Negotiating a Fragmented World: What Do We Know, How Do We Know It, and Where Do We Go from Here?
Abstract
:1. Introduction
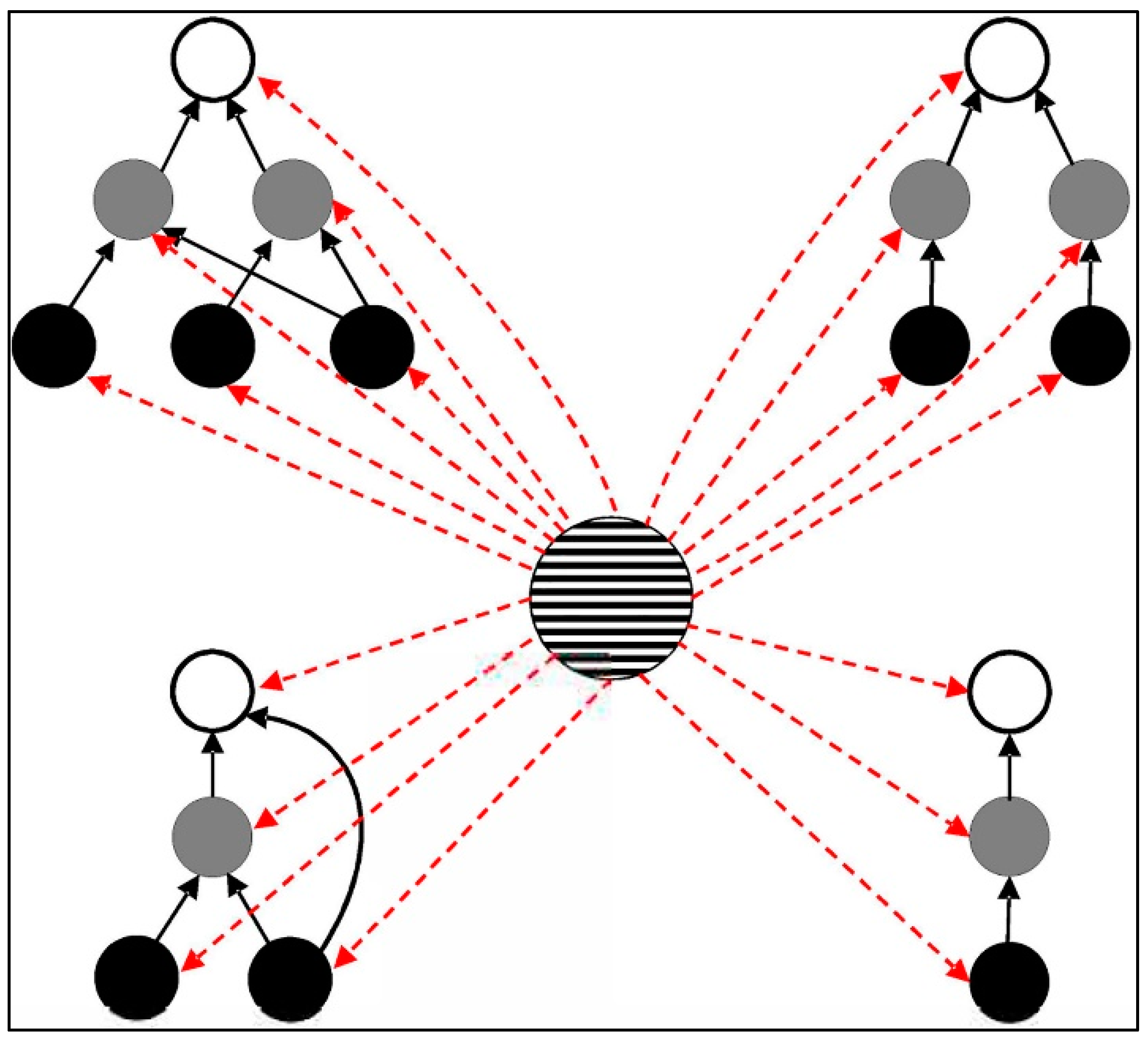
2. Habitat Spatial Structure and the Metapopulation Concept
2.1. Metapopulation Theory
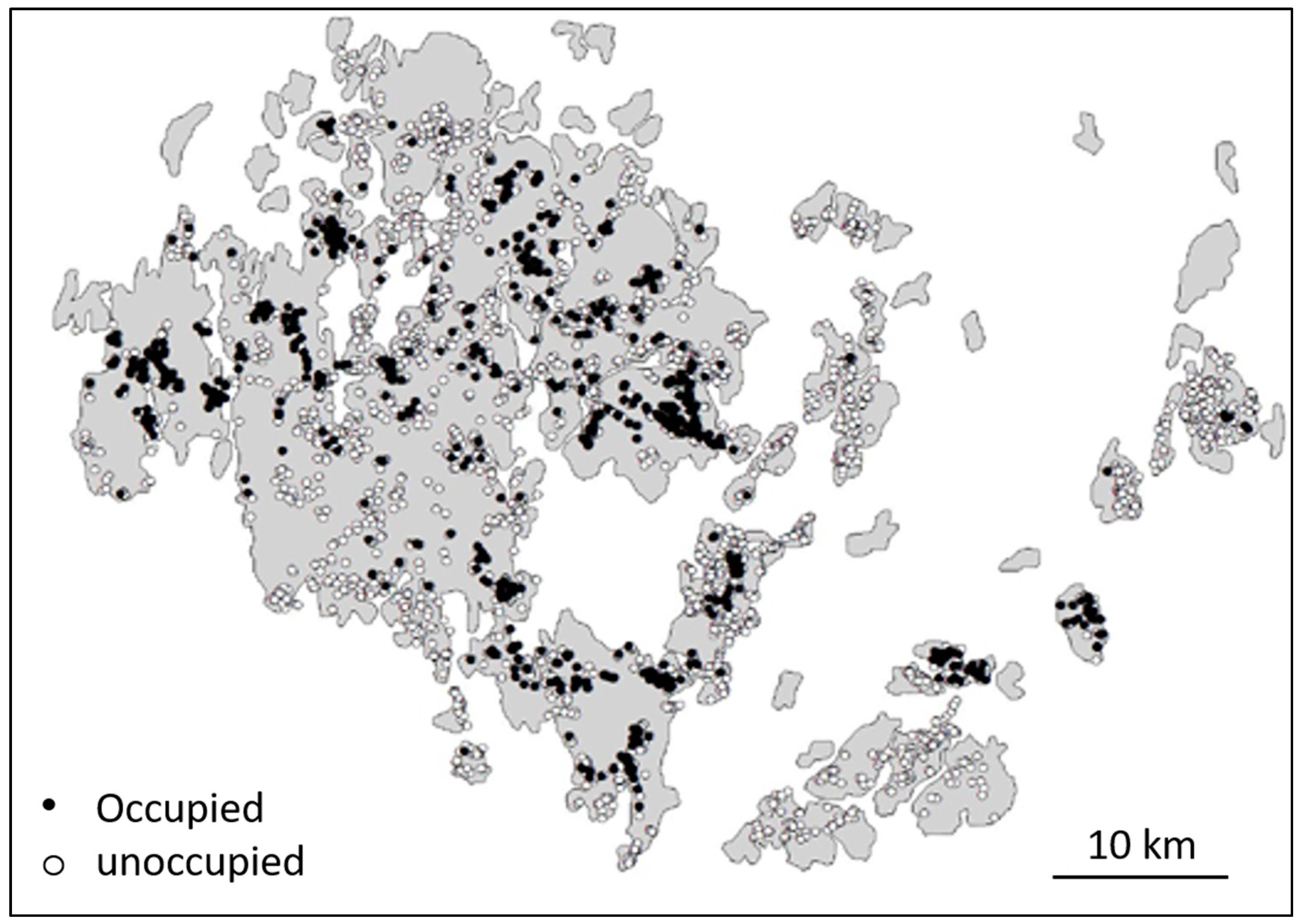
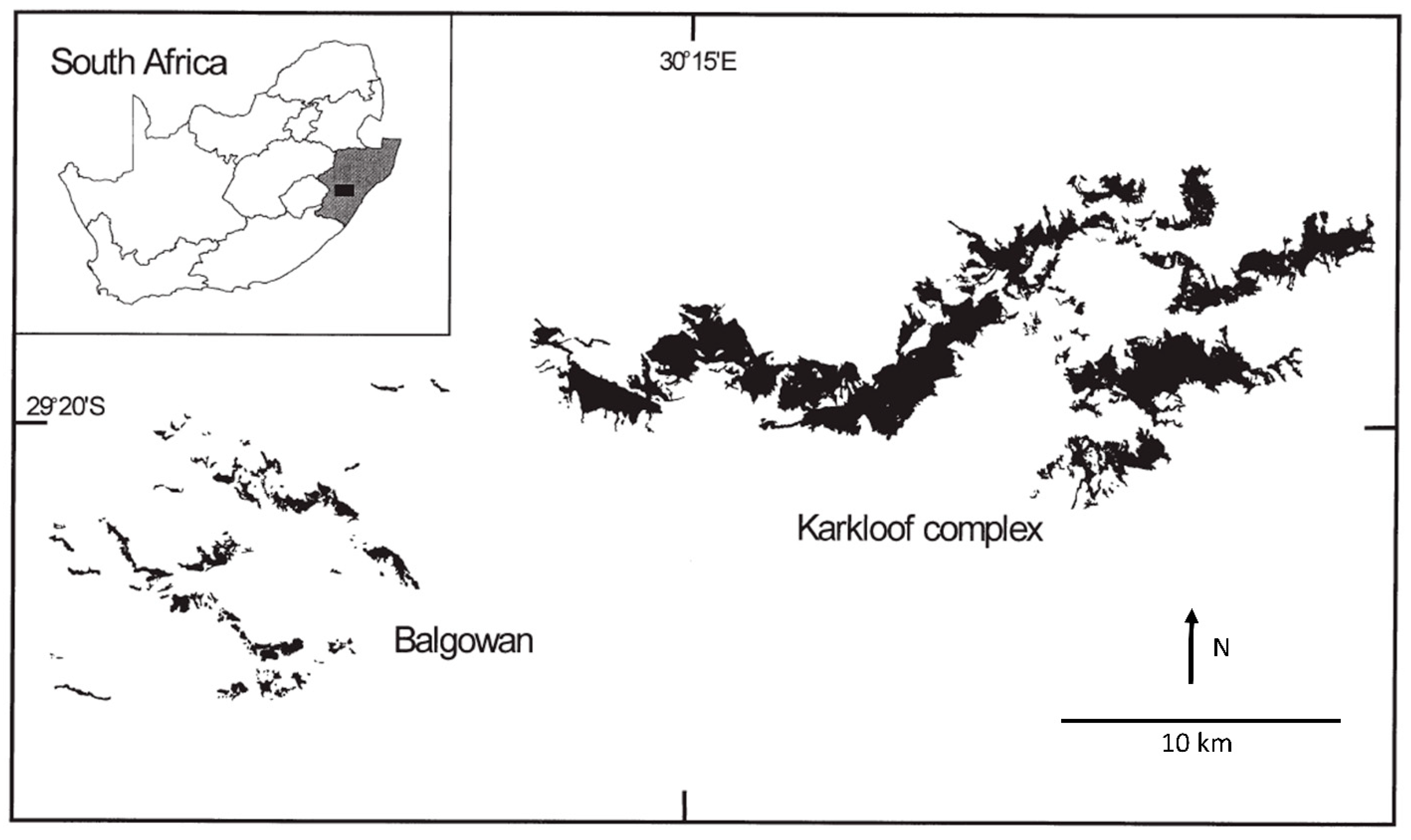
2.2. Tests of Metapopulation Theory
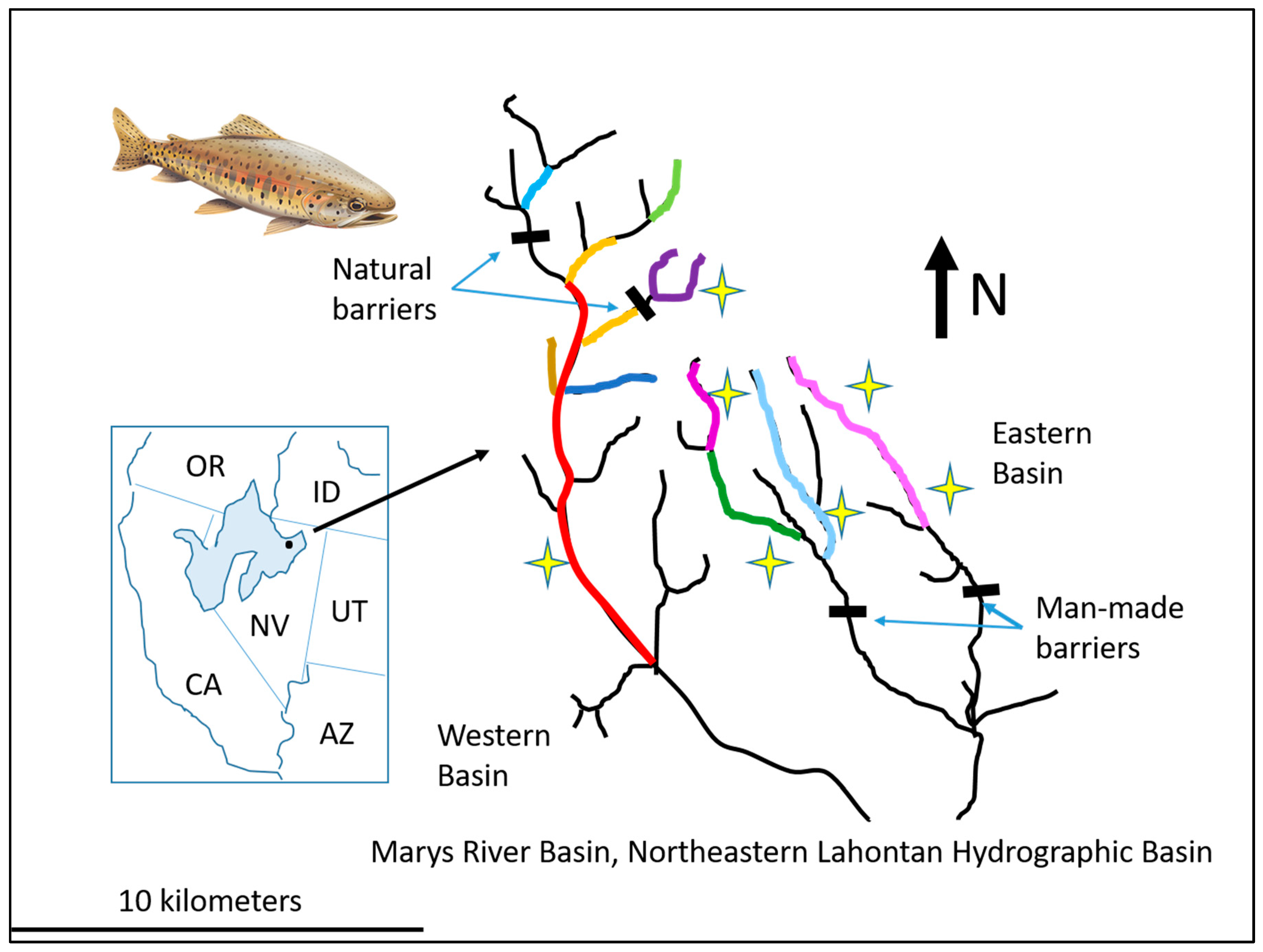
2.2.1. Classic Metapopulations
2.2.2. Mainland–Island Metapopulations
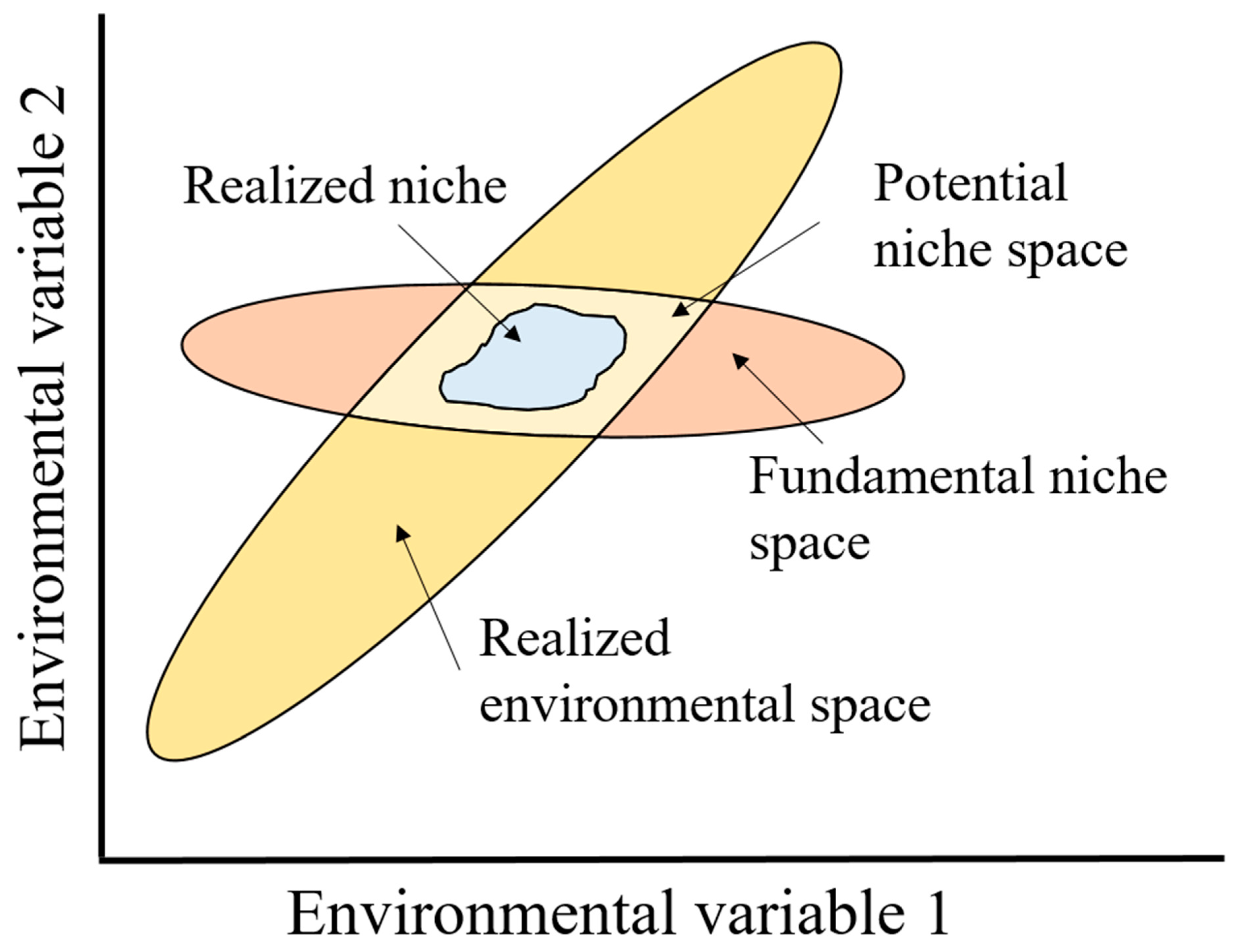
2.2.3. When Is Fragmented Habitat Fragmented? A Species Perspective
2.2.4. Metapopulation Theory and the Real World
2.3. Genetic Implications of Metapopulation Structure
2.4. Limitations of a Metapopulation Approac
3. Food Webs, Biodiversity and Fragmented Habitats
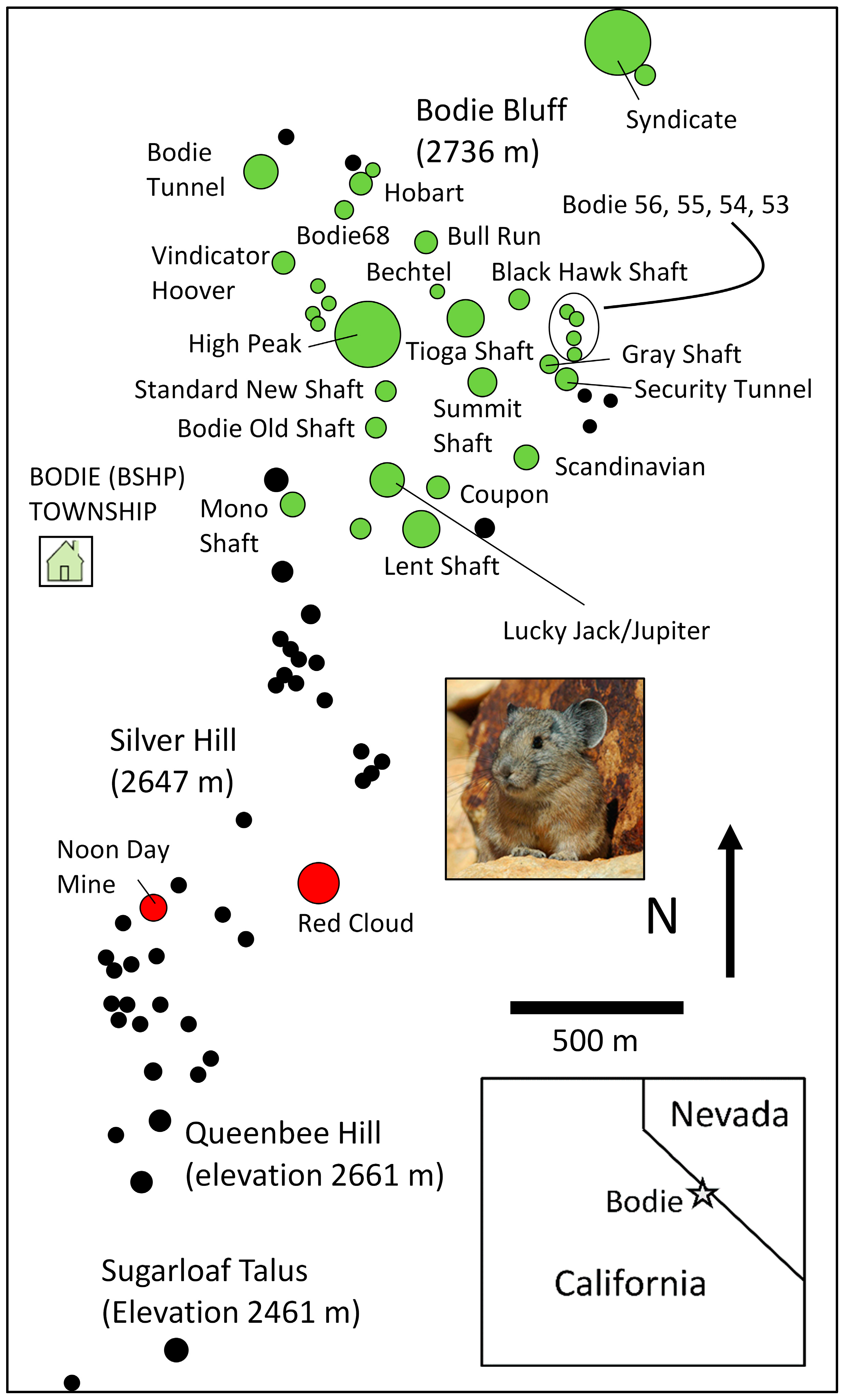
3.1. Foundation Species
3.2. Keystone Species
3.3. Keystone Communities
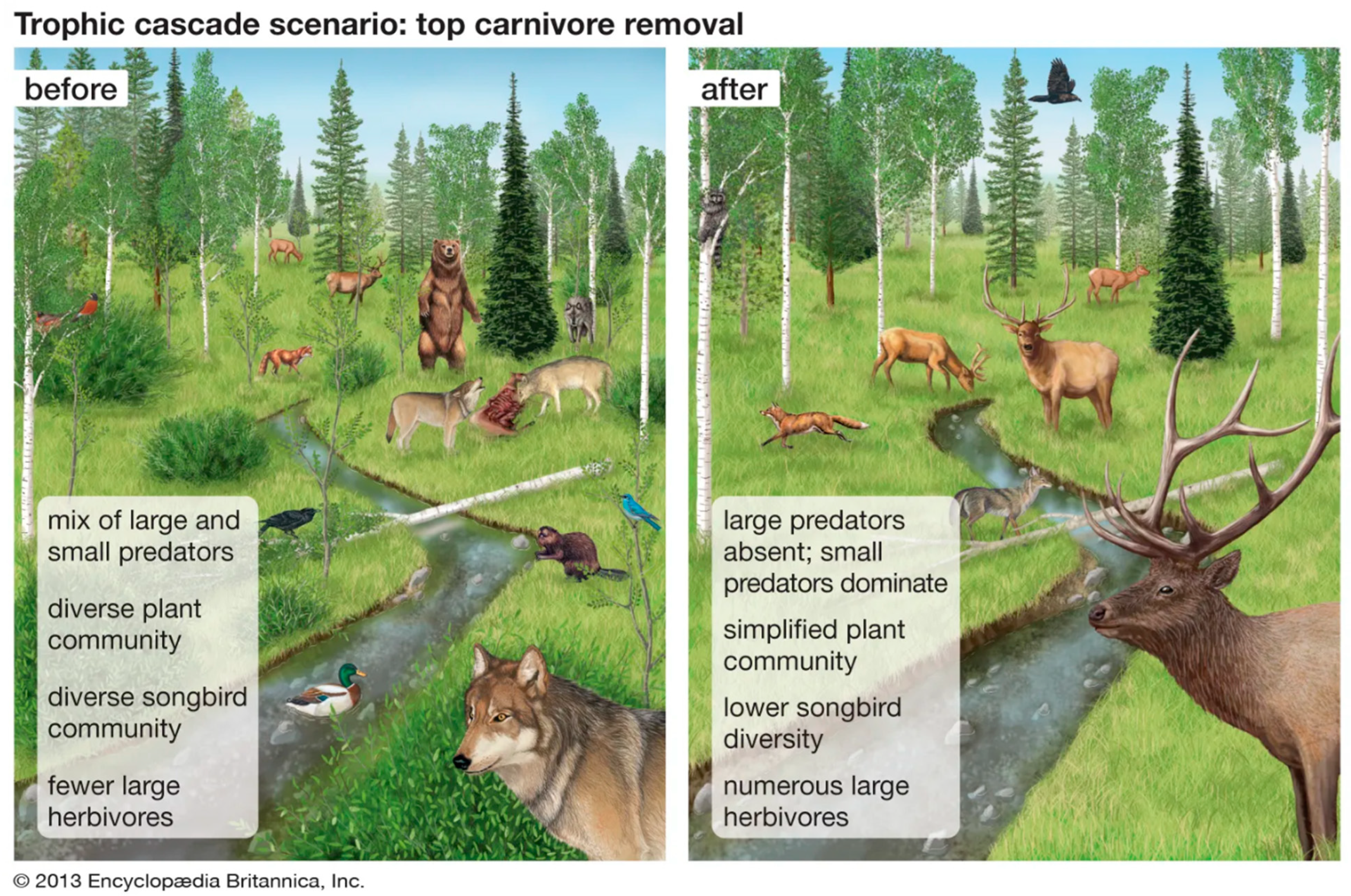
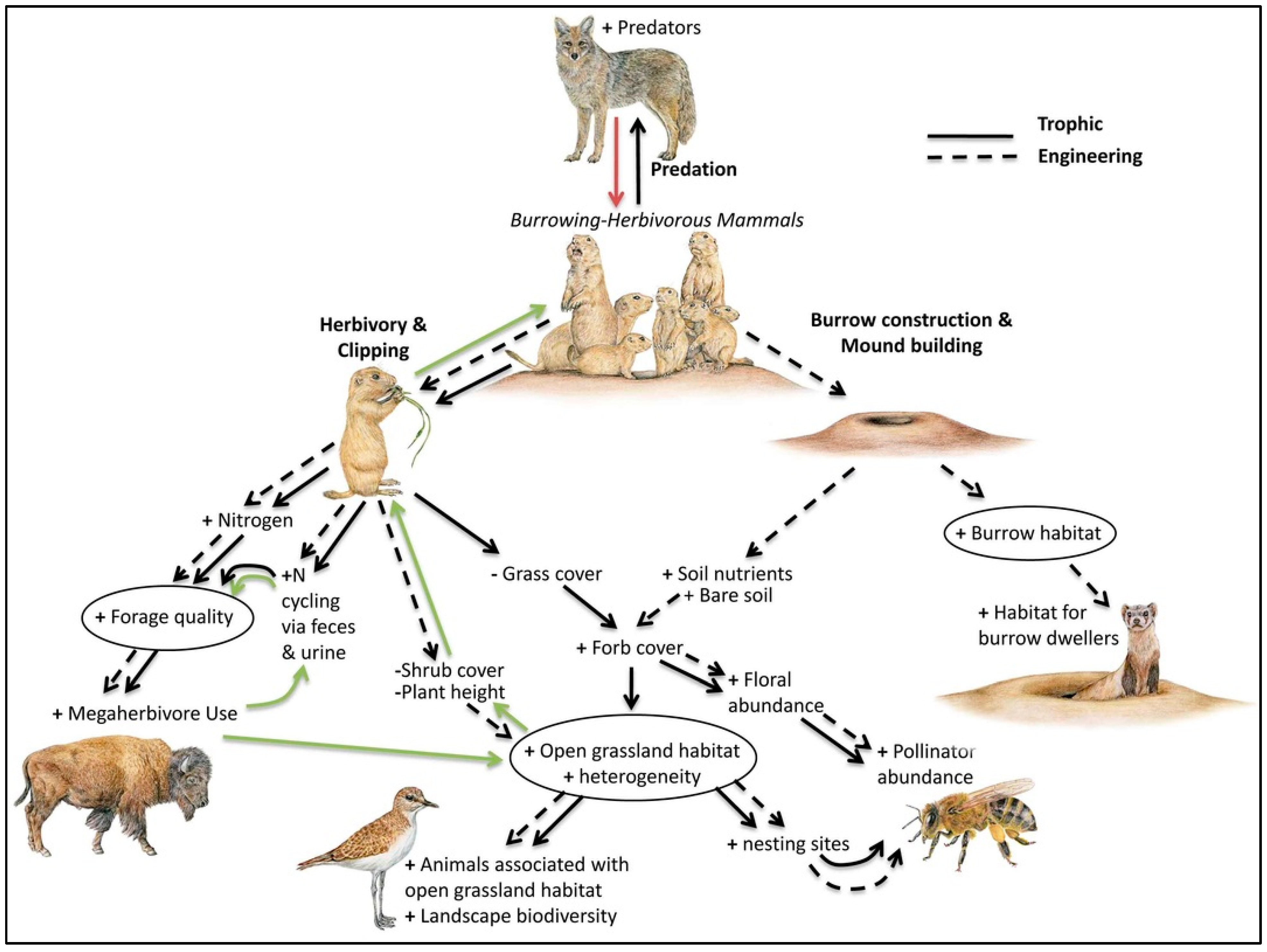
3.4. Habitat Fragmentation and Trophic Cascades—The Black-Tailed Prairie Dog
4. Ecosystem Function and Biodiversity in Fragmented Landscapes
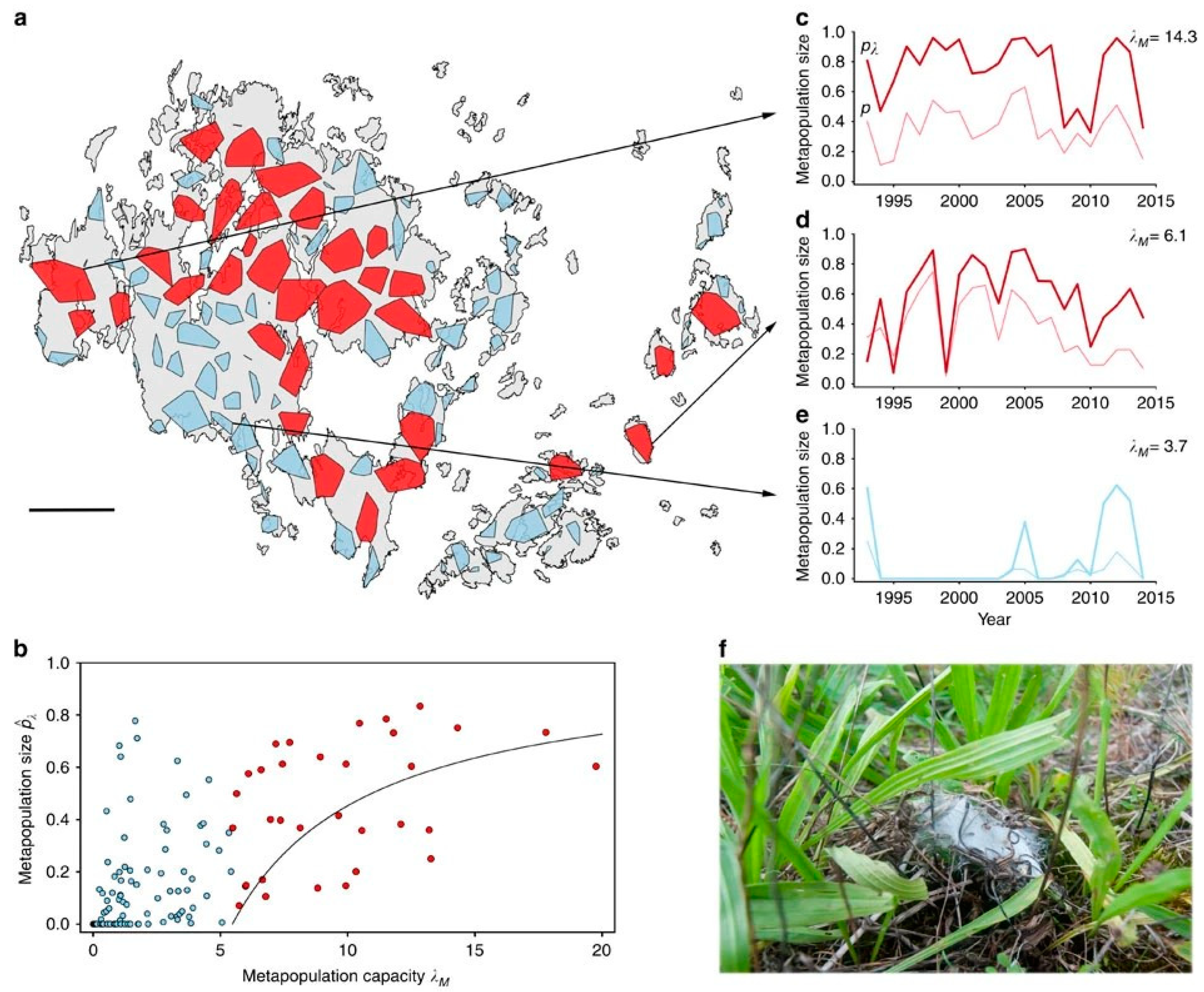
5. Biodiversity and Metapopulation Capacity
6. Landscape Genetics/Genomics and Biodiversity in a Fragmented World: Integration
7. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Avise, J.C.; Hamrick, J.L. (Eds.) Conservation Genetics: Case Histories from Nature; Chapman & Hall: New York, NY, USA, 1996. [Google Scholar]
- Hedrick, P.W. Recent developments in conservation genetics. Forest Ecol. Manag. 2004, 197, 3–19. [Google Scholar] [CrossRef]
- Frankham, R. Where are we in conservation genetics and where do we need to go? Conserv. Genet. 2010, 11, 661–663. [Google Scholar] [CrossRef]
- Kardos, M. Conservation genetics. Curr. Biol. 2021, 31, R1185–R1190. [Google Scholar] [CrossRef]
- Fahrig, L. Effects of habitat fragmentation on biodiversity. Annu. Rev. Ecol. Evol. Syst. 2003, 34, 487–515. [Google Scholar] [CrossRef]
- Fischer, J.; Lindenmayer, D.B. Landscape modification and habitat fragmentation: A synthesis. Global. Ecol. Biogeogr. 2007, 16, 265–280. [Google Scholar] [CrossRef]
- Berry, P.; Ogawa-Onishi, Y.; McVey, A. The vulnerability of threatened species: Adaptive capability and adaptation opportunity. Biology 2013, 2, 872–893. [Google Scholar] [CrossRef]
- Lindenmayer, D.B.; Fischer, J. Habitat Fragmentation and Landscape Change: An Ecological and Conservation Synthesis; Island Press: Washington, DC, USA, 2013. [Google Scholar]
- Haddad, N.M.; Brudvig, L.A.; Clobert, J.; Davies, K.F.; Gonzalez, A.; Holt, R.D.; Lovejoy, T.E.; Sexton, J.O.; Austin, M.P.; Collins, C.D.; et al. Habitat fragmentation and its lasting impact on Earth’s ecosystems. Sci. Adv. 2015, 1, e1500052. [Google Scholar] [CrossRef]
- Wilson, M.C.; Chen, X.Y.; Corlett, R.T.; Didham, R.K.; Ding, P.; Holt, R.D.; Holyoak, M.; Hu, G.; Hughes, A.C.; Jaing, L.; et al. Habitat fragmentation and biodiversity conservation: Key findings and future challenges. Landscape Ecol. 2016, 31, 219–227. [Google Scholar] [CrossRef]
- Fletcher, R.J., Jr.; Betts, M.G.; Damschen, E.I.; Hefley, T.J.; Hightower, J.; Smith, T.A.; Fortin, M.-J.; Haddad, N.M. Addressing the problem of scale that emerges with habitat fragmentation. Global. Ecol. Biogeogr. 2023, 32, 828–841. [Google Scholar] [CrossRef]
- Lienert, J. Habitat fragmentation effects on fitness of plant populations–a review. J. Nat. Conserv. 2004, 12, 53–72. [Google Scholar] [CrossRef]
- Stewart, J.R.; Lister, A.M.; Barnes, I.; Dalén, L. Refugia revisited: Individualistic responses of species in space and time. Proc. Roy. Soc. B-Biol. Sci. 2010, 277, 661–671. [Google Scholar] [CrossRef] [PubMed]
- Hannah, L.; Flint, L.; Syphard, A.D.; Moritz, M.A.; Buckley, L.B.; McCullough, I.M. Fine-grain modeling of species’ response to climate change: Holdouts, stepping-stones, and microrefugia. Trends Ecol. Evol. 2014, 29, 390–397. [Google Scholar] [CrossRef]
- Klingler, K.B.; Jahner, J.P.; Parchman, T.L.; Ray, C.; Peacock, M.M. Genomic variation in the American pika: Signatures of geographic isolation and implications for conservation. BMC Ecol. Evol. 2021, 21, 2. [Google Scholar] [CrossRef] [PubMed]
- Cullingham, C.I.; Stephens, T.R.; Swan, K.D.; Wilson, S.C.; Janes, J.K.; Matchett, M.R.; Griebel, R.; Moehrenschlager, A. Genetic analysis reveals hidden threats and new motivation for conservation translocation of black-tailed prairie dogs at the northern limit of their range. Glob. Ecol. Conserv. 2023, 46, e02591. [Google Scholar] [CrossRef]
- Keppel, G.; Stralberg, D.; Morelli, T.L.; Bátori, Z. Managing climate-change refugia to prevent extinctions. Trends Ecol. Evol. 2024, 39, 800–808. [Google Scholar] [CrossRef]
- Johansson, M.; Primmer, C.R.; Merilä, J. Does habitat fragmentation reduce fitness and adaptability? A case study of the common frog (Rana temporaria). Mol. Ecol. 2007, 16, 2693–2700. [Google Scholar] [CrossRef]
- Leimu, R.; Vergeer, P.; Angeloni, F.; Ouborg, N.J. Habitat fragmentation, climate change, and inbreeding in plants. Ann. Ny. Acad. Sci. 2010, 1195, 84–98. [Google Scholar] [CrossRef] [PubMed]
- Fraser, D.J.; Debes, P.V.; Bernatchez, L.; Hutchings, J.A. Population size, habitat fragmentation, and the nature of adaptive variation in a stream fish. Proc. Roy. Soc. B-Biol. Sci. 2014, 281, 20140370. [Google Scholar] [CrossRef] [PubMed]
- Cheptou, P.O.; Hargreaves, A.L.; Bonte, D.; Jacquemyn, H. Adaptation to fragmentation: Evolutionary dynamics driven by human influences. Philos. Trans. Roy. Soc. B 2017, 372, 20160037. [Google Scholar] [CrossRef]
- Mable, B.K. Conservation of adaptive potential and functional diversity: Integrating old and new approaches. Conserv. Genet. 2019, 20, 89–100. [Google Scholar] [CrossRef]
- Taylor Aiken, G.; Mabon, L. Where next for managed retreat: Bringing in history, community and under-researched places. Area 2024, 56, e12890. [Google Scholar] [CrossRef]
- Cincotta, R.P.; Wisnewski, J.; Engelman, R. Human population in the biodiversity hotspots. Nature 2000, 404, 990–992. [Google Scholar] [CrossRef] [PubMed]
- Hanski, I. Habitat loss, the dynamics of biodiversity, and a perspective on conservation. Ambio 2011, 40, 248–255. [Google Scholar] [CrossRef]
- Crist, E.; Mora, C.; Engelman, R. The interaction of human population, food production, and biodiversity protection. Science 2017, 356, 260–264. [Google Scholar] [CrossRef] [PubMed]
- Wilting, H.C.; Schipper, A.M.; Bakkenes, M.; Meijer, J.R.; Huijbregts, M.A. Quantifying biodiversity losses due to human consumption: A global-scale footprint analysis. Environ. Sci. Technol. 2017, 51, 3298–3306. [Google Scholar] [CrossRef]
- Elisha, O.D.; Felix, M.J. The loss of biodiversity and ecosystems: A threat to the functioning of our planet, economy and human society. IJEEDS 2020, 1, 30–44. [Google Scholar]
- Cafaro, P.; Hansson, P.; Götmark, F. Overpopulation is a major cause of biodiversity loss and smaller human populations are necessary to preserve what is left. Biol. Conserv. 2022, 272, 109646. [Google Scholar] [CrossRef]
- World Wildlife Fund. Living Planet Report 2024—A System in Peril; WWF: Gland, Switzerland, 2024. [Google Scholar]
- Yuan, R.; Zhang, N.; Zhang, Q. The impact of habitat loss and fragmentation on biodiversity in global protected areas. Sci. Total Environ. 2024, 931, 173004. [Google Scholar] [CrossRef]
- McCallum, H.; Dobson, A. Disease, habitat fragmentation and conservation. Proc. Roy. Soc. B-Biol. Sci. 2002, 269, 2041–2049. [Google Scholar] [CrossRef]
- Trudeau, K.M.; Britten, H.B.; Restani, M. Sylvatic plague reduces genetic variability in black-tailed prairie dogs. J. Wildlife Dis. 2004, 40, 205–211. [Google Scholar] [CrossRef]
- Ewers, R.M.; Didham, R.K. Confounding factors in the detection of species responses to habitat fragmentation. Biol. Rev. 2006, 81, 117–142. [Google Scholar] [CrossRef] [PubMed]
- Laurance, W.F.; Nascimento, H.E.; Laurance, S.G.; Andrade, A.; Ewers, R.M.; Harms, K.E.; Luizão, R.C.C.; Ribeiro, J.E. Habitat fragmentation, variable edge effects, and the landscape-divergence hypothesis. PLoS ONE 2007, 2, e1017. [Google Scholar] [CrossRef]
- Moilanen, A.; Wintle, B.A. The boundary-quality penalty: A quantitative method for approximating species responses to fragmentation in reserve selection. Conserv. Biol. 2007, 21, 355–364. [Google Scholar] [CrossRef]
- Gillespie, T.R.; Chapman, C.A. Forest fragmentation, the decline of an endangered primate, and changes in host–parasite interactions relative to an unfragmented forest. Am. J. Primatol. 2008, 70, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Porensky, L.M.; Young, T.P. Edge-effect interactions in fragmented and patchy landscapes. Conserv. Biol. 2013, 27, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Kiene, F.; Andriatsitohaina, B.; Ramsay, M.S.; Rakotondramanana, H.; Rakotondravony, R.; Radespiel, U.; Strube, C. Forest edges affect ectoparasite infestation patterns of small mammalian hosts in fragmented forests in Madagascar. Int. J. Parasitol. 2020, 50, 299–313. [Google Scholar] [CrossRef]
- DeWoody, J.A.; Harder, A.M.; Mathur, S.; Willoughby, J.R. The long-standing significance of genetic diversity in conservation. Mol. Ecol. 2021, 30, 4147–4154. [Google Scholar] [CrossRef]
- Johnson, K.H. Trophic-dynamic considerations in relating species diversity to ecosystem resilience. Biol. Rev. 2000, 75, 347–376. [Google Scholar]
- Oliver, T.H.; Heard, M.S.; Isaac, N.J.; Roy, D.B.; Procter, D.; Eigenbrod, F.; Freckleton, R.; Hector, A.; Orme, C.D.L.; Petchy, O.L.; et al. Biodiversity and resilience of ecosystem functions. Trends Ecol. Evol. 2015, 30, 673–684. [Google Scholar] [CrossRef]
- Truchy, A.; Angeler, D.G.; Sponseller, R.A.; Johnson, R.K.; McKie, B.G. Linking biodiversity, ecosystem functioning and services, and ecological resilience: Towards an integrative framework for improved management. Adv. Ecol. Res. 2015, 53, 55–96. [Google Scholar]
- Brussard, P.F. Update: Minimum viable populations: How many are too few? Restor. Manag. Notes 1985, 3, 21–25. [Google Scholar] [CrossRef]
- Soulé, M.E. (Ed.) Viable Populations for Conservation; Cambridge University Press: Cambridge, UK, 1987. [Google Scholar]
- Nunney, L.; Campbell, K.A. Assessing minimum viable population size: Demography meets population genetics. Trends Ecol. Evol. 1993, 8, 234–239. [Google Scholar] [CrossRef]
- Reed, D.H.; O’Grady, J.J.; Brook, B.W.; Ballou, J.D.; Frankham, R. Estimates of minimum viable population sizes for vertebrates and factors influencing those estimates. Biol. Conserv. 2003, 113, 23–34. [Google Scholar] [CrossRef]
- Hanski, I.; Gilpin, M.E. Metapopulation dynamics: Brief history and conceptual domain. Biol. J. Linn. Soc. 1991, 42, 3–16. [Google Scholar] [CrossRef]
- Simberloff, D. Habitat fragmentation and population extinction of birds. IBIS 1995, 137, S105–S111. [Google Scholar] [CrossRef]
- Rieman, B.E.; Dunham, J.B. Metapopulations and salmonids: A synthesis of life history patterns and empirical observations. Ecol. Freshw. Fish 2000, 9, 51–64. [Google Scholar] [CrossRef]
- MacNally, R.; Bennett, A.F. Species-specific predictions of the impact of habitat fragmentation: Local extinction of birds in the box-ironbark forests of central Victoria, Australia. Biol. Conserv. 1997, 82, 147–155. [Google Scholar] [CrossRef]
- Hill, M.F.; Caswell, H. Habitat fragmentation and extinction thresholds on fractal landscapes. Ecol. Lett. 1999, 2, 121–127. [Google Scholar] [CrossRef]
- Fahrig, L. Effect of habitat fragmentation on the extinction threshold: A synthesis. Ecol. Appl. 2002, 12, 346–353. [Google Scholar]
- Gu, W.; Heikkilä, R.; Hanski, I. Estimating the consequences of habitat fragmentation on extinction risk in dynamic landscapes. Landsc. Ecol. 2002, 17, 699–710. [Google Scholar] [CrossRef]
- Reed, D.H. Extinction risk in fragmented habitats. In Animal Conservation Forum; Cambridge University Press: Cambridge, UK, 2004; Volume 7, pp. 181–191. [Google Scholar]
- Levins, R. Evolution in Changing Environments; Princeton University Press: Princeton, NJ, USA, 1969. [Google Scholar]
- Levins, R. Extinction. Lect. Notes Math. 1970, 2, 75–107. [Google Scholar]
- Hanski, I. Metapopulation ecology. In Population Dynamics in Ecological Space and Time; Rhodes, O.E., Jr., Chesser, R.K., Smith, M.H., Eds.; University of Chicago Press: Chicago, IL, USA, 1996; Volume 1, p. 13. [Google Scholar]
- Hanski, I. Metapopulation Ecology; Oxford University Press: Oxford, UK, 1999. [Google Scholar]
- Hanski, I.; Simberloff, D. The metapopulation approach, its history, conceptual domain, and application to conservation. In Metapopulation Biology; Hanski, I., Gilpin, M.E., Eds.; Academic Press: San Diego, CA, USA, 1997; pp. 5–26. [Google Scholar]
- Harrison, S. Local extinction in a metapopulation context: An empirical evaluation. Biol. J. Linn. Soc. 1991, 42, 73–88. [Google Scholar] [CrossRef]
- Harrison, S.; Taylor, A.D. Empirical evidence for metapopulation dynamics. In Metapopulation Biology; Hanski, I., Gilpin, M.E., Eds.; Academic Press: San Diego, CA, USA, 1997; pp. 27–42. [Google Scholar]
- Lande, R. Risks of population extinction from demographic and environmental stochasticity and random catastrophes. Am. Nat. 1993, 142, 911–927. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.H.; Kepler, C.B.; Snyder, N.F.; Derrickson, S.R.; Dein, F.J.; Wiley, J.W.; Wunderle, J.M., Jr.; Lugo, A.E.; Graham, D.L.; Toone, W.D. Puerto Rican parrots and potential limitations of the metapopulation approach to species conservation. Conserv. Biol. 1994, 8, 114–123. [Google Scholar] [CrossRef]
- Elmhagen, B.; Angerbjörn, A. The applicability of metapopulation theory to large mammals. Oikos 2001, 94, 89–100. [Google Scholar] [CrossRef]
- Baguette, M. The classical metapopulation theory and the real, natural world: A critical appraisal. Basic Appl. Ecol. 2004, 5, 213–224. [Google Scholar] [CrossRef]
- Driscoll, D.A. How to find a metapopulation. Can. J. Zool. 2007, 85, 1031–1048. [Google Scholar] [CrossRef]
- Tao, Y.; Hastings, A.; Lafferty, K.D.; Hanski, I.; Ovaskainen, O. Landscape fragmentation overturns classical metapopulation thinking. Proc. Natl. Acad. Sci. USA 2024, 121, e2303846121. [Google Scholar] [CrossRef]
- Hanski, I. Effects of landscape pattern on competitive interactions. In Mosaic Landscapes and Ecological Processes; Forman, T.T., Ed.; Cambridge University Press: Cambridge, UK, 1995; pp. 203–224. [Google Scholar]
- Moilanen, A.; Hanski, I. Metapopulation dynamics: Effects of habitat quality and landscape structure. Ecology 1998, 79, 2503–2515. [Google Scholar] [CrossRef]
- Fleishman, E.; Ray, C.; Sjögren-Gulve, P.; Boggs, C.L.; Murphy, D.D. Assessing the roles of patch quality, area, and isolation in predicting metapopulation dynamics. Conserv. Biol. 2002, 16, 706–716. [Google Scholar] [CrossRef]
- Bohonak, A.J.; Jenkins, D.G. Ecological and evolutionary significance of dispersal by freshwater invertebrates. Ecol. Lett. 2003, 6, 783–796. [Google Scholar] [CrossRef]
- Pellet, J.; Fleishman, E.; Dobkin, D.S.; Gander, A.; Murphy, D.D. An empirical evaluation of the area and isolation paradigm of metapopulation dynamics. Biol. Conserv. 2007, 136, 483–495. [Google Scholar] [CrossRef]
- Vandewoestijne, S.; Schtickzelle, N.; Baguette, M. Positive correlation between genetic diversity and fitness in a large, well-connected metapopulation. BMC Biol. 2008, 6, 46. [Google Scholar] [CrossRef] [PubMed]
- Guiney, M.S.; Andow, D.A.; Wilder, T.T. Metapopulation structure and dynamics of an endangered butterfly. Basic Appl. Ecol. 2010, 11, 354–362. [Google Scholar] [CrossRef]
- Lamy, T.; Pointier, J.P.; Jarne, P.; David, P. Testing metapopulation dynamics using genetic, demographic and ecological data. Mol. Ecol. 2012, 21, 1394–1410. [Google Scholar] [CrossRef]
- Holyoak, M.; Lawler, S.P. Persistence of an extinction-prone predator-prey interaction through metapopulation dynamics. Ecology 1996, 77, 1867–1879. [Google Scholar] [CrossRef]
- Peacock, M.M.; Smith, A.T. The effect of habitat fragmentation on dispersal patterns, mating behavior, and genetic variation in a pika (Ochotona princeps) metapopulation. Oecologia 1997, 112, 524–533. [Google Scholar] [CrossRef]
- Moilanen, A.; Smith, A.T.; Hanski, I. Long-term dynamics in a metapopulation of the American pika. Am. Nat. 1998, 152, 530–542. [Google Scholar] [CrossRef]
- Sweanor, L.L.; Logan, K.A.; Hornocker, M.G. Cougar dispersal patterns, metapopulation dynamics, and conservation. Conserv. Biol. 2000, 14, 798–808. [Google Scholar] [CrossRef]
- Lambin, X.; Aars, J.; Piertney, S.B.; Telfer, S. Inferring pattern and process in small mammal metapopulations: Insights from ecological and genetic data. In Ecology, Genetics and Evolution of Metapopulations; Hanski, I., Gaggiotti, O.E., Eds.; Elsevier Academic Press: London, UK, 2004; pp. 515–540. [Google Scholar]
- Harrison, S.; Ray, C. Plant population viability and metapopulation-level processes. In Population Viability Analysis; Beissenger, S.R., McCullough, D.R., Eds.; University of Chicago Press: Chicago, IL, USA, 2002; pp. 109–122. [Google Scholar]
- Verheyen, K.; Vellend, M.; Van Calster, H.; Peterken, G.; Hermy, M. Metapopulation dynamics in changing landscapes: A new spatially realistic model for forest plants. Ecology 2004, 85, 3302–3312. [Google Scholar] [CrossRef]
- Dahlgren, J.P.; Ehrlén, J. Distribution patterns of vascular plants in lakes–the role of metapopulation dynamics. Ecography 2005, 28, 49–58. [Google Scholar] [CrossRef]
- Meulebrouck, K.; Verheyen, K.; Brys, R.; Hermy, M. Metapopulation viability of an endangered holoparasitic plant in a dynamic landscape. Ecography 2009, 32, 1040–1050. [Google Scholar] [CrossRef]
- Vermaat, J.E.; Vigneau, N.; Omtzigt, N. Viability of metapopulations of wetland birds in a fragmented landscape: Testing the key-patch approach. Biodivers. Conserv. 2008, 17, 2263–2273. [Google Scholar] [CrossRef]
- Huang, R.; Pimm, S.L.; Giri, C. Using metapopulation theory for practical conservation of mangrove endemic birds. Conserv. Biol. 2020, 34, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Neville, H.M.; Dunham, J.B.; Peacock, M.M. Landscape attributes and life history variability shape genetic structure of trout populations in a stream network. Landsc. Ecol. 2006, 21, 901–916. [Google Scholar] [CrossRef]
- Neville, H.; Dauwalter, D.; Peacock, M. Monitoring demographic and genetic responses of a threatened inland trout to habitat reconnection. Trans. Am. Fish. Soc. 2016, 145, 610–626. [Google Scholar] [CrossRef]
- Vera-Escalona, I.; Senthivasan, S.; Habit, E.; Ruzzante, D.E. Past, present, and future of a freshwater fish metapopulation in a threatened landscape. Conserv. Biol. 2018, 32, 849–859. [Google Scholar] [CrossRef]
- Snead, A.A.; Tatarenkov, A.; Taylor, D.S.; Marson, K.; Earley, R.L. Centrality to the metapopulation is more important for population genetic diversity than habitat area or fragmentation. Biology Lett. 2024, 20, 20240158. [Google Scholar] [CrossRef]
- Hanski, I.; Pakkala, T.; Kuussaari, M.; Lei, G. Metapopulation persistence of an endangered butterfly in a fragmented landscape. Oikos 1995, 72, 21–28. [Google Scholar] [CrossRef]
- Hanski, I.; Moilanen, A.; Pakkala, T.; Kuussaari, M. The quantitative incidence function model and persistence of an endangered butterfly metapopulation. Conserv. Biol. 1996, 10, 578–590. [Google Scholar] [CrossRef]
- Hanski, I. Metapopulation dynamics: From concepts and observations to predictive models. In Metapopulation Biology; Hanski, I., Gilpin, M.E., Eds.; Academic Press: San Diego, CA, USA, 1997; pp. 69–91. [Google Scholar]
- Hanski, I.; Ovaskainen, O. The metapopulation capacity of a fragmented landscape. Nature 2000, 404, 755–758. [Google Scholar] [CrossRef] [PubMed]
- Sjögren, P.E.R. Extinction and isolation gradients in metapopulations: The case of the pool frog (Rana lessonae). Biol. J. Linn. Soc. 1991, 42, 135–147. [Google Scholar] [CrossRef]
- Gulve, P.S. Distribution and extinction patterns within a northern metapopulation of the pool frog, Rana lessonae. Ecology 1994, 75, 1357–1367. [Google Scholar] [CrossRef]
- Sjögren-Gulve, P. Spatial movement patterns in frogs: Target-oriented dispersal in the pool frog, Rana lessonae. Ecoscience 1998, 5, 31–38. [Google Scholar] [CrossRef]
- Kuussaari, M.; Nieminen, M.; Hanski, I. An experimental study of migration in the Glanville fritillary butterfly Melitaea cinxia. J. Anim. Ecol. 1996, 65, 791–801. [Google Scholar] [CrossRef]
- Hanski, I.; Singer, M.C. Extinction-colonization dynamics and host-plant choice in butterfly metapopulations. Am. Nat. 2001, 158, 341–353. [Google Scholar] [CrossRef]
- Hanski, I.; Saastamoinen, M.; Ovaskainen, O. Dispersal-related life-history trade-offs in a butterfly metapopulation. J. Anim. Ecol. 2006, 75, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Hanski, I.; Alho, J.; Moilanen, A. Estimating the parameters of survival and migration of individuals in metapopulations. Ecology 2000, 81, 239–251. [Google Scholar] [CrossRef]
- Hanski, I.; Thomas, C.D. Metapopulation dynamics and conservation: A spatially explicit model applied to butterflies. Biol. Conserv. 1994, 68, 167–180. [Google Scholar] [CrossRef]
- Lewis, O.; Thomas, C.; Hill, J.; Brookes, M.; Crane, T.P.; Graneau, Y.; Mallet, J.; Rose, O. Three ways of assessing metapopulation structure in the butterfly Plebejus argus. Ecol. Entomol. 1997, 22, 283–293. [Google Scholar] [CrossRef]
- Schtickzelle, N.; Choutt, J.; Goffart, P.; Fichefet, V.; Baguette, M. Metapopulation dynamics and conservation of the marsh fritillary butterfly: Population viability analysis and management options for a critically endangered species in Western Europe. Biol. Conserv. 2005, 126, 569–581. [Google Scholar] [CrossRef]
- Griffiths, R.A.; Sewell, D.; McCrea, R.S. Dynamics of a declining amphibian metapopulation: Survival, dispersal and the impact of climate. Biol. Conserv. 2010, 143, 485–491. [Google Scholar] [CrossRef]
- Fronhofer, E.A.; Kubisch, A.; Hilker, F.M.; Hovestadt, T.; Poethke, H.J. Why are metapopulations so rare? Ecology 2012, 93, 1967–1978. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.; Murphy, D.D.; Ehrlich, P.R. Distribution of the bay checkerspot butterfly, Euphydryas editha bayensis: Evidence for a metapopulation model. Am. Nat. 1988, 132, 360–382. [Google Scholar] [CrossRef]
- Lawes, M.J.; Mealin, P.E.; Piper, S.E. Patch occupancy and potential metapopulation dynamics of three forest mammals in fragmented afromontane forest in South Africa. Conserv. Biol. 2000, 14, 1088–1098. [Google Scholar] [CrossRef]
- Onorato, D.P.; Hellgren, E.C.; Van Den Bussche, R.A.; Doan-Crider, D.L. Phylogeographic patterns within a metapopulation of black bears (Ursus americanus) in the American Southwest. J. Mammal. 2004, 85, 140–147. [Google Scholar] [CrossRef]
- Lawes, M.J.; Fly, S.; Piper, S.E. Gamebird vulnerability to forest fragmentation: Patch occupancy of the Crested guineafowl (Guttera edouardi) in Afromontane forests. Anim. Conserv. 2006, 9, 67–74. [Google Scholar] [CrossRef]
- Åström, J.; Bengtsson, J. Patch size matters more than dispersal distance in a mainland–island metacommunity. Oecologia 2011, 167, 747–757. [Google Scholar] [CrossRef] [PubMed]
- MacPherson, J.L.; Bright, P.W. Metapopulation dynamics and a landscape approach to conservation of lowland water voles (Arvicola amphibius). Landsc. Ecol. 2011, 26, 1395–1404. [Google Scholar] [CrossRef]
- Glorvigen, P.; Andreassen, H.P.; Ims, R.A. Local and regional determinants of colonisation-extinction dynamics of a riparian mainland-island root vole metapopulation. PLoS ONE 2013, 8, e56462. [Google Scholar] [CrossRef]
- Murphy, D.D.; Weiss, S.B. Ecological studies and the conservation of the bay checkerspot butterfly, Euphydryas editha bayensis. Biol. Conserv. 1988, 46, 183–200. [Google Scholar] [CrossRef]
- Ross, J.V. Stochastic models for mainland-island metapopulations in static and dynamic landscapes. Bull. Math. Biol. 2006, 68, 417–449. [Google Scholar] [CrossRef] [PubMed]
- Klingler, K.B.; Nichols, L.B.; Hekkala, E.R.; Stewart, J.A.; Peacock, M.M. Life on the edge—A changing genetic landscape within an iconic American pika metapopulation over the last half century. PeerJ 2023, 11, e15962. [Google Scholar] [CrossRef] [PubMed]
- Matthysen, E.; Adriaensen, F.; Dhondt, A.A. Dispersal distances of nuthatches, Sitta europaea, in a highly fragmented forest habitat. Oikos 1995, 72, 375–381. [Google Scholar] [CrossRef]
- Stow, A.J.; Sunnucks, P.; Briscoe, D.A.; Gardner, M. The impact of habitat fragmentation on dispersal of Cunningham’s skink (Egernia cunninghami): Evidence from allelic and genotypic analyses of microsatellites. Mol. Ecol. 2001, 10, 867–878. [Google Scholar] [CrossRef]
- Parmesan, C.; Singer, M.C. Mosaics of climatic stress across species’ ranges: Tradeoffs cause adaptive evolution to limits of climatic tolerance. Philos. Trans. Roy. Soc. B 2022, 377, 20210003. [Google Scholar] [CrossRef]
- Rodhouse, T.J.; Jeffress, M.R.; Sherrill, K.R.; Mohren, S.R.; Nordensten, N.J.; Magnuson, M.L.; Schwalm, D.; Castillo, J.A.; Shinderman, M.; Epps, C.W. Geographical variation in the influence of habitat and climate on site occupancy turnover in American pika (Ochotona princeps). Divers. Distrib. 2018, 24, 1506–1520. [Google Scholar] [CrossRef]
- Steffens, T.S.; Lehman, S.M. Lemur species-specific metapopulation responses to habitat loss and fragmentation. PLoS ONE 2018, 13, e0195791. [Google Scholar] [CrossRef]
- Driscoll, D. The frequency of metapopulations, metacommunities and nestedness in a fragmented landscape. Oikos 2008, 117, 297–309. [Google Scholar] [CrossRef]
- Pantel, J.H.; Lamy, T.; Dubart, M.; Pointier, J.P.; Jarne, P.; David, P. Metapopulation dynamics of multiple species in a heterogeneous landscape. Ecol. Monogr. 2022, 92, e1515. [Google Scholar] [CrossRef]
- Thomas, C.D. Dispersal and extinction in fragmented landscapes. Proc. Roy. Soc. B-Biol. Sci. 2000, 267, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Dunham, J.B.; Vinyard, G.L.; Rieman, B.E. Habitat fragmentation and extinction risk of Lahontan cutthroat trout. N. Am. J. Fish. Manag. 1997, 17, 1126–1133. [Google Scholar] [CrossRef]
- Dunham, J.B.; Rieman, B.E. Metapopulation structure of bull trout: Influences of physical, biotic, and geometrical landscape characteristics. Ecol. Appl. 1999, 9, 642–655. [Google Scholar] [CrossRef]
- Loehle, C. Effect of ephemeral stepping stones on metapopulations on fragmented landscapes. Ecol. Complex. 2007, 4, 42–47. [Google Scholar] [CrossRef]
- Kramer-Schadt, S.; Kaiser, T.S.; Frank, K.; Wiegand, T. Analyzing the effect of stepping stones on target patch colonisation in structured landscapes for Eurasian lynx. Landsc. Ecol. 2011, 26, 501–513. [Google Scholar] [CrossRef]
- Peacock, M.M.; Dochtermann, N.A. Evolutionary potential but not extinction risk of Lahontan cutthroat trout (Oncorhynchus clarkii henshawi) is associated with stream characteristics. Can. J. Fish. Aquat. Sci. 2012, 69, 615–626. [Google Scholar] [CrossRef]
- Edgardo, B.; Brandão, N.B.; Ribeiro, M.C.; Vieira, M.V. Dispersal movement through fragmented landscapes: The role of stepping stones and perceptual range. Landsc. Ecol. 2021, 36, 3249–3267. [Google Scholar]
- García-Antón, A.; Garza, V.; Traba, J. Connectivity in Spanish metapopulation of Dupont’s lark may be maintained by dispersal over medium-distance range and stepping stones. PeerJ 2021, 9, e11925. [Google Scholar] [CrossRef]
- Kindvall, O. Habitat heterogeneity and survival in a bush cricket metapopulation. Ecology 1996, 77, 207–214. [Google Scholar] [CrossRef]
- Graniero, P.A. The influence of landscape heterogeneity and local habitat effects on the response to competitive pressures in metapopulations. Ecol. Model. 2007, 203, 349–362. [Google Scholar] [CrossRef]
- Schooley, R.L.; Branch, L.C. Spatial heterogeneity in habitat quality and cross-scale interactions in metapopulations. Ecosystems 2007, 10, 846–853. [Google Scholar] [CrossRef]
- Shen, G.; He, F.; Waagepetersen, R.; Sun, I.F.; Hao, Z.; Chen, Z.S.; Yu, M. Quantifying effects of habitat heterogeneity and other clustering processes on spatial distributions of tree species. Ecology 2013, 94, 2436–2443. [Google Scholar] [CrossRef] [PubMed]
- Szacki, J. Spatially structured populations: How much do they match the classic metapopulation concept? Landsc. Ecol. 1999, 14, 369–379. [Google Scholar] [CrossRef]
- Vandermeer, J.; Carvajal, R. Metapopulation dynamics and the quality of the matrix. Am. Nat. 2001, 158, 211–220. [Google Scholar] [CrossRef]
- Ricketts, T.H. The matrix matters: Effective isolation in fragmented landscapes. Am. Nat. 2001, 158, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Jules, E.S.; Shahani, P. A broader ecological context to habitat fragmentation: Why matrix habitat is more important than we thought. J. Veg. Sci. 2003, 14, 459–464. [Google Scholar] [CrossRef]
- Prugh, L.R.; Hodges, K.E.; Sinclair, A.R.; Brashares, J.S. Effect of habitat area and isolation on fragmented animal populations. Proc. Natl. Acad. Sci. USA 2008, 105, 20770–20775. [Google Scholar] [CrossRef]
- Akçakaya, H.R.; Mills, G.; Doncaster, C.P. The role of metapopulations in conservation. Key Topics Cons. Biol. 2007, 1, 64–84. [Google Scholar]
- Hanski, I.; Schulz, T.; Wong, S.C.; Ahola, V.; Ruokolainen, A.; Ojanen, S.P. Ecological and genetic basis of metapopulation persistence of the Glanville fritillary butterfly in fragmented landscapes. Nat. Commun. 2017, 8, 14504. [Google Scholar] [CrossRef]
- Booy, G.; Hendriks, R.J.J.; Smulders, M.J.M.; Van Groenendael, J.M.; Vosman, B. Genetic diversity and the survival of populations. Plant biol. 2000, 2, 379–395. [Google Scholar] [CrossRef]
- Battey, C.J.; Ralph, P.L.; Kern, A.D. Space is the place: Effects of continuous spatial structure on analysis of population genetic data. Genetics 2020, 215, 193–214. [Google Scholar] [CrossRef] [PubMed]
- Lande, R. Genetics and demography in biological conservation. Science 1988, 241, 1455–1460. [Google Scholar] [CrossRef] [PubMed]
- Gilpin, M. The genetic effective size of a metapopulation. Biol. J. Linn. Soc. 1991, 42, 165–175. [Google Scholar] [CrossRef]
- Korn, H. Genetic, demographic, spatial, environmental and catastrophic effects on the survival probability of small populations of mammals. In Minimum Animal Populations; Remmert, H., Ed.; Springer: Berlin/Heidelberg, Germany, 1994; pp. 33–49. [Google Scholar]
- Pannell, J.R.; Charlesworth, B. Neutral genetic diversity in a metapopulation with recurrent local extinction and recolonization. Evolution 1999, 53, 664–676. [Google Scholar] [CrossRef] [PubMed]
- Pannell, J.R.; Charlesworth, B. Effects of metapopulation processes on measures of genetic diversity. Philos. Trans. Roy. Soc. B 2000, 355, 1851–1864. [Google Scholar] [CrossRef]
- Frankham, R. Effective population size/adult population size ratios in wildlife: A review. Genet Res. 1995, 66, 95–107. [Google Scholar] [CrossRef]
- Johnson, J.A.; Bellinger, M.R.; Toepfer, J.E.; Dunn, P. Temporal changes in allele frequencies and low effective population size in greater prairie-chickens. Mol. Ecol. 2004, 13, 2617–2630. [Google Scholar] [CrossRef]
- Johnson, W.E.; Onorato, D.P.; Roelke, M.E.; Land, E.D.; Cunningham, M.; Belden, R.C.; McBride, R.; Jansen, D.; Lotz, M.; Shindle, D.; et al. Genetic restoration of the Florida panther. Science 2010, 329, 1641–1645. [Google Scholar] [CrossRef]
- Waples, R.S.; Antao, T.; Luikart, G. Effects of overlapping generations on linkage disequilibrium estimates of effective population size. Genetics 2014, 197, 769–780. [Google Scholar] [CrossRef]
- Senneville, S.; Beaulieu, J.; Daoust, G.; Deslauriers, M.; Bousquet, J. Evidence for low genetic diversity and metapopulation structure in Canada yew (Taxus canadensis): Considerations for conservation. Can. J. Forest Res. 2001, 31, 110–116. [Google Scholar] [CrossRef]
- Gerlach, G.; Hoeck, H.N. Islands on the plains: Metapopulation dynamics and female biased dispersal in hyraxes (Hyracoidea) in the Serengeti National Park. Mol. Ecol. 2001, 10, 2307–2317. [Google Scholar] [CrossRef]
- Ortego, J.; Aparicio, J.M.; Cordero, P.J.; Calabuig, G. Individual genetic diversity correlates with the size and spatial isolation of natal colonies in a bird metapopulation. Proc. Roy. Soc. B-Biol. Sci. 2008, 275, 2039–2047. [Google Scholar] [CrossRef] [PubMed]
- Kunz, F.; Klinga, P.; Sittenthaler, M.; Schebeck, M.; Stauffer, C.; Grünschachner-Berger, V.; Hackländer, K.; Nopp-Mayr, U. Assessment of drivers of spatial genetic variation of a ground-dwelling bird species and its implications for conservation. Ecol. Evol. 2022, 12, e8460. [Google Scholar] [CrossRef]
- Fountain, T.; Nieminen, M.; Sirén, J.; Wong, S.C.; Lehtonen, R.; Hanski, I. Predictable allele frequency changes due to habitat fragmentation in the Glanville fritillary butterfly. Proc. Natl. Acad. Sci. USA 2016, 113, 2678–2683. [Google Scholar] [CrossRef]
- Saccheri, I.; Kuussaari, M.; Kankare, M.; Vikman, P.; Fortelius, W.; Hanski, I. Inbreeding and extinction in a butterfly metapopulation. Nature 1998, 392, 491–494. [Google Scholar] [CrossRef]
- DiLeo, M.F.; Nair, A.; Kardos, M.; Husby, A.; Saastamoinen, M. Demography and environment modulate the effects of genetic diversity on extinction risk in a butterfly metapopulation. Proc. Natl. Acad. Sci. USA 2024, 121, e2309455121. [Google Scholar] [CrossRef] [PubMed]
- Berendonk, T.U.; Spitze, K.; Kerfoot, W.C. Ephemeral metapopulations show high genetic diversity at regional scales. Ecology 2009, 90, 2670–2675. [Google Scholar] [CrossRef]
- Morrissey, M.B.; de Kerckhove, D.T. The maintenance of genetic variation due to asymmetric gene flow in dendritic metapopulations. Am. Nat. 2009, 174, 875–889. [Google Scholar] [CrossRef]
- Hand, B.K.; Muhlfeld, C.C.; Wade, A.A.; Kovach, R.P.; Whited, D.C.; Narum, S.R.; Matala, A.P.; Ackerman, M.W.; Garner, B.A.; Kimball, J.S.; et al. Climate variables explain neutral and adaptive variation within salmonid metapopulations: The importance of replication in landscape genetics. Mol. Ecol. 2016, 25, 689–705. [Google Scholar] [CrossRef]
- Huntsman, B.M.; Petty, J.T.; Sharma, S.; Merriam, E.R. More than a corridor: Use of a main stem stream as supplemental foraging habitat by a brook trout metapopulation. Oecologia 2016, 182, 463–473. [Google Scholar] [CrossRef]
- Ardren, W.R.; Bernall, S.R. Dams impact westslope cutthroat trout metapopulation structure and hybridization dynamics. Conserv. Genet. 2017, 18, 297–312. [Google Scholar] [CrossRef]
- Peacock, M.M.; Hekkala, E.R.; Kirchoff, V.S.; Heki, L.G. Return of a giant: DNA from archival museum samples helps to identify a unique cutthroat trout lineage formerly thought to be extinct. Roy. Soc. Open Sci. 2017, 4, 171253. [Google Scholar] [CrossRef] [PubMed]
- Arntzen, J.W.; van Belkom, J. ‘Mainland-island’ population structure of a terrestrial salamander in a forest-bocage landscape with little evidence for in situ ecological speciation. Sci. Rep. 2020, 10, 1700. [Google Scholar]
- Moncrief, N.D.; Roberts, J.H.; Hallerman, E.M.; Van Den Bussche, R.A.; Porter, J.H.; Dueser, R.D. Landscape genetics of a raccoon (Procyon lotor) metapopulation in an undeveloped coastal island system. J. Mammal. 2017, 98, 1137–1155. [Google Scholar] [CrossRef]
- Stacey, P.B.; Taper, M.L.; Johnson, V.A. Migration within metapopulations: The impact upon local population dynamics. In Metapopulation Biology; Hanski, I., Gilpin, M.E., Eds.; Academic Press: San Diego, CA, USA, 1997; pp. 267–291. [Google Scholar]
- Sonsthagen, S.A.; McClaren, E.L.; Doyle, F.I.; Titus, K.; Sage, G.K.; Wilson, R.E.; Gust, J.R.; Talbot, S.L. Identification of metapopulation dynamics among Northern Goshawks of the Alexander Archipelago, Alaska, and coastal British Columbia. Conserv. Genet. 2012, 13, 1045–1057. [Google Scholar] [CrossRef]
- Severaid, J.H. The Natural History of the Pikas (Mammalian Genus Ochotona). Doctoral Dissertation, University of California, Berkeley, CA, USA, 1955. [Google Scholar]
- Smith, A.T. The distribution and dispersal of pikas: Consequences of insular population structure. Ecology 1974, 55, 1112–1119. [Google Scholar] [CrossRef]
- Peacock, M.M. Determining natal dispersal patterns in a population of North American pikas (Ochotona princeps) using direct mark-resight and indirect genetic methods. Behav. Ecol. 1997, 8, 340–350. [Google Scholar] [CrossRef]
- Nichols, L. Fecal pellets of American pikas (Ochotona princeps) provide a crude chronometer for dating patch occupancy. West. N. Am. Naturalist. 2011, 70, 500–507. [Google Scholar] [CrossRef]
- Nichols, L.B.; Klingler, K.B.; Peacock, M.M. American pikas (Ochotona princeps) extirpated from the historic Masonic Mining District of eastern California. West. N. Am. Naturalist. 2016, 76, 163–171. [Google Scholar] [CrossRef]
- Reiss, J.; Bridle, J.R.; Montoya, J.M.; Woodward, G. Emerging horizons in biodiversity and ecosystem functioning research. Trends Ecol. Evol. 2009, 24, 505–514. [Google Scholar] [CrossRef]
- Villard, M.A.; Metzger, J.P. Beyond the fragmentation debate: A conceptual model to predict when habitat configuration really matters. J. Appl. Ecol. 2014, 51, 309–318. [Google Scholar] [CrossRef]
- Tilman, D.; Clark, M.; Williams, D.R.; Kimmel, K.; Polasky, S.; Packer, C. Future threats to biodiversity and pathways to their prevention. Nature 2017, 546, 73–81. [Google Scholar] [CrossRef]
- Brose, U.; Hillebrand, H. Biodiversity and ecosystem functioning in dynamic landscapes. Philos. Trans. Roy. Soc. B 2016, 371, 20150267. [Google Scholar] [CrossRef] [PubMed]
- Diamond, J.M. The island dilemma: Lessons of modern biogeographic studies for the design of natural reserves. Biol. Conserv. 1975, 7, 129–146. [Google Scholar] [CrossRef]
- Quinn, J.F.; Harrison, S.P. Effects of habitat fragmentation and isolation on species richness: Evidence from biogeographic patterns. Oecologia 1988, 75, 132–140. [Google Scholar] [CrossRef]
- Baz, A.; Garcia-Boyero, A. The SLOSS dilemma: A butterfly case study. Biodivers. Conserv. 1996, 5, 493–502. [Google Scholar] [CrossRef]
- Tjørve, E. How to resolve the SLOSS debate: Lessons from species-diversity models. Theor. Popul. Biol. 2010, 264, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Fahrig, L. Ecological responses to habitat fragmentation per se. Annu. Rev. Ecol. Evol. Syst. 2017, 48, 1–23. [Google Scholar] [CrossRef]
- Fahrig, L. Why do several small patches hold more species than few large patches? Global Ecol. Biogeogr. 2020, 29, 615–628. [Google Scholar] [CrossRef]
- Riva, F.; Fahrig, L. The disproportionately high value of small patches for biodiversity conservation. Conserv. Lett. 2022, 15, e12881. [Google Scholar] [CrossRef]
- Chase, J.M.; Liebergesell, M.; Sagouis, A.; May, F.; Blowes, S.A.; Berg, Å.; Bernard, E.; Brosi, B.J.; Cadotte, M.W.; Cayuela, L.; et al. FragSAD: A database of diversity and species abundance distributions from habitat fragments. Ecology 2019, 100, 2861. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wilson, M.; Hu, G.; Liu, J.; Wu, J.; Yu, M. How does habitat fragmentation affect the biodiversity and ecosystem functioning relationship? Landsc. Ecol. 2018, 33, 341–352. [Google Scholar] [CrossRef]
- Swihart, R.K.; Gehring, T.M.; Kolozsvary, M.B.; Nupp, T.E. Responses of ‘resistant’ vertebrates to habitat loss and fragmentation: The importance of niche breadth and range boundaries. Divers Distrib. 2003, 9, 1–18. [Google Scholar] [CrossRef]
- Layman, C.A.; Quattrochi, J.P.; Peyer, C.M.; Allgeier, J.E. Niche width collapse in a resilient top predator following ecosystem fragmentation. Ecol. Lett. 2007, 10, 937–944. [Google Scholar] [CrossRef]
- Birand, A.; Vose, A.; Gavrilets, S. Patterns of species ranges, speciation, and extinction. Am Nat. 2012, 179, 1–21. [Google Scholar] [CrossRef]
- Cagnolo, L.; Valladares, G.; Salvo, A.; Cabido, M.; Zak, M. Habitat fragmentation and species loss across three interacting trophic levels: Effects of life-history and food-web traits. Conserv. Biol. 2009, 23, 1167–1175. [Google Scholar] [CrossRef]
- Hagen, M.; Kissling, W.D.; Rasmussen, C.; De Aguiar, M.A.; Brown, L.E.; Carstensen, D.W.; Alves-Dos-Santos, I.; Dupont, Y.L.; Edwards, F.K.; Genini, J.; et al. Biodiversity, species interactions and ecological networks in a fragmented world. Adv. Ecol. Res. 2012, 46, 89–210. [Google Scholar]
- Valladares, G.; Cagnolo, L.; Salvo, A. Forest fragmentation leads to food web contraction. Oikos 2012, 121, 299–305. [Google Scholar] [CrossRef]
- Ellison, A.M.; Bank, M.S.; Clinton, B.D.; Colburn, E.A.; Elliott, K.; Ford, C.R.; Foster, D.R.; Kloeppel, B.D.; Knoepp, J.D.; Lovett, G.M.; et al. Loss of foundation species: Consequences for the structure and dynamics of forested ecosystems. Front. Ecol. Environ. 2005, 3, 479–486. [Google Scholar] [CrossRef]
- Baiser, B.; Whitaker, N.; Ellison, A.M. Modeling foundation species in food webs. Ecosphere 2013, 4, 1–14. [Google Scholar] [CrossRef]
- Castorani, M.C.; Reed, D.C.; Miller, R.J. Loss of foundation species: Disturbance frequency outweighs severity in structuring kelp forest communities. Ecology 2018, 99, 2442–2454. [Google Scholar] [CrossRef]
- Ellison, A.M. Foundation species, non-trophic interactions, and the value of being common. Iscience 2019, 13, 254–268. [Google Scholar] [CrossRef] [PubMed]
- Bangert, R.K.; Lonsdorf, E.V.; Wimp, G.M.; Shuster, S.M.; Fischer, D.; Schweitzer, J.A.; Allan, G.J.; Bailey, J.K.; Whitham, T.G. Genetic structure of a foundation species: Scaling community phenotypes from the individual to the region. Heredity 2008, 100, 121–131. [Google Scholar] [CrossRef]
- Yando, E.S.; Osland, M.J.; Jones, S.F.; Hester, M.W. Jump-starting coastal wetland restoration: A comparison of marsh and mangrove foundation species. Restor. Ecol. 2019, 27, 1145–1154. [Google Scholar] [CrossRef]
- Narwani, A.; Reyes, M.; Pereira, A.L.; Penson, H.; Dennis, S.R.; Derrer, S.; Spaak, P.; Matthews, B. Interactive effects of foundation species on ecosystem functioning and stability in response to disturbance. Proc. R. Soc. Ser. B Biol. Sci. 2019, 286, 20191857. [Google Scholar] [CrossRef]
- Thomsen, M.S.; Altieri, A.H.; Angelini, C.; Bishop, M.J.; Bulleri, F.; Farhan, R.; Frühling, V.M.M.; Gribben, P.E.; Harrison, S.B.; He, Q.; et al. Heterogeneity within and among co-occurring foundation species increases biodiversity. Nat. Commun. 2022, 13, 581. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Jiang, Q.; Shen, Y.; Wang, H.; Yao, X. Using landscape genomics to assess local adaptation of fruit trees to current and future climatic conditions. Fruit Res. 2024, 4, e003. [Google Scholar] [CrossRef]
- Sorte, C.J.; Davidson, V.E.; Franklin, M.C.; Benes, K.M.; Doellman, M.M.; Etter, R.J.; Hannigan, R.E.; Lubchenco, J.; Menge, B.A. Long-term declines in an intertidal foundation species parallel shifts in community composition. Global Change Biol. 2017, 23, 341–352. [Google Scholar] [CrossRef]
- Sarà, G.; Giommi, C.; Giacoletti, A.; Conti, E.; Mulder, C.; Mangano, M.C. Multiple climate-driven cascading ecosystem effects after the loss of a foundation species. Sci. Total Environ. 2021, 770, 144749. [Google Scholar] [CrossRef]
- Paine, R.T. Food web complexity and species diversity. Am. Nat. 1966, 100, 65–75. [Google Scholar] [CrossRef]
- Paine, R.T. A note on trophic complexity and community stability. Am. Nat. 1969, 103, 91–93. [Google Scholar] [CrossRef]
- Powers, M.E.; Tilman, D.; Estes, J.A.; Menge, B.A.; Bond, W.J.; Mills, L.S.; Daily, G.; Castilla, J.C.; Lubchenco, J.; Paine, R.T. Challenges in the quest for keystones: Identifying keystone species is difficult—But essential to understanding how loss of species will affect ecosystems. BioScience 1996, 46, 609–620. [Google Scholar] [CrossRef]
- Davic, R.D. Linking keystone species and functional groups: A new operational definition of the keystone species concept. Conserv Ecol. 2003, 7, r11. Available online: http://www.consecol.org/vol7/iss1/resp11/ (accessed on 8 March 2025). [CrossRef]
- Paine, R.T. A conversation on refining the concept of keystone species. Conserv. Biol. 1995, 9, 962–964. [Google Scholar] [CrossRef]
- Mills, L.S.; Soulé, M.E.; Doak, D.F. The keystone-species concept in ecology and conservation. BioScience 1993, 43, 219–224. [Google Scholar] [CrossRef]
- Bond, W.J. Keystone species. In Biodiversity and Ecosystem Function; Schulze, E., Mooney, H.A., Eds.; Springer: Berlin, Germany, 1994; pp. 237–253. [Google Scholar]
- Jordán, F. Keystone species and food webs. Philos. Trans. Roy. Soc. B 2009, 364, 1733–1741. [Google Scholar] [CrossRef]
- Valls, A.; Coll, M.; Christensen, V. Keystone species: Toward an operational concept for marine biodiversity conservation. Ecol. Monogr. 2015, 85, 29–47. [Google Scholar] [CrossRef]
- Luther, D.A.; Cooper, W.J.; Wolfe, J.D.; Bierregaard, R.O.; Gonzalez, A.; Lovejoy, T.E. Tropical forest fragmentation and isolation: Is community decay a random process? Glob. Ecol. Conserv. 2020, 23, e01168. [Google Scholar] [CrossRef]
- Navarrete, S.A.; Menge, B.A. Keystone predation and interaction strength: Interactive effects of predators on their main prey. Ecol. Monogr. 1996, 66, 409–429. [Google Scholar] [CrossRef]
- Suzuki, S.S.; Baba, Y.G.; Toju, H. Dynamics of species-rich predator-prey networks and seasonal alternations of core species. Nat. Ecol. Evol. 2023, 7, 1432–1443. [Google Scholar] [CrossRef]
- Roffler, G.H.; Eriksson, C.E.; Allen, J.M.; Levi, T. Recovery of a marine keystone predator transforms terrestrial predator-prey dynamics. Proc. Natl. Acad. Sci. USA 2023, 120, e2209037120. [Google Scholar] [CrossRef] [PubMed]
- Vandermeer, J.; Maruca, S. Indirect effects with a keystone predator: Coexistence and chaos. Theor. Popul. Biol. 1998, 54, 38–43. [Google Scholar] [CrossRef]
- Barrios-O’Neill, D.; Bertolini, C.; Collins, P.C. Trophic cascades and the transient keystone concept. Biol. Conserv. 2017, 212, 191–195. [Google Scholar] [CrossRef]
- Wallach, A.D.; Dekker, A.H.; Lurgi, M.; Montoya, J.M.; Fordham, D.A.; Ritchie, E.G. Trophic cascades in 3D: Network analysis reveals how apex predators structure ecosystems. Methods Ecol. Evol. 2017, 8, 135–142. [Google Scholar] [CrossRef]
- Traveset, A.; Tur, C.; Eguíluz, V.M. Plant survival and keystone pollinator species in stochastic coextinction models: Role of intrinsic dependence on animal-pollination. Sci. Rep. 2017, 7, 6915. [Google Scholar] [CrossRef]
- Easton-Calabria, A.; Demary, K.C.; Oner, N.J. Beyond pollination: Honey bees (Apis mellifera) as zootherapy keystone species. Front. Ecol. Evol. 2019, 6, 161. [Google Scholar] [CrossRef]
- Simpson, D.T.; Weinman, L.R.; Genung, M.A.; Roswell, M.; MacLeod, M.; Winfree, R. Many bee species, including rare species, are important for function of entire plant–pollinator networks. Proc. Roy. Soc. B-Biol. Sci. 2022, 289, 20212689. [Google Scholar] [CrossRef]
- Gove, A.D.; Majer, J.D.; Dunn, R.R. A keystone ant species promotes seed dispersal in a “diffuse” mutualism. Oecologia 2007, 153, 687–697. [Google Scholar] [CrossRef]
- Longland, W.S.; Ostoja, S.M. Ecosystem services from keystone species: Diversionary seeding and seed-caching desert rodents can enhance Indian rice grass seedling establishment. Restor. Ecol. 2013, 21, 285–291. [Google Scholar] [CrossRef]
- Mello, M.A.R.; Rodrigues, F.A.; Costa, L.D.F.; Kissling, W.D.; Şekercioğlu, Ç.H.; Marquitti, F.M.D.; Kalko, E.K.V. Keystone species in seed dispersal networks are mainly determined by dietary specialization. Oikos 2015, 124, 1031–1039. [Google Scholar] [CrossRef]
- Vitali, A.; Sasal, Y.; Vázquez, D.P.; Miguel, M.F.; Rodríguez-Cabal, M.A. The disruption of a keystone interaction erodes pollination and seed dispersal networks. Ecology 2022, 103, e03547. [Google Scholar] [CrossRef] [PubMed]
- Byers, J.E.; Cuddington, K.; Jones, C.G.; Talley, T.S.; Hastings, A.; Lambrinos, J.G.; Crooks, J.A.; Wilson, W.G. Using ecosystem engineers to restore ecological systems. Trends Ecol. Evol. 2006, 21, 493–500. [Google Scholar] [CrossRef]
- de Visser, S.; Thébault, E.; de Ruiter, P.C. Ecosystem engineers, keystone species. In Ecological Systems: Selected Entries from the Encyclopedia of Sustainability Science and Technology; Leemans, R., Ed.; Springer: New York, NY, USA, 2012; pp. 59–68. [Google Scholar]
- Johnson, S.A.; Ober, H.K.; Adams, D.C. Are keystone species effective umbrellas for habitat conservation? A spatially explicit approach. J. Nat. Conserv. 2017, 37, 47–55. [Google Scholar] [CrossRef]
- Lomolino, M.V.; Smith, G.A. Terrestrial vertebrate communities at black-tailed prairie dog (Cynomys ludovicianus) towns. Biol. Conserv. 2004, 115, 89–100. [Google Scholar] [CrossRef]
- Ishida, Y.; Gugala, N.A.; Georgiadis, N.J.; Roca, A.L. Evolutionary and demographic processes shaping geographic patterns of genetic diversity in a keystone species, the African forest elephant (Loxodonta cyclotis). Ecol. Evol. 2018, 8, 4919–4931. [Google Scholar] [CrossRef] [PubMed]
- Boswell, G.P.; Britton, N.F.; Franks, N.R. Habitat fragmentation, percolation theory and the conservation of a keystone species. Proc. Roy. Soc. B-Biol. Sci. 1998, 265, 1921–1925. [Google Scholar] [CrossRef]
- Williams, D.A.; Wang, Y.; Borchetta, M.; Gaines, M.S. Genetic diversity and spatial structure of a keystone species in fragmented pine rockland habitat. Biol. Conserv. 2007, 138, 256–268. [Google Scholar] [CrossRef]
- Ripple, W.J.; Beschta, R.L. Trophic cascades in Yellowstone: The first 15 years after wolf reintroduction. Biol. Conserv. 2012, 145, 205–213. [Google Scholar] [CrossRef]
- Kareiva, P.; Estes, J.A.; Marvier, M. Restore protected status for gray wolves. Science 2021, 373, 632. [Google Scholar] [CrossRef]
- Ripple, W.J.; Beschta, R.L.; Wolf, C.; Painter, L.E.; Wirsing, A.J. The strength of the Yellowstone trophic cascade after wolf reintroduction. Glob. Ecol. Conserv. 2025, 58, e03428. [Google Scholar] [CrossRef]
- Kitchell, J.F.; Boggs, C.H.; He, X.; Walters, C.J. Keystone predators in the central Pacific. In Ecosystem Approaches for Fisheries Management; Proceedings of the Symposium on Ecosystem Considerations in Fisheries Management; 1999; pp. 665–683. Available online: https://repository.library.noaa.gov/ (accessed on 8 March 2025).
- Griffith, G.P.; Strutton, P.G.; Semmens, J.M.; Fulton, E.A. Identifying important species that amplify or mitigate the interactive effects of human impacts on marine food webs. Conserv. Biol. 2019, 33, 403–412. [Google Scholar] [CrossRef]
- Motivarash Yagnesh, B.; Fofandi Durga, C.; Dabhi Raj, M.; Makrani Rehanavaz, A.; Tanna Poojaben, D. Importance of sharks in ocean ecosystem. J. Entomol. Zool Stud. 2020, 8, 611–613. [Google Scholar]
- Banks-Leite, C.; Ewers, R.M.; Folkard-Tapp, H.; Fraser, A. Countering the effects of habitat loss, fragmentation, and degradation through habitat restoration. One Earth 2020, 3, 672–676. [Google Scholar] [CrossRef]
- Lindenmayer, D.B.; Westgate, M.J. Are flagship, umbrella and keystone species useful surrogates to understand the consequences of landscape change? Curr. Landsc. Ecol. Rep. 2020, 5, 76–84. [Google Scholar] [CrossRef]
- Christianou, M.; Ebenman, B. Keystone species and vulnerable species in ecological communities: Strong or weak interactors? J. Theor. Biol. 2005, 235, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Ebenman, B.; Jonsson, T. Using community viability analysis to identify fragile systems and keystone species. Trends Ecol. Evol. 2005, 20, 568–575. [Google Scholar] [CrossRef]
- Steneck, R.S.; Graham, M.H.; Bourque, B.J.; Corbett, D.; Erlandson, J.M.; Estes, J.A.; Tegner, M.J. Kelp forest ecosystems: Biodiversity, stability, resilience and future. Environ. Conserv. J. 2002, 29, 436–459. [Google Scholar] [CrossRef]
- Foster, M.S.; Schiel, D.R. Loss of predators and the collapse of southern California kelp forests: Alternatives, explanations and generalizations. J. Exp. Mar. Biol. Ecol. 2010, 393, 59–70. [Google Scholar] [CrossRef]
- Beschta, R.L.; Ripple, W.J. Riparian vegetation recovery in Yellowstone: The first two decades after wolf reintroduction. Biol. Conserv. 2016, 198, 93–103. [Google Scholar] [CrossRef]
- Atkins, J.L.; Long, R.A.; Pansu, J.; Daskin, J.H.; Potter, A.B.; Stalmans, M.E.; Tarnita, C.E.; Pringle, R.M. Cascading impacts of large-carnivore extirpation in an African ecosystem. Science 2019, 364, 173–177. [Google Scholar] [CrossRef]
- Polis, G.A.; Strong, D.R. Food web complexity and community dynamics. Am. Nat. 1996, 147, 813–846. [Google Scholar] [CrossRef]
- Petchey, O.L.; Downing, A.L.; Mittelbach, G.G.; Persson, L.; Steiner, C.F.; Warren, P.H.; Woodward, G. Species loss and the structure and functioning of multitrophic aquatic systems. Oikos 2004, 10, 467–478. [Google Scholar] [CrossRef]
- Oro, D.; Martínez-Abraín, A. Ecological non-equilibrium and biological conservation. Biol. Conserv. 2023, 286, 110258. [Google Scholar] [CrossRef]
- Mouquet, N.; Gravel, D.; Massol, F.; Calcagno, V. Extending the concept of keystone species to communities and ecosystems. Ecol. Lett. 2013, 16, 1–8. [Google Scholar] [CrossRef]
- Loreau, M.; Mouquet, N.; Gonzalez, A. Biodiversity as spatial insurance in heterogeneous landscapes. Proc. Natl. Acad. Sci. USA 2003, 100, 12765–12770. [Google Scholar] [CrossRef]
- Leibold, M.A.; Holyoak, M.; Mouquet, N.; Amarasekare, P.; Chase, J.M.; Hoopes, M.F.; Holt, R.D.; Shurin, J.B.; Law, R.; Tilman, D.; et al. The metacommunity concept: A framework for multi-scale community ecology. Ecol. Lett. 2004, 7, 601–613. [Google Scholar] [CrossRef]
- Leibold, M.A.; Norberg, J. Biodiversity in metacommunities: Plankton as complex adaptive systems? Limnol. Oceanogr. 2004, 49, 1278–1289. [Google Scholar] [CrossRef]
- Jamoneau, A.; Chabrerie, O.; Closset-Kopp, D.; Decocq, G. Fragmentation alters beta-diversity patterns of habitat specialists within forest metacommunities. Ecography 2012, 35, 124–133. [Google Scholar] [CrossRef]
- Willig, M.R.; Presley, S.J.; Cullerton, E.I.A. Canonical metacommunity structure over 3 decades: Ecologically consistent but spatially dynamic patterns in a hurricane-prone montane forest. Oecologia 2021, 196, 919–933. [Google Scholar] [CrossRef]
- Resetarits, E.J.; Cathey, S.E.; Leibold, M.A. Testing the keystone community concept: Effects of landscape, patch removal, and environment on metacommunity structure. Ecology 2018, 99, 57–67. [Google Scholar] [CrossRef]
- Yang, X.; Tan, J.; Sun, K.H.; Jiang, L. Experimental demonstration of the importance of keystone communities for maintaining metacommunity biodiversity and ecosystem functioning. Oecologia 2020, 193, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Davidson, A.D.; Thiesen Brum, F.; Houts, M.; Menefee, M.; Williamson, M.; Sterling Krank, L.; Van Pelt, B.; Augustine, D.J. Potential landscapes for conservation of the black-tailed prairie dog ecosystem. Divers. Distrib. 2025, 31, e13945. [Google Scholar] [CrossRef]
- Sierra–Corona, R.; Davidson, A.; Fredrickson, E.L.; Luna-Soria, H.; Suzan-Azpiri, H.; Ponce-Guevara, E.; Ceballos, G. Black-tailed prairie dogs, cattle, and the conservation of North America’s arid grasslands. PLoS ONE 2015, 10, e0118602. [Google Scholar] [CrossRef]
- Magle, S.B.; Ruell, E.W.; Antolin, M.F.; Crooks, K.R. Population genetic structure of black-tailed prairie dogs, a highly interactive species, in fragmented urban habitat. J. Mammal. 2010, 91, 326–335. [Google Scholar] [CrossRef]
- Parker, R.A.; Duchardt, C.J.; Dwyer, A.M.; Painter, C.; Pierce, A.K.; Michels, T.J.; Wunder, M.B. Trophic ecology warrants multispecies management in a grassland setting: Proposed species interactions on black-tailed prairie dog colonies. Rangelands 2019, 41, 135–144. [Google Scholar] [CrossRef]
- Roach, J.L.; Stapp, P.; Van Horne, B.; Antolin, M.F. Genetic structure of a metapopulation of black-tailed prairie dogs. J. Mammal. 2001, 82, 946–959. [Google Scholar] [CrossRef]
- Lomolino, M.V.; Smith, G.A.; Vidal, V. Long-term persistence of prairie dog towns: Insights for designing networks of prairie reserves. Biol. Conserv. 2004, 115, 111–120. [Google Scholar] [CrossRef]
- Jones, P.H.; Britten, H.B. The absence of concordant population genetic structure in the black-tailed prairie dog and the flea, Oropsylla hirsuta, with implications for the spread of Yersinia pestis. Mol. Ecol. 2010, 19, 2038–2049. [Google Scholar] [CrossRef]
- Collinge, S.K.; Johnson, W.C.; Ray, C.; Matchett, R.; Grensten, J.; Cully, J.F., Jr.; Gage, K.L.; Kosoy, M.Y.; Loye, J.E.; Martin, A.P. Landscape structure and plague occurrence in black-tailed prairie dogs on grasslands of the western USA. Landsc. Ecol. 2005, 20, 941–955. [Google Scholar] [CrossRef]
- George, D.B.; Webb, C.T.; Pepin, K.M.; Savage, L.T.; Antolin, M.F. Persistence of black-tailed prairie-dog populations affected by plague in northern Colorado, USA. Ecology 2013, 94, 1572–1583. [Google Scholar] [CrossRef]
- Hoogland, J.L. The Black-Tailed Prairie Dog: Social Life of a Burrowing Mammal; Chicago University Press: Chicago, IL, USA, 1995. [Google Scholar]
- Goguen, C.B. Habitat use by mountain plovers in prairie dog colonies in northeastern New Mexico. J. Field Ornithol. 2012, 83, 154–165. [Google Scholar] [CrossRef]
- Baker, B.W.; Augustine, D.J.; Sedgwick, J.A.; Lubow, B.C. Ecosystem engineering varies spatially: A test of the vegetation modification paradigm for prairie dogs. Ecography 2013, 36, 230–239. [Google Scholar] [CrossRef]
- Duchardt, C.J.; Beck, J.L.; Augustine, D.J. Mountain plover habitat selection and nest survival in relation to weather variability and spatial attributes of black-tailed prairie dog disturbance. Condor 2020, 122, duz059. [Google Scholar] [CrossRef]
- Haun, A.J.; Dreelin, R.A.; Boyce, A.J. Prairie dog towns increase grassland bird diversity at the landscape scale. Wilson J. Ornithol. 2024, 136, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Shaughnessy, M.J., Jr.; Cifelli, R.L. Influence of black-tailed prairie dog towns (Cynomys ludovicianus) on carnivore distributions in the Oklahoma panhandle. West. N. Am. Naturalist. 2004, 64, 184–192. [Google Scholar]
- Shipley, B.K.; Reading, R.P. A comparison of herpetofauna and small mammal diversity on black-tailed prairie dog (Cynomys ludovicianus) colonies and non-colonized grasslands in Colorado. J. Arid Environ. 2006, 66, 27–41. [Google Scholar] [CrossRef]
- Bangert, R.K.; Slobodchikoff, C.N. Conservation of prairie dog ecosystem engineering may support arthropod beta and gamma diversity. J. Arid Environ. 2006, 67, 100–115. [Google Scholar] [CrossRef]
- Goodrich, J.M.; Buskirk, S.W. Spacing and ecology of North American badgers (Taxidea taxus) in a prairie-dog (Cynomys leucurus) complex. J. Mammal. 1998, 79, 171–179. [Google Scholar] [CrossRef]
- Eads, D.A.; Biggins, D.E. Aboveground predation by an American badger (Taxidea taxus) on black-tailed prairie dogs (Cynomys ludovicianus). West. N. Am. Naturalist. 2008, 68, 396–401. [Google Scholar] [CrossRef]
- Grassel, S.M.; Rachlow, J.L. When generalists behave as specialists: Local specialization by American badgers (Taxidea taxus). Can. J. Zool. 2018, 96, 592–599. [Google Scholar] [CrossRef]
- Livieri, T.M.; Forrest, S.C.; Matchett, M.R.; Breck, S. Conserving endangered black-footed ferrets: Biological threats, political challenges, and lessons learned. In Reference Module in Earth Systems and Environmental Sciences; Elsevier: New York, NY, USA, 2021. [Google Scholar]
- Rocke, T.E. 13 Plague and distemper threats to black-footed ferret conservation. In Wildlife Disease and Health in Conservation; Jessup, D.A., Radcliff, R.W., Eds.; John Hopkins University Press: Baltimore, MD, USA, 2023; pp. 217–236. [Google Scholar]
- Davidson, A.D.; Detling, J.K.; Brown, J.H. Ecological roles and conservation challenges of social, burrowing, herbivorous mammals in the world’s grasslands. Front. Ecol. Environ. 2012, 10, 477–486. [Google Scholar] [CrossRef]
- Klute, D.S.; Ayers, L.W.; Green, M.T.; Howe, W.H.; Jones, S.L.; Sohaffer, J.A.; Sheffield, S.R.; Zimmerman, T.S. Status Assessment and Conservation Plan for the Western Burrowing Owl in the United States; Biological Technical Publication FWS/BTP-R6001-2003; U.S. Department of Interior, Fish and Wildlife Service: Washington, DC, USA, 2003.
- Conway, C.J. Spatial and temporal patterns in population trends and burrow usage of burrowing owls in North America. J. Raptor. Res. 2018, 52, 129–142. [Google Scholar] [CrossRef]
- Johnson, W.C.; Collinge, S.K. Landscape effects on black-tailed prairie dog colonies. Biol. Conserv. 2004, 115, 487–497. [Google Scholar] [CrossRef]
- Windell, R.M.; Bailey, L.L.; Livieri, T.M.; Eads, D.A.; Biggins, D.E.; Breck, S.W. Coyote use of prairie dog colonies is most frequent in areas used by American badgers. J. Mammal. 2024, 105, 1309–1321. [Google Scholar] [CrossRef]
- Butler, A.R.; Bly, K.L.S.; Harris, H.; Inman, R.M.; Moehrenschlager, A.; Schwalm, D.; Jachowski, D.S. Life on the edge: Habitat fragmentation limits expansion of a restored carnivore. Anim. Conserv. 2021, 24, 108–119. [Google Scholar] [CrossRef]
- Sackett, L.C.; Cross, T.B.; Jones, R.T.; Johnson, W.C.; Ballare, K.; Ray, C.; Collinge, S.K.; Martin, A.P. Connectivity of prairie dog colonies in an altered landscape: Inferences from analysis of microsatellite DNA variation. Conserv. Genet. 2012, 13, 407–418. [Google Scholar] [CrossRef]
- Castellanos-Morales, G.; Gasca-Pineda, J.; Ceballos, G.; Ortega, J. Genetic variation in a peripheral and declining population of black-tailed prairie dogs (Cynomys ludovicianus) from Mexico. J. Mammal. 2014, 95, 467–479. [Google Scholar] [CrossRef]
- Vaughn, C.C. Biodiversity losses and ecosystem function in freshwaters: Emerging conclusions and research directions. BioScience 2010, 60, 25–35. [Google Scholar] [CrossRef]
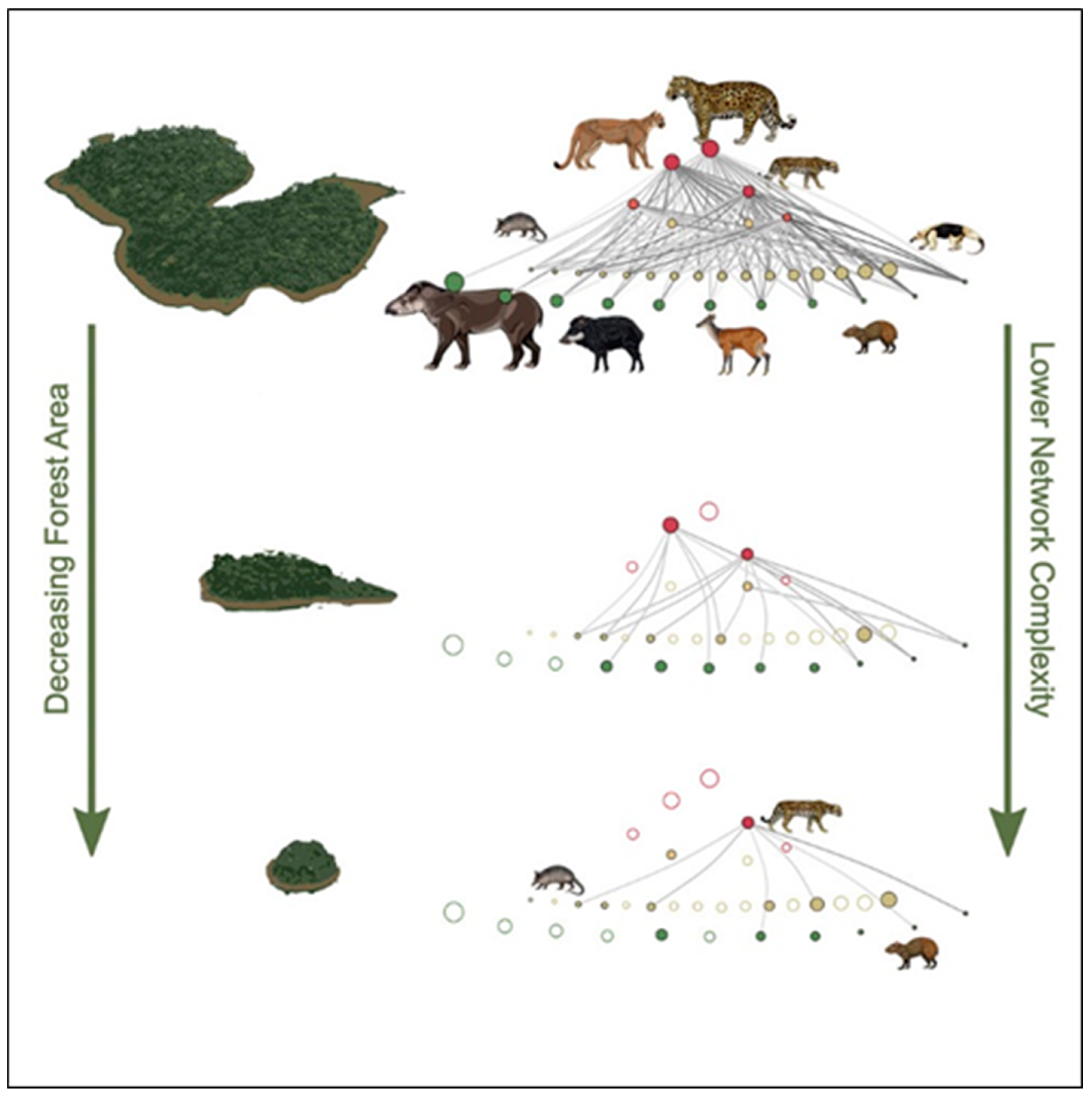
- Pires, M.M.; Benchimol, M.; Cruz, L.R.; Peres, C.A. Terrestrial food web complexity in Amazonian forests decays with habitat loss. Curr. Biol. 2023, 33, 389–396. [Google Scholar] [CrossRef]
- Biggs, C.R.; Yeager, L.A.; Bolser, D.G.; Bonsell, C.; Dichiera, A.M.; Hou, Z.; Keyser, S.R.; Khursigara, A.J.; Lu, K.; Muth, A.F.; et al. Does functional redundancy affect ecological stability and resilience? A review and meta-analysis. Ecosphere 2020, 11, e03184. [Google Scholar] [CrossRef]
- Hitchman, S.M.; Mather, M.E.; Smith, J.M.; Fencl, J.S. Identifying keystone habitats with a mosaic approach can improve biodiversity conservation in disturbed ecosystems. Glob. Change Biol. 2018, 24, 308–321. [Google Scholar] [CrossRef] [PubMed]
- Regolin, A.L.; Ribeiro, M.C.; Martello, F.; Melo, G.L.; Sponchiado, J.; Campanha, L.F.D.C.; Sugae, L.S.M.; Silva, T.S.F.; Cáceres, N.C. Spatial heterogeneity and habitat configuration overcome habitat composition influences on alpha and beta mammal diversity. Biotropica 2020, 52, 969–980. [Google Scholar] [CrossRef]
- Dufour, A.; Gadallah, F.; Wagner, H.H.; Guisan, A.; Buttler, A. Plant species richness and environmental heterogeneity in a mountain landscape: Effects of variability and spatial configuration. Ecography 2006, 29, 573–584. [Google Scholar] [CrossRef]
- Costanza, J.K.; Moody, A.; Peet, R.K. Multi-scale environmental heterogeneity as a predictor of plant species richness. Landsc. Ecol. 2011, 26, 851–864. [Google Scholar] [CrossRef]
- Udy, K.; Fritsch, M.; Meyer, K.M.; Grass, I.; Hanß, S.; Hartig, F.; Kneib, T.; Kreft, H.; Kukunda, C.B.; Pe’er, G.; et al. Environmental heterogeneity predicts global species richness patterns better than area. Glob. Ecol. Biogeogr. 2021, 30, 842–851. [Google Scholar] [CrossRef]
- Agra, J.; Cornelissen, T.; Viana-Junior, A.B.; Callisto, M. A global synthesis and meta-analysis of the environmental heterogeneity effects on the freshwater biodiversity. Oikos 2024, e10186. [Google Scholar] [CrossRef]
- Tews, J.; Brose, U.; Grimm, V.; Tielbörger, K.; Wichmann, M.C.; Schwager, M.; Jeltsch, F. Animal species diversity driven by habitat heterogeneity/diversity: The importance of keystone structures. J. Biogeogr. 2004, 31, 79–92. [Google Scholar] [CrossRef]
- Tamme, R.; Hiiesalu, I.; Laanisto, L.; Szava-Kovats, R.; Pärtel, M. Environmental heterogeneity, species diversity and co-existence at different spatial scales. J. Veg. Sci. 2010, 21, 796–801. [Google Scholar] [CrossRef]
- Stein, A. Environmental heterogeneity–species richness relationships from a global perspective. Front. Biogeogr. 2015, 7, 4. [Google Scholar]
- Seiferling, I.; Proulx, R.; Wirth, C. Disentangling the environmental-heterogeneity–species-diversity relationship along a gradient of human footprint. Ecology 2014, 95, 2084–2095. [Google Scholar] [CrossRef]
- de Lima Filho, J.A.; Vieira, R.J.; de Souza, C.A.; Ferreira, F.F.; de Oliveira, V.M. Effects of habitat fragmentation on biodiversity patterns of ecosystems with resource competition. Phys. A Stat. Mech. Its Appl. 2021, 564, 125497. [Google Scholar] [CrossRef]
- Wan, J.Z.; Wang, C.-J.; Marquet, P.A. Environmental heterogeneity as a driver of terrestrial biodiversity on a global scale. Prog. Phys. Geog. 2023, 47, 912–930. [Google Scholar] [CrossRef]
- Albrecht, J.; Peters, M.K.; Becker, J.N.; Behler, C.; Classen, A.; Ensslin, A.; Ferger, S.W.; Gebert, F.; Gerschlauer, F.; Helbig-Bonitz, M.; et al. Species richness is more important for ecosystem functioning than species turnover along an elevational gradient. Nat. Ecol. Evol. 2021, 5, 1582–1593. [Google Scholar] [CrossRef]
- Feld, C.K.; Martins da Silva, P.; Paulo Sousa, J.; De Bello, F.; Bugter, R.; Grandin, U.; Hering, D.; Lavorel, S.; Mountford, O.; Pardo, I.; et al. Indicators of biodiversity and ecosystem services: A synthesis across ecosystems and spatial scales. Oikos 2009, 118, 1862–1871. [Google Scholar] [CrossRef]
- Gonzalez, A.; Germain, R.M.; Srivastava, D.S.; Filotas, E.; Dee, L.E.; Gravel, D.; Thompson, P.L.; Isbell, F.; Wang, S.; Kéfi, S.; et al. Scaling-up biodiversity-ecosystem functioning research. Ecol. Lett. 2020, 23, 757–776. [Google Scholar] [CrossRef] [PubMed]
- Thompson, P.L.; Kéfi, S.; Zelnik, Y.R.; Dee, L.E.; Wang, S.; de Mazancourt, C.; Loreau, M.; Gonzalez, A. Scaling up biodiversity–ecosystem functioning relationships: The role of environmental heterogeneity in space and time. Proc. Roy. Soc. B-Biol. Sci. 2021, 288, 20202779. [Google Scholar] [CrossRef]
- Fahrig, L.; Nuttle, W.K. Population ecology in spatially heterogeneous environments. In Ecosystem Function in Heterogeneous Landscapes; Lovett, G.M., Turner, M.G., Jones, C.G., Weathers, K.C., Eds.; Springer: New York, NY, USA, 2005; pp. 95–118. [Google Scholar]
- Laanisto, L.; Tamme, R.; Hiiesalu, I.; Szava-Kovats, R.; Gazol, A.; Pärtel, M. Microfragmentation concept explains non-positive environmental heterogeneity–diversity relationships. Oecologia 2013, 171, 217–226. [Google Scholar] [CrossRef]
- Foster, E.; Love, J.; Rader, R.; Reid, N.; Drielsma, M.J. Integrating a generic focal species, metapopulation capacity, and connectivity to identify opportunities to link fragmented habitat. Landsc. Ecol. 2017, 32, 1837–1847. [Google Scholar] [CrossRef]
- Wang, S.; Brose, U.; van Nouhuys, S.; Holt, R.D.; Loreau, M. Metapopulation capacity determines food chain length in fragmented landscapes. Proc. Natl. Acad. Sci. USA 2021, 118, e2102733118. [Google Scholar] [CrossRef]
- Brodie, J.F.; Mohd-Azlan, J.; Schnell, J.K. How individual links affect network stability in a large-scale, heterogeneous metacommunity. Ecology 2016, 97, 1658–1667. [Google Scholar] [CrossRef]
- Loreau, M.; Mouquet, N.; Holt, R.D. From metacommunities to metaecosystems. In Metacommunities: Spatial Dynamics and Ecological Communities; Holyoak, M., Leibold, M.A., Holt, R.D., Eds.; University of Chicago Press: Chicago, IL, USA, 2005; pp. 418–438. [Google Scholar]
- Clark, W. Principles of Landscape Ecology. In Nature Education Knowledge; Island Press: Washington, DC, USA, 2010; Volume 3, p. 34. Available online: https://www.nature.com/scitable/knowledge/ (accessed on 8 March 2025).
- Holderegger, R.; Wagner, H.H. Landscape genetics. Bioscience 2008, 58, 199–207. [Google Scholar] [CrossRef]
- Richardson, J.L.; Brady, S.P.; Wang, I.J.; Spear, S.F. Navigating the pitfalls and promise of landscape genetics. Mol. Ecol. 2016, 25, 849–863. [Google Scholar] [CrossRef] [PubMed]
- Borokini, I.T.; Klingler, K.B.; Peacock, M.M. Life in the desert: The impact of geographic and environmental gradients on genetic diversity and population structure of Ivesia webberi. Ecol. Evol. 2021, 11, 17537–17556. [Google Scholar] [CrossRef]
- Manel, S.; Joost, S.; Epperson, B.K.; Holderegger, R.; Storfer, A.; Rosenberg, M.S.; Scribner, K.T.; Bonin, A.; Fortin, M.J. Perspectives on the use of landscape genetics to detect genetic adaptive variation in the field. Mol. Ecol. 2010, 19, 3760–3772. [Google Scholar] [CrossRef]
- Manel, S.; Holderegger, R. Ten years of landscape genetics. Trends Ecol. Evol. 2013, 28, 614–621. [Google Scholar] [CrossRef] [PubMed]
- Vellend, M.; Geber, M.A. Connections between species diversity and genetic diversity. Ecol. Lett. 2005, 8, 767–781. [Google Scholar] [CrossRef]
- Struebig, M.J.; Kingston, T.; Petit, E.J.; Le Comber, S.C.; Zubaid, A.; Mohd-Adnan, A.; Rossiter, S.J. Parallel declines in species and genetic diversity in tropical forest fragments. Ecol. Lett. 2011, 14, 582–590. [Google Scholar] [CrossRef]
- Bragg, J.G.; Supple, M.A.; Andrew, R.L.; Borevitz, J.O. Genomic variation across landscapes: Insights and applications. New Phytol. 2015, 207, 953–967. [Google Scholar] [CrossRef]
- Balkenhol, N.; Dudaniec, R.Y.; Krutovsky, K.V.; Johnson, J.S.; Cairns, D.M.; Segelbacher, G.; Selkoe, K.A.; von der Hayden, S.; Wang, I.J.; Selmoni, O.; et al. Landscape genomics: Understanding relationships between environmental heterogeneity and genomic characteristics of populations. In Population Genomics: Concepts, Approaches and Applications; Rajora, O.P., Ed.; Springer: New York, NY, USA, 2019; pp. 261–322. [Google Scholar]
- Hand, B.K.; Lowe, W.H.; Kovach, R.P.; Muhlfeld, C.C.; Luikart, G. Landscape community genomics: Understanding eco-evolutionary processes in complex environments. Trends Ecol. Evol. 2015, 30, 161–168. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Mao, R.L.; Yang, J.; Miao, C.Y.; Li, Z.; Qiu, Y.X. Ten years of landscape genomics: Challenges and opportunities. Front. Plant Sci. 2017, 8, 2136. [Google Scholar] [CrossRef]
- Segelbacher, G.; Cushman, S.A.; Epperson, B.K.; Fortin, M.J.; Francois, O.; Hardy, O.J.; Holderegger, R.; Taberlet, P.; Waits, L.P.; Manel, S. Applications of landscape genetics in conservation biology: Concepts and challenges. Conserv. Genet. 2010, 11, 375–385. [Google Scholar] [CrossRef]
- Chambers, E.A.; Bishop, A.P.; Wang, I.J. Individual-based landscape genomics for conservation: An analysis pipeline. Mol. Ecol. Resour. 2023. Available online: https://doi-org.unr.idm.oclc.org/10.1111/1755-0998.13884 (accessed on 8 March 2025). [CrossRef] [PubMed]
- Alahuhta, J.; Tukiainen, H.; Toivanen, M.; Ala-Hulkko, T.; Farrahi, V.; Hjort, J.; Ikäheimo, T.M.; Lankila, T.; Maliniemi, T.; Puhakka, S.; et al. Acknowledging geodiversity in safeguarding biodiversity and human health. Lancet. Planet Health 2022, 6, e987–e992. [Google Scholar] [CrossRef]
- Crisp, J.R.; Ellison, J.C.; Fischer, A.; Tan, J.S. Geodiversity inclusiveness in biodiversity assessment. Prog. Phys. Geog. 2023, 47, 414–437. [Google Scholar] [CrossRef]
- Hjort, J.; Gordon, J.E.; Gray, M.; Hunter, M.L., Jr. Why geodiversity matters in valuing nature’s stage. Conserv. Biol. 2015, 29, 630–639. [Google Scholar] [CrossRef]
- Sutton, P.C.; Anderson, S.J.; Costanza, R.; Kubiszewski, I. The ecological economics of land degradation: Impacts on ecosystem service values. Ecol. Econ. 2016, 129, 182–192. [Google Scholar] [CrossRef]
- Haines-Young, R.; Potschin, M. The links between biodiversity, ecosystem services and human well-being. In Ecosystem Ecology: A New Synthesis; Raffaelli, D.G., Frid, C.L.J., Eds.; Cambridge University Press: Cambridge, UK, 2010; pp. 110–139. [Google Scholar]
- Mestre, F. Synergistic effects of climate change and habitat fragmentation on species range shifts and metapopulation persistence. Front. Biogeogr. 2018, 9. [Google Scholar] [CrossRef]
- Mantyka-Pringle, C.S.; Martin, T.G.; Rhodes, J.R. Interactions between climate and habitat loss effects on biodiversity: A systematic review and meta-analysis. Glob. Change Biol. 2012, 18, 1239–1252. [Google Scholar] [CrossRef]
- Gahlawat, I.N.; Lakra, P. Global Climate change and its effects. JISS 2020, 7, 14–23. [Google Scholar]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peacock, M.M. Negotiating a Fragmented World: What Do We Know, How Do We Know It, and Where Do We Go from Here? Diversity 2025, 17, 200. https://doi.org/10.3390/d17030200
Peacock MM. Negotiating a Fragmented World: What Do We Know, How Do We Know It, and Where Do We Go from Here? Diversity. 2025; 17(3):200. https://doi.org/10.3390/d17030200
Chicago/Turabian StylePeacock, Mary M. 2025. "Negotiating a Fragmented World: What Do We Know, How Do We Know It, and Where Do We Go from Here?" Diversity 17, no. 3: 200. https://doi.org/10.3390/d17030200
APA StylePeacock, M. M. (2025). Negotiating a Fragmented World: What Do We Know, How Do We Know It, and Where Do We Go from Here? Diversity, 17(3), 200. https://doi.org/10.3390/d17030200






