Abstract
The natricine snake genus Smithophis Giri, Gower, Das, Lalremsanga, Lalronunga, Captain, and Deepak, 2019, is represented by four species, three of which are distributed in northeast India and Bangladesh, and a single species in Yunnan and Myanmar. In the past, S. bicolor (Blyth, 1855) was said to be widespread across northeast India and Myanmar; however, recent studies have shown it to be a species complex. Here, we describe a new species of the complex from the Indian state of Mizoram that resembles S. bicolor. The new species differs in bearing a patterned dorsum, a darker venter, and moderately keeled sacral scales. Re-examination of types of S. arunachalensis Das, Deepak, Captain, Wade, and Gower, 2020, shows the presence of strongly keeled sacral keels in males, which is an important diagnostic character. A revised key to members of the genus is presented with notes on S. arunachalensis.
1. Introduction
The natricine snake genus Smithophis Giri, Gower, Das, Lalremsanga, Lalronunga, Captain, Deepak, 2019, is represented by four species distributed across northeast India, Bangladesh, Myanmar, and Yunnan in China [1,2,3,4]. The genus was described to accommodate S. atemporalis Giri, Gower, Das, Lalremsanga, Lalronunga, Captain, and Deepak, 2019, from Mizoram, and S. bicolor (Blyth, 1855), distributed across Meghalaya and Mizoram, India [4,5,6]. The population of S. bicolor from Arunachal Pradesh was subsequently described as a new species, S. arunachalensis Das, Deepak, Captain, Wade, and Gower, 2020, by Das et al. [4]. Furthermore, the Yunnan population, referred to as S. bicolor, was also described as S. linearis Vogel, Chen, Deepak, Gower, Shi, Ding, and Hou, 2020, by Vogel et al. [1]. At the same time, while resolving the status of the population of S. bicolor, Das et al. [4] hinted at the presence of additional species within the broadly distributed S. bicolor. The species S. bicolor was described from Meghalaya, and a population of the species occurs in Mizoram, whose taxonomic status was morphologically assessed by Ruatpuii et al. [3], who identified two different morphs of S. bicolor. However, the study failed to resolve the taxonomic status of these two morphs as it lacked molecular data.
We examined material referred to as S. bicolor from Mizoram state, India, to assess its taxonomic status. Based on Ruatpuii et al. [3], we identified one form that was banded (yellow bands) and another dark brown morph. The morph with yellow bands is most similar to S. bicolor sensu stricto; however, they differ in bearing dorsal bands [7]. The specimens assigned to the brown morph yielded three specimens that bore indistinct reticulate patterns on an olive background. This population resembled S. bicolor sensu lato in scalation but differed in several other aspects. Furthermore, molecular data suggest that the population is substantially divergent from S. bicolor, warranting recognition as a distinct species. Here, we describe a new species of the genus Smithophis and provide additional notes on the genus.
2. Materials and Methods
2.1. Field Collection and Morphology
Specimens were collected in the field between September and October 2021 by J.C. Lalmuanawma and H.T. Lalremsanga under permit No. A.33011/2/99-CWLW/225 issued by the Department of Environment, Forests, and Climate Change, Government of Mizoram, India. The specimens were collected by hand, photographed, and euthanized with halothane. Subsequently, they were preserved in 70% ethanol. The type specimens are stored at the Bombay Natural History Society, Mumbai, India.
2.2. Morphology
The dorsal scale rows were counted at the 10th ventral, midbody, and one head length above the vent. Subcaudal scale counts do not include the terminal scute. The number of supralabials in contact with the eye is given in brackets next to the number of supralabials. Values for symmetric head characters are given in right-to-left order. The description style follows Patel et al. [8] and Mirza et al. [9] with some modifications. Scalation and other comparable characters are described as ventral scales (VS); subcaudal scales (SC); dorsal rows of scales, counted at midbody and at one head length after the head and before vent (DSR); supralabial scales (SL); loreal scales (LS); preocular scales (PreO); postocular scales (PostO); temporal scales (T); infralabial scales (IL); scales touching parietals, scales counted from the anterior temporal along the border of the parietals to the anterior temporal of the other side (STP); snout–vent length (SVL); tail length (TaL); total length (TL); head length (HL); head width (HW). Measurements were taken with the help of a digital caliper to the nearest 0.1 mm, and those for snout to vent length (SVL) and tail length (TaL) were taken with the aid of a piece of non-elastic string, which was then measured using a scale. The descriptive terminology of the morphology of the hemipenis was as stated by Dowling and Savage [10] and Zaher [11].
Acronyms for institutions where comparative material was examined are BNHS—Bombay Natural History Society, Mumbai, India; MZMU—Departmental Museum of Zoology, Mizoram University, Aizawl, Mizoram, India; NHMUK—Natural History Museum, London, U.K.; and ZSIK—Zoological Survey of India, Kolkata, India.
2.3. Nomenclature Acts
The electronic edition of this article conforms to the requirements of the amended International Code of Zoological Nomenclature. Hence, the new names contained herein are available under that code in the electronic edition of this article. This published work and the nomenclatural acts it contains have been registered in ZooBank, the online registration system for the ICZN [12]. The ZooBank LSIDs (Life Science Identifiers) can be resolved, and the associated information is viewed through any standard web browser by appending the LSID to the prefix “http://zoobank.org/.” The LSID for this publication is urn:lsid:zoobank.org:pub:3F207083-C7BC-42B6-BE4C-B4D8866A6D35.
2.4. Molecular Methods
Genomic DNA was extracted from liver tissue using the Qiagen DNeasy Kit following the manufacturer’s protocols. A fragment of the mitochondrial cytochrome b (cyt b) gene and 16S rRNA was amplified using previously published primers (L14910 5′-GACCTGTGATMTGAAAAACCAYCGTTGT -3′ and H16064 5′-CTTTGGTTTACAAGAACAATGCTTTA -3′) [13] and (16Sa 5′-CGCCTGTTTATCAAAAACAT -3′) and (16Sb 5′- CCGGTCTGAACTCAGATCACGT -3′), respectively. The PCR protocol for amplification consisted of an initial 3 min at 94 °C for initial denaturation, followed by 35 cycles of 30 s at 94 °C for denaturation, 30 s for annealing at 50.3 °C for cyt b, and 45 °C for 16S, elongation for 1 min at 72 °C, and a final elongation for 5 min at 72 °C. The generated sequence was aligned with other congeners of the genus Smithophis, Opisthotropis Günther, 1872, and was rooted with Trimerodytes percarinatus (Boulenger, 1899) species based on results from Deepak et al. [6] in Mega X [14] with CLUSTALW [15] with default parameters. Sequences of focal taxa for the mitochondrial gene NADH dehydrogenase subunit 4 (ND4), nuclear oocyte maturation factor (cmos), neurotrophin 3 (NT3), Brain-Derived Neurotrophic Factor (BDNF), and Recombination activating gene 1 (RAG-1) were also downloaded and concatenated with data generated in the present work. The aligned dataset was subjected to phylogenetic analysis through Bayesian Inference (BI) and Maximum Likelihood (ML) phylogeny to elucidate the placement of Smithophis in Mizoram. The sequence substitution model was selected with ModelFinder [16]. The BI phylogenetic analysis was run in MrBayes [17] and ML on the IQ-TREE (http://iqtree.cibiv.univie.ac.at/) online portal [18]. The BI analysis was run for 1 million generations with a tree recorded every 1000th iteration, and the run was terminated after reaching a split frequency of 0.01. The analysis was implemented with a partition model for the concatenated dataset. The ML analysis was run with an ultrafast bootstrapping option for 1000 iterations to assess clade support. Uncorrected pairwise p-distance (% sequence divergence) was calculated in Mega X with pairwise deletions of missing data and gaps. Sequences used in phylogenetic analysis are listed in Table S1, and the sequence evolution model for individual analysis is presented in Table S2.
3. Results
3.1. Molecular Phylogeny
The concatenated mitochondrial (16S, cyt b, ND4) and nuclear (BDNF, cmos, NT3, RAG1) gene dataset comprised 4825 bp. The BI as well as ML phylogeny, did not recover the monophyly of the genus Opisthotropis. Smithophis was found to be nested within the genus Opisthotropis. BI and ML analysis resulted in trees with different topologies, and relationships across the tree have poor support at the deeper nodes (ML < 60 Supporting Figure S1) to moderate support in BI (Figure 1). The relationships across species of Smithophis are well supported (BI posterior probability > 0.97, ML bootstrap > 86). Smithophis linearis was recovered sister to a clade containing S. atemporalis, S. bicolor, and the population of Smithophis from Mizoram with high support (BI posterior probability 1, ML bootstrap 99). The species S. atemporalis was recovered sister to S. bicolor, and the population of Smithophis from Mizoram had high support (BI posterior probability 1, ML bootstrap 100). The new species shows a pairwise sequence divergence of 10–14% for cyt b from other congeners (Table S3). Intraspecific sequence divergence is 0–2% (n = 4).
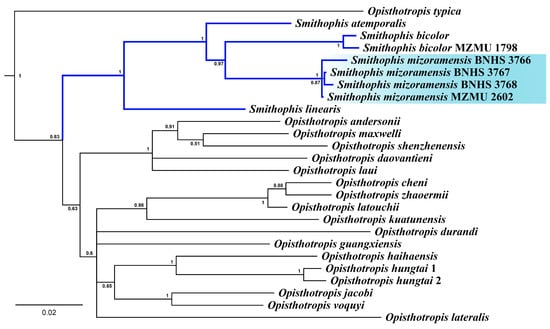
Figure 1.
BI phylogeny of the genus Smithophis and Opisthotropis based on concatenated mitochondrial (16S, cyt b, ND4) and nuclear genes (BDNF, cmos, NT3, RAG-1). Numbers at nodes indicate posterior probabilities. For the complete tree, see Figure S1.
3.2. Morphology
In its general appearance, the population of Smithophis from Mizoram differs from S. bicolor s. s. in lacking a uniform, and unpatterned dorsum. Furthermore, it differs from S. bicolor s. l. in bearing moderately keeled sacral scales. We propose a new name for this population based on support from molecular data and non-overlapping morphological characters.
3.3. Systematics
Smithophis mizoramensis sp. nov.
urn:lsid:zoobank.org:act:333B3711-FACE-4391-8C97-B9ED2606CC16
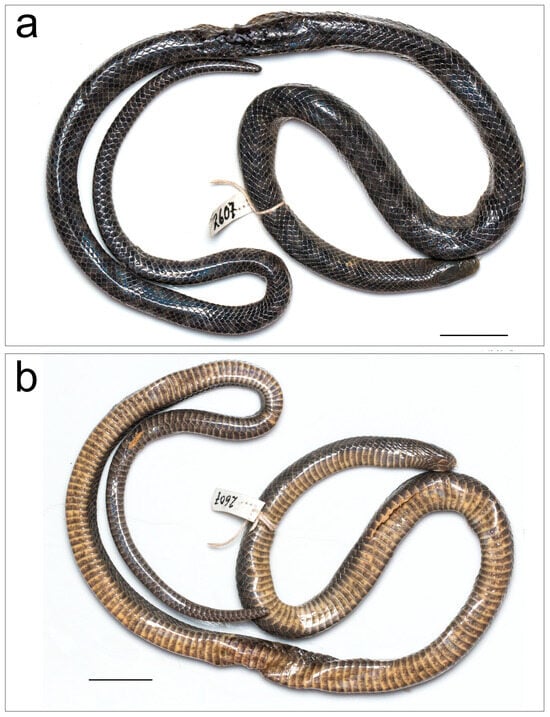
Figure 2.
Smithophis mizoramensis sp. nov. holotype male BNHS 3766 (ex-MZMU 2607) (a) dorsal view, (b) ventral view. Scale bar 20 mm.
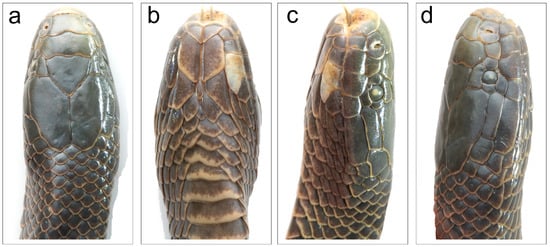
Figure 3.
Smithophis mizoramensis sp. nov. holotype male BNHS 3766 showing head scalation (a) dorsal view, (b) ventral view, (c) left lateral view, (d) right lateral view.
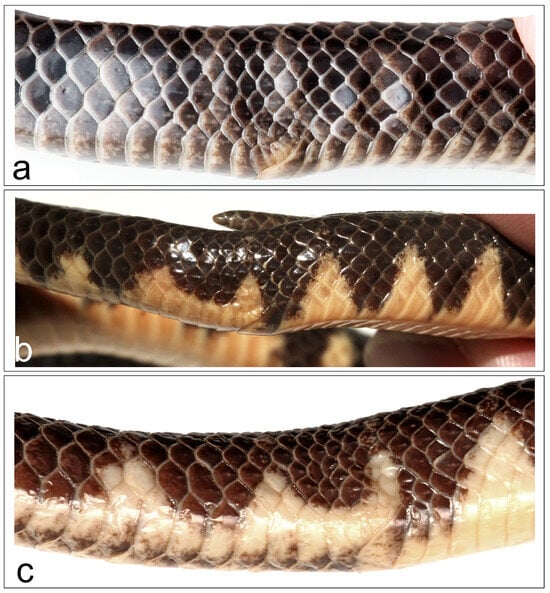
Figure 4.
Sacral region of male Smithophis, showing keeled scales (a) S. mizoramensis sp. nov. holotype BNHS 3766; (b) S. arunachalensis paratype NHMUK 1935.10.12.10; (c) S. arunachalensis ZSIK 27216. Note that the head is to the right.
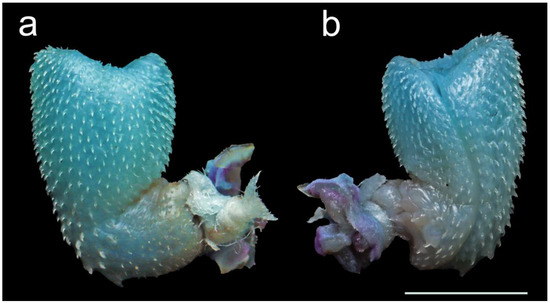
Figure 5.
Hemipenis of male Smithophis mizoramensis sp. nov. paratype BNHS 3768, (a) asulcul view, (b) sulcul view. Scale bar 5 mm.

Figure 6.
Smithophis mizoramensis sp. nov. in life, (a) BNHS 3767, photo by Jignesh Patel; (b) an uncollected individual from Tam Dil National Wetland, Saitual district, Mizoram, photo by Prashant Vaghela.

Table 1.
Morphological and meristic data for type specimens of Smithophis mizoramensis sp. nov., all measurements in mm.
Holotype: BNHS 3766 (ex-MZMU 2607), an adult male from under boulders in Rawkawn stream near Suangpuilawn village, Saitual District (23.936435° N, 92.849410° E, 837 m asl) Mizoram. India, collected by J.C. Lalmuanawma and H.T. Lalremsanga on 9 September 2021.
Paratype: two adults collected at the same locality as the holotype, female BNHS 3767 (ex-MZMU 2603), collected on 24 October 2021 by J.C. Lalmuanawma, and male BNHS 3768 (ex-MZMU 2549), collected on 20 September 2021 by J.C. Lalmuanawma.
Additional referred material: juvenile female MZMU 2602, collected on 20 October 2021 by J.C. Lalmuanawma from the same locality as the holotype.
Diagnosis: A Smithophis with 17 smooth dorsal scales throughout the body and moderately keeled sacral scales in males. Temporal scales are present. Circum-orbital scales 4–5. Dorsal coloration is a shade of olive with indistinct dark reticulate patterns in life, overall black with faint gray reticulate markings or bands on the body in preservative. Each ventral scale is darker anteriorly edged with a yellowish/off-white border; the tail is ventrally darker compared to the ventral scales. Sequence divergence of 10–14% from other Smithophis for the gene cyt b.
Comparison: Smithophis mizoramensis sp. nov. differs from its congeners in bearing the following diagnostic and non-overlapping characters: sacral keels present in males (vs. absent in S. atemporalis, S. bicolor s. l., and S. linearis); temporal scales present (vs. absent in S. atemporalis); dorsal coloration shade of olive with indistinct dark reticulate patterns in life (vs. black dorsally with white or yellow subtriangular and triangular patches on the lateral aspect in S. atemporalis and S. arunachalensis, dorsally in a shade of brown lacking any pattern and a light belly in S. bicolor, dorsally brown with thin longitudinal stripes in S. linearis), circumorbital scales 4–5 (vs. 6–7 in S. linearis); sacral scales moderately keeled (vs. sacral scales strongly keeled appearing tuberculate in nature in S. arunachalensis); ventral scales median 213 in the new species (vs. 199 in S. arunachalensis n = 7).
Etymology: The specific epithet refers to the Indian state of Mizoram, where the new species was discovered, and that has yielded many notable herpetological discoveries. The type and all other currently known localities of the new species are in Mizoram. This is the first snake to be named after the state. As individuals were mostly encountered in and around small hill streams, we suggest the common names Mizo Brook Snake (English) and Tuithiangrul (Mizo).
Description of male holotype BNHS 3766 (Figure 2a,b):
The specimen is damaged in several places around the midbody. The anterior and posterior parts of the snake are undamaged and in a good state of preservation.
Head short, 13.3 mm; high, 5.4 mm, with mildly domed snout in lateral view; upper jaw visible from ventral side. Head barely distinct from neck. Snout tapering to blunt, rounded tip in dorsal view (Figure 3a). Rostral visible in dorsal view (high 1.9 mm); nearly twice as wide as deep (4.2 mm). Nostrils are large, crescent-shaped, and situated dorsolaterally within a large, incompletely divided nasal scale that has a suture below the nares. A single large internasal, broader (median width 3.8 mm) than long (1.6 mm); smaller than prefrontal. Prefrontal single, length almost twice (median width 5.1 mm) that breadth (2.1 mm).
Frontal pentagonal, approximately triangular, 3.6 mm at the widest anterior border, equal width at suture with supraocular median length 3.7 mm. Parietals 6.7 mm long 3.4 mm at their widest anterior end. Temporals 1 + 1, anterior temporal long (3.7 mm long, 1.1 mm wide), posterior one small (1.8 mm long, 1.4 mm wide). STP, eleven scales border the parietals (excluding parietals). Supraocular slightly larger than preocular; preocular taller than broad. Loreal slightly longer than high. One postocular. Eye circular, 1.1 mm diameter, with a round pupil. Five supralabials, SL, not in contact with orbit (Figure 3c,d). First supralabial narrowest in width and contacts the rostral, nasal, and second supralabial. Second supralabial in contact with nasal, loreal, first and third supralabials. Third supralabial largest, fourth and fifth much wider than high. Third supralabial in contact with preocular, loreal, two suboculars, second and fourth supralabials. Fifth supralabial largest and longest. Mental short, broad, triangular, wider, 1.6 mm than 1.8 mm long. Infralabials 8/7, (Figure 3b). First four infralabials in contact with anterior chin shields. Fourth infralabial largest and broadest. All head shields punctate.
Body not rounded, ventral surface slightly flattened. Dorsal scales in 17-17-17 rows are smooth throughout the body, except for the scales at the sacral region (Figure 4a). Ventral scales: 214 + 4 preventrals. Anal shield divided, slightly larger than last ventral scale, and its posterior margin overlaps irregular scales on each side, in addition to a pair of larger subcaudals medially. Subcaudals paired, 73 in number. Tail terminates in a slightly blunt, tapering apical spine. Total length: 611.8 mm; SVL: 477.8 mm; tail length: 134 mm; tail/total length ratio: 0.219.
Hemipenis (based on male paratype BNHS 3768): The hemipenis is almost fully everted (distal tip not fully everted) and expanded. The organ is short and stout, very shallowly bilobed, noncalyculate, and noncapitate; lobes extend to about 1/10 of the hemipenis (Figure 5). The organ is ornamented throughout with spines and spinules; the spines are larger in size near the trunk more densely packed on the asulcul side; 36 to 38 spines around the trunk; the apical area on crotch not protruding; sulcus spermaticus single deep extends to the inner right side of the lobe; sulcus lip well developed but not raised covered with spines; hemipenial base with few small spinnules and a large hook-like spine. The hemipenis reaches up to 11 SC examined before hemipenis preparation.
Coloration: In preservative. Black to dark brown with indistinct lighter reticular markings or thin bars. Head, dorsally and laterally uniform bluish-gray; ventrally brown, each scale with an off-white or yellowish border. The belly is yellowish to off-white, with the anterior half of each ventral scale smeared with brown to gray. The tail is ventrally darker than the ventral scales. In life. Coloration based on paratype female BNHS 3767. Yellowish-brown to olive overall with a purplish-gray vertebral stripe from neck to tail tip and with irregular two-scale wide blotches on either side of the vertebral stripe; the blotches form indistinct bands on the paler background. The dark markings on the tail form a broken reticulate pattern on the yellowish-brown or olive background color (Figure 6a). The head is slightly darker than the dorsal body color and lacks defined markings; the lower half of supralabial five is yellow and not as dark as the other head scales. Color variation. Individuals may be darker in appearance, as seen in an uncollected individual from Tam Dil National Wetland (Figure 6b).
Variation: The paratypes match the male holotype in most regards; however, they differ in the female paratype, which lacks keeled sacral scales. The supralabials do not touch the orbit in the male holotype; however, the 3rd SL is in contact with the orbit in the female paratypes. See Table 1 for a summary of characters in the type series.
Natural history and distribution: The species was collected from in and around riparian habitats with dominant vegetation comprising Macropanax dispermus, Tetrameles nudiflora, Musa sp., Melocanna baccifera, Dendrocalamus hamiltonii, Pterris sp., and Phryninum capitatum. Suangpuilawn is located ca. 41 km in the northeast direction from the state capital, Aizawl, in the aerial distance (Figure 7). In winter, the temperature varies from 14 °C to 19 °C, while in summer it ranges between 20 °C and 28 °C. The vegetation of the area falls under the tropical semi-evergreen forest as per the classification by Singh et al. [15], corresponding to the semi-evergreen 2B/C2 forest type [16], and the average annual rainfall of this area is 2593.4 mm. The species appears to be nocturnal; it is a ground dweller, and all current records show it is distributed above 700 m elevation near water resources and is mostly active during the monsoons. In captivity, it was seen feeding on earthworms. Nothing much is known about the biology of the species. Other serpents found in the type locality include Bungarus fasciatus, Fowlea piscator, Herpetoreas xenura, and Trimeresurus erythrurus.
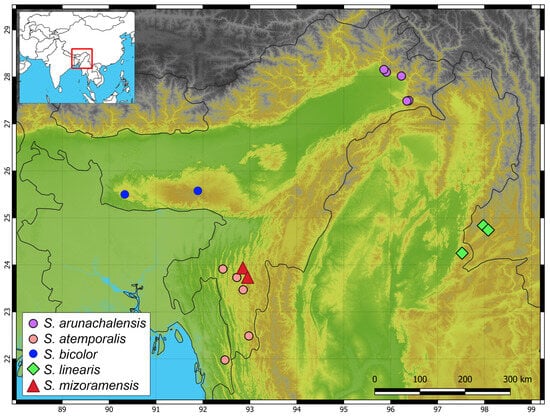
Figure 7.
Map of northeast India and the adjoining region shows the distribution of Smithophis species. Distribution localities from Meghalaya represent S. bicolor s. s.; inset map shows the region of interest in the Indo-Burma region of Asia.
Smithophis arunachalensis Das, Deepak, Captain, Wade, and Gower, 2020
Figure 4b,c
Material examined: Holotype female BNHS 3650, Koronu, Lower Dibang Valley, Arunachal Pradesh, India; paratype male NHMUK 1935.10.12.10, Dening, Mishmi Hills, “Assam,” currently in Lohit District, Arunachal Pradesh, India; male ZSIK 27216, Namdapha Tiger Reserve, Arunachal Pradesh. See Table S4 for a list of comparative material.
Remarks: The species was diagnosed as bearing 17 smooth scales throughout the body. A re-examination of the male paratype NHMUK 1935.10.12.10 showed the presence of strongly keeled scales near the sacral region (Figure 4b). A male ZSIK 27216 specimen also bore similar keeled scales (Figure 4c). The female holotype BNHS 3650 and paratype female ZSIK 23875 (stated as male by Das et al. [4]) lack these keeled scales. This character state is sexually dimorphic and an informative character to diagnose it from other Smithophis.
4. Discussion and Conclusions
The genus Smithophis was described by Giri et al. [5] to accommodate S. bicolor and S. atemporalis based on molecular data as well as scalation data. The genus was diagnosed with Opisthotropis, bearing smooth dorsal scales, fused internasals, and the prefrontal. However, the genus was found to be embedded with the genus Opisthotropis in a robust phylogeny of natricine snakes by Deepak et al. [19] as well as in the present work.
Furthermore, the genus Smithophis, as currently demonstrated in the present work, also bears keeled scales, as observed in certain species of Opisthotropis. Molecular data for S. arunachalensis are currently lacking, which is a major hurdle in assessing the validity of the genus Smithophis. The genus may be treated as a subgenus or merged with Opisthotropis if the current phylogenetic relationships are consistent in future analysis.
Based on the concatenated mitochondrial and nuclear genes, the BI and ML phylogeny recovered the new species as a sister taxon to S. bicolor. The new species does look similar to S. bicolor; however, based on morphology, it is more similar to S. arunachalensis, which shares the presence of keeled sacral scales. The lack of molecular data for the latter species provides an avenue for future investigations to test relationships within the species and the validity of the genus. The nearest known locality for S. arunachalensis is Namdapha National Park in Arunachal Pradesh, which is >500 km from the type locality of the new species.
Mizoram lies south of the Brahmaputra River along the western front of the Indo-Burma biodiversity hotspot [20,21], with most parts of the state under forest cover. Two species of the genus Smithophis are reported from the state [4], and the present work adds yet another species. Smithophis has a patchy distribution across northeast India and Indo-Burma (Figure 7) (see Figure 3 of Das et al. [4]), and it is likely that sampling across these distribution gaps may yield additional species. As scanty information is available on the distribution and natural history of this species, we suggest that it is currently likely to be considered as Data Deficient based on criteria for the Red List of Threatened Species. The description of the new species merely reflects the poor reptile documentation of the Indo-Burma region. Several new species of snakes and lizards have been described from the Indo-Burma region in the last couple of years [22,23,24,25,26,27,28], warranting dedicated efforts to document the snake fauna of the region.
| Key to members of the genus Smithophis |
| 1a. Temporal scales absent..................................................................................... S. atemporalis |
| 1b. Temporal scales present........................................................................................................2 |
| 2a. Sacral scales keeled in males.................................................................................................3 |
| 2b. Sacral scales smooth in both sexes.......................................................................................4 |
| 3a. Dorsum black, venter yellow with a zig-zag ventrolateral pattern; sacral scales strongly keeled................................................................................................... S. arunachalensis |
| 3b. Dorsal in shades of olive (never black) with indistinct brown reticulate patterns; sacral scales moderately keeled.......................................................................S. mizoramensis sp. nov. |
| 4a. Dorsum brown or grayish-brown without any markings; circumorbital scales 4–5..........................................................................................................................................S. bicolor |
| 4b. Dorsum brown with narrow dark longitudinal stripes; circumorbital scales 6–7.........................................................................................................................................S. linearis |
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/d16080480/s1, Table S1: GenBank accession numbers and voucher codes used in the phylogenetic analysis. Numbers in bold indicate sequences generated in the present work; Table S2: Sequence evolution model used in the ML and BI phylogenetic analyses; Table S3: Uncorrected p-distance for members of the genus Smithophis and Opisthotropis for the gene cytochrome b; Table S4. Examined comparative matierial; Figure S1: BI phylogeny of the genus Smithophis and Opisthotropis based on concatenated mitochondrial (16S, cyt b, ND4) and nuclear genes (BDNF, cmos, NT3, RAG-1). Numbers at nodes indicate posterior probabilities; Figure S2: ML phylogeny of the genus Smithophis and Opisthotropis based on concatenated mitochondrial (16S, cyt b, ND4) and nuclear genes (BDNF, cmos, NT3, RAG-1). Numbers at nodes indicate bootstrap support.
Author Contributions
Conceptualization, Z.A.M., H.T.L. and H.P.; methodology, Z.A.M., V.K.B., H.T.L. and M.V.; formal analysis, Z.A.M., V.K.B., H.T.L. and A.C.; resources, H.T.L., J.C.L., G.C., A.Z. and M.V.; writing—original draft preparation, Z.A.M.; writing—review and editing, H.T.L., A.C. and H.P.; funding acquisition, Z.A.M., H.T.L. and M.V. All authors have read and agreed to the published version of the manuscript.
Funding
Z.A.M. was supported through the Max Planck Society’s IMPRS from Molecules to Organisms program and V.K.B. through the CSIR-JRF fellowship. The financial grants from DST-SERB, New Delhi (DST Nos. EEQ/2021/000243, EEQ/2023/000877, and CRG/2023/000805) and the Chicago Board of Trade (CBOT) Endangered Species Fund USA to H.T.L. and M.V. are acknowledged.
Institutional Review Board Statement
The animal study protocol was approved by the Institutional Ethics Committee of Mizoram University (proposal code MZUIAEC 18-19-12 and approved on 26 March 2018). Field collection of specimens was approved by the Chief Wildlife Warden, Forest Department of Mizoram, permit number A.33011/2/99-CWLW/225.
Data Availability Statement
The holotype, two paratypes and one juvenile specimen collected for the study have been deposited at the Bombay Natural History Society’s herpetological collection in Mumbai and are accessible to researchers globally. DNA sequences generated in the present work have been submitted to GenBank, operated by the US National Centre for Biotechnology Information.
Acknowledgments
Special thanks to the Chief Wildlife Warden, Forest Department of Mizoram, for permission (No. A.33011/2/99-CWLW/225) to conduct herpetofaunal surveys. Lalmuansanga, Malsawmdawngliana Fanai, and Lalbiakzuala, Department of Zoology, Mizoram University, for their laboratory assistance in specimen processing and hemipenial preparation. Rahul Khot, Saunak Pal, and Vithoba Hegde (BNHS), Dhriti Banerjee, Pratyush P. Mohapatra (ZSIK), and Patrick Campbell (NHMUK) for hosting and granting access to specimens in their respective museum collections. Special thanks to three anonymous reviewers for their lucid comments, which greatly benefited the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Vogel, G.; Chen, Z.; Deepak, V.; Gower, D.J.; Jingsong, S.H.I.; Ding, L.; Mian, H.O.U. A new species of the genus Smithophis (Squamata: Serpentes: Natricidae) from Southwestern China and northeastern Myanmar. Zootaxa 2020, 4803, 51–74. [Google Scholar] [CrossRef] [PubMed]
- Uetz, P.; Hošek, J. The Reptile Database. Available online: http://www.reptile-database.org (accessed on 2 July 2024).
- Ruatpuii, R.; Biakzuala, L.; Santra, V.; Lalremsanga, H.T. Additional notes on morphology and distributional records of the snake genus Smithophis Giri, Gower, Das, Lalremsanga, Lalronunga, Captain Et Deepak, 2019 (Squamata: Serpentes: Natricidae) from north-east India. Russ. J. Herpetol. 2022, 29, 331–340. [Google Scholar] [CrossRef]
- Das, A.; Deepak, V.; Captain, A.; Wade, E.O.Z.; Gower, D.J. Description of a new species of Smithophis Giri et al. 2019 (Serpentes: Colubridae: Natricinae) from Arunachal Pradesh, India. Zootaxa 2020, 4860, 267–283. [Google Scholar] [CrossRef] [PubMed]
- Giri, V.B.; Gower, D.J.; Das, A.; Lalremsanga, H.T.; Lalronunga, S.; Captain, A.; Deepak, V. A new genus and species of natricine snake from Northeast India. Zootaxa 2019, 4603, 241–264. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.A. Fauna of British India, Ceylon and Burma, Including the Whole of the Indo-Chinese Sub-Region. Reptilia and Amphibia. Vol. 3. Serpentes; Taylor and Francis: London, UK, 1943; p. 440. [Google Scholar]
- Chandramouli, S.R.; Karthik, P.; Naveen, R.; Babu, S.; Karunakaran, P.; Kumara, H. A Two-Colored Forestsnake, Smithophis bicolor (Blyth 1855) (Reptilia: Natricidae), from the Khasi Hills, Meghalaya, India. Reptil. Amphib. 2021, 28, 24–25. [Google Scholar] [CrossRef]
- Patel, H.; Thackeray, T.; Campbell, P.D.; Mirza, Z.A. Systematic assessment of Hebius beddomei (Günther, 1864) (Serpentes: Colubridae: Natricinae) with description of a new genus and a new allied species from the Western Ghats, India. Taxonomy 2023, 3, 415–434. [Google Scholar] [CrossRef]
- Mirza, Z.A.; Vyas, R.; Patel, H.; Maheta, J.; Sanap, R. A new Miocene-Divergent lineage of Old World Racer snake from India. PLoS ONE 2016, 11, e0154301. [Google Scholar] [CrossRef] [PubMed]
- Dowling, H.; Savage, J. A guide to the snakes hemipenis: A survey of basic structure and systematic characteristics. Zoologica 1960, 45, 17–31. [Google Scholar] [CrossRef]
- Zaher, H. Hemipenial morphology of the South American Xenodontine Snakes, with a proposal for a monophyletic Xenodontinae and a reappraisal of colubrid hemipenes. Bull. Am. Museum Nat. Hist. 1999, 240, 1–240. [Google Scholar]
- International Commission on Zoological Nomenclature. International Code of Zoological Nomenclature; International Trust for Zoological Nomenclature: London, UK, 1999. [Google Scholar]
- Burbrink, F.T.; Lawson, R.; Slowinski, J.B. Mitochondrial DNA phylogeography of the polytypic North American rat snake (Elaphe obsoleta): A critique of the subspecies concept. Evolution 2000, 54, 2107–2118. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.; Higgins, D.; Gibson, T. ClustalW: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef] [PubMed]
- Kalyaanamoorthy, S.; Minh, B.; Wong, T.; Von Haeseler, A.; Jermiin, L. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Huelsenbeck, J. MrBayes 3: Bayesian Phylogenetic Inference under Mixed Models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef] [PubMed]
- Minh, B.; Schmidt, H.; Chernomor, O.; Schrempf, D.; Woodhams, M.; von Haeseler, A.; Lanfear, R. IQ-TREE 2: New models and efficient methods for Phylogenetic Inference in the genomic era. Mol. Biol. Evol. 2020, 37, 1530–1534. [Google Scholar] [CrossRef] [PubMed]
- Deepak, V.; Cooper, N.; Poyarkov, N.A.; Kraus, F.; Burin, G.; Das, A.; Narayanan, S.; Streicher, J.W.; Smith, S.-J.; Gower, D.J. Multilocus phylogeny, natural history traits and classification of natricine snakes (Serpentes: Natricinae). Zool. J. Linn. Soc. 2022, 195, 279–298. [Google Scholar] [CrossRef]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; da Fonseca, G.A.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef]
- Pawar, S.S.; Birand, A.C.; Ahmed, M.F.; Sengupta, S.; Raman, T.R.S. Conservation biogeography in north-east India: Hierarchical analysis of cross-taxon distributional congruence. Divers. Distrib. 2007, 13, 53–65. [Google Scholar] [CrossRef]
- Lalremsanga, H.T.; Bal, A.K.; Vogel, G.; Biakzuala, L. Molecular phylogenetic analyses of lesser known colubrid snakes reveal a new species of Herpetoreas (Squamata: Colubridae: Natricinae), and new insights into the systematics of Gongylosoma scriptum and its allies from northeastern India. Salamandra 2022, 58, 101–115. [Google Scholar]
- Lalremsanga, H.T.; Muansanga, L.; Vabeiryureilai, M.; Mirza, Z.A. A new species of parachute gecko of the subgenus Ptychozoon (Sauria: Gekkonidae: Gekko) from the Indo-Burma Region. Salamandra 2023, 59, 125–135. [Google Scholar]
- Idiiatullina, S.S.; Van Nguyen, T.; Pawangkhanant, P.; Suwannapoom, C.; Chanhome, L.; Mirza, Z.A.; David, P.; Vogel, G.; Poyarkov, N.A. An integrative taxonomic revision of the Trimeresurus popeiorum Group of pitvipers (Reptilia: Serpentes: Viperidae) with descriptions of two new species from the Indo-Burma Biodiversity Hotspot. Vertebr. Zool. 2024, 74, 303–342. [Google Scholar] [CrossRef]
- Agarwal, I.; Mahony, S.; Giri, V.B.; Chaitanya, R.; Bauer, A.M. Six new Cyrtodactylus (Squamata: Gekkonidae) from Northeast India. Zootaxa 2018, 4524, 501–535. [Google Scholar] [CrossRef] [PubMed]
- Lalronunga, S.; Lalhmangaiha, K.; Zosangliana, I.; Lalhmingliani, E.; Gower, D.J.; Das, A.; Deepak, V. A new species of Stoliczkia Jerdon, 1870 (Serpentes: Xenodermidae) from Mizoram, India. Zootaxa 2021, 4996, 555–568. [Google Scholar] [CrossRef]
- Rathee, Y.S.; Purkayastha, J.; Lalremsanga, H.T.; Dalal, S.; Biakzuala, L.; Muansanga, L.; Mirza, Z.A. A new cryptic species of green pit viper of the genus Trimeresurus Lacépède, 1804 (Serpentes, Viperidae) from Northeast India. PLoS ONE 2022, 17, e0268402. [Google Scholar] [CrossRef] [PubMed]
- Purkayastha, J.; Lalremsanga, H.T.; Bohra, S.C.; Biakzuala, L.; Decemson, H.; Muansanga, L.; Vabeiryureilai, M.; Chauhan, S.; Rathee, Y.S. Four new bent-toed geckos (Cyrtodactylus Gray: Squamata: Gekkonidae) from Northeast India. Zootaxa 2021, 4980, 451–489. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).