Resprouting Control of Ailanthus altissima by Means of Cut and Stump Covering: Experimental Evidence for a Promising Technique
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experiment Setup
2.2. Measurements
2.3. Statistical Analysis
3. Results
3.1. Plant Phenology
3.2. Sprout Number
3.3. Sprout Biomass
3.4. Sprouting Model
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kota, N.L.; Landenberger, R.E.; McGraw, J.B. Germination and early growth of Ailanthus and tulip poplar in three levels of forest disturbance. Biol. Invasions 2007, 9, 197–211. [Google Scholar] [CrossRef]
- Enescu, C.M.; Houston Durrant, T.; Caudullo, G. Ailanthus altissima in Europe: Distribution, habitat, usage and threats. In European Atlas of Forest Tree Species; San-Miguel-Ayanz, J., de Rigo, D., Caudullo, G., Houston Durrant, T., Mauri, A., Eds.; Publication Office of the European Union: Luxembourg, 2016; p. 61. Available online: https://ies-ows.jrc.ec.europa.eu/efdac/download/Atlas/pdf/Ailanthus_altissima.pdf (accessed on 25 June 2024).
- Walker, G.A.; Gaertner, M.; Robertson, M.P.; Richardson, D.M. The prognosis for Ailanthus altissima (Simaroubaceae; tree of heaven) as an invasive species in South Africa; insights from its performance elsewhere in the world. S. Afr. J. Bot. 2017, 112, 283–289. [Google Scholar] [CrossRef]
- Kowarik, I.; Säumel, I. Biological flora of Central Europe: Ailanthus altissima (Mill.) Swingle. Perspect. Plant Ecol. 2007, 8, 207–237. [Google Scholar] [CrossRef]
- Montagnani, C.; Gentili, R.; Brundu, G.; Celesti-Grapow, L.; Galasso, G.; Lazzaro, L.; Armeli Minicante, S.; Carnevali, L.; Acosta, A.T.R.; Agrillo, E.; et al. Specie esotiche invasive di rilevanza unionale in Italia: Aggiornamenti e integrazioni. In Valutazione e Classificazione Degli Impatti e Distribuzione Delle Specie Alloctone in Italia; Armeli Minicante, S., Celesti-Grapow, L., Galasso, G., Lazzaro, L., Montagnani, C., Brundu, G., Eds.; Notiziario della Società Botanica Italiana: Milano, Italy, 2022; Volume 6, pp. 19–20. ISSN 2532-8034. Available online: http://notiziario.societabotanicaitaliana.it/wp-content/uploads/2022/08/Notiziario-6_1_2022-compresso.pdf (accessed on 25 June 2024).
- Knapp, L.B.; Canham, C.D. Invasion of an old-growth forest in New York by Ailanthus altissima: Sapling growth and recruitment in canopy gaps. J. Torrey Bot. Soc. 2000, 127, 307–315. [Google Scholar] [CrossRef]
- Celesti-Grapow, L.; Blasi, C. The role of alien and native weeds in the deterioration of archaeological remains in Italy. Weed Technol. 2004, 18, 1508–1513. [Google Scholar]
- Badalamenti, E.; La Mantia, T. Stem-injection of herbicide for control of Ailanthus altissima (Mill.) Swingle: A practical source of power for drilling holes in stems. iForest 2013, 6, 123–126. [Google Scholar] [CrossRef]
- Fogliatto, S.; Milan, M.; Vidotto, F. Control of Ailanthus altissima using cut stump and basal bark herbicide applications in an eighteenth-century fortress. Weed Res. 2020, 60, 425–434. [Google Scholar] [CrossRef]
- Brooks, R.K.; Barney, J.N.; Salom, S.M. The invasive tree, Ailanthus altissima, impacts understory nativity, not seedbank nativity. Forest Ecol. Manag. 2021, 489, 119025. [Google Scholar] [CrossRef]
- Terzi, M.; Fontaneto, D.; Casella, F. Effects of Ailanthus altissima invasion and removal on high-biodiversity Mediterranean grasslands. Environ. Manag. 2021, 68, 914–927. [Google Scholar] [CrossRef] [PubMed]
- Sladonja, B.; Sušek, M.; Guillermic, J. Review on invasive Tree of Heaven (Ailanthus altissima (Mill.) Swingle) conflicting values: Assessment of its ecosystem services and potential biological threat. Environ. Manag. 2015, 56, 1009–1034. [Google Scholar] [CrossRef] [PubMed]
- Greer, G.K.; Dietrich, M.A.; Lincoln, J.M. Ailanthus altissima stimulates legume nodulation in Trifolium pratense via root exudates: A novel mechanism facilitating invasion? Int. J. Plant Sci. 2016, 177, 400–408. [Google Scholar] [CrossRef]
- European Commission. Regulation (EU) No 1143/2014 of the European Parliament and of the Council of 22 October 2014 on the Prevention and Management of the Introduction and Spread of Invasive Alien Species. Off. J. Eur. Union 2014, 317, 35–55. [Google Scholar]
- Badalamenti, E.; Barone, E.; La Mantia, T. Seasonal effects on mortality rates and resprouting of stems treated with glyphosate in the invasive tree of heaven (Ailanthus altissima (Mill.) Swingle). Arboric. J. 2015, 37, 180–195. [Google Scholar] [CrossRef]
- Motard, E.; Muratet, A.; Clair-Maczulajtys, D.; Machon, N. Does the invasive species Ailanthus altissima threaten floristic diversity of temperate peri-urban forests? Comptes Rendus Biol. 2015, 334, 872–879. [Google Scholar] [CrossRef] [PubMed]
- Di Tomaso, J.M.; Kyser, G.B. Control of Ailanthus altissima using stem herbicide application techniques. Arboric. Urban. For. 2007, 33, 55–63. [Google Scholar] [CrossRef]
- Constán-Nava, S.; Bonet, A.; Pastor, E.; Lledó, M.J. Long-term control of the invasive tree Ailanthus altissima: Insights from Mediterranean protected forests. For. Ecol. Manag. 2010, 260, 1058–1064. [Google Scholar] [CrossRef]
- Burch, P.L.; Zedaker, S.M. Removing the invasive tree Ailanthus altissima and restoring natural cover. J. Arboric. 2003, 29, 18–24. [Google Scholar] [CrossRef]
- Feret, P.P. Ailanthus: Variation, cultivation, and frustration. J. Arboric. 1985, 11, 361–369. [Google Scholar] [CrossRef]
- Harris, P.T.; Cannon, G.H.; Smith, N.E.; Muth, N.Z. Assessment of plant community restoration following Tree-of-Heaven (Ailanthus altissima) control by Verticillium albo-atrum. Biol. Invasions 2013, 15, 1887–1893. [Google Scholar] [CrossRef]
- Pisuttu, C.; Marchica, A.; Bernardi, R.; Calzone, A.; Cotrozzi, L.; Nali, C.; Pellegrini, E.; Lorenzini, G. Verticillium wilt of Ailanthus altissima in Italy caused by V. dahliae: New outbreaks from Tuscany. iForest 2020, 13, 238–245. [Google Scholar] [CrossRef]
- European Community. Directive 2009/128/EC of the European Parliament and of the Council of 21 October 2009 establishing a framework for Community action to achieve the sustainable use of pesticides. Off. J. Eur. Union 2009, L 309/71, 1–16. [Google Scholar]
- Hoshovsky, M.C. Element Stewardship Abstract for Ailanthus altissima; The Nature Conservancy: Arlington, VA, USA, 1988; 13p, Available online: https://www.invasive.org/gist/esadocs/documnts/ailaalt.pdf (accessed on 2 August 2024).
- Clarke, P.J.; Lawes, M.J.; Midgley, J.J.; Lamont, B.B.; Ojeda, F.; Burrows, G.E.; Enright, N.J.; Knox, K.J.E. Resprouting as a key functional trait: How buds, protection and resources drive persistence after fire. New Phytol. 2013, 197, 19–35. [Google Scholar] [CrossRef] [PubMed]
- Wildy, D.T.; Pate, J.S. Quantifying above- and below-ground growth responses of the Western Australian oil mallee, Eucalyptus kochii subsp. pienissima, to contrasting decapitation regimes. Ann. Bot. 2002, 90, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Schneider, A.; Godin, C.; Boudon, F.; Demotes-Mainard, S.; Sakr, S.; Bertheloot, J. Light regulation of axillary bud outgrowth along plant axes: An overview of the roles of sugars and hormones. Front. Plant Sci. 2019, 10, 1296. [Google Scholar] [CrossRef] [PubMed]
- Girault, T.; Abidi, F.; Sigogne, M.; Pelleschi-Travier, S.; Boumaza, R.; Sakr, S.; Leduc, N. Sugars are under light control during bud burst in Rosa sp. Plant Cell Environ. 2010, 33, 1339–1350. [Google Scholar] [CrossRef] [PubMed]
- Choubane, D.; Rabot, A.; Mortreau, E.; Legourrierec, J.; Péron, T.; Foucher, F.; Ahcène, Y.; Pelleschi-Travier, S.; Leduc, N.; Hamama, L.; et al. Photocontrol of bud burst involves gibberellin biosynthesis in Rosa sp. J. Plant Physiol. 2012, 169, 1271–1280. [Google Scholar] [CrossRef] [PubMed]
- Tree Diseases Articles. Available online: https://www.72tree.com/how-to-kill-stop-tree-stumps-growing-back/ (accessed on 18 July 2024).
- Knüsel, S.; Wunder, J.; Moos, C.; Dorren, L.; Schwarz, M.; Gurtner, D.; Conedera, M. L’ailanto nei Boschi Svizzeri. Ecologia e Opzioni Gestionali. Notizie per la Pratica; WSL Birmensdorf: Birmensdorf, Switzerland, 2020; pp. 1–12. [Google Scholar]
- Köppen, W. Das geographische System der Klimate. In Handbuch der Klimatologie, 1st ed.; Koppen, W., Geiger, R., Eds.; Verlag von Gebürder Borntraeger: Berlin, Germany, 1936; Volume 1, pp. 1–44. [Google Scholar]
- Usda, Natural Resource Conservation Service. Available online: https://www.nrcs.usda.gov/resources/guides-and-instructions/soil-taxonomy (accessed on 25 June 2024).
- Berry, Z.C.; Ávila-Lovera, E.; De Guzman, M.E.; O’Keefe, K.; Emery, N.C. Beneath the bark: Assessing woody stem water and carbon fluxes and its prevalence across climates and the woody plant phylogeny. Front. Glob. Change 2021, 4, 675299. Available online: https://www.frontiersin.org/articles/10.3389/ffgc.2021.675299/full (accessed on 25 June 2024). [CrossRef]
- Cline, M.G. Concepts and terminology of apical dominance. Am. J. Bot. 1997, 84, 1064–1069. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Yoda, K.; Suzuki, M.; Suzuki, H. Vascular tissue in the stem and roots of woody plants conduct light. J. Exp. Bot. 2003, 54, 1627–1635. [Google Scholar] [CrossRef] [PubMed]
- Canadell, J.; López-Soria, L. Lignotuber reserves support regrowth following clipping of two Mediterranean shrubs. Funct. Ecol. 1998, 12, 31–38. [Google Scholar] [CrossRef]
- Kowarik, I. Clonal growth in Ailanthus altissima on a natural site in West Virginia. J. Veg. Sci. 1995, 6, 853–856. [Google Scholar] [CrossRef]
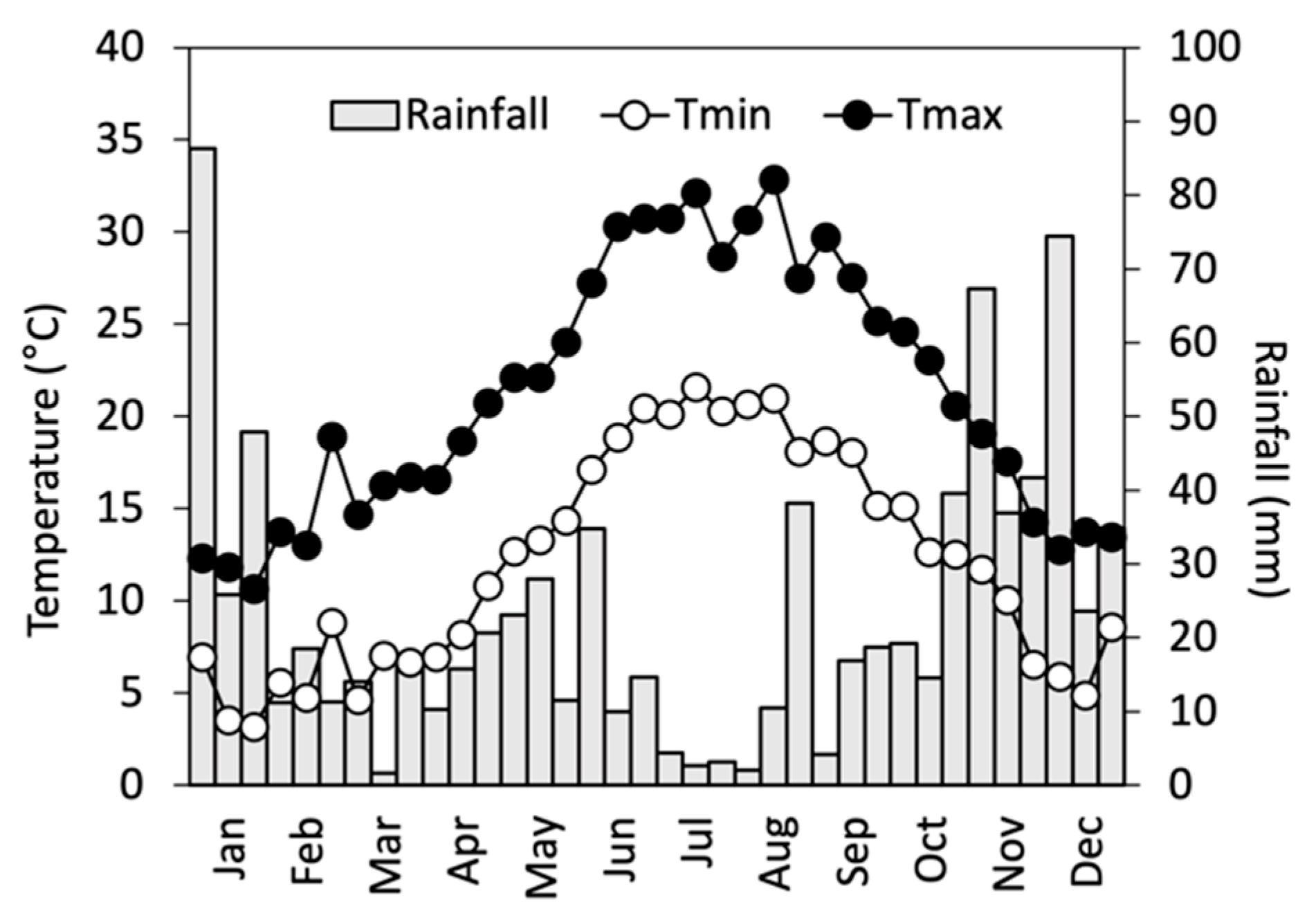


| Plant Stage | Date | ||
|---|---|---|---|
| Winter dormancy of shoots (control tree leaves completely dropped) | 1 November 2021 | 12 October 2022 | 25 October 2023 |
| Brake of winter dormancy (leaf buds swollen in C; in the first season also, stem buds swollen on cut stumps) | 16 March 2022 | 12 March 2023 | 18 March 2024 |
| First emergence of root sprouts in cut stems | 1 June 2022 | 17 April 2023 | 22 April 2024 |
| Full vegetative growth (1st leaves of C plants unfolded) | 1 June 2022 | 6 June 2023 | 31 May 2024 |
| Full vegetative growth of sprouts (1st leaves unfolded) | 25 July 2022, cut I | 21 July 2023, cut III | - |
| Leaves of control trees started yellowing | 12 September 2022 | 25 September 2023 | - |
| Leaves of sprouts started yellowing | 3 October 2022, cut II | 23 October 2023, cut IV | - |
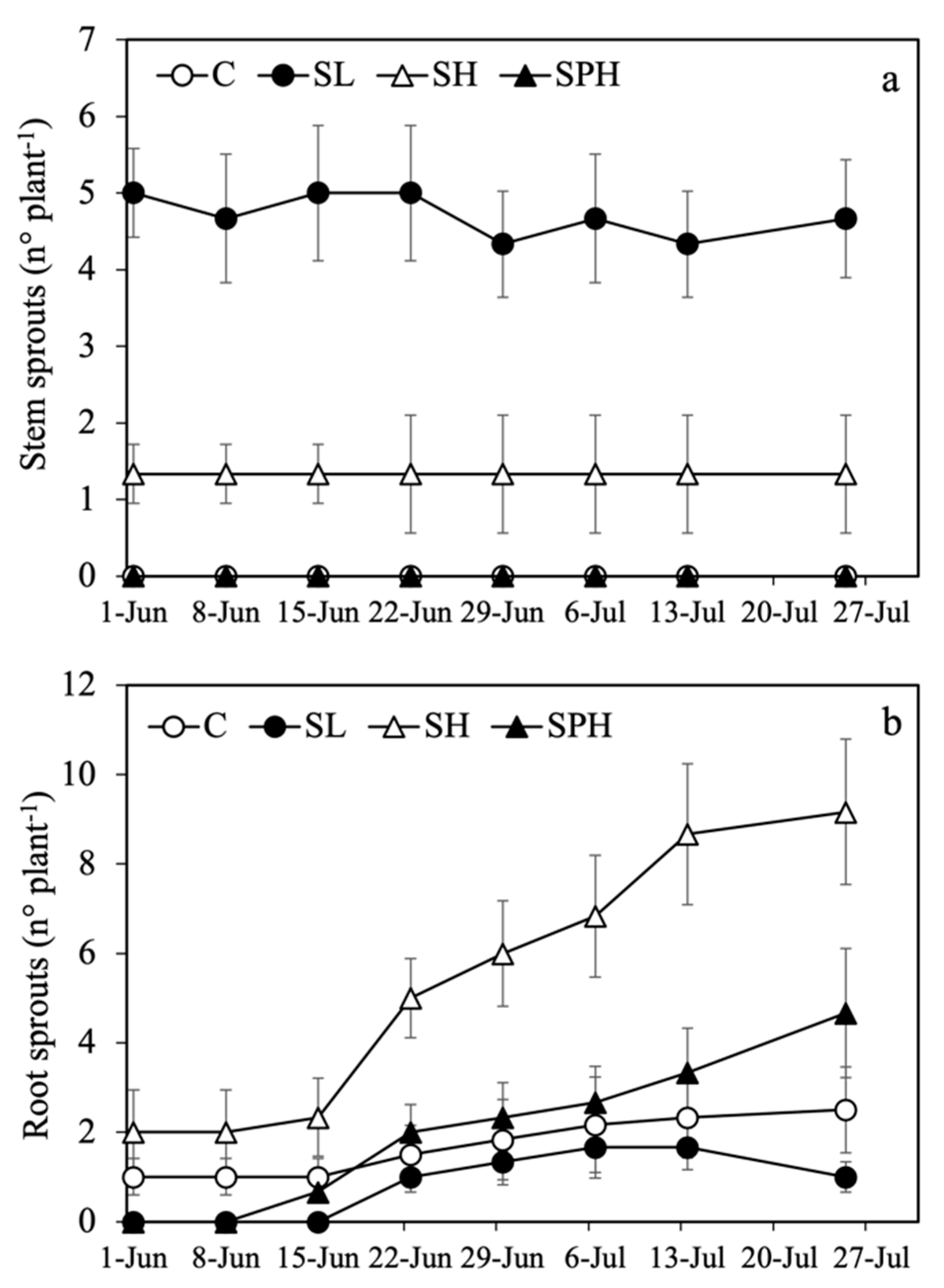
| Sprouting Plants (%) | N° of Sprouts per Plant | ||||
|---|---|---|---|---|---|
| Treatments | Stem | Roots | Stem | Root | Total |
| Cover removal (1 June 2022) | |||||
| C | 0 | 50 | -- | 1.0 ± 0.41 a | 1.0 ± 0.41 b |
| SL | 100 | 0 | 5.0 ± 0.58 a | -- | 5.0 ± 0.58 a |
| SH | 67 | 50 | 1.3 ± 0.39 b | 2.0 ± 0.94 a | 3.3 ± 1.22 ab |
| SPH | 0 | 0 | -- | -- | -- |
| Cut I (25 July 2022) | |||||
| C | 0 | 67 | - | 2.5 ± 0.97 b | 2.5 ± 0.97 b |
| SL | 100 | 67 | 4.7 ± 0.77 a | 1.0 ± 0.33 b | 5.7 ± 0.84 ab |
| SH | 50 | 100 | 1.3 ± 0.77 b | 9.2 ± 1.62 a | 10.5 ± 2.34 a |
| SPH | 0 | 67 | - | 4.7 ± 1.45 ab | 4.7 ± 1.45 ab |
| Cut II (3 October 2022) | |||||
| C | 0 | 50 | -- | 0.5 ± 0.20 b | 0.5 ± 0.20 b |
| SL | 50 | 50 | 1.0 ± 0.47 | 8.0 ± 3.30 a | 9.0 ± 3.70 a |
| SH | 0 | 0 | -- | -- | -- |
| SPH | 0 | 67 | -- | 1.3 ± 0.45 b | 1.3 ± 0.45 b |
| Cut III (21 July 2023) | |||||
| C | 0 | 100 | -- | 8.5 ± 1.87 ab | 8.5 ± 1.87 ab |
| SL | 0 | 50 | -- | 10.5 ± 4.78 a | 10.5 ± 4.78 a |
| SH | 0 | 0 | -- | -- | -- |
| SPH | 0 | 33 | -- | 2.5 ± 1.45 b | 2.5 ± 1.45 b |
| Cut IV (23 October 2023) | |||||
| C | 0 | 0 | -- | -- | -- |
| SL | 0 | 50 | -- | 7.0 ± 3.19 a | 7.0 ± 3.19 a |
| SH | 0 | 0 | -- | -- | -- |
| SPH | 0 | 33 | -- | 0.83 ± 0.50 b | 0.83 ± 0.50 b |
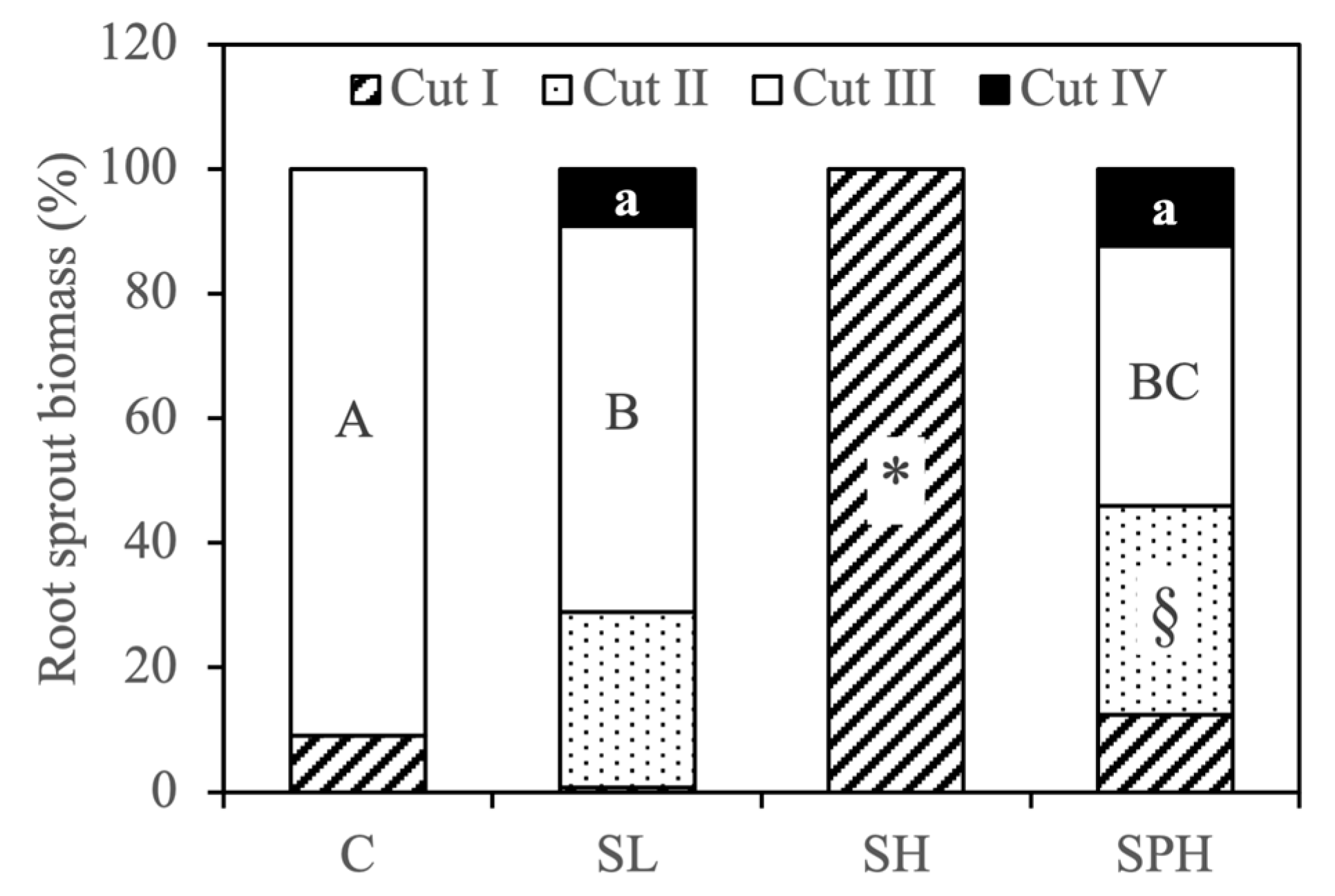
| Stem | Root | Stem | Root | Total | |
|---|---|---|---|---|---|
| Treatments | g dw Sprout−1 | g dw Plant−1 | |||
| Cut I (25 July 2022) | |||||
| C | -- | 0.02 ± 0.002 a | -- | 0.06 ± 0.024 a | 0.06 ± 0.024 b |
| SL | 8.5 ± 0.41 a | 0.09 ± 0.026 a | 39.5 ± 5.95 a | 0.09 ± 0.051 a | 39.5 ± 5.96 a |
| SH | 3.3 ± 0.63 b | 0.87 ± 0.328 a | 4.4 ± 2.56 b | 8.0 ± 4.54 a | 12.4 ± 6.98 b |
| SPH | -- | 0.24 ± 0.102 a | -- | 1.1 ± 0.75 a | 1.1 ± 0.75 b |
| Cut II (3 October 2022) | |||||
| C | -- | -- | -- | -- | -- |
| SL | 0.82 ± 0.373 | 0.40 ± 0.093 b | 0.82 ± 0.373 | 3.2 ± 1.37 a | 4.0 ± 1.74 a |
| SH | -- | -- | -- | -- | -- |
| SPH | -- | 2.3 ± 0.54 a | -- | 3.1 ± 1.41 a | 3.1 ± 1.41 a |
| Cut III (21 July 2023) | |||||
| C | -- | 0.07 ± 0.005 a | -- | 0.6 ± 0.10 a | 0.6 ± 0.10 a |
| SL | -- | 0.67 ± 0.143 a | -- | 7.0 ± 3.09 a | 7.0 ± 3.09 a |
| SH | -- | -- | -- | -- | -- |
| SPH | -- | 1.5 ± 0.30 a | -- | 3.9 ± 2.23 a | 3.9 ± 2.23 a |
| Cut IV (23 October 2023) | |||||
| C | -- | -- | -- | -- | -- |
| SL | -- | 0.15 ± 0.032 b | -- | 1.0 ± 0.46 a | 1.1 ± 0.46 a |
| SH | -- | -- | -- | -- | -- |
| SPH | -- | 1.4 ± 0.26 a | -- | 1.2 ± 0.69 a | 1.2 ± 0.69 a |
| p-Value | |||
|---|---|---|---|
| Root Sprouts (g Plant−1) | |||
| Contrasts | Cut I | Cut III | |
| C | SL + SH + SPH | ns | ns |
| SL | C + SH + SPH | ns | 0.0314 |
| SH | C + SL + SPH | 0.0173 | ns |
| SPH | C + SL + SH | ns | ns |
| SH + SPH | C + SL | ns | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arduini, I.; Pampana, S.; Alessandrini, V. Resprouting Control of Ailanthus altissima by Means of Cut and Stump Covering: Experimental Evidence for a Promising Technique. Diversity 2024, 16, 471. https://doi.org/10.3390/d16080471
Arduini I, Pampana S, Alessandrini V. Resprouting Control of Ailanthus altissima by Means of Cut and Stump Covering: Experimental Evidence for a Promising Technique. Diversity. 2024; 16(8):471. https://doi.org/10.3390/d16080471
Chicago/Turabian StyleArduini, Iduna, Silvia Pampana, and Viola Alessandrini. 2024. "Resprouting Control of Ailanthus altissima by Means of Cut and Stump Covering: Experimental Evidence for a Promising Technique" Diversity 16, no. 8: 471. https://doi.org/10.3390/d16080471








