Understanding Balanophyllia regia Distribution in the Canary Islands: Effects of Environmental Factors and Methodologies for Future Monitoring
Abstract
:1. Introduction
2. Materials and Methods
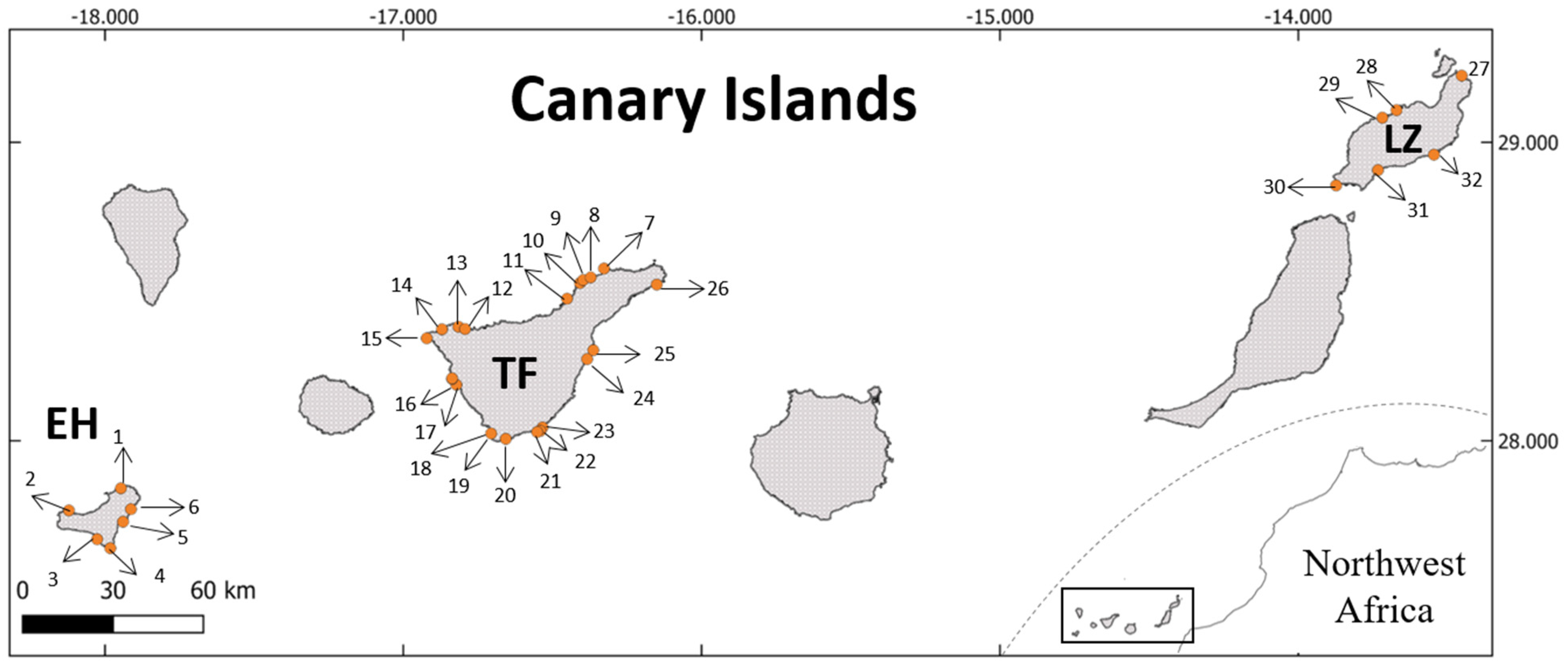
2.1. Sampling Sites and Local-Scale Environmental Drivers
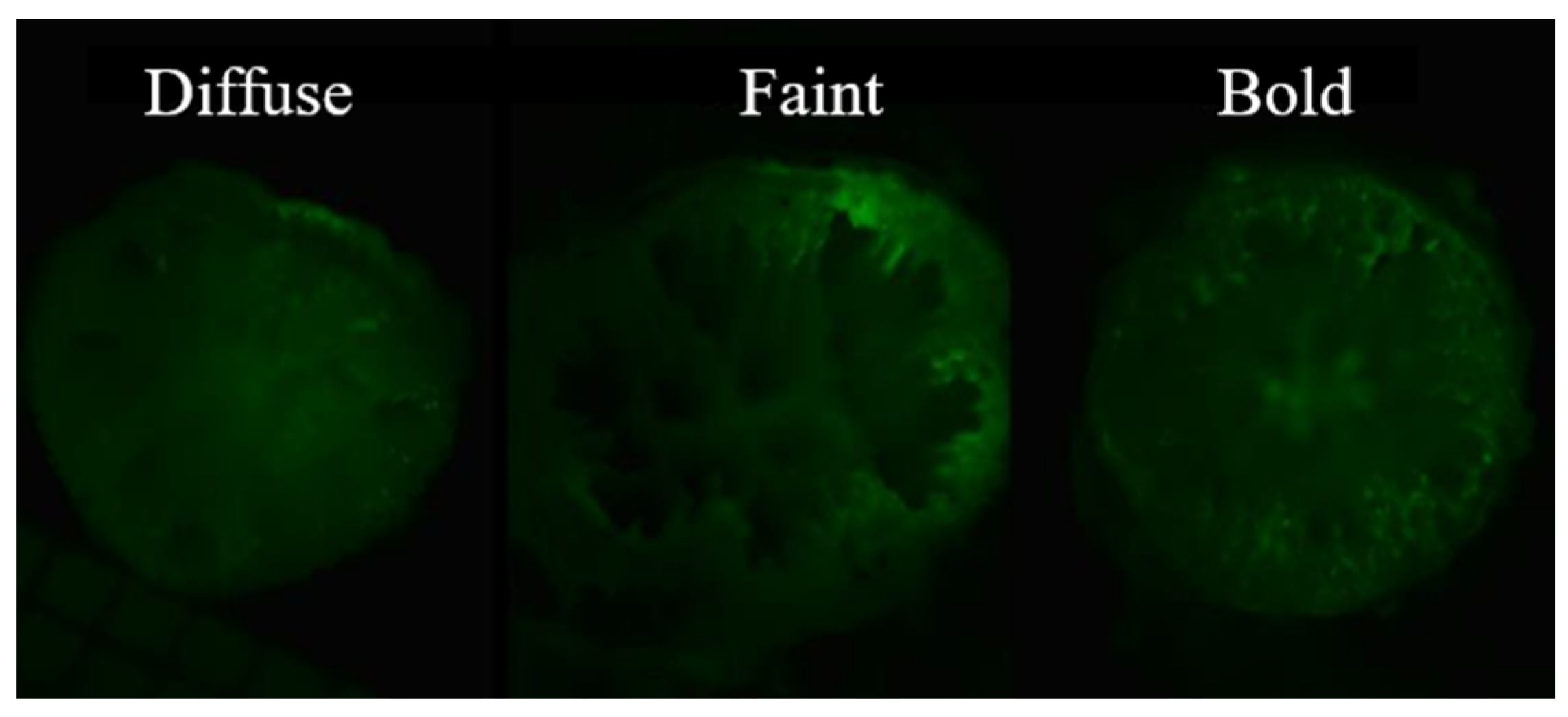
2.2. Calcein Tagging
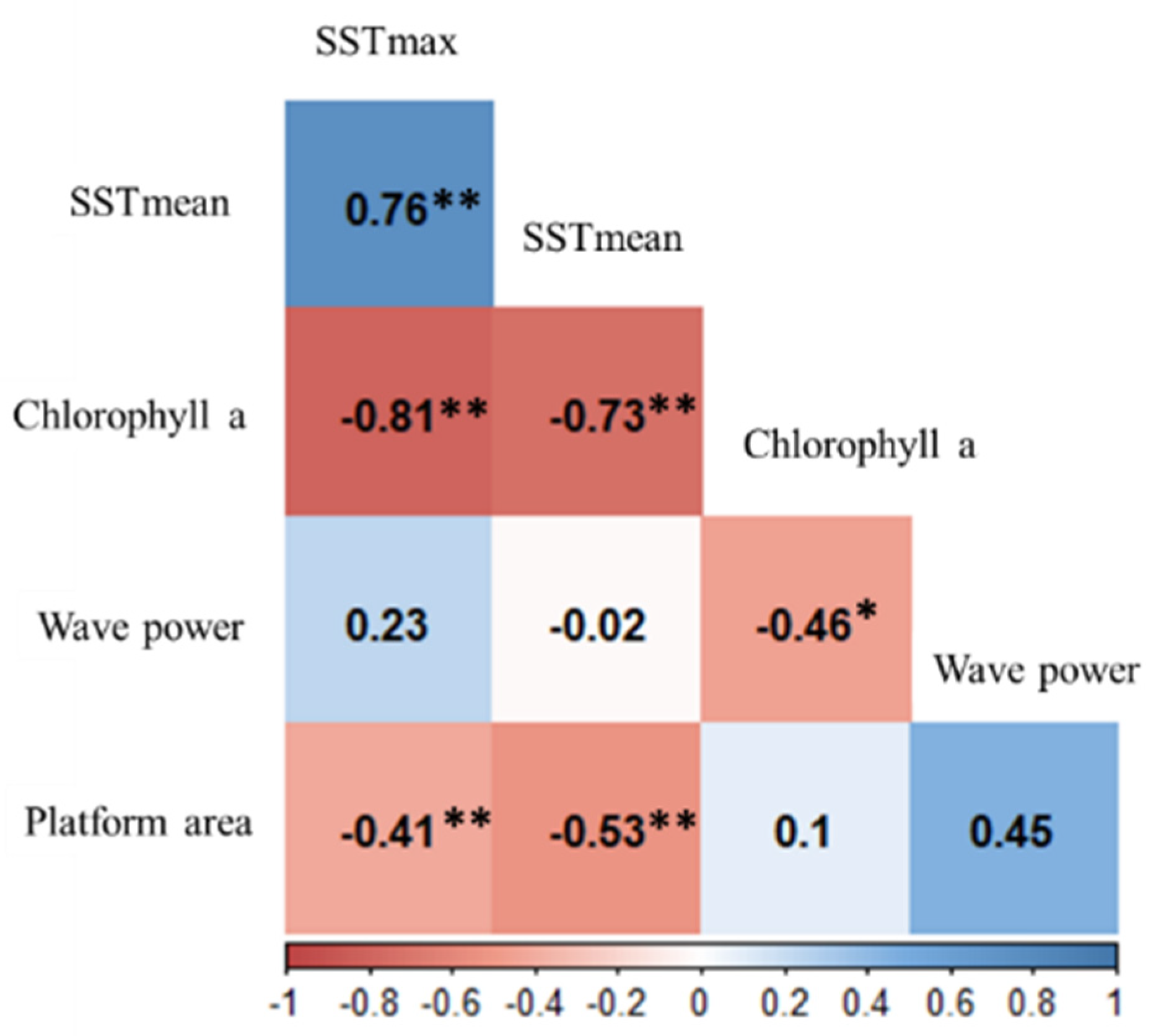
2.3. Data Analyses
3. Results
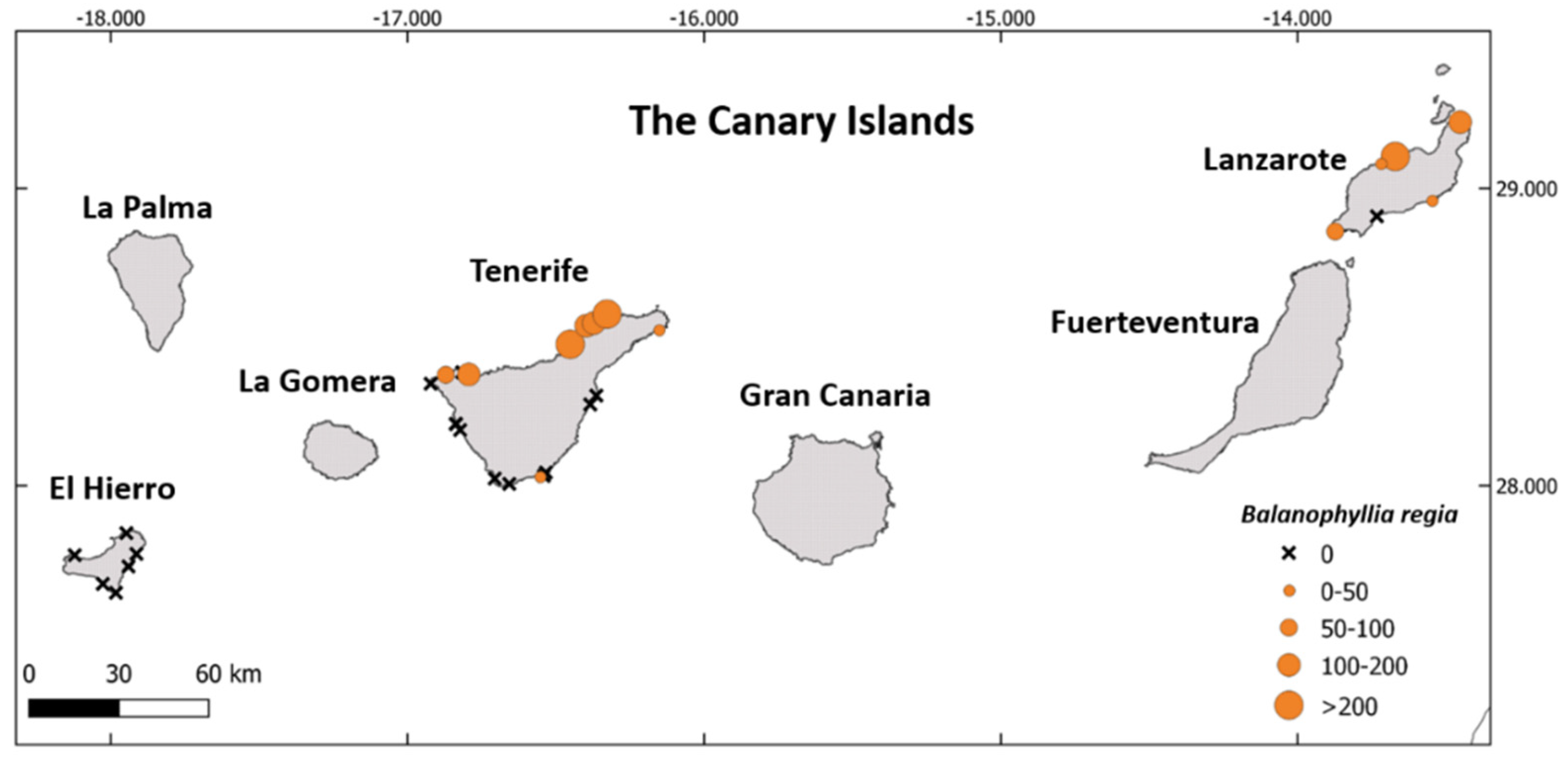
3.1. Distribution and Abundance of Balanophyllia regia
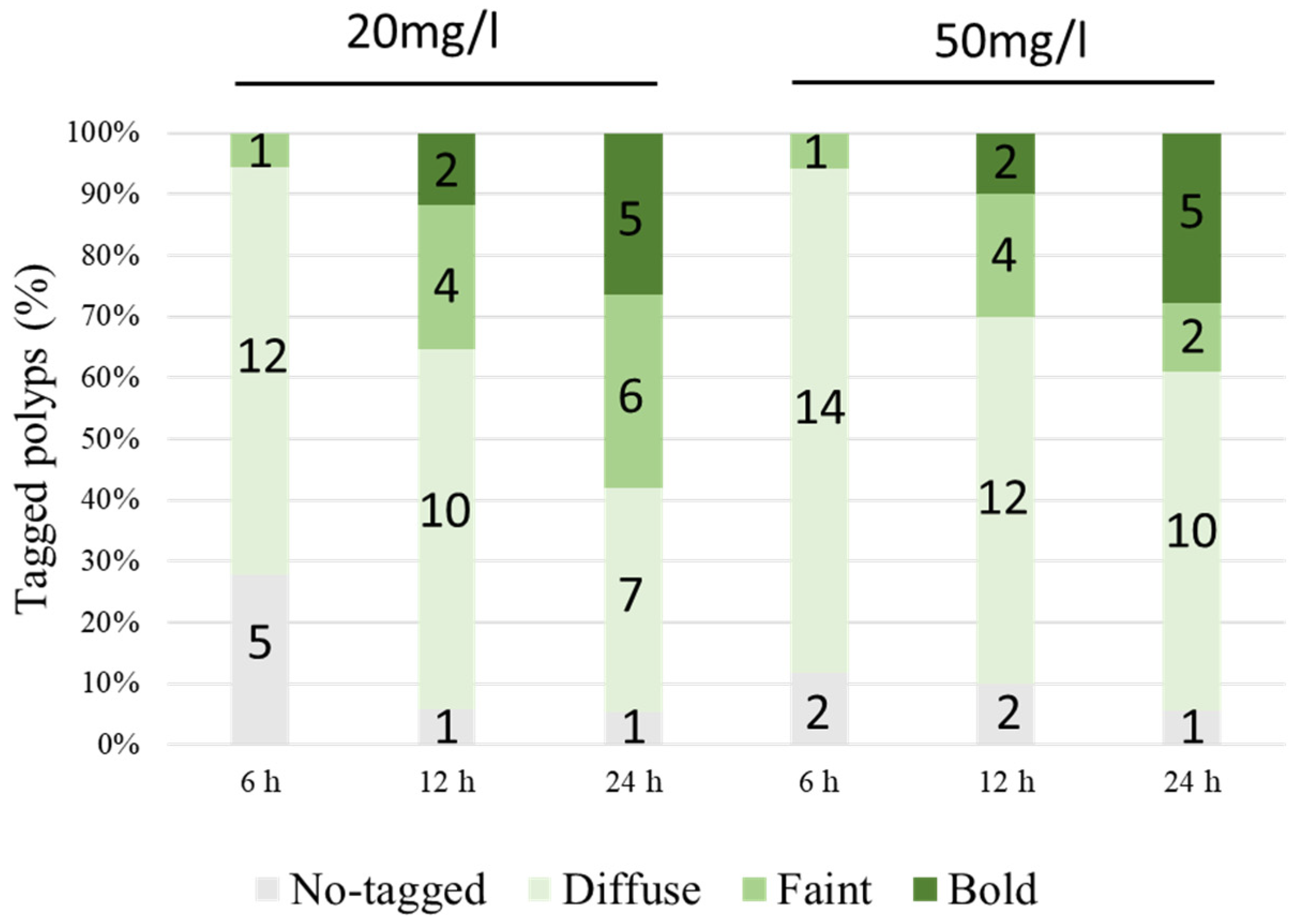
3.2. Calcein Labeling Experiment
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Walther, G.-R.; Roques, A.; Hulme, P.E.; Sykes, M.T.; Pyšek, P.; Kühn, I.; Zobel, M.; Bacher, S.; Botta-Dukát, Z.; Bugmann, H.; et al. Alien species in a warmer world: Risk and opportunities. Trends Ecol. Evol. 2009, 24, 686–693. [Google Scholar] [CrossRef]
- Horta e Costa, B.; Gonçalves, E.J. First occurrence of the monrovia doctorfish Acanthurus monroviae (Perciformes: Acanthuridae) in European Atlantic waters. Mar. Biodivers. Rec. 2013, 6, e20. [Google Scholar] [CrossRef]
- Vergés, A.; Doropoulos, C.; Malcolm, H.A.; Skye, M.; Garcia-Pizá, M.; Marzinelli, E.M.; Campbell, A.H.; Ballesteros, E.; Hoey, A.S.; Vila-Concejo, A.; et al. Long-term empirical evidence of ocean warming leading to tropicalization of fish communities, increased herbivory, and loss of kelp. Proc. Natl. Acad. Sci. USA 2016, 113, 13791–13796. [Google Scholar] [CrossRef]
- López, C.; Moreno-Borges, S.; Alvarez, O.; Brito, A.; Clemente, S. Distribution of zooxanthellate zo-antharians in the Canary Islands: Potential indicators of ocean warming. Estuar. Coast. Shelf Sci. 2020, 233, 106519. [Google Scholar] [CrossRef]
- Poloczanska, E.S.; Burrows, M.T.; Brown, C.J.; García Molinos, J.C.; Halpern, B.S.; Ehoegh-Guldberg, O.; Kappel, C.V.; Moore, P.J.; Richardson, A.J.; Schoeman, D.S.; et al. Responses of marine organisms to climate change across oceans. Front. Mar. Sci. 2016, 3, 62. [Google Scholar] [CrossRef]
- Yapici, S.; Filiz, H.; Bilge, G. Northwards range expansion of Sparisoma cretense (Linnaeus, 1758) in the Turkish Aegean Sea. J. Aquac. Eng. Fish. Res. 2016, 2, 201–207. [Google Scholar] [CrossRef]
- Hughes, T.P.; Baird, A.H.; Bellwood, D.R.; Card, M.; Connolly, S.R.; Folke, C.; Grosberg, R.; Hoegh-Guldberg, O.; Jackson, J.B.C.; Kleypas, J.; et al. Climate change, human impacts, and the resilience of coral reefs. Science 2003, 301, 929–933. [Google Scholar] [CrossRef]
- Harley, C.D.G.; Hughes, A.R.; Hultgren, K.M.; Miner, B.G.; Sorte, C.J.B.; Thornber, C.S.; Rodriguez, L.F.; Tomanek, L.; Williams, S.L. The impacts of climate change in coastal marine systems. Ecol. Lett. 2006, 9, 228–241. [Google Scholar] [CrossRef] [PubMed]
- Doney, S.C.; Ruckelshaus, M.; Duffy, J.E.; Barry, J.P.; Chan, F.; English, C.A.; Galindo, H.M.; Grebmeier, J.M.; Hollowed, A.B.; Knowlton, N.; et al. Climate Change impacts on marine ecosystems. Annu. Rev. Mar. Sci. 2012, 4, 11–37. [Google Scholar] [CrossRef]
- Rastrick, S.P.S.; Collier, V.; Graham, H.; Strohmeier, T.; Whiteley, N.M.; Strand, Ø. Feeding plasticity more than metabolic rate drives the productivity of economically important filter feeders in response to elevated CO2 and reduced salinity. ICES J. Mar. Sci. 2018, 75, 2117–2128. [Google Scholar] [CrossRef]
- Kroeker, K.J.; Kordas, R.L.; Crim, R.; Hendriks, I.E.; Ramajo, L.; Singh, G.S.; Duarte, C.M.; Gattuso, J. Impacts of ocean acidification on marine organisms: Quantifying sensitivities and interaction with warming. Glob. Change Biol. 2013, 19, 1884–1896. [Google Scholar] [CrossRef] [PubMed]
- Kuffner, I.B.; Andersson, A.J.; Jokiel, P.L.; Rodgers, K.S.; Mackenzie, F.T. Decreased abundance of crustose coralline algae due to ocean acidification. Nat. Geosci. 2008, 1, 77–140. [Google Scholar] [CrossRef]
- Gil-Díaz, T.; Haroun, R.; Tuya, F.; Betancor, S.; Viera-Rodríguez, M.A. Effects of ocean acidification on the brown alga Padina pavonica: Decalcification due to acute and chronic events. PLoS ONE 2014, 9, e108630. [Google Scholar] [CrossRef] [PubMed]
- Vélez, P.; González, M.; Pérez, M.D.; Hernández, A. Open ocean temperature and salinity trends in the Canary Current large marine ecosystem. In Oceanographic and Biological Features in the Canary Current Large Marine Ecosystem; Valdés, J.L., Déniz, G.I., Eds.; IOC Technical Series; IOC-UNESCO: Paris, France, 2015. [Google Scholar]
- Falcón, J.M.; Bortone, S.A.; Brito, A.; Bundrick, C.M. Structure of and relationships within and between the littoral, rock-substrate fish communities off four islands in the Canarian Archipelago. Mar. Biol. 1996, 125, 215–231. [Google Scholar] [CrossRef]
- Tuya, F.; Haroun, R.J. Phytogeography of the Lusitanian Macaronesia: Biogeographic affinities in species richness and assemblage composition. Eur. J. Phycol. 2009, 44, 405–413. [Google Scholar] [CrossRef]
- Clemente, S.; Rodríguez, A.; Brito, A.; Ramos, A.; Monterroso, Ó.; Hernández, J.C. On the occurrence of the hydrocoral Millepora (Hydrozoa: Milleporidae) in the subtropical eastern Atlantic (Canary Islands): Is the colonization related to climatic events? Coral Reefs 2011, 30, 237–240. [Google Scholar] [CrossRef]
- López, C.; Reimer, J.D.; Brito, A.; Simón, D.; Clemente, S.; Hernández, M. Diversity of zoantharian species and their symbionts from the Macaronesian and Cape Verde ecoregions demonstrates their widespread distribution in the Atlantic Ocean. Coral Reefs 2019, 38, 269–283. [Google Scholar] [CrossRef]
- Espino, F.; Tuya, F.; del Rosario, A.; Bosch, N.; Coca, J.; González-Ramos, A.J.; del Rosario, F.; Otero-Ferrer, F.J.; Moreno, A.C.; Haroun, R. Geographical range extension of the Spotfin burrfish, Chilomycterus reticulatus L. 1758, in the Canary Islands: Response to ocean warming? Diversity 2020, 11, 230. [Google Scholar] [CrossRef]
- Valdazo, J.; Viera-Rodríguez, M.A.; Espino, F.; Haroun, R.; Tuya, F. Massive decline of Cystoseira abies-marina forests in Gran Canaria Island (Canary Islands, eastern Atlantic). Sci. Mar. 2017, 81, 499–507. [Google Scholar] [CrossRef]
- Álvarez-Canali, D.; Sangil, C.; Reyes, J.; Sansón, M. Local variations in environmental parameters govern 50 years of the decline of Fucus guiryi populations in the Canary Islands (eastern Atlantic). J. Sea Res. 2019, 155, 101823. [Google Scholar] [CrossRef]
- Zibrowius, H. Les Scléractiniaires de la Méditerranée et de l’Atlantique Nord-Oriental; Mémoires de l’Institut Océanograhique, Monaco; Institut Océanographique: Monaco, Monaco, 1980; Volume 11, p. 391. [Google Scholar]
- Cairns, S.D. A generic revision and phylogenetic analysis of the Dendrophylliidae (Cnidaria: Scleractinia). Smithson. Contrib. Zool. 2001, 615, 75. [Google Scholar] [CrossRef]
- Crook, E.D.; Cooper, H.; Potts, D.C.; Lambert, T.; Paytan, A. Impacts of food availability and pCO2 on planulation, juvenile, survival, and calcification of the azooxanthellate scleractinian coral Balanophyllia elegans. Biogeosciences 2013, 10, 7599–7608. [Google Scholar] [CrossRef]
- Gómez, C.E.; Paul, V.J.; Ritson-Williams, R.; Muehllehner, N.; Langdon, C.; Sánchez, J.A. Responses of the tropical gorgonian coral Eunicea fusca to ocean acidification conditions. Coral Reefs 2015, 34, 451–460. [Google Scholar] [CrossRef]
- Ohno, Y.; Akira, I.; Chuya, S.; Mikako, G.; Mayuri, I. Calcification process dynamics in coral primary polyps as observed using a calcein incubation method. Biochem. Biophys. Rep. 2019, 9, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Venn, A.A.; Bernardet, C.; Chabenat, A.; Tambutté, E.; Tambutté, S. Paracellular transport to the coral calcifying medium: Effects of environmental parameters. J. Exp. Biol. 2020, 223, jeb227074. [Google Scholar] [CrossRef] [PubMed]
- Brahmi, C.; Meibom, A.; Smith, D.C.; Stolarski, J.; Auzoux-Bordenave, S.; Nouet, J.; Doumenc, D.; Djediat, C.; Domart-Coulon, I. Skeletal growth, ultrastructure and composition of the azooxanthellate scleractinian coral Balanophyllia regia. Coral Reefs 2010, 29, 175–189. [Google Scholar] [CrossRef]
- Assis, J.; Tyberghein, L.; Bosh, S.; Verbruggen, H.; Serrão, E.A.; De Clerck, O. Bio-ORACLE v2.0: Extending marine data layers for bioclimatic modelling. Glob. Ecol. Biogeogr. 2017, 27, 277–284. [Google Scholar] [CrossRef]
- Tyberghein, L.; Verbruggen, H.; Pauly, K.; Troupin, C.; Mineur, F.; De Clerck, O. Bio-ORACLE: A global environmental dataset for marine species distribution modelling. Glob. Ecol. Biogeogr. 2012, 21, 272–281. [Google Scholar] [CrossRef]
- Buckley, Y.M. Generalised linear models. In Ecological Statistics; Fox, G.A., Negrete-Yankelevich, S., Sosa, V.J., Eds.; Oxford University: Oxford, UK, 2014. [Google Scholar]
- Quinn, G.P.; Keough, M.J. Experimental Design and Data Analysis for Biologists; Cambridge University Press: Cambridge, UK, 2002; 537p. [Google Scholar]
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S.; Christensen, R.H.B.; Singmann, H.; Dai, B.; Grothendieck, G. Convergence. In Package ‘lme4’; 2015; pp. 12–13. Available online: https://www.researchgate.net/publication/279236477_Package_Lme4_Linear_Mixed-Effects_Models_Using_Eigen_and_S4 (accessed on 5 March 2024).
- Fox, J.; Weisberg, S. An R Companion to Applied Regression; Sage Publications: Thousand Oaks, CA, USA, 2019; Available online: https://socialsciences.mcmaster.ca/jfox/Books/Companion (accessed on 5 March 2024).
- Bartoń, K. MuMIn: Multi-Model Inference, R Package Version 1.43.6; R Core Team: Vienna, Austria, 2019. Available online: https://cran.r-project.org/web/packages/MuMIn(accessed on 5 March 2024).
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S, 4th ed.; Springer: Berlin/Heidelberg, Germany, 2022. [Google Scholar]
- Acosta, A.L.; Giannini, T.C.; Imperatriz-Fonseca, V.L.; Saraiva, A.M. Worldwide alien invasion: A methodological approach to forecast the potential spread of a highly invasive pollinator. PLoS ONE 2016, 11, e0148295. [Google Scholar] [CrossRef]
- Durante, L.M.; Cruz, I.C.S.; Lotufo, T.M.C. The effect of climate change on the distribution of a tropical zoanthid (Palythoa caribaeorum) and its ecological implications. PeerJ 2018, 6, e4777. [Google Scholar] [CrossRef]
- Tuya, F.; Boyra, A.; Sánchez-Jerez, P.; Haroun, R.J.; Barberá, C. Relationships between rocky-reef fish assemblages, the sea urchin Diadema antillarum and macroalgae throughout the Canarian Archipelago. Mar. Ecol. Prog. Ser. 2004, 278, 157–169. [Google Scholar] [CrossRef]
- Ocaña, O.; Brito, A.; González, G. The genus Actinia in the Macaronesian archipelagos: A general perspective of the North-oriental Atlantic and the Mediterranean species (Actiniaria: Actiniidae). Vieraea 2005, 33, 477–494. [Google Scholar]
- Falcón, J.M.; Herrera, R.; Ayza, O.; Brito, A.; New species of tropical litoral fish found in Canarian waters. Oil platforms as a central introduction vector. Rev. Acad. Canar. Cienc. 2015, 27, 67–82. [Google Scholar]
- Tuya, F.R.; Sánchez-Jerez, C.J.; González-Ramos, A.J.P.; Haroun, R.J. Coastal resources exploitation can mask bottom–up mesoscale regulation of intertidal populations. Hydrobiologia 2006, 553, 337–344. [Google Scholar] [CrossRef]
- Hernández-León, S.; Gómez, M.; Arístegui, J. Mesozooplankton in the Canary Current System: The coastal-ocean transition zone. Progr. Oceanogr. 2007, 74, 397–421. [Google Scholar] [CrossRef]
- Van De Poll, W.H.; Kulk, G.; Timmermans, K.R.; Brussaard, C.P.D.; Van Der Woerd, H.J.; Kehoe, M.J.; Mojica, K.D.A.; Visser, R.J.W.; Rozema, P.D.; Buma, A.G.J. Phytoplankton chlorophyll a biomass, composition, and productivity along a temperature and stratification gradient in the northeast Atlantic Ocean. Biogeosciences 2013, 10, 4227–4240. [Google Scholar] [CrossRef]
- Tuya, F.; Haroun, R.J. Spatial patterns and response to wave exposure of shallow water algal assemblages across the Canarian Archipelago: A multi-scaled approach. Mar. Ecol. Prog. Ser. 2006, 311, 15–28. [Google Scholar] [CrossRef]
- Dubinsky, Z.; Stambler, N. (Eds.) Coral Reefs: An Ecosystem in Transition; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Muller-Parker, G.; D’Elia, C.F.; Cook, C.B. Interactions between corals and their symbiotic algae. In Coral Reefs in the Anthropocene; Birkeland, C., Ed.; Springer: Dordrecht, The Netherlands, 2015. [Google Scholar]
- Palazzo, Q.; Prada, F.; Steffens, T.; Fermani, S.; Samorì, C.; Bernardi, G.; Terrón-Sigler, A.; Sparla, F.; Falini, G.; Goffredo, S. The skeleton of Balanophyllia coral species suggests adaptive traits linked to the onset of mixotrophy. Sci. Total Environ. 2021, 795, 148778. [Google Scholar] [CrossRef]
- Navarro, P.G.; Ramírez, R.; Tuya, F.; Sánchez-Jerez, C.; Fernández-Gil, P.; Haroun, R.J. Hierarchical analysis of spatial distribution patterns of Patellid limpets in the Canary Islands. J. Molluscan Stud. 2005, 71, 67–73. [Google Scholar] [CrossRef]
- Ramírez, R.; Tuya, F.; Sánchez-Jerez, P.; Fernández-Gil, C.; Bergasa, O.; Haroun, R.J.; Hernández-Brito, J.J. Estructura poblacional y distribución espacial de los moluscos gasterópodos Osilinus atrata (Wood, 1828) y Osilinus sauciatus (Koch, 1845) en el intermareal rocoso de las Islas Canarias (Atlántico centro—Oriental). Cienc. Mar. 2005, 31, 697–706. [Google Scholar] [CrossRef]
- Mitchell, N.C.; Dade, W.B.; Masson, D.G. Erosion of the submarine flanks of the Canary Islands. J. Geophys. Res. 2003, 108, 6002. [Google Scholar] [CrossRef]
- Tuya, F.; Aguilar, R.; Espino, F.; Bosch, N.E.; Meyers, E.K.M.; Jiménez-Alvarado, D.; Castro, J.J.; Otero-Ferrer, F.; Haroun, R. Differences in the occurrence and abundance of batoids across an oceanic archipelago using complementary data sources: Implications for conservation. Ecol. Evol. 2021, 11, 16704–16715. [Google Scholar] [CrossRef] [PubMed]
- Coyer, J.A.; Ambrose, R.F.; Engle, J.M.; Carroll, J.C. Interactions between corals and algae on a temperate zone rocky reef: Mediation by sea urchins. J. Exp. Mar. Biol. Ecol. 1993, 167, 21–37. [Google Scholar] [CrossRef]
- Wards, S. Two patterns of energy allocation for growth, reproduction and lipid storage in the scleractinian coral Pocillopora damicornis. Coral Reefs 1994, 14, 87–90. [Google Scholar] [CrossRef]
- Thébault, J.; Chauvaud, L.; Clavier, J.; Fichez, R.; Morize, E. Evidence of a 2-day periodicity of striae formation in the tropical scallop Comptopallium radula using calcein marking. Mar. Biol. 2006, 149, 257–267. [Google Scholar] [CrossRef]
- Van der Geest, M.; van Gils, J.A.; van der Meer, J.; Olff, H.; Piersma, T. Suitability of calcein as an in situ growth marker in burrowing bivalves. J. Exp. Mar. Biol. Ecol. 2011, 399, 1–7. [Google Scholar] [CrossRef]
- Russell, M.P.; Urbaniak, L.M. Does calcein affect estimates of growth rates in sea urchins? In Echinoderms: Munchen, Proceedings of the 11th International Echinoderm Conference, Munich, Germany, 6-10 October 2003; A.A. Balkema Publisher: Rotterdam, The Netherlands, 2004. [Google Scholar]
- Rodríguez, A.; Hernández, J.C.; Clemente, S. Efficiency of calcein tagging on juveniles of the sea urchins Diadema africanum and Paracentrotus lividus. Mar. Ecol. 2016, 37, 463–469. [Google Scholar] [CrossRef]
- Venti, A.; Andersson, A.; Langdon, C. Multiple driving factors explain spatial and temporal variability in coral calcification rates on the Bermuda platform. Coral Reefs 2014, 33, 979–997. [Google Scholar] [CrossRef]
- Lovett, G.M.; Burns, D.A.; Driscoll, C.T.; Jenkins, J.C.; Mitchell, M.J.; Rustad, L.; Shanley, J.B.; Likens, G.E.; Haeuber, R. Who needs environmental monitoring? Front. Ecol. Environ. 2007, 5, 253–260. [Google Scholar] [CrossRef]
- Hinkel, J.; Klein, R.J.T. Integrating knowledge for assessing coastal vulnerability to climate change. In Managing Coastal Vulnerability; Mc Fadden, L., Nicholls, R.J., Penning-Rowsell, E., Eds.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 61–77. [Google Scholar]
- Fitzer, S.C.; Torres-Gabarda, S.; Daly, L.; Hughes, B.; Dove, M.; O’Connor, W.; Potts, J.; Scanes, P.; Byrne, M. Coastal acidification impacts on shell mineral structure of bivalve mollusks. Eco. Evol. 2018, 8, 8973–8984. [Google Scholar] [CrossRef]
- Iwasaki, S.; Inoue, M.; Suzuki, A.; Sasaki, O.; Kano, H.; Iguchi, A.; Sakai, K.; Kawahata, H. The role of symbiotic algae in the formation of the coral polyp skeleton: 3-D morphological study based on X-ray microcomputed tomography. Geochem. Geophys. Geosyst. 2016, 17, 3629–3637. [Google Scholar] [CrossRef]
- Gerhardt, A. Bioindicator species and their use in biomonitoring. In Environmental Monitoring; Encyclopedia of Life Support Systems (EOLSS): Abu Dhabi, United Arab Emirates, 2002; Volume I. [Google Scholar]
- Rice, J.; Rochet, M.J. A framework for selecting a suite of indicators for fisheries management. J. Mar. Sci. 2005, 62, 516–527. [Google Scholar] [CrossRef]
- Dulvy, N.K.; Rogers, S.I.; Jennings, S.; Stelzenmüller, V.; Dye, S.R.; Skjoldal, H.R. Climate change and deepening of the North Sea fish assemblage: A biotic indicator of warming seas. J. Appl. Ecol. 2008, 45, 1029–1039. [Google Scholar] [CrossRef]
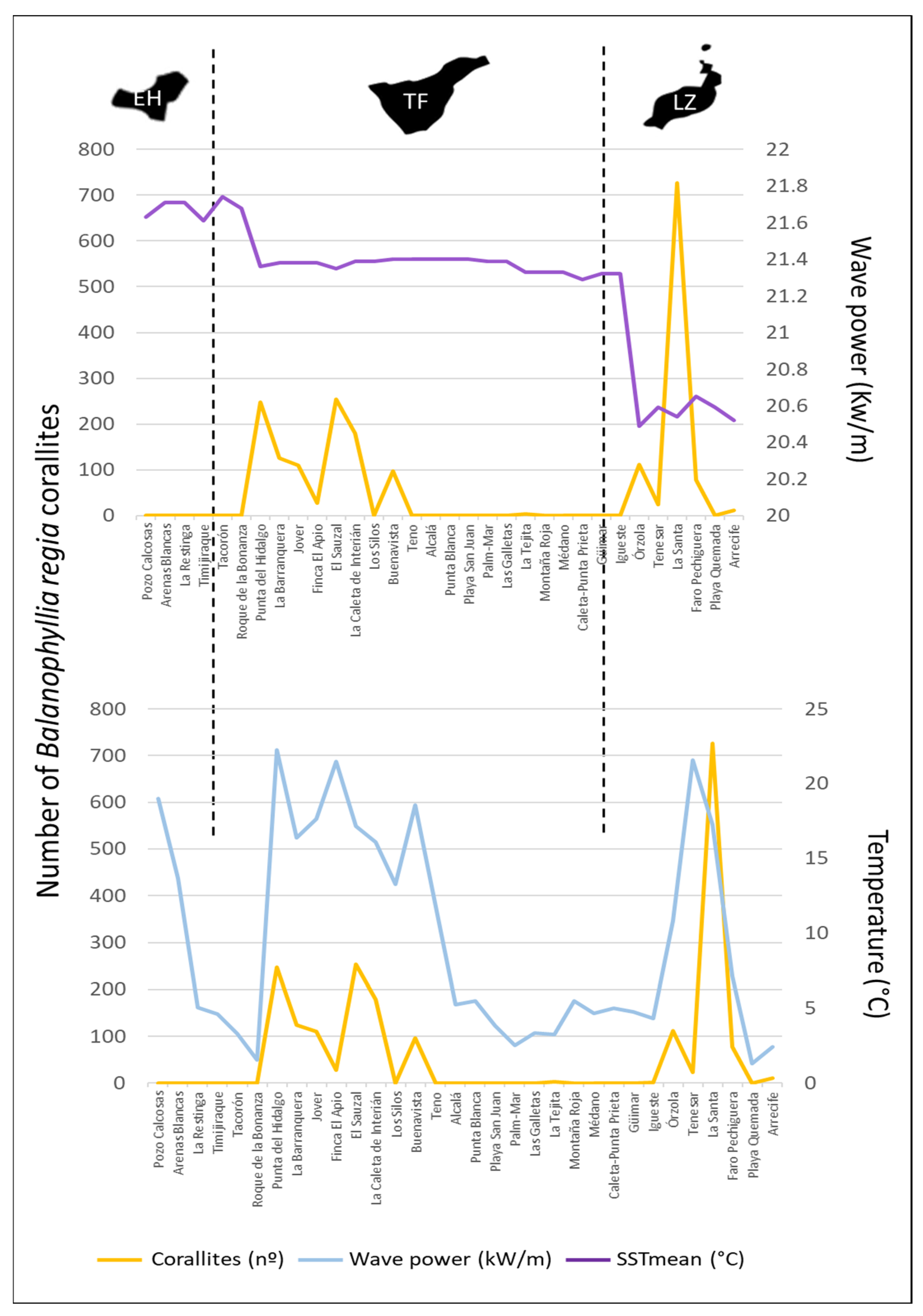

| Model | df | logLik | AICc | Weight |
|---|---|---|---|---|
| Model 1: SSTmean, Wave power | 4 | −133.78 | 2685.50 | 1 |
| Model 2: Wave power | 3 | −178.37 | 3574.30 | 0 |
| Model 3: SSTmean | 3 | −235.88 | 4724.50 | 0 |
| Predictor | Estimate (Coefficient) | Adjusted SE | z Value | Pr (>|z|) | Relative Importance |
|---|---|---|---|---|---|
| 0.16 | 0.0041 | 37.44 | <2 × 10−16 | 1 | |
| SSTmean | −1.61 | 0.0549 | 29.27 | <2 × 10−16 | 1 |
| Mark Intensity | Intercept | 50 mg/L | 12 h | 24 h | 50 mg/L * 12 h | 50 mg/L * 24 h |
|---|---|---|---|---|---|---|
| Diffuse | 0.86 | 10.70 | 1.43 | 1.07 | −1.58 | −0.71 |
| Faint | −16.08 | 0.92 | 2.99 * | 3.40 * | −1.61 | −20.13 |
| Bold | −122.80 | −25.67 | 12.97 | 13.89 | 1.88 | 25.68 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López, C.; Tuya, F.; Clemente, S. Understanding Balanophyllia regia Distribution in the Canary Islands: Effects of Environmental Factors and Methodologies for Future Monitoring. Diversity 2024, 16, 475. https://doi.org/10.3390/d16080475
López C, Tuya F, Clemente S. Understanding Balanophyllia regia Distribution in the Canary Islands: Effects of Environmental Factors and Methodologies for Future Monitoring. Diversity. 2024; 16(8):475. https://doi.org/10.3390/d16080475
Chicago/Turabian StyleLópez, Cataixa, Fernando Tuya, and Sabrina Clemente. 2024. "Understanding Balanophyllia regia Distribution in the Canary Islands: Effects of Environmental Factors and Methodologies for Future Monitoring" Diversity 16, no. 8: 475. https://doi.org/10.3390/d16080475






