Pollination Strategies and Reproductive Biology of Fritillaria imperialis L. (Liliaceae): Insights from Erzincan, Türkiye
Abstract
:1. Introduction
2. Materials and Methods
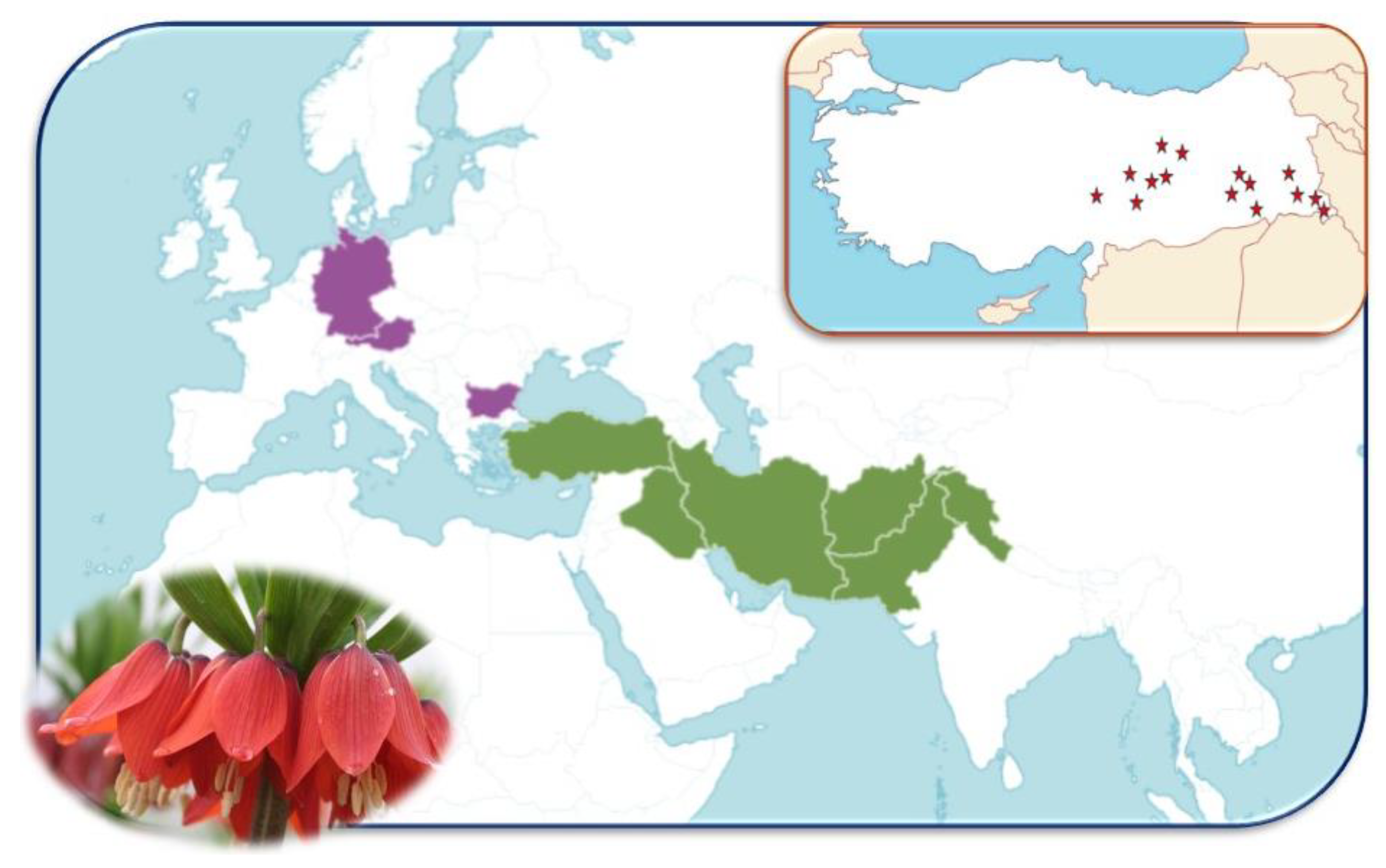
2.1. Study Site and Plant Material
2.2. Detection of Pollination Strategies
2.3. Detection of Flowering Dynamics
2.4. Detection of Pollen Viability and Stigma Receptivity
2.5. Detection of the Pollen/Ovule Ratio (P/O) and Self-Incompatibility Index (SII)
2.6. Detection of Germination and Seed Viability
2.7. Detection of Floral Visitors
3. Results
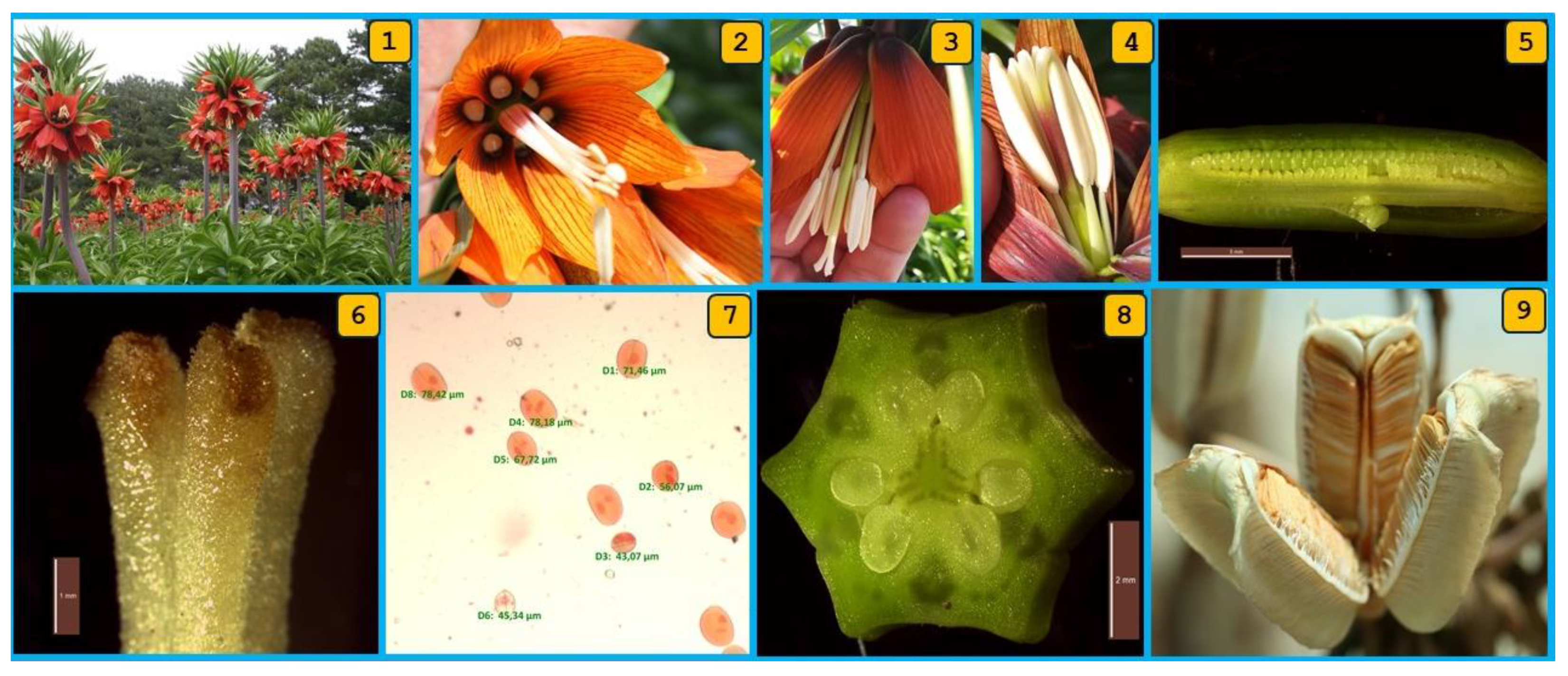
3.1. Floral Features and Reproductive Phenology of F. imperialis
3.2. Flowering Phenology of F. imperialis
3.3. Pollen/Ovule (P/O) Ratio and Self-Incompatibility Index (SII) of F. imperialis
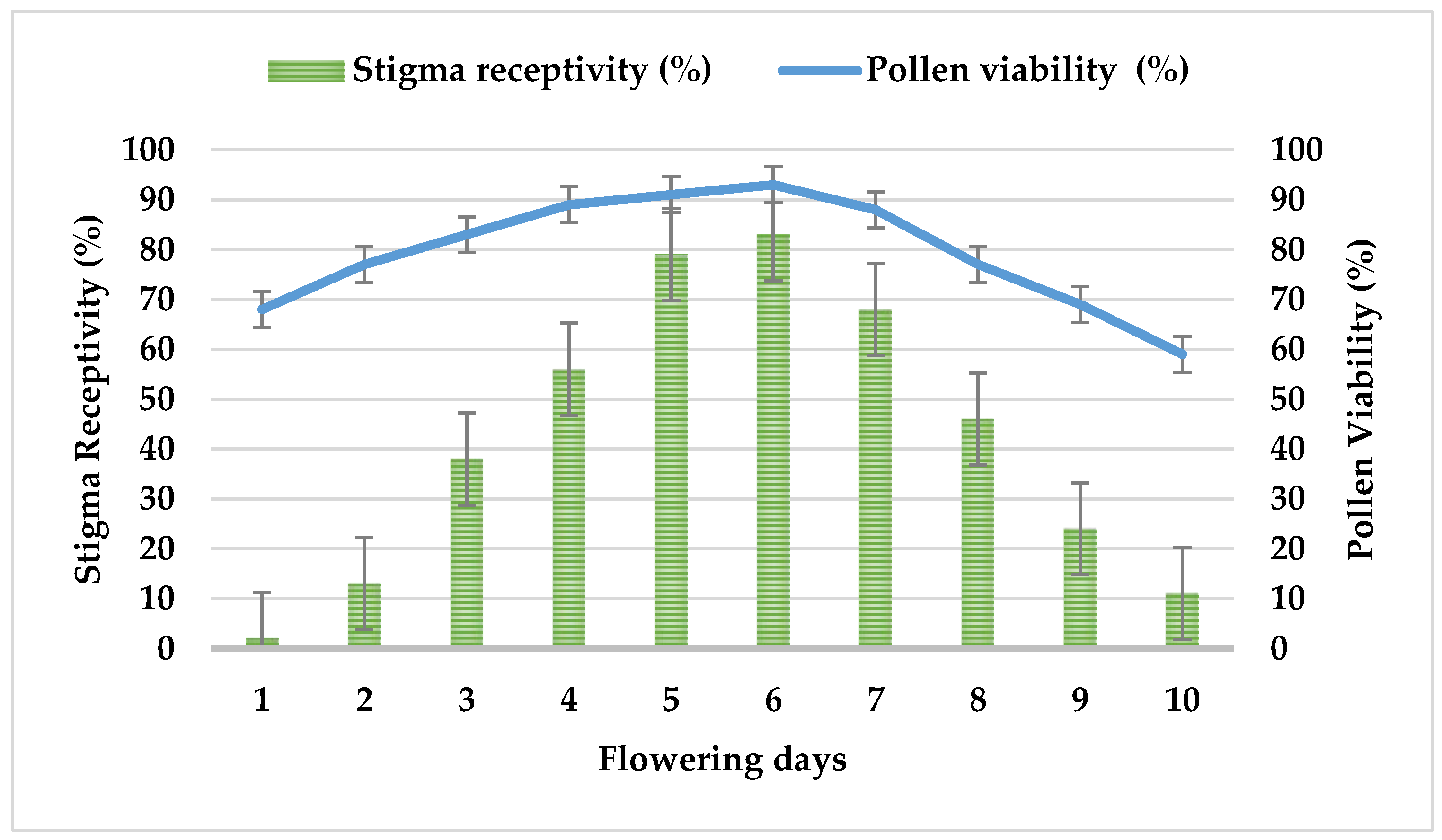
3.4. Stigma Receptivity and Pollen Viability of F. imperialis
3.5. Pollination Experiments of F. imperialis
3.6. Seed Viability of F. imperialis
3.7. Pollinator Observations of F. imperialis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Körner, C. Alpine Plant Life Functional Plant Ecology of High Mountain Ecosystems, 2nd ed.; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 2003. [Google Scholar] [CrossRef]
- Kaya, O.; Kose, C.; Donderalp, V.; Gecim, T.; Taskın, S. Last updates on cell death point, bud death time and exothermic characteristics of flower buds for deciduous fruit species by using differential thermal analysis. Sci. Hortic. 2020, 270, 109403. [Google Scholar] [CrossRef]
- Tsiftsis, S.; Djordjević, V. Modelling sexually deceptive orchid species distributions under future climates: The importance of plant pollinator interactions. Sci. Rep. 2020, 10, 10623. [Google Scholar] [CrossRef] [PubMed]
- Razanajatovo, M.; Fischer, L.; van Kleunen, M. Do floral traits and the selfing capacity of Mimulus guttatus plastically respond to experimental temperature changes? Oecologia 2020, 192, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Cooper, E.J.; Dullinger, S.; Semenchuk, P. Late snowmelt delays plant development and results in lower reproductive success in the High Arctic. Plant Sci. 2011, 180, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Yıldız, F.; Aslay, M.; Kandemir, A.; Kaya, O. Reproductive Biology of Fritillaria aurea Schott (Liliaceae), a Rare Species Endemic to Turkey. Diversity 2022, 14, 1052. [Google Scholar] [CrossRef]
- Gopalakrishnan, K.K.; Thomas, T.D. Reproductive biology of Pittosporum dasycaulon Miq., (Family Pittosporaceae) a rare medicinal tree endemic to Western Ghats. Bot. Stud. 2014, 55, 15. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Liu, X.; Li, Z.; Ma, H.; Wan, Y.; Liu, X.; Fu, L. Study on Reproductive Biology of Rhododendron longipedicellatum: A Newly Discovered and Special Threatened Plant Surviving in Limestone Habitat in Southeast Yunnan, China. Front. Plant Sci. 2018, 9, 33. [Google Scholar] [CrossRef] [PubMed]
- Moza, M.K.; Bhatnagar, A.K. Plant reproductive biology studies crucial for conservation. Curr. Sci. 2007, 92, 1207. [Google Scholar]
- Roguz, K.; Bajguz, A.; Gołębiewska, A.; Chmur, M.; Hill, L.; Kalinowski, P.; Schönenberger, J.; Stpiczyńska, M.; Zych, M. Functional diversity of nectary structure and nectar composition in the genus Fritillaria (Liliaceae). Front. Plant Sci. 2018, 9, 1246. [Google Scholar] [CrossRef] [PubMed]
- Ono, A.; Dohzono, I.; Sugawara, T. Bumblebee pollination and reproductive biology of Rhododendron semibarbatum (Ericaceae). J. Plant Res. 2008, 121, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Stout, J.C. Pollination of invasive Rhododendron ponticum (Ericaceae) in Ireland. Apidologie 2007, 38, 198–206. [Google Scholar] [CrossRef]
- Kuswantoro, F. Flower-insect visitor interaction: Case study on Rhododendron inundatum Sleumer in Bali Botanic Garden. J. Trop. Biodivers. Biotechnol. 2017, 2, 35–38. [Google Scholar] [CrossRef]
- Sutherland, S. Floral sex ratios, fruit set, and resource allocation in plants. Ecology 1986, 67, 991–1001. [Google Scholar] [CrossRef]
- Bawa, K.S.; Webb, C.J. Flower, fruit and seed abortion in tropical forest trees: Implications for the evolution of paternal and maternal reproductive patterns. Am. J. Bot. 1984, 71, 736–751. [Google Scholar] [CrossRef]
- Humphreys, A.M.; Govaerts, R.; Ficinski, S.Z.; Nic Lughadha, E.; Vorontsova, M.S. Global dataset shows geography and life form predict modern plant extinction and rediscovery. Nat. Ecol. Evol. 2019, 3, 1043–1047. [Google Scholar] [CrossRef] [PubMed]
- Nic Lughadha, E.; Bachman, S.P.; Leão, T.C.; Forest, F.; Halley, J.M.; Moat, J.; Acedo, C.; Bacon, K.L.; Brewer, R.F.A.; Gâteblé, G.; et al. Extinction risk and threats to plants and fungi. Plants People Planet 2020, 2, 389–408. [Google Scholar] [CrossRef]
- Tekşen, M.; Aytaç, Z. The revision of the genus Fritillaria L. (Liliaceae) in the Mediterranean region (Turkey). Turk. J. Bot. 2011, 35, 447–478. [Google Scholar] [CrossRef]
- Hill, L. 847. Fritillaria Kiusiana: Liliaceae. Curtis’s Bot. Mag. 2016, 33, 275–285. [Google Scholar] [CrossRef]
- Kiani, M.; Mohammadi, S.; Babaei, A.; Sefidkon, F.; Naghavi, M.R.; Ranjbar, M.; Razavi, S.A.; Saeidi, K.; Jafari, H.; Asgari, D.; et al. Iran supports a great share of biodiversity and floristic endemism for Fritillaria spp. (Liliaceae): A review. Plant Divers. 2017, 39, 245–262. [Google Scholar] [CrossRef] [PubMed]
- Tamura, M.N. Liliaceae. In The Families and Genera of Vascular Plants; Kubitzki, K., Ed.; Springer: Berlin/Heidelberg, Germany, 1998; Volume 3, pp. 343–353. [Google Scholar] [CrossRef]
- Rønsted, N.; Law, S.; Thornton, H.; Fay, M.F.; Chase, M.W. Molecular phylogenetic evidence for the monophyly of Fritillaria and Lilium (Liliaceae; Liliales) and the infrageneric classification of Fritillaria. Mol. Phylogenetics Evol. 2005, 35, 509–527. [Google Scholar] [CrossRef]
- Tomović, G.; Vukojičić, S.; Niketić, M.; Zlatković, B.; Stevanović, V. Fritillaria (Liliaceae) in Serbia: Distribution, habitats and some taxonomic notes. Phytol. Balc. 2007, 13, 359–370. [Google Scholar]
- Day, P.D.; Berger, M.; Hill, L.; Fay, M.F.; Leitch, A.R.; Leitch, I.J.; Kelly, L.J. Evolutionary relationships in the medicinally important genus Fritillaria L. (Liliaceae). Mol. Phylogenetics Evol. 2014, 80, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Özhatay, N. Fritillaria L. In Flora of Turkey and the East Aegean Islands; Davis, P.H., Mill, R.R., Eds.; Edinburgh University Press: Edinburgh, UK, 2000; Volume 11, pp. 243–245. [Google Scholar]
- Rix, M.; Strange, K. Fritillaria sororum Liliaceae. Curtis’s Bot. Mag. 2014, 31, 214–222. [Google Scholar] [CrossRef]
- IUCN. IUCN Red List Categories and Criteria; Version 3.1; IUCN Species Survival Commission: Gland, Switzerland, 2001. [Google Scholar]
- Zych, M.; Stpiczyńska, M.; Roguz, K. Pollination biology and breeding system of European Fritillaria meleagris L. (Liliaceae). Plant Reprod. 2014, 27, 147–163. [Google Scholar] [CrossRef]
- Rix, E.M. Fritillaria L. In Flora of Turkey and the East Aegean Islands, 1st ed.; Davis, P.H., Ed.; Edinburgh University Press: Edinburgh, UK, 1975; Volume 8, pp. 284–302. [Google Scholar]
- Tekşen, M. Fritillaria L. In Resimli Türkiye Florası; Güner, A., Kandemir, A., Menemen, Y., Yıldırım, H., Aslan, S., Ekşi, G., Güner, I., Çimen, A.Ö., Eds.; Ali Nihat Gökyiğit Vakfı Nezahat Gökyiğit Botanik Bahçesi Yayınları: Istanbul, Turkey, 2018; Volume 2, pp. 800–879. (In Turkish) [Google Scholar]
- Yildirim, H.; Tekşen, M. Fritillaria arsusiana (Lilieae, Liliaceae), a new species from southern Anatolia. Phytotaxa 2021, 502, 149–159. [Google Scholar] [CrossRef]
- Eker, İ.; Tekşen, M. Fritillaria umitkaplanii (Liliaceae), a new species from south Anatolia. Nord. J. Bot. 2022, 2023, e03803. [Google Scholar] [CrossRef]
- Adıgüzel, N.; Bani, B.; Pınar, S.M. Rare and endemic species from Van and Hakkari provinces, East Anatolia/Turkey and their threat categories. In Plant, Fungal and Habitat Diversity Investigation and Conservation, Proceedings of the IV Balkan Botanical Congress, Sofia, Bulgaria, 20–26 June 2006; Institute of Botany: Botany, NSW, Australia, 2009; p. 229. [Google Scholar]
- Burquez, A. Blue tits, Parus caeruleus, as pollinators of the crown imperial, Fritillaria imperialis in Britain. Oikos 1989, 55, 335–340. [Google Scholar] [CrossRef]
- Roguz, K.; Hill, L.; Koethe, S.; Lunau, K.; Roguz, A.; Zych, M. Visibility and attractiveness of Fritillaria (Liliaceae) flowers to potential pollinators. Sci. Rep. 2021, 11, 11006. [Google Scholar] [CrossRef] [PubMed]
- Peters, W.S.; Pirl, M.; Gottsberger, G.; Peters, D.S. Pollination of the crown imperial Fritillaria imperialis by great tits Parus major. J. Für Ornithol. 1995, 136, 207–212. [Google Scholar] [CrossRef]
- Dafni, A. Pollination Ecology: A Practical Approach; University Press: New York, NY, USA, 1992. [Google Scholar]
- Ma, Y.; Cui, Z.; Cheng, C.Y.; Li, R.; Wu, H.; Jin, L.; Ma, Y.; Wang, Z. Flowering characteristics and mating system of Fritillaria cirrhosa (Liliaceae), an endangered plant in China. Braz. J. Bot. 2022, 45, 1307–1318. [Google Scholar] [CrossRef]
- Dafni, A.; Maues, J. A rapid and simple procedure to determine stigma receptivity. Sex. Plant Reprod. 1998, 11, 177–180. [Google Scholar] [CrossRef]
- Shivanna, K.R.; Tandon, R. Reproductive Ecology of Flowering Plants: A Manual; Springer: New Delhi, India, 2014; pp. 107–123. [Google Scholar] [CrossRef]
- Cruden, R.W. Pollen-ovule ratios: A conservative indicator of breeding systems in flowering plants. Evolution 1977, 31, 32–46. [Google Scholar] [CrossRef] [PubMed]
- Zapata, T.R.; Arroyo, M.T.K. Plant reproductive ecology of a secondary deciduous tropical forest in Venezuela. Biotropica 1978, 10, 221–230. [Google Scholar] [CrossRef]
- Aslay, M.; Ünlü, M.H.; Kadıoğlu, Z.; Tuncer, S.; Kaya, E. Development of New Varieties from Turkeys Endemic Species—Fritillaria michailovskyi Fomın. In Researches in Landscape and Ornamental Plants; Gece Kitaplıgı: Ankara, Turkey, 2019; pp. 27–49. [Google Scholar]
- Roguz, K.; Hill, L.; Roguz, A.; Zych, M. Evolution of bird and insect flower traits in Fritillaria L. (Liliaceae). Front. Plant Sci. 2021, 12, 656783. [Google Scholar] [CrossRef]
- Tekşen, M.; Aytaç, Z. Fritillaria mughlae (Liliaceae), a new species from Turkey. In Annales Botanici Fennici; Finnish Zoological and Botanical Publishing Board: Helsinki, Finland, 2008; Volume 45, pp. 141–147. [Google Scholar]
- Sonay, V.; Tekşen, M.; Yıldırım, H.; Balos, M.M.; Akan, H. Fritillaria karakocanensis (Liliaceae), a new species of the F. crassifolia group from Anatolia (Turkey). Nord. J. Bot. 2023, 2023, e03903. [Google Scholar] [CrossRef]
- Tekşen, M.; Aytac, Z.; Pinar, N.M. Pollen morphology of the genus Fritillaria L.(Liliaceae) in Turkey. Turk. J. Bot. 2010, 34, 397–416. [Google Scholar] [CrossRef]
- Zych, M.; Stpiczyńska, M. Neither protogynous nor obligatory out-crossed: Pollination biology and breeding system of the European Red List Fritillaria meleagris L.(Liliaceae). Plant Biol. 2012, 14, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Lei, F.W.; Wu, Y.M.; Shen, X.L.; Xia, X.F.; Zhang, D.H.; Mu, X.Y.; Zhang, Z.X. Multiple reproductive strategies of a spring ephemeral plant, Fritillaria maximowiczii, enable its adaptation to harsh environments. Plant Species Biol. 2022, 37, 38–51. [Google Scholar] [CrossRef]
- Zhang, X.; Cui, D.; Zong, X.; Ren, R.; Wei, J.; Situ, L.; Zhang, Y. Pollination biology and breeding system of Fritillaria ussuriensis Maxim. Acta Bot. Boreali-Occident. Sin. 2010, 30, 1404–1408. [Google Scholar]
- Gao, Y.Q.; Zhang, L.X.; Wang, M.R.; Song, B. Pollination biology of Fritillaria delavayi. China J. Chin. Mater. Medica 2014, 39, 1795–1798. [Google Scholar]
- Gao, Y.; Wang, C.; Song, B.; Du, F. Corolla retention after pollination facilitates the development of fertilized ovules in Fritillaria delavayi (Liliaceae). Sci. Rep. 2019, 9, 729. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xu, B.Q.; Guo, F.X.; Bai, G.; Zhang, J.F.; Zhang, Y. Studies of the floral organ characteristics and sexual breeding system of Fritillaria unibracteata. Acta Prataculturae Sin. 2017, 26, 90–98. [Google Scholar]
- Anderson, G.J. Systematics and reproductive biology. In Experimental and Molecular Approaches to Plant Systematics; Hoch, P.C., Stephenson, A.G., Eds.; Monographs in Systematic Botany from the Missouri Botanical Garden; Missouri Botanical Garden: St. Louis, MO, USA, 1995; pp. 263–272. [Google Scholar]
- Bernardello, G.; Anderson, G.J.; Lopez, S.P.; Cleland, M.A.; Stuessy, T.F.; Crawford, D.J. Reproductive biology of Lactoris fernandeziana (Lactoridaceae). Am. J. Bot. 1999, 86, 829–840. [Google Scholar] [CrossRef] [PubMed]
- Seifert, T.; Muller-Starck, G. Impacts of fructification on biomass production and correlated genetic effects in Norway spruce (Picea abies [L.] Karst.). Eur. J. For. Res. 2009, 128, 155–169. [Google Scholar] [CrossRef]
- Yashima, T.; Kinoshita, E.; Shimizu, T. Flowering phenology and self-incompatibility in Fritillaria camtschatcensis (L.) Ker-Gawl. J. Phytogeogr. Taxon. 1997, 45, 129–133. [Google Scholar]
- Aslay, M.; Yıldız, F.; Kaya, O.; Bita-Nicolae, C. Reproductive biology and pollination ecology of Fritillaria michailovskyi Fomin (Liliaceae), endemic to East Anatolia (Turkey). Diversity 2023, 15, 414. [Google Scholar] [CrossRef]
- Nyman, Y. Pollination mechanisms in six Campanula species (Campanulaceae). Plant Syst. Evol. 1992, 181, 97–108. [Google Scholar] [CrossRef]
- Barrett, S.C.H.; Cruzan, M.B. Incompatibility in heterostylous plants. In Genetic Control of Self Incompatibility and Reproductive Development in Flowering Plants; Williams, E.G., Clarke, A.E., Knox, R.B., Eds.; Kluwer: Dordrecht, The Netherlands, 1994; pp. 189–219. [Google Scholar]
- Brys, R.; Jacquemyn, H.; De Bruyn, L.; Hermy, M. Pollination success and reproductive output in experimental populations of the self-incompatible Primula vulgaris. Int. J. Plant Sci. 2007, 168, 571–578. [Google Scholar] [CrossRef]
- Culley, T.M.; Weller, S.G.; Sakai, A.K. The evolution of wind pollination in angiosperms. Trends Ecol. Evol. 2002, 17, 361–369. [Google Scholar] [CrossRef]
- Friedman, J.; Barrett, S.C. Wind of change: New insights on the ecology and evolution of pollination and mating in wind-pollinated plants. Ann. Bot. 2009, 103, 1515–1527. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, E.; Peruzzi, L. Male individuals in cultivated Fritillaria persica L. (Liliaceae): Real androdioecy or gender disphasy? Turk. J. Bot. 2010, 34, 435–440. [Google Scholar] [CrossRef]
- Kawano, S.; Masuda, J.; Hayashi, K. Life-history monographs of Japanese plants. 10: Fritillaria koidzumiana Ohwi (Liliaceae). Plant Species Biol. 2008, 23, 51–57. [Google Scholar] [CrossRef]
- Peruzzi, L.; Mancuso, E.; Gargano, D. Males are cheaper, or the extreme consequence of size/age-dependent sex allocation: Sexist gender diphasy in Fritillaria montana (Liliaceae). Bot. J. Linn. Soc. 2012, 168, 323–333. [Google Scholar] [CrossRef]
- Dafni, A.; Firmage, D. Pollen viability and longevity: Practical, ecological and evolutionary implications. Plant Syst. Evol. 2000, 222, 113–132. [Google Scholar] [CrossRef]
- He, G.; Hu, F.; Ming, J.; Liu, C.; Yuan, S. Pollen viability and stigma receptivity in Lilium during anthesis. Euphytica 2017, 213, 231. [Google Scholar] [CrossRef]
- Stanley, R.G.; Linskens, H.F. Pollen: Biology Biochemistry and Management; Springer: Berlin/Heidelberg, Germany, 1974. [Google Scholar] [CrossRef]
- Colling, G.; Reckinger, C.; Matthies, D. Effects of pollen quantity and quality on reproduction and offspring vigour in the rare plant Scorzonera humilis (Asteraceae). Am. J. Bot. 2004, 91, 1774–1782. [Google Scholar] [CrossRef] [PubMed]
- Rocha, O.J.; Aguilar, G. Reproductive biology of the dry forest tree Enterolobium cyclocarpum (Guanacaste) in Costa Rica: A comparison between trees left in pastures and in continuous forest. Am. J. Bot. 2001, 88, 1607–1614. [Google Scholar] [CrossRef] [PubMed]
- Dudash, M.R.; Fenster, C.B. Inbreeding and outbreeding depression in fragmented populations. In Genetics, Demography and Viability of Fragmented Populations; Young, A., Clarke, G., Eds.; Cambridge University Press: Cambridge, UK, 2000; pp. 35–53. [Google Scholar] [CrossRef]
- Byers, D.L. Pollen quantity and quality as explanations for low seed set in small populations exemplified by Eupatorium (Asteraceae). Am. J. Bot. 2004, 82, 1000–1006. [Google Scholar] [CrossRef]
- Westerkamp, C. Honeybees are poor pollinators—Why? Plant Syst. Evol. 1991, 177, 71–75. [Google Scholar] [CrossRef]
- Knuth, P. Handbuch der Blutenbiologie, Band II, Teil 2: Lobeliaceae bis Gnetaceae; Verlag von Wilhelm Engelmann: Leipzig, Germany, 1899. [Google Scholar]
- Rix, E.M.; Rast, D. Nectar sugars and subgeneric classification in Fritillaria. Biochem. Syst. Ecol. 1975, 2, 207–209. [Google Scholar] [CrossRef]
- Huang, T.; Song, B.; Chen, Z.; Sun, H.; Niu, Y. Pollinator shift ensures reproductive success in a camouflaged alpine plant. Ann. Bot. 2024, 134, 325–336. [Google Scholar] [CrossRef]




| Procedure Code | Procedure | Measured Strategy | Possibility of Self-Pollination | Possibility of Cross-Pollination | Fruit/Seed- Setting Status |
|---|---|---|---|---|---|
| C | Free exposure (control group) | Natural Pollination | Likely | Likely | + |
| P1 | Bagged | Spontaneous autogamy | Likely | No | − |
| P2 | Bagged, hand self-pollinated | Assisted autogamy | Likely | No | − |
| P3 | Emasculated, bagged, hand self-pollinated | Geitonogamy | Likely | No | − |
| P4 | Emasculated, free exposure | Spontaneous allogamy | No | Likely | + |
| P5 | Emasculated, bagged, hand cross-pollinated | Assisted allogamy | No | Likely | + |
| P6 | Emasculated, bagged | Apomixis | No | No | − |
| Character | Unit | Observations | Mean Value |
|---|---|---|---|
| Flowering period | month | March–April | - |
| Inflorescence | term | Umbella | - |
| Bulb | term | Ovoid, perennial | - |
| Perigon | term | Broadly campanulate | - |
| Plant length | cm | 28–122 (min and max) | 58 ± 0.9 |
| Flower | term | Hermaphrodite, weakly zygomorhic | - |
| Flower color | term | orange to red, rarely yellow | - |
| Flower number | number | 3–21 (min and max) | 8 ± 1.2 |
| Flower length | mm | 36–68 (min and max) | 52 ± 3.4 |
| Odor | term | Nil | - |
| Nectar | term | White, drop-like, dense | - |
| Anther dehiscence | term | Bursting inward by slits, extrorse | - |
| Anthers per flower | number | 6 | 6 ± 0.5 |
| Pollen grains per flower | number | 612,000–775,000 (min–max) | 702.000 ± 12.36 |
| Ovules/ovary per flower | number | 168–240 (min and max) | 228 ± 5.3 |
| Length of petal | mm | 35–75 (min and max) | 54 ± 1.5 |
| Length of filament | mm | 18–64 (min and max) | 41 ± 2.4 |
| Length of anther | mm | 11–22 (min and max) | 18 ± 1.6 |
| Length of style | mm | 16–46 (min and max) | 36 ± 1.3 |
| Pollen surface | term | Granulate | - |
| Pollen shape | term | Subprolate | - |
| Pollen size | μm | 42–74 (min and max) | 56 ± 4.3 |
| Pollen cell | number | 2-celled | 2 ± 0.4 |
| Pollen viability | % | 83–97 (min and max) | 93% |
| Stigma shape | term | Trifid or trilobate | - |
| Pollen–ovule ratio | number | 2898–3220 (min and max) | 3080 ± 10.9 |
| Seed viability | % | 71–92 (min and max) | 86.3 ± 1.2 |
| Stigma surface | term | Wet | - |
| Diameter of ovary | mm | 1.8–4.2 (min and max) | 3.4 ± 0.5 |
| Ovary placentation type | term | Axile | - |
| Ovary capsule | number | 6-lobed | 6 ± 1.1 |
| Ovary status | term | Hypogin | - |
| Stamen type | term | Singenesis stamen | - |
| Filament type | term | Trichomes at the base | - |
| Perianth type | term | Campanulate perigon tepal | - |
| Perianth estivation | term | Imbricate-alternate | - |
| Anther type | term | Basifix | - |
| Corolla type | term | Campanulate, bell-shaped | - |
| Code | No. of Flowers | No. to Recycle * | Fruit Set Rate % | Seed Set Rate % | Seed Viability (%) |
|---|---|---|---|---|---|
| C | 50 | 44 | 63.6 ± 3.2 | 75.2 ± 1.9 | 89.3 ± 2.3 |
| P1 | 40 | 36 | 0 | 0 | - |
| P2 | 30 | 25 | 0 | 0 | - |
| P3 | 30 | 26 | 0 | 0 | - |
| P4 | 30 | 27 | 77.7 ± 1.5 | 85.0 ± 1.5 | 83.4 ± 1.5 |
| P5 | 30 | 23 | 69.5 ± 2.4 | 81.4 ± 1.8 | 86.1 ± 0.9 |
| P6 | 30 | 26 | 0 | 0 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yildiz, F.; Aslay, M.; Kaya, O. Pollination Strategies and Reproductive Biology of Fritillaria imperialis L. (Liliaceae): Insights from Erzincan, Türkiye. Diversity 2024, 16, 455. https://doi.org/10.3390/d16080455
Yildiz F, Aslay M, Kaya O. Pollination Strategies and Reproductive Biology of Fritillaria imperialis L. (Liliaceae): Insights from Erzincan, Türkiye. Diversity. 2024; 16(8):455. https://doi.org/10.3390/d16080455
Chicago/Turabian StyleYildiz, Faruk, Meral Aslay, and Ozkan Kaya. 2024. "Pollination Strategies and Reproductive Biology of Fritillaria imperialis L. (Liliaceae): Insights from Erzincan, Türkiye" Diversity 16, no. 8: 455. https://doi.org/10.3390/d16080455








