C.; Nielsen, P.; Timmermann, K. The use of shellfish for eutrophication control. Aquac. Int. 2015, 24, 857–878. [Google Scholar] [CrossRef]
- Rice, M. Environmental Impacts of Shellfish Aquaculture: Filter Feeding to Control Eutrophication. In Marine Aquaculture and the Marine Environment: A Meeting for the Stakeholders in the Northeast; Cape Cod Press: Falmouth, MA, USA, 2001; pp. 77–86. [Google Scholar]
- Shi, Y.; Fan, H.; Cui, X.; Pan, L.; Li, S.; Song, X. Overview on seagrasses and related research in China. Chin. J. Oceanol. Limnol. 2010, 28, 329–339. [Google Scholar] [CrossRef]
- Theuerkauf, S.J.; Eggleston, D.B.; Puckett, B.J. Integrating ecosystem services considerations within a GIS-based habitat suitability index for oyster restoration. PLoS ONE 2019, 14, e0210936. [Google Scholar] [CrossRef]
- van den Burg, S.W.K.; Termeer, E.E.W.; Skirtun, M.; Poelman, M.; Veraart, J.A.; Selnes, T. Exploring mechanisms to pay for ecosystem services provided by mussels, oysters and seaweeds. Ecosyst. Serv. 2022, 54, 101407. [Google Scholar] [CrossRef]
- Smart, S.M.; Henrys, P. Nectar plant diversity; an indicator of the ‘regulating’ ecosystem service of pollination. In An Integrated Assessment of Countryside Survey Data to Investigate Ecosystem Services in Great Britain; Centre for Ecology & Hydrology: Wallingford, UK, 2010; p. 108. [Google Scholar]
- Howe, H.F.; Miriti, M.N. When Seed Dispersal Matters. Bioscience 2004, 54, 651. [Google Scholar] [CrossRef]
- Levin, S.A.; Muller-Landau, H.C.; Nathan, R.; Chave, J. The Ecology and Evolution of Seed Dispersal: A Theoretical Perspective. Annu. Rev. Ecol. Evol. Syst. 2003, 34, 575–604. [Google Scholar] [CrossRef]
- Garcia, D.; Zamora, R.; Amico, G.C. Birds as Suppliers of Seed Dispersal in Temperate Ecosystems: Conservation Guidelines from Real-World Landscapes. Conserv. Biol. 2010, 24, 1070–1079. [Google Scholar] [CrossRef]
- Kremen, C. Managing ecosystem services: What do we need to know about their ecology? Ecol. Lett. 2005, 8, 468–479. [Google Scholar] [CrossRef] [PubMed]
- Free, J.B.; Williams, I.H. Pollination as a factor limiting the yield of field beans (Vicia faba L.). J. Agric. Sci. 1976, 87, 395–399. [Google Scholar] [CrossRef]
- Isaacs, R.; Williams, N.; Ellis, J.; Pitts-Singer, T.L.; Bommarco, R.; Vaughan, M. Integrated Crop Pollination: Combining strategies to ensure stable and sustainable yields of pollination-dependent crops. Basic Appl. Ecol. 2017, 22, 44–60. [Google Scholar] [CrossRef]
- Jackson, J.F.; Clarke, G.R. Gene flow in an almond orchard. Theor. Appl. Genet. 1991, 82, 169–173. [Google Scholar] [CrossRef]
- Kremen, C.; Williams, N.M.; Thorp, R.W. Crop pollination from native bees at risk from agricultural intensification. Proc. Natl. Acad. Sci. USA 2002, 99, 16812–16816. [Google Scholar] [CrossRef]
- Richards, A.J. Does Low Biodiversity Resulting from Modern Agricultural Practice Affect Crop Pollination and Yield? Ann. Bot. 2001, 88, 165–172. [Google Scholar] [CrossRef]
- Steffan-Dewenter, I.; Tscharntke, T. Effects of habitat isolation on pollinator communities and seed set. Oecologia 1999, 121, 432–440. [Google Scholar] [CrossRef] [PubMed]
- Bohn, G.W.; Mann, L.K. Nectarless, a yield-reducing mutant character in the muskmelon. Proc. Am. Soc. Hortic. Sci. 1960, 76, 455–459. [Google Scholar]
- Cane, J.H.; Schiffhauer, D. Pollinator genetics and pollination: Do honey bee colonies selected for pollen-hoarding field better pollinators of cranberry Vaccinium macrocarpon? Ecol. Entomol 2001, 26, 117–123. [Google Scholar] [CrossRef]
- Kubisova, S.; Haslbachova, H. Pollination of male-sterile green pepper line (Capsicum annuum L.) by honeybees. Acta Hortic. 1991, 288, 364–370. [Google Scholar] [CrossRef]
- Steffan-dewenter, I. Seed set of male-sterile and male-fertile oilseed rape (Brassica napus) in relation to pollinator density. Apidologie 2003, 34, 227–235. [Google Scholar] [CrossRef][Green Version]
- Suso, M.J.; Moreno, M.T.; Mondragao-Rodrigues, F.; Cubero, J.I. Reproductive biology of Vicia faba: Role of pollination conditions. Field Crops Res. 1996, 46, 81–91. [Google Scholar] [CrossRef]
- Tuxill, J. Agrarian Change and Crop Diversity in Mayan Milpas of Yucatan, Mexico: Implications for On-Farm Conservation; Yale University: New Haven, NY, USA, 2005. [Google Scholar]
- Willmer, P.G.; Bataw, A.A.M.; Hughes, J.P. The superiority of bumblebees to honeybees as pollinators: Insect visits to raspberry flowers. Ecol. Entomol 1994, 19, 271–284. [Google Scholar] [CrossRef]
- Benthien, O.; Bober, J.; Castens, J.; Stolter, C. Seed dispersal capacity of sheep and goats in a near-coastal dry grassland habitat. Basic Appl. Ecol. 2016, 17, 508–515. [Google Scholar] [CrossRef]
- FAO. Livestock Keepers—Guardians of Biodiversity. Animal Production and Health Paper. No. 167; FAO: Rome, Italy, 2009. [Google Scholar] [CrossRef]
- Manzano, P.; Malo, J.E. Extreme long-distance seed dispersal via sheep. Front. Ecol. Environ. 2006, 4, 244–248. [Google Scholar] [CrossRef]
- Pauliuk, F.; Müller, J.; Heinken, T. Bryophyte dispersal by sheep on dry grassland. Nova Hedwigia 2011, 92, 327–341. [Google Scholar] [CrossRef]
- de Groot, R.S.; Wilson, M.A.; Boumans, R.M.J. A typology for the classification, description and valuation of ecosystem functions, goods and services. Ecol. Econ. 2002, 41, 393–408. [Google Scholar] [CrossRef]
- Falkenmark, M.; Lannerstad, M. Consumptive water use to feed humanity—Curing a blind spot. Hydrol. Earth Syst. Sci. 2005, 9, 15–28. [Google Scholar] [CrossRef]
- Martin-Ortega, J.; Ferrier, R.C.; Gordon, I.J.; Khan, S. Water Ecosystem Services: A Global Perspective, Water Ecosystem Services: A Global Perspective; Cambridge University Press: Cambridge, UK, 2015. [Google Scholar] [CrossRef]
- Isbell, F.; Adler, P.R.; Eisenhauer, N.; Fornara, D.; Kimmel, K.; Kremen, C.; Letourneau, D.K.; Liebman, M.; Polley, H.W.; Quijas, S.; et al. Benefits of increasing plant diversity in sustainable agroecosystems. J. Ecol. 2017, 105, 871–879. [Google Scholar] [CrossRef]
- Atta-Krah, K.; Kindt, R.; Skilton, J.N.; Amaral, W. Managing biological and genetic diversity in tropical agroforestry. Agrofor. Syst. 2004, 61–62, 183–194. [Google Scholar] [CrossRef]
- Beillouin, D.; Ben-Ari, T.; Malézieux, E.; Seufert, V.; Makowski, D. Positive but variable effects of crop diversification on biodiversity and ecosystem services. Glob. Chang. Biol. 2021, 27, 4697–4710. [Google Scholar] [CrossRef] [PubMed]
- Biswakarma, N.; Bag, K.; Tigga, P.; Sarkar, S. Cover Crops under Conservation Agriculture. Food Sci. Rep. 2022, 3, 48–51. [Google Scholar]
- Blum, A. Sorghum physiology. In Physiology and Biotechnology Integration for Plant Breeding; Nguyen, H.T., Blum, A., Eds.; Marcel Dekker, Inc.: New York, NY, USA, 2004; pp. 141–223. [Google Scholar]
- Brainard, D.; Henshaw, B.; Snapp, S. Hairy vetch varieties and Bi-Cultures influence cover crop services in strip-tilled sweet corn. Agron J. 2012, 104, 629–638. [Google Scholar] [CrossRef]
- Carroll, Z.L.; Bird, S.B.; Emmett, B.A.; Reynolds, B.; Sinclair, F.L. Can tree shelterbelts on agricultural land reduce flood risk? Soil Use Manag. 2004, 20, 357–359. [Google Scholar] [CrossRef]
- Dawson, I.K.; Guariguata, M.R.; Loo, J.; Weber, J.C.; Lengkeek, A.; Bush, D.; Cornelius, J.; Guarino, L.; Kindt, R.; Orwa, C.; et al. What is the relevance of smallholders’ agroforestry systems for conserving tropical tree species and genetic diversity in circa situm, in situ and ex situ settings? A review. Biodivers. Conserv. 2013, 22, 301–324. [Google Scholar] [CrossRef]
- Dawson, I.K.; Vinceti, B.; Weber, J.C.; Neufeldt, H.; Russell, J.; Lengkeek, A.G.; Kalinganire, A.; Kindt, R.; Lillesø, J.-P.B.; Roshetko, J.; et al. Climate change and tree genetic resource management: Maintaining and enhancing the productivity and value of smallholder tropical agroforestry landscapes. A review. Agrofor. Syst. 2010, 81, 67–78. [Google Scholar] [CrossRef]
- Di Falco, S.; Chavas, J.P. On the role of crop biodiversity in the management of environmental ris. In Biodiversity Economics; Kontoleon, A., Pascual, U., Swanson, T., Eds.; Cambridge University Press: Cambridge, UK, 2007; pp. 581–594. [Google Scholar]
- Hesse, C.; Anderson, S.; Cotula, L.; Skinner, J.; Toulmin, C. Managing the Boom and Bust: Supporting Climate Resilient Livelihoods in the Sahel; Hesse, C., Anderson, S., Cotula, L., Skinner, J., Toulmin, C., Eds.; Issue November. IIED Issue Paper; IIED: London, UK, 2013. [Google Scholar]
- Kelly, B.A.; Hardy, O.J.; Bouvet, J.M. Temporal and spatial genetic structure in Vitellaria paradoxa (shea tree) in an agroforestry system in southern Mali. Mol. Ecol. 2004, 13, 1231–1240. [Google Scholar] [CrossRef] [PubMed]
- Lengkeek, A.G.; Mwangi, A.M.; Agufa, C.A.C.; Ahenda, J.O.; Dawson, I.K. Comparing genetic diversity in agroforestry systems with natural forest: A case study of the important timber tree Vitex fischeri in central Kenya. Agrofor. Syst. 2006, 67, 293–300. [Google Scholar] [CrossRef]
- Sadiki, M. Diversity of Moroccan local faba bean landraces for reaction to drought stress. In Enhancing Crop Genetic Diversity to Manage Abiotic Stress. 23–27 May 2005, Budapest, Hungary; Jarvis, D., Mar, I., Sears, L., Eds.; Bioversity International: Rome, Italy, 2006; pp. 11–17. [Google Scholar]
- Sawadogo, M.; Balma, D.; Some, L.; Fadda, C.; Devra, J. Management of the agrobiodiversity under the clinal variation of rainfall pattern in Burkina Faso: The example of okra drought resistance. In Enhancing Crop Genetic Diversity to Manage Abiotic Stress. 23–27 May 2005, Budapest, Hungary; Jarvis, D., Mar, I., Sears, L., Eds.; Bioversity International: Rome, Italy, 2006; pp. 18–24. [Google Scholar]
- Silva, E.M.; Delate, K. A decade of progress in organic cover crop-based reduced tillage practices in the upper Midwestern USA. Agriculture 2017, 7, 44. [Google Scholar] [CrossRef]
- Singh, R.; Ghildyal, B.P. Soil Submergence Effects on Nutrient Uptake, Growth, and Yield of Five Corn Cultivars 1. Agron. J. 1980, 72, 737–741. [Google Scholar] [CrossRef]
- Wayman, S.; Cogger, C.; Benedict, C.; Burke, I.; Collins, D.; Bary, A. The influence of cover crop variety, termination timing and termination method on mulch, weed cover and soil nitrate in reduced-tillage organic systems. Renew. Agric. Food Syst. 2015, 30, 450–460. [Google Scholar] [CrossRef]
- Weber, J.C.; Montes, C.S.; Vidaurre, H.; Dawson, I.K.; Simons, A.J. Participatory domestication of agroforestry trees: An example from the Peruvian Amazon. Dev. Pract. 2001, 11, 425–433. [Google Scholar] [CrossRef]
- Weltzien, E.; Rattunde, H.F.W.; Clerget, B.; Siart, S.; Toure, A.; Sagnard, F. Sorghum diversity and adaptation to drought in West Africa. In Enhancing the Use of Crop Genetic Diversity to Manage Abiotic Sress in Agricultural Production Systems. 23-27 May, Budapest, Hungary; Jarvis, D., Mar, I., Sears, L., Eds.; Bioversity International: Rome, Italy, 2006; pp. 31–38. [Google Scholar]
- Naskar, S.; Gowane, G.R.; Chopra, A.; Paswan, C.; Prince, L.L.L. Genetic adaptability of livestock to environmental stresses. In Environmental Stress and Amelioration in Livestock Production; Springer: Berlin/Heidelberg, Germany, 2012; pp. 317–378. [Google Scholar] [CrossRef]
- Schlink, A.C.; Nguyen, M.L.; Viljoen, G.J. Water requirements for livestock production: A global perspective. OIE Rev. Sci. Tech. 2010, 29, 603–619. [Google Scholar] [CrossRef] [PubMed]
- Zander, K.K.; Drucker, A.G. Conserving what’s important: Using choice model scenarios to value local cattle breeds in East Africa. Ecol. Econ. 2008, 68, 34–45. [Google Scholar] [CrossRef]
- Bengtsson, J.; Bullock, J.M.; Egoh, B.; Everson, C.; Everson, T.; O’Connor, T.; O’Farrell, P.J.; Smith, H.G.; Lindborg, R. Grasslands—More important for ecosystem services than you might think. Ecosphere 2019, 10, e02582. [Google Scholar] [CrossRef]
- King, J.M. Livestock Water Needs in Pastoral Africa in Relation to Climate and Forage; International Livestock Centre for Africa (ILCA): Addis Ababa, Ethiopia, 1983. [Google Scholar]
- Leroy, G.; Boettcher, P.; Joly, F.; Looft, C.; Baumung, R. Multifunctionality and provision of ecosystem services by livestock species and breeds at global level. Animal 2024, 18, 101048. [Google Scholar] [CrossRef]
- Hoffmann, I. Adaptation to climate change—Exploring the potential of locally adapted breeds. Animal 2013, 7 (Suppl. 2), 346–362. [Google Scholar] [CrossRef] [PubMed]
- Turpie, J.K.; Marais, C.; Blignaut, J.N. The working for water programme: Evolution of a payments for ecosystem services mechanism that addresses both poverty and ecosystem service delivery in South Africa. Ecol. Econ. 2008, 65, 788–798. [Google Scholar] [CrossRef]
- Guerra, C.A.; Pinto-Correia, T.; Metzger, M.J. Mapping Soil Erosion Prevention Using an Ecosystem Service Modeling Framework for Integrated Land Management and Policy. Ecosystems 2014, 17, 878–889. [Google Scholar] [CrossRef]
- Guerra, C.A.; Maes, J.; Geijzendorffer, I.; Metzger, M.J. An assessment of soil erosion prevention by vegetation in Mediterranean Europe: Current trends of ecosystem service provision. Ecol. Indic. 2016, 60, 213–222. [Google Scholar] [CrossRef]
- Lal, R. Soil conservation and ecosystem services. Int. Soil Water Conserv. Res. 2014, 2, 36–47. [Google Scholar] [CrossRef]
- Pereira, P.; Bogunovic, I.; Muñoz-Rojas, M.; Brevik, E.C. Soil ecosystem services, sustainability, valuation and management. Curr. Opin. Environ. Sci. Health 2018, 5, 7–13. [Google Scholar] [CrossRef]
- Reganold, J.P.; Elliott, L.F.; Unger, Y.L. Long-term effects of organic and conventional farming on soil erosion. Nature 1987, 330, 370–372. [Google Scholar] [CrossRef]
- Zougmore, R.; Kambou, F.N.; Ouattara, K.; Guillobez, S. Sorghum-cowpea Intercropping: An Effective Technique Against Runoff and Soil Erosion in the Sahel (Saria, Burkina Faso). Arid Soil Res. Rehabil. 2000, 14, 329–342. [Google Scholar] [CrossRef]
- Gauchan, D.; Smale, M. Comparing the choices of farmers and breeders: The value of rice landraces in Nepal. In Managing Biodiversity in Agricultural Ecosystems; Jarvis, D., Padoch, C., Cooper, H.D., Eds.; Columbia University Press: New York, NY, USA; Chichester, UK; West Sussex, UK, 2007; pp. 407–425. [Google Scholar] [CrossRef]
- Jackson, M.B.; Ram, P.C. Physiological and Molecular Basis of Susceptibility and Tolerance of Rice Plants to Complete Submergence. Ann. Bot. 2003, 91, 227–241. [Google Scholar] [CrossRef]
- Thinlay, X. Rice Blast, Caused by Magnaporthe Grisea, in Bhutan and Development of Strategies for Resistance Breeding and Management; Swiss Federal Institute of Technology: Zurich, Switzerland, 1998. [Google Scholar]
- Ghaley, B.B.; Porter, J.R.; Sandhu, H.S. Soil-based ecosystem services: A synthesis of nutrient cycling and carbon sequestration assessment methods. Int. J. Biodivers. Sci. Ecosyst. Serv. Manag. 2014, 10, 177–186. [Google Scholar] [CrossRef]
- Schröder, J.J.; Schulte, R.P.O.; Creamer, R.E.; Delgado, A.; van Leeuwen, J.; Lehtinen, T.; Rutgers, M.; Spiegel, H.; Staes, J.; Tóth, G.; et al. The elusive role of soil quality in nutrient cycling: A review. Soil Use Manag. 2016, 32, 476–486. [Google Scholar] [CrossRef]
- Wilcke, W.; Boy, J.; Hamer, U.; Potthast, K.; Rollenbeck, R.; Valarezo, C. Current Regulating and Supporting Services: Nutrient Cycles. In Ecosystem Services, Biodiversity and Environmental Change in a Tropical Mountain Ecosystem of South Ecuador; Springer: Berlin/Heidelberg, Germany, 2013; pp. 141–151. [Google Scholar] [CrossRef]
- Liu, X.; Herbert, S.J.; Hashemi, A.M.; Zhang, X.; Ding, G. Effects of agricultural management on soil organic matter and carbon transformation—A review. Plant Soil Environ. 2006, 52, 531–543. [Google Scholar] [CrossRef]
- Nesper, M.; Kueffer, C.; Krishnan, S.; Kushalappa, C.G.; Ghazoul, J. Simplification of shade tree diversity reduces nutrient cycling resilience in coffee agroforestry. J. Appl. Ecol. 2019, 56, 119–131. [Google Scholar] [CrossRef]
- Pervaiz, Z.H.; Iqbal, J.; Zhang, Q.; Chen, D.; Wei, H.; Saleem, M. Continuous Cropping Alters Multiple Biotic and Abiotic Indicators of Soil Health. Soil Syst. 2020, 4, 59. [Google Scholar] [CrossRef]
- Yang, T.; Siddique, K.H.M.; Liu, K. Cropping systems in agriculture and their impact on soil health—A review. Glob. Ecol. Conserv. 2020, 23, e01118. [Google Scholar] [CrossRef]
- Altieri, M.A. The ecological role of biodiversity in agroecosystems. Agric. Ecosyst. Environ. 1999, 74, 19–31. [Google Scholar] [CrossRef]
- Chatzistathis, T.; Therios, I. How Soil Nutrient Availability Influences Plant Biomass and How Biomass Stimulation Alleviates Heavy Metal Toxicity in Soils: The Cases of Nutrient Use Efficient Genotypes and Phytoremediators, Respectively. In Biomass Now—Cultivation and Utilization; Matovic, M.D., Ed.; InTechOpen: Rijeka, Croatia, 2013; pp. 427–448. [Google Scholar] [CrossRef]
- Harrison, J.; Brugière, N.; Phillipson, B.; Ferrario-Mery, S.; Becker, T.; Limami, A.; Hirel, B. Manipulating the pathway of ammonia assimilation through genetic engineering and breeding: Consequences to plant physiology and plant development. Plant Soil 2000, 221, 81–93. [Google Scholar] [CrossRef]
- Jun, D.J.; Allen, E.B. Physiological Responses of 6 Wheatgrass Cultivars to Mycorrhizae. J. Range Manag. 1991, 44, 336. [Google Scholar] [CrossRef][Green Version]
- Krishna, K.R.; Shetty, K.G.; Dart, P.J.; Andrews, D.J. Genotype dependent variation in mycorrhizal colonization and response to inoculation of pearl millet. Plant Soil 1985, 86, 113–125. [Google Scholar] [CrossRef]
- Madritch, M.D.; Hunter, M.D. Intraspecific litter diversity and nitrogen deposition affect nutrient dynamics and soil respiration. Oecologia 2003, 136, 124–128. [Google Scholar] [CrossRef]
- Msairi, S.; Chliyeh, M.; Artib, M.; Elgabardi, S.; Selmaoui, K.; Touhami, A.O.; Benkirane, R.; Douira, A. Effect of Endomycorrhizal Inoculum on the Growth and Protection of Olive Plants Against Phytophthora palmivora. Tree Planters’ Notes 2020, 63, 19–28. [Google Scholar]
- Copland, J.W.; Alders, R.G. The comparative advantages of village or smallholder poultry in rural development. In Village Chickens, Poverty Alleviation and the Sustainable Control of Newcastle Disease; ACIAR Proceedings; Australian Centre for International Agricultural Research: Bruce, ACT, Australia, 2009; Volume 131, pp. 11–14. [Google Scholar]
- Freire, J.P.B.; Dias, R.I.M.; Cunha, L.F.; Aumaitre, A. The effect of genotype and dietary fibre level on the caecal bacterial enzyme activity of young piglets: Digestive consequences. Anim. Feed Sci. Technol. 2003, 106, 119–130. [Google Scholar] [CrossRef]
- Krätli, S. Cattle breeding, complexity and mobility in a structurally unpredictable environment: The WoDaaBe herders of niger. Nomadic People 2008, 12, 11–41. [Google Scholar] [CrossRef]
- Mottet, A.; de Haan, C.; Falcucci, A.; Tempio, G.; Opio, C.; Gerber, P. Livestock: On our plates or eating at our table? A new analysis of the feed/food debate. Glob. Food Secur. 2017, 14, 1–8. [Google Scholar] [CrossRef]
- Potter, P.; Ramankutty, N.; Bennett, E.M.; Donner, S.D. Characterizing the Spatial Patterns of Global Fertilizer Application and Manure Production. Earth Interact. 2010, 14, 1–22. [Google Scholar] [CrossRef]
- Román-Trufero, A.; García-Prieto, V.; Martínez, A.; Osoro, K.; Celaya, R. Beef steer production from two local breeds under two management systems differing in the utilisation of mountain pastures. Ital. J. Anim. Sci. 2019, 18, 1174–1185. [Google Scholar] [CrossRef]
- Román-Trufero, A.; Osoro, K.; Celaya, R. Performance of two local beef cattle breeds in Cantabrian mountain pastures. In Mountain Pastures and Livestock Farming Facing Uncertainty: Environmental, Technical and Socio-Economic Challenges; Casasús, I., Lombardi, G., Eds.; Options Méditerranéennes. Series A: Mediterranean Seminars; Centre International de Hautes Etudes Agronomiques Méditerranéennes: Montpellier, France, 2016; pp. 153–156. [Google Scholar]
- Sollenberger, L.E.; Kohmann, M.M.; Dubeux, J.C.B.; Silveira, M.L. Grassland Management Affects Delivery of Regulating and Supporting Ecosystem Services. Crop Sci. 2019, 59, 441–459. [Google Scholar] [CrossRef]
- Abreu, M.H.; Varela, D.A.; Henríquez, L.; Villarroel, A.; Yarish, C.; Sousa-Pinto, I.; Buschmann, A.H. Traditional vs. integrated multi-trophic aquaculture of Gracilaria chilensis CJ Bird, J. McLachlan & EC Oliveira: Productivity and physiological performance. Aquaculture 2009, 293, 211–220. [Google Scholar] [CrossRef]
- Augyte, S.; Yarish, C.; Redmond, S.; Kim, J.K. Cultivation of a morphologically distinct strain of the sugar kelp, Saccharina latissima forma angustissima, from coastal Maine, USA, with implications for ecosystem services. J. Appl. Phycol. 2017, 29, 1967–1976. [Google Scholar] [CrossRef]
- Higgins, C.B.; Stephenson, K.; Brown, B.L. Nutrient Bioassimilation Capacity of Aquacultured Oysters: Quantification of an Ecosystem Service. J. Environ. Qual. 2011, 40, 271–277. [Google Scholar] [CrossRef]
- Lotze, H.K.; Milewski, I.; Fast, J.; Kay, L.; Worm, B. Ecosystem-based management of seaweed harvesting. Bot. Mar. 2019, 62, 395–409. [Google Scholar] [CrossRef]
- Dobson, A.; Lodge, D.; Alder, J.; Cumming, G.S.; Keymer, J.; Mcglade, J.; Mooney, H.; Rusak, J.A.; Sala, O.; Wolters, V.; et al. Habitat loss, trophic collapse, and the decline of ecosystem services. Ecology 2006, 87, 1915–1924. [Google Scholar] [CrossRef]
- Harrington, R.; Anton, C.; Dawson, T.P.; de Bello, F.; Feld, C.K.; Haslett, J.R.; Kluvánkova-Oravská, T.; Kontogianni, A.; Lavorel, S.; Luck, G.W.; et al. Ecosystem services and biodiversity conservation: Concepts and a glossary. Biodivers. Conserv. 2010, 19, 2773–2790. [Google Scholar] [CrossRef]
- Altieri, M.A. The Ecological Impacts of Large-Scale Agrofuel Monoculture Production Systems in the Americas. Bull. Sci. Technol. Soc. 2009, 29, 236–244. [Google Scholar] [CrossRef]
- Azhar, B.; Puan, C.L.; Zakaria, M.; Hassan, N.; Arif, M. Effects of monoculture and polyculture practices in oil palm smallholdings on tropical farmland birds. Basic Appl. Ecol. 2014, 15, 336–346. [Google Scholar] [CrossRef]
- Fitzherbert, E.B.; Struebig, M.J.; Morel, A.; Danielsen, F.; Brühl, C.A.; Donald, P.F.; Phalan, B. How will oil palm expansion affect biodiversity? Trends Ecol. Evol. 2008, 23, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Iezzi, M.E.; Cruz, P.; Varela, D.; De Angelo, C.; Di Bitetti, M.S. Tree monocultures in a biodiversity hotspot: Impact of pine plantations on mammal and bird assemblages in the Atlantic Forest. Ecol. Manag. 2018, 424, 216–227. [Google Scholar] [CrossRef]
- Karp, D.S.; Rominger, A.J.; Zook, J.; Ranganathan, J.; Ehrlich, P.R.; Daily, G.C. Intensive agriculture erodes β-diversity at large scales. Ecol. Lett. 2012, 15, 963–970. [Google Scholar] [CrossRef]
- Kline, O.; Joshi, N.K. Mitigating the effects of habitat loss on solitary bees in agricultural ecosystems. Agriculture 2020, 10, 115. [Google Scholar] [CrossRef]
- Ponisio, L.C.; M’Gonigle, L.K.; Kremen, C. On-farm habitat restoration counters biotic homogenization in intensively managed agriculture. Glob. Chang. Biol. 2016, 22, 704–715. [Google Scholar] [CrossRef]
- Potapov, A.M.; Dupérré, N.; Jochum, M.; Dreczko, K.; Klarner, B.; Barnes, A.D.; Krashevska, V.; Rembold, K.; Kreft, H.; Brose, U.; et al. Functional losses in ground spider communities due to habitat structure degradation under tropical land-use change. Ecology 2020, 101, e02957. [Google Scholar] [CrossRef] [PubMed]
- Celaya, R.; Ferreira, L.M.M.; García, U.; Rosa García, R.; Osoro, K. Heavy grazing by horses on heathlands of different botanical composition. EAAP Sci. Ser. 2012, 132, 219–226. [Google Scholar] [CrossRef]
- Celaya, R.; Jáuregui, B.M.; García, R.R.; Benavides, R.; García, U.; Osoro, K. Changes in heathland vegetation under goat grazing: Effects of breed and stocking rate. Appl. Veg. Sci. 2010, 13, 125–134. [Google Scholar] [CrossRef]
- Dajić Stevanović, Z.; Peeters, A.; Vrbničanin, S.; Šoštarić, I.; Aćić, S. Long term grassland vegetation changes: Case study Nature Park Stara Planina (Serbia). Community Ecol. 2008, 9, 23–31. [Google Scholar] [CrossRef]
- Dash, S.K.; Sethi, B.P.; Rao, P.K.; Prakash, B. Characterization and prospective of Chilika buffalo—A unique germplasm of Eastern India. In Livestock Biodiversity Journal; Society for Conservation of Domestic Animal Biodiversity: Karnal, India, 2010. [Google Scholar]
- Elias, D.; Tischew, S. Goat pasturing—A biological solution to counteract shrub encroachment on abandoned dry grasslands in Central Europe? Agric. Ecosyst. Environ. 2016, 234, 98–106. [Google Scholar] [CrossRef]
- Kleijn, D.; Rundlöf, M.; Scheper, J.; Smith, H.G.; Tscharntke, T. Does conservation on farmland contribute to halting the biodiversity decline? Trends Ecol. Evol. 2011, 26, 474–481. [Google Scholar] [CrossRef] [PubMed]
- Laiolo, P.; Dondero, F.; Ciliento, E.; Rolando, A. Consequences of pastoral abandonment for the structure and diversity of the alpine avifauna. J. Appl. Ecol. 2004, 41, 294–304. [Google Scholar] [CrossRef]
- López, C.L.; García, R.R.; Ferreira, L.M.M.; García, U.; Osoro, K.; Celaya, R. Impacts of horse grazing on botanical composition and diversity in different types of heathland. Rangel. J. 2017, 39, 375–385. [Google Scholar] [CrossRef]
- Lovreglio, R.; Meddour-Sahar, O.; Leone, V. Goat grazing as a wildfire prevention tool: A basic review. IForest 2014, 7, 260–268. [Google Scholar] [CrossRef]
- Patro, B.N.; Mishra, P.K.; Rao, P.K. Chilika buffaloes in Orissa: A unique germplasm. Anim. Genet. Resour. Inf. 2003, 33, 73–79. [Google Scholar] [CrossRef][Green Version]
- Sharma, L.N.; Vetaas, O.R.; Chaudhary, R.P.; Måren, I.E. Pastoral Abandonment, Shrub Proliferation and Landscape Changes: A Case Study from Gorkha, Nepal. Landsc. Res. 2014, 39, 53–69. [Google Scholar] [CrossRef]
- Targetti, S.; Staglianò, N.; Messeri, A.; Argenti, G. A state-and-transition approach to alpine grasslands under abandonment. IForest 2010, 3, 44–51. [Google Scholar] [CrossRef]
- Alleway, H.K.; Gillies, C.L.; Bishop, M.J.; Gentry, R.R.; Theuerkauf, S.J.; Jones, R. The Ecosystem Services of Marine Aquaculture: Valuing Benefits to People and Nature. BioScience 2019, 69, 59–68. [Google Scholar] [CrossRef]
- Barrett, L.T.; Theuerkauf, S.J.; Rose, J.M.; Alleway, H.K.; Bricker, S.B.; Parker, M.; Petrolia, D.R.; Jones, R.C. Sustainable growth of non-fed aquaculture can generate valuable ecosystem benefits. Ecosyst. Serv. 2022, 53, 101396. [Google Scholar] [CrossRef]
- Cerco, C.F.; Noel, M.R. Can oyster restoration reverse cultural eutrophication in Chesapeake Bay? Estuaries Coasts 2007, 30, 331–343. [Google Scholar] [CrossRef]
- Forbes, H.; Shelamoff, V.; Visch, W.; Layton, C. Farms and forests: Evaluating the biodiversity benefits of kelp aquaculture. J. Appl. Phycol. 2022, 34, 3059–3067. [Google Scholar] [CrossRef]
- Gentry, R.R.; Alleway, H.K.; Bishop, M.J.; Gillies, C.L.; Waters, T.; Jones, R. Exploring the potential for marine aquaculture to contribute to ecosystem services. Rev. Aquac. 2020, 12, 499–512. [Google Scholar] [CrossRef]
- Martínez-Baena, F.; Lanham, B.S.; McLeod, I.; Taylor, M.D.; McOrrie, S.; Bishop, M.J. De novo reefs: Fish habitat provision by oyster aquaculture varies with farming method. Aquac. Environ. Interact. 2022, 14, 71–84. [Google Scholar] [CrossRef]
- Petrolia, D.R.; Nyanzu, F.; Cebrian, J.; Harri, A.; Amato, J.; Walton, W.C. Eliciting expert judgment to inform management of diverse oyster resources for multiple ecosystem services. J. Environ. Manag. 2020, 268, 110676. [Google Scholar] [CrossRef]
- Rose, J.M.; Bricker, S.B.; Tedesco, M.A.; Wikfors, G.H. A role for shellfish aquaculture in coastal nitrogen management. Environ. Sci. Technol. 2014, 48, 2519–2525. [Google Scholar] [CrossRef]
- SIFT. Seaweed Cultivation in Scotland—A Guide for Community Participation in Seaweed Farm Applications; Sustainable Inshore Fisheries Trust: Edinburgh, Scotland, UK, 2021. [Google Scholar]
- Theuerkauf, S.J.; Barrett, L.T.; Alleway, H.K.; Costa-Pierce, B.A.; St. Gelais, A.; Jones, R.C. Habitat value of bivalve shellfish and seaweed aquaculture for fish and invertebrates: Pathways, synthesis and next steps. Rev. Aquac. 2022, 14, 54–72. [Google Scholar] [CrossRef]
- Veggerby, K.B.; Scheuerell, M.D.; Sanderson, B.L.; Kiffney, P.M. Stable isotopes reveal intertidal fish and crabs use bivalve farms as foraging habitat in Puget Sound, Washington. Front. Mar. Sci. 2024, 10, 1282225. [Google Scholar] [CrossRef]
- Duval, A.; Mijatovic, D.; Hodgkin, T. The Contribution of Biodiversity for Food and Agriculture to the Resilience of Production Systems, Thematic Study for The State of the World’s Biodiversity for Food and Agriculture; FAO: Rome, Italy, 2019. [Google Scholar]
- Zahoor, I.; Mushtaq, A. Water pollution from agricultural activities: A critical global review. Int. J. Chem. Biochem. Sci. 2023, 23, 164–176. [Google Scholar]
- Meneses, I.; Santelices, B. Strain selection and genetic variation in Gracilaria chilensis (Gracilariales, Rhodophyta). J. Appl. Phycol. 1999, 11, 241–246. [Google Scholar] [CrossRef]
- Primavera, J.H. Overcoming the impacts of aquaculture on the coastal zone. Ocean Coast. Manag. 2006, 49, 531–545. [Google Scholar] [CrossRef]
- Sadiki, M.; Jarvis, D.; Rijal, D.; Bajracharya, J.; Hue, N.N.; Camacho-Villa, T.C.; Burgos-May, L.A.; Sawadogo, M.; Balma, D.; Lope, D.; et al. 3. Variety Names: An Entry Point to Crop Genetic Diversity and Distribution in Agroecosystems? In Managing Biodiversity in Agricultural Ecosystems; Devra, J., Ed.; Columbia University Press: New York, NY, USA; Chichester, UK; West Sussex, UK, 2007; pp. 34–76. [Google Scholar]
- Hall, S.J.G.; Bradley, D.G. Conserving livestock breed biodiversity. Trends Ecol. Evol. 1995, 10, 267–270. [Google Scholar] [CrossRef]
- Notter, D.R.; Scherf, B.; Hoffmann, I. Breeding of Animals. Encycl. Biodivers. 2013, 1, 533–545. [Google Scholar] [CrossRef]
- Dumont, B.; Fortun-Lamothe, L.; Jouven, M.; Thomas, M.; Tichit, M. Prospects from agroecology and industrial ecology for animal production in the 21st century. Animal 2013, 7, 1028–1043. [Google Scholar] [CrossRef]
- Brown, A. The genetic structure of crop landraces and the challenge to conserve them in situ on farms. In Genes in the Field; IDRC: Ottawa, ON, Canada, 1999. [Google Scholar] [CrossRef]
- Thomas, M.; Verzelen, N.; Barbillon, P.; Coomes, O.T.; Caillon, S.; McKey, D.; Elias, M.; Garine, E.; Raimond, C.; Dounias, E.; et al. A Network-Based Method to Detect Patterns of Local Crop Biodiversity: Validation at the Species and Infra-Species Levels. Adv. Ecol. Res 2015, 53, 259–320. [Google Scholar] [CrossRef]
- Boettcher, P.J.; Hoffmann, I. Conserve Livestock Genetic Resources, Too. Science 2009, 326, 365. [Google Scholar] [CrossRef]
- Sponenberg, D.P.; Beranger, J.; Martin, A.M.; Couch, C.R. Conservation of rare and local breeds of livestock. Rev. Sci. Tech. 2018, 37, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Bennett, E.M.; Peterson, G.D.; Gordon, L.J. Understanding relationships among multiple ecosystem services. Ecol. Lett. 2009, 12, 1394–1404. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.H.D. The disease damage, genetic diversity, genetic vulnerability diagram—Some reflections. In Damage, Diversity and Genetic Vulnerability: The Role of Crop Genetic Diversity in the Agricultural Production System to Reduce Pest and Disease Damage, Proceedings of the International Symposium, Rabat, Morocco, 15–17 February 2011; Jarvis, D.I., De Santis, P., Thompson, J., Eds.; Bioversity International: Rome Italy, 2012; pp. 318–329. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).