Substrate Preference and Seasonal Distribution of Bdelloid Rotifers in Mosses in a Primary Forest in Thailand
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Sample Collection
2.2. Sample Preparation and Species Identification and Count
2.3. Data Analysis
2.3.1. Species Richness and Diversity
2.3.2. Species Composition
2.3.3. Species–Environment Relationship
2.3.4. Molecular Analysis
2.3.5. Species Delimitation
3. Results
3.1. Molecular Taxonomy
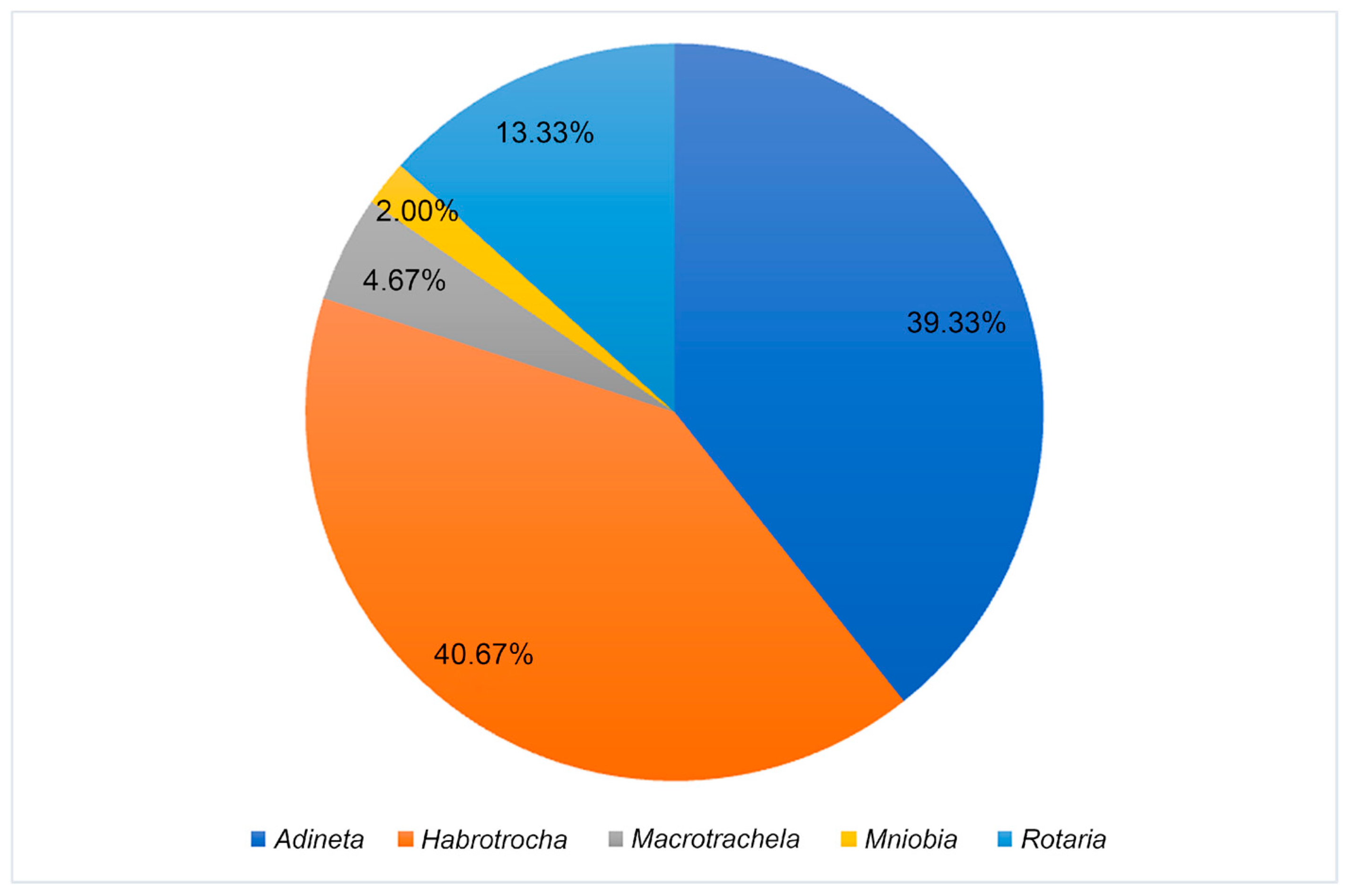
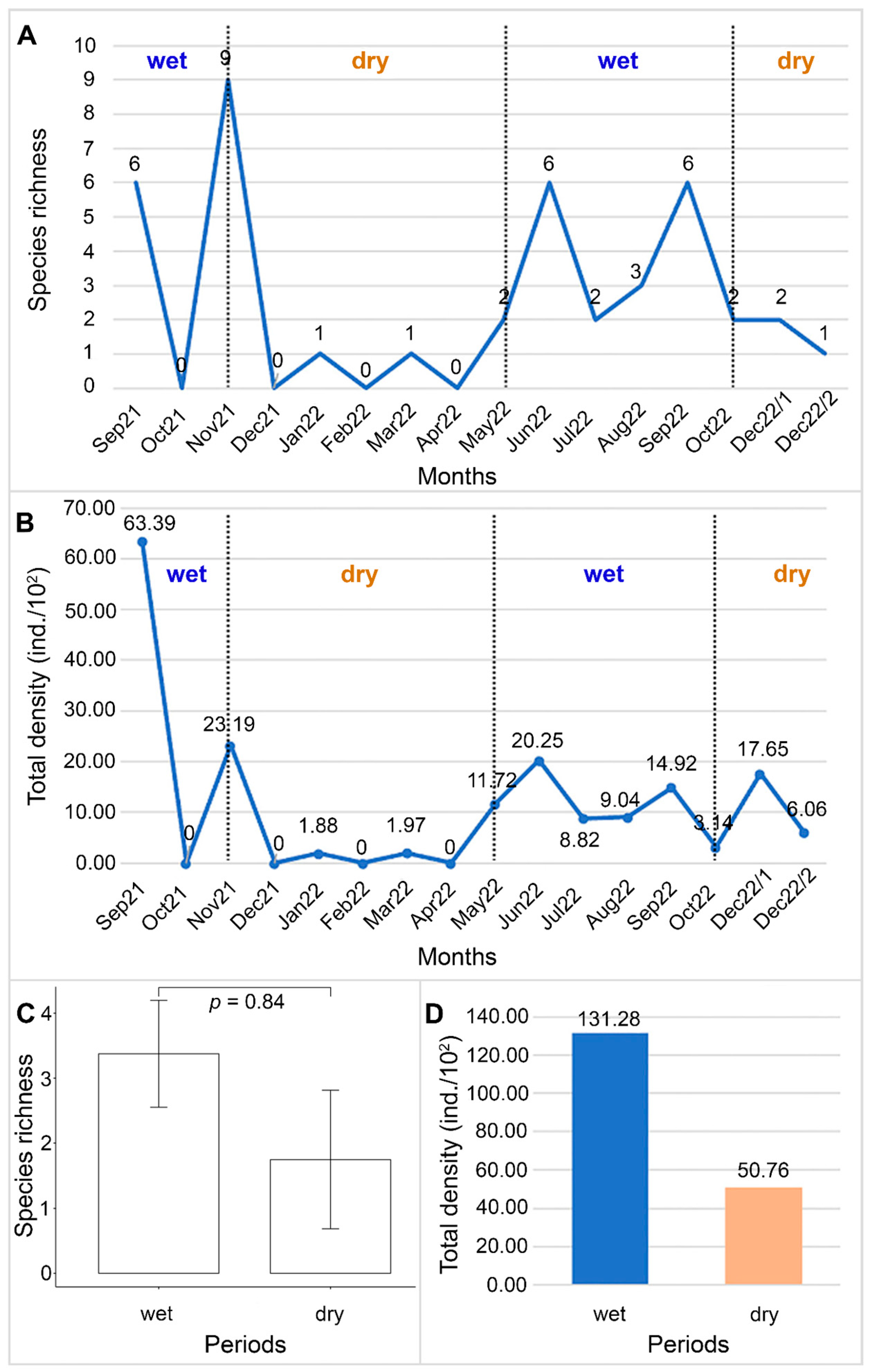
3.2. Species Diversity
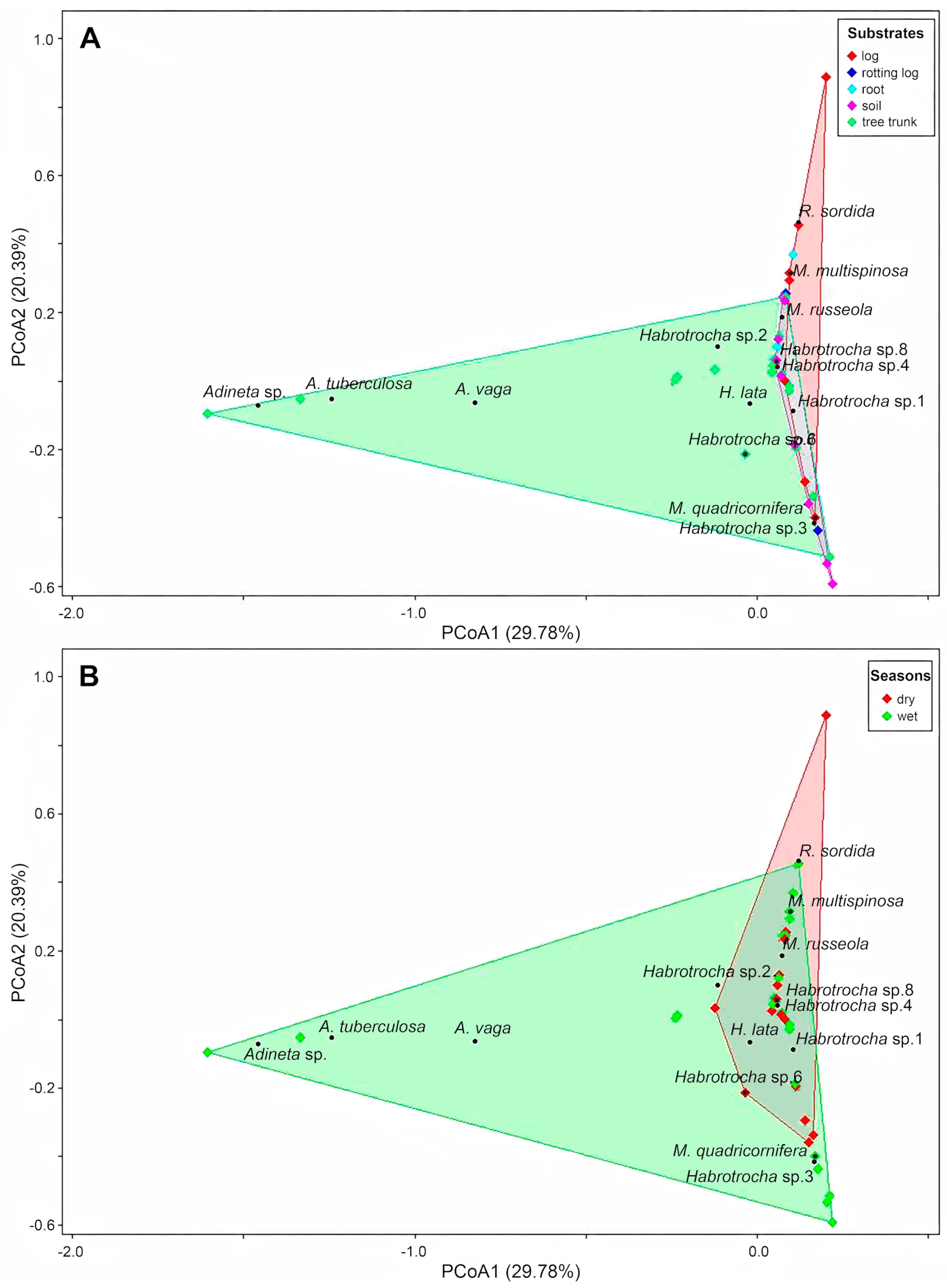
3.3. Bdelloid Rotifer Community in Substrate Type of Mosses
3.4. Distribution of Bdelloid Rotifers in Each Period
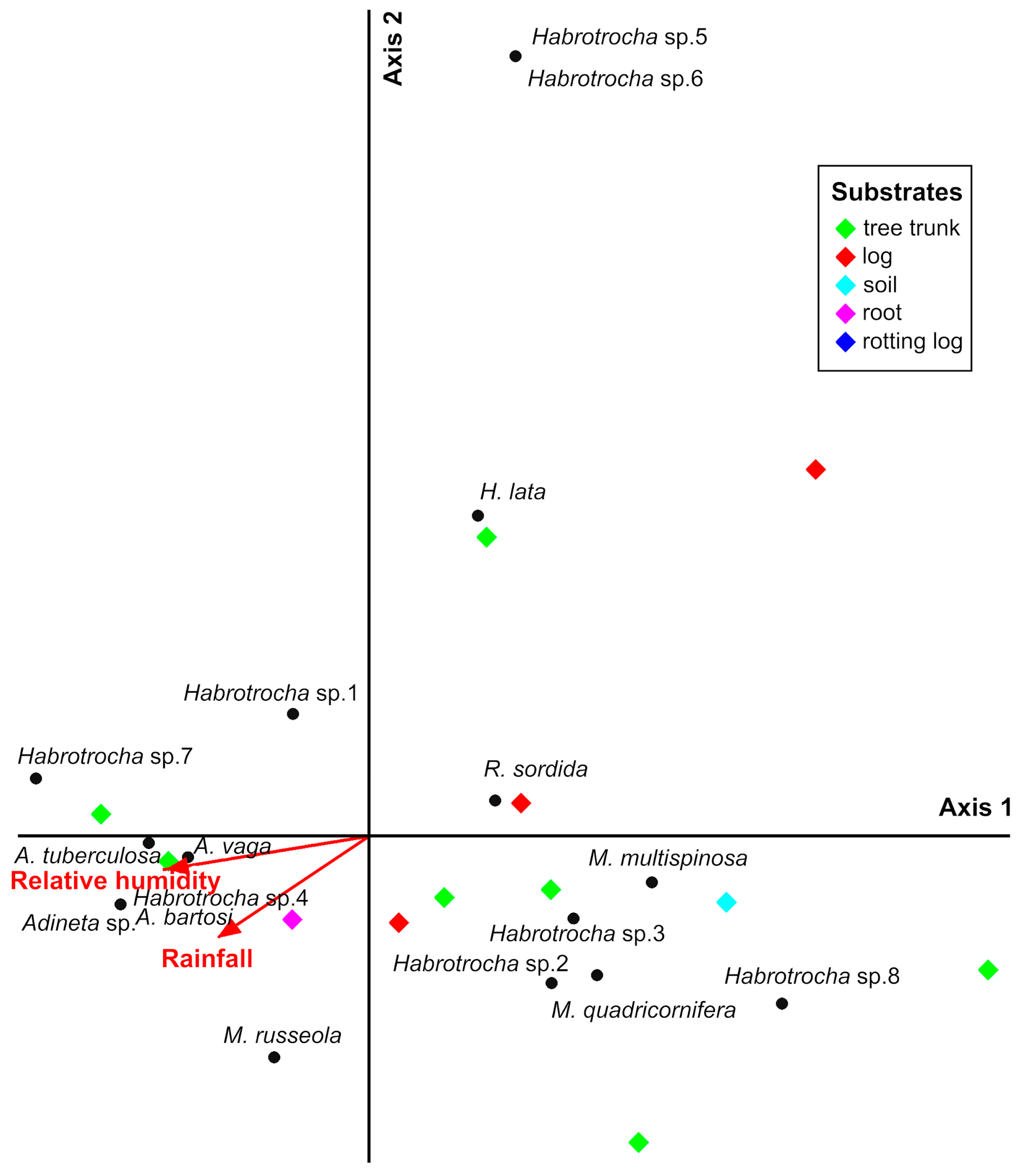
3.5. Influences of Environmental Variables on Bdelloid Rotifer
4. Discussion
4.1. Species Diversity
4.2. Ecological and Temporal Distributions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Duan, X.; Wang, Z.; Tian, S. Effect of streambed substrate on macroinvertebrate biodiversity. Front. Environ. Sci. Eng. China 2008, 2, 122–128. [Google Scholar] [CrossRef]
- Pereira, T.d.S.; Pio, J.F.G.; Calor, A.R.; Copatti, C.E. Can the substrate influence the distribution and composition of benthic macroinvertebrates in streams in northeastern Brazil? Limnologica 2017, 63, 27–30. [Google Scholar] [CrossRef]
- Meradi, K.; Bounar, R.; Benzina, I.; Meradi, S.; Bachir, A.S.; Céréghino, R. How do substrate types affect the seasonal richness and functional feeding groups variation of benthic insects in an arid region (northeastern Algeria)? Biologia 2024, 79, 1749–1759. [Google Scholar] [CrossRef]
- Minshall, G.W.; Petersen, R.C.; Cummins, K.W. Interbiome comparison of stream ecosystems. In Stream Ecology: Application and Testing of General Ecological Theory; Barnes, J.R., Minshall, G.W., Eds.; Springer: New York, NY, USA, 1983; pp. 126–143. [Google Scholar]
- Kaya, M.; Erdoğan, S. Testing the habitat selectivity of bdelloid rotifers in a restricted area. Turk. J. Zool. 2015, 39, 1132–1141. [Google Scholar] [CrossRef]
- Gething, K.J.; Ripley, M.C.; Mathers, K.L.; Chadd, R.P.; Wood, P.J. The influence of substrate type on macroinvertebrate assemblages within agricultural drainage ditches. Hydrobiologia 2020, 847, 4273–4284. [Google Scholar] [CrossRef]
- Stark, J.D.; Maxted, J.R. The influence of substrate on the diversity of stream invertebrates. N. Z. J. Mar. Freshw. Res. 2006, 40, 23–32. [Google Scholar]
- Allan, J.D. Stream Ecology: Structure and Function of Running Waters; Springer: Dordrecht, The Netherlands, 1995; p. 388. [Google Scholar]
- Wallace, J.B.; Eggert, S.L.; Meyer, J.L.; Webster, J.R. Effects of resource limitation on a detrital-based ecosystem. Ecol. Monogr. 1999, 69, 409–442. [Google Scholar] [CrossRef]
- Fisher, S.G.; Grimm, N.B.; Marti, E. Hydrochemical factors influencing stream invertebrates. J. N. Am. Benthol. Soc. 1982, 1, 181–195. [Google Scholar]
- Mavromati, E.; Kemitzoglou, D.; Tsiaoussi, V. Does littoral substrate affect macroinvertebrate assemblages in Mediterranean lakes? Aquat. Ecol. 2023, 57, 667–679. [Google Scholar] [CrossRef]
- McLay, C.L.; McDonald, R.I.; Whitfield, A.K. Substrate characteristics and their effects on aquatic invertebrate communities: A global review. Freshw. Biol. 2021, 66, 1002–1015. [Google Scholar]
- Sabo, J.L.; Soykan, C.U.; Keller, A. Functional roles of leaf litter detritus in terrestrial food webs. In Theoretical Ecology Series, Dynamic Food Webs; Ruiter, P.D., Wolters, V., Moore, J.C., Melville-Smith, K., Eds.; Academic Press: Cambridge, MA, USA, 2005; Volume 3, pp. 211–222. [Google Scholar]
- Hou, J.; Cao, R.; Li, F.; Wang, Z.; Li, X.; Wang, Q.; Yang, W. The interactions between soil invertebrates and microbes mediate litter decomposition in the rainy zone of western China. Plant Soil 2024, 501, 491–508. [Google Scholar] [CrossRef]
- Zhao, Y.-Y.; Li, Z.-T.; Xu, T.; Lou, A.-R. Leaf litter decomposition characteristics and controlling factors across two contrasting forest types. J. Plant Ecol. 2022, 15, 1285–1301. [Google Scholar] [CrossRef]
- Frouz, J.; Livečková, M.; Albrechtová, J.; Chroňáková, A.; Cajthaml, T.; Pižl, V.; Háněl, L.; Starý, J.; Baldrian, P.; Lhotáková, Z.; et al. Is the effect of trees on soil properties mediated by soil fauna? A case study from post-mining sites. For. Ecol. Manag. 2013, 309, 87–95. [Google Scholar] [CrossRef]
- Averill, C.; Waring, B. Nitrogen limitation of decomposition and decay: How can it occur? Glob. Change Biol. 2018, 24, 1417–1427. [Google Scholar] [CrossRef]
- Li, M.; Gao, F.; Zhu, L.; Li, J.; Xiang, J.; Xi, Y.; Xiang, X. Geographic origin shapes the adaptive divergences of Rotaria rotatoria (Rotifera, Bdelloidea) to thermal stress: Insights from ecology and transcriptomics. Ecol. Evol. 2024, 14, e11307. [Google Scholar] [CrossRef]
- Ricci, C.; Melone, G.; Santo, N.; Caprioli, M. Morphological response of a bdelloid rotifer to desiccation. J. Morphol. 2003, 257, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Nowell, R.W.; Almeida, P.; Wilson, C.G.; Smith, T.P.; Fontaneto, D.; Crisp, A.; Micklem, G.; Tunnacliffe, A.; Boschetti, C.; Barraclough, T.G. Comparative genomics of bdelloid rotifers: Insights from desiccating and nondesiccating species. PLoS Biol. 2018, 16, e2004830. [Google Scholar] [CrossRef] [PubMed]
- Gladyshev, E.; Meselson, M. Extreme resistance of bdelloid rotifers to ionizing radiation. Proc. Natl. Acad. Sci. USA 2008, 105, 5139–5144. [Google Scholar] [CrossRef]
- Örstan, A. Factors affecting long-term survival of dry bdelloid rotifers: A preliminary study. Hydrobiologia 1998, 387, 327–331. [Google Scholar] [CrossRef]
- Pejler, B.; Bērziņš, B. On choice of substrate and habitat in bdelzoid rotifers. Hydrobiologia 1993, 255, 333–338. [Google Scholar] [CrossRef]
- Hamil, S.; Chikha, C.; Alili, M.; Arab, S.; Essahran, W.; Baha, M.; Arab, A. Testing the Habitat Selectivity of Bdelloid Rotifers in a Humid Area; National Park of Chrea (Algeria). In Recent Advances in Environmental Science from the Euro-Mediterranean and Surrounding Regions, 3rd ed.; Ksibi, M., Negm, A., Hentati, O., Ghorbal, A., Sousa, A., Rodrigo-Comino, J., Panda, S., Velho, J.L., El-Kenawy, A.M., Perilli, N., Eds.; Advances in Science, Technology & Innovation; Springer: Cham, Switzerland, 2024; pp. 715–718. [Google Scholar]
- Donner, J. Ordnung Bdelloidea (Rotifera, Rädertiere); Akademie Verlag: Berlin, Germany, 1965. [Google Scholar]
- Ricci, C.; Melone, G. Key to the identification of the genera of bdelloid rotifers. Hydrobiologia 2000, 418, 73–80. [Google Scholar] [CrossRef]
- Segers, H. Annotated checklist of the rotifers (Phylum Rotifera), with notes on nomenclature, taxonomy and distribution. Zootaxa 2007, 1564, 1–104. [Google Scholar] [CrossRef]
- Sa-Ardrit, P.; Pholpunthin, P.; Segers, H. A checklist of the freshwater rotifer fauna of Thailand (Rotifera, Monogononta, Bdelloidea). J. Limnol. 2013, 72, 361–375. [Google Scholar] [CrossRef][Green Version]
- Montero-Pau, J.; Gómez, A.; Muñoz, J. Application of an inexpensive and high-throughput genomic DNA extraction method for the molecular ecology of zooplanktonic diapausing eggs. Limnol. Oceanogr. Methods 2008, 6, 218–222. [Google Scholar] [CrossRef]
- Folmer, O.; Black, M.B.; Hoeh, W.; Lutz, R.; Vrijenhoek, R.C. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar] [PubMed]
- RStudio Team. RStudio: Integrated Development for R; RStudio, PBC: Boston, MA, USA, 2022; Available online: http://www.rstudio.com/ (accessed on 24 February 2025).
- Bloom, S.A. Similarity indices in community studies: Potential Pitfalls. Mar. Ecol. Prog. Ser. 1981, 5, 125–128. [Google Scholar] [CrossRef]
- McCune, B.; Mefford, M.J. PC-ORD. Multivariate Analysis of Ecological Data, Version 7.0 for Windows; MjM Software Design: Gleneden Beach, OR, USA, 2016. [Google Scholar]
- ter Braak, C.J.F.; Verdonschot, P.F.M. Canonical correspondence analysis and related multivariate methods in aquatic ecology. Aquat. Sci. 1995, 57, 255–289. [Google Scholar] [CrossRef]
- Technelysium Pty Ltd. Chromas Version 2.6.6; Technelysium Pty Ltd.: South Brisbane, QLD, Australia, 2018. [Google Scholar]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Maddison, W.P.; Maddison, D.R. Mesquite: A Modular System for Evolutionary Analysis, version 3.70; Mesquite Software, Inc.: Austin, TX, USA, 2021. [Google Scholar]
- Villesen, P. FaBox: An online toolbox for fasta sequences. Mol. Ecol. Notes 2007, 7, 965–968. [Google Scholar] [CrossRef]
- Miller, M.A.; Pfeiffer, W.; Schwartz, T. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In Proceedings of the Gateway Computing Environments Workshop (GCE), New Orleans, LA, USA, 14 November 2010; pp. 1–8. [Google Scholar]
- Drummond, A.J.; Rambaut, A. BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol. Biol. 2007, 7, 214. [Google Scholar] [CrossRef] [PubMed]
- Drummond, A.J.; Suchard, M.A.; Xie, D.; Rambaut, A. Bayesian Phylogenetics with BEAUti and the BEAST 1.7. Mol. Biol. Evol. 2012, 29, 1969–1973. [Google Scholar] [CrossRef] [PubMed]
- Rambaut, A.; Drummond, A.J.; Xie, D.; Baele, G.; Suchard, M.A. Posterior summarization in Bayesian phylogenetics using Tracer 1.7. Syst. Biol. 2018, 67, 901–904. [Google Scholar] [CrossRef] [PubMed]
- Puillandre, N.; Lambert, A.; Brouillet, S.; Achaz, G. ABGD, Automatic Barcode Gap Discovery for primary species delimitation. Mol. Ecol. 2012, 21, 1864–1877. [Google Scholar] [CrossRef]
- Zhang, J.; Kapli, P.; Pavlidis, P.; Stamatakis, A. A general species delimitation method with applications to phylogenetic placements. Bioinformatics 2013, 29, 2869–2876. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, T.; Barraclough, T.G. Delimiting species using single-locus data and the generalized mixed yule coalescent approach: A revised method and evaluation on simulated data sets. Syst. Biol. 2013, 62, 707–724. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Jersabek, C.D.; Leitner, M.F. Rotifer World Catalog. World Wide Web Electronic Publication. Available online: http://www.rotifera.hausdernatur.at/ (accessed on 9 February 2024).
- Maiphae, S. Species Diversity of Meiofauna (Rotifers, Cladocerans and Copepods) in Peatswamps, Thailand; Kasetsart University: Bangkok, Thailand, 2017; pp. 1–44. [Google Scholar]
- Jaturapruek, R.; Fontaneto, D.; Meksuwan, P.; Pholpunthin, P.; Maiphae, S. Planktonic and periphytic bdelloid rotifers from Thailand reveal a species assemblage with a combination of cosmopolitan and tropical species. Syst. Biodivers. 2018, 16, 128–141. [Google Scholar] [CrossRef]
- Jaturapruek, R.; Fontaneto, D.; Mammola, S.; Maiphae, S. Potential niche displacement in species of aquatic bdelloid rotifers between temperate and tropical areas. Hydrobiologia 2021, 848, 4903–4918. [Google Scholar] [CrossRef]
- Devetter, M. Spatiotemporal dynamics of soil rotifers in a South-Bohemian beech forest. Pesq. Agropec. Bras. 2009, 44, 1027–1032. [Google Scholar] [CrossRef]
- Jattupan, S.; Jaturapruek, R.; Sa-ardrit, P.; Inuthai, J.; Ngernsaengsaruay, C.; Maiphae, S. Diversity and habitat preferences of bdelloid rotifers in mosses and liverworts from beach forest along sand dunes in Thailand. PeerJ 2024, 12, e18721. [Google Scholar] [CrossRef] [PubMed]
- Ricci, C. Anhydrobiotic capabilities of bdelloid rotifers. Hydrobiologia 1998, 387, 321–326. [Google Scholar] [CrossRef]
- Eyres, I.; Boschetti, C.; Crisp, A.; Smith, T.P.; Fontaneto, D.; Tunnacliffe, A.; Barraclough, T.G. Horizontal gene transfer in bdelloid rotifers is ancient, ongoing and more frequent in species from desiccating habitats. BMC Biol. 2015, 13, 90. [Google Scholar] [CrossRef]
- Hirschfelder, A.; Koste, W.; Zucchi, H. Bdelloid rotifers in aerophytic mosses: Influence of habitat structure and habitat age on species composition. Hydrobiologia 1993, 255, 343–344. [Google Scholar] [CrossRef]
- Ricci, C. Ecology of bdelloids: How to be successful. Hydrobiologia 1987, 147, 117–127. [Google Scholar] [CrossRef]
- Fontaneto, D.; Bunnefeld, N.; Westberg, M. Long-Term Survival of Microscopic Animals Under Desiccation Is Not So Long. Astrobiology 2012, 12, 863–869. [Google Scholar] [CrossRef]
- Lukashanets, D.A. Fauna and taxonomy of the subclass Bdelloidea Hudson (Class Eurotatoria De Ridder; phylum Rotifera Cuvier) in Belarus, the Eastern Europe region. Zool. Ecol. 2018, 28, 36–45. [Google Scholar] [CrossRef]
- Wang, W.; Yang, Y.; Cui, Z.; Chen, M.; Ma, X.; Wang, Q. High diversity and strong habitat preference of bdelloid rotifers in the moss and leaf litter from a small area of urban plain and adjacent hill in China. Biodivers. Conserv. 2023, 32, 2769–2789. [Google Scholar] [CrossRef]
- Kaya, M. Terrestrial bdelloid rotifers from Erzurum (eastern part of Turkey). Turk. J. Zool. 2013, 37, 413–418. [Google Scholar] [CrossRef]
- Fontaneto, D.; Ricci, C. Spatial gradients in species diversity of microscopic animals: The case of bdelloid rotifers at high altitude. J. Biogeogr. 2006, 33, 1305–1313. [Google Scholar] [CrossRef]
- Jaturapruek, R.; Fontaneto, D.; Maiphae, S. The influence of environmental variables on bdelloid rotifers of the genus Rotaria in Thailand. J. Trop. Ecol. 2020, 36, 267–274. [Google Scholar] [CrossRef]
- Verberk, W. Explaining general patterns in species abundance and distributions. Nat. Educ. Knowl. 2011, 3, 38. [Google Scholar]
- Örstan, A. Desiccation survival of the eggs of the rotifer Adleta vaga (Davis, 1873). Hydrobiologia 1995, 313, 373–375. [Google Scholar] [CrossRef]
- Hespeels, B.; Penninckx, S.; Cornet, V.; Bruneau, L.; Bopp, C.; Baumlé, V.; Redivo, B.; Heuskin, A.-C.; Moeller, R.; Fujimori, A.; et al. Iron Ladies—How Desiccated Asexual Rotifer Adineta vaga Deal With X-Rays and Heavy Ions? Front. Microbiol. 2020, 11, 1792. [Google Scholar] [CrossRef] [PubMed]
- Hespeels, B.; Fontaneto, D.; Cornet, V.; Penninckx, S.; Berthe, J.; Bruneau, L.; Larrick, J.W.; Rapport, E.; Bailly, J.; Debortoli, N.; et al. Back to the roots, desiccation and radiation resistances are ancestral characters in bdelloid rotifers. BMC Biol. 2023, 21, 72. [Google Scholar] [CrossRef]


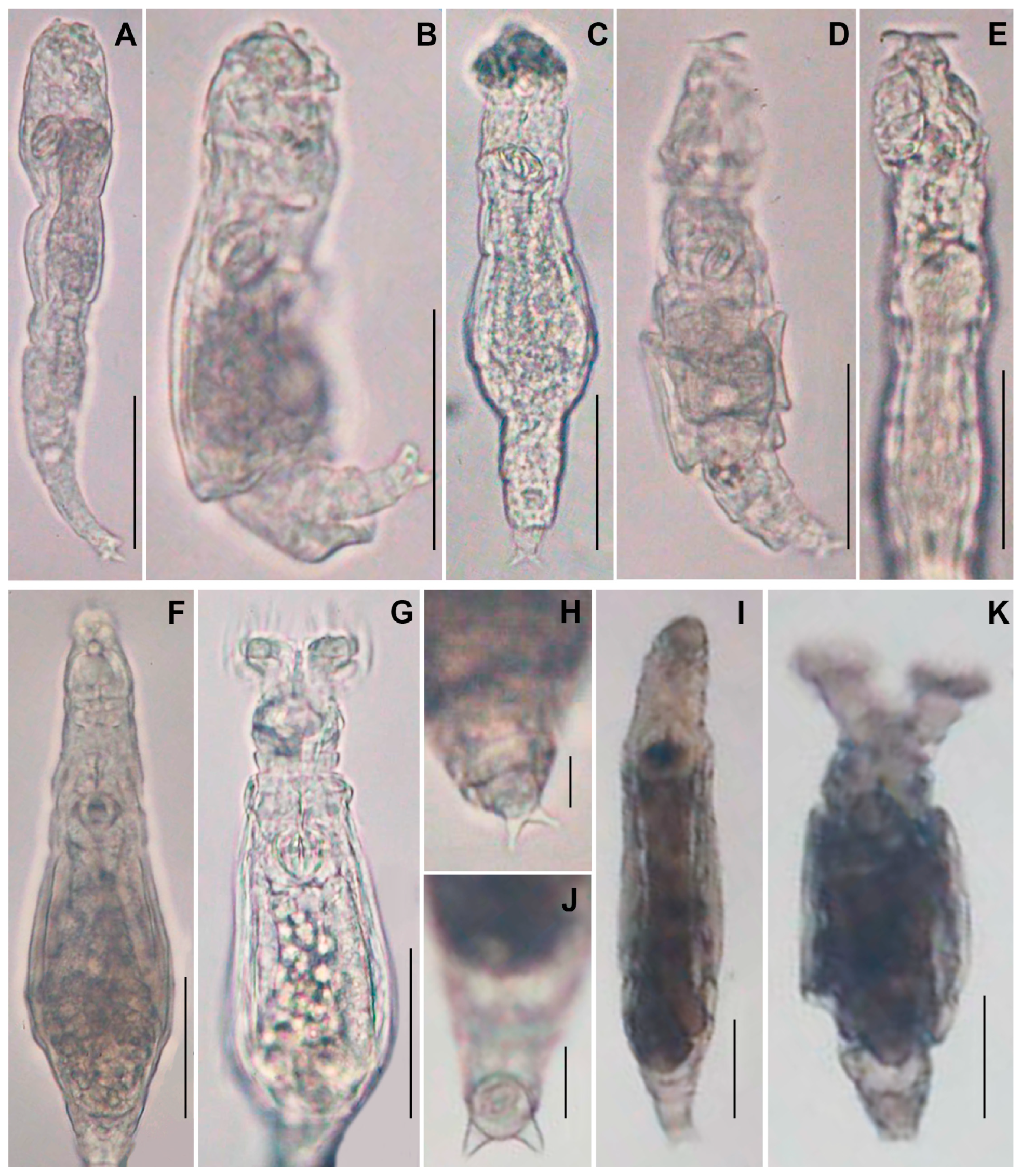

| Code | GenBank Accession Number | Species | Country | Substrates of Moss |
|---|---|---|---|---|
| SP120_MS | Habrotrocha sp.8 | Thailand | soil | |
| SP123_ML | Macrotrachela quadricornifera | Thailand | log | |
| SP130_MT | Habrotrocha lata | Thailand | tree trunk | |
| SP138_ML | Rotaria sordida | Thailand | log | |
| SP147_ML | Rotaria sordida | Thailand | log | |
| SP148_MT | Habrotrocha sp.1 | Thailand | tree trunk | |
| SP149_MT | Habrotrocha sp.2 | Thailand | tree trunk | |
| SP155_MRO | Rotaria sordida | Thailand | root | |
| SP157_MRO | Mniobia russeola | Thailand | root | |
| SP158_MT | Mniobia russeola | Thailand | tree trunk | |
| SP159_MT | Mniobia russeola | Thailand | tree trunk | |
| SP176_MRL | Habrotrocha sp.7 | Thailand | rotting log | |
| SP180_MT | Habrotrocha sp.1 | Thailand | tree trunk | |
| SP181_MT | unidentified species | Thailand | tree trunk | |
| SP182_MT | Habrotrocha sp.1 | Thailand | tree trunk | |
| SP190_MS | Rotaria sordida | Thailand | soil | |
| SP191_MS | Habrotrocha sp.2 | Thailand | soil | |
| SP193_MT | Rotaria sordida | Thailand | tree trunk | |
| SP196_MS | Rotaria sordida | Thailand | soil | |
| SP198_ML | Rotaria sordida | Thailand | log | |
| SP199_ML | Rotaria sordida | Thailand | log | |
| SP200_ML | Rotaria sordida | Thailand | log | |
| SP201_ML | Rotaria sordida | Thailand | log | |
| SP202_ML | Rotaria sordida | Thailand | log | |
| SP203_ML | Rotaria sordida | Thailand | log | |
| SP204_ML | Rotaria sordida | Thailand | log | |
| SP205_ML | Macrotrachela multispinosa | Thailand | log | |
| SP206_ML | Rotaria sordida | Thailand | log | |
| SP207_ML | Rotaria sordida | Thailand | log | |
| SP209_MS | Habrotrocha sp.8 | Thailand | soil | |
| SP210_MS | Habrotrocha sp.8 | Thailand | soil | |
| SP211_MS | Habrotrocha sp.8 | Thailand | soil | |
| M. quadricornifera_EF650621_IT | EF650621 | Macrotrachela quadricornifera | Italy | |
| M. quadricornifera_EF650557_UK | EF650557 | Macrotrachela quadricornifera | UK | |
| M. quadricornifera_EU751278_UK | EU751278 | Macrotrachela quadricornifera | UK | |
| M. quadricornifera_FJ426419_FR | FJ426419 | Macrotrachela quadricornifera | France | |
| M. quadricornifera_MH251758 | MH251758 | Macrotrachela quadricornifera | Switzerland | |
| M. quadricornifera_FJ426414_TU | FJ426414 | Macrotrachela quadricornifera | Turkey | |
| M. multispinosa_EF650498_AU | EF650498 | Macrotrachela multispinosa | Australia | |
| M. multispinosa_EU751094_UK | EU751094 | Macrotrachela multispinosa | UK | |
| M. multispinosa_EU751089_UK | EU751089 | Macrotrachela multispinosa | UK | |
| M. multispinosa_EU751099_UK | EU751099 | Macrotrachela multispinosa | UK | |
| M. multispinosa_EF650497_IT | EF650497 | Macrotrachela multispinosa | Italy | |
| M. multispinosa_EU751100_UK | EU751100 | Macrotrachela multispinosa | UK | |
| R. sordida_DQ656852_IT | DQ656852 | Rotaria sordida | Italy | |
| R. sordida_EF173268_IT | EF173268 | Rotaria sordida | Italy | |
| R. sordida_EF173269_IT | EF173269 | Rotaria sordida | Italy | |
| R. sordida_JQ309635_A783RS1 | JQ309635 | Rotaria sordida | UK | |
| R. sordida_DQ656854_CH | DQ656854 | Rotaria sordida | Switzerland | |
| R. sordida_EU751109_UK | EU751109 | Rotaria sordida | UK | |
| R. sordida_EU076899_TW | EU076899 | Rotaria sordida | Taiwan | |
| R. sordida_EU751110_UK | EU751110 | Rotaria sordida | UK | |
| R. sordida_DQ656855_FR | DQ656855 | Rotaria sordida | France | |
| R. sordida_EU751121_TU | EU751121 | Rotaria sordida | Turkey | |
| R. sordida_EU751162_TU | EU751162 | Rotaria sordida | Turkey | |
| Mni. russeola_KM043198_A953 | KM043198 | Mniobia russeola | - | |
| Mni. russeola_EF650513_IT | EF650513 | Mniobia russeola | Italy | |
| Mni. magna_KM043197_645a | KM043197 | Mniobia magna | - | |
| Mni. incrassata_EF650571_AN | EF650571 | Mniobia incrassata | Antarctica | |
| Mni. incrassata_EF650573_AN | EF650573 | Mniobia incrassata | Antarctica | |
| Mni. incrassata_EF650576_AN | EF650576 | Mniobia incrassata | Antarctica | |
| H. elusa_KM043193_HB001 | KM043193 | Habrotrocha elusa | - | |
| H. elusa_EF650487_UK | EF650487 | Habrotrocha elusa | UK | |
| H. ligula_KM043194_HB003 | KM043194 | Habrotrocha ligula | - | |
| H. constricta_KM043192_HB005 | KM043192 | Habrotrocha constricta | - | |
| H. bidens_EF650488_UK | EF650488 | Habrotrocha bidens | UK | |
| H. antarctica_MF503418_R6 | MF503418 | Habrotrocha antarctica | Antarctica | |
| H. antarctica_MF503419_R7 | MF503419 | Habrotrocha antarctica | Antarctica | |
| H. antarctica_MF503420_R8 | MF503420 | Habrotrocha antarctica | Antarctica | |
| H. lata_MH251808_D05 | MH251808 | Habrotrocha lata | Switzerland | |
| H. lata_MH251811_D06 | MH251811 | Habrotrocha lata | Switzerland | |
| Lecane bulla_JX216667 | JX216667 | Lecane bulla | Mexico |
| Taxa | Haplotype Number | Within Taxa | Between Taxa | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | |||
| 1. Mniobia russeola | 5 | 0.15 | ||||||||||||||||
| 2. Mniobia magna | 1 | n/c | 0.21 | |||||||||||||||
| 3. Mniobia incrassata | 2 | 0.00 | 0.23 | 0.24 | ||||||||||||||
| 4. Habrotrocha sp.1 | 4 | 0.00 | 0.23 | 0.25 | 0.15 | |||||||||||||
| 5. Macrotrachela multispinosa | 7 | 0.12 | 0.25 | 0.27 | 0.19 | 0.15 | ||||||||||||
| 6. Macrotrachela quadricornifera | 7 | 0.12 | 0.24 | 0.24 | 0.17 | 0.14 | 0.15 | |||||||||||
| 7. Habrotrocha antarctica | 3 | 0.00 | 0.23 | 0.25 | 0.18 | 0.16 | 0.16 | 0.15 | ||||||||||
| 8. Habrotrocha elusa | 2 | 0.13 | 0.22 | 0.26 | 0.17 | 0.15 | 0.16 | 0.15 | 0.11 | |||||||||
| 9. Habrotrocha lata | 3 | 0.14 | 0.22 | 0.24 | 0.17 | 0.16 | 0.17 | 0.15 | 0.15 | 0.14 | ||||||||
| 10. Habrotrocha ligula | 1 | n/c | 0.23 | 0.26 | 0.17 | 0.14 | 0.17 | 0.15 | 0.15 | 0.13 | 0.14 | |||||||
| 11. Habrotrocha sp.2 | 1 | n/c | 0.27 | 0.29 | 0.19 | 0.17 | 0.19 | 0.17 | 0.16 | 0.17 | 0.17 | 0.18 | ||||||
| 12. Habrotrocha sp.7 | 1 | n/c | 0.24 | 0.28 | 0.17 | 0.12 | 0.16 | 0.14 | 0.14 | 0.14 | 0.17 | 0.16 | 0.15 | |||||
| 13. Habrotrocha constricta | 1 | n/c | 0.25 | 0.29 | 0.19 | 0.12 | 0.16 | 0.14 | 0.13 | 0.15 | 0.15 | 0.13 | 0.16 | 0.13 | ||||
| 14. Habrotrocha sp.8 | 4 | 0.03 | 0.26 | 0.26 | 0.19 | 0.19 | 0.22 | 0.17 | 0.18 | 0.19 | 0.18 | 0.20 | 0.18 | 0.16 | 0.16 | |||
| 15. Habrotrocha bidens | 1 | n/c | 0.25 | 0.26 | 0.18 | 0.16 | 0.19 | 0.16 | 0.14 | 0.16 | 0.15 | 0.16 | 0.18 | 0.16 | 0.14 | 0.16 | ||
| 16. Rotaria sordida | 25 | 0.13 | 0.26 | 0.28 | 0.20 | 0.18 | 0.20 | 0.18 | 0.19 | 0.18 | 0.18 | 0.18 | 0.20 | 0.19 | 0.16 | 0.21 | 0.19 | |
| Bdelloid Species | Substrates | Periods |
|---|---|---|
| Adineta bartosi Wulfert 1960 * | TT | wet |
| Adineta tuberculosa Janson, 1893 * | TT | wet, dry |
| Adineta vaga (Davis, 1873) | TT | wet, dry |
| Adineta sp. | TT | wet |
| Habrotrocha lata (Bryce, 1892) * | TT, S | wet, dry |
| Habrotrocha sp.1 | TT, L, S | wet, dry |
| Habrotrocha sp.2 | TT, S | wet |
| Habrotrocha sp.3 | TT, L, S, RL | wet, dry |
| Habrotrocha sp.4 | TT | wet |
| Habrotrocha sp.5 | TT | wet, dry |
| Habrotrocha sp.6 | TT | dry |
| Habrotrocha sp.7 | RL | wet |
| Habrotrocha sp.8 | S | wet, dry |
| Macrotrachela multispinosa Thompson, 1892 | TT, L, S, RL | wet, dry |
| Macrotrachela quadricornifera Milne, 1886 | L | wet |
| Mniobia russeola (Zelinka, 1891) * | TT, RL | wet |
| Rotaria sordida (Western, 1893) | TT, L, S, R, RL | wet, dry |
| Species Richness | Shannon Diversity Index | |
|---|---|---|
| Substrates | ||
| tree trunk | 14 (1.72 ± 0.83) | 2.15 |
| log | 5 (1.43 ± 0.53) | 1.14 |
| soil | 7 (1.18 ± 0.40) | 1.45 |
| root | 3 (1.50 ± 0.71) | 1.04 |
| rotting log | 3 (1.50 ± 0.71) | 0.99 |
| Seasons | ||
| wet period | 16 (3.86 ± 4.14) | 2.29 |
| dry period | 10 (3.50 ± 14.33) | 1.91 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pokpongmongkol, P.; Jaturapruek, R.; Sa-ardrit, P.; Maiphae, S. Substrate Preference and Seasonal Distribution of Bdelloid Rotifers in Mosses in a Primary Forest in Thailand. Diversity 2025, 17, 171. https://doi.org/10.3390/d17030171
Pokpongmongkol P, Jaturapruek R, Sa-ardrit P, Maiphae S. Substrate Preference and Seasonal Distribution of Bdelloid Rotifers in Mosses in a Primary Forest in Thailand. Diversity. 2025; 17(3):171. https://doi.org/10.3390/d17030171
Chicago/Turabian StylePokpongmongkol, Poomipat, Rapeepan Jaturapruek, Phannee Sa-ardrit, and Supiyanit Maiphae. 2025. "Substrate Preference and Seasonal Distribution of Bdelloid Rotifers in Mosses in a Primary Forest in Thailand" Diversity 17, no. 3: 171. https://doi.org/10.3390/d17030171
APA StylePokpongmongkol, P., Jaturapruek, R., Sa-ardrit, P., & Maiphae, S. (2025). Substrate Preference and Seasonal Distribution of Bdelloid Rotifers in Mosses in a Primary Forest in Thailand. Diversity, 17(3), 171. https://doi.org/10.3390/d17030171






