Oxygen Concentration and Its Implications for Microbial Structure and Metabolism: A Case Study in a Deep Tropical Reservoir
Abstract
:1. Introduction
2. Material and Methods
3. Results
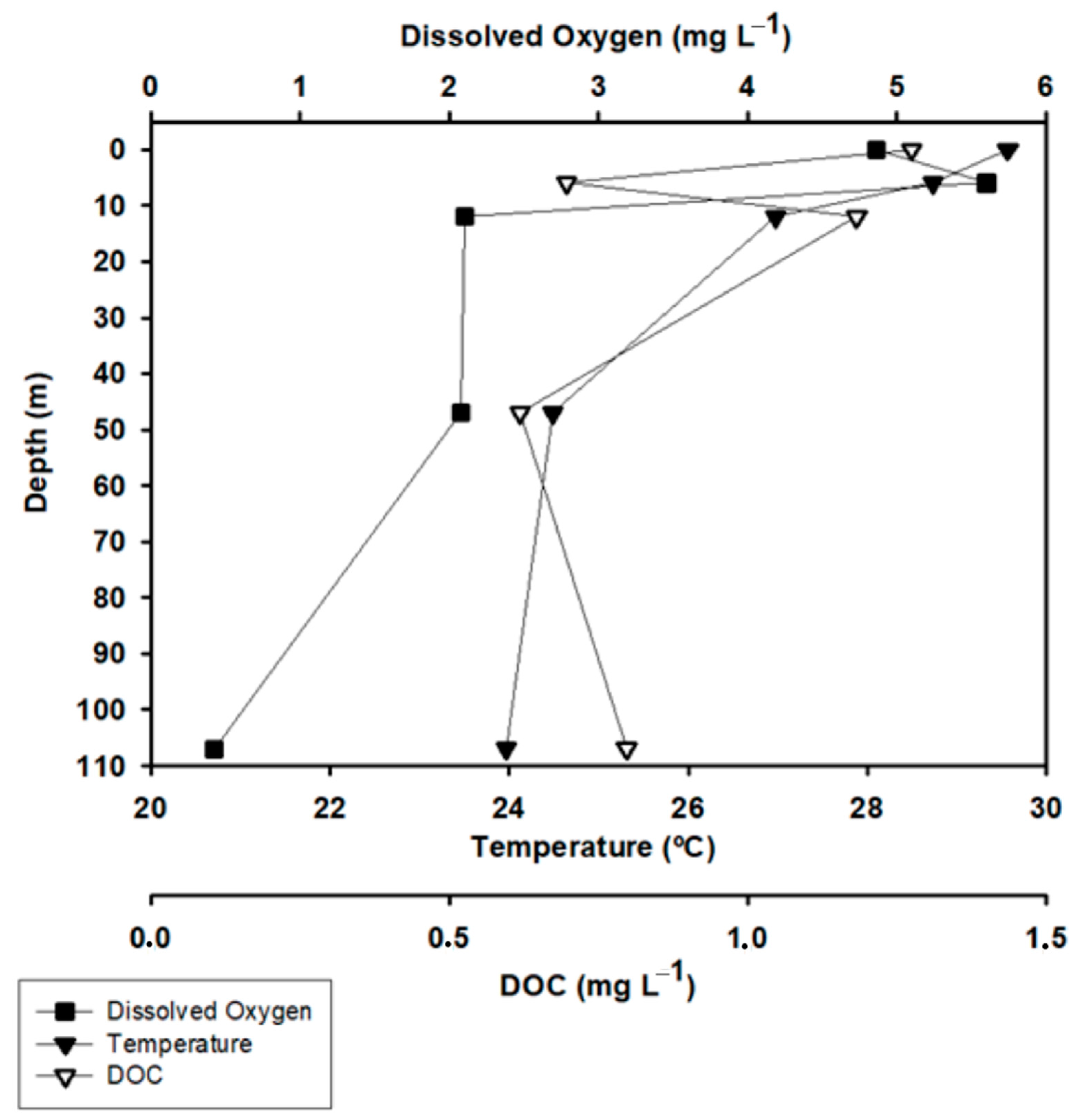
3.1. Oxygen and Temperature Profiles
3.2. Nutrient Concentrations
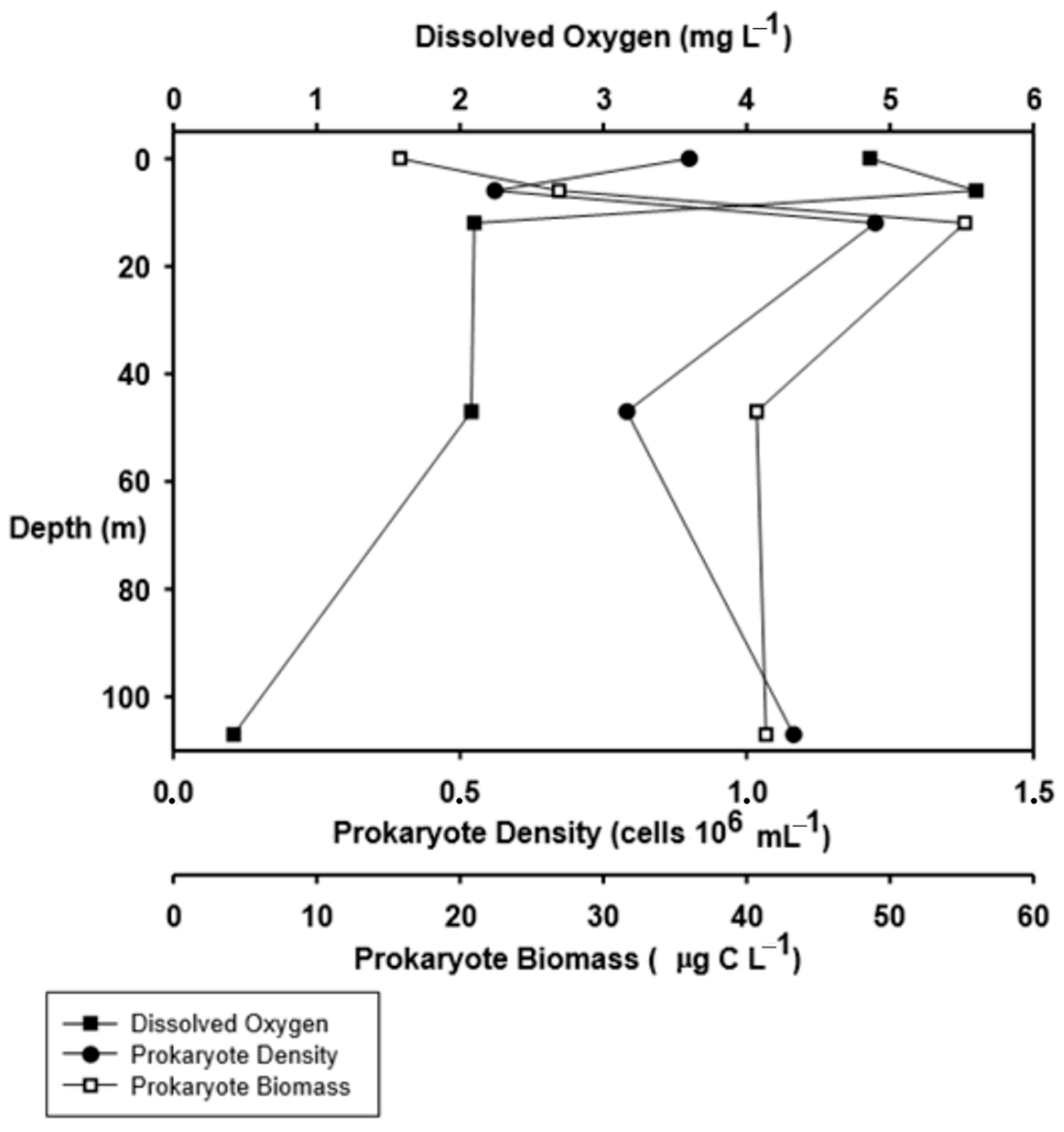
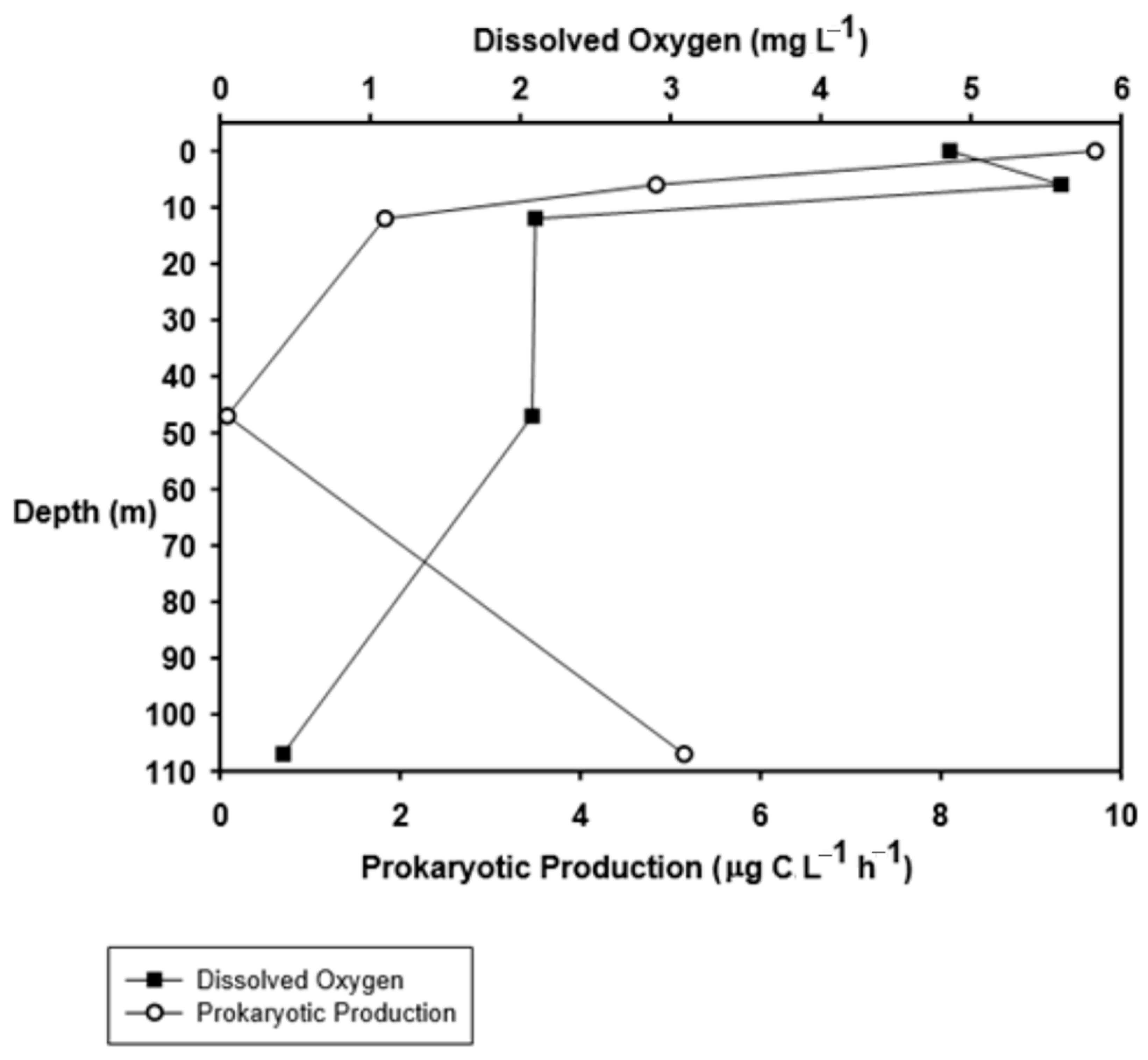
3.3. Density, Biomass, and Production of Prokaryotes
3.4. Microbial Community Composition
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carey, C.C.; Hanson, P.C.; Thomas, R.Q.; Gerling, A.B.; Hounshell, A.G.; Lewis, A.S.L.; Lofton, M.E.; McClure, R.P.; Wander, H.L.; Woelmer, W.M.; et al. Anoxia Decreases the Magnitude of the Carbon, Nitrogen, and Phosphorus Sink in Freshwaters. Glob. Chang. Biol. 2022, 28, 4861–4881. [Google Scholar] [CrossRef] [PubMed]
- Hounshell, A.G.; McClure, R.P.; Lofton, M.E.; Carey, C.C. Whole-ecosystem Oxygenation Experiments Reveal Substantially Greater Hypolimnetic Methane Concentrations in Reservoirs during Anoxia. Limnol. Oceanogr. Lett. 2021, 6, 33–42. [Google Scholar] [CrossRef]
- Yue, Y.; Cai, L.; Tang, Y.; Zhang, Y.; Yang, M.; Wang, F. Vertical Distribution of Bacterial Community in Water Columns of Reservoirs With Different Trophic Conditions During Thermal Stratification. Front. Environ. Sci. 2021, 9, 632089. [Google Scholar] [CrossRef]
- Luo, X.; Xiang, X.; Huang, G.; Song, X.; Wang, P.; Fu, K. Bacterial Abundance and Physicochemical Characteristics of Water and Sediment Associated with Hydroelectric Dam on the Lancang River China. Int. J. Environ. Res. Public Health 2019, 16, 2031. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Kasahara, T.; Chiwa, M.; Fujimoto, N. Effects of Dams and Reservoirs on Organic Matter Decomposition in Forested Mountain Streams in Western Japan. River Res. Apps 2020, 36, 1257–1266. [Google Scholar] [CrossRef]
- Kirchman, D.L. The Ecology of Cytophaga–Flavobacteria in Aquatic Environments. FEMS Microbiol. Ecol. 2002, 39, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Amaral, V.; Graeber, D.; Calliari, D.; Alonso, C. Strong Linkages between DOM Optical Properties and Main Clades of Aquatic Bacteria. Limnol. Oceanogr. 2016, 61, 906–918. [Google Scholar] [CrossRef]
- Palatinszky, M.; Herbold, C.; Jehmlich, N.; Pogoda, M.; Han, P.; Von Bergen, M.; Lagkouvardos, I.; Karst, S.M.; Galushko, A.; Koch, H.; et al. Cyanate as an Energy Source for Nitrifiers. Nature 2015, 524, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Lehtovirta-Morley, L.E. Ammonia Oxidation: Ecology, Physiology, Biochemistry and Why They Must All Come Together. FEMS Microbiol. Lett. 2018, 365, fny058. [Google Scholar] [CrossRef]
- Lu, S.; Liu, X.; Liu, C.; Cheng, G.; Shen, H. Influence of Photoinhibition on Nitrification by Ammonia-Oxidizing Microorganisms in Aquatic Ecosystems. Rev. Environ. Sci. Biotechnol. 2020, 19, 531–542. [Google Scholar] [CrossRef]
- Freixa, A.; Rubol, S.; Carles-Brangarí, A.; Fernàndez-Garcia, D.; Butturini, A.; Sanchez-Vila, X.; Romaní, A.M. The Effects of Sediment Depth and Oxygen Concentration on the Use of Organic Matter: An Experimental Study Using an Infiltration Sediment Tank. Sci. Total Environ. 2016, 540, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Toneatti, D.M.; Albarracín, V.H.; Flores, M.R.; Polerecky, L.; Farías, M.E. Stratified Bacterial Diversity along Physico-Chemical Gradients in High-Altitude Modern Stromatolites. Front. Microbiol. 2017, 8, 646. [Google Scholar] [CrossRef] [PubMed]
- Baricz, A.; Chiriac, C.M.; Andrei, A.; Bulzu, P.; Levei, E.A.; Cadar, O.; Battes, K.P.; Cîmpean, M.; Șenilă, M.; Cristea, A.; et al. Spatio-temporal Insights into Microbiology of the Freshwater-to-hypersaline, Oxic-hypoxic-euxinic Waters of Ursu Lake. Environ. Microbiol. 2021, 23, 3523–3540. [Google Scholar] [CrossRef]
- Lau, M.P.; Hupfer, M.; Grossart, H. Reduction-oxidation Cycles of Organic Matter Increase Bacterial Activity in the Pelagic Oxycline. Environ. Microbiol. Rep. 2017, 9, 257–267. [Google Scholar] [CrossRef]
- Suominen, S.; Dombrowski, N.; Sinninghe Damsté, J.S.; Villanueva, L. A Diverse Uncultivated Microbial Community Is Responsible for Organic Matter Degradation in the Black Sea Sulphidic Zone. Environ. Microbiol. 2021, 23, 2709–2728. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.J.; Konwar, K.M.; Hallam, S.J. Microbial Ecology of Expanding Oxygen Minimum Zones. Nat. Rev. Microbiol. 2012, 10, 381–394. [Google Scholar] [CrossRef]
- Pajares, S.; Soto-Jiménez, M.F.; Merino-Ibarra, M. Molecular and Isotopic Evidence of the Distribution of Nitrogen-Cycling Microbial Communities in the Oxygen Minimum Zone of the Tropical Mexican Pacific. FEMS Microbiol. Ecol. 2019, 95, fiz143. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.; Ng, S.-K.; Lim, C.K.; Lu, H.; Jia, Y.; Lee, P.K.H. Physiological and Metagenomic Characterizations of the Synergistic Relationships between Ammonia- and Nitrite-Oxidizing Bacteria in Freshwater Nitrification. Front. Microbiol. 2018, 9, 280. [Google Scholar] [CrossRef] [PubMed]
- Röske, K.; Sachse, R.; Scheerer, C.; Röske, I. Microbial Diversity and Composition of the Sediment in the Drinking Water Reservoir Saidenbach (Saxonia, Germany). Syst. Appl. Microbiol. 2012, 35, 35–44. [Google Scholar] [CrossRef]
- Soares, L.A.; Santos, A.C.A.D.; Duarte, I.C.S.; Romagnoli, E.M.; Calijuri, M.D.C. Distribution of Archaeal and Bacterial Communities in a Subtropical Reservoir. Acta Limnol. Bras. 2015, 27, 411–420. [Google Scholar] [CrossRef]
- Pierangeli, G.M.F.; Domingues, M.R.; Choueri, R.B.; Hanisch, W.S.; Gregoracci, G.B.; Benassi, R.F. Spatial Variation and Environmental Parameters Affecting the Abundant and Rare Communities of Bacteria and Archaea in the Sediments of Tropical Urban Reservoirs. Microb. Ecol. 2023, 86, 297–310. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-G.; Lee, B.; Heo, T.-Y.; Cheon, A.-I.; Jun, H.-B. Metagenomics Approach and Canonical Correspondence Analysis of Novel Nitrifiers and Ammonia-Oxidizing Archaea in Full Scale Anaerobic-Anoxic-Oxic (A2/O) and Oxidation Ditch Processes. Bioresour. Technol. 2021, 319, 124205. [Google Scholar] [CrossRef] [PubMed]
- Van Kessel, M.A.H.J.; Speth, D.R.; Albertsen, M.; Nielsen, P.H.; Op Den Camp, H.J.M.; Kartal, B.; Jetten, M.S.M.; Lücker, S. Complete Nitrification by a Single Microorganism. Nature 2015, 528, 555–559. [Google Scholar] [CrossRef] [PubMed]
- Santoro, A.E. The Do-It-All Nitrifier. Science 2016, 351, 342–343. [Google Scholar] [CrossRef] [PubMed]
- Ai, Y.; Huang, T.; Duan, C.; Huang, D.; Gong, Y.; Cheng, H. Knowledge Domain of Greenhouse Gas Emissions from Hydropower Reservoirs: Hotspots, Frontiers and Future Perspectives. Front. Environ. Sci. 2022, 10, 1055891. [Google Scholar] [CrossRef]
- Roland, F.; Cimbleris, A.C.P.; Lobão, L.M.; Vidal, L.O. Bacterioplankton Metabolism in Hydroelectric Reservoirs. Oecologia Aust. 2011, 15, 605–617. [Google Scholar] [CrossRef]
- Cardoso, S.J.; Vidal, L.O.; Mendonça, R.F.; Tranvik, L.J.; Sobek, S.; Fábio, R. Spatial Variation of Sediment Mineralization Supports Differential CO2 Emissions from a Tropical Hydroelectric Reservoir. Front. Microbiol. 2013, 4, 101. [Google Scholar] [CrossRef] [PubMed]
- De Filippo, R.; Gomes, E.L.; Lenz-César, J.; Soares, C.B.P.; Menezes, C.F.S. As Alterações Na Qualidade Da Água Durante o Enchimento Do Reservatório de UHE Serra Da Mesa—GO. In Ecologia de Reservatórios: Estrutura, Função e Aspectos Sociais; Henry, R., Ed.; Fapesp–Fundibio: Botucatu, Brazil, 1999; pp. 323–345. [Google Scholar]
- De Souza Dias, V.; Pereira Da Luz, M.; Medero, G.; Tarley Ferreira Nascimento, D. An Overview of Hydropower Reservoirs in Brazil: Current Situation, Future Perspectives and Impacts of Climate Change. Water 2018, 10, 592. [Google Scholar] [CrossRef]
- Ometto, J.P.H.B.; Pacheco, F.S.; Cimbleris, A.C.P.; Stech, J.L.; Lorenzzetti, J.; Assireu, A.T.; Santos, M.A.; Matvienko, B.; Rosa, L.P.; Sadigisgalli, C.; et al. Carbon Dynamic and Emissions in Brazilian Hydropower Reservoirs. In Energy Resources: Development, Distribution and Exploitation; Alcantara, E., Ed.; Nova Science Publishers: Hauppauge, NY, USA, 2011; pp. 155–188. [Google Scholar]
- Barros, N.; Cole, J.J.; Tranvik, L.J.; Prairie, Y.T.; Bastviken, D.; Huszar, V.L.M.; Del Giorgio, P.; Roland, F. Carbon Emission from Hydroelectric Reservoirs Linked to Reservoir Age and Latitude. Nat. Geosci. 2011, 4, 593–596. [Google Scholar] [CrossRef]
- Roland, F.; Caraco, N.F.; Cole, J.J.; Del Giorgio, P. Rapid and Precise Determination of Dissolved Oxygen by Spectrophotometry: Evaluation of Interference from Color and Turbidity. Limnol. Oceanogr. 1999, 44, 1148–1154. [Google Scholar] [CrossRef]
- Wetzel, R.G.; Likens, G.E. Limnological Analyses; Springer: New York, NY, USA, 2000; ISBN 978-1-4419-3186-3. [Google Scholar]
- Kirchman, D. Measuring Bacterial Biomass Production and Growth Rates from Leucine Incorporation in Natural Aquatic Environments. In Methods in Microbiology; Elsevier Inc.: Amsterdam, The Netherlands, 2001; Volume 30, pp. 227–237. ISBN 978-0-12-521530-5. [Google Scholar]
- Cottrell, M.T.; Kirchman, D.L. Community Composition of Marine Bacterioplankton Determined by 16S rRNA Gene Clone Libraries and Fluorescence In Situ Hybridization. Appl. Environ. Microbiol. 2000, 66, 5116–5122. [Google Scholar] [CrossRef] [PubMed]
- Massana, R.; Gasol, J.M.; Bjørnsen, P.K.; Blackburn, N.; Hagström, Å.; Hietanen, S.; Hygum, B.H.; Kuparinen, J.; Pedrós-Alió, C. Measurement of Bacterial Size via Analysis of Epifluorescence Preparations: Description of an Inexpensive System and Solutions to Some of the Most Common Problems. Sci. Mar. 1997, 61, 397–407. [Google Scholar]
- Norland, S. The Relationship between Biomass and Volume of Bacteria. In Handbook of Methods in Aquatic Microbial Ecology; Kemp, P., Sherr, B., Sherr, E., Cole, J., Eds.; CRC Press: Boca Raton, FL, USA, 1993; pp. 303–307, 525. [Google Scholar]
- O’Boyle, S.; Nolan, G. The Influence of Water Column Stratification on Dissolved Oxygen Levels in Coastal and Shelf Waters Around Ireland. Biol. Environ. Proc. R. Ir. Acad. 2011, 110, 195–209. [Google Scholar] [CrossRef]
- Gautreau, E.; Volatier, L.; Nogaro, G.; Gouze, E.; Mermillod-Blondin, F. The Influence of Bioturbation and Water Column Oxygenation on Nutrient Recycling in Reservoir Sediments. Hydrobiologia 2020, 847, 1027–1040. [Google Scholar] [CrossRef]
- Bush, T.; Diao, M.; Allen, R.J.; Sinnige, R.; Muyzer, G.; Huisman, J. Oxic-Anoxic Regime Shifts Mediated by Feedbacks between Biogeochemical Processes and Microbial Community Dynamics. Nat. Commun. 2017, 8, 789. [Google Scholar] [CrossRef] [PubMed]
- Spietz, R.L.; Williams, C.M.; Rocap, G.; Horner-Devine, M.C. A Dissolved Oxygen Threshold for Shifts in Bacterial Community Structure in a Seasonally Hypoxic Estuary. PLoS ONE 2015, 10, e0135731. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; He, R.; Song, A.; Huang, Y.; Jin, Z.; Liang, Y.; Li, Q.; Wang, X.; Müller, W.E.G.; Cao, J. Spatial and Temporal Dynamics of Bacterioplankton Community Composition in a Subtropical Dammed Karst River of Southwestern China. MicrobiologyOpen 2019, 8, e00849. [Google Scholar] [CrossRef] [PubMed]
- Pajares, S.; Varona-Cordero, F.; Hernández-Becerril, D.U. Spatial Distribution Patterns of Bacterioplankton in the Oxygen Minimum Zone of the Tropical Mexican Pacific. Microb. Ecol. 2020, 80, 519–536. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Robledo, E.; Padilla, C.C.; Aldunate, M.; Stewart, F.J.; Ulloa, O.; Paulmier, A.; Gregori, G.; Revsbech, N.P. Cryptic Oxygen Cycling in Anoxic Marine Zones. Proc. Natl. Acad. Sci. USA 2017, 114, 8319–8324. [Google Scholar] [CrossRef]
- Okazaki, Y.; Nakano, S. Vertical Partitioning of Freshwater Bacterioplankton Community in a Deep Mesotrophic Lake with a Fully Oxygenated Hypolimnion (Lake Biwa, Japan). Environ. Microbiol. Rep. 2016, 8, 780–788. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, S.; Shi, H. Impacts of Dams and Reservoirs on Local Climate Change: A Global Perspective. Environ. Res. Lett. 2021, 16, 104043. [Google Scholar] [CrossRef]
- Yan, Q.; Bi, Y.; Deng, Y.; He, Z.; Wu, L.; Van Nostrand, J.D.; Shi, Z.; Li, J.; Wang, X.; Hu, Z.; et al. Impacts of the Three Gorges Dam on Microbial Structure and Potential Function. Sci. Rep. 2015, 5, 8605. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhang, W.; Li, Y.; Wu, H.; Yang, N.; Hui, C. Dams Shift Microbial Community Assembly and Imprint Nitrogen Transformation along the Yangtze River. Water Res. 2021, 189, 116579. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.M.; Chavez, F.P.; Francis, C.A. Ammonium Uptake by Phytoplankton Regulates Nitrification in the Sunlit Ocean. PLoS ONE 2014, 9, e108173. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.S.; Sheng, H.-X.; Dai, M.; Zhang, Y.; Shi, D.; Trull, T.W.; Zhu, Y.; Lomas, M.W.; Kao, S.-J. Ambient Nitrate Switches the Ammonium Consumption Pathway in the Euphotic Ocean. Nat. Commun. 2018, 9, 915. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Zhang, C.; Wang, L.; Li, Y.; Zhang, W.; Niu, L.; Zhang, H.; Wang, L. Nitrogen Cycling Processes and the Role of Multi-Trophic Microbiota in Dam-Induced River-Reservoir Systems. Water Res. 2021, 206, 117730. [Google Scholar] [CrossRef] [PubMed]
- Bryant, J.A.; Stewart, F.J.; Eppley, J.M.; DeLong, E.F. Microbial Community Phylogenetic and Trait Diversity Declines with Depth in a Marine Oxygen Minimum Zone. Ecology 2012, 93, 1659–1673. [Google Scholar] [CrossRef] [PubMed]
- Giering, S.L.C.; Evans, C. Overestimation of Prokaryotic Production by Leucine Incorporation—And How to Avoid It. Limnol. Oceanogr. 2022, 67, 726–738. [Google Scholar] [CrossRef]
- Landry, M.R.; Rivera, S.R.; Stukel, M.R.; Selph, K.E. Comparison of Bacterial Carbon Production Estimates from Dilution and 3H-leucine Methods across a Strong Gradient in Ocean Productivity. Limnol. Ocean. Methods 2023, 21, 295–306. [Google Scholar] [CrossRef]
- Lau, M.P.; Del Giorgio, P. Reactivity, Fate and Functional Roles of Dissolved Organic Matter in Anoxic Inland Waters. Biol. Lett. 2020, 16, 20190694. [Google Scholar] [CrossRef]
- Amado, A.M.; Meirelles-Pereira, F.; Vidal, L.O.; Sarmento, H.; Suhett, A.L.; Farjalla, V.F.; Cotner, J.B.; Roland, F. Tropical Freshwater Ecosystems Have Lower Bacterial Growth Efficiency than Temperate Ones. Front. Microbiol. 2013, 4, 167. [Google Scholar] [CrossRef] [PubMed]
- Bertagnolli, A.D.; Stewart, F.J. Microbial Niches in Marine Oxygen Minimum Zones. Nat. Rev. Microbiol. 2018, 16, 723–729. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yu, X.; He, Q.; Deng, T.; Guan, X.; Lian, Y.; Xu, K.; Shu, L.; Wang, C.; Yan, Q.; et al. Niche Differentiation among Comammox (Nitrospira Inopinata) and Other Metabolically Distinct Nitrifiers. Front. Microbiol. 2022, 13, 956860. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Li, Y.; Lin, L.; Zhang, W.; Wang, L.; Niu, L.; Zhang, H. Dam-Induced Flow Velocity Decrease Leads to the Transition from Heterotrophic to Autotrophic System through Modifying Microbial Food Web Dynamics. Environ. Res. 2022, 212, 113568. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Zhang, B.; Liu, J.; Fang, Y.; Wang, A. Spatiotemporal Dynamics in Microbial Communities Mediating Biogeochemical Cycling of Nutrients across the Xiaowan Reservoir in Lancang River. Sci. Total Environ. 2022, 813, 151862. [Google Scholar] [CrossRef]
- Nie, W.-B.; Xie, G.-J.; Tan, X.; Ding, J.; Lu, Y.; Chen, Y.; Yang, C.; He, Q.; Liu, B.-F.; Xing, D.; et al. Microbial Niche Differentiation during Nitrite-Dependent Anaerobic Methane Oxidation. Environ. Sci. Technol. 2023, 57, 7029–7040. [Google Scholar] [CrossRef] [PubMed]
- Samad, M.S.; Lee, H.J.; Cerbin, S.; Meima-Franke, M.; Bodelier, P.L.E. Niche Differentiation of Host-Associated Pelagic Microbes and Their Potential Contribution to Biogeochemical Cycling in Artificially Warmed Lakes. Front. Microbiol. 2020, 11, 582. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Lu, L.; Guo, J.; Yang, J.; Zhang, J.; He, B.; Xu, L. Responses of Spatial-Temporal Dynamics of Bacterioplankton Community to Large-Scale Reservoir Operation: A Case Study in the Three Gorges Reservoir, China. Sci. Rep. 2017, 7, 42469. [Google Scholar] [CrossRef] [PubMed]
- Sakami, T. Seasonal and Spatial Variation of Bacterial Community Structure in River-Mouth Areas of Gokasho Bay, Japan. Microb. Environ. 2008, 23, 277–284. [Google Scholar] [CrossRef]
- Langenheder, S.; Sobek, S.; Tranvik, L.J. Changes in Bacterial Community Composition along a Solar Radiation Gradient in Humic Waters. Aquat. Sci. 2006, 68, 415–424. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, L.; Hong, Y.; Wu, J.; Zhu, G.; Ye, F.; Li, Z. Divergent Responses of Taxonomic and Predicted Functional Profiles of Bacterioplankton to Reservoir Impoundment. Environ. Res. 2020, 182, 109083. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-González, C.; Proia, L.; Ferrera, I.; Gasol, J.M.; Sabater, S. Effects of Large River Dam Regulation on Bacterioplankton Community Structure. FEMS Microbiol. Ecol. 2013, 84, 316–331. [Google Scholar] [CrossRef] [PubMed]
- Carlson, C.A.; Hansell, D.A. DOM Sources, Sinks, Reactivity, and Budgets. In Biogeochemistry of Marine Dissolved Organic Matter; Elsevier Inc.: Amsterdam, The Netherlands, 2015; pp. 65–126. ISBN 978-0-12-405940-5. [Google Scholar]
- Guerrero-Feijóo, E.; Nieto-Cid, M.; Sintes, E.; Dobal-Amador, V.; Hernando-Morales, V.; Álvarez, M.; Balagué, V.; Varela, M.M. Optical Properties of Dissolved Organic Matter Relate to Different Depth-Specific Patterns of Archaeal and Bacterial Community Structure in the North Atlantic Ocean. FEMS Microbiol. Ecol. 2017, 93, fiw224. [Google Scholar] [CrossRef] [PubMed]
- Goto, S.; Tada, Y.; Suzuki, K.; Yamashita, Y. Evaluation of the Production of Dissolved Organic Matter by Three Marine Bacterial Strains. Front. Microbiol. 2020, 11, 584419. [Google Scholar] [CrossRef] [PubMed]
- Baetge, N.; Bolaños, L.M.; Penna, A.D.; Gaube, P.; Liu, S.; Opalk, K.; Graff, J.R.; Giovannoni, S.J.; Behrenfeld, M.J.; Carlson, C.A. Bacterioplankton Response to Physical Stratification Following Deep Convection. Elem. Sci. Anthr. 2022, 10, 00078. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Y.; Wang, X. Spatiotemporal Variation of Bacterial and Archaeal Communities in Sediments of a Drinking Reservoir, Beijing, China. Appl. Microbiol. Biotechnol. 2017, 101, 3379–3391. [Google Scholar] [CrossRef] [PubMed]
- Medina-Silva, R.; De Oliveira, R.R.; Pivel, M.A.G.; Borges, L.G.A.; Simão, T.L.L.; Pereira, L.M.; Trindade, F.J.; Augustin, A.H.; Valdez, F.P.; Eizirik, E.; et al. Microbial Diversity from Chlorophyll Maximum, Oxygen Minimum and Bottom Zones in the Southwestern Atlantic Ocean. J. Mar. Syst. 2018, 178, 52–61. [Google Scholar] [CrossRef]
- Vergara, C.; Muñoz, R.; Campos, J.L.; Seeger, M.; Jeison, D. Influence of Light Intensity on Bacterial Nitrifying Activity in Algal-Bacterial Photobioreactors and Its Implications for Microalgae-Based Wastewater Treatment. Int. Biodeterior. Biodegrad. 2016, 114, 116–121. [Google Scholar] [CrossRef]
- Lehours, A.-C.; Bardot, C.; Thenot, A.; Debroas, D.; Fonty, G. Anaerobic Microbial Communities in Lake Pavin, a Unique Meromictic Lake in France. Appl. Environ. Microbiol. 2005, 71, 7389–7400. [Google Scholar] [CrossRef]
- Lin, X.; Wakeham, S.G.; Putnam, I.F.; Astor, Y.M.; Scranton, M.I.; Chistoserdov, A.Y.; Taylor, G.T. Comparison of Vertical Distributions of Prokaryotic Assemblages in the Anoxic Cariaco Basin and Black Sea by Use of Fluorescence In Situ Hybridization. Appl. Environ. Microbiol. 2006, 72, 2679–2690. [Google Scholar] [CrossRef]
- Parsons, R.J.; Nelson, C.E.; Carlson, C.A.; Denman, C.C.; Andersson, A.J.; Kledzik, A.L.; Vergin, K.L.; McNally, S.P.; Treusch, A.H.; Giovannoni, S.J. Marine Bacterioplankton Community Turnover within Seasonally Hypoxic Waters of a Subtropical Sound: D Evil’s H Ole, B Ermuda. Environ. Microbiol. 2015, 17, 3481–3499. [Google Scholar] [CrossRef] [PubMed]
- Lentini, V.; Gugliandolo, C.; Maugeri, T.L. Vertical Distribution of Archaea and Bacteria in a Meromictic Lake as Determined by Fluorescent In Situ Hybridization. Curr. Microbiol. 2012, 64, 66–74. [Google Scholar] [CrossRef]
- Humayoun, S.B.; Bano, N.; Hollibaugh, J.T. Depth Distribution of Microbial Diversity in Mono Lake, a Meromictic Soda Lake in California. Appl. Environ. Microbiol. 2003, 69, 1030–1042. [Google Scholar] [CrossRef] [PubMed]
- Aldunate, M.; De La Iglesia, R.; Bertagnolli, A.D.; Ulloa, O. Oxygen Modulates Bacterial Community Composition in the Coastal Upwelling Waters off Central Chile. Deep. Sea Res. Part II Top. Stud. Oceanogr. 2018, 156, 68–79. [Google Scholar] [CrossRef]
- La Ferla, R.; Maimone, G.; Caruso, G.; Azzaro, F.; Azzaro, M.; Decembrini, F.; Cosenza, A.; Leonardi, M.; Paranhos, R. Are Prokaryotic Cell Shape and Size Suitable to Ecosystem Characterization? Hydrobiologia 2014, 726, 65–80. [Google Scholar] [CrossRef]
- Pernthaler, J. Freshwater Microbial Communities. In The Prokaryotes; Rosenberg, E., DeLong, E.F., Lory, S., Stackebrandt, E., Thompson, F., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 97–112. ISBN 978-3-642-30122-3. [Google Scholar]
- Oikonomou, A.; Pachiadaki, M.; Stoeck, T. Protistan Grazing in a Meromictic Freshwater Lake with Anoxic Bottom Water. FEMS Microbiol. Ecol. 2014, 87, 691–703. [Google Scholar] [CrossRef]
- Nürnberg, G.K. Quantification of Anoxia and Hypoxia in Water Bodies. In Encyclopedia of Water; Maurice, P., Ed.; Wiley: Hoboken, NJ, USA, 2019; pp. 1–9. ISBN 978-1-119-30075-5. [Google Scholar]

| Probes | Specificity | Sequence (5′-3′) |
|---|---|---|
| ARCH915 | Archaea | GTG CTC CCC CGC CAA TTC CT |
| Alf968 | Alphaproteobacteria | GGT AAG GTT CTG CGC GTT |
| Bet42a | Betaproteobacteria | GCC TTC CCA CAT CGT TT |
| Gam42a | Gammaproteobacteria | GCC TTC CCA CAT CGT TT |
| CF319a | Cytophaga–Flavobacter | TGG TCC GTG TCT CAG TAC |
| Depth (m) | Prokaryotes Abundance (cells 106 mL−1) | Archaea | Alpha | Beta | Gamma | Cytophaga–Flavobacter | Other Groups |
|---|---|---|---|---|---|---|---|
| Proteobacteria (% of Total Bacterial Abundance) | |||||||
| 0 | 0.9 | 9 ± 2.5 | 10 ± 2.2 | 10 ± 6.5 | <0.1 | 10 ± 3.4 | 61 |
| 6 | 0.6 | 7 ± 0.4 | 9 ± 5.6 | 4 ± 0.4 | 9 ± 9.7 | 3 ± 2.0 | 68 |
| 12 | 1.2 | 1 ± 0.5 | 10 ± 1.8 | 2 ± 2.4 | 8 ± 5.0 | 12 ± 2.0 | 67 |
| 47 | 0.8 | 6 ± 4.3 | 4 ± 1.0 | 6 ± 1.7 | 3 ± 1.7 | 5 ± 0.6 | 76 |
| 107 | 1.1 | 33 ± 9.0 | 16 ± 1.3 | 9 ± 1.0 | 11 ± 7.8 | 14 ± 4.1 | 17 |
| Depth (m) | Prokaryotes Biomass (μg C mL−1) | Archaea | Alpha | Beta | Gamma | Cytophaga–Flavobacter | Other Groups |
|---|---|---|---|---|---|---|---|
| Proteobacteria (% of Total Bacterial Biomass) | |||||||
| 0 | 16 | 21 | 15 | 20 | <0.1 | 20 | 24 |
| 6 | 27 | 2.2 | 9.2 | 9.2 | 4.9 | 2.7 | 72 |
| 12 | 55 | 2.2 | 9.3 | 1.5 | 5.5 | 11 | 71 |
| 47 | 41 | 3.4 | 7.5 | 6.0 | 1.5 | 4.8 | 77 |
| 107 | 41 | 24 | 23 | 9.1 | 13 | 13 | 18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Del’Duca, A.; Janiques, A.M.d.S.; Azevedo, R.d.S.; Roland, F.; Cesar, D.E. Oxygen Concentration and Its Implications for Microbial Structure and Metabolism: A Case Study in a Deep Tropical Reservoir. Diversity 2024, 16, 444. https://doi.org/10.3390/d16080444
Del’Duca A, Janiques AMdS, Azevedo RdS, Roland F, Cesar DE. Oxygen Concentration and Its Implications for Microbial Structure and Metabolism: A Case Study in a Deep Tropical Reservoir. Diversity. 2024; 16(8):444. https://doi.org/10.3390/d16080444
Chicago/Turabian StyleDel’Duca, Alessandro, Amanda Meirelles de Sá Janiques, Raiza dos Santos Azevedo, Fábio Roland, and Dionéia Evangelista Cesar. 2024. "Oxygen Concentration and Its Implications for Microbial Structure and Metabolism: A Case Study in a Deep Tropical Reservoir" Diversity 16, no. 8: 444. https://doi.org/10.3390/d16080444






