Finding Isolated Aquatic Habitat: Can Beggars Be Choosers?
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites
2.2. Local Environmental Variables
2.3. Odonate Data
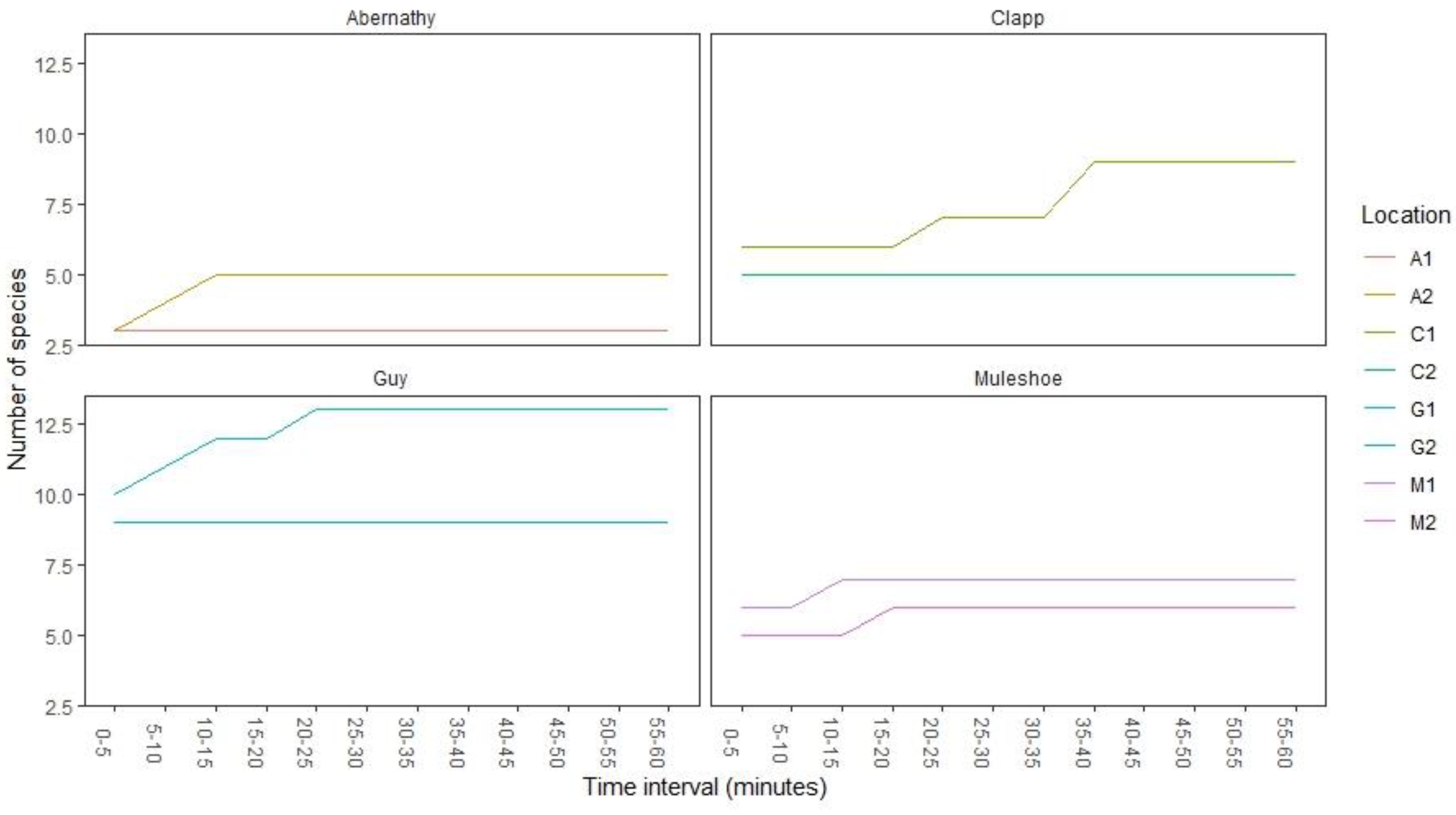
2.4. Analyses
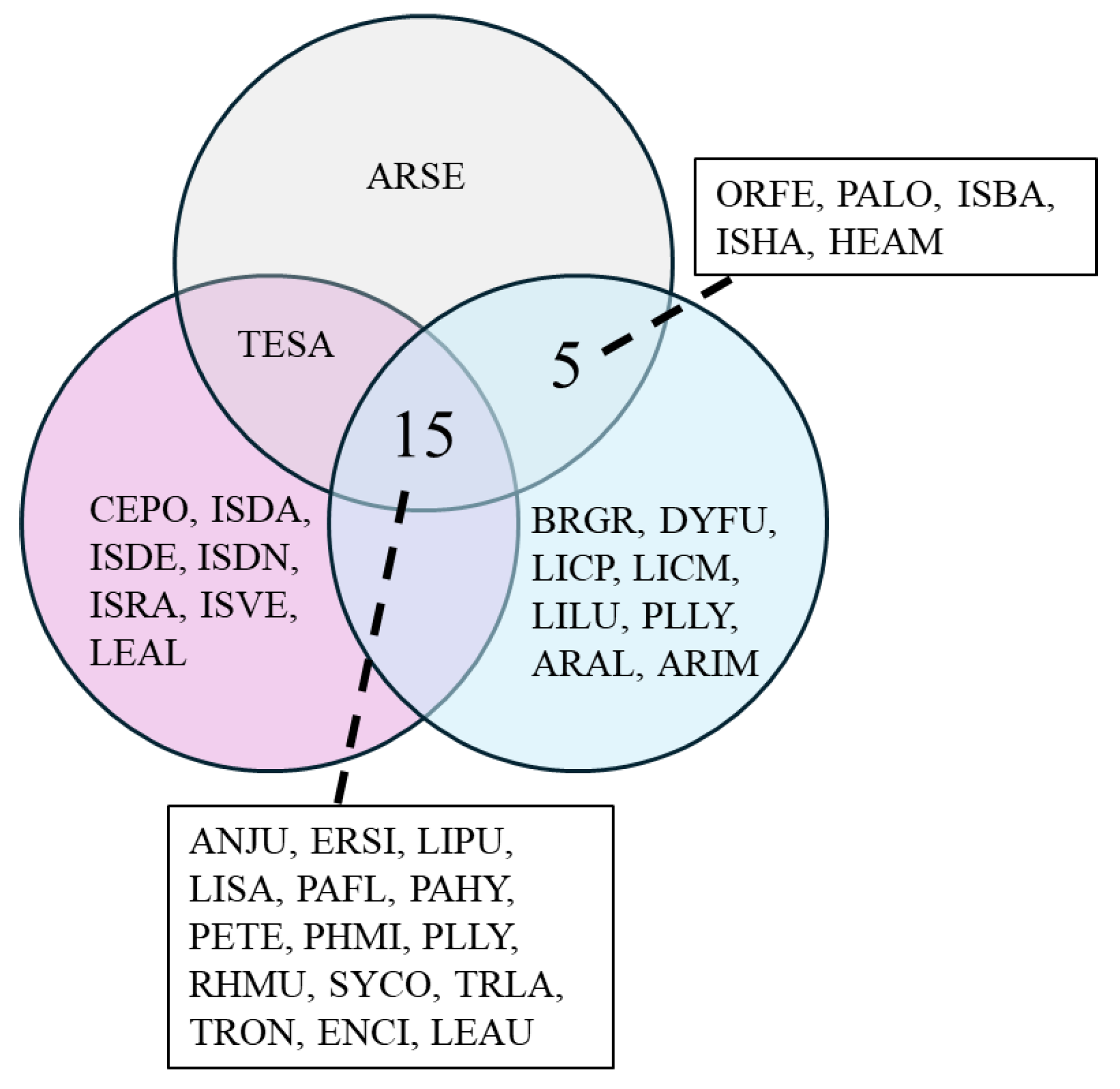
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Galbraith, H.S.; Vaughn, C.C.; Meier, C.K. Environmental variables interact across spatial scales to structure Trichopteran assemblages in Ouachita mountain rivers. Hydrobiologia 2008, 596, 401–411. [Google Scholar] [CrossRef]
- Thornhill, I.; Batty, L.; Death, R.G.; Friberg, N.R.; Ledger, M.E. Local and landscape scale determinants of macroinvertebrate assemblages and their conservation value in ponds across an urban land-use gradient. Biodiv. Conserv. 2017, 26, 1065–1086. [Google Scholar] [CrossRef] [PubMed]
- Collen, B.; Whitton, F.; Dyer, E.E.; Baillie, J.E.M.; Cumberlidge, N.; Darwall, W.R.T.; Pollock, C.; Richman, N.I.; Soulsby, A.-M.; Böhm, M. Global patterns of freshwater species diversity, threat and endemism. Glob. Ecol. Biogeogr. 2014, 23, 40–51. [Google Scholar] [CrossRef]
- Collier, K.J.; Probert, P.K.; Jeffries, M. Conservation of aquatic invertebrates: Concerns, challenges, conundrums. Aquat. Conserv. Mar. Freshw. Ecosyst. 2016, 26, 817–837. [Google Scholar] [CrossRef]
- Reid, A.J.; Carlson, A.K.; Creed, I.F.; Eliason, E.J.; Gell, P.A.; Johnson, P.T.J.; Kidd, K.A.; MacCormack, T.J.; Olden, J.D.; Ormerod, S.J.; et al. Emerging threats and persistent conservation challenges for freshwater biodiversity. Biol. Rev. 2019, 94, 849–873. [Google Scholar] [CrossRef]
- Heino, J.; Mykrä, H. Control of stream insect assemblages: Roles of spatial configuration and local environmental factors. Ecol. Entomol. 2008, 33, 614–622. [Google Scholar] [CrossRef]
- Juen, L.; De Marco, P., Jr. Odonate biodiversity in terra-firme forest streamlets in Central Amazonia: On the relative effects of neutral and niche drivers at small geographical extents. Insect Conserv. Div. 2011, 4, 265–274. [Google Scholar] [CrossRef]
- Arribas, P.; Velasco, J.; Abellán, P.; Sánchez-Fernández, D.; Andújar, C.; Calosi, P.; Millán, A.; Ribera, I.; Bilton, D.T. Dispersal ability rather than ecological tolerance drives differences in range size between lentic and lotic water beetles (Coleoptera: Hydrophilidae). J. Biogeogr. 2012, 39, 984–994. [Google Scholar] [CrossRef]
- Bispo, P.C.; Zanini Branco, C.C.; Carneiro Novaes, M.; Costa, L.S.M.; Yokoyama, E.; Barfield, M.; Holt, R.D. Partitioning and drivers of aquatic insect beta diversity in mountain streams. Freshw. Sci. 2021, 40, 644–658. [Google Scholar] [CrossRef]
- Reece, B.A.; McIntyre, N.E. Odonata of playas in the Southern High Plains, Texas. Southwest. Nat. 2009, 54, 96–99. [Google Scholar] [CrossRef]
- Reece, B.A.; McIntyre, N.E. Community assemblage patterns of odonates inhabiting a wetland complex influenced by anthropogenic disturbance. Insect Conserv. Div. 2009, 2, 73–80. [Google Scholar] [CrossRef]
- McIntyre, N.E.; Collins, S.D.; Heintzman, L.J.; Starr, S.M.; van Gestel, N. The challenge of assaying landscape connectivity in a changing world: A 17-year case study in the Southern Great Plains (USA) playa network. Ecol. Indic. 2018, 91, 607–616. [Google Scholar] [CrossRef]
- Smith, L. Playas of the Great Plains; University of Texas Press: Austin, TX, USA, 2003. [Google Scholar]
- Heintzman, L.J.; Starr, S.M.; Mulligan, K.R.; Barbato, L.S.; McIntyre, N.E. Using satellite imagery to examine the relationship between surface-water dynamics of the salt lakes of western Texas and Ogallala Aquifer depletion. Wetlands 2017, 37, 1055–1065. [Google Scholar] [CrossRef]
- Husband, D.M. Odonate Assemblages of Four Wetland Types Varying in Salinity, Hydroperiod, and Water Chemistry in the Texas Panhandle. Master’s Thesis, Texas Tech University, Lubbock, TX, USA, 2022. [Google Scholar]
- Crumrine, P.W.; Switzer, P.V.; Crowley, P.H. Structure and dynamics of odonate communities: Accessing habitat, responding to risk, and enabling reproduction. In Dragonflies and Damselflies: Model Organisms for Ecological and Evolutionary Research, 1st ed.; Córdoba-Aguilar, A., Ed.; Oxford University Press: New York, NY, USA, 2008; pp. 21–38. [Google Scholar]
- Pires, M.M.; Stenert, C.; Maltchik, L. Partitioning beta-diversity through different pond hydroperiod lengths reveals predominance of nestedness in assemblages of immature odonates. Entomol. Sci. 2017, 20, 318–326. [Google Scholar] [CrossRef]
- Chang, X.; Zhai, B.; Wang, M.; Wang, B. Relationship between exposure to an insecticide and fluctuating asymmetry in a damselfly (Odonata, Coenagriidae). Hydrobiologia 2007, 586, 213–220. [Google Scholar] [CrossRef]
- Al-Shami, S.A.; Hisamuddin, S.N.; Rawi, C.S.M.; Abdul, N.H.; Ahmad, A.H. Developmental instability in Odonata larvae in relation to water quality of Serdang River, Kedah, Malaysia. Life Sci. J. 2014, 11, 152–159. [Google Scholar]
- Stoks, R.; Debecker, S.; Van, K.D.; Janssens, L. Integrating ecology and evolution in aquatic toxicology: Insights from damselflies. Freshw. Sci. 2015, 34, 1032–1039. [Google Scholar] [CrossRef]
- Mangahas, R.S.; Murry, R.L.; McCauley, S.J. Chronic exposure to high concentrations of road salt decreases immune response of dragonfly larvae. Front. Ecol. Evol. 2019, 7, 376. [Google Scholar] [CrossRef]
- Beketov, M.A. Ammonia toxicity to larvae of Erythromma najas (Hansemann), Lestes sponsa (Hansemann) and Sympetrum flaveolum (Linnaeus) (Zygoptera: Coenagrionidae, Lestidae; Anisoptera: Libellulidae). Odonatologica 2002, 31, 297–304. [Google Scholar]
- Osborn, R. Odonata as indicators of habitat quality at lakes in Louisiana, United States. Odonatologica 2005, 34, 259–270. [Google Scholar]
- Ishak, M.; Norhisham, A.R.; Thomas, S.M.; Nurhidayu, S.; Ghazali, A.; Azhar, B. Physicochemical properties as driver of odonata diversity in oil palm waterways. Front. For. Glob. Chang. 2021, 4, 613064. [Google Scholar] [CrossRef]
- Rembsurg, A.J.; Turner, M.G. Aquatic and terrestrial drivers of dragonfly (Odonata) assemblages within and among north-temperate lakes. J. N. Am. Benthol. Soc. 2009, 28, 44–56. [Google Scholar]
- Brito, J.S.; Michelin, T.S.; Juen, L. Aquatic macrophytes are important substrates for Libellulidae (Odonata) larvae and adults. Limnology 2021, 22, 139–149. [Google Scholar] [CrossRef]
- Schindler, M.; Fesl, C.; Chovanec, A. Dragonfly associations (Insecta: Odonata) in relation to habitat variables: A multivariate approach. Hydrobiologia 2003, 497, 169–180. [Google Scholar] [CrossRef]
- Janssen, A.; Hunger, H.; Konold, W.; Pufal, G.; Staab, M. Simple pond restoration measures increase dragonfly (Insecta: Odonata) diversity. Biodiv. Conserv. 2018, 27, 2311–2325. [Google Scholar] [CrossRef]
- Perron, M.A.C.; Richmond, I.C.; Pick, F.R. Plants, water quality and land cover as drivers of Odonata assemblages in urban ponds. Sci. Total Environ. 2021, 773, 145467. [Google Scholar] [CrossRef]
- Worthen, W.B.; Fravel, R.K.; Horne, C.P. Downstream changes in odonate (Insecta: Odonata) communities along a suburban to urban gradient: Untangling natural and anthropogenic effects. Insects 2021, 12, 201. [Google Scholar] [CrossRef] [PubMed]
- Jeanmougin, M.; Leprieur, F.; Loïs, G.; Clergeau, P. Fine-scale urbanization affects Odonata species diversity in ponds of a megacity (Paris, France). Acta Oecol. 2014, 59, 26–34. [Google Scholar] [CrossRef]
- Corbet, P.S. Dragonflies: Behavior and Ecology of Odonata; Comstock Publishing Associates: New York, NY, USA, 1999. [Google Scholar]
- Abbott, J.C. Distribution of dragonflies and damselflies (Odonata) in Texas. Trans. Am. Entomol. Soc. 2001, 127, 199–228. [Google Scholar]
- Hernandez, K.M.; Reece, B.A.; McIntyre, N.E. Effects of anthropogenic land use on Odonata in playas of the Southern High Plains. West. N. Am. Nat. 2006, 66, 273–278. [Google Scholar] [CrossRef]
- Craig, C.N.; Reece, B.A.; McIntyre, N.E. Nestedness in playa odonates as a function of area and surrounding land-use. Wetlands 2008, 28, 995–1003. [Google Scholar] [CrossRef]
- Oertli, B.; Parris, K.M. Review: Toward management of urban ponds for freshwater biodiversity. Ecosphere 2019, 10, e02810. [Google Scholar] [CrossRef]
- Callaghan, C.T.; Borda-de-Água, L.; van Klink, R.; Rozzi, R.; Pereira, H.M. Unveiling global species abundance distributions. Nature Ecol. Evol. 2023, 7, 1600–1609. [Google Scholar] [CrossRef] [PubMed]
- Holtmann, L.; Juchem, M.; Brüggeshemke, J.; Möhlmeyer, A.; Fartmann, T. Stormwater ponds promote dragonfly (Odonata) species richness and density in urban areas. Ecol. Eng. 2018, 118, 1–11. [Google Scholar] [CrossRef]
- Perron, M.A.C.; Pick, F.R. Stormwater ponds as habitat for Odonata in urban areas: The importance of obligate wetland plant species. Biodiv. Conserv. 2020, 29, 913–931. [Google Scholar] [CrossRef]
- Vilenica, M.; Brigić, A.; Štih Koren, A.; Koren, T.; Sertić Perić, M.; Schmidt, B.; Bužan, T.; Gottstein, S. Odonata assemblages in urban semi-natural wetlands. Insects 2024, 15, 207. [Google Scholar] [CrossRef] [PubMed]
- Maciel Barbosa dos Santos, F.; Juen, L.; Cajaiba, R.L.; Pereira de Sousa, J.R. Distribution of the Odonata assemblages along an environmental gradient in the streams of the legal Amazonia region in western Maranhão (Brazil). J. Insect Conserv. 2024. [Google Scholar] [CrossRef]
- Cezário, R.R.; Pena Firme, P.; Pestana, G.C.; Vilela, D.S.; June, L.; Cordero-Rivera, A.; Guillermo, R. Sampling methods for dragonflies and damselflies. In Measuring Arthropod Biodiversity; Santos, J.C., Fernandes, G.W., Eds.; Springer: Cham, Switzerland, 2021; pp. 223–240. [Google Scholar]
- Bried, J.T.; Hager, B.J.; Hunt, P.D.; Fox, J.N.; Jensen, H.J.; Vowels, K.M. Bias of reduced-effort community surveys for adult Odonata of lentic waters. Insect Conserv. Div. 2011, 5, 213–222. [Google Scholar] [CrossRef]
- McPeek, M.A. Determination of species composition in the Enallagma damselfly assemblages of permanent lakes. Ecology 1990, 71, 83–98. [Google Scholar] [CrossRef]
- Rouquette, J.R.; Thompson, D.J. Patterns of movement and dispersal in an endangered damselfly and the consequences for its management. J. Appl. Ecol. 2007, 44, 692–701. [Google Scholar] [CrossRef]
- Keller, D.; Holderegger, R. Damselflies use different movement strategies for short- and long-distance dispersal. Insect Conserv. Div. 2013, 6, 590–597. [Google Scholar] [CrossRef]
- Bried, J.T.; Hinchliffe, R.P. Improving Taxonomic resolution in large-scale freshwater biodiversity monitoring: An example using wetlands and Odonata. Insect Conserv. Div. 2019, 12, 9–17. [Google Scholar] [CrossRef]
- Šigatová, H.; Dolný, A.; Samways, M.J.; Hardersen, S.; Oliveira-Junior, J.M.B.; June, L.; Van Dinh, K.; Bried, J.T. Odonata as indicators of pollution, habitat quality, and landscape disturbance. In Dragonflies & Damselflies: Model Organisms for Ecological and Evolutionary Research, 2nd ed.; Córdoba-Aguilar,, A., Beatty, C.D., Bried, J.T., Eds.; Oxford University Press: Oxford, UK, 2023; pp. 371–399. [Google Scholar]
- Medina-Espinoza, E.F.; June, L.; Calvão, L.B.; Cruz, G.A. Variations in the Odonata assemblages: How do the dry season and water bodies influence them? Neotrop. Entomol. 2024, 53, 630–640. [Google Scholar] [CrossRef] [PubMed]
- Raebel, E.M.; Merckx, T.; Riordan, P.; Macdonald, D.W.; Thompson, D.J. The dragonfly delusion: Why it is essential to sample exuviae to avoid biased surveys. J. Insect Conserv. 2010, 14, 523–533. [Google Scholar] [CrossRef]
- Bried, J.T.; DAmico, F.; Samways, M.J. A critique of the dragonfly delusion hypothesis: Why sampling exuviae does not avoid bias. Insect Conserv. Div. 2012, 5, 398–402. [Google Scholar] [CrossRef]
- Patten, M.A.; Bried, J.T.; Smith-Patten, B.D. Survey data matter: Predicted niche of adult vs breeding Odonata. Freshw. Sci. 2015, 34, 1114–1122. [Google Scholar] [CrossRef]
- Kutcher, T.E.; Bried, J.T. Adult Odonata conservatism as an indicator of freshwater wetland condition. Ecol. Indic. 2014, 38, 31–39. [Google Scholar] [CrossRef]
- De, K.; Dey, D.; Shruti, M.; Uniyal, V.P.; Adhikari, B.S.; Johnson, J.A.; Hussain, S.A. β-diversity of odonate community of the Ganga River: Partitioning and insights from local and species contribution. Wetlands Ecol. Manag. 2023, 31, 899–912. [Google Scholar] [CrossRef]
- Tennessen, K.J. Dragonfly Nymphs of North America: An Identification Guide; Springer: Cham, Switzerland, 2019. [Google Scholar]
- Silva, L.F.R.; Castro, D.M.P.; June, L.; Callisto, M.; Hughes, R.M.; Hermes, M.G. A matter of suborder: Are Zygoptera and Anisoptera larvae influenced by riparian vegetation in Neotropical savanna streams? Hydrobiologia 2021, 848, 4433–4443. [Google Scholar] [CrossRef]
- Posit Team. RStudio: Integrated Development Environment for R; Posit Software; PBC: Boston, MA, USA, 2023; Available online: http://www.posit.co/ (accessed on 23 July 2024).
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; OHara, R.B.; Simpson, G.L.; Solvmos, P.; et al. Package Vegan, R Package Version 2.6-4. 2022. Available online: https://CRAN.R-project.org/package=vegan (accessed on 23 July 2024).
- Maechler, M.; Rousseeuw, P.; Struyf, A.; Hubert, M.; Hornik, K. cluster: Cluster Analysis Basics and Extensions, R Package Version 2.1.4. 2022. Available online: https://CRAN.R-project.org/package=cluster (accessed on 23 July 2024).
- Altman, N.; Krzywinski, M. Clustering. Nat. Methods 2017, 14, 545–546. [Google Scholar] [CrossRef]
- Legendre, P.; Gallagher, E.D. Ecologically meaningful transformations for ordination of species data. Oecologia 2001, 129, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Poos, M.S.; Jackson, D.A. Addressing the removal of rare species in multivariate bioassessments: The impact of methodological choices. Ecol. Indic. 2012, 18, 82–90. [Google Scholar] [CrossRef]
- Wellborn, G.A.; Skelly, D.K.; Werner, E.E. Mechanisms creating community structure across a freshwater habitat gradient. Ann. Rev. Ecol. Syst. 1996, 27, 337–363. [Google Scholar] [CrossRef]
- City of Lubbock. City of Lubbock, Texas Master Drainage Plan: 2010 Update. Available online: https://ci.lubbock.tx.us/storage/images/sqXjFEEizzaq7V49ynM1VuXzWgRCxDavguVrt8Zv.pdf (accessed on 4 February 2024).
- Beisel, J.-N.; Usseglio-Polatera, P.; Thomas, S.; Moreteau, J.-C. Stream community structure in relation to spatial variation: The influence of mesohabitat characteristics. Hydrobiologia 1998, 389, 73–88. [Google Scholar] [CrossRef]
- Villalobos-Jiménez, G.; Dunn, A.M.; Hassall, C. Dragonflies and damselflies (Odonata) in urban ecosystems: A review. Eur. J. Entomol. 2016, 113, 217–232. [Google Scholar] [CrossRef]
- Husband, D.M.; McIntyre, N.E. Urban areas create refugia for odonates in a semi-arid region. Insects 2021, 12, 431. [Google Scholar] [CrossRef] [PubMed]
- Goertzen, D.; Suhling, F. Promoting dragonfly diversity in cities: Major determinants and implications for urban pond design. J. Insect Conserv. 2013, 17, 399–409. [Google Scholar] [CrossRef]
- Larsen, R.R. Notes on the fragile habitat, distribution, and ecology of the bleached skimmer (Libellula composita). Argia 2008, 20, 19–20. [Google Scholar]
- Swanson, G.A.; Winter, T.C.; Adomaitis, V.A.; LaBaugh, J.W. Chemical Characteristics of Prairie Lakes in South-Central North Dakota–Their Potential for Influencing Use by Fish and Wildlife; U.S. Fish and Wildlife Service, Fish and Wildlife Technical Report; US Department of the Interior, Fish and Wildlife Service: Washington, DC, USA, 1988; Volume 18, p. 44.
- Halse, S.A.; Shiel, R.J.; Williams, W.D. Aquatic invertebrates of Lake Gregory, Northwestern Australia, in relation to salinity and ionic composition. Hydrobiologia 1998, 381, 15–29. [Google Scholar] [CrossRef]
- Carver, S.; Storey, A.; Spafford, H.; Lynas, J.; Chandler, L.; Weinstein, P. Salinity as a driver of aquatic invertebrate colonization behavior and distribution in the wheatbelt of Western Australia. Hydrobiologia 2009, 617, 75–90. [Google Scholar] [CrossRef]
- Preston, T.M.; Borgreen, M.J.; Ray, A.M. Effects of brine contamination from energy development on wetland macroinvertebrate community structure in the Prairie Pothole Region. Environ. Pollut. 2018, 239, 722–732. [Google Scholar] [CrossRef] [PubMed]
- Hudak, P.F. Sulfate and chloride concentrations in Texas aquifers. Environ. Int. 2000, 26, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Mengel, K. Iron availability in plant tissues—Iron chlorosis on calcareous soils. Plant Soil 1994, 165, 275–283. [Google Scholar] [CrossRef]
- Natural Resources Conservation Service. Soils of Texas, Texas Almanac. 2021. Available online: https://www.texasalmanac.com/articles/soils-of-texas (accessed on 4 February 2024).
- Dolný, A.; Pyszko, P.; Šigutová, H. Community changes in odonate monitoring: Why are long-term studies so relevant? Insect Conserv. Div. 2021, 14, 597–608. [Google Scholar] [CrossRef]
- Smith, L.M.; Haukos, D.A.; McMurry, S.T.; LaGrange, T.; Willis, D. Ecosystem services provided by playas in the High Plains: Potential for USDA conservation programs. Ecol. Applic. 2011, 21, S82–S92. [Google Scholar] [CrossRef]
- Belden, J.B.; Hanson, B.R.; McMurry, S.T.; Smith, L.M.; Haukos, D.A. Assessment of the effects of farming and conservation programs on pesticide deposition in High Plains wetlands. Environ. Sci. Technol. 2012, 46, 3424–3432. [Google Scholar] [CrossRef] [PubMed]
- Rosen, D.J.; Caskey, A.D.; Conway, W.C.; Haukos, D.A. Vascular flora of saline lakes in the Southern High Plains of Texas and eastern New Mexico. J. Bot. Res. Inst. Tex. 2013, 7, 595–602. [Google Scholar]
- Harabiš, F.; Dolný, A. Human altered ecosystems: Suitable habitats as well as ecological traps for dragonflies (Odonata): The matter of scale. J. Insect Conserv. 2012, 16, 121–130. [Google Scholar] [CrossRef]
- Ball-Damerow, J.E.; M’Gonigle, L.K.; Resh, V.H. Changes in occurrence, richness, and biological traits of dragonflies and damselflies (Odonata) in California and Nevada over the past century. Biodiv. Conserv. 2014, 23, 2107–2126. [Google Scholar] [CrossRef]
- Rocha-Ortega, M.; Rodríguez, P.; Córdoba-Aguilar, A. Spatial and temporal effects of land use change as potential drivers of odonate community composition but not species richness. Biodiv. Conserv. 2018, 28, 451–466. [Google Scholar] [CrossRef]
- Suárez-Tovar, C.M.; Castillo-Pérez, E.U.; Sandoval-García, I.A.; Schondube, J.E.; Cano-Santana, Z.; Córdoba-Aguilar, A. Resilient dragons: Exploring Odonata communities in an urbanization gradient. Ecol. Indic. 2022, 141, 109134. [Google Scholar] [CrossRef]
- Catling, P.M. A potential for the use of dragonfly (Odonata) diversity as bioindicators of the efficiency of sewage lagoons. Can. Field-Nat. 2005, 119, 233–236. [Google Scholar] [CrossRef]
- Barbosa de Oliveira-Junior, J.M.; De Marco Junior, P.; Dias-Silva, K.; Leitão, R.P.; Leal, C.G.; Pompeu, P.S.; Gardner, T.A.; Hughes, R.M.; June, L. Effects of human disturbance and riparian conditions on Odonata (Insecta) assemblages in eastern Amazon Basin streams. Limnologica 2017, 66, 31–39. [Google Scholar] [CrossRef]
- Thompson, A.L. The Life History of Anax junius (Drury) in Minnesota: Determining Instars, Growth Development Pathways, Emergence Phenology, and the Effect of Temperature on Development. Ph.D. Thesis, University of Minnesota, Minneapolis, MN, USA, December 2019. [Google Scholar]
- Paulson, D. Damselflies and Dragonflies of the West; Princeton University Press: Princeton, NJ, USA, 2009. [Google Scholar]
- Abbott, J.C. Dragonflies of Texas: A Field Guide; University of Texas Press: Austin, TX, USA, 2015. [Google Scholar]
- Carrillo-Lara, D.E.; Novelo-Guitérrez, R. The life cycle of Orthemis ferruginea (Fabricius, 1775) (Odonata: Libellulidae). Int. J. Odonatol. 2021, 24, 275–299. [Google Scholar] [CrossRef] [PubMed]
- Wissinger, S.A. Effects of food availability on larval development and inter-instar predation among larvae of Libellula lydia and Libellula luctuosa (Odonata: Anisoptera). Can. J. Zool. 1988, 66, 543–549. [Google Scholar] [CrossRef]
- Smith, S.G.F.; Smith, D.L. Salinity tolerance of Erythemis simplicicollis Say (Odontata: Anisoptera, Libellulidae). In Proceedings of the Sixth Symposium on the Natural History of the Bahamas, San Salvador, The Bahamas, 9–13 June 1996; pp. 139–142. [Google Scholar]
- Hilsenhoff, W.L. Aquatic Insects of Wisconsin: Keys to Wisconsin Genera and Notes on Biology, Habitat, Distribution and Species; Publication# G3648; University of Wisconsin, Natural History Museums Council: Madison, WI, USA, 1995; Volume 3, pp. 1–77. [Google Scholar]
- Hedlund, J.S.U.; Lv, H.; Lehmann, P.; Hu, G.; Anderson, R.C.; Chapman, J.W. Unraveling the world’s longest non-stop migration: The Indian Ocean crossing of the globe skimmer dragonfly. Front. Ecol. Evol. 2021, 9, 698128. [Google Scholar] [CrossRef]
- Catling, P.M. Climate warming as an explanation for the recent northward range extension of two dragonflies, Pachydiplax longipennis and Perithemis tenera, into the Ottawa Valley, Eastern Ontario. Ott. Field-Nat. Club 2016, 130, 122–132. [Google Scholar] [CrossRef]
- Zia, A.; Din, A.U.; Azam, I.; Munir, A.; Afsheen, S. Effect of salinity gradients on species composition of Odonata naiads. Arthropods 2018, 7, 11–25. [Google Scholar]
- Schilling, E.G.; Lawrenz, R.; Kundel, H. An assessment of the geographic distribution and status of a rare dragonfly, Rhionaeschna mutata, at the northwestern edge of its range. Northeast. Nat. 2019, 26, 523–536. [Google Scholar] [CrossRef]
- Bick, G.H.; Bick, J.C. Demography and behavior of the damselfly, Argia apicalis (Say), (Odonata: Coenagriidae). Ecology 1965, 46, 461–472. [Google Scholar] [CrossRef]
- Tennessen, K.J. Reproductive Behavior and Isolation of Two Sympatric Coenagrionid Damselflies in Florida. Ph.D. Thesis, University of Florida, Gainesville, FL, USA, 1975. [Google Scholar]
- Córdoba-Aguilar, A.; Rocha-Ortega, M. Damselfly (Odonata: Calopterygidae) population decline in an urbanizing watershed. J. Insect Sci. 2019, 19, 30. [Google Scholar] [CrossRef] [PubMed]
- Norling, U. Growth, winter preparations and timing of emergence in temperate zone Odonata: Control by a succession of larval response patterns. Int. J. Odonatol. 2021, 24, 1–36. [Google Scholar] [CrossRef] [PubMed]
- Runck, C.; Blinn, D.W. Secondary production of Telebasis salva (Odonata) in a thermally constant aquatic ecosystem. J. N. Am. Benthol. Soc. 1993, 12, 136–147. [Google Scholar] [CrossRef]




| Site Name | Site Code | Species Richness |
|---|---|---|
| Abernathy Community Park, Abernathy * | U1 | 12 |
| Buster Long Park, Lubbock, * | U2 | 5 |
| Clifford H. Andrews Park, Lubbock * | U3 | 7 |
| Caudle Lake Park, Hale Center * | U4 | 9 |
| K.N. Clapp Park, Lubbock * | U5 | 16 |
| George W. Dupree Park, Lubbock * | U6 | 7 |
| Edgar & Essie Givens Park, Plainview * | U7 | 8 |
| Earl Crow Park, Lubbock * | U8 | 6 |
| Charles A. Guy Park, Lubbock * | U9 | 10 |
| Frank Higinbotham Park, Lubbock * | U10 | 6 |
| Phil Hoel Park, Lubbock * | U11 | 3 |
| Jack Stevens Park, Lubbock * | U12 | 4 |
| Jan Jennings Park, Lubbock * | U13 | 8 |
| Leftwich Park, Lubbock * | U14 | 9 |
| Leroy Elmore Park, Lubbock * | U15 | 2 |
| Lobo Lark Park, Levelland * | U16 | 9 |
| George Mahon Park, Lubbock * | U17 | 3 |
| Maxey Park, Lubbock * | U18 | 4 |
| McAlister Park, Lubbock * | U19 | 5 |
| McCullough Park, Lubbock * | U20 | 9 |
| Muleshoe City Park, Muleshoe * | U21 | 10 |
| O.W. Ribble Park, Lubbock * | U22 | 4 |
| Brookdale Remington Park, Lubbock * | U23 | 3 |
| Travis Trussel Park, Plainview * | U24 | 8 |
| Southeast Park, Amarillo ~ | U25 | 5 |
| Southeast Park, Canyon ~ | U26 | 5 |
| Tahoka Lake, Wilson * | S1 | 30 |
| Bean, Gaines Co. ~ | F2 | 9 |
| McKenzie Lake, Gaines Co. * | S2 | 20 |
| Mound Lark, Terry Co. ~ | F1 | 7 |
| Coyote Lake, Bailey Co. | Coyote | -- |
| Baileyboro Lake, Bailey Co. | Baileyboro | -- |
| Goose Lake, Muleshoe National Wildlife Refuge ~ | S4 | 7 |
| Paul’s Lake, Muleshoe National Wildlife Refuge ~ | F3 | 10 |
| Rich Lake, Terry Co. ~ | S3 | 16 |
| Shafter Lake, Andrews Co. | Shafter | -- |
| White Lake, Muleshoe National Wildlife Refuge ~ | F4 | 9 |
| B1, Floyd Co. ~ | P1 | 4 |
| B2, Floyd Co. ~ | P2 | 5 |
| Castro1, Castro Co. ~ | P3 | 3 |
| Cotton Center, Hale Co. ~ | P4 | 8 |
| D2C, Dawson Co. ~ | D2C | -- |
| DarrylB, Castro Co. ~ | P5 | 2 |
| Dport, Floyd Co. ~ | Dport | -- |
| Floyd1, Floyd Co. ~ | P6 | 1 |
| Floyd2, Floyd Co. ~ | P7 | 2 |
| Mil, Floyd Co. ~ | Mil | -- |
| Mur, Floyd Co. ~ | P8 | 2 |
| TTU Range Barn, Lubbock ~ | P9 | 2 |
| S26G, Swisher Co. ~ | S26G | -- |
| Floyd Co. 1 ~ | P10 | 4 |
| Floyd Co. 2 ~ | P11 | 6 |
| Floyd Co. 3 ~ | P12 | 7 |
| SPart, Floyd Co. ~ | SPart | -- |
| SW2007_3C, Swisher Co. ~ | 3C | -- |
| SW2007_4C, Swisher Co. ~ | 4C | -- |
| Wson, Floyd Co. ~ | Wson | -- |
| Wten, Floyd Co. ~ | P13 | 5 |
| Cluster | Site Code | Hp | NO3 | SO2 | PO4 | NO2 | NTU | Veg | Salinity | pH | Macrophytes | Summary |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F2 | Short | −0.2218 | 1.8451 | −1.3979 | −2 | 0.5391 | 0.4636 | 2.9996 | 0.9227 | Y | Mostly long- |
| F3 | Short | 0 | 1.8451 | −0.9208 | −1.1549 | 0.4786 | 0.1002 | 2.9996 | 0.9299 | Y | hydroperiod | |
| F4 | Short | 0.1614 | 1.6434 | −0.4750 | −1.2596 | 0.6042 | 0 | 2.8261 | 0.9199 | N | wetlands of | |
| P10 | Short | 0.2553 | 0.6990 | 0.3979 | −2.2218 | 0.5587 | 1.1071 | 2.1673 | 1.1464 | N | moderate | |
| S3 | Long | −0.3979 | 1.8451 | −0.1938 | −2.2218 | 1.4082 | 1.5708 | 2.9996 | 0.8998 | Y | turbidity, | |
| U1 | Long | −0.5229 | 1.2430 | −0.7959 | −1.7825 | 1.5900 | 0.6590 | 1.9496 | 0.9212 | N | salinity, and | |
| U3 | Long | −0.5229 | 1.1139 | −1.0969 | −1.8239 | 1.6735 | 0.2774 | 2.1156 | 0.9106 | N | acidity, with | |
| U4 | Long | −0.6990 | 1.1461 | −0.5528 | −1.6778 | 1.4836 | 0.3218 | 2.2516 | 0.9268 | N | relatively little | |
| U10 | Long | −0.5229 | 1.1761 | −0.5607 | −2.6990 | 1.6170 | 0.1588 | 2.0354 | 0.9248 | N | vegetation or | |
| U11 | Long | −0.1871 | 1.2041 | 0.1383 | −1.8697 | 1.5838 | 0 | 2.0523 | 0.9281 | N | macrophytes. | |
| U13 | Long | 0.2304 | 1.0212 | −0.8861 | −2.2219 | 2.1472 | 0.1588 | 2.0288 | 0.9219 | N | Moderate-to- | |
| U16 | Long | 0.0969 | 1.1903 | −0.6383 | −1.7959 | 1.3420 | 0.0708 | 2.0288 | 0.9348 | N | high sulphate, | |
| U19 | Long | −0.5229 | 0.6021 | −1.3468 | −2.5229 | 1.5250 | 0.3614 | 2.0032 | 0.9292 | N | generally low | |
| U20 | Long | −0.2596 | 1.5315 | 0.1688 | −2.5229 | 1.4150 | 0 | 2.3374 | 0.9012 | Y | phosphate and | |
| U21 | Long | −1.2596 | 1.8451 | 0.1222 | −2.3010 | 1.2146 | 0.5220 | 2.6928 | 0.9395 | Y | nitrate, variable | |
| U23 | Long | −0.5229 | 1.6128 | −0.1427 | −2.1249 | 1.6360 | 0.1002 | 2.0344 | 0.9261 | N | in nitrite. | |
| 2 | F1 | Short | −0.5229 | 1.8452 | 0.1399 | −1.9586 | 1.1461 | 0.3218 | 0.7910 | 0.8932 | Y | Mostly long |
| P4 | Short | 0.9868 | 1.4472 | 0.3979 | −1.8239 | 1.0682 | 1.5708 | 1.9460 | 0.8751 | N | hydroperiods, | |
| P6 | Short | 1.0531 | 0.6021 | 0.3979 | −1.8861 | 2.6618 | 1.5708 | 2.1644 | 0.9375 | N | moderate-to- | |
| P7 | Short | 0.9445 | 1.8451 | 0.3979 | −1.9208 | 1.3560 | 1.5708 | 1.9841 | 0.8774 | N | high nitrate, | |
| P9 | Short | 0.6232 | 0.6990 | 0.3979 | −1.3979 | 2.1335 | 1.5708 | 2.1106 | 0.8669 | N | sulphate, | |
| S1 | Long | −0.1154 | 1.8451 | −0.7280 | −2.2730 | 2.2586 | 1.1283 | 0.4909 | 0.8850 | Y | nitrite, | |
| S2 | Long | 0.5502 | 1.8451 | 0.1658 | −1.8239 | 1.8334 | 0.7854 | 0.7734 | 0.8834 | Y | phosphate, and | |
| S4 | Long | 0.2304 | 1.8451 | 0.3424 | −2.6990 | 0.7505 | 0.9377 | 0 | 0.8739 | N | turbidity. Low- | |
| U2 | Long | 0.2671 | 1.3222 | 0.1761 | −2.1871 | 1.6080 | 0.3218 | 2.2678 | 0.9261 | N | to-moderate | |
| U6 | Long | 0.0969 | 1.3979 | 0.1903 | −1.5452 | 1.8414 | 0.3977 | 2.1492 | 0.8822 | N | salinity and | |
| U7 | Long | 0.1903 | 0.8129 | 0.3284 | −2.4559 | 1.6656 | 0.6590 | 2.0548 | 0.8618 | Y | acidity. | |
| U8 | Long | 0.1461 | 0.6990 | 0.3979 | −2.3979 | 1.4948 | 0.1588 | 1.9652 | 0.8859 | N | Variable with | |
| U9 | Long | 1.1973 | 0.9778 | 0.1688 | −2.6990 | 2.6994 | 0.1588 | 2.2454 | 0.9513 | Y | respect to | |
| U12 | Long | −0.2218 | 1.2788 | 0.1320 | −2.0220 | 1.5960 | 0.1588 | 2.0006 | 0.8993 | N | vegetation | |
| U14 | Long | 0.1614 | 0.8129 | 0.2467 | −2.3468 | 0.9437 | 0.2255 | 2.0737 | 0.9031 | N | and | |
| U15 | Long | 0.3802 | 1.6434 | 0.1123 | −2.0969 | 1.3128 | 0.1588 | 2.0498 | 0.9101 | N | macrophytes. | |
| U17 | Long | 0.2041 | 1.3222 | 0.1446 | −0.5935 | 1.6232 | 1.2094 | 1.8283 | 0.8727 | N | ||
| U18 | Long | −0.0223 | 0.8862 | 0.9164 | −1.7328 | 1.2304 | 0.2255 | 1.8831 | 0.9028 | N | ||
| U22 | Long | −0.0223 | 1.5119 | 0.3979 | −1.9031 | 1.7392 | 0.1002 | 2.0660 | 0.9149 | N | ||
| U24 | Long | 0.2788 | 1.3892 | 0.1303 | −1.8136 | 1.4208 | 0.6330 | 2.0030 | 0.9199 | Y | ||
| U25 | Long | 0.3010 | 1.7404 | 0.3979 | −2.6990 | 0.9768 | 1.1071 | 2.5391 | 0.8675 | N | ||
| U26 | Long | −0.0969 | 1.5315 | −0.0809 | −2.3010 | 1.3541 | 0.9377 | 2.0864 | 0.8976 | N | ||
| 3 | P1 | Short | −0.3010 | 0.3010 | 0.3979 | −2.6990 | 0.7275 | 1.5708 | 2.3096 | 0.8621 | N | Mostly short |
| P2 | Short | −0.5228 | 0.4771 | 0.3979 | −1.9586 | 1.6253 | 1.5708 | 2.2672 | 0.8445 | N | hydroperiod | |
| P3 | Short | −0.5228 | 0.3010 | −0.1308 | −2.6990 | 3 | 1.5708 | 2.0719 | 0.8825 | N | with shoreline | |
| P5 | Short | −0.5228 | 0.3010 | 0.3979 | −3 | 0.7566 | 1.5708 | 2.1931 | 0.8432 | N | vegetation but | |
| P8 | Short | −0.5228 | 0.3010 | −0.0555 | −2.6990 | 2.1761 | 0.9377 | 1.9921 | 0.9222 | N | few floating | |
| P11 | Short | 0.1461 | 0.9542 | 0.3979 | −2.3979 | 1.0414 | 1.1731 | 2.0128 | 0.8561 | N | macrophytes. | |
| P12 | Short | −0.2218 | 0.3010 | 0.4116 | −2.6990 | 0.6042 | 1.0472 | 2.1173 | 0.8887 | Y | Generally low | |
| P13 | Short | −0.5228 | 0.3010 | 0.1173 | −2.6990 | 2.8222 | 1.2490 | 1.9186 | 0.8859 | N | in nitrate, | |
| U5 | Long | 0.1461 | 0.7803 | 0.2480 | −2.6021 | 0.5734 | 0.9912 | 2.1432 | 0.8420 | Y | sulphate, nitrite, | |
| and acidity. | ||||||||||||
| Generally high | ||||||||||||
| in phosphate, | ||||||||||||
| moderate | ||||||||||||
| salinity. | ||||||||||||
| Variable in | ||||||||||||
| turbidity. |
| SITE | CLUSTER | ANJU | BRGR | CEPO | DYFU | ERSI | LICP | LICM | LILU | LIPU | LISA | ORFE | PALO | PAFL | PAHY | PETE | PHMI | PLLY | PLSU | RHMU | SYCO | TESA | TRLA | TRON | ARAL | ARIM | ARSE | ENCI | ISBA | ISDA | ISDE | ISDN | ISHA | ISRA | ISVE | LEAL | LEAU | HEAM |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F2 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| F3 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| F4 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| S3 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| U1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| U3 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| U4 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| U11 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| U13 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| U16 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| U19 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| U20 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| U21 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| U23 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| P10 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| F1 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| P4 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| P6 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| P7 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| P9 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| S1 | 2 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 1 |
| S2 | 2 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| S4 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| U2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| U6 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| U7 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| U8 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| U9 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| U10 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| U12 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| U14 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| U15 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| U17 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| U18 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| U22 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| U24 | 2 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| U25 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| U26 | 2 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 |
| P1 | 3 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| P2 | 3 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| P3 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| P5 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| P8 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| P11 | 3 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| P12 | 3 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| P13 | 3 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| U5 | 3 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cluster | Number of Wetlands | Number of Odonate Species |
|---|---|---|
| 1 | 16 | 23 |
| 2 | 22 | 32 |
| 3 | 9 | 17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Husband, D.M.; McIntyre, N.E. Finding Isolated Aquatic Habitat: Can Beggars Be Choosers? Diversity 2024, 16, 468. https://doi.org/10.3390/d16080468
Husband DM, McIntyre NE. Finding Isolated Aquatic Habitat: Can Beggars Be Choosers? Diversity. 2024; 16(8):468. https://doi.org/10.3390/d16080468
Chicago/Turabian StyleHusband, Danielle M., and Nancy E. McIntyre. 2024. "Finding Isolated Aquatic Habitat: Can Beggars Be Choosers?" Diversity 16, no. 8: 468. https://doi.org/10.3390/d16080468
APA StyleHusband, D. M., & McIntyre, N. E. (2024). Finding Isolated Aquatic Habitat: Can Beggars Be Choosers? Diversity, 16(8), 468. https://doi.org/10.3390/d16080468






