Variation in Phenology and Morphological Traits of Seed-Propagated Laggera alata and Laggera crispata Forms
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. Data Collection
2.2.1. Phenological Events
2.2.2. Morphological Traits
2.3. Statistical Analysis
3. Results
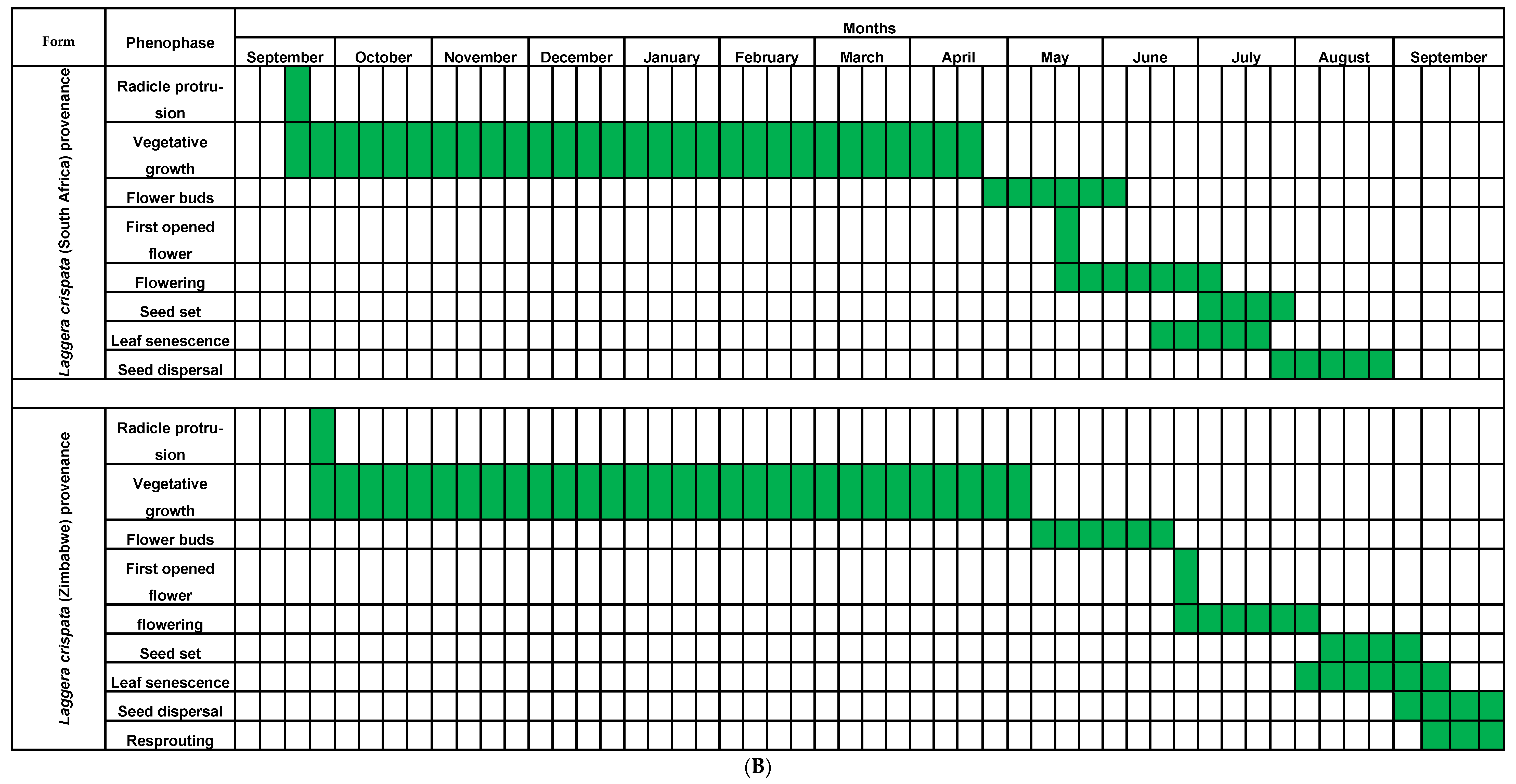
3.1. Phenology
3.2. Qualitative Traits
3.3. Leaf Size
3.4. Leaf Chlorophyll Content
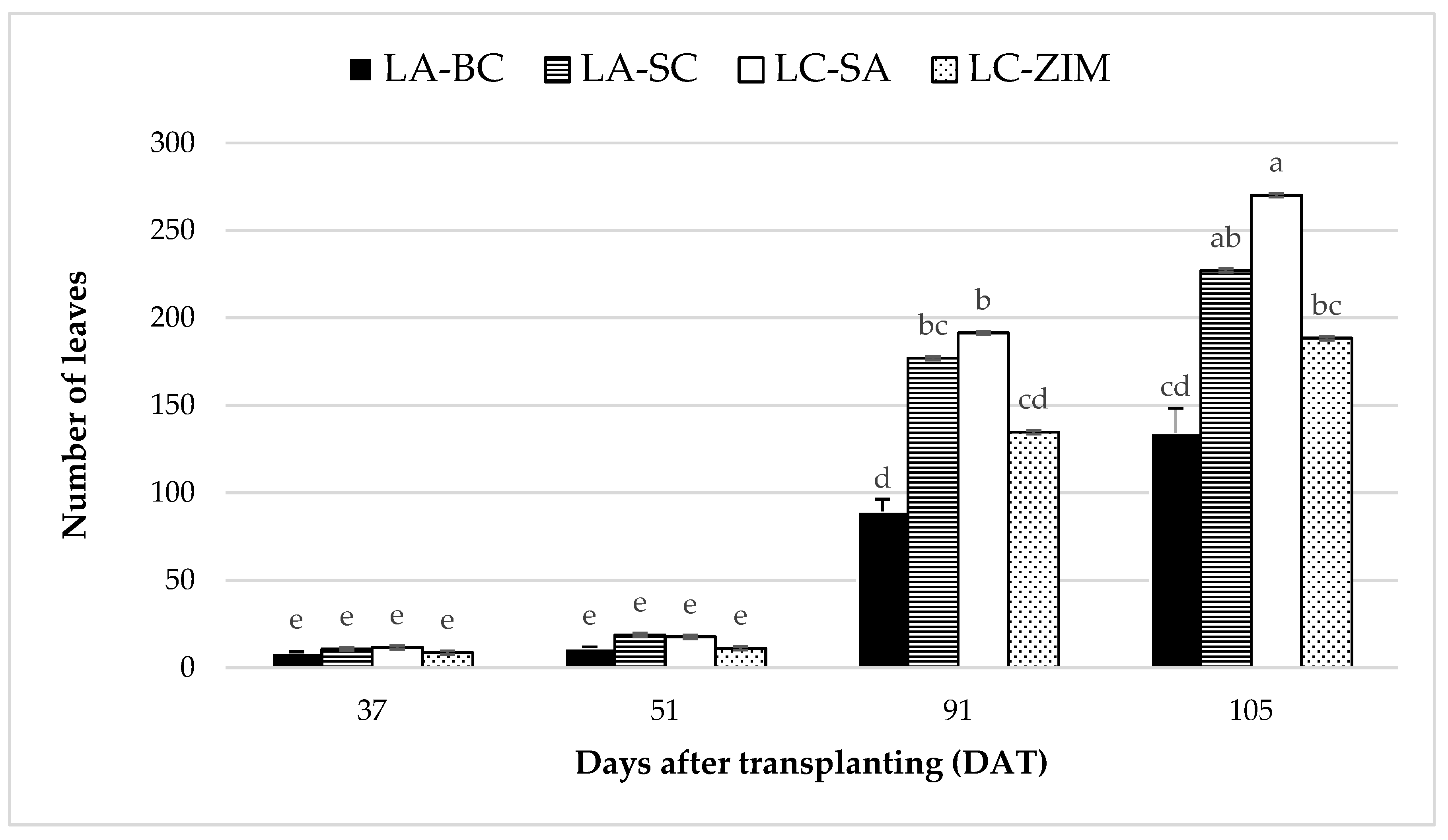
3.5. Number of Leaves per Plant
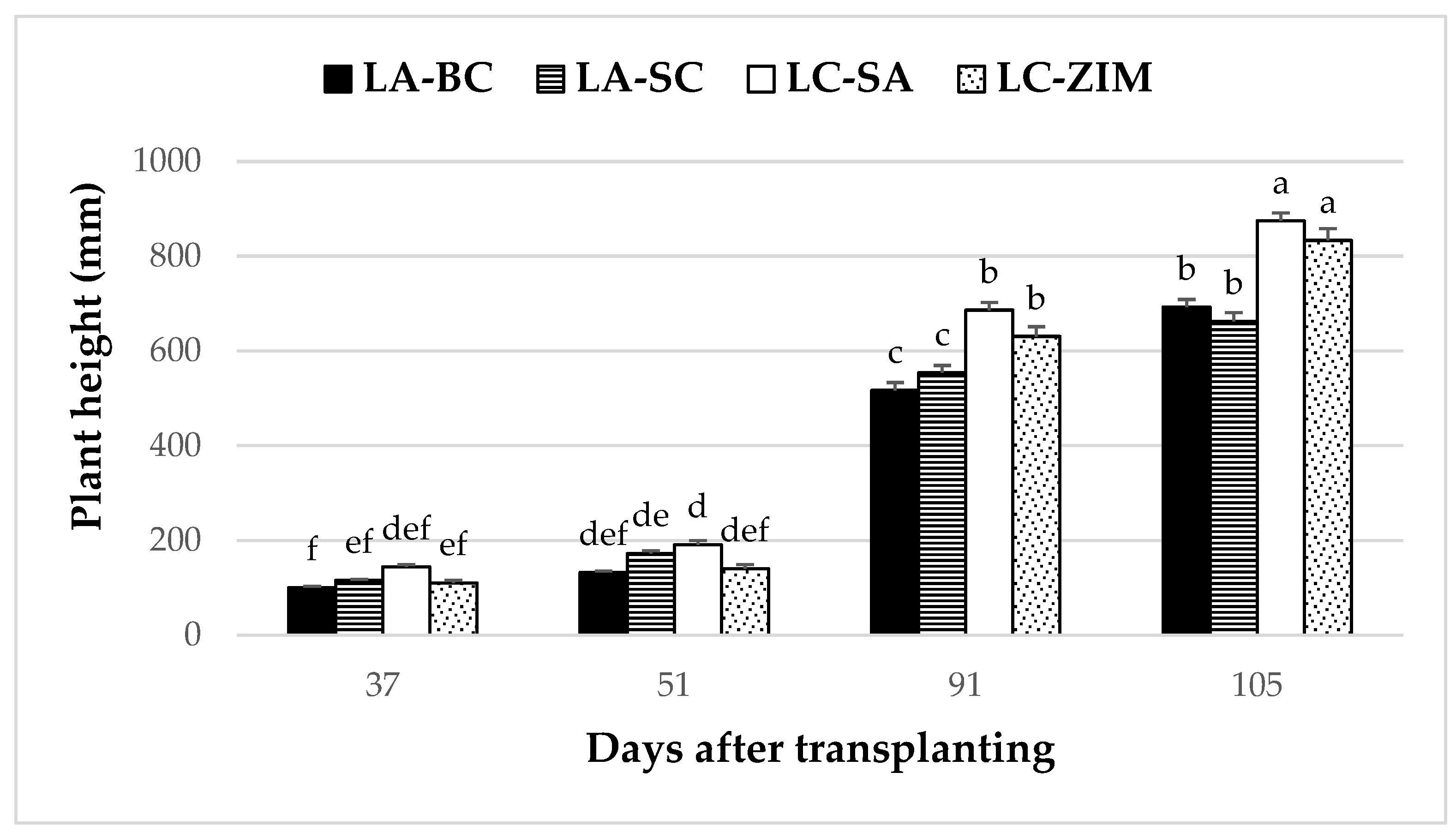
3.6. Stem Diameter and Plant Height
4. Discussion
4.1. Phenology
4.2. Qualitative Traits
4.3. Leaf Traits
4.4. Stem Diameter and Plant Height
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Hegazy, A.K.; Fahmy, G.M.; Ali, M.I.; Gomaa, N.H. Growth and Phenology of Eight Common Weed Species. J. Arid Environ. 2005, 61, 171–183. [Google Scholar] [CrossRef]
- Weber, A. What Is Morphology and Why Is It Time for Its Renaissance in Plant Systematics? In Deep Morphology: Toward a Renaissance of Morphology in Plant Systematics; Stuessy, T.F., Mayer, V., Horandl, E., Eds.; A R G Gantner Verlag K G: Koenigstein, Germany, 2003; Volume 141, pp. 3–32. [Google Scholar]
- Susetyarini, E.; Wahyono, P.; Latifa, R.; Nurrohman, E. The Identification of Morphological and Anatomical Structures of Pluchea Indica. J. Phys. Conf. Ser. 2020, 1539, 012001. [Google Scholar] [CrossRef]
- Endress, P.K.; Baas, P.; Gregory, M. Systematic Plant Morphology and Anatomy-50 Years of Progress. Taxon 2000, 49, 401–434. [Google Scholar] [CrossRef]
- Nakar, R.N.; Jadeja, B.A. Flowering and Fruiting Phenology of Some Herbs, Shrubs and Undershrubs from Girnar Reserve Forest, Gujarat, India. Curr. Sci. 2015, 108, 111–118. [Google Scholar]
- Torres, C.; Galetto, L. Flowering Phenology of Co-Occurring Asteraceae: A Matter of Climate, Ecological Interactions, Plant Attributes or of Evolutionary Relationships among Species? Org. Divers. Evol. 2011, 11, 9–19. [Google Scholar] [CrossRef]
- Mbatudde, M.; Mucunguzi, P.; Lye, K.A. Phenology of Asteraceae in Selected Districts of Central Uganda. Afr. J. Ecol. 2007, 45, 67–72. [Google Scholar] [CrossRef]
- Hidayat, I.W.; Suhendri, Y. Observation Series of Flowering and Fruiting Phenology of Mischocarpus Pentapetalus (Roxb.) Radlk. (Sapindaceae) in Cibodas Botanic Gardens, 2014–2018. Bul. Kebun Raya 2020, 23, 169–209. [Google Scholar] [CrossRef]
- Chauhan, H.K.; Gallacher, D.; Bisht, A.K.; Bhatt, I.D.; Bhatt, A.; Dhyani, P.; Kewlani, P. Variations in Phytochemistry, Morphology, and Population Structure in Trillium Govanianum (Melanthiaceae). Botany 2021, 99, 651–664. [Google Scholar] [CrossRef]
- Kuiate, J.-R.; Bessière, J.-M.; Amvam Zollo, P.-H. Composition of the Essential Oils from Three Laggera Spp. from Cameroon: Essential Oils of Laggera spp. Flavour Fragr. J. 2002, 17, 105–108. [Google Scholar] [CrossRef]
- Krichen, L.; Audergon, J.-M.; Trifi-Farah, N. Variability of Morphological Characters among Tunisian Apricot Germplasm. Sci. Hortic. 2014, 179, 328–339. [Google Scholar] [CrossRef]
- Anderberg, A.A.; Bengtson, A. Taxonomic Novelties in the Asteraceae–Inuleae with the Description of a New Genus, Galgera Separate from Laggera. Willdenowia Ann. Bot. Gard. Bot. Mus. Berl. 2022, 52, 373–386. [Google Scholar] [CrossRef]
- Wild, H. The Compositae of the Flora Zambesiaca Area. Kirkia 1968, 7, 121–135. [Google Scholar]
- Anderberg, A.A. Taxonomy and Phylogeny of the Tribe Plucheeae (Asteraceae). Plant Syst. Evol. 1991, 176, 145–177. [Google Scholar] [CrossRef]
- Meo, A.A.; Khan, M.A. Pollen Morphology of Rare Taxa Laggera Alata and Its Related Species Pluchea Lanceolata of Tribe Plucheeae (Asteraceae). Pak. J. Bot. 2009, 41, 1539–1544. [Google Scholar]
- Espinosa, F.; Castro, M.P. On the Use of Herbarium Specimens for Morphological and Anatomical Research. Bot. Lett. 2018, 165, 361–367. [Google Scholar] [CrossRef]
- Parnell, J.; Rich, T.; McVeigh, A.; Lim, A.; Quigley, S.; Morris, D.; Wong, Z. The Effect of Preservation Methods on Plant Morphology. Taxon 2013, 62, 1259–1265. [Google Scholar] [CrossRef]
- Pornpongrungrueng, P.; Borchsenius, F.; Gustafsson, M.H. Relationships within Blumea (Inuleae, Asteraceae) and the Utility of the 5SNTS in Specieslevel Phylogeny Reconstruction. Taxon 2009, 58, 1181–1193. [Google Scholar] [CrossRef]
- Pramanik, K.; Mohapatra, P.P.; Jena, C.; Kumar, D.S. Application of 3-4-5 Rule in Agriculture: A Review. Curr. J. Appl. Sci. Technol. 2020, 39, 37–41. [Google Scholar] [CrossRef]
- Khatiwada, A.; Adhikari, P. Effect of Various Organic Fertilizers on Seedling Health and Vigour of Different Varieties of Cucumber in Rautahat Condition. Malays. J. Sustain. Agric. 2020, 4, 81–85. [Google Scholar] [CrossRef]
- Kaur, G.; Singh, B.P.; Nagpal, A.K. Phenology of Some Phanerogams (Trees and Shrubs) of Northwestern Punjab, India. J. Bot. 2013, 2013, 712405. [Google Scholar] [CrossRef]
- Ștef, R.; Carabeț, A.; Manea, D.; Sala, F. Characterization of Leaves Geometry in Asclepias Syriaca L. Species. Appl. Ecol. Environ. Res. 2023, 21, 3095–3108. [Google Scholar] [CrossRef]
- Asaduzzaman, M.; Wu, H.; Koetz, E.; Hopwood, M.; Shepherd, A. Phenology and Population Differentiation in Reproductive Plasticity in Feathertop Rhodes Grass (Chloris Virgata Sw.). Agronomy 2022, 12, 736. [Google Scholar] [CrossRef]
- Wang, C.; Zhou, J.; Xiao, H.; Liu, J.; Wang, L. Variations in Leaf Functional Traits among Plant Species Grouped by Growth and Leaf Types in Zhenjiang, China. J. For. Res. 2017, 28, 241–248. [Google Scholar] [CrossRef]
- Egli, D.B. Time and the Productivity of Agronomic Crops and Cropping Systems. Agron. J. 2011, 103, 743–750. [Google Scholar] [CrossRef]
- Guo, Y.; Ren, G.; Zhang, K.; Li, Z.; Miao, Y.; Guo, H. Leaf Senescence: Progression, Regulation, and Application. Mol. Hortic. 2021, 1, 5. [Google Scholar] [CrossRef] [PubMed]
- Lusa, M.G.; Loeuille, B.F.P.; Ciccarelli, D.; Appezzato-da-Glória, B. Evolution of Stem and Leaf Structural Diversity: A Case Study in Lychnophorinae (Asteraceae). Bot. Rev. 2018, 84, 203–241. [Google Scholar] [CrossRef]
- Angst, Š.; Mudrák, O.; Frouz, J.; Jílková, V.; Schnablová, R.; Straková, P.; Veselá, H.; Angst, G. The Effect of Dead Standing (Marcescent) Biomass on Litter Decomposition in Herbaceous Flora Is Governed by Plant Functional Group. Funct. Ecol. 2024, 38, 1309–1319. [Google Scholar] [CrossRef]
- Mudrák, O.; Angst, Š.; Angst, G.; Veselá, H.; Schnablová, R.; Herben, T.; Frouz, J. Ecological Significance of Standing Dead Phytomass: Marcescence as a Puzzle Piece to the Nutrient Cycle in Temperate Ecosystems. J. Ecol. 2023, 111, 2245–2256. [Google Scholar] [CrossRef]
- Chondol, T.; Korznikov, K.A.; Doležal, J. Ecological Significance of Marcescence in Himalayan Plants: Why Is Standing Dead Phytomass More Important in Demanding, Resource-Limited Environments? Funct. Ecol. 2024, 38, 942–954. [Google Scholar] [CrossRef]
- Kindscher, K.; Price, D.; Castle, L. Resprouting of Echinacea Angustifolia Augments Sustainability of Wild Medicinal Plant Populations. Econ. Bot. 2008, 62, 139–147. [Google Scholar] [CrossRef]
- Pausas, J.G.; Pratt, R.B.; Keeley, J.E.; Jacobsen, A.L.; Ramirez, A.R.; Vilagrosa, A.; Paula, S.; Kaneakua-Pia, I.N.; Davis, S.D. Towards Understanding Resprouting at the Global Scale. New Phytol. 2016, 209, 945–954. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-L.; Yu, H.; Luo, H.-M.; Wu, Q.; Li, C.-F.; Steinmetz, A. Conservation and Sustainable Use of Medicinal Plants: Problems, Progress, and Prospects. Chin. Med. 2016, 11, 37. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.D. Flora of India; Botanical Survey of India: Kolkata, India, 1993; ISBN 978-81-958726-1-9. [Google Scholar]
- Bai, S.; Xu, J.; Lv, Y.; Shi, X.; Tan, D. Evaluation of the Potential Seed Dispersal Effectiveness of Malus Sieversii (Lebed.) M. Roem. by Cattle. Diversity 2023, 15, 1205. [Google Scholar] [CrossRef]
- Bastida, F.; Talavera, S. Temporal and Spatial Patterns of Seed Dispersal in Two Cistus Species (Cistaceae). Ann. Bot. 2002, 89, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Schupp, E.W.; Zwolak, R.; Jones, L.R.; Snell, R.S.; Beckman, N.G.; Aslan, C.; Cavazos, B.R.; Effiom, E.; Fricke, E.C.; Montaño-Centellas, F.; et al. Intrinsic and Extrinsic Drivers of Intraspecific Variation in Seed Dispersal Are Diverse and Pervasive. AoB Plants 2019, 11, plz067. [Google Scholar] [CrossRef] [PubMed]
- Sogbohossou, E.O.D.; Kortekaas, D.; Achigan-Dako, E.G.; Maundu, P.; Stoilova, T.; Van Deynze, A.; de Vos, R.C.H.; Schranz, M.E. Association between Vitamin Content, Plant Morphology and Geographical Origin in a Worldwide Collection of the Orphan Crop Gynandropsis Gynandra (Cleomaceae). Planta 2019, 250, 933–947. [Google Scholar] [CrossRef] [PubMed]
- Aliyeva, G.N.; Mammadova, Z.A.; Ojaghi, J.M.; Pourbabaei, H. Inter- and Intrapopulation Variation in Leaf Morphological and Functional Traits of Q. Petraea Subsp. Iberica Were Mainly Influenced by Ecological Factors in Azerbaijan. Plant Fungal Res. 2020, 2, 61–68. [Google Scholar] [CrossRef]
- Getahun, T.; Sharma, V.; Gupta, N. The Genus Laggera (Asteraceae)—Ethnobotanical and Ethnopharmacological Information, Chemical Composition as Well as Biological Activities of Its Essential Oils and Extracts: A Review. Chem. Biodivers. 2019, 16, e1900131. [Google Scholar] [CrossRef]
- Moussaoui, A.E.; Bourhia, M.; Jawhari, F.Z.; Khalis, H.; Chedadi, M.; Agour, A.; Salamatullah, A.M.; Alzahrani, A.; Alyahya, H.K.; Alotaibi, A.; et al. Responses of Withania Frutescens (L.) Pauquy (Solanaceae) Growing in the Mediterranean Area to Changes in the Environmental Conditions: An Approach of Adaptation. Front. Ecol. Evol. 2021, 9, 666005. [Google Scholar] [CrossRef]
- Verma, R.S.; Padalia, R.C.; Chanotiya, C.S.; Chauhan, A.; Yadav, A. Chemical Investigation of the Essential Oil of Laggera Crispata (Vahl) Hepper & Wood from India. J. Serbian Chem. Soc. 2011, 76, 523–528. [Google Scholar]
- Li, Y.; Zheng, Y.; Ratkowsky, D.A.; Wei, H.; Shi, P. Application of an Ovate Leaf Shape Model to Evaluate Leaf Bilateral Asymmetry and Calculate Lamina Centroid Location. Front. Plant Sci. 2022, 12, 822907. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.; Yang, M.; Wang, C.; Tian, C. Leaf Phenotypic Variation and Its Response to Environmental Factors in Natural Populations of Eucommia Ulmoides. BMC Plant Biol. 2023, 23, 562. [Google Scholar] [CrossRef] [PubMed]
- Sims, D.A.; Gamon, J.A. Relationships between Leaf Pigment Content and Spectral Reflectance across a Wide Range of Species, Leaf Structures and Developmental Stages. Remote Sens. Environ. 2002, 81, 337–354. [Google Scholar] [CrossRef]
- Aschenbrenner, A.-K.; Amrehn, E.; Bechtel, L.; Spring, O. Trichome Differentiation on Leaf Primordia of Helianthus Annuus (Asteraceae): Morphology, Gene Expression and Metabolite Profile. Planta 2015, 241, 837–846. [Google Scholar] [CrossRef] [PubMed]
- Bombo, A.B.; Appezzato-da-Glória, B.; Aschenbrenner, A.-K.; Spring, O. Capitate Glandular Trichomes in Aldama Discolor (Heliantheae—Asteraceae): Morphology, Metabolite Profile and Sesquiterpene Biosynthesis. Plant Biol. 2016, 18, 455–462. [Google Scholar] [CrossRef]
- Liu, B.; Wu, J.; Li, H.; Ma, Y.-F.; Luo, Y.-P.; Huang, G.-L.; Tian, X.; Chen, Y. Constituents of the Glandular Trichome Exudate on the Leaves of Laggera Pterodonta. Chem. Nat. Compd. 2016, 52, 902–903. [Google Scholar] [CrossRef]
- Chautá, A.; Kumar, A.; Mejia, J.; Stashenko, E.E.; Kessler, A. Defensive Functions and Potential Ecological Conflicts of Floral Stickiness. Sci. Rep. 2022, 12, 19848. [Google Scholar] [CrossRef] [PubMed]
- Randeria, A.J. The Composite Genus Blumea, a Taxonomic Revision. Blumea Biodivers. Evol. Biogeogr. Plants 1960, 10, 176–317. [Google Scholar]
- Zacchaeus, O.S.; Anuoluwa, I.A.; Adeyemi, A.F.; Uyaboerigha, D.I. Synergistic Efficacy of Phytochemical, Antioxidant and Bactericidal Properties of the Aerial Essential Oil of Laggera Crispata. Pharmacogn. J. 2021, 13, 1304–1311. [Google Scholar] [CrossRef]
- Wang, C.; He, J.; Zhao, T.-H.; Cao, Y.; Wang, G.; Sun, B.; Yan, X.; Guo, W.; Li, M.-H. The Smaller the Leaf Is, the Faster the Leaf Water Loses in a Temperate Forest. Front. Plant Sci. 2019, 10, 58. [Google Scholar] [CrossRef]
- Dong, N.; Prentice, I.C.; Wright, I.J.; Evans, B.J.; Togashi, H.F.; Caddy-Retalic, S.; McInerney, F.A.; Sparrow, B.; Leitch, E.; Lowe, A.J. Components of Leaf-Trait Variation along Environmental Gradients. New Phytol. 2020, 228, 82–94. [Google Scholar] [CrossRef]
- Campetella, G.; Chelli, S.; Wellstein, C.; Farris, E.; Calvia, G.; Simonetti, E.; Borsukiewicz, L.; Vanderplank, S.; Marignani, M. Contrasting Patterns in Leaf Traits of Mediterranean Shrub Communities along an Elevation Gradient: Measurements Matter. Plant Ecol. 2019, 220, 765–776. [Google Scholar] [CrossRef]
- Schrader, J.; Shi, P.; Royer, D.L.; Peppe, D.J.; Gallagher, R.V.; Li, Y.; Wang, R.; Wright, I.J. Leaf Size Estimation Based on Leaf Length, Width and Shape. Ann. Bot. 2021, 128, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Shangguan, Z.; Xi, W. Seasonal Variations of Leaf Traits and Drought Adaptation Strategies of Four Common Woody Species in South Texas, USA. J. For. Res. 2019, 30, 1715–1725. [Google Scholar] [CrossRef]
- Mudibu, J.; Nkongolo, K.K.C.; Kalonji-Mbuyi, A. Morphovariability and Agronomic Characteristics of Soybean Accessions from the Democratic Republic of Congo (DR-Congo) Gene Pool. J. Plant Breed. Crop. Sci. 2011, 3, 260–268. [Google Scholar]
- Whitman, T.; Aarssen, L.W. The Leaf Size/Number Trade-off in Herbaceous Angiosperms. J. Plant Ecol. 2010, 3, 49–58. [Google Scholar] [CrossRef]
- Scott, S.L.; Aarssen, L.W. Within-Species Leaf Size-Number Trade-Offs in Herbaceous Angiosperms: Botany. Botany 2012, 90, 223–235. [Google Scholar] [CrossRef]
- Roshanak, S.; Behbahani, B.A.; Shahidi, F.; Yazdi, F.T.; Vasiee, A.R.; Norouzi, N. Evaluation of Chlorophyll Content, Antioxidant Activity and Antimicrobial Effect of Dandelion Leaves Extract. Iran. Food Sci. Technol. Res. J. 2021, 17, 63–72. [Google Scholar] [CrossRef]
- Doddavarapu, B.; Crasta, G.L.; Shankar, M. Comparative Studies on Chlorophyll Concentration in Some Important Plant Families. J. Pharmacogn. Phytochem. 2021, 10, 214–220. [Google Scholar] [CrossRef]
- Amrani-Allalou, H.; Boulekbache-Makhlouf, L.; Mapelli-Brahm, P.; Sait, S.; Tenore, G.C.; Benmeziane, A.; Kadri, N.; Madani, K.; Jesús Meléndez Martínez, A. Antioxidant Activity, Carotenoids, Chlorophylls and Mineral Composition from Leaves of Pallenis Spinosa : An Algerian Medicinal Plant. J. Complement. Integr. Med. 2020, 17, 20170081. [Google Scholar] [CrossRef]
- Li, Y.; He, N.; Hou, J.; Xu, L.; Liu, C.; Zhang, J.; Wang, Q.; Zhang, X.; Wu, X. Factors Influencing Leaf Chlorophyll Content in Natural Forests at the Biome Scale. Front. Ecol. Evol. 2018, 6, 64. [Google Scholar] [CrossRef]
- Loewe-Muñoz, V.; del Río, R.; Delard, C.; Balzarini, M. Short-Term Stem Diameter Variations in Irrigated and Non-Irrigated Stone Pine (Pinus Pinea L.) Trees in a Xeric Non-Native Environment. Ann. For. Sci. 2021, 78, 99. [Google Scholar] [CrossRef]
- Kambiré, D.A.; Brice Boti, J.; Yapi, T.A.; Ouattara, Z.A.; Paoli, M.; Bighelli, A.; Tomi, F.; Casanova, J. Composition and Intraspecific Chemical Variability of Leaf Essential Oil of Laggera Pterodonta from Côte d’Ivoire. Chem. Biodivers. 2020, 17, e1900504. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Nicolás, M.; Colinas-León, T.; Alia-Tejacal, I.; Peña-Ortega, G.; González-Andrés, F.; Beltrán-Rodríguez, L. Morphological Variation in Scarlet Plume (Euphorbia Fulgens Karw Ex Klotzsch, Euphorbiaceae), an Underutilized Ornamental Resource of Mexico with Global Importance. Plants 2021, 10, 2020. [Google Scholar] [CrossRef] [PubMed]


| Month | 2021/2022 Season | 2022/2023 Season | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TMX | TMN | Tt | RHP | RAIN | SRAD | TMX | TMN | Tt | RHP | RAIN | SRAD | |
| Sept. | 26.1 | 15.2 | 10.7 | 58 | 134.6 | 16.3 | 28 | 16.6 | 12.3 | 59 | 47.9 | 16.6 |
| Oct. | 25.9 | 15.6 | 10.8 | 67 | 102.7 | 16.1 | 29.6 | 18.9 | 14.3 | 62 | 92.8 | 16.9 |
| Nov. | 27.6 | 18.1 | 12.9 | 65 | 43 | 19.5 | 28 | 18.6 | 13.3 | 67 | 358.5 | 18.7 |
| Dec. | 29.5 | 20.1 | 14.8 | 68 | 194.9 | 18.9 | 29.4 | 20.1 | 14.8 | 70 | 194 | 19 |
| Jan. | 31.1 | 20.7 | 15.9 | 66 | 102.4 | 23.4 | 32.6 | 20.6 | 16.6 | 57 | 86.4 | 26.1 |
| Feb. | 31.4 | 21.1 | 16.3 | 65 | 94.8 | 22.9 | 29.8 | 21.4 | 15.6 | 78 | 246.9 | 17.4 |
| Mar. | 30.3 | 19.7 | 15.0 | 62 | 102.7 | 19.6 | 30 | 20.6 | 15.3 | 69 | 82.7 | 18.1 |
| Apr. | 26 | 17.4 | 11.7 | 74 | 356 | 12.8 | 28.7 | 17.7 | 13.2 | 62 | 34.4 | 15.8 |
| May | 25.4 | 15.3 | 10.4 | 64 | 66.1 | 12.9 | 25.7 | 16 | 10.9 | 68 | 128.6 | 10.6 |
| Jun. | 24 | 11.8 | 7.9 | 54 | 71.4 | 11.7 | 25.5 | 13.2 | 9.4 | 54 | 10 | 11.8 |
| Jul. | 25.5 | 13.4 | 9.5 | 53 | 67.3 | 11.4 | 24.1 | 12.3 | 8.2 | 56 | 81.7 | 11.3 |
| Aug. | 25.2 | 13.8 | 9.5 | 54 | 35.7 | 13.5 | 25.2 | 13.7 | 9.5 | 57 | 4.6 | 14.1 |
| Sept. | 28 | 16.6 | 12.3 | 59 | 47.9 | 16.6 | 28.2 | 15.8 | 12.0 | 54 | 37.9 | 16.7 |
| Mean | 27.4 | 16.8 | 12.1 | 62.2 | 109.2 | 16.6 | 28.1 | 17.4 | 12.7 | 62.5 | 108.2 | 16.4 |
| Qualitative Traits | LA-SC | LA-BC | LC-SA | LC-ZIM |
|---|---|---|---|---|
| Stem | Profusely branched, continuous, and entire wings | Profusely branched, continuous, and entire wings | Profusely branched, interrupted, and toothed wings | Profusely branched, interrupted, and toothed wings |
| Young leaves | Maroon pigment on the leaf edges | Maroon pigment on the leaf edges | No pigmentation | No pigmentation |
| Leaf petiole | Absent | Absent | Absent | Absent |
| Leaf orientation | Alternate | Alternate | Alternate | Alternate |
| Plant scent | Very strong | Moderately strong | Sweet-like odor | Sweet-like odor |
| Leaf shape | Oblong | Ovate | Lanceolate | Lanceolate and curly |
| Leaf apex | Acute | Acute | Acute | Acute |
| Leaf color | Greyish green | Dark green | Shiny light green | Shiny light green |
| Leaf trichome stickiness | Less sticky | Less sticky | Moderately sticky | Very sticky |
| Leaf margins | Serrulate | Serrulate | Serrate | Serrate |
| Flower color | Pink | Pink | Pink | Pink |
| Seed color | White | White | White | White |
| Leaf Traits | DAT | LA-BC | LA-SC | LC-SA | LC-ZIM |
|---|---|---|---|---|---|
| Leaf length (mm) | 37 | 96.20 def | 91.80 ef | 83.80 f | 89.40 ef |
| 51 | 99.80 de | 99.20 de | 97.00 def | 101.47 cde | |
| 91 | 123.73 a | 107.00 bcd | 118.73 ab | 120.73 ab | |
| 105 | 126.80 a | 103.40 cde | 115.40 abc | 125.20 a | |
| Leaf width (mm) | 37 | 43.20 de | 40.00 def | 34.73 f | 37.33 ef |
| 51 | 45.93 cd | 44.73 de | 45.47 d | 44.60 de | |
| 91 | 60.60 ab | 40.73 def | 44.33 de | 53.47 bc | |
| 105 | 62.80 a | 37.20 ef | 45.27 d | 55.53 ab | |
| Leaf area (mm2) | 37 | 2085 def | 1865 ef | 1457 f | 1678 ef |
| 51 | 2309 de | 2207 de | 2204 de | 2246 de | |
| 91 | 3720 ab | 2161 de | 2603 cd | 3212 bc | |
| 105 | 3964 a | 1915 def | 2583 cd | 3457 ab |
| DAT | LA-BC | LA-SC | LC-SA | LC-ZIM |
|---|---|---|---|---|
| 37 | 0.47 e | 0.42 e | 0.36 e | 0.36 e |
| 51 | 0.54 e | 0.52 e | 0.45 e | 0.47 e |
| 91 | 1.17 bcd | 1.02 cd | 1.00 d | 1.17 bcd |
| 105 | 1.32 ab | 1.20 bc | 1.29 ab | 1.45 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nkosi, N.N.; Zharare, G.E.; Zimudzi, C.; Stedje, B.; Ntuli, N.R. Variation in Phenology and Morphological Traits of Seed-Propagated Laggera alata and Laggera crispata Forms. Diversity 2024, 16, 466. https://doi.org/10.3390/d16080466
Nkosi NN, Zharare GE, Zimudzi C, Stedje B, Ntuli NR. Variation in Phenology and Morphological Traits of Seed-Propagated Laggera alata and Laggera crispata Forms. Diversity. 2024; 16(8):466. https://doi.org/10.3390/d16080466
Chicago/Turabian StyleNkosi, Noluthando Nonjabulo, Godfrey Elijah Zharare, Clemence Zimudzi, Brita Stedje, and Nontuthuko Rosemary Ntuli. 2024. "Variation in Phenology and Morphological Traits of Seed-Propagated Laggera alata and Laggera crispata Forms" Diversity 16, no. 8: 466. https://doi.org/10.3390/d16080466
APA StyleNkosi, N. N., Zharare, G. E., Zimudzi, C., Stedje, B., & Ntuli, N. R. (2024). Variation in Phenology and Morphological Traits of Seed-Propagated Laggera alata and Laggera crispata Forms. Diversity, 16(8), 466. https://doi.org/10.3390/d16080466







