A Contribution to the Study of the Flora and Vegetation of Mnemba Island, Zanzibar
Abstract
1. Introduction
2. Methods
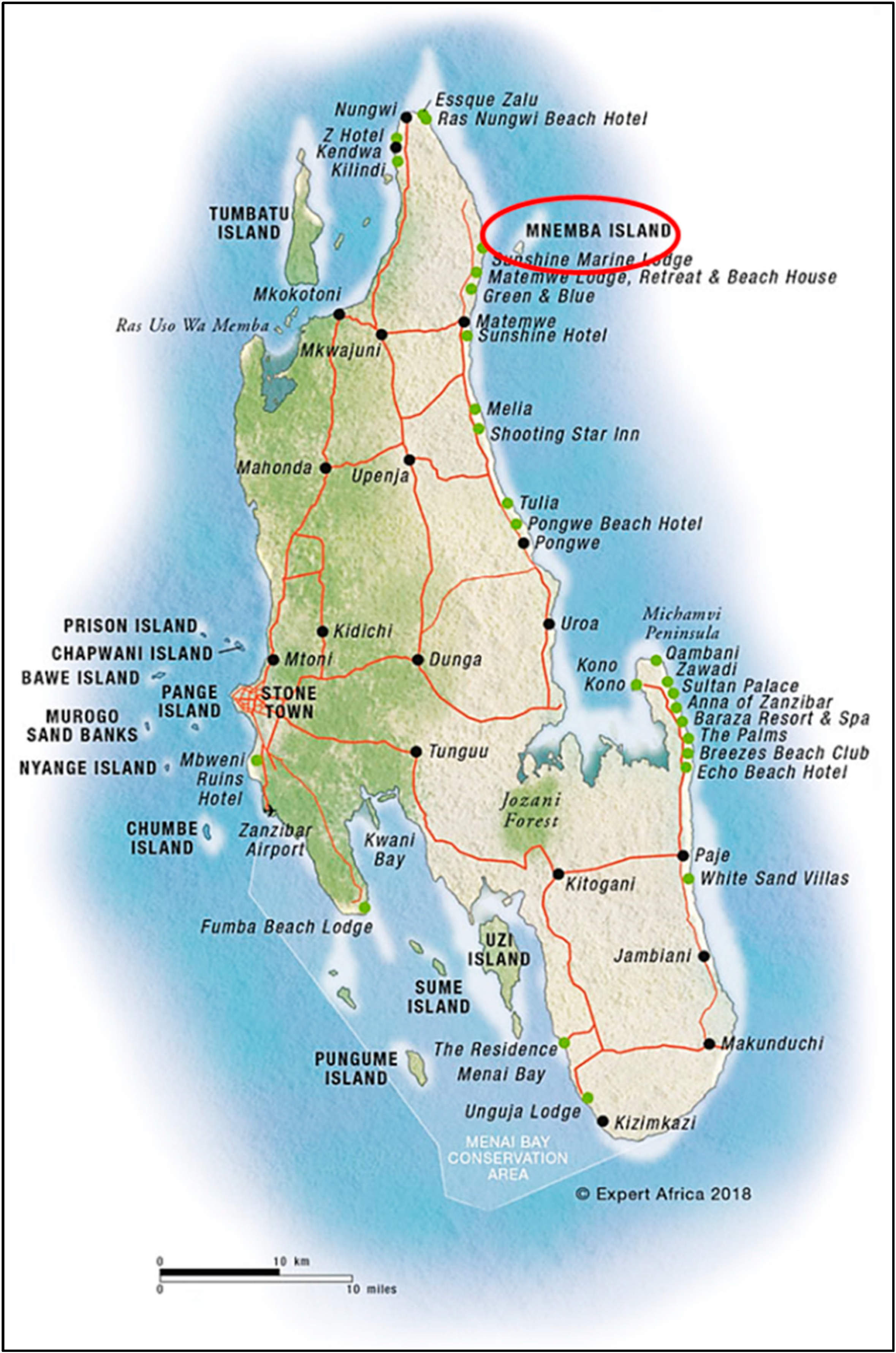
2.1. Study Area
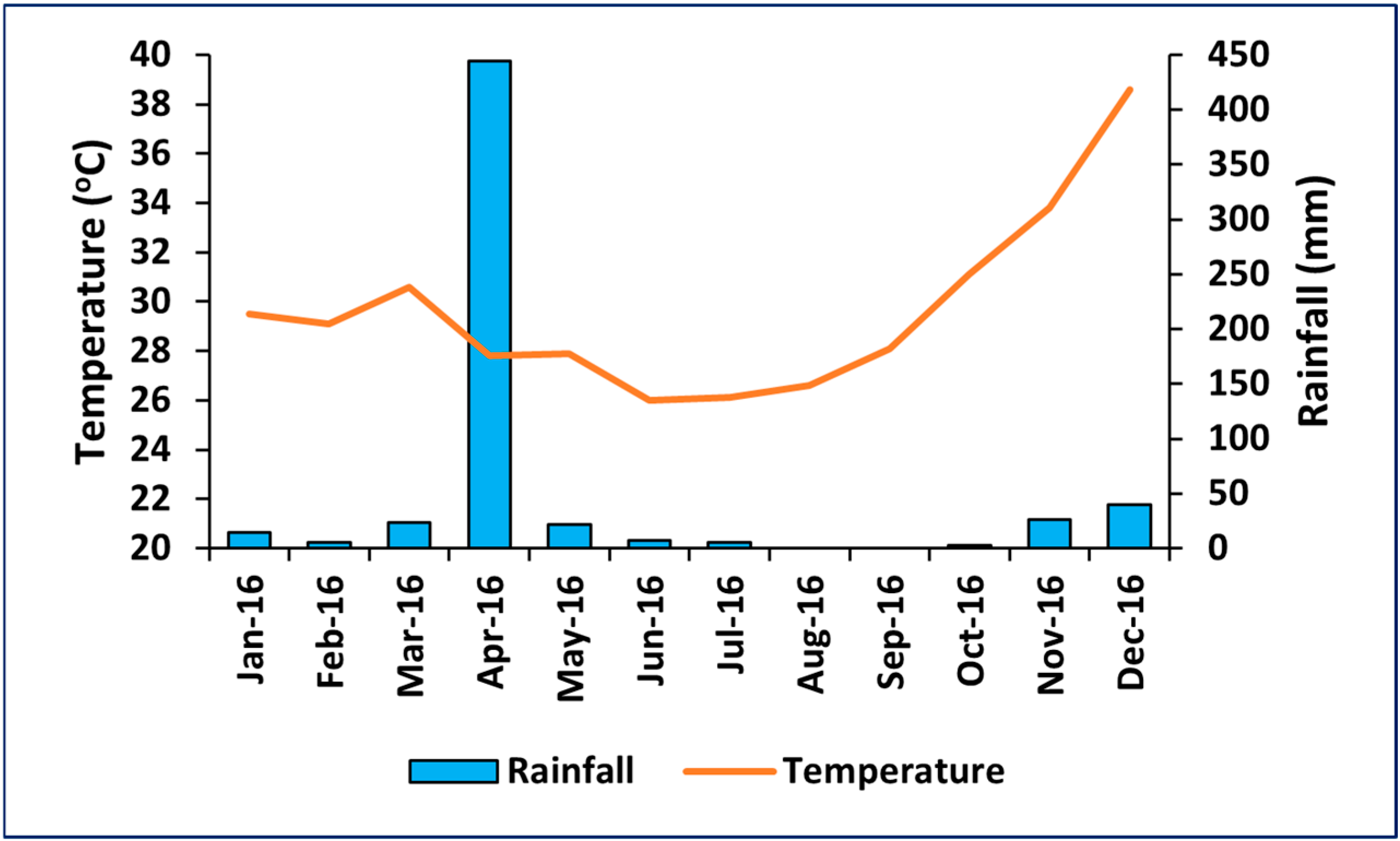
2.2. Climate
2.3. Vegetation Sampling
- TH = Tree height
- LS = Length of the stick
- DT = Distance to the tree
- DE = Distance to the eye.
2.4. Data Analysis
- = Shannon–Weiner Index;
- ∑ = summation;
- = proportion of individuals belonging to the i-th species in the community; and
- = natural logarithm.
- = evenness;
- = Shannon–Weiner Index;
- = maximum possible value of the Shannon–Weiner Index.
- D = density, expressed as individuals per unit area (ind/ha);
- N = number of individuals counted in all sample plots of a plant community × 10,000 (i.e., p/ha);
- A = area size of plots within which the individuals were counted.
3. Results
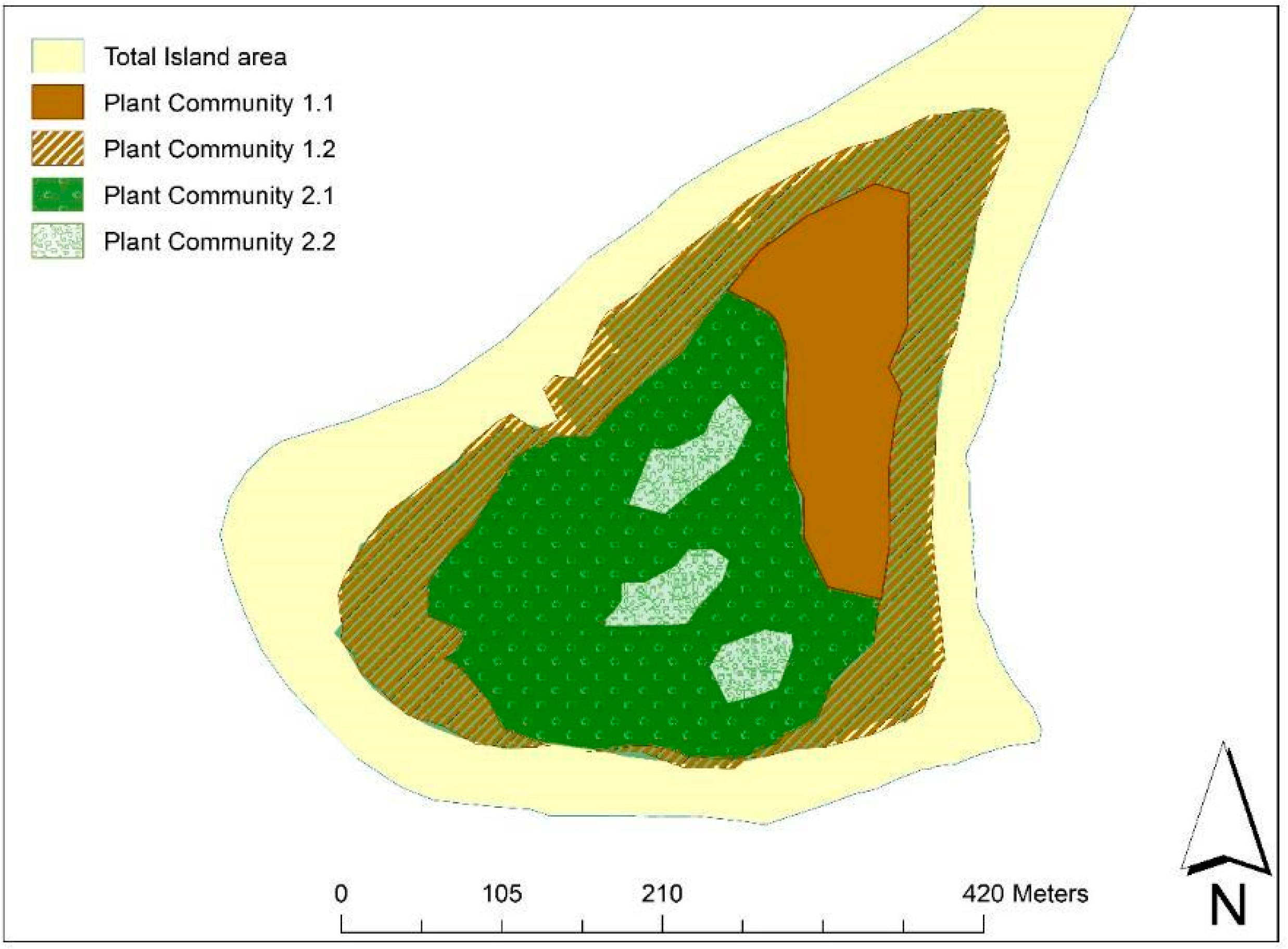
3.1. Vegetation Classification and Description
- The Casuarina cunninghamiana–Suriana maritima open to closed sandy dry coastal forest:
- Casuarina cunninghamiana–Suriana maritima–Enteropogon macrostachyus sandy dry coastal forest community;
- Casuarina cunninghamiana–Suriana maritima–Chrysothrix sp. open sandy dry coastal forest.
- The Eugenia capensis–Mimusops obtusifolia coastal forest:
- Eugenia capensis–Mimusops obtusifolia–Scutia myrtina coastal forest;
- Eugenia capensis–Mimusops obtusifolia–Clerodendrum glabrum coastal scrub.
3.1.1. The Casuarina cunninghamiana–Suriana maritime Open to Closed Sandy Dry Coastal Forest
The Casuarina cunninghamiana–Suriana maritima–Enteropogon macrostachyus Sandy Dry Coastal Forest Community
The Casuarina cunninghamiana–Suriana maritime–Chrysothrix Open Sandy Dry Coastal Forest
3.1.2. The Eugenia capensis–Mimusops obtusifolia Coastal Forest
The Eugenia capensis–Mimusops obtusifolia–Scutia myrtina Coastal Forest
The Eugenia capensis–Mimusops obtusifolia–Clerodendrum glabrum Coastal Scrub
3.2. Plant Species Not Recorded in Sample Relevés
3.3. Floristic Characteristics
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kombo, Y.H. Zanzibar Forest Biodiveristy versus Energy Crisis and Climate Change: Toward Zanzibar Environmental Policy Formulation; The Department of Commercial Crops, Fruits and Forestry: Zanzibar, Tanzania, 2010. [Google Scholar]
- Bronkhorst, L.R. Ecology of the Aders’ Duiker (Cephalophus adersi) on Mnemba Island, Zanzibar. Ph.D. Thesis, University of South Africa, Pretoria, South Africa, 2020. [Google Scholar]
- Prendergast, M.E.; Rouby, H.; Punnwong, P.; Marchant, R.; Crowther, A.; Kourampas, N.; Shipton, C.; Walsh, M.; Lambeck, K.; Boivin, N.L. Continental Island Formation and the Archaeology of Defaunation on Zanzibar, Eastern Africa. PLoS ONE 2016, 11, e0149565. [Google Scholar] [CrossRef] [PubMed]
- Woodroffe, C.; Biribo, N. Atolls. In Encyclopedia of Modern Coral Reefs: Structure, Form and Process; Hopley, D., Ed.; Springer: Dordrecht, The Netherlands, 2011; pp. 51–71. [Google Scholar] [CrossRef]
- Goldberg, W. Atolls of the world: Revisiting the Original Checklist. Atoll Res. Bull. 2016, 610, 1–47. [Google Scholar] [CrossRef]
- Yu, A.D.; Lei, S.A. Equilibrium Theory of Island Biogeography: A Review. In Shrubland Ecosystem Genetics and Biodiversity; U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station: Fort Collins, CO, USA, 2001; pp. 163–171. [Google Scholar]
- The Theory of Island Biogeography Revisited; Losos, J.B., Ricklefs, R.E., Eds.; Princeton University Press: Princeton, NJ, USA, 2010. [Google Scholar] [CrossRef]
- Hnatiuk, R.; Merton, L. Vegetation of Aldabra, a reassessment. Atoll Res. Bull. 1979, 239, 1–21. [Google Scholar] [CrossRef][Green Version]
- Wickens, G.E. Speculations on Seed Dispersal and the Flora of the Aldabra Archipelago. Philos. Trans. R. Soc. Lond. 1979, 286, 85–97. [Google Scholar] [CrossRef]
- Stoddart, D.R.; Walsh, R.P.D. Long-term climatic change in the western Indian Ocean. Philos. Trans. R. Soc. Lond. 1979, 286, 11–23. [Google Scholar] [CrossRef]
- Yamano, H.; Kayanne, H.; Chikamori, M. An Overview of The Nature and Dynamics of Reef Islands. Glob. Environ. Res. 2005, 9, 9–20. [Google Scholar]
- Taylor, J.; Braithwaite, C.; Peake, J.; Arnold, E. Terrestrial faunas and habitats of Aldabra during the late Pleistocene. Philos. Trans. R. Soc. Lond. 1979, 286, 47–66. [Google Scholar] [CrossRef]
- Brown, L.; Du Preez, P.; Bezuidenhout, H.; Bredenkamp, G.; Mostert, T.; Collins, N. Guidelines for phytosociological classifications and descriptions of vegetation in southern Africa. Koedoe 2013, 55, 1–10. [Google Scholar] [CrossRef]
- Ngoile, M. Ecological baseline surveys of coral reefs and intertidal zones around Mnemba Island and Zanzibar Town. Commission of Lands and Environment. Zanzibar ZNZ Environ. Study Ser. 1990, 8, 1–87. [Google Scholar]
- IUCN SSC Antelope Specialist Group. Cephalophus adersi. The IUCN Red List of Threatened Species 2017: E.T4137A50182159. 2017. Available online: https://www.iucnredlist.org/species/4137/50182159 (accessed on 31 October 2023). [CrossRef]
- Expert Africa. Map of Tanzania’s Coastline, Showing Zanzibar, Pemba and Mafia Islands. 2012. Available online: www.expertafrica.com/tanzania/zanzibar-island/reference-map (accessed on 17 October 2018).
- Swanepoel, C. Effect of Coral Reefs on Wave Attenuation and Erosion: Mnemba Island, Zanzibar. Master’s Dissertation, University of Kwazulu Natal, Natal, South Africa, 2017. [Google Scholar]
- John, M.S.T.; John, O.S.T. Scuba Diving at the Mnemba Island in Zanzibar. 2017. Available online: www.drinkteatravel.com/diving-mnemba-island-zanzibar/ (accessed on 4 November 2018).
- Revolutionary Government of Zanzibar. Mnemba Island and Chwaka Bay Conservation Areas: A Preliminary Situational Assessment (July 2005); EcoAfrica Environmental Consultants for the Department of Fisheries and Marine Resources: Tshwane, South Africa, 2005.
- Grellman, K.A. Disappearing Island: An Impact Assessment of Coastal Erosion on the Mnemba Island House Reef, Zanzibar. Independent Study Project (ISP). Collection. 2870. 2018. Available online: https://digitalcollections.sit.edu/isp_collection/2870 (accessed on 20 November 2018).
- Batianoff, G.; Naylor, G.; Fensham, R.; Neldner, V. Characteristics of Coral Cay Soils at Coringa-Herald Coral Sea Islands, Australia. Pac. Sci. 2010, 64, 335–347. [Google Scholar] [CrossRef][Green Version]
- Eyes on Africa. Mnemba Island Lodge. 2016. Available online: www.eyesonafrica.net/african-safari-tanzania/mnemba-island-lodge.htm (accessed on 22 November 2018).
- Peet, R.K.; Roberts, D.W. Classification of Natural and Semi-natural Vegetation. In Vegetation Ecology, 2nd ed.; Van Der Maarel, E., Franklin, J., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2013. [Google Scholar] [CrossRef]
- McNellie, M.; Dorrough, J.; Oliver, I. Species abundance distributions should underpin ordinal cover-abundance transformations. Appl. Veg. Sci. 2019, 22, 361–372. [Google Scholar] [CrossRef]
- Avery, T.E.; Burkhart, H.E. Forest Measurements, 5th ed.; McGraw-Hill: New York, NY, USA, 2001. [Google Scholar]
- Dansereau, P. Description and recording of vegetation upon a structural basis. Ecology 1951, 32, 172–229. [Google Scholar] [CrossRef]
- Roleček, J.; Tichý, L.; Zelený, D.; Chytry, M. Modified TWINSPAN classification in which the hierarchy respects cluster heterogeneity. J. Veg. Sci. 2009, 20, 596–602. [Google Scholar] [CrossRef]
- Westhoff, V.; Van Der Maarel, E. The Braun-Blanquet approach. In Classification of Plant Communities; Whittaker, R., Ed.; Springer: Dordrecht, The Netherlands, 1978; pp. 289–399. [Google Scholar] [CrossRef]
- Grobler, C.H.; Bredenkamp, G.J.; Brown, L.R. Primary grassland communities of urban open spaces in Gauteng, South Africa. S. Afr. J. Bot. 2006, 72, 367–377. [Google Scholar] [CrossRef][Green Version]
- Mligo, C. Conservation of plant species diversity based on richness and evenness criteria in the Coast Forests of Tanzania. J. Environ. Ecol. 2015, 6, 1–20. [Google Scholar] [CrossRef][Green Version]
- Walker, T. Pisonia islands of the Great Barrier Reef. Part I. The distribution, abundance and dispersal by seabirds of Pisonia grandis. Atoll Res. Bull. 1991, 350, 1–23. [Google Scholar] [CrossRef]
- Beentje, H. Botanical Assessment of Ngesi Forest, Pemba; Zanzibar Forestry Development Project of FINNIDA and the Finnish National Board of Forestry; CHICOP: Zanzibar, Tanzania, 1990. [Google Scholar]
- Potgieter, L.J.; Richardson, D.M.; Wilson, J.R.U. Casuarina: Biogeography and ecology of an important tree genus in a changing world. Biol. Invasions 2014, 16, 609–633. [Google Scholar] [CrossRef]
- Hammerton, J.L. Casuarinas in the Bahamas: A clear and present danger. Bahamas. J. Sci. 2001, 9, 2–14. [Google Scholar]
- Stamps, N. Out of the quagmire of plant defense hypotheses. Q. Rev. Biol. 2003, 78, 23–55. [Google Scholar] [CrossRef]
- Niering, W. Observations on Puluwat and Gaferut, Caroline Islands with historical and climatic information on Gaferut Island. Atoll Res. Bull. 1956, 76, 1–10. [Google Scholar] [CrossRef][Green Version]
- Asseid, B.; Haji, T.; Khamis, M.; Klein, R.; Mzee, A.; Sitari, T. Practical Measures to Tackle Climate Change: Coastal Forest Buffer Zones and Shoreline Change in Zanibar, Tanzania; Mustelin, J., Ed.; Report No.: 13; Turku University Department of Geography Publications: Turku, Finland, 2009. [Google Scholar]
- Sharples, J.; Cairney, J. Assimilation of inorganic nitrogen by a mycobiont isolated from Pisonia grandis R. Br. (Nyctaginaceae) mycorrhiza. Mycorrhiza 1998, 7, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Woodroffe, C.D.; Morrison, R.J. Reef-island accretion and soil development on Makin, Kiribati, central Pacific. Catena 2001, 44, 245–261. [Google Scholar] [CrossRef]
- Dubi, A. Coastal Erosion, Chapter 1. In Tanzania Coastal Management Partnership: The Present State of Knowledge of Marine Science in Tanzania Synthesis Report; Ngusaru, A., Ed.; Tanzania Coastal Management Partnership Support Unit, Science and Technical Working Group: Zanzibar, Tanzania, 2000; pp. 5–42. [Google Scholar]
- Odada, E. The problem of coastal erosion and flooding in Eastern Africa. In Proceedings of the Workshop and Policy Conference on Integrated Coastal Zone Management in East Africa, Arusha, Tanzania, 21–23 April 1993. [Google Scholar]
- Kindt, R.; Van Breugel, P.; Orwa, C.; Lillesø, J.-P.B.; Graudal, L.; Jamnadass, R. Useful Tree Species for Eastern Africa: A Species Selection Tool Based on the VECEA Map V2.0. 2013. Available online: https://www.worldagroforestry.org/publication/useful-tree-species-eastern-africa-species-selection-tool-based-vecea-map (accessed on 21 November 2018).
- Van Breugel, P.; Kindt, R.; Lillesø, J.P.B.; Van Breugel, M. Environmental Gap Analysis to Prioritize Conservation Efforts in Eastern Africa. PLoS ONE 2015, 10, e0121444. [Google Scholar] [CrossRef] [PubMed]
- Van Breugel, P.; Kindt, R.; Lillesø, J.P.B.; Bingham, M.; Demissew, S.; Dudley, C.; Friis, I.; Gachathi, F.; Kalema, J.; Mbago, F. Potential Natural Vegetation Map of Eastern Africa (Burundi, Ethiopia, Kenya, Malawi, Rwanda, Tanzania, Uganda and Zambia) V2.0. 2015. Available online: http://vegetationmap4africa.org (accessed on 28 November 2018).
- Vesey-Fitzgerald, D. Further Studies of the Vegetation on Islands in the Indian Ocean. J. Ecol. 1942, 30, 1–16. [Google Scholar] [CrossRef]
- Gwynne, M.; Wood, D. Plants collected on Islands in the Western Indian Ocean during a cruise of the M.F.R.V. ‘MANIHINE,’ Sept.–Oct. 1967. Atoll Res. Bull. 1969, 134, 1–15. [Google Scholar] [CrossRef][Green Version]
- Barnes, R.; Smith, D.; Barnes, D.; Gerlach, J. Variation in the distribution of supralittoral vegetation around an atoll cay: Desroches (Amirante Islands, Seychelles). Atoll Res. Bull. 2008, 565, 1–6. [Google Scholar] [CrossRef][Green Version]
- Airy Shaw, H. On the Distribution of Pisonia grandis R. BR. (Nyctaginaceae), with Special Reference to Malaysia. Kew Bull. 1952, 7, 87–97. [Google Scholar] [CrossRef]
- Stoddart, D.; Fosberg, R. Phytogeography and vegetation of the reef islands of the Northern Great Barrier Reef. Atoll Res. Bull. 1991, 349, 1–619. [Google Scholar] [CrossRef][Green Version]
- Hill, M.; Vel, T.; Holm, K.; Parr, S.; Shah, N. Cousin. Atoll Res. Bull. 2002, 495, 49–72. [Google Scholar] [CrossRef]


| Scale | Description | Predicted Mean (PM) [24] |
|---|---|---|
| r | One or a few individuals with less than 1% cover of the total sample relevés area. | 0.01 |
| + | Occasional and less than 1% cover of the total sample relevés area. | 0.49 |
| 1 | Abundant with low cover or less abundant but with higher cover; 1–5% cover of the total sample relevés area. | 0.74 |
| 2a | 6–12.5% cover. | 8.95 |
| 2b | 12.6–25% cover. | 17.5 |
| 3 | 26–50% cover of the total sample relevés area, irrespective of the number of individuals. | 38.77 |
| 4 | 51–75% cover of the total sample relevés area, irrespective of the number of individuals. | 62.43 |
| 5 | 76–100% cover of the total sample relevés area, irrespective of the number of individuals. | 81.24 |
| Community Number | 1 | 2 | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1.1 | 1.2 | 2.1 | 2.2 | ||||||||||||||||||||||
| % Constancy | |||||||||||||||||||||||||
| Relevé numbers | 1 | 1 | 1 | 1 | 2 | 2 | 2 | 2 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | Main | |||||||||
| 3 | 4 | 7 | 6 | 3 | 2 | 5 | 1 | 7 | 0 | 2 | 4 | 1 | 6 | 8 | 9 | 3 | 9 | 5 | 2 | 1 | 4 | 0 | 8 | Community | |
| Species group A | |||||||||||||||||||||||||
| Casuarina cunninghamiana (*) | 5 | 4 | 1 | 2b | 1 | 5 | 4 | 5 | r | r | 100% | ||||||||||||||
| Suriana maritima (***) | + | + | + | r | 2b | 3 | 2a | 2a | 100% | ||||||||||||||||
| Enteropogon macrostachyus (***) | + | 2a | 1 | + | + | + | + | r | r | 88% | |||||||||||||||
| Evernia prunastri (***) | + | + | + | + | + | r | 63% | ||||||||||||||||||
| Brachiaria leersioides (***) | + | + | 25% | ||||||||||||||||||||||
| Ricinus communis (***) | r | r | + | 1 | + | r | + | + | + | + | + | 46% | |||||||||||||
| Rhynchosia sublobata (***) | + | + | + | + | r | r | + | + | + | 38% | |||||||||||||||
| Aneilema indehiscence (***) | + | + | + | r | + | r | + | + | 33% | ||||||||||||||||
| Dactyloctenium aegyptium (***) | + | + | + | r | + | + | + | r | 33% | ||||||||||||||||
| Polyporus tubaeformis (***) | + | + | + | + | + | + | + | 63% | |||||||||||||||||
| Species group B | |||||||||||||||||||||||||
| Melanthera biflora (***) | 1 | 5 | 50% | ||||||||||||||||||||||
| Panicum repens (***) | + | + | + | 75% | |||||||||||||||||||||
| Ipomoea pes-caprae (***) | r | r | r | 50% | |||||||||||||||||||||
| Scaevola sericea (***) | + | 25% | |||||||||||||||||||||||
| Kyllinga erecta (***) | + | 25% | |||||||||||||||||||||||
| Sesbania bispinosa (***) | 2a | 25% | |||||||||||||||||||||||
| Gymnosphoria heterophylla (***) | r | + | r | 50% | |||||||||||||||||||||
| Species group C | |||||||||||||||||||||||||
| Bougainvillea spectabilis (**) | r | r | r | 75% | |||||||||||||||||||||
| Chrysothrix sp. (***) | 1 | + | + | 50% | |||||||||||||||||||||
| Fomitopsis pinicola (***) | + | 25% | |||||||||||||||||||||||
| Bidens pilosa (**) | r | 25% | |||||||||||||||||||||||
| Panicum maximum (***) | + | r | 25% | ||||||||||||||||||||||
| Species group D | |||||||||||||||||||||||||
| Eugenia capensis (***) | + | 2a | 3 | 2b | 2a | 2a | 1 | 3 | 2b | 3 | 2a | 4 | 4 | 2b | 2b | 2a | 2a | 100% | |||||||
| Mimusops obtusifolia (***) | + | + | 3 | 3 | 2a | 1 | + | + | + | 2b | 2b | + | 1 | 2a | 2b | 1 | 1 | 1 | 100% | ||||||
| Pandanus kirkii (***) | r | 2a | 2a | 2b | 2b | 1 | + | 2a | + | 2b | 1 | 2a | 1 | 1 | + | + | + | + | 1 | 100% | |||||
| Secamone puntulata (***) | r | + | 1 | 2a | + | 2a | + | 1 | 1 | 1 | + | + | 1 | 1 | + | + | 1 | 100% | |||||||
| Cassytha filiformis (***) | + | 1 | 1 | + | 1 | + | 1 | 1 | + | 2a | + | + | + | 81% | |||||||||||
| Grewia glandulosa (***) | r | r | r | r | + | + | r | + | + | + | + | 2a | 1 | 2a | 1 | 1 | 1 | 81% | |||||||
| Maerua triphylla (***) | r | + | + | + | + | + | + | + | 1 | r | r | + | + | 75% | |||||||||||
| Synaptolepis kirkii (***) | r | + | + | + | r | + | + | + | 1 | + | + | + | + | 1 | 75% | ||||||||||
| Pisonia grandis (*****) | r | r | + | 3 | + | 2a | + | r | + | 44% | |||||||||||||||
| Ecbolium ligustrinum (***) | 1 | + | + | + | + | 1 | r | r | + | + | 2b | 1 | 63% | ||||||||||||
| Suregada zanibariensis (***) | r | + | + | r | + | + | + | + | + | + | + | + | + | 63% | |||||||||||
| Ficus scassellati (***) | r | + | + | 19% | |||||||||||||||||||||
| Afroligusticum linderi (***) | + | r | 13% | ||||||||||||||||||||||
| Hypoxidia sp. (***) | r | r | 13% | ||||||||||||||||||||||
| Adenia gummifera (***) | r | r | 13% | ||||||||||||||||||||||
| Species group E | |||||||||||||||||||||||||
| Scutia myrtina (***) | 2b | + | + | + | + | 44% | |||||||||||||||||||
| Pavetta stenosepala (***) | + | + | + | + | + | 44% | |||||||||||||||||||
| Bourreria petiolaris (***) | + | + | + | r | 33% | ||||||||||||||||||||
| Ficus lutea (***) | r | + | r | r | r | 44% | |||||||||||||||||||
| Psychotria psychotrioides (***) | r | + | + | r | 33% | ||||||||||||||||||||
| Ficus polita (***) | + | r | + | 22% | |||||||||||||||||||||
| Conyza newii (***) | r | 11% | |||||||||||||||||||||||
| Justicia capensis (***) | 1 | 11% | |||||||||||||||||||||||
| Auricularia auricular-judae (***) | + | 11% | |||||||||||||||||||||||
| Xanthoria sp. (***) | + | 11% | |||||||||||||||||||||||
| Ludia mauritiana (***) | + | 11% | |||||||||||||||||||||||
| Phaeotrametes decipiens (***) | + | 11% | |||||||||||||||||||||||
| Daldinia concentrica (***) | r | 11% | |||||||||||||||||||||||
| Solanum viarum (*) | r | 11% | |||||||||||||||||||||||
| Species group F | |||||||||||||||||||||||||
| Clerodendrum glabrum (***) | r | 1 | + | + | + | + | + | 1 | 100% | ||||||||||||||||
| Daedaleopsis confragosa (***) | r | + | + | + | r | + | + | + | + | 100% | |||||||||||||||
| Pavonia sp. (***) | r | + | 1 | + | + | 57% | |||||||||||||||||||
| Phyllanthus amarus (**) | + | + | r | + | 57% | ||||||||||||||||||||
| Polysphaeria parvifolia (***) | + | + | + | + | + | + | 57% | ||||||||||||||||||
| Abutilon sp. (***) | + | r | + | + | + | 57% | |||||||||||||||||||
| Ipomoea sp. (***) | + | r | 29% | ||||||||||||||||||||||
| Morinda citrifolia (****) | r | + | r | + | + | r | r | 29% | |||||||||||||||||
| Kyllinga platyphylla (***) | + | + | + | + | 17% | ||||||||||||||||||||
| Acacia auriculiformis (**) | r | r | r | r | 17% | ||||||||||||||||||||
| Ehretia amoena (***) | + | 14% | |||||||||||||||||||||||
| Parmelia sulcata (***) | + | 14% | |||||||||||||||||||||||
| Pycnoporus sp. (***) | + | 14% | |||||||||||||||||||||||
| Trametes hirsuta (***) | + | 14% | |||||||||||||||||||||||
| Boerhavia sp. (***) | + | 14% | |||||||||||||||||||||||
| Morinda citrifolia (****) | + | 14% | |||||||||||||||||||||||
| Coccinia grandis (***) | r | 14% | |||||||||||||||||||||||
| Pupalia lappacea (***) | r | + | + | r | 17% | ||||||||||||||||||||
| Species group G | |||||||||||||||||||||||||
| Sideroxylon inerme (***) | + | 2a | 1 | + | + | r | + | 2b | + | 1 | + | + | 2a | 1 | + | 1 | r | + | + | r | + | + | 92% | ||
| Capparis viminea var. orthacantha (***) | + | 1 | + | + | + | + | + | + | + | + | r | + | + | + | + | r | + | r | + | + | + | 88% | |||
| Cyphostemma adenocaule (***) | + | 1 | 1 | 1 | + | + | + | + | r | r | r | + | + | + | + | + | + | + | 1 | 1 | 83% | ||||
| Eragrostis ciliaris (***) | + | r | + | r | r | 21% | |||||||||||||||||||
| Terminalia catappa (****) | r | r | r | r | r | 21% | |||||||||||||||||||
| Dalechampia scandens (***) | + | + | 1 | 2a | 2a | 1 | 1 | + | + | + | + | + | + | + | + | + | 1 | + | + | + | + | 88% | |||
| Family | Genera | Species | Family | Genera | Species |
|---|---|---|---|---|---|
| Acanthaceae | 1 | 1 | Fomitopsidaceae | 1 | 1 |
| Amaranthaceae | 1 | 1 | Goodeniaceae | 1 | 1 |
| Apocynaceae | 1 | 1 | Malvaceae | 1 | 1 |
| Asclepiadaceae | 1 | 1 | Moraceae | 1 | 1 |
| Asteraceae | 2 | 2 | Myrtaceae | 1 | 1 |
| Capparaceae | 2 | 2 | Nyctaginaceae | 3 | 3 |
| Casuarinaceae | 1 | 1 | Pandanaceae | 1 | 1 |
| Celastraceae | 1 | 1 | Parmeliaceae | 1 | 1 |
| Chrysothricaceae | 1 | 1 | Poaceae | 5 | 6 |
| Combretaceae | 1 | 1 | Polyporaceae | 1 | 1 |
| Commelinaceae | 1 | 1 | Sapotaceae | 2 | 2 |
| Convolvulaceae | 1 | 1 | Surianaceae | 1 | 1 |
| Cyperaceae | 1 | 2 | Thymelaeaceae | 1 | 1 |
| Euphorbiaceae | 3 | 3 | Tiliaceae | 1 | 1 |
| Fabaceae | 3 | 3 | Vitaceae | 1 | 1 |
| Family | Genera | Species | Family | Genera | Species |
|---|---|---|---|---|---|
| Acanthaceae | 2 | 2 | Myrtaceae | 1 | 1 |
| Amaranthaceae | 1 | 1 | Nyctaginaceae | 3 | 3 |
| Apiaceae | 1 | 1 | Palmae | 1 | 1 |
| Apocynaceae | 1 | 1 | Pandanaceae | 1 | 1 |
| Araucariaceae | 1 | 1 | Parmeliaceae | 2 | 2 |
| Asteraceae | 1 | 1 | Passifloraceae | 1 | 1 |
| Boraginaceae | 2 | 2 | Phyllanthaceae | 1 | 1 |
| Capparaceae | 2 | 2 | Poaceae | 4 | 4 |
| Casuarinaceae | 1 | 1 | Polyporaceae | 5 | 5 |
| Celastraceae | 1 | 1 | Rhamnaceae | 1 | 1 |
| Chrysothricaceae | 1 | 1 | Rubiaceae | 4 | 4 |
| Combretaceae | 1 | 1 | Salicaceae | 1 | 1 |
| Commelinaceae | 1 | 1 | Sapotaceae | 2 | 2 |
| Convolvulaceae | 1 | 1 | Solanaceae | 1 | 1 |
| Curbitaceae | 1 | 1 | Teloschistaceae | 1 | 1 |
| Cyperaceae | 1 | 1 | Thymelaeaceae | 1 | 1 |
| Euphorbiaceae | 3 | 3 | Tiliaceae | 1 | 1 |
| Fabaceae | 2 | 2 | Verbenaceae | 1 | 1 |
| Lauraceae | 1 | 1 | Vitaceae | 1 | 1 |
| Malvaceae | 2 | 2 | Xylariaceae | 1 | 1 |
| Moraceae | 3 | 3 |
| Community 1 | Community 2 | ||
|---|---|---|---|
| Family | Cover (%) | Family | Cover (%) |
| Casuarinaceae | 41% | Myrtaceae | 23% |
| Asteraceae | 21% | Sapotaceae | 12% |
| Surianaceae | 10% | Pandanaceae | 5% |
| Euphorbiaceae | 3% | Nyctaginaceae | 3% |
| Pandanaceae | 2% | Apocynaceae | 8% |
| Fabaceae | 2% | Acanthaceae | 1% |
| Rhamnaceae | 1% | ||
| Plant Community | Shannon–Weiner Index (H) | Evenness (E) |
|---|---|---|
| 1 | 2.24 | 0.97 |
| 1.1 | 1.84 | 0.95 |
| 1.2 | 1.06 | 0.96 |
| 2 | 2.54 | 0.94 |
| 2.1 | 2.53 | 0.96 |
| 2.2 | 2.53 | 0.88 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barrett, A.; Bronkhorst, L.R.; Brown, L. A Contribution to the Study of the Flora and Vegetation of Mnemba Island, Zanzibar. Diversity 2024, 16, 579. https://doi.org/10.3390/d16090579
Barrett A, Bronkhorst LR, Brown L. A Contribution to the Study of the Flora and Vegetation of Mnemba Island, Zanzibar. Diversity. 2024; 16(9):579. https://doi.org/10.3390/d16090579
Chicago/Turabian StyleBarrett, Alan, Lorraine Raby Bronkhorst, and Leslie Brown. 2024. "A Contribution to the Study of the Flora and Vegetation of Mnemba Island, Zanzibar" Diversity 16, no. 9: 579. https://doi.org/10.3390/d16090579
APA StyleBarrett, A., Bronkhorst, L. R., & Brown, L. (2024). A Contribution to the Study of the Flora and Vegetation of Mnemba Island, Zanzibar. Diversity, 16(9), 579. https://doi.org/10.3390/d16090579







