Abstract
Lianas play a crucial role in forest species diversity, biomass, nutrient cycling, and vegetation restoration. To date, numerous studies on the stoichiometric characteristics of liana leaves have predominantly focused on temperate, subtropical, and tropical forests. However, there remains a lack of comprehensive understanding regarding the nutrient concentrations and their stoichiometric characteristics in lianas growing in rocky desertification habitats. In the present study, we investigated six leaf nutrient concentrations and three stoichiometric ratios across 20 liana species in various subtropical rocky desertification habitats. The results indicated that lianas in habitats with severe rocky desertification exhibited significantly higher potassium concentrations in their leaves compared to those in habitats with moderate rocky desertification. Within habitats characterized by moderate rocky desertification, there were notable positive correlations observed between the nitrogen, phosphorus, and potassium concentrations in liana leaves. However, in habitats with severe rocky desertification, phosphorus demonstrated significant positive correlations with both magnesium and potassium. Principal component analysis further revealed that lianas in severely desertified habitats tended to possess higher concentrations of leaf potassium and calcium, whereas those in moderately desertified habitats exhibited an opposite trend. The findings of this research provide crucial theoretical insights that can guide vegetation restoration efforts in different rocky desertification regions.
1. Introduction
Ecological stoichiometry is a common method for revealing alterations in the relative proportions of various interacting chemical elements within terrestrial ecosystems and their subsequent impacts on material cycling, energy flow, coupling mechanisms, nutrient limitations, and plant habitat adaptation [1,2]. In recent decades, many previous studies have concentrated on the stoichiometric characteristics of plants across diverse geographical scales, ecosystems, and biological types [1,3,4]. Lianas (wood vines) are a diverse and abundant interlayer and canopy plant group within tropical and subtropical forest ecosystems that serve as integral structural parasites within these habitats [5,6]. However, to date, few studies have examined the leaf stoichiometric characteristics of lianas [7,8,9]; therefore, the concentrations of nutrients and their stoichiometric characteristics within the leaves of liana growing in rocky desertification forest ecosystems remain unknown.
Carbon (C), nitrogen (N), phosphorus (P), potassium (K), calcium (Ca), and magnesium (Mg) are key elements in the growth, development, photosynthesis, and physiological regulation of plants [10]. The research on ecological stoichiometric characteristics across diverse ecosystems predominantly concentrates on plant C, N, and P concentrations [1,2,4,11]. Many studies have shown that the coupling between the C, N, and P concentrations in plant leaves reflects the plant’s adaptive mechanisms, regulatory processes, and environmental feedback mechanisms [12,13,14]. Specifically, the C:N and C:P ratios are associated with a plant’s carbon sequestration capacity, N and P use efficiency, and growth rate [12,15,16]. Additionally, the N:P ratio is an marker of nutrient limitation and can indicate nutrient deficiency or the plant’s health status during growth [17,18]. The K element plays a crucial role in activating various enzymes, thereby modulating physiological plant functions such as cell osmotic potential and stomatal movement [19,20,21]. The Ca and Mg elements are associated with stress resistance and photosynthesis in plants [22,23]. However, research on the ecological stoichiometry of plants often ignores K, Ca, and Mg. In addition, the C, N, P, K, Ca, and Mg concentrations and their stoichiometric characteristics in liana leaves from rocky desertification areas are rarely reported upon.
Lianas constitute a diverse and abundant group of plants in virtually all tropical and subtropical forests [5,24]. Typically, they comprise less than 25% of the total rooted lianas within forest ecosystems [25] and account for approximately 5% of the overall forest biomass [26]. The abundance and diversity of lianas significantly contribute to forest biomass and litter accumulation, while also playing a pivotal role in carbon sequestration processes and nutrient cycling dynamics [27,28,29]. Furthermore, the fruits or seeds of numerous liana species serve as essential food resources for animals, thereby helping to sustain the biodiversity of forest fauna [30,31,32].
Karst rocky desertification mountainous areas are distinctive due to their high rock exposure, thin soil layers, limited nutrient availability, and low water retention capacity. Consequently, plant growth in forests affected by rocky desertification is persistently impacted by drought and soil nutrient deficiencies [33,34,35,36]. Previous studies on subtropical rocky desertification forests have revealed that plant growth is generally limited by P [37,38,39], N [40,41], or both [42,43]. Additionally, there are studies that have reported that plant growth is often limited by the availability of K [44,45]. Lianas constitute a diverse and abundant group within karst rocky desertification forests [46,47]; however, although some prior studies have indicated that liana growth in subtropical forests is limited by N [48] or P [8,49], the nutritional limitations and stoichiometric characteristics of liana species in rocky desertification forests remain poorly understood, necessitating further study.
In this study, we investigated the concentrations and stoichiometric ratios of C, N, P, K, Ca, and Mg in the leaves of 12 liana species from moderately rocky desertification habitats and 8 liana species from severely rocky desertification habitats. The aim was to understand the variations in the leaf nutritional statuses and stoichiometric characteristics of these lianas across different levels of rocky desertification. Previous studies have shown that plant growth in rocky desertification habitats is limited by N, P, or a combination of N, P, and K [50,51,52]. Furthermore, it has been observed that soil P concentrations decline as the severity of rocky desertification increases [53]. Based on these findings, we expect that the growth of lianas in moderately rocky desertified habitats to be co-limited by deficiencies in both N and P, whereas in severely rocky desertified habitats, the primary limitation would be due to P deficiency.
2. Materials and Methods
2.1. Study Sites
Based on the established criteria for rocky desertification classification, habitats with vegetation coverage ranging from 30% to 50%, rock exposure between 50% and 70%, and a soil thickness of 20 cm to 40 cm are categorized as exhibiting moderate rocky desertification. Habitats with vegetation coverage of 10% to 30%, rock exposure of 70% to 90%, and a soil thickness of less than 20 cm are classified as exhibiting severe rocky desertification [54,55].
The moderate rocky desertification sample site (Figure 1) was located in the middle and upper reaches of the Chishui River, at the border of the Guizhou and Sichuan provinces in southwestern China (105°13′19″–106°58′34″ E, 27°13′16″–28°45′58″ N; 1000–1600 m a.s.l.) [56]. The average annual temperature of this site is 14.7 ◦C. The average annual precipitation is approximately 749 to 1286 mm. The predominant soil types are limestone soil and yellow-brown soil; all soil types have a pH value exceeding 7.0 [57]. The severe rocky desertification sample site (Figure 1) was located within the Maolan National Nature Reserve, a subtropical forest in Guizhou Province in southwestern China (107°52′10″–108°45′40″ E, 25°09′20″–25°20′50″ N; 450–790 m a.s.l.) [58]. This area has an average annual temperature of 15.3 °C. The average annual precipitation is approximately 1752 mm. The main soil type is limestone soil that has a pH value greater than 7.0 [40]. The soil has abundant organic matter, potassium, and nitrogen in this region [59].
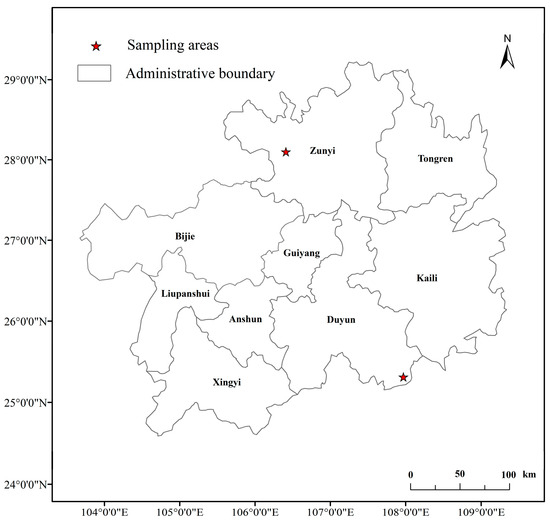
Figure 1.
Location of the study sites.
2.2. Sampling
In August 2021, we identified and selected 8 species of liana from the Maolan subtropical forest region, which is characterized by severe rocky desertification (Table 1). In August 2023, we identified and selected 12 species of lianas from the Chishui River Basin habitat, which exhibits moderate subtropical rocky desertification (Table 1). For each of the selected liana species, we chose 3 to 5 individuals that possessed a diameter at breast height greater than 1 cm. Then, we collected mature, healthy, intact leaves that were exposed to sunlight.

Table 1.
Species information of the lianas sampled in this study.
2.3. Element Measurements
The leaf samples were placed in envelopes and subsequently dried in an oven at 70 °C for 48 h. Following the drying process, the leaf samples were ground into powder using a crusher and then sieved through a mesh with a pore size of 0.25 mm. The C and N concentrations were analyzed using a Dumas-type combustion C-N elemental analyzer (Vario MAX CN, Elementar Analysensysteme GmbH, Hanau, Germany). Additionally, the concentrations of P and K were determined using an inductively coupled plasma atomic-emission spectrometer (iCAP 7400, Thermo Fisher Scientific, Bremen, Germany). The ratios of C:N and C:P were calculated as indicators of the plant growth rate and N and P use efficiency [13,15]. The N:P ratio was calculated and served as a proxy for nutrient limitation [60].
2.4. Data Analyses
The nutrient concentrations and their stoichiometric ratio in liana leaves were averaged across species. The data were log10-transformed before analyses to improve the normality distribution. We employed a t-test to examine differences in leaf nutrient concentration among lianas in various subtropical rocky desertification forests. We used Pearson’s correlation analysis to evaluate the relationships between leaf nutrients and their stoichiometric ratios. Furthermore, principal component analysis (PCA) was conducted to analyze trait associations. Notably, previous research in karst rocky desertification forests has indicated that interspecific variation in plant nutrients is not controlled by phylogeny [61,62]. Therefore, the effects of phylogeny on nutrient variation were not taken into account in our data analysis. All statistical analyses were performed using R-4.4.0 software (R Core Team 2024).
3. Results
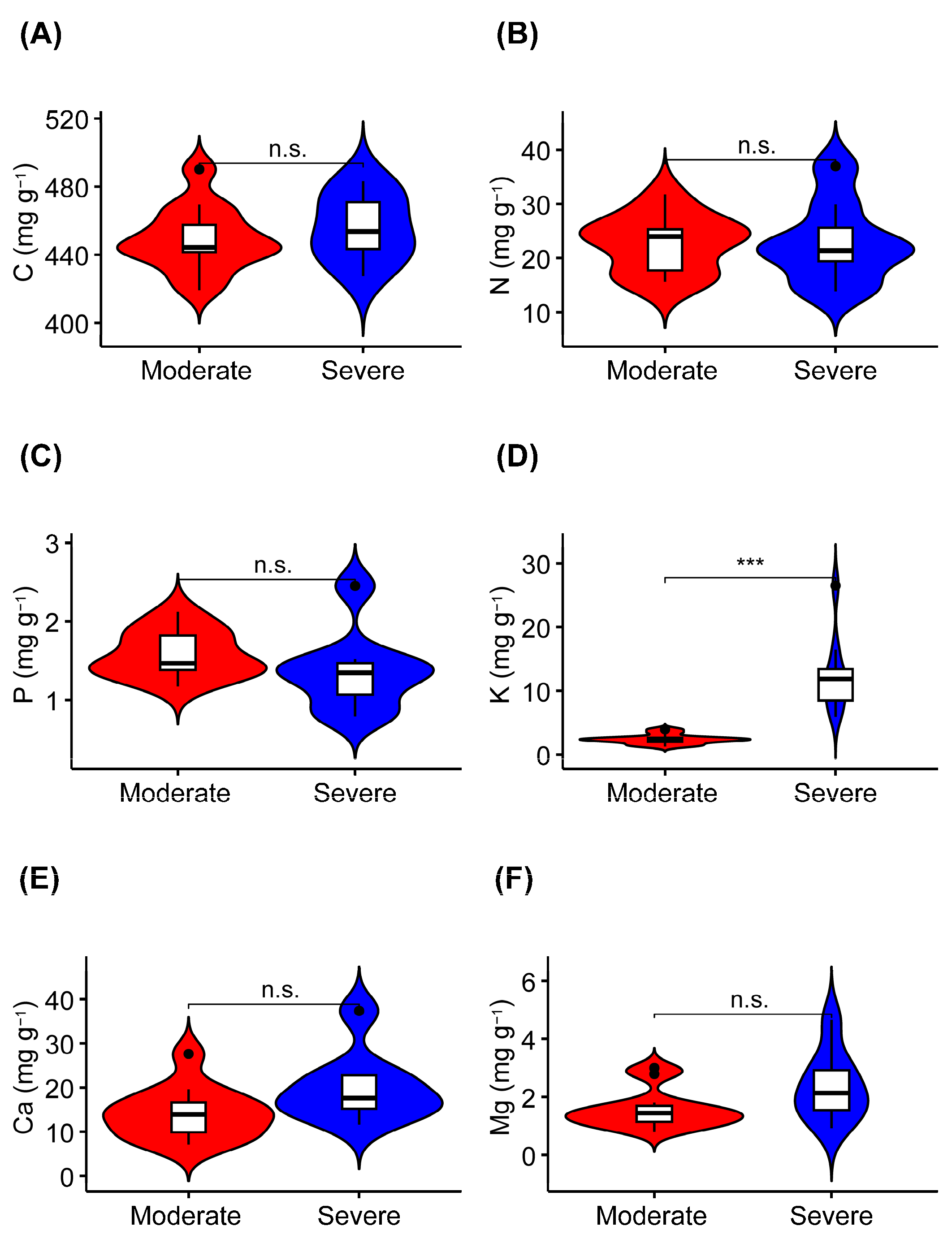
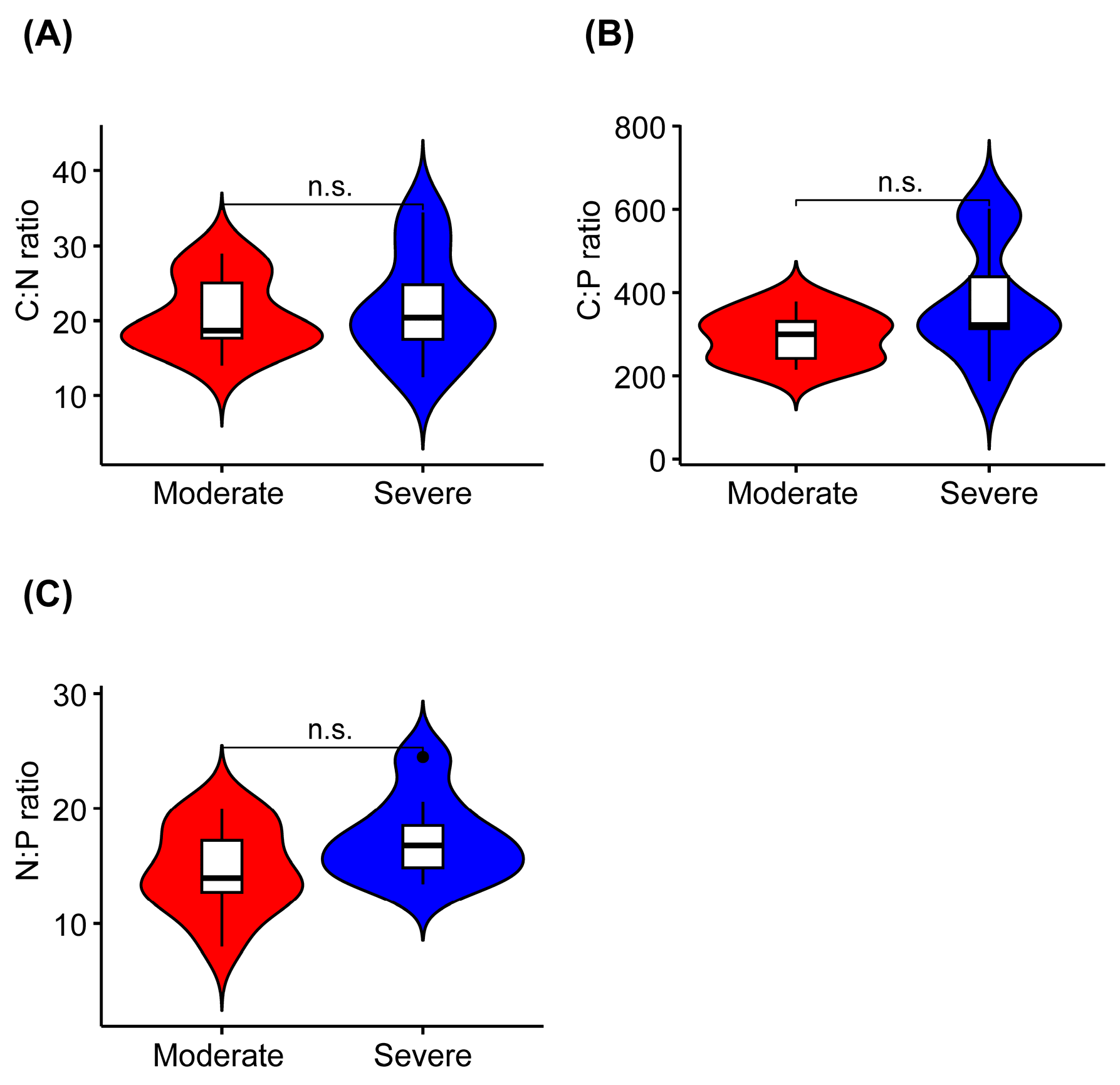
The K concentration was significantly higher in liana leaves from the severe rocky desertification habitat than it was in liana leaves from the moderate rocky desertification habitat (Figure 2D, Table 2). The leaf C, N, P, Ca, and Mg concentrations did not show significant differences between the two habitats (Figure 2A–C,E,F). In terms of the stoichiometric characteristics of liana leaves, the C:N, C:P, and N:P ratios were not significantly different between the leaves collected from the two habitats (Figure 3).
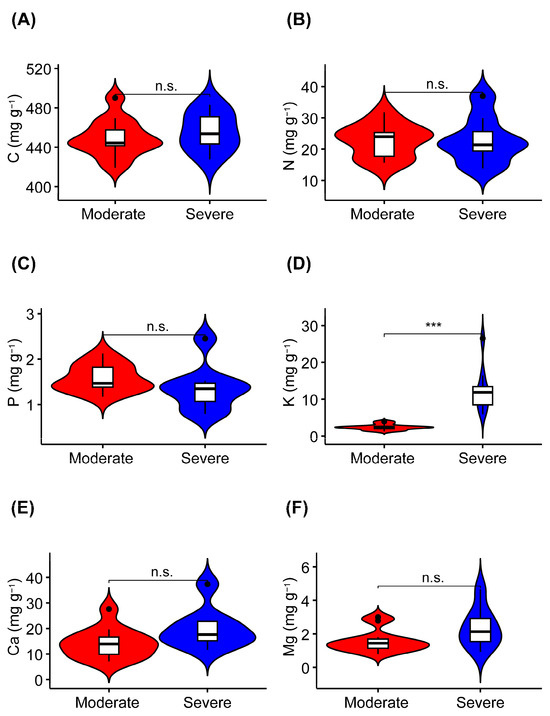
Figure 2.
Differences in the concentrations of leaf carbon (C), nitrogen (N), phosphorus (P), potassium (K), calcium (Ca), and magnesium (Mg) in lianas from moderate or severe rocky desertification habitats. (A–F) indicates the serial number of figures. n.s., p > 0.05; ***, p < 0.001.

Table 2.
The average nutrient concentration values and their stoichiometric characteristics in liana leaves from moderate and severe rocky desertification habitats (mean ± standard error).
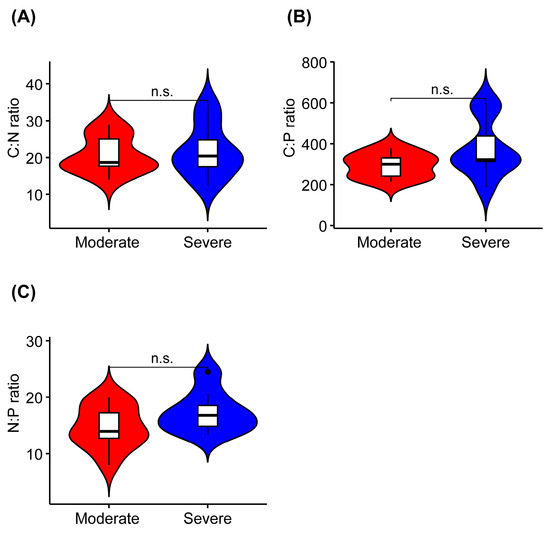
Figure 3.
Differences in leaf carbon/nitrogen (C:N), carbon/phosphorus (C:P), and nitrogen/phosphorus (N:P) ratios in lianas from moderate or severe rocky desertification habitats. (A–C) indicates the serial number of figures. n.s., p > 0.05.
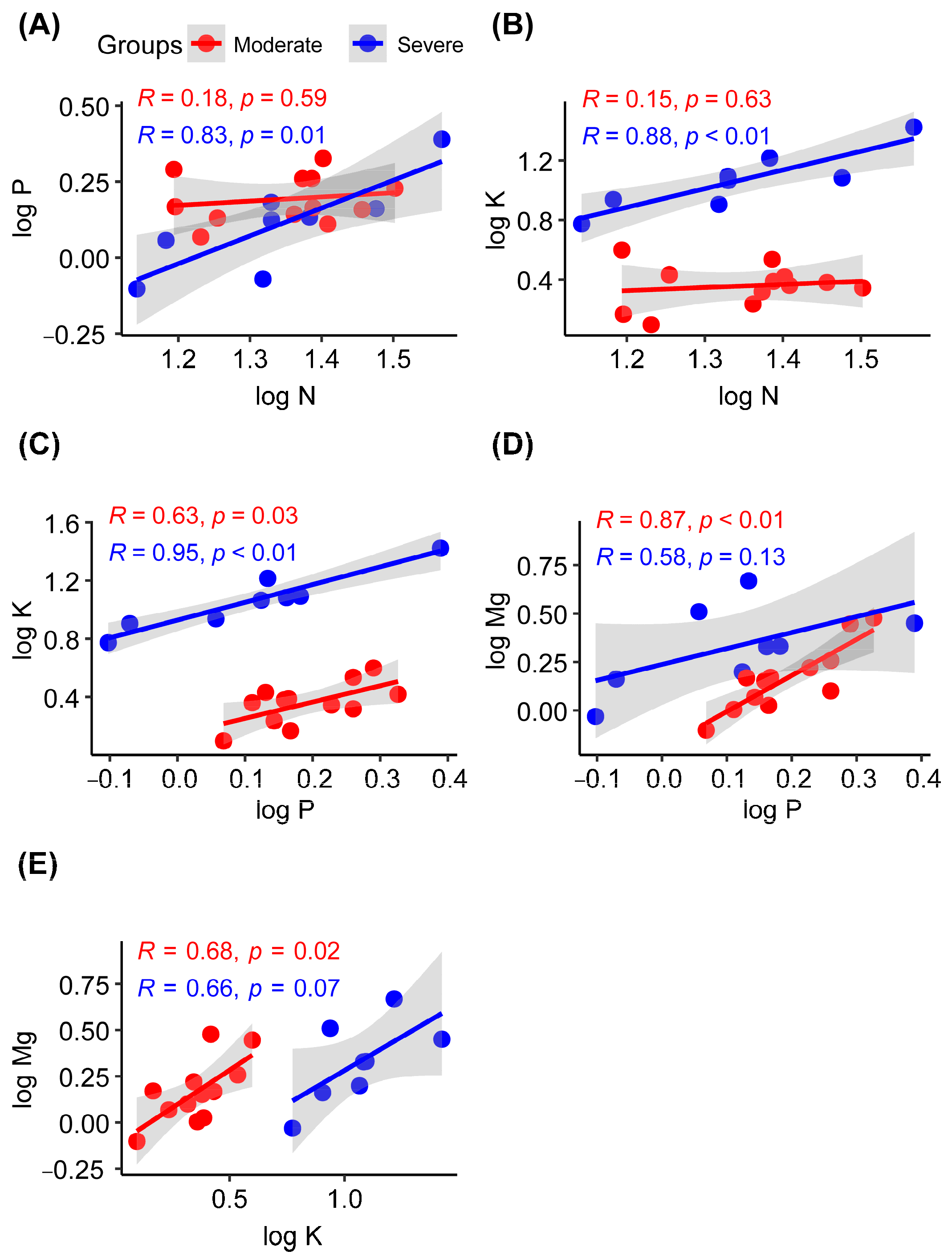
In the severe rocky desertification habitat, the liana leaf N concentration showed significant positive correlations with the P (R = 0.83; p = 0.01) and K (R = 0.88; p < 0.01) concentrations, whereas no significant correlations were observed in the moderate rocky desertification habitat (Figure 4A,B). The P and K concentrations in liana leaves showed significant positive correlations in both moderate (R = 0.63; p = 0.03) and severe (R = 0.95; p < 0.01) rocky desertification habitats (Figure 4C). In the moderate rocky desertification habitat, the liana leaf Mg concentration showed significant positive correlations with the P (R = 0.87; p < 0.01) and K (R = 0.68; p = 0.02) concentrations, whereas no significant correlations were observed in severe rocky desertification habitats (Figure 4D,E).
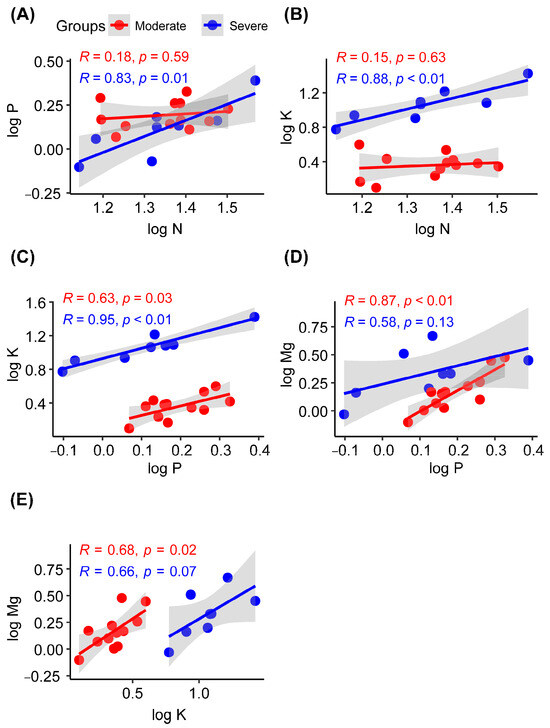
Figure 4.
Pearson correlations between leaf nutrients in lianas from moderate or severe rocky desertification habitats. N, nitrogen concentration; P, phosphorus concentration; K, potassium concentration; Mg, magnesium concentration. (A–E) indicates the serial number of figures.
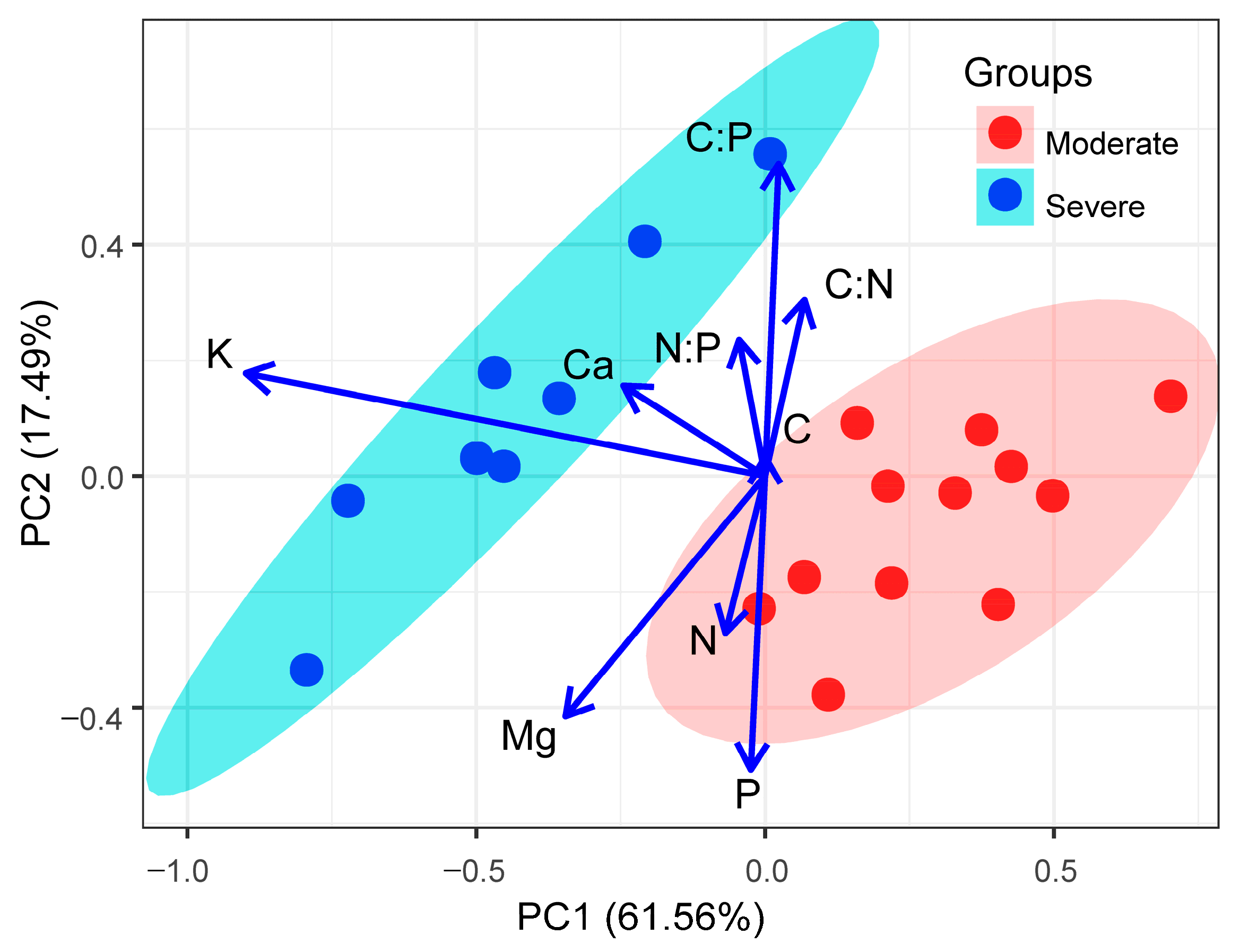
The results of the PCA, based on the concentrations of six leaf nutrients and three stoichiometric characteristics from 20 liana species, are shown in Figure 5. The first component accounted for 61.56% of the total variance and was negatively correlated with the leaf K and Ca concentrations. The second component accounted for 17.49% of the total variance and was positively correlated with the leaf C:N, N:P, and C:P ratios but negatively correlated with the leaf N, P, and Mg concentrations. The liana species from moderate rocky desertification habitats do not overlap with those from severe rocky desertification habitats in the multivariate nutrient space, indicating that liana species from different habitats have different nutrient strategies (Figure 5).
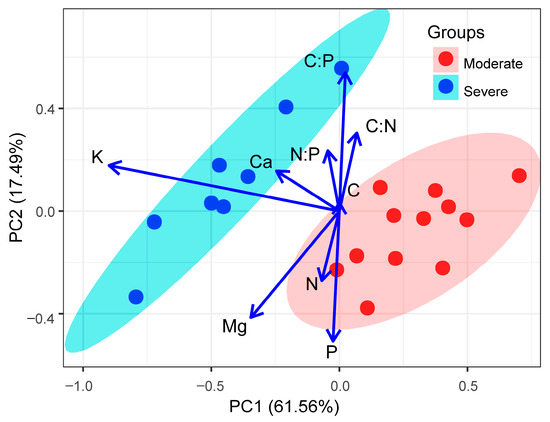
Figure 5.
The biplot of the first two axes of the principal component analysis (PCA) for the relationships of leaf nutrients and stoichiometric characteristics and the loadings of 12 liana species in moderate rocky desertification habitats and 8 liana species in severe rocky desertification habitats. See the text for trait abbreviations.
4. Discussion
The results showed that the concentration of K was significantly higher in liana leaves from severe rocky desertification habitats than it was in liana leaves from moderate rocky desertification habitats. There were no obvious differences in the concentrations of C, N, P, Ca, and Mg in liana leaves between the two types of rocky desertification habitat.
The C and N concentrations in liana leaves were not significantly different between habitats with moderate and severe rocky desertification (Figure 2, Table 2). The harsh environmental conditions of karst rocky desertification habitats (such as drought and poor soil) lead plants to accumulate more structural and storage substances, thereby enabling them to build protective structures for adaptation to the adverse environmental conditions [38,63]. We infer that lianas adopt similar C strategies to adapt to the harsh environmental conditions associated with varying degrees of karst rocky desertification. Furthermore, in rocky desertification habitats, factors such as high rock exposure rates, severe wind erosion, and intense rainfall weaken the soil’s ability to retain N, ultimately leading to a relatively low soil N concentration and consequently influencing the N concentration in plant leaves [40,58,64]. The P concentration in liana leaves was significantly different between the moderate (1.58 mg g⁻1) and severe (1.36 mg g⁻1) rocky desertification habitats (Figure 2, Table 2). The P concentration in liana leaves across different rocky desertification habitats approximates other plant species within the Maolan rocky desertification forest in Guizhou province (1.49 mg g⁻1) [65], yet it is lower than the average P concentration reported for global terrestrial plant leaves (1.99 mg g⁻1) [8]. Our results are consistent those reported by Han et al. (2005), where the P concentration of terrestrial plants in China was lower than that of terrestrial plants in other parts of the world [66]. This is possibly due to soil phosphorus deficiencies in forests affected by karst rocky desertification [58,67]. Notably, the K concentration of liana leaves in severe rocky desertification habitats (12.71 mg g⁻1) was significantly higher than that in moderate rocky desertification habitats (2.38 mg g⁻1) (Figure 2, Table 2). Under stressful conditions such as drought or high temperature or salinity, high K concentrations promote chloroplast integrity and light absorption efficiency, while also mitigating the formation of reactive oxygen species (ROS). These effects promote photosynthetic CO2 assimilation and improve nutrient absorption and utilization [68,69,70]. Additionally, high K concentrations regulate stomatal opening during drought conditions and promote transpiration, thereby ensuring efficient gas and water exchange, absorption, and utilization within plant organs [71,72,73]. Lastly, high K concentrations enhance enzymic activity, increasing the accumulation of organic penetrants to regulate osmotic balance and promote the biosynthesis of specific amino acids (such as proline) to facilitate adaptation to environmental stress [21,70,74]. This may explain the higher K concentrations observed in the leaves of liana species in habitats with severe rocky desertification. The Ca concentration in liana leaves did not exhibit a notable difference between moderate and severe rocky desertification habitats (Figure 2, Table 2). This may have resulted from the ample availability of Ca in the soil and water resources of karst rocky desertification habitats, which enables plants to accumulate Ca within their tissues as an adaptive mechanism to the high-Ca environment [75]. The Mg concentration in liana leaves also showed no obvious difference between moderate (1.64 mg g⁻1) and severe (2.37 mg g⁻1) rocky desertification habitats. However, these Mg concentrations are lower than those found in other plant species within the Maolan National Nature Reserve (4.78 mg g⁻1) [51]. A possible explanation for this observation could be the differential Mg absorption rates exhibited by various plant species [76].
The C:N and C:P ratios reflect the growth rate and utilization efficiency of N and P in plants. Generally, high C:N and C:P ratios in plants indicate a high nutrient (N and P) utilization efficiency but low growth rate [15,77]. We did not find significant differences in the C:N and C:P ratios of liana leaves across habitats with different levels of rocky desertification (Figure 2, Table 2). As an indicator of nutrient limitation, the N:P ratio can suggest N limitation (N:P < 14), P limitation (N:P > 16), both N and P limitation, or none of these [60]. The N:P ratio of liana leaves in moderate rocky desertification habitats was 14.72. This suggests that the growth of lianas in rocky desertification habitats may be limited by both N and P elements. A previous study also found that plant growth in rocky desertification regions was limited by the amounts of both N and P [65]. The N:P ratio of liana leaves in severe rocky desertification habitats was 17.38. This means that the growth of lianas may be limited by P in these areas. These results are consistent with previous studies carried out in rocky desertification forests in the Yunnan and Guizhou provinces [38,78].
Regarding the correlations among nutrients, the N concentration was positively correlated with the P concentration in habitats with severe rocky desertification (Figure 3). This finding is consistent with previous studies conducted on lianas and other plant species [7,49,66,79]. During plant growth, protein synthesis requires adenosine triphosphate (ATP); this explains the synergistic effect of N and P absorption in plants [80]. The N concentration was positively associated with the K concentration in habitats with severe rocky desertification. It is interesting that the K concentration was also positively correlated with the P concentration in both moderate and severe rocky desertification habitats. These findings indicate that the absorption of N, P, and K in plant leaves has a synergistic relationship in severe desertification habitats and that any deficiency in these essential elements can adversely impact plant growth and development [52,65,81]. In moderate rocky desertification habitats, P and K showed significant positive correlations with Mg (Figure 4). This indicates that moderate rocky desertification habitats have a synergistic effect on the absorption of P, K and Mg in lianas [39].
5. Conclusions
In summary, our study compared the concentrations of six nutrients and three stoichiometric characteristics across 12 liana species in habitats with moderate rocky desertification and 8 liana species in habitats with severe rocky desertification. Among the leaf element concentrations in these lianas, significant differences in K concentration were observed between the two different levels of rocky desertification. The absorption of N, P, and K by lianas exhibited a synergistic effect in severe rocky desertification habitats, whereas the absorption of P, K, and Mg showed a synergistic effect in moderate rocky desertification habitats. Lianas employ distinct nutrient combination strategies in different habitats to adapt to their environment. The findings of this study have certain guiding implications for vegetation restoration in rocky desertification areas.
Author Contributions
Conceptualization, X.B. and W.L.; investigation, X.B.; T.F.; Y.C. and W.L.; methodology, X.B., T.F. and S.Z.; formal analysis: X.B., Y.C. and B.H.; writing—original draft preparation, X.B. and W.L.; writing—review and editing, X.B. and W.L. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financially supported by the Bijie Science and Technology Project (bikelianhe[2023]23), the Bijie Science and Technology Project (bikelianhe[2023]22), the Bijie Science and Technology Project (bikelianhe[2023]10), the Project of Guizhou Science and Technology Fund (qiankehejichu-ZK-[2024]key077), the Bijie Talent Team of Biological Protection and Ecological Restoration in Liuchong River Basin (202112), the Guizhou Key Laboratory of Plateau Wetland Conservation and Restoration (qiankehepingtai[2025]015), and the Regional First-class Discipline of Ecology in Guizhou Province (XKTJ[2020]22).
Data Availability Statement
The original contributions presented in this study are included in the article; further inquiries can be directed to the corresponding author.
Acknowledgments
Thanks to the Public Technology Service Center of Xishuangbanna Tropical Botanical Garden, Chinese Academy of Sciences for analyzing the foliar nutrient concentrations.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sardans, J.; Janssens, I.A.; Ciais, P.; Obersteiner, M.; Peñuelas, J. Recent advances and future research in ecological stoichiometry. Perspect. Plant Ecol. Evol. Syst. 2021, 50, 125611. [Google Scholar] [CrossRef]
- Elser, J.J.; Fagan, W.F.; Denno, R.F.; Dobberfuhl, D.R.; Folarin, A.; Huberty, A.; Interlandi, S.; Kilham, S.S.; McCauley, E.; Schulz, K.L.; et al. Nutritional constraints in terrestrial and freshwater food webs. Nature 2000, 408, 578–580. [Google Scholar] [CrossRef] [PubMed]
- Dibar, D.T.; Zhang, K.; Yuan, S.; Zhang, J.; Zhou, Z.; Ye, X. Ecological stoichiometric characteristics of Carbon (C), Nitrogen (N) and phosphorus (P) in leaf, root, stem, and soil in four wetland plants communities in Shengjin Lake, China. PLoS ONE 2020, 15, e0230089. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, J.; Wang, L. Seasonal variations in C/N/P/K stoichiometric characteristics in different plant organs in the various forest types of Sygera Mountain. Front. Plant Sci. 2024, 15, 1293934. [Google Scholar] [CrossRef] [PubMed]
- Schnitzer, S.A.; Bongers, F. The ecology of lianas and their role in forests. Trends Ecol. Evol. 2002, 17, 223–230. [Google Scholar] [CrossRef]
- Tang, Y.; Kitching, R.L.; Cao, M. Lianas as structural parasites: A re-evaluation. Chin. Sci. Bull. 2012, 57, 307–312. [Google Scholar] [CrossRef]
- Wang, J.Y.; Lan, J.C.; Long, T.; Xie, Y.J.; Lu, X.M.; Lei, L.Q.; Zhu, H.G.; Wen, Y.G. Ecological stoichiometry characteristics of nitrogen, phosphorus and potassium in liana leaf of evergreen broad-leaf forest. J. South. Agric. 2013, 5, 815–818. [Google Scholar]
- Huang, X.B.; Liu, W.D.; Su, J.R.; Li, S.F.; Lang, X.D. Stoichiometry of leaf C, N and P across152 woody species of a monsoon broad-leaved evergreen forest in Pu’er, Yunnan province. Chin. J. Ecol. 2016, 35, 567–575. [Google Scholar]
- Collins, C.G.; Wright, S.J.; Wurzburger, N. Root and leaf traits reflect distinct resource acquisition strategies in tropical lianas and trees. Oecologia 2016, 180, 1037–1047. [Google Scholar] [CrossRef]
- Bhatla, S.C.; Lal, M.A. Plant Physiology, Development and Metabolism; Springer: Singapore, 2023. [Google Scholar]
- Costa, M.G.; dos Santos Sarah, M.M.; de Mello Prado, R.; Palaretti, L.F.; de Cássia Piccolo, M.; de Souza Júnior, J.P. Impact of Si on C, N, and P stoichiometric homeostasis favors nutrition and stem dry mass accumulation in sugarcane cultivated in tropical soils with different water regimes. Front. Plant Sci. 2022, 13, 949909. [Google Scholar] [CrossRef]
- Ågren, G.I. The CN:P stoichiometry of autotrophs–theory and observations. Ecol. Lett. 2004, 7, 185–191. [Google Scholar] [CrossRef]
- McGroddy, M.E.; Daufresne, T.; Hedin, L.O. Scaling of C:N:P stoichiometry in forests worldwide: Implications of terrestrial redfield-type ratios. Ecology 2004, 85, 2390–2401. [Google Scholar] [CrossRef]
- Song, Z.; Zuo, X.; Zhao, X.; Li, X.; Hu, Y.; Qiao, J.; Yue, P.; Chen, M.; Wang, S.; Sardans, J.; et al. Plant functional traits modulate effects of drought on C: N: P stoichiometry of plant, litter, and soil microbe in an arid grassland. J. Soil Sci. Plant Nutr. 2024, 24, 7228–7241. [Google Scholar] [CrossRef]
- Herbert, D.A.; Williams, M.; Rastetter, E.B. A model analysis of N and P limitation on carbon accumulation in Amazonian secondary forest after alternate land-use abandonment. Biogeochemistry 2003, 65, 121–150. [Google Scholar] [CrossRef]
- Minden, V.; Kleyer, M. Internal and external regulation of plant organ stoichiometry. Plant Biol. 2014, 16, 897–907. [Google Scholar] [CrossRef]
- Elser, J.J.; Bracken, M.E.S.; Cleland, E.E.; Gruner, D.S.; Harpole, W.S.; Hillebrand, H.; Ngai, J.T.; Seabloom, E.W.; Shurin, J.B.; Smith, J.E. Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecol. Lett. 2007, 10, 1135–1142. [Google Scholar] [CrossRef]
- Vitousek, P.M.; Porder, S.; Houlton, B.Z.; Chadwick, O.A. Terrestrial phosphorus limitation: Mechanisms, implications, and nitrogen–phosphorus interactions. Ecol. Appl. 2010, 20, 5–15. [Google Scholar] [CrossRef]
- Andrés, Z.; Pérez-Hormaeche, J.; Leidi, E.O.; Schlücking, K.; Steinhorst, L.; McLachlan, D.H.; Schumacher, K.; Hetherington, A.M.; Kudla, J.; Cubero, B.; et al. Control of vacuolar dynamics and regulation of stomatal aperture by tonoplast potassium uptake. Proc. Natl. Acad. Sci. USA 2014, 111, E1806–E1814. [Google Scholar] [CrossRef]
- Battie-Laclau, P.; Laclau, J.P.; Domec, J.C.; Christina, M.; Bouillet, J.P.; de Cassia, P.M.; de Moraes Gonçalves, J.L.; Moreira, R.M.; Krusche, A.V.; Bouvet, J.M.; et al. Effects of potassium and sodium supply on drought-adaptive mechanisms in Eucalyptus grandis plantations. New Phytol. 2014, 203, 401–413. [Google Scholar] [CrossRef]
- Chen, B.; Fang, J.; Piao, S.; Ciais, P.; Black, T.A.; Wang, F.; Niu, S.; Zeng, Z.; Luo, Y. A meta-analysis highlights globally widespread potassium limitation in terrestrial ecosystems. New Phytol. 2024, 241, 154–165. [Google Scholar] [CrossRef]
- White, P.J.; Broadley, M.R. Calcium in plants. Ann. Bot. 2003, 92, 487–511. [Google Scholar] [CrossRef] [PubMed]
- Verbruggen, N.; Hermans, C. Physiological and molecular responses to magnesium nutritional imbalance in plants. Plant Soil 2013, 368, 87–99. [Google Scholar] [CrossRef]
- Pandi, V. Ecology of lianas: Diversity and distribution. In Taxonomy and Ecology of Climbers: Climbing Plants of India; Springer Nature Singapore: Singapore, 2023; pp. 57–70. [Google Scholar]
- Schnitzer, S.A.; Mangan, S.A.; Dalling, J.W.; Baldeck, C.A.; Hubbell, S.P.; Ledo, A.; Muller-Landau, H.; Tobin, M.F.; Aguilar, S.; Brassfield, D.; et al. Liana abundance, diversity, and distribution on Barro Colorado Island, Panama. PLoS ONE 2012, 7, e52114. [Google Scholar] [CrossRef]
- Dossa, G.G.; Li, H.L.; Pan, B.; Ling, T.C.; Schaefer, D.A.; Roeder, M.; Njoroge, D.M.; Zuo, J.; Song, L.; Ofosu-Bamfo, B.; et al. Effects of lianas on forest biogeochemistry during their lives and afterlives. Glob. Chang. Biol. 2024, 30, e17274. [Google Scholar] [CrossRef] [PubMed]
- Kusumoto, B.; Enoki, T. Contribution of a liana species, Mucuna macrocarpa Wall., to litterfall production and nitrogen input in a subtropical evergreen broad-leaved forest. J. For. Res. 2008, 13, 35–42. [Google Scholar] [CrossRef]
- van der Heijden, G.M.; Schnitzer, S.A.; Powers, J.S.; Phillips, O.L. Liana impacts on carbon cycling, storage and sequestration in tropical forests. Biotropica 2013, 45, 682–692. [Google Scholar] [CrossRef]
- Meunier, F.; Visser, M.D.; Shiklomanov, A.; Dietze, M.C.; Guzmán Q., J.A.; Sanchez-Azofeifa, G.A.; De Deurwaerder, H.P.T.; Moorthy, S.M.K.; Schnitzer, S.A.; Marvin, D.C.; et al. Liana optical traits increase tropical forest albedo and reduce ecosystem productivity. Glob. Chang. Biol. 2022, 28, 227–244. [Google Scholar] [CrossRef] [PubMed]
- Kilgore, A.; Lambert, T.D.; Adler, G.H. Lianas influence fruit and seed use by rodents in a tropical forest. Trop. Ecol. 2010, 51, 265. [Google Scholar]
- Michel, N.L.; Douglas Robinson, W.; Sherry, T.W. Liana–bird relationships: A review. Ecol. Lianas 2015, 362–397. [Google Scholar]
- Schnitzer, S.A.; Michel, N.L.; Powers, J.S.; Robinson, W.D. Lianas maintain insectivorous bird abundance and diversity in a neotropical forest. Ecology 2020, 101, e03176. [Google Scholar] [CrossRef]
- Su, W.; Zhu, W.; Xiong, K. Stone desertification and eco-economics improving model in Guizhou karst mountain. Carsol. Sin. 2002, 21, 21–26. [Google Scholar]
- Li, Y.; Tan, Q.; Wang, S. Current status, problems analysis and basic framework of karst rocky desertification research. Sci. Soil Water Conserv. 2005, 3, 27–34. [Google Scholar]
- Jiang, Z.; Lian, Y.; Qin, X. Rocky desertification in Southwest China: Impacts, causes, and restoration. Earth-Sci. Rev. 2014, 132, 1–12. [Google Scholar] [CrossRef]
- Xiong, K.; Chi, Y. The problems in southern China karst ecosystem in southern of China and its countermeasures. Ecol. Econ. 2015, 31, 23–30. [Google Scholar]
- Cai, G.J.; Suo, P.C.; Zhang, L.M.; Fu, Y.H.; Li, A.D. C, N, P stoichiometric characteristics in different organs of three constructive plants in Karst peak-cluster depressions in southern Guizhou, Southwest China. J. Guizhou Norm. Univ. Nat. Sci. 2021, 39, 36–44. [Google Scholar]
- Du, J.; Cai, G.; Zhang, H.; Li, A. Response of plant Leaf C, N, P stoichiometry characteristics to climatic environment and soil nutrients in karst areas of Guizhou. Ecol. Environ. Sci. 2023, 32, 2154–2165. [Google Scholar]
- Liu, Q.; Long, C.; He, Q.; Yuan, R.; Li, J. Nitrogen and phosphorous stoichiometry of leaves of Cyclobalanopsis glauca forest in Maolan National Nature Reserve. J. Guizhou Norm. Univ. Nat. Sci. 2024, 1–8. [Google Scholar]
- Yu, Y.F.; Wei, J.H.; Hu, J.M.; Zhang, J.H.; Li, T.T.; Zheng, F.H.; Zhang, Y.; Su, L.R.; He, T.G. Nitrogen and phosphorus stoichiometric homoeostasis in different organs of shrubs and herbs in degraded vegetation communities in the karst area of northwestern Guangxi. Acta Ecol. Sin. 2024, 44, 5367–5376. [Google Scholar]
- Liu, Q.; Wang, Z. Nutrient characteristics of typical plant leaves in karst and non-karst regions of southwest China. Hunan Shengtai Kexue Xuebao 2024, 11, 10–17. [Google Scholar]
- Pi, F.; Yuan, C.; Yu, L.; Yan, L.; Wu, L.; Yang, R. Ecological stoichiometry characteristics of plant leaves from the main dominant species of natural secondary forest in the central of Guizhou. Ecol. Environ. Sci. 2016, 25, 801–807. [Google Scholar]
- Yu, Y.H.; Zhong, X.P.; Zheng, W.; Chen, Z.X.; Wang, J.X. Species diversity, functional traits, stoichiometry and correlation of plant community in different succession stages of karst forest. Acta Ecol. Sin. 2021, 41, 2408–2417. [Google Scholar]
- Lu, X.; Yang, W.; Ding, F.; Ding, H.; Wu, J.; Cao, M.; Cui, P.; Xu, H. Dynamics of litterfall and nutrient recycling in virgin forest in Maolan karst region. J. Ecol. Rural Environ. 2014, 30, 614–619. [Google Scholar]
- Wu, P.; Zhou, H.; Cui, Y.C.; Zhao, W.J.; Hou, Y.J.; Zhu, J.; Ding, F.J. Stoichiometric characteristics of leaf nutrients in Karst plant species during natural restoration in Maolan national nature reserve, Guizhou, China. J. Sustain. Forest. 2023, 42, 95–119. [Google Scholar] [CrossRef]
- Lu, F.; Li, X.K.; Wang, B.; Li, D.X.; Huang, F.Z.; Li, J.X.; Chen, T.; Lu, S.H.; Guo, Y.L.; Wen, S.J.; et al. Spatial pattern of lianas of Litsea dilleniifolia community and its relationship with main tree species in Nonggang, Guangxi. Acta Ecol. Sin. 2021, 41, 6191–6202. [Google Scholar]
- Wang, Y.S.; Chen, L.J.; Li, Y.H.; He, L.X.; Li, Z.Z.; Qing, R.B.; Yang, X.J. Analysis of species composition and attribute characteristics of related traits of lianas growing in the karst areas of south China. Pratacult. Sci. 2020, 37, 126–138. [Google Scholar]
- Bai, X.L.; Yang, D.; Sher, J.; Zhang, Y.B.; Zhang, K.Y.; Liu, Q.; Wen, H.D.; Zhang, J.L.; Slot, M. Divergences in stem and leaf traits between lianas and coexisting trees in a subtropical montane forest. J. Plant Ecol. 2024, 17, rtad037. [Google Scholar] [CrossRef]
- Xu, X.; Li, W.; Zhou, X.; Lv, S.; Bai, K. Leaf ecological stoichiometry in understory plants with different life forms in a subtropical evergreen broad-leaved forest. J. Trop. Subtrop. Bot. 2024, 32, 725–736. [Google Scholar]
- Luo, X.; Zhang, G.; Du, X.; Wang, S.; Yang, H.; Huang, T. Characteristics of element contents and ecological stoichiometry in leaves of common calcicole species in Maolan karst forest. Ecol. Environ. Sci. 2014, 23, 1121–1129. [Google Scholar]
- Pi, F.; Shu, L.; Yu, L.; Zhou, C.; Wu, Z.; Yuan, C. Study on ecological stoichiometry characteristics and correlation of plants within different organs of 10 dominant tree species in karst region of central Guizhou. Environ. Sci. 2017, 26, 628–634. [Google Scholar]
- Hu, Q.J.; Sheng, M.Y.; Yin, J.; Bai, Y.X. Stoichiometric characteristics of fine roots and rhizosphere soil of Broussonetia papyrifera adapted to the karst rocky desertification environment in southwest China. Chin. J. Plant Ecol. 2020, 44, 962–972. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Zhang, M.; Bai, Y.; Huang, X.; Zhang, Y.; Zhang, W.; Jiang, T. Analysis of soil nutrient conditions under different rocky desertification grades in Guizhou karst region by the bibliometrics method. Soil Fert. Sci. China 2019, 171–180. [Google Scholar]
- Li, R.L.; Wang, S.J.; Xiong, K.N.; Li, F.Q. A study on rocky desertification evaluation index system-a case study of Guizhou Province. Trop. Geogr. 2004, 24, 145–149. [Google Scholar]
- Li, S.; Dong, Y.; Wang, J. Re-discussion on the concept and classification of rocky desertification. Carsol. Sin. 2007, 26, 179–284. [Google Scholar]
- Yang, S.; An, Y.; Wang, P.; Ma, L.; Hu, F.; Sun, Q. Study of ecological red-line zones in Guizhou Chishui River Basin. Resour. Environ. Yangtze Basin 2015, 24, 1405–1411. [Google Scholar]
- Xiao, Y.; Huang, Z.; Li, Y.; Zhang, Y.; Wang, M. Soil microbial community structure and diversity of typical vegetation types in Chishui River Basin. Sci. Soil Water Conserv. 2022, 29, 275–283. [Google Scholar]
- Wu, P. Study on Ecological Stoichiometric Characteristics of Plant Leaf-Litter-Soil in the Process of Natural Restoration in Maolan Karst Forest; Chinese Academy of Forestry: Beijing, China, 2017. [Google Scholar]
- Yu, J.; An, M.; Zhang, Y.; Tian, L.; Wang, K. Vertical distribution characteristics and environmental interpretation of plant species richness in Maolan karst forest. Acta Bot. Boreal.-Occident. Sin. 2023, 43, 326–334. [Google Scholar]
- Koerselman, W.; Meuleman, A.F. The vegetation N:P ratio: A new tool to detect the nature of nutrient limitation. J. Appl. Ecol. 1996, 33, 1441–1450. [Google Scholar] [CrossRef]
- Bai, K.; Lv, S.; Ning, S.; Zeng, D.; Guo, Y.; Wang, B. Leaf nutrient concentrations associated with phylogeny, leaf habit and soil chemistry in tropical karst seasonal rainforest tree species. Plant Soil 2019, 434, 305–326. [Google Scholar] [CrossRef]
- Li, Y.; He, W.; Wu, J.; Zhao, P.; Chen, T.; Zhu, L.; Ouyang, L.; Hölscher, D. Leaf stoichiometry is synergistically-driven by climate, site, soil characteristics and phylogeny in karst areas, Southwest China. Biogeochemistry 2021, 155, 283–301. [Google Scholar] [CrossRef]
- Onoda, Y.; Hikosaka, K.; Hirose, T. Allocation of nitrogen to cell walls decreases photosynthetic nitrogen-use efficiency. Funct. Ecol. 2004, 18, 419–425. [Google Scholar] [CrossRef]
- Galloway, J.N.; Townsend, A.R.; Erisman, J.W.; Bekunda, M.; Cai, Z.C.; Freney, J.R.; Martinellj, L.A.; Seitzinger, S.P.; Sutton, M.A. Transformation of the nitrogen cycle: Recent trends, questions, and potential solutions. Science 2008, 320, 889–892. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Cui, Y.C.; Zhao, W.J.; Hou, Y.J.; Zhu, J.; Ding, F.J.; Yang, W.B. Leaf stoichiometric characteristics of 68 typical plant species in Maolan National Nature Reserve, Guizhou, China. Acta Ecol. Sin. 2020, 40, 5063–5080. [Google Scholar]
- Han, W.; Fang, J.; Guo, D.; Zhang, Y. Leaf nitrogen and phosphorus stoichiometry across 753 terrestrial plant species in China. New Phytol. 2005, 168, 377–385. [Google Scholar] [CrossRef]
- Wen, P.; Wang, L.; Sheng, M. Research progress in ecological stoichiometry of karst forest ecosystem in Southwest China. Word For. Res. 2018, 31, 66–71. [Google Scholar]
- Egilla, J.N.; Davies, J.F.T.; Boutton, T.W. Drought stress influences leaf water content, photosynthesis, and water-use efficiency of Hibiscus rosa-sinensis at three potassium concentrations. Photosynthetica 2005, 43, 135–140. [Google Scholar] [CrossRef]
- Lemoine, R.; Camera, S.L.; Atanassova, R.; Dédaldéchamp, F.; Allario, T.; Pourtau, N.; Bonnemain, J.L.; Laloi, M.; Coutos-Thévenot, P.; Maurousset, L.; et al. Source-to-sink transport of sugar and regulation by environmental factors. Front. Plant Sci. 2013, 4, 272. [Google Scholar] [CrossRef] [PubMed]
- Sardans, J.; Peñuelas, J. Potassium control of plant functions: Ecological and agricultural implications. Plants 2021, 10, 419. [Google Scholar] [CrossRef] [PubMed]
- Tränker, M.; Tavakol, A.; Jákli, B. Functioning of potassium and magnesium in photosynthesis, photosynthate translocation and photoprotection. Physiol. Plant. 2018, 163, 414–431. [Google Scholar] [CrossRef]
- Srivastava, A.K.; Shankar, A.; CHandran, A.K.N.; Sharma, M.; Jung, K.H.; Suprasanna, P.; Pandey, G.K. Emerging concepts of potassium homeostasis in plants. J. Exp. Bot. 2019, 71, 608–619. [Google Scholar] [CrossRef]
- Johnson, R.; Vishwakarma, K.; Hossen, M.S.; Kumar, V.; Shackira, A.M.; Puthur, J.T.; Abdi, G.; Sarraf, M.; Hasanuzzaman, M. Potassium in plants: Growth regulation, signaling, and environmental stress tolerance. Plant Physiol. Biochem. 2022, 172, 56–69. [Google Scholar] [CrossRef]
- Oddo, E.; Inzerillo, S.; La Bella, F.; Grisafi, F.; Salleo, S.; Nardini, A.; Goldstein, G. Short-term effectsof potassium fertilization on the hydraulic conductance of Laurus nobilis L. Tree Physiol. 2011, 31, 131–138. [Google Scholar] [CrossRef]
- Ji, F.T.; Li, N.; Deng, X. Calcium contents and high calcium adaptation of plants in karst areas of China. Chin. J. Plant Ecol. 2009, 33, 926–935. [Google Scholar]
- White, P.J.; Broadley, M.R.; El-Serehy, H.A.; George, T.S.; Neugebauer, K. Linear relationships between shoot magnesium and calcium concentrations among angiosperm species are associated with cell wall chemistry. Ann. Bot. 2018, 122, 221–226. [Google Scholar] [CrossRef]
- Müller, M.; Oelmann, Y.; Schickhoff, U.; Böhner, J.; Scholten, T. Himalayan treeline soil and foliar C: N: P stoichiometry indicate nutrient shortage with elevation. Geoderma 2017, 291, 21–32. [Google Scholar] [CrossRef]
- Chen, K.; Du, H.; Liu, C. Characteristics of leaf ecological stoichiometry in typical plant communities in karst fault-depression basins of Yunnan Province. Carsol. Sin. 2020, 39, 883–893. [Google Scholar]
- Wang, J.; Liang, Y.; Wang, G.; Lin, X.; Liu, J.; Wang, H.; Chen, Z.; Wu, B. Leaf nitrogen and phosphorus stoichiometry and its response to geographical and climatic factors in a tropical region: Evidence from Hainan Island. Agronomy 2023, 13, 411. [Google Scholar] [CrossRef]
- Wright, I.J.; Reich, P.B.; Cornelissen, J.H.; Falster, D.S.; Garnier, E.; Hikosaka, K.; Lamont, B.B.; Lee, W.; Oleksyn, J.; Osada, N.; et al. Assessing the generality of global leaf trait relationships. New Phytol. 2005, 166, 485–496. [Google Scholar] [CrossRef]
- Zhen, L.; Yu, L.; Dai, P.; Xue, Y.; Long, P. Nutrient characteristics and adaptability of plant leaves in Tiankeng Complex of Dashiwei, Guangxi, China. Chin. J. Plant Ecol. 2024, 48, 872–887. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).