Updated and Corrected List of Hosts of European Pinnotherids (Crustacea, Decapoda, and Pinnotheridae): Relationship between Number of Hosts and Distribution
Abstract
:1. Introduction
2. Materials and Methods
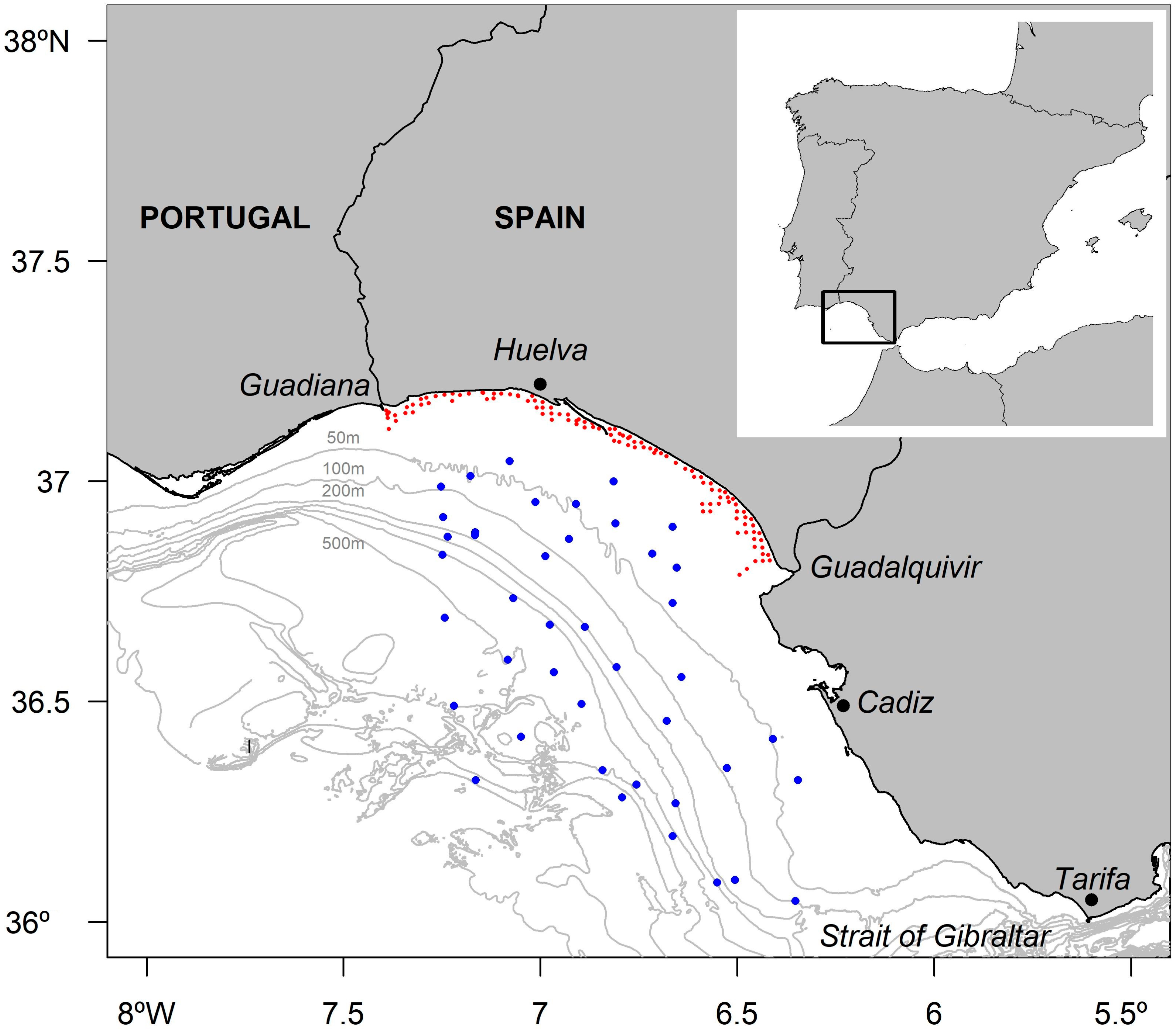
2.1. Study Area and Sample Collection
2.2. Environmental Variables
2.3. Species Identification
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Perez-Miguel, M.; Drake, P.; García Raso, J.E.; Maman Menéndez, L.; Navas, J.I.; Cuesta, J.A. European Pinnotheridae (Crustacea, Decapoda, Brachyura): Species, distribution, host use and DNA barcodes. Mar. Biodiver. 2019, 49, 57–68. [Google Scholar] [CrossRef]
- Cuesta, J.A.; García Raso, J.E.; Abelló, P.; Marco-Herrero, E.; Silva, L.; Drake, P. A new species of pea crab from south-western Europe (Crustacea, Decapoda, Brachyura): Species description, geographical distribution and population structure with an identification key to European Pinnotheridae. J. Mar. Biol. Assoc. U. K. 2019, 99, 1141–1152. [Google Scholar] [CrossRef]
- Becker, C.; Türkay, M. Taxonomy and morphology of European pea crabs (Crustacea: Brachyura: Pinnotheridae). J. Nat. Hist. 2010, 44, 1555–1575. [Google Scholar] [CrossRef]
- Marco-Herrero, E.; Galimany, E.; Abelló, P.; Cuesta, J.A.; Drake, P.; Ramon, M. Updating hosts and distribution range of the pea crab Pinnotheres bicristatus (Brachyura: Pinnotheridae). Med. Mar. Sci. 2020, 21, 499–505. [Google Scholar] [CrossRef]
- Moreno, E.; Bacallado, J.J.; Pérez, J.M. Nueva contribución al conocimiento de los crustáceos decápodos de las islas Canarias. In Actas del II Simposio Ibérico de Estudio del Bentos Marino; Ros, J., Niell, F.X., Eds.; Cuadernos de Biología Marina del C.R.I.S.: Barcelona, Spain, 1982; Volume 3, pp. 213–219. [Google Scholar]
- Triay-Portella, R.; Perez-Miguel, M.; González, J.A.; Cuesta, J.A. On the presence of Pinnotheres pisum (Brachyura, Pinnotheridae) in the Canary Islands (NE Atlantic), its southernmost distribution limit. Crustaceana 2018, 91, 1397–1402. [Google Scholar] [CrossRef]
- de Gier, W.; Becker, C. A review of the ecomorphology of Pinnotherine pea crabs (Brachyura: Pinnotheridae), with an updated list of symbiont-host associations. Diversity 2020, 12, 431. [Google Scholar] [CrossRef]
- Delgado, M.; Silva, L.; Román, S.; Llorens, S.; Rodríguez-Rua, A.; Cojan, M. Spatial distribution patterns of striped venus clam (Chamelea gallina, L. 1758) natural beds in the Gulf of Cádiz (SW Spain): Influence of environmental variables and management considerations. Reg. Stud. Mar. Sci. 2023, 63, 103024. [Google Scholar] [CrossRef]
- Sobrino, I.; Jiménez, M.P.; Ramos, F.; Baro, J. Descripción de las Pesquerías Demersales de la Región Suratlántica Española; Technical report; IEO: Madrid, Spain, 1994; 151p. [Google Scholar]
- Muñoz, I. Colección de Crustáceos Marinos (CRUST-IEOCD). Version 1.31; Occurrence Dataset; Instituto Español de Oceanografía. Centro Oceanográfico de Cádiz (CSIC): Cádiz, Spain, 2024. [Google Scholar] [CrossRef]
- Blott, S.J.; Pye, K. Gradistat: A grain size distribution and statistics package for the analysis of unconsolidated sediments. Earth Surf. Process. Landf. 2001, 26, 1237–1248. [Google Scholar] [CrossRef]
- Folk, R.L.; Ward, W.C. Brazos River bar: A study in the significance of grain size parameter. J. Sediment. Petrol. 1957, 27, 3–27. [Google Scholar] [CrossRef]
- Muñoz, J.L.; Sánchez, J.; Jiménez, F.; Martínez, M.; Torres, J.; Meneses, V. Guía de los Moluscos Marinos y Continentales del Campo de Gibraltar; de Andalucía, J., Ed.; Consejería de Medio ambiente y Ordenación del Territorio: Ornitour, Spain, 2018; 405p. [Google Scholar]
- Gofas, S.; Moreno, D.; Salas, C. Moluscos Marinos de Andalucía; Universidad de Málaga: Málaga, Spain, 2011; 800p. [Google Scholar]
- Pesta, O. Die Decapodenfauna der Adria; Deuticke, F., Ed.; Smithsonian Libraries and Archives: Liepzig, Germany, 1918; 500p. [Google Scholar]
- Bourdon, R. Inventaire de la faune marine de Roscoff. Decapodes-Stomatopodes; Editions de la Station Biologique de Roscoff: Roscoff, France, 1965; 45p. [Google Scholar]
- Adema, J.P.H.M. De Krabben van Nederland en België (Crustacea, Decapoda, Brachyura); Nationaal Natuurhistorisch Museum: Leiden, The Netherlands, 1991; 244p. [Google Scholar]
- Moro, L.; Herrera, R.; Ortea, J.; Riera, R.; Bacallado, J.J.; Martín, J. Aportaciones al conocimiento y distribución de los decápodos y estomatópodos (Crustacea: Malacostraca) de las islas Canarias. Rev. Acad. Canaria Cienc. 2014, 26, 33–82. [Google Scholar]
- Palacios Theil, E.; Cuesta, J.A.; Felder, D.L. Molecular evidence for non-monophyly of the pinnotheroid crabs (Crustacea: Brachyura: Pinnotheroidea), warranting taxonomic reappraisal. Inv. Syst. 2016, 30, 1–27. [Google Scholar] [CrossRef]
- Marco-Herrero, E.; Ramírez-Amaro, S.; Díaz, A.J.; Ordines, F.; Massutí, E. A new host record for the pea crab Pinnotheres pisum (Linnaeus, 1767) (Decapoda: Pinnotheridae) in the western Mediterranean, with an update on host species. Med. Mar. Sci. 2023, 24, 419–425. [Google Scholar] [CrossRef]
- Becker, C.; Türkay, M. Host specificity and feeding in European pea crabs (Brachyura, Pinnotheridae). Crustaceana 2017, 90, 819–844. [Google Scholar] [CrossRef]
- Perez Miguel, M. Efectos del Cangrejo Afropinnotheres monodi Manning, 1993 sobre las Especies de Bivalvos de Interés Comercial de la Península Ibérica. Ph.D Thesis, University of Cádiz, Cádiz, Spain, 2018. [Google Scholar]
- DecaNet. World Register of Marine Species. Available online: https://www.marinespecies.org (accessed on 8 May 2024).
- Perez-Miguel, M.; Drake, P.; Cuesta, J.A. Temperature effect on the African pea crab Afropinnotheres monodi: Embryonic and larval developments, fecundity and adult survival. J. Exp. Mar. Biol. Ecol. 2020, 527, 151380. [Google Scholar] [CrossRef]
- Drake, P.; Marco-Herrero, E.; Subida, M.D.; Arias, A.M.; Cuesta, J.A. Host use pattern of the pea crab Afropinnotheres monodi: Potential effects on its reproductive success and geographical expansion. Mar. Ecol. Prog. Ser. 2014, 498, 203–215. [Google Scholar] [CrossRef]
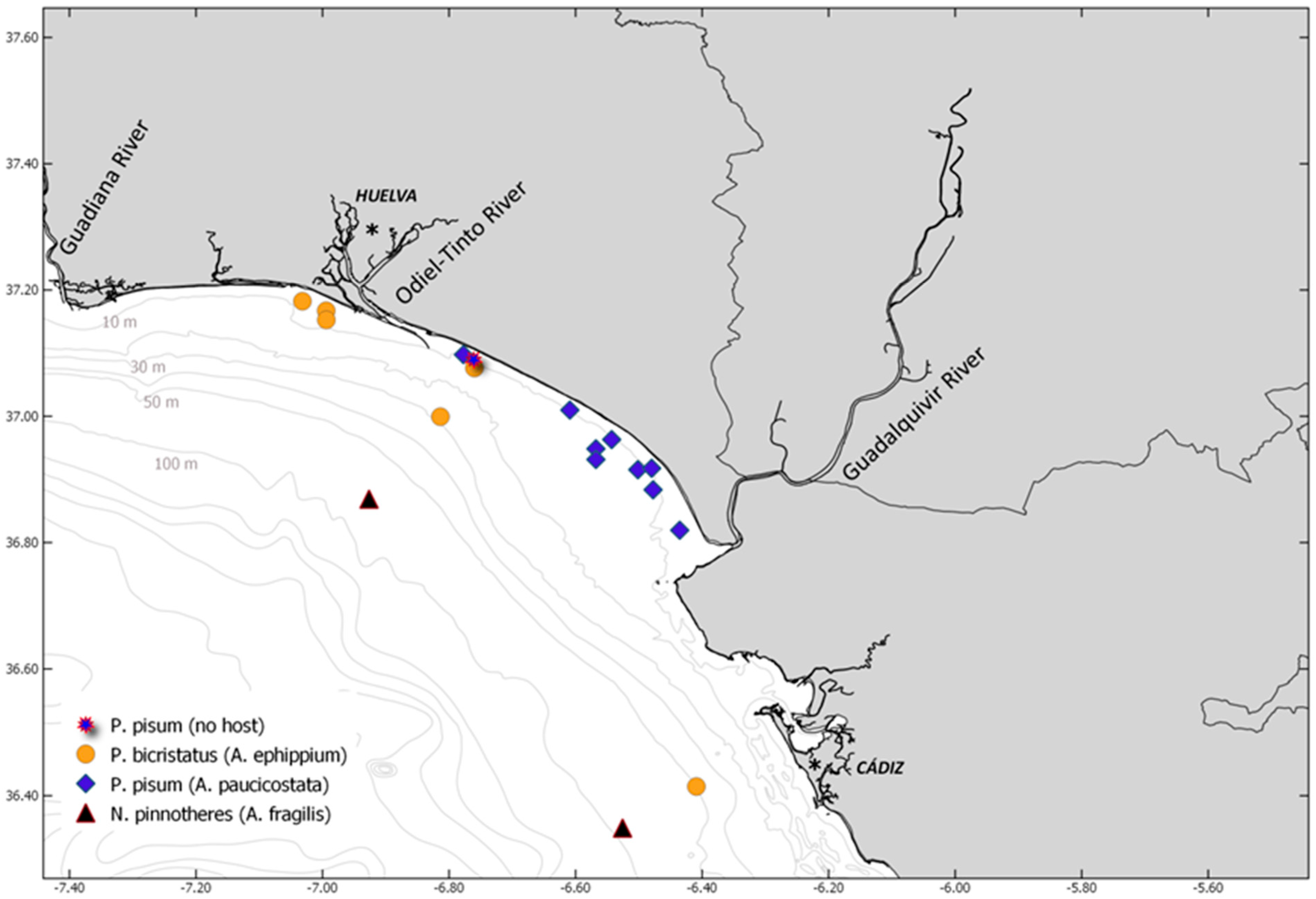
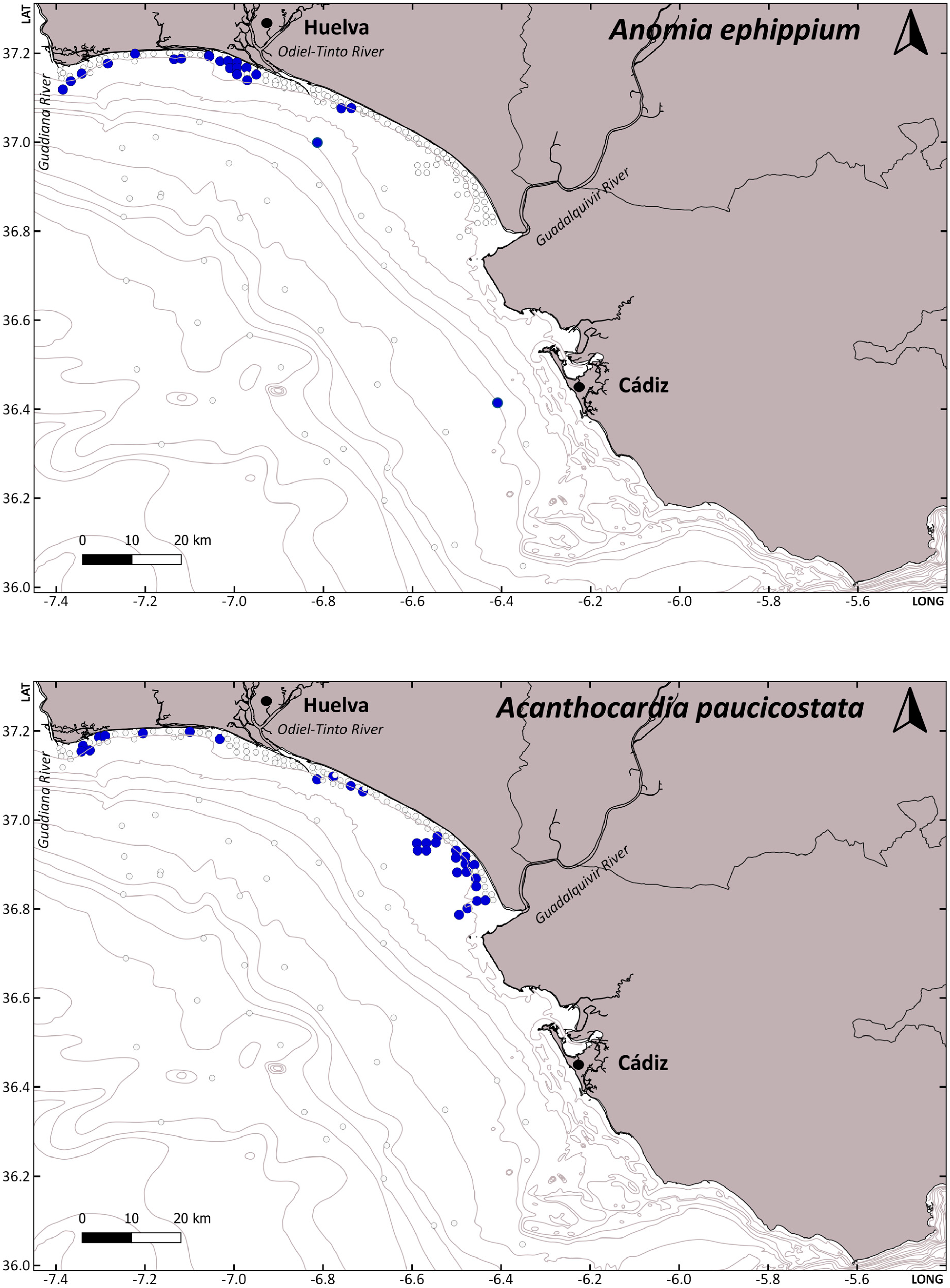
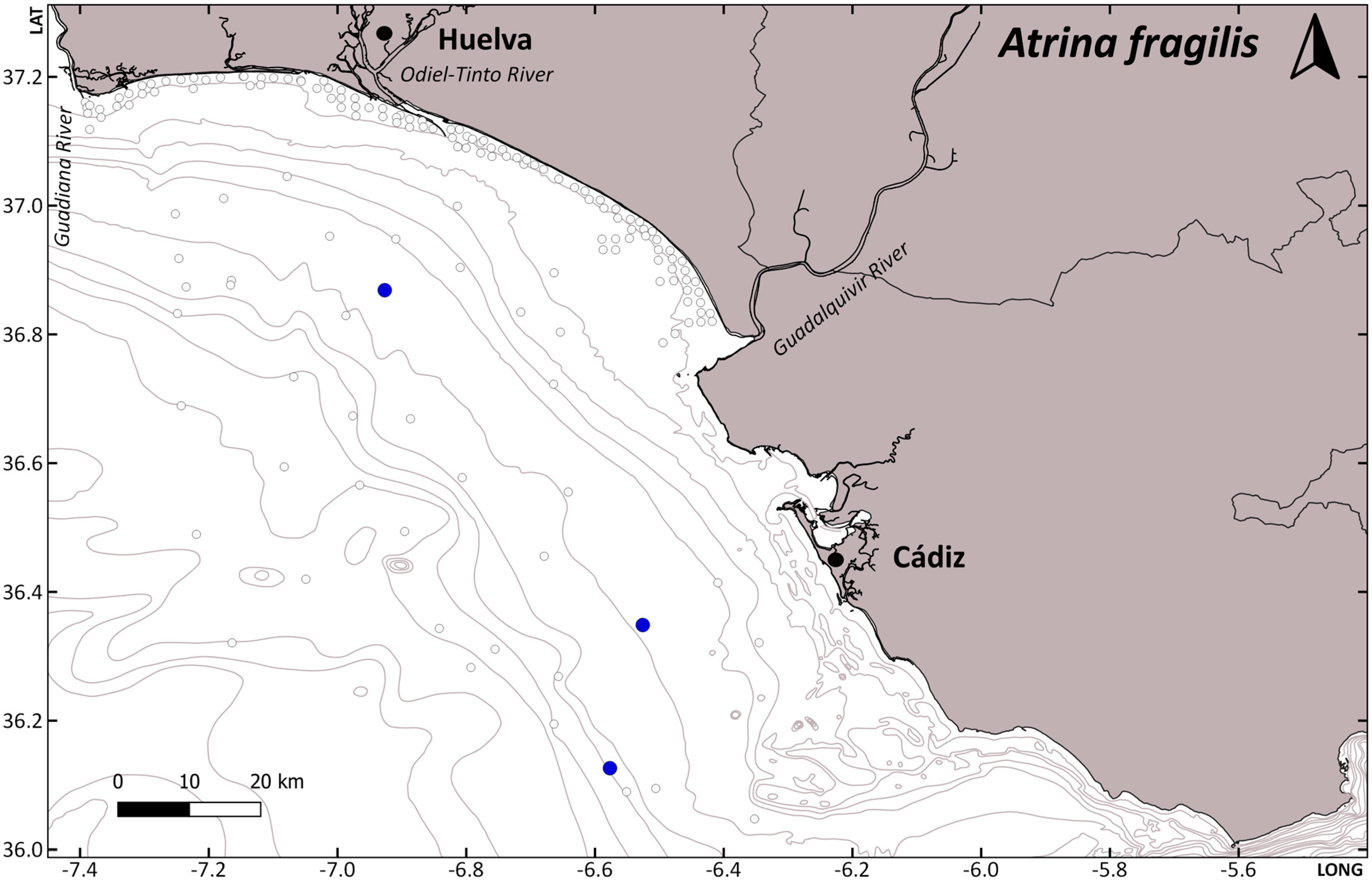
| Species | Code | Host | N | Survey | St | Date | Depth | T | S | MGS | Sed |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Nepinnotheres pinnotheres | CRUST_PINN/3940 | Atrina fragilis | 1 | ARSA0318 | 31 | 26 February 2018 | 83 | - | - | - | - |
| Nepinnotheres pinnotheres | CRUST_PINN/3941 | Atrina fragilis | 1 | ARSA0318 | 31 | 26 February 2018 | 83 | - | - | - | - |
| Nepinnotheres pinnotheres | CRUST_PINN/3942 | Atrina fragilis | 1 | ARSA0318 | 40 | 3 March 2018 | 94 | - | - | - | - |
| Nepinnotheres pinnotheres | CRUST_PINN/3943 | Atrina fragilis | 1 | ARSA0318 | 40 | 3 March 2018 | 94 | - | - | - | - |
| Nepinnotheres pinnotheres | CRUST_PINN/3944 | Atrina fragilis | 1 | ARSA0318 | 40 | 3 March 2018 | 94 | - | - | - | - |
| Nepinnotheres pinnotheres | CRUST_PINN/3938 | Atrina fragilis | 1 | ARSA0319 | 3 | 19 February 2019 | 259 | - | - | - | - |
| Pinnotheres bicristatus | CRUST_PINN/3927 | Anomia ephippium | 1 | ACUVEN-3 | 60 | 11 May 2019 | 9.75 | 15.55 | 35.91 | 2.03 | FS |
| Pinnotheres bicristatus | CRUST_PINN/3930 | Anomia ephippium | 1 | ACUVEN-3 | 60 | 11 May 2019 | 9.75 | 15.55 | 35.91 | 2.03 | FS |
| Pinnotheres bicristatus | CRUST_PINN/3937 | Anomia ephippium | 2 | ACUVEN-3 | 69 | 12 May 2019 | 8 | 16.22 | 35.91 | 1.19 | MS |
| Pinnotheres bicristatus | CRUST_PINN/3931 | Anomia ephippium | 1 | ACUVEN-3 | 114 | 15 May 2019 | 10.8 | 19.61 | 35.93 | 2.54 | FS |
| Pinnotheres bicristatus | CRUST_PINN/3932 | Anomia ephippium | 4 | ACUVEN-3 | 114 | 15 May 2019 | 10.8 | 19.61 | 35.93 | 2.54 | FS |
| Pinnotheres bicristatus | CRUST_PINN/3933 | Anomia ephippium | 3 | ACUVEN-3 | 70 | 12 May 2019 | 9.5 | 16.39 | 35.91 | 0.67 | CS |
| Pinnotheres bicristatus | Anomia ephippium | 27 | ARSA0318 | 1 | 19 February 2018 | 52 | - | - | - | - | |
| Pinnotheres bicristatus | Anomia ephippium | 13 | ARSA0318 | 24 | 25 February 2018 | 29 | - | - | - | - | |
| Pinnotheres pisum | CRUST_PINN/3925 | Acanthocardia paucicostata | 1 | ACUVEN-3 | 109 | 16 May 2019 | 9.6 | 19.05 | 35.91 | 3.16 | VFS |
| Pinnotheres pisum | CRUST_PINN/3926 | Out of the host | 1 | ACUVEN-3 | 113 | 15 May 2019 | 10.4 | 20.11 | 35.94 | 2.78 | FS |
| Pinnotheres pisum | Acanthocardia paucicostata | 1 | ACUVEN-3 | 147 | 9 | 16.55 | 35.93 | 0.63 | CS | ||
| Pinnotheres pisum | CRUST_PINN/3928 | Acanthocardia paucicostata | 1 | ACUVEN-3 | 167 | 25 May 2019 | 14.15 | 19.05 | 35.97 | 3.34 | VFS |
| Pinnotheres pisum | CRUST_PINN/3929 | Acanthocardia paucicostata | 3 | ACUVEN-3 | 168 | 27 May 2019 | 15.5 | 18.61 | 35.96 | 3.19 | VFS |
| Pinnotheres pisum | Acanthocardia paucicostata | 1 | ACUVEN-3 | 173 | 10.4 | 18.78 | 35.98 | 3.01 | VFS | ||
| Pinnotheres pisum | CRUST_PINN/3936 | Acanthocardia paucicostata | 1 | ACUVEN-3 | 189 | 24 May 2019 | 11.75 | 19.75 | 36.00 | 3.63 | VFS |
| Pinnotheres pisum | Acanthocardia paucicostata | 1 | ACUVEN-3 | 195 | 12.35 | 20.70 | 36.04 | 3.53 | VFS | ||
| Pinnotheres pisum | CRUST_PINN/3939 | Acanthocardia paucicostata | 1 | ACUVEN-2 | 189 | 18 November 2020 | 12 | 18.40 | 37.20 | 2.71 | FS |
| Pinnotheres pisum | CRUST_PINN/3934 | Acanthocardia paucicostata | 2 | ACUVEN-2 | 193 | 18 November 2020 | 10 | 18.30 | 37.16 | 3.30 | VFS |
| Pinnotheres pisum | CRUST_PINN/3935 | Acanthocardia paucicostata | 1 | ACUVEN-2 | 244 | 20 November 2020 | 8.55 | 18.60 | 36.88 | 3.33 | VFS |
| Species: | Afropinnotheres monodi | Nepinnotheres pinnotheres | |
|---|---|---|---|
| Host species: | Cerastoderma edule (Linnaeus, 1758) | Ascidia mentula Müller, 1776 | |
| Cerastoderme glaucum (Bruguière, 1789) | Ascidia virginea? Müller, 1776 | ||
| Chamelea gallina (Linnaeus, 1758) | Atrina fragilis (Pennant, 1777) | ||
| Donax trunculus (Linnaeus, 1758) | Atrina pectinata (Linnaeus, 1767) | ||
| Eastonia rugosa (Helbling, 1779) | Halocynthia papillosa (Linnaeus, 1767) | ||
| Mactra stultorum (Linnaeus, 1758) | Microcosmos spp. | ||
| Magallana gigas (Thunberg, 1793) | Phallusia mammillata? (Cuvier, 1815) | ||
| Mytilus galloprovincialis (Lamarck, 1819) | Pinna nobilis (Linnaeus, 1758) | ||
| Polititapes aureus (Gmelin, 1791) | Pinna rudis (Linnaeus, 1758) | ||
| Ruditapes decussatus (Linnaeus, 1758) | |||
| Scobricularia plana (da Costa, 1778) | |||
| Spisula solida (Linnaeus, 1758) | |||
| Venerupis corrugata (Gmelin, 1791) | |||
| Species: | Pinnotheres bicristatus | Pinnotheres pectunculi | Pinnotheres pisum |
| Host species: | Anomia ephippium Linnaeus, 1758 | Chamalea gallina | Acanthocardia echinate (Linnaeus, 1758) |
| Ostrea edulis Linnaeus, 1758 | Clausinella fasciata (da Costa, 1778) | Acanthocardia paucicostata (G. B. Sowerby II, 1834) | |
| Glycymeris glycimeris (Linnaeus, 1758) | Arctica islandica (Linnaeus, 1767) | ||
| Venus casina Linnaeus, 1758 | Arenomya arenaria (Linnaeus, 1758) | ||
| Venus verrucosa Linnaeus, 1758 | Atrina pectinata | ||
| Chamelea gallina | |||
| Chamelea striatula (da Costa, 1778) | |||
| Cerastoderma edule | |||
| Cerastoderma glaucum | |||
| Donax trunculus | |||
| Donax variegatus (Gmelin, 1791) | |||
| Donax vetustus Poli, 1795 | |||
| Donax vittatus (da Costa, 1778) | |||
| Fulvia fragilis (Forsskål, 1775) | |||
| Gari fervensis (Gmelin, 1791) | |||
| Glossus humanus (Linnaeus, 1758) | |||
| Laevicardium crissum (Gmelin, 1791) | |||
| Lutraria lutraria (Linnaeus, 1758) | |||
| Mactra stultorum | |||
| Modiolus modiolus (Linnaeus, 1758) | |||
| Mytilus edulis Linnaeus, 1758 | |||
| Mytilus galloprovincialis | |||
| Ostrea edulis | |||
| Pinna nobilis | |||
| Ruditapes decussatus | |||
| Spisula solida | |||
| Spisula elliptica (T. Brown, 1827) | |||
| Spisula subtruncata (da Costa, 1778) | |||
| Venus verrucosa |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cuesta, J.A.; Román, S.; Muñoz, I.; Rodríguez de la Rúa, A.; Farias, C.; Silva, L.; Delgado, M. Updated and Corrected List of Hosts of European Pinnotherids (Crustacea, Decapoda, and Pinnotheridae): Relationship between Number of Hosts and Distribution. Diversity 2024, 16, 470. https://doi.org/10.3390/d16080470
Cuesta JA, Román S, Muñoz I, Rodríguez de la Rúa A, Farias C, Silva L, Delgado M. Updated and Corrected List of Hosts of European Pinnotherids (Crustacea, Decapoda, and Pinnotheridae): Relationship between Number of Hosts and Distribution. Diversity. 2024; 16(8):470. https://doi.org/10.3390/d16080470
Chicago/Turabian StyleCuesta, Jose A., Sara Román, Isabel Muñoz, Ana Rodríguez de la Rúa, Carlos Farias, Luis Silva, and Marina Delgado. 2024. "Updated and Corrected List of Hosts of European Pinnotherids (Crustacea, Decapoda, and Pinnotheridae): Relationship between Number of Hosts and Distribution" Diversity 16, no. 8: 470. https://doi.org/10.3390/d16080470






