Biodiversity, Ecological Status and Ecosystem Attributes of Agricultural Ditches Based on the Analysis of Macroinvertebrate Communities
Abstract
:1. Introduction
2. Materials and Methods
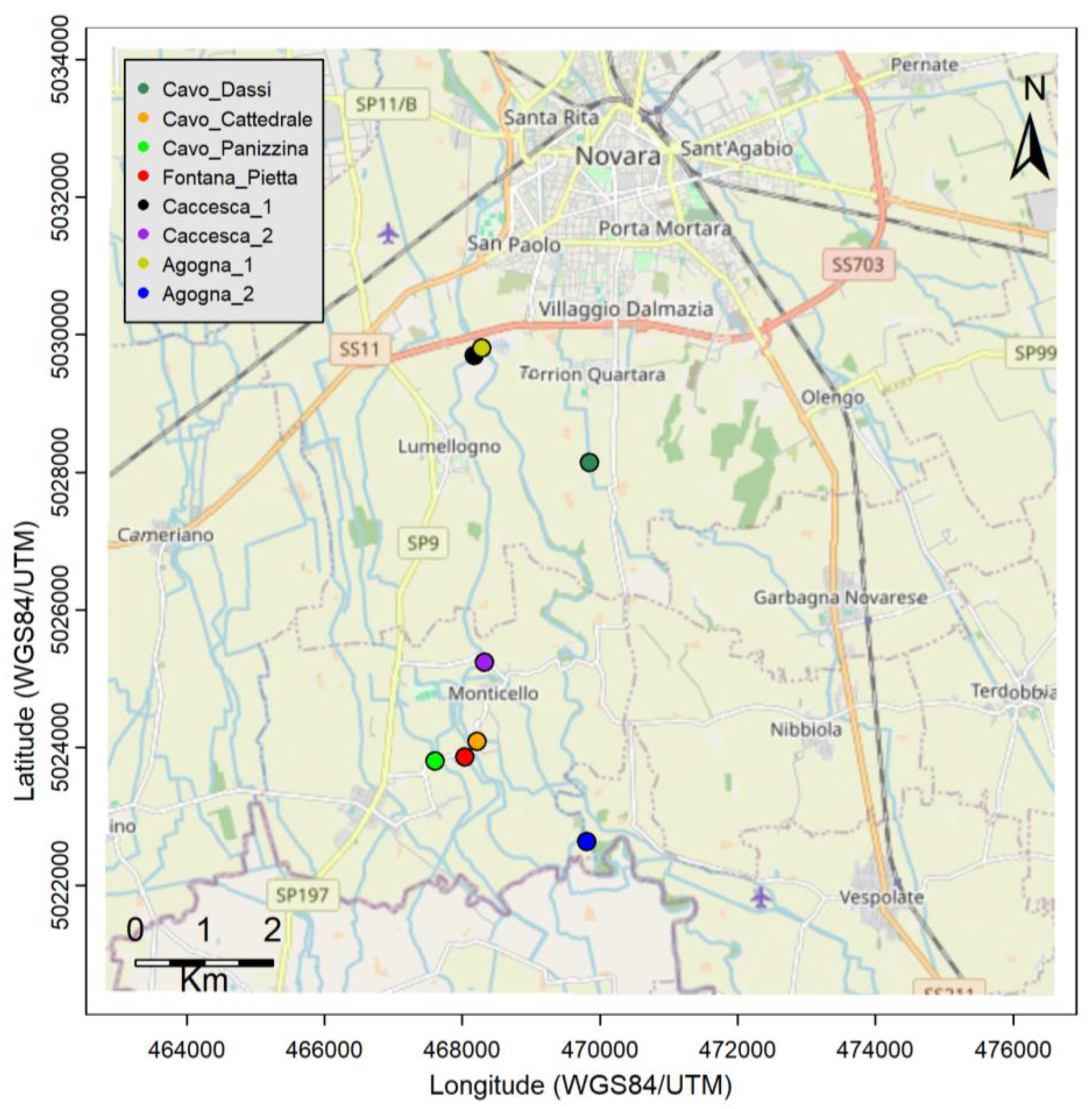
2.1. Area of Study
2.2. Data Collection
2.3. Statistical Analyses
3. Results
3.1. Environmental Parameters
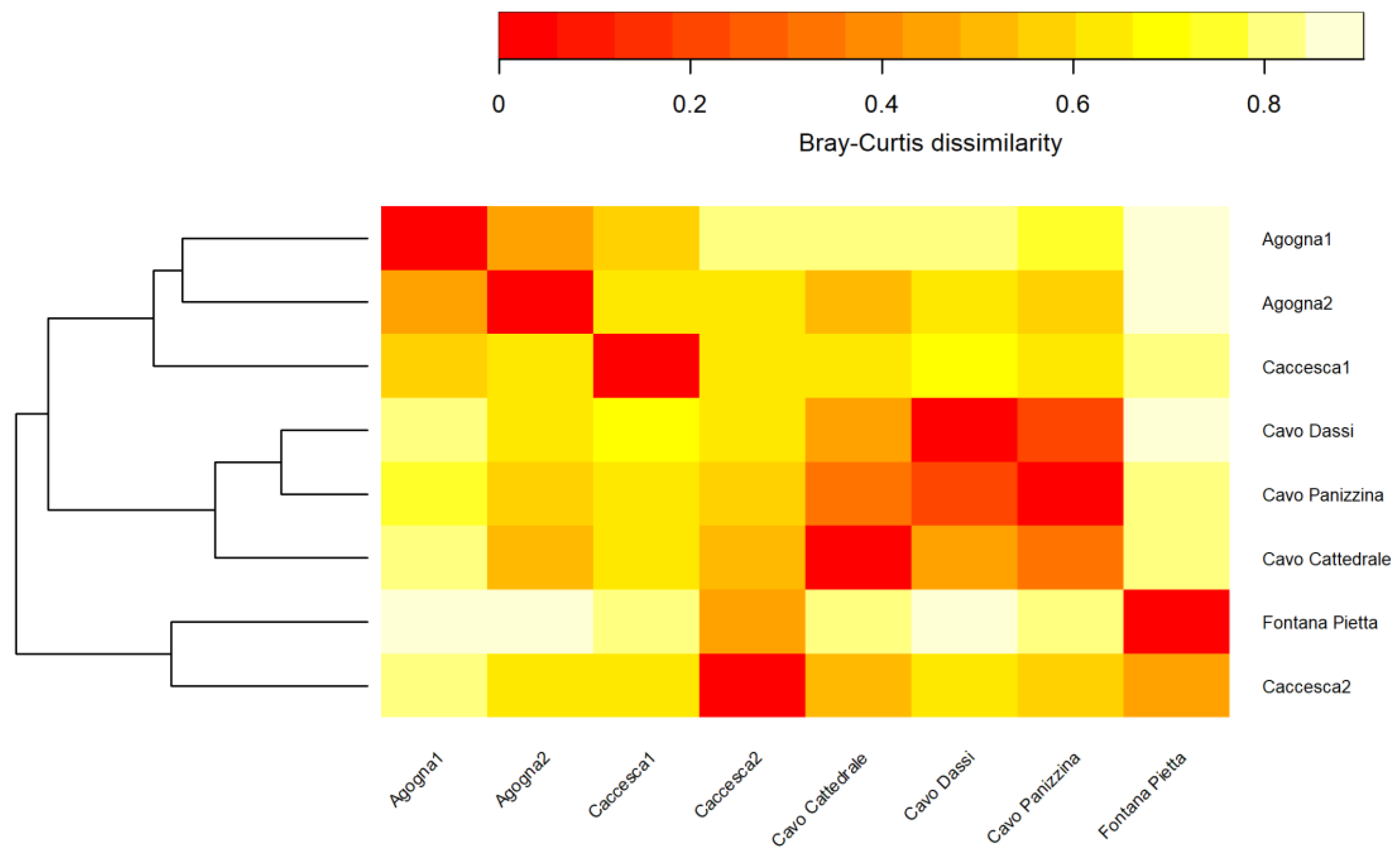
3.2. Macroinvertebrates
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Needelman, B.A.; Kleinman, P.J.; Strock, J.S.; Allen, A.L. Drainage Ditches: Improved management of agricultural drainage ditches for water quality protection: An overview. J. Soil Water Conserv. 2007, 62, 171–178. [Google Scholar]
- Dollinger, J.; Dagès, C.; Bailly, J.S.; Lagacherie, P.; Voltz, M. Managing ditches for agroecological engineering of landscape. A review. Agron. Sustain. Dev. 2015, 35, 999–1020. [Google Scholar] [CrossRef]
- Williams, P.; Whitfield, M.; Biggs, J.; Bray, S.; Fox, G.; Nicolet, P.; Sear, D. Comparative biodiversity of rivers, streams, ditches and ponds in an agricultural landscape in Southern England. Biol. Conserv. 2004, 115, 329–341. [Google Scholar] [CrossRef]
- Herzon, I.; Helenius, J. Agricultural drainage ditches, their biological importance and functioning. Biol. Conserv. 2008, 141, 1171–1183. [Google Scholar] [CrossRef]
- Death, R.G. Disturbance and riverine benthic communities: What has it contributed to general ecological theory? River. Res. Appl. 2010, 26, 15–25. [Google Scholar] [CrossRef]
- De Meester, L.; Declerck, S.; Stoks, R.; Louette, G.; Van De Meutter, F.; De Bie, T.; Michels, E.; Brendonck, L. Ponds and pools as model systems in conservation biology, ecology and evolutionary biology. Aquat. Conserv. 2005, 15, 715–725. [Google Scholar] [CrossRef]
- Iwamoto, H.; Tahara, D.; Yoshida, T. Contrasting metacommunity patterns of fish and aquatic insects in drainage ditches of paddy fields. Ecol. Res. 2022, 37, 635–646. [Google Scholar] [CrossRef]
- Gething, K.J.; Little, S. The importance of artificial drains for macroinvertebrate biodiversity in reclaimed agricultural landscapes. Hydrobiologia 2020, 847, 3129–3138. [Google Scholar] [CrossRef]
- Ward-Campbell, B.; Cottenie, K.; Mandrak, N.E.; McLaughlin, R. Fish assemblages in agricultural drains are resilient to habitat change caused by drain maintenance. Can. J. Fish. Aquat. Sci. 2017, 74, 1538–1548. [Google Scholar] [CrossRef]
- Leslie, A.W.; Lamp, W.O. Burrowing macroinvertebrates alter phosphorus dynamics in drainage ditch sediments. Aquat. Sci. 2019, 81, 23. [Google Scholar] [CrossRef]
- Linares, M.S.; dos Santos, L.B.; Callisto, M.; Santos, J.C. Physical habitat condition as a key tool to maintain freshwater biodiversity in neotropical artificial ponds. Water Biol. Secur. 2023, 2, 100–189. [Google Scholar] [CrossRef]
- Uno, H.; Power, M.E. Mainstem-tributary linkages by mayfly migration help sustain salmonids in a warming river network. Ecol. Lett. 2015, 18, 1012–1020. [Google Scholar] [CrossRef]
- Huryn, A.D.; Wallace, J.B. Life history and production of stream insects. Annu. Rev. Entomol. 2000, 45, 83–110. [Google Scholar] [CrossRef]
- Cummins, K.W. Combining taxonomy and function in the study of stream macroinvertebrates. J. Limnol. 2016, 75, 235–241. [Google Scholar] [CrossRef]
- Sitati, A.; Masese, F.O.; Yegon, M.J.; Achieng, A.O.; Agembe, S.W. Abundance-and biomass-based metrics of functional composition of macroinvertebrates as surrogates of ecosystem attributes in Afrotropical streams. Aquat. Sci. 2021, 83, 73. [Google Scholar] [CrossRef]
- Buss, D.F.; Carlisle, D.M.; Chon, T.S.; Culp, J.; Harding, J.S.; Keizer-Vlek, H.E.; Wayne, A.R.; Strachan, S.; Thirion, C.; Hughes, R.M. Stream biomonitoring using macroinvertebrates around the globe: A comparison of large-scale programs. Environ. Monit. Assess. 2015, 187, 4132. [Google Scholar] [CrossRef] [PubMed]
- Tornwall, B.; Sokol, E.; Skelton, J.; Brown, B.L. Trends in stream biodiversity research since the river continuum concept. Diversity 2015, 7, 16–35. [Google Scholar] [CrossRef]
- Bo, T.; Doretto, A.; Alex, L.; Bona, F.; Stefano, F.; Fenoglio, S.; Laini, A. Biomonitoring with macroinvertebrate communities in Italy: What happened to our past and what is the future? J. Limnol. 2017, 76, 21–28. [Google Scholar] [CrossRef]
- Pelloli, C. The long rice story. A comparison of rice’s introduction into Italy and Japan. Asian Archaeol. 2024, 8, 37–58. [Google Scholar] [CrossRef]
- Doretto, A.; Bo, T.; Bona, F.; Fenoglio, S. Efficiency of Surber net under different substrate and flow conditions: Insights for macroinvertebrates sampling and river biomonitoring. Knowl. Manag. Aquat. Ecosyst. 2020, 421, 10. [Google Scholar] [CrossRef]
- Hering, D.; Buffagni, A.; Moog, O.; Sandin, L.; Sommerhäuser, M.; Stubauer, I.; Feld, C.; Johnson, R.; Pinto, P.; Skoulikidis, N.; et al. The development of a system to assess the ecological quality of streams based on macroinvertebrates–design of the sampling program within the AQEM project. Int. Rev. Hydrobiol. 2003, 88, 345–361. [Google Scholar] [CrossRef]
- Campaioli, S.; Ghetti, P.F.; Minelli, A.; Ruffo, S. Manuale per Il Riconoscimento Dei Macroinvertebrati Delle Acque Dolci Italiane; Provincia Autonoma di Trento: Trento, Italy, 1994; Volume I. [Google Scholar]
- Campaioli, S.; Ghetti, P.F.; Minelli, A.; Ruffo, S. Manuale per Il Riconoscimento Dei Macroinvertebrati Delle Acque Dolci Italiane; Provincia Autonoma di Trento: Trento, Italy, 1999; Volume II. [Google Scholar]
- Erba, S.; Cazzola, M.; Belfiore, C.; Buffagni, A. Macroinvertebrate metrics responses to morphological alteration in Italian rivers. Hydrobiologia 2020, 847, 2169–2191. [Google Scholar] [CrossRef]
- Bo, T.; Doretto, A.; Marino, A.; Laini, A.; Candiotto, A. Taxonomic and functional responses of macroinvertebrate communities to dam construction in a non-wadeable river. Knowl. Manag. Aquat. Ecosyst. 2023, 424, 18. [Google Scholar] [CrossRef]
- Armitage, P.D.; Moss, D.; Wright, J.F.; Furse, M.T. The performance of a new biological water quality score system based on macroinvertebrates over a wide range of unpolluted running-water sites. Water Res. 1983, 17, 333–347. [Google Scholar] [CrossRef]
- Alba-Tercedor, J.; Sánchez-Ortega, A. Un método rápido y simple para evaluar la calidad biológica de las aguas corrientes basado en el de Hellawell (1978). Limnetica 1988, 4, 51–56. [Google Scholar] [CrossRef]
- O’Callaghan, P.; Kelly-Quinn, M. Distribution and structure of lotic macroinvertebrate communities and the influence of environmental factors in a tropical cloud forest, Cusuco National Park, Honduras. J. Limnol. 2017, 76, 148–160. [Google Scholar] [CrossRef]
- Fenoglio, S.; Doretto, A. Monitoring of neotropical streams using macroinvertebrate communities: Evidence from Honduras. Environments 2021, 8, 27. [Google Scholar] [CrossRef]
- Cummins, K.W.; Merritt, R.W.; Andrade, P.C. The use of invertebrate functional groups to characterize ecosystem attributes in selected streams and rivers in south Brazil. Stud. Neotrop. Fauna Environ. 2005, 40, 69–89. [Google Scholar] [CrossRef]
- Cummins, K.W.; Wilzbach, M.; Kolouch, B.; Merritt, R. Estimating macroinvertebrate biomass for stream ecosystem assessments. Int. J. Environ. Res. Public Health 2022, 19, 3240. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.R-project.org/ (accessed on 30 June 2024).
- Laini, A.; Guareschi, S.; Bolpagni, R.; Burgazzi, G.; Bruno, D.; GutiérrezCánovas, C.; Miranda, R.; Mondy, C.; Várbíró, G.; Cancellario, T. Biomonitor: An R package for calculating taxonomic and functional indices for river biomonitoring. PeerJ 2022, 10, e14183. [Google Scholar] [CrossRef]
- Warnes, M.G.R.; Bolker, B.; Bonebakker, L.; Gentleman, R.; Huber, W.; Liaw, A. Package ‘gplots’; Various R Programming Tools for Plotting Data; 2016; pp. 112–119. Available online: https://cran.r-project.org/web/packages/gplots/gplots.pdf (accessed on 30 June 2024).
- Wickham, H.; Chang, W.; Wickham, M.H. Package ‘ggplot2’. Version 2 (1); Create Elegant Data Visualisations Using the Grammar of Graphi Graphics; 2016; pp. 1–189. Available online: https://ggplot2.tidyverse.org/reference/ggplot2-package.html (accessed on 30 June 2024).
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. R Package; Version 2; Vegan: Community Ecology Package; 2015; Available online: http://CRAN.Rproject.org/package=vegan (accessed on 30 June 2024).
- Shaw, R.F.; Johnson, P.J.; Macdonald, D.W.; Feber, R.E. Enhancing the biodiversity of ditches in intensively man-aged UK farmland. PLoS ONE 2015, 10, e0138306. [Google Scholar] [CrossRef]
- Pieri, L.; Ventura, F.; Ventura, M.; Tagliavini, M.; Ponti, M.; Pistocchi, R.; Albertazzi, S.; Vignudelli, M.; Pisa, P.R. Chemical and biological indicators of water quality in three agricultural watersheds of the Po valley, Italy. Ital. J. Agron. 2011, 6, 29–38. [Google Scholar] [CrossRef]
- Gething, K.J. Physicochemical drivers of managed river and agricultural drainage channel macroinvertebrate communities. River. Res. Appl. 2021, 37, 675–680. [Google Scholar] [CrossRef]
- Leslie, A.W.; Smith, R.F.; Ruppert, D.E.; Bejleri, K.; Mcgrath, J.M.; Needelman, B.A.; Lamp, W.O. Environmental factors structuring benthic macroinvertebrate communities of agricultural ditches in Maryland. Environ. Entomol. 2012, 41, 802–812. [Google Scholar] [CrossRef]
- Gething, K.J.; Ripley, M.C.; Mathers, K.L.; Chadd, R.P.; Wood, P.J. The influence of substrate type on macroinvertebrate assemblages within agricultural drainage ditches. Hydrobiologia 2020, 847, 4273–4284. [Google Scholar] [CrossRef]
- Vallefuoco, F.; Vanek, M.; Scotti, A.; Bottarin, R. Effect of management strategies and substrate composition on functional and taxonomic macroinvertebrate communities in lowland ditches of Alto Adige/Südtirol. Gredleriana 2023, 23, 87–1015. [Google Scholar]
- Davies, B.R.; Biggs, J.; Williams, P.J.; Lee, J.T.; Thompson, S. A comparison of the catchment sizes of rivers, streams, ponds, ditches and lakes: Implications for protecting aquatic biodiversity in an agricultural landscape. In Pond Conservation in Europe; Springer: Berlin/Heidelberg, Germany, 2010; pp. 7–17. [Google Scholar]
- Armitage, P.D.; Szoszkiewicz, K.; Blackburn, J.H.; Nesbitt, I. Ditch communities: A major contributor to flood-plain biodiversity. Aquat. Conserv. 2003, 13, 165–185. [Google Scholar] [CrossRef]
- Simon, T.N.; Travis, J. The contribution of man-made ditches to the regional stream biodiversity of the new river watershed in the Florida panhandle. Hydrobiologia 2011, 661, 163–177. [Google Scholar] [CrossRef]
- Heino, J.; Melo, A.S.; Siqueira, T.; Soininen, J.; Valanko, S.; Bini, L.M. Metacommunity organisation, spatial extent and dispersal in aquatic systems: Patterns, processes and prospects. Freshw. Biol. 2015, 60, 845–869. [Google Scholar] [CrossRef]
- Li, F.; Tonkin, J.D.; Haase, P. Dispersal capacity and broad-scale landscape structure shape benthic invertebrate communities along stream networks. Limnologica 2018, 71, 68–74. [Google Scholar] [CrossRef]
- Beisel, J.N.; Usseglio-Polatera, P.; Thomas, S.; Moreteau, J.C. Stream community structure in relation to spatial variation: The influence of mesohabitat characteristics. Hydrobiologia 1998, 389, 73–88. [Google Scholar] [CrossRef]
- Mesa, L.M. Hydraulic parameters and longitudinal distribution of macroinvertebrates in a subtropical andean basin. Interciencia 2010, 35, 759–764. [Google Scholar]
- Rempel, L.L.; Richardson, J.S.; Healey, M.C. Macroinvertebrate community structure along gradients of hydraulic and sedimentary conditions in a large gravel-bed river. Freshw. Biol. 2000, 45, 57–73. [Google Scholar] [CrossRef]
- Doretto, A.; Receveur, J.P.; Baker, E.A.; Benbow, M.E.; Scribner, K.T. Nested analysis of macroinvertebrate diversity along a river continuum: Identifying relevant spatial scales for stream communities. River. Res. Appl. 2022, 38, 334–344. [Google Scholar] [CrossRef]
- Verdonschot, R.C.; Keizer-vlek, H.E.; Verdonschot, P.F. Biodiversity value of agricultural drainage ditches: A comparative analysis of the aquatic invertebrate fauna of ditches and small lakes. Aquat. Conserv. 2011, 21, 715–727. [Google Scholar] [CrossRef]
- Guareschi, S.; Laini, A.; Sanchez-Montoya, M.M. How do low-abundance taxa affect river biomonitoring? Exploring the response of different macroinvertebrate-based indices. J. Limnol. 2016, 76, 9–20. [Google Scholar] [CrossRef]
- Jones, J.I.; Lloyd, C.E.; Murphy, J.F.; Arnold, A.; Duerdoth, C.P.; Hawczak, A.; Pretty, J.L.; Johnes, P.J.; Freer, J.E.; Stirling, M.W.; et al. What do macroinvertebrate indices measure? Stressor-specific stream macroinvertebrate indices can be confounded by other stressors. Freshw. Biol. 2023, 68, 1330–1345. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.; McAree, C.; Mulville, W.; Sullivan, T. The problem of agricultural ‘diffuse’pollution: Getting to the point. Sci. Total Environ. 2019, 677, 700–717. [Google Scholar] [CrossRef] [PubMed]
- Keizer-Vlek, H.E.; Verdonschot, P.F.; Verdonschot, R.C.; Goedhart, P.W. Quantifying spatial and temporal variability of macroinvertebrate metrics. Ecol. Indic. 2012, 23, 384–393. [Google Scholar] [CrossRef]
- Verdonschot, R.C.; Keizer-Vlek, H.E.; Verdonschot, P.F. Development of a multimetric index based on macroinvertebrates for drainage ditch networks in agricultural areas. Ecol. Indic. 2012, 13, 232–242. [Google Scholar] [CrossRef]
- Leslie, A.W.; Lamp, W.O. Taxonomic and functional group composition of macroinvertebrate assemblages in agricultural drainage ditches. Hydrobiologia 2017, 787, 99–110. [Google Scholar] [CrossRef]
- Harvey, E.; Altermatt, F. Regulation of the functional structure of aquatic communities across spatial scales in a major river network. Ecology 2019, 100, e02633. [Google Scholar] [CrossRef] [PubMed]
- Bonada, N.; Prat, N.; Resh, V.H.; Statzner, B. Developments in aquatic insect biomonitoring: A comparative analysis of recent approaches. Annu. Rev. Entomol. 2006, 51, 495–523. [Google Scholar] [CrossRef] [PubMed]
- Salmaso, F.; Crosa, G.; Espa, P.; Quadroni, S. Climate change and water exploitation as co-impact sources on river benthic macroinvertebrates. Water 2021, 13, 2778. [Google Scholar] [CrossRef]



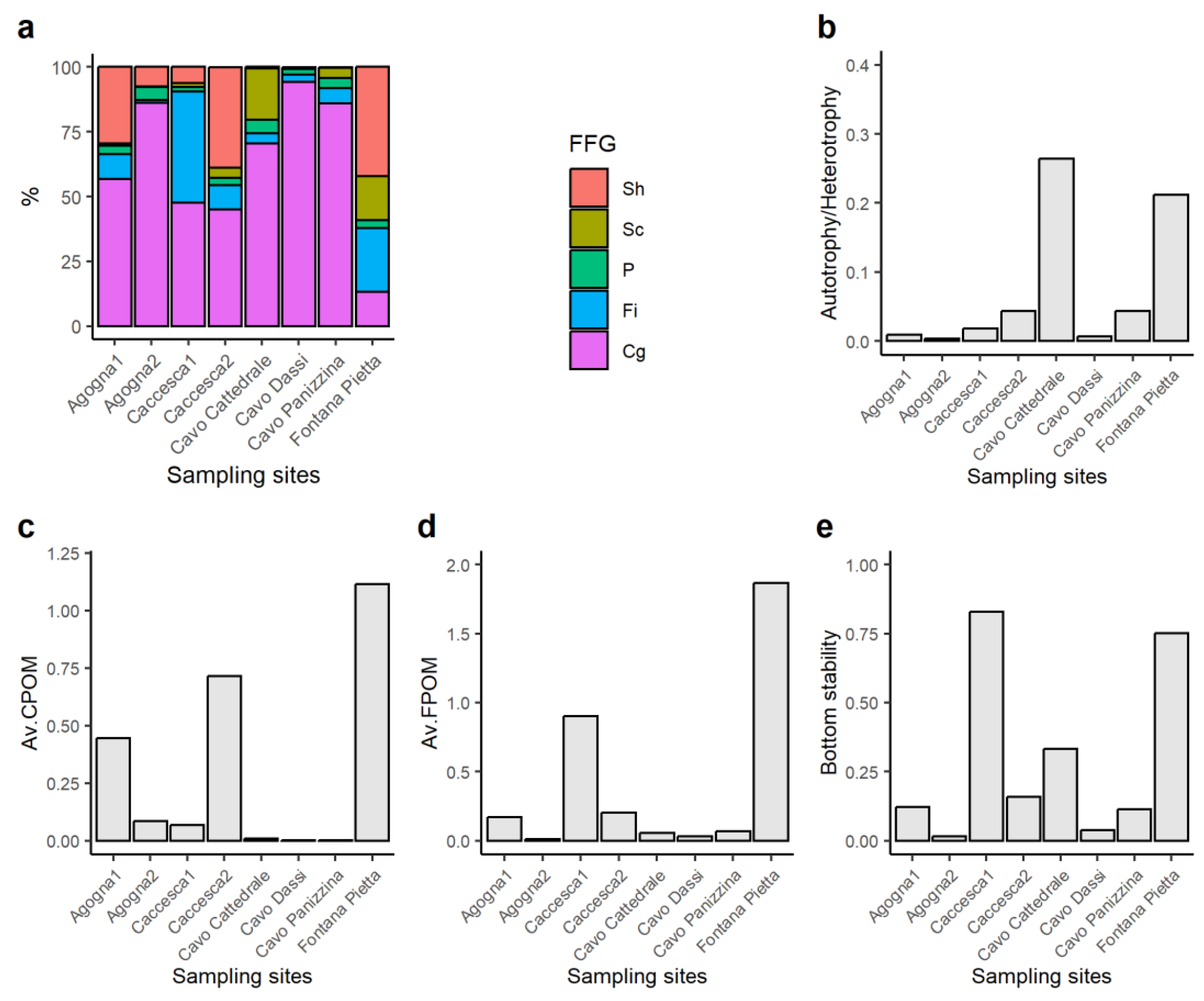
| Ecosystem Attribute | Ratio | Description | Threshold |
|---|---|---|---|
| Autotrophy/heterotrophy | Sc/(Sh + Cg + Fi) | Prevalence of instream production or detritus food-chain based on the feeding strategy of macroinvertebrates | >0.75 = Autotrophic |
| <0.75 = Heterotrophic | |||
| Av.CPOM | Sh/(Cg + Fi) | Availability of CPOM to FPOM | >0.50 = Abundant food for shredders |
| <0.50 = Spare food for shredders | |||
| Av.FPOM | Fi/Cg | Availability of FPOM in transport | >0.50 = Abundant food for filterers |
| <0.50 = Low food for filterers | |||
| Bottom stability | (Sc + Fi)/(Sh + Cg) | Stream bottom stability based on the feeding strategies of macroinvertebrates | >0.50 = Stable bottom dominates |
| <0.50 = Stable bottom limiting |
| Index or Metric | PC1 | PC2 |
|---|---|---|
| ASPT | 0.809 * | 0.500 |
| N. Families | 0.716 * | 0.185 |
| N. Fam. EPT | 0.530 | 0.001 |
| Sel(EPTD + 1) | 0.239 | −0.072 |
| 1-GOLD | −0.071 | −0.429 |
| Shannon | −0.262 | −0.714 |
| STAR_ICMi | 0.429 | −0.024 |
| BMWP | 0.778 * | 0.503 |
| %Gg | −0.095 | 0.381 |
| %Fi | −0.190 | −0.143 |
| %P | 0.524 | −0.357 |
| %Sc | 0.635 | 0.108 |
| %Sh | 0.048 | −0.785 * |
| Autotrophy/Heterotrophy | 0.667 | 0.143 |
| Av.CPOM | 0.048 | −0.786 * |
| Av.FPOM | 0.024 | −0.143 |
| Bottom stability | −0.047 | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiorino, M.; Spreafico, C.; Solazzo, D.; Doretto, A. Biodiversity, Ecological Status and Ecosystem Attributes of Agricultural Ditches Based on the Analysis of Macroinvertebrate Communities. Diversity 2024, 16, 558. https://doi.org/10.3390/d16090558
Chiorino M, Spreafico C, Solazzo D, Doretto A. Biodiversity, Ecological Status and Ecosystem Attributes of Agricultural Ditches Based on the Analysis of Macroinvertebrate Communities. Diversity. 2024; 16(9):558. https://doi.org/10.3390/d16090558
Chicago/Turabian StyleChiorino, Martina, Cristina Spreafico, Davide Solazzo, and Alberto Doretto. 2024. "Biodiversity, Ecological Status and Ecosystem Attributes of Agricultural Ditches Based on the Analysis of Macroinvertebrate Communities" Diversity 16, no. 9: 558. https://doi.org/10.3390/d16090558
APA StyleChiorino, M., Spreafico, C., Solazzo, D., & Doretto, A. (2024). Biodiversity, Ecological Status and Ecosystem Attributes of Agricultural Ditches Based on the Analysis of Macroinvertebrate Communities. Diversity, 16(9), 558. https://doi.org/10.3390/d16090558







