Abstract
One of the eight shortfalls in European wild bee data is the knowledge of the flowering plants they favor. This knowledge is of particular importance for bee gardens and wildflower strips initiatives aiming to provide forage for the wild bees. The aim of this study is to provide a list of plants that are used for food by certain taxa of wild bees in Bulgaria and to evaluate their potential for cultivation in bee gardens and wildflower strips. In relation to this, we discuss the food plant specialization of the wild bees. We summarize our observations on the pollination of certain plants and faunistic records considering the food plants in several grassland habitats on the territory of Bulgaria at altitudes between 0 and 1500 m above sea level, during the last 30 years. More than 54 taxa of wild bees are listed. They belong to the families Apidae, Andrenidae, Colletidae, Halictidae, and Megachilidae. Some of these bees are identified to the species level, and others to the genus or family level. Among the recorded wild bees are observed eight oligolectic species (22.2%). The listed bees are flower visitors of 60 plant taxa belonging to 20 families, which offer nectar and/or pollen. The wild bees’ food plants are predominantly from the families Fabaceae (15 species), Lamiaceae (14 species), Asteraceae (9 species), etc. The perennials are 67%, while annuals are 9%, annual or biennial 6%, biennials 5%, etc. We discuss the seed germination specifics of these plants. More studies are needed in this field. The conservation of wild bees may be supported by wildflower restoration activities, but the process depends on many factors, including seed germination difficulties. Therefore, the natural grassland habitats must be preserved and protected.
1. Introduction
One of the reasons for the global decline in wild bees is the insufficiency of food plants that provide pollen and/or nectar [1,2,3,4,5]. The intensive agriculture contributes considerably to this deficit [6]. The vast agricultural blocks leave no strips of entomophilous flowering plants in-between. Additionally, the farmers kill the bees’ food plants with herbicides. For example, the lavender plantations in the agricultural land in Dobruja (SE Bulgaria) are found to have very little activity and diversity of wild bees. At the same time, the ornamental plantation in the Rusalka resort, which is close to Natura 2000 steppe habitats, is characterized with a high activity and diversity of wild bees [7]. Also, in Bulgaria, in the last decade, land use has often changed from meadows to intensive agriculture. In recent years, on the Balkans, a new hazard has emerged for the floral food resources. It might turn into the next threatening factor for wild bees’ decline. Vast photovoltaic power stations (solar parks of many hectares) replace wild ecosystems, such as shrub and grasslands. The owners tend to “kill the grass” beneath the panels (using herbicides or other methods) in order to avoid the necessity of mowing [personal communication with photovoltaic power station owners]. This equals to a loss of flowering food resources. However, solar parks, managed as wildflower meadows, provide bumble bees with foraging and nesting opportunities [8]. Another hazard, related to the flowering food resources loss, is the change in the landscape. In Bulgaria, for the period of 2007–2017, a decline in livestock production was reported by more than 20% [9]. This is a significant issue concerning semi-artificial open habitats, such as pastures and meadows. In mountainous and low-mountainous regions, these habitats are often abandoned. The consequence is thriving of shrubs followed by gradual afforestation [10,11]. Where maintenance is practiced, it frequently involves intensive techniques aimed at the deliberate removal of shrubs and certain herbaceous species through mechanical treatments and pesticides and/or the enhancement in economically valuable species through fertilization. Intensive maintenance increases the economic value of pastures and meadows [11]. However, these practices simultaneously lead to a significant reduction in the biological diversity and habitats’ sustainability.
The decline in the pollination service results in agricultural loss [12,13,14]. It is shown that reducing land-use intensity on agricultural grasslands benefits bee diversity and pollination service delivery. Biodiversity-friendly management on grasslands results in improved outcomes for growers through positive effects on pollination service delivery for sunflowers and apples [15,16]. Maintaining landscape heterogeneity and improving the quality of semi-natural habitats to ensure resource diversity and continuity is fundamental to pollinator conservation [17].
Lately bee gardens [18] and wildflower strips have been implemented on farmlands to provide forage for insect pollinators [19,20,21,22,23,24,25,26]. The composition of the seed mixes and, hence, the obtained floral food resources for wild bees are of great importance. They determine the success of agri-environmental schemes, wildflower strips, and restoration activities [27,28,29,30]. Which plants species should be used for seed mix preparation is known as a result of several observational and experimental studies as well as good practices [17,29,31,32,33,34,35]. It is well demonstrated that the choice of species is important at the local level. An increase in species richness and abundance of wild bees and butterflies is recorded in wildflower strips initiated with seeds of regional origin [36]. In some European countries, phacelias are recommended among the other food plants [19,37]. However, Phacelia tanacetifolia Benth. is recognized as potentially invasive [38] as well as P. campanularia A. Gray [39]. Therefore, the composition of the seed mixes used for wildflower strips and restoration activities should be based on locally orientated research of plant–pollinators relationships. Many of the wild bees are polylectic and not that “picky” regarding the flowering food resources. However, even though the polylectic bees are flexible in their food choices, they still have preferences. For example, although bumblebees are known as polylectic, the long-tongued species have more specialized diets compared to short-tongued ones [2]. It is shown that the persisting abundant generalist pollinators buffer against the rapid loss of interaction diversity upon landscape simplification [40]. On the other hand, many species of wild bees are specialized in certain food plants. For example, Hylaeus punctulatissimus Smith, 1842, and Colletes carinatus Radoszkowski, 1891, are oligolectic on Allium sp. div., while Colletes fodiens (Geoffroy in Fourcroy, 1785) is specialized on Asteraceae (e.g., Tanacetum) [41], Eucera macroglossa uses the pollen of Malva alcea, and Osmia adunca is oligolectic on Echium vulgare [40]. The lack of the certain food plants for the oligolectic bees is hazardous for their existence. For sustainable wildflower strips establishment, it is necessary to know the local plant–pollinator networks. This topic has been the focus of scholars for many years, and considerable data on plant–pollinator networks in various regions and habitats are available [42,43,44,45,46]. Despite this, such research is still needed [26].
One of the eight shortfalls in European wild bee data is the Eltonian shortfall. It concerns the knowledge of the visitation of flowering plants to collect pollen or nectar (measured by observing interactions in situ and recording every time a bee is seen to be visiting a certain plant species) [47]. In Bulgaria, to date, the knowledge about wild bees’ flower choices based on the local flora and vegetation is scarce. Some records of certain plants and their wild bee pollinators are available [7,48,49,50,51,52,53,54,55,56,57].
The aim of this study is to provide a list of plants that are used for food by certain taxa of wild bees in Bulgaria and to evaluate their potential for cultivation in bee gardens and wildflower strips. In relation to this, the food plant specialization of the wild bees and propagation specifics of these plants are discussed.
2. Materials and Methods
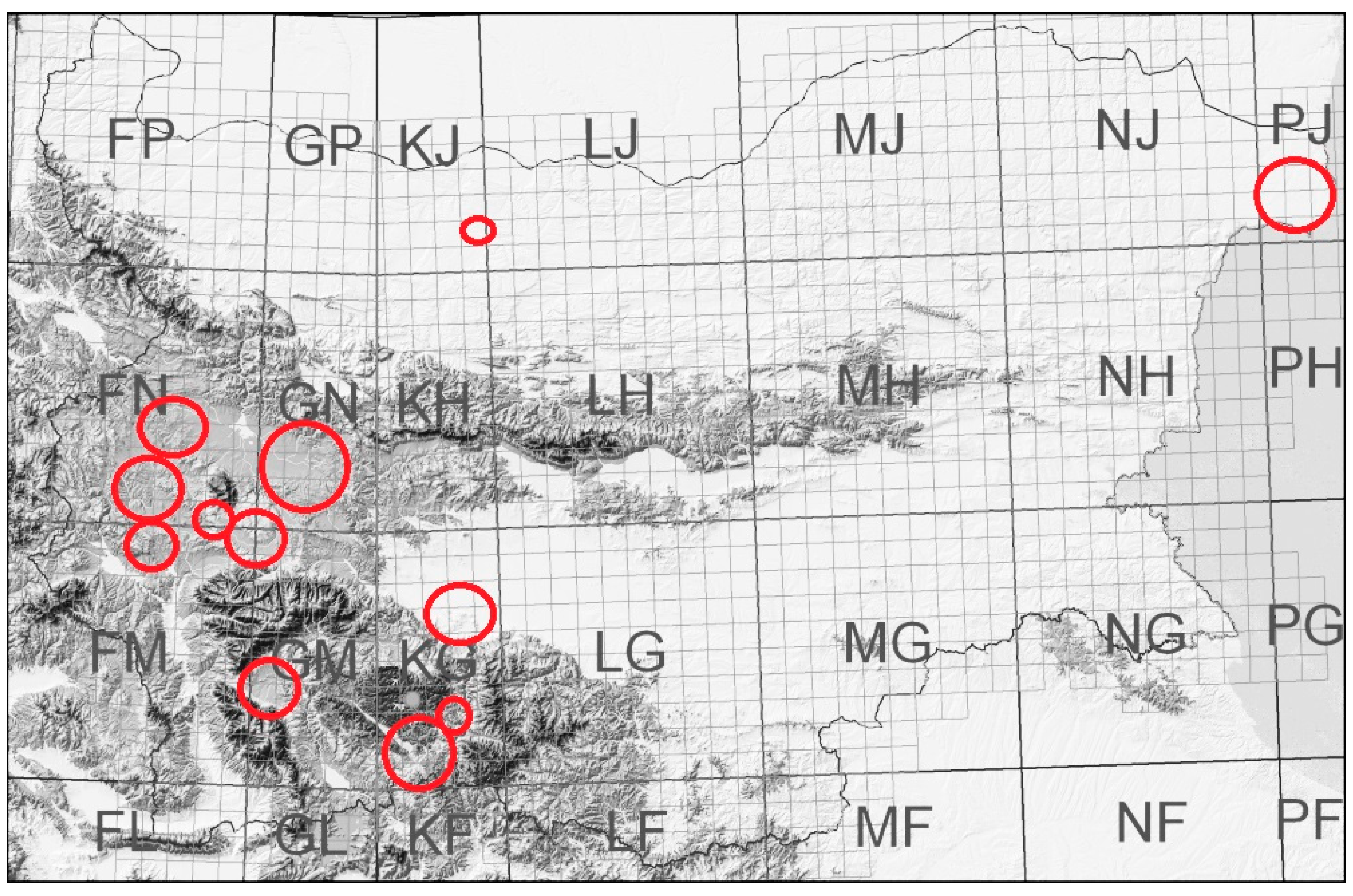
We summarize our observations on the pollination of certain plants and faunistic records considering the food plants during the last 30 years. These observations were performed in 50 study sites in several grassland habitats of 12 regions (Figure 1) on the territory of Bulgaria at an altitude between 0 and 1300 m above sea level. We chose the study sites in habitats that were less destroyed by agricultural activity and, at the same time, in regions with agriculture. We used the site-transect method to record the pollinators of Astragalus dasyanthus Pall., A. alopecurus Pall., Lavandula angustifolia L., Sideritis scardica Griseb., Gypsophila trichotoma Wend., Gentiana cruciata L. wild populations, and the sympatric and simultaneously flowering plants there [7,48,49,50,51,52,55,56,57] (Figure 1). We also used the transect method in the grassland communities in the rest of the study sites [50] (Kozuharova unpublished data, Trifonov unpublished data, Figure 1 (Map)). The bees were either photographed (OLYMPUS E500 with a macro-lens) and identified at least to the genus level, or captured for detailed identification. We followed Plants of the World Online and Euro+Med Plant Base [58,59] for the accepted plant taxa names (Table 1) and floristic analysis was presented by families (Figure 2). The food specialization and preferences of the bees were taken from published sources (Table 2) as well as the life forms of plants (Figure 3, Table 3). The plant propagation specifics were summarized from published sources (Table 3). Our preliminary experiments for the germination of Paeonia peregrina Mill. seeds (n = 30) include scarification and stratification.
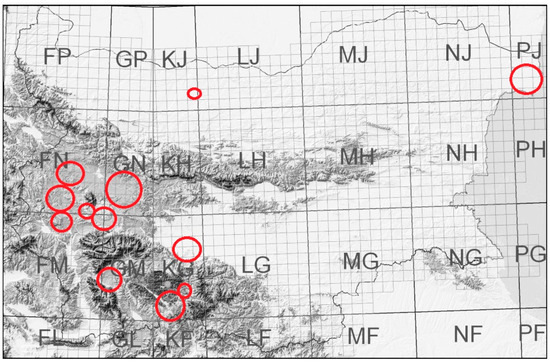
Figure 1.
Regions where the study sites are located.
3. Results
3.1. Wild Bees and Their Food Plants in Bulgaria—Floristic Analysis
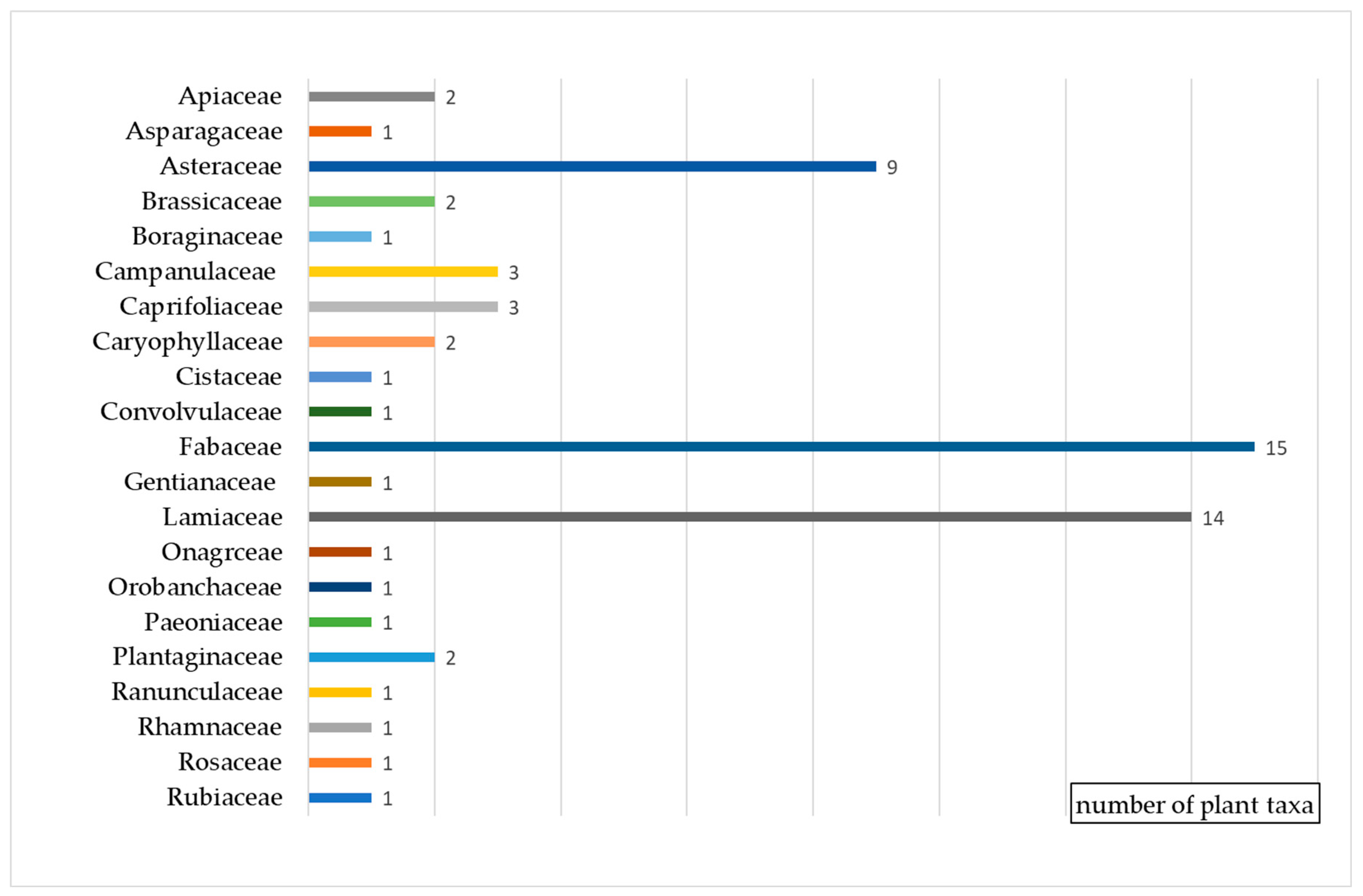
The wild bees were recorded on the flowers of 64 plant taxa, mostly from the families Fabaceae (15 species), Lamiaceae (14 species), and Asteraceae (9 species) (Figure 2, Table 1).

Table 1.
Wild bees recorded in the flowers of plant species using them as a source of pollen and/or nectar.
Table 1.
Wild bees recorded in the flowers of plant species using them as a source of pollen and/or nectar.
| Plant Family | Plant Species | Bee Taxa | Bee Family |
|---|---|---|---|
| Apiaceae | Eryngium campestre L. | Colletes carinatus Radoszkowski, 1891 | Colletidae |
| Colletes hylaeformis Eversmann, 1852 | Colletidae | ||
| Hylaeus meridionalis Förster, 1871 | Colletidae | ||
| Icteranthidium grohmanni (Spinola, 1838) | Megachilidae | ||
| Megachile argentata (Fabricius, 1793) | Megachilidae | ||
| Scandix pecten-veneris L. | Andrena danuvia E. Stoeckhert, 1950 | Andrenidae | |
| Asparagaceae | Ornithogalum montanum Cirillo | Osmia bischoffi Atanassov, 1938 | Megachilidae |
| Asteraceae | Achillea clypeolata Sm. | Hylaeus kahri Förster, 1871 | Colletidae |
| Calendula officinalis L. | Xylocopa cf. violacea | Apidae | |
| Centaurea diffusa Lam. | Megachile argentata (Fabricius, 1793) | Megachilidae | |
| Centaurea salonitana Vis. | Andrena albopunctata (Rossi, 1792) | Andrenidae | |
| Bombus armeniacus Radoszkowski, 1877 | Apidae | ||
| Lithurgus chrysurus Fonscolombe, 1834 | Megachilidae | ||
| Pseudoanthidium melanurum (Klug, 1832) | Megachilidae | ||
| Cirsium ligulare Boiss. | Bombus cf. lapidarius | Apidae | |
| Bombus cf. terrestis | Apidae | ||
| Bombus cf. hortorum | Apidae | ||
| Crepis biennis L. | Genus species | Megachilidae | |
| Leontodon biscutellifolius DC. | Andrena sp. div. | Andrenidae | |
| Onopordum tauricum Willd. | Xylocopa cf. violacea | Apidae | |
| Bombus cf. lapidarius | Apidae | ||
| Megachile cf. melanopyga | Megachilidae | ||
| Xeranthemum annuum L. | Osmia spinigera Latreille, 1811 | Megachilidae | |
| Brassicaceae | Mutarda nigra (L.) Bernh. | Andrena erberi Morawitz, 1871 | Andrenidae |
| Osmia bischoffi Atanassov, 1938 | Megachilidae | ||
| Sisymbrium orientale L. | Andrena pilipes Fabricius 1781 | Andrenidae | |
| Boraginaceae | Echium vulgare L. | Bombus pratorum (Linnaeus, 1761) | Apidae |
| Campanulaceae | Campanula bononiensis L. | Hylaeus sp. | Colletidae |
| Campanula rapunculoides L. | Hylaeus sp. | Colletidae | |
| Campanula trachelium L. | Hylaeus sp. | Colletidae | |
| Caprifoliaceae | Cephalaria transsilvanica (L.) Roem. & Schult. | Megachile melanopyga (Costa, 1863) | Megachilidae |
| Icteranthidium grohmanni (Spinola, 1838) | Megachilidae | ||
| Knautia arvensis (L.) Coult. | Icteranthidium grohmanni (Spinola, 1838) | Megachilidae | |
| Lomelosia argentea (L.) Greuter & Burdet | Trachusa integra (Eversmann, 1852) | Megachilidae | |
| Caryophyllaceae | Gypsophila glomerata Pall. ex Adams | Hylaeus meridionalis Förster, 1871 | Colletidae |
| Gypsophila trichotoma Wend. | Andrena sp. div. | Andrenidae | |
| Eucera sp. div. | Apidae | ||
| Ceratina cyanea (Kirby, 1802) | Apidae | ||
| Hylaeus communis Nylander, 1852 | Colletidae | ||
| Hylaeus gibbus Saunders, 1850 | Colletidae | ||
| Hylaeus moricei (Friese, 1898) | Colletidae | ||
| Hylaeus sp. | Colletidae | ||
| Lasioglossum sp. div. | Halictidae | ||
| Lasioglossum malachurum (Kirby, 1802) | Halictidae | ||
| Lasioglossum duckei (Alfken, 1909) | Halictidae | ||
| Lasioglossum nitidulum (Fabricius, 1804) | Halictidae | ||
| Lasioglossum politum (Schenck, 1853) | Halictidae | ||
| Cistaceae | Helianthemum nummularium (L.) Miller | Bombus pratorum (Linnaeus, 1761) | Apidae |
| Convolvulaceae | Convolvulus cantabrica L. | Hoplitis perezi (Ferton, 1895) | Megachilidae |
| Fabaceae | Astragalus alopecurus Pall. | Bombus pascuorum (Scopoli, 1763) | Apidae |
| Bombus cf. hortorum | Apidae | ||
| Astragalus dasyanthus Pall. | Bombus argillaceus (Scopoli, 1763) | Apidae | |
| Bombus cf. lapidarius | Apidae | ||
| Bombus cf. terrestris | Apidae | ||
| Astragalus glycyphyllos L. | Eucera sp. | Apidae | |
| Bombus cf. lapidarius | Apidae | ||
| Bombus pascuorum (Scopoli, 1763) | Apidae | ||
| Coronilla varia L. | Andrena sp. div. | Andrenidae | |
| Bombus cf. lapidarius | Apidae | ||
| Bombus pratorum (Linnaeus, 1761) | Apidae | ||
| Halictus sp. div. | Halictidae | ||
| Genus species | Megachilidae | ||
| Megachile argentata (Fabricius, 1793) | Megachilidae | ||
| Lathyrus pratensis L. | Bombus cf. hortorum | Apidae | |
| Lotus corniculatus L. | Bombus cf. lapidarius | Apidae | |
| Bombus cf. terrestris | Apidae | ||
| Medicago falcata L. | Andrena sp. div. | Andrenidae | |
| Onobrychis alba (Waldst. et Kit.) Desv. | Andrena sp. div. | Andrenidae | |
| Genus species | Megachilidae | ||
| Onobrychis arenaria W.D.J.Koch | Osmia bischoffi Atanassov, 1939 | Megachilidae | |
| Osmia rufohirta Latreille, 1811 | Megachilidae | ||
| Trifolium alpestre Crantz | Bombus argillaceus (Scopoli, 1763) | Apidae | |
| Trifolium medium L. | Bombus pascuorum (Scopoli, 1763) | Apidae | |
| Trifolium pratense L. | Andrena sp. div. | Andrenidae | |
| Bombus cf. veteranus | Apidae | ||
| Vicia dumetorum L. | Andrena sp. div. | Andrenidae | |
| Bombus cf. veteranus | Apidae | ||
| Vicia grandiflora Scop. | Eucera sp. | Apidae | |
| Vicia tenuifolia Roth. | Bombus haematurus Kriechbaumer, 1870 | Apidae | |
| Gentianaceae | Gentiana cruciata L. | Bombus argillaceus (Scopoli, 1763) | Apidae |
| Bombus hortorum (Linnaeus 1761) | Apidae | ||
| Bombus pascuorum (Scopoli, 1763) | Apidae | ||
| Bombus pomorum (Panzer, 1805) | Apidae | ||
| Hylaeus dilatatus (Kirby, 1802) | Colletidae | ||
| Halictus sp. div. | Halictidae | ||
| Lamiaceae | Ajuga chia Schreb. | Osmia andrenoides Spinola, 1808 | Megachilidae |
| Osmia bischoffi Atanassov, 1939 | Megachilidae | ||
| Osmia rufohirta Latreille, 1811 | Megachilidae | ||
| Ballota nigra L. | Andrena sp. div. | Andrenidae | |
| Bombus cf. hortorum | Apidae | ||
| Bombus cf. lapidarius | Apidae | ||
| Bombus cf. veteranus | Apidae | ||
| Bombus cf. veteranus | Apidae | ||
| Bombus pascuorum (Scopoli, 1763) | Apidae | ||
| Ceratina chalibea Chevrier, 1872 | Apidae | ||
| Xylocopa cf. violacea | Apidae | ||
| Hylaeus sp. div. | Colletidae | ||
| Halictus sp. div. | Halictidae | ||
| Lasioglossum sp. div. | Halictidae | ||
| Anthidium cf. manicatum | Megachilidae | ||
| Clinopodium vulgare L. | Bombus pratorum (Linnaeus, 1761) | Apidae | |
| Lamium garganicum L. | Bombus cf. hortorum | Apidae | |
| Lamium maculatum (L.) L. | Bombus sp. | Apidae | |
| Lavandula angustifolia L. | Andrena sp. div. | Andrenidae | |
| Eucera sp. | Apidae | ||
| Amegilla sp. | Apidae | ||
| Bombus argillaceus (Scopoli, 1763) | Apidae | ||
| Bombus cf. terrestris | Apidae | ||
| Bombus haematurus (Kriechbaumer 1870) | Apidae | ||
| Bombus pascuorum (Scopoli, 1763) | Apidae | ||
| Thyreus sp. | Apidae | ||
| Hylaeus sp. div. | Colletidae | ||
| Halictus sp. div. | Halictidae | ||
| Lasioglossum sp. div. | Halictidae | ||
| Anthidium cf. manicatum | Megachilidae | ||
| Megachile cf. centucularis | Megachilidae | ||
| Megachile cf. ericetorum | Megachilidae | ||
| Megachile melanopyga (Costa, 1863) | Megachilidae | ||
| Osmia sp. | Megachilidae | ||
| Stelis cf. nasuta | Megachilidae | ||
| Mentha spicata L. | Halictus sp. div. | Halictidae | |
| Lasioglossum sp. div. | Halictidae | ||
| Salvia pratensis L. | Andrena sp. div. | Andrenidae | |
| Genus species | Megachilidae | ||
| Salvia virgata Jacq. | Andrena sp. div. | Andrenidae | |
| Genus species | Megachilidae | ||
| Satureja coerulea Janka | Anthidium manicatum (Linnaeus, 1758) | Megachilidae | |
| Sideritis scardica Griseb. | Bombus cf. terrestris | Apidae | |
| Bombus pascuorum (Scopoli, 1763) | Apidae | ||
| Xylocopa cf. violacea | Apidae | ||
| Stachys germanica L. | Bombus cf. lapidarius | Apidae | |
| Bombus pascuorum (Scopoli, 1763) | Apidae | ||
| Teucrium chamaedrys L. | Bombus argillaceus (Scopoli, 1763) | Apidae | |
| Bombus cf. terrestris | Apidae | ||
| Rhodanthidium septemdentatum (Latreille, 1809) | Megachilidae | ||
| Teucrium capitatum L. | Trachusa integra (Eversmann, 1852) | Megachilidae | |
| Onagrceae | Epilobium angustifolium L. | Bombus cf. lapidarius | Apidae |
| Orobanchaceae | Rhinanthus wagneri Degen | Bombus cf. lapidarius | Apidae |
| Bombus pratorum (Linnaeus, 1761) | Apidae | ||
| Paeoniaceae | Paeonia peregrina Mill. | Bombus cf. lapidarius | Apidae |
| Plantaginaceae | Digitalis lanata Ehrh. | Bombus argillaceus (Scopoli, 1763) | Apidae |
| Anthidium manicatum (Linnaeus, 1758) | Megachilidae | ||
| Digitalis viridiflora Lindl. | Bombus cf. hortorum | Apidae | |
| Bombus cf. terrestris | Apidae | ||
| Ranunculacea | Pulsatilla vulgaris Mill. | Bombus cf. terrestris | Apidae |
| Rhamnaceae | Paliurus spina-christi Mill. | Andrena erberi Morawitz, 1872 | Andrenidae |
| Hylaeus duckei (Alfken, 1904) | Colletidae | ||
| Rosaceae | Rosa pendulina L | Bombus cf. lapidarius | Apidae |
| Rubiaceae | Galium verum L. | Hylaeus sp. div. | Halictidae |
| Lasioglossum sp. div. | Halictidae |
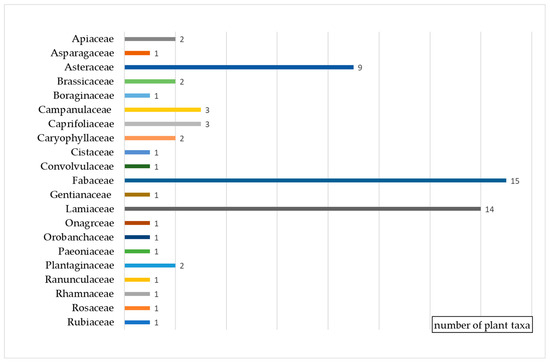
Figure 2.
Wild bees’ preferences of food plants in Bulgaria grouped by families.
Figure 2.
Wild bees’ preferences of food plants in Bulgaria grouped by families.
The plants listed in Table 1 are predominantly perennials (Figure 3). The life form of the food plants that are pollen and/or nectar sources for the wild bees is an important factor. It should be taken into account when bee gardens or wildflower strips are designed. This biological feature is strongly related to the propagation specifics of the plants.
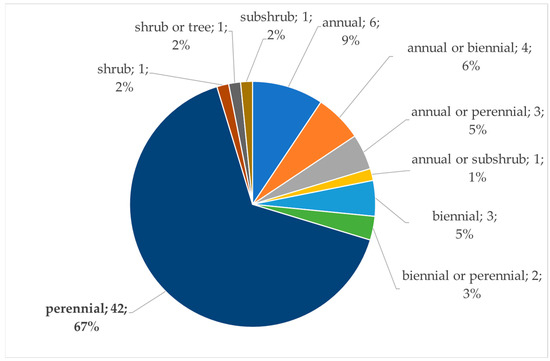
Figure 3.
Life forms of the food plants of the wild bees in Bulgaria.
Figure 3.
Life forms of the food plants of the wild bees in Bulgaria.
3.2. Flowering Phenology and Floral Food Rewards
Some of the listed plants (Table 1) bloom early in spring—for example, Pulsatilla vulgaris Mill., Lamium maculatum (L.) L., and Lamium garganicum L.—and they are very important food for the bumblebee queens, which are busy to establish the colonies at that time of the season (Figure 4). Many of the listed plants (Table 1) provide nectar (Figure 5a) and pollen in early or late summer. Few plants remain flowering later in the autumn, such as Cirsium ligulare Boiss. and Epilobium angustifolium L. Late flowering plants are very important to support the bees until the end of the season.

Figure 4.
Early spring food plants: (a) Bombus cf. terrestris on Pulsatilla vulgaris; (b) Bombus sp. on Lamium maculatum (photo: E. Kozuharova).

Figure 5.
Nectar-collecting bees: (a) Eucera sp. on Vicia grandiflora; (b) Bombus haematurus on Vicia tenuifolia and Astragalus dasyanthus (photo: E. Kozuharova).
Figure 5.
Nectar-collecting bees: (a) Eucera sp. on Vicia grandiflora; (b) Bombus haematurus on Vicia tenuifolia and Astragalus dasyanthus (photo: E. Kozuharova).

Many of the bumblebee workers are seen with full pollen baskets and often they are observed to transfer the pollen grains adhered to their bodies into the baskets during the flights from flower to flower (Figure 5b and Figure 6a). Megachile species are observed to collect both nectar and pollen (Table 1).

Figure 6.
Nectar- and pollen-collecting bees: (a) Bombus cf. lapidaries on Onopordum tauricum; (b) Megachile cf. melanopyga on Onopordum tauricum (photo: E. Kozuharova).
3.3. Diversity of Wild Bees
4. Discussion
4.1. Specialization and Food Preferences of the Wild Bees
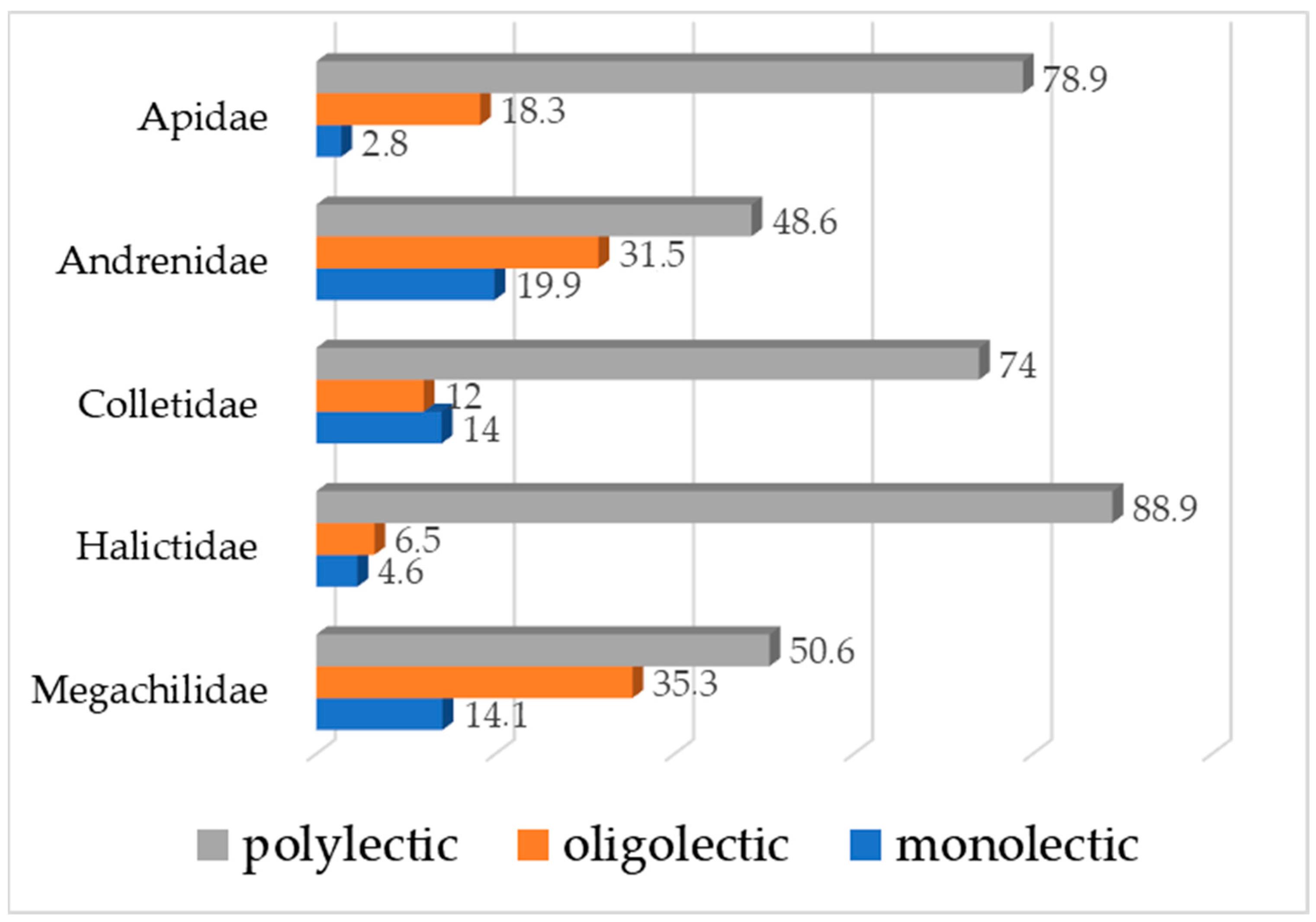
Some of the flower visitors are identified to the family or genus level (Table 1), and their specialization to the food sources cannot be specified. Most of the European wild bees are polylectic as follows in terms of families (Figure 7): Apidae—78.9%, Andrenidae—48.6%, Colletidae—74.0%, Halictidae—88.9%, and Megachilidae—50.6% [60], while almost all European species from the family Melittidae are oligolectic [61]. This aligns with the findings of previous studies, showing that the majority of wild bees are generalists [62]. Polylectic species are important for maintaining pollination services across diverse ecosystems, as they provide flexibility in response to the availability of different plants.
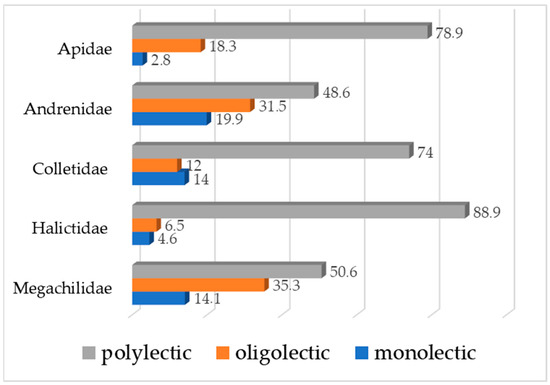
Figure 7.
Food socialization of the wild bees—percentage of taxa in each category (polylectic, oligolectic, or monolectic), according to Michez and co-authors [61]. Legend: x-axis—percent values, y-axis—wild bee families.
Amongst Apidae, bumblebees are known to be polylectic [63]. However, they also have food plant preferences. The short-tongued bumblebee species that emerge early in the season have less specialized diets compared to long-tongued ones [2] and they remain abundant.
Similar to the European pattern, we found that the Bulgarian wild bees listed in this study are preliminary polylectic. Some species of Andrena are polylectic, while others are oligolectic (Table 2). Polylectic are 28 wild bee species (77.8%). Eight wild bee species (22.2%) are oligolectic to certain plant genera, broadly oligolectic or polylectic with strong preferences to certain plant genera (Table 2). The food preferences of few of them are not known (Table 2). The majority of the observed oligolectic species are broad specialists on the family Asteraceae or forage specifically on certain genera from the family, such as Lithurgus chrysurus, Osmia spinigera, and Hylaeus duckei (Table 2).

Table 2.
Food preferences and specialization of the wild bees recorded in the study sites in Bulgaria.
Table 2.
Food preferences and specialization of the wild bees recorded in the study sites in Bulgaria.
| Bee Taxa | Food Preferences | References |
|---|---|---|
| Amegilla sp. | Deficient data | |
| Andrena albopunctata (Rossi, 1792) | Deficient data | |
| Andrena erberi Morawitz, 1871 | Deficient data | |
| Andrena danuvia E. Stoeckhert, 1950 | Polylectic | [41] |
| Andrena pilipes Fabricius 1781 | Polylectic | [64] |
| Andrena sp. div. | Polylectic/oligolectic | |
| Anthidium cf. manicatum | Polylectic | [65] |
| Anthidium manicatum (Linnaeus, 1758) | Polylectic | [65] |
| Bombus argillaceus (Scopoli, 1763) | Polylectic | [66] |
| Bombus armeniacus Radoszkowski, 1877 | Polylectic | [2,63] |
| Bombus cf. hortorum | Polylectic | [2,63] |
| Bombus cf. lapidarius | Polylectic | [2,63] |
| Bombus cf. terrestris | Polylectic | [2,63] |
| Bombus cf. veteranus | Polylectic | [2,63] |
| Bombus haematurus (Kriechbaumer 1870) | Polylectic | [2,63] |
| Bombus pascuorum (Scopoli, 1763) | Polylectic | [2,63] |
| Bombus pratorum (Linnaeus, 1761) | Polylectic | [2,63] |
| Ceratina chalybea Chevrier, 1872 | Polylectic | [67] |
| Ceratina cyanea (Kirby, 1802) | Polylectic | [67] |
| Colletes carinatus Radoszkowski, 1891 | Polylectic with a strong preference for Allium | [68] |
| Colletes hylaeformis Eversmann, 1852 | Narrowly oligolectic (Eryngium) | [68] |
| Eucera sp. div. | Polylectic/oligolectic | |
| Halictus sp. div. | Deficient data | |
| Hoplitis perezi (Ferton, 1895) | Oligolectic on Convolvulaceae | [69] |
| Hylaeus communis Nylander, 1852 | Polylectic | [70] |
| Hylaeus duckei (Alfken, 1904) | Broadly oligolectic on Asteraceae | [70] |
| Hylaeus gibbus Saunders, 1850 | Polylectic | [70] |
| Hylaeus meridionalis Förster, 1871 | Probably polylectic | [71] |
| Hylaeus moricei (Friese, 1898) | Polylectic | [70] |
| Hylaeus sp. div. | Deficient data | |
| Icteranthidium grohmanni (Spinola, 1838) | Polylectic | [65] |
| Lasioglossum duckei (Alfken, 1909) | Deficient data | |
| Lasioglossum malachurum (Kirby, 1802) | Polylectic | [72] |
| Lasioglossum nitidulum (Fabricius, 1804) | Deficient data | |
| Lasioglossum politum (Schenck, 1853) | Deficient data | |
| Lasioglossum sp. div. | Deficient data | |
| Lithurgus chrysurus Fonscolombe, 1834 | Oligolectic on Centaurea | [73] |
| Megachile argentata (Fabricius, 1793) | Polylectic | [74] |
| Megachile cf. centucularis | Polylectic | [67] |
| Megachile cf. ericetorum | Probably oligolectic on Fabaceae | [67] |
| Megachile melanopyga (Costa, 1863) | Polylectic pollen harvested from Centaurea and Cardueae, Atraceae | [67,75] |
| Megachilidae Genus species | Polylectic/oligolectic | [75] |
| Osmia andrenoides Spinola, 1808 | Polylectic | [76] |
| Osmia bischoffi Atanassov, 1938 | Polylectic | [77] |
| Osmia rufohirta Latreille, 1811 | Polylectic | [77] |
| Osmia sp. | Polylectic/oligolectic | |
| Osmia spinigera Latreille, 1811 | Oligolectic on Asteraceae | [78] |
| Pseudoanthidium melanurum (Klug, 1832) | Oligolectic on Carduoideae | [65] |
| Rhodanthidium septemdentatum (Latreille, 1809) | Polylectic | [79] |
| Stelis cf. nasuta | Parasitic, nectar visits on Lamiaceae | [80] |
| Thyreus sp. | ||
| Trachusa integra (Eversmann, 1852) | Observed on Jasione (Campanulaceae) | [81] |
| Xylocopa cf. violacea | Polylectic | [67] |
4.2. Floral Rewards
Many plants from the families Lamiaceae, Asteraceae, Fabaceae, and others are good sources of nectar [31,82,83,84]. Basically, plants with numerous stamens are considered a good source of pollen. For example, Paeonia peregrina has plenty of stamens, and we have observed honeybees and Bombus cf. lapidarius worker (Table 1) to gather pollen in its flowers. This species is among the peonies with the least quantity of nectar [85]. Interestingly, most authors consider Pulsatilla vulgaris to be a polleniferous plant due to the large number of stamens, which do not produce nectar; however, it has been shown that the flowers have staminodial nectaries [86]. Although rich in protein and thus an important food for the larvae, the pollen appears to be a tricky resource. Many flowers offer small pollen quantities [87]. Also, plants have adaptations to protect their pollen. They develop mechanical and chemical barriers that reduce the pollen exploitation. Although dandelions are listed amongst important pollen source for wild bees [29], Taraxacum pollen is pointed out as an example of chemical defense, which explains why Asteraceae pollen is rare in generalist bee diets [68,88]. This is interesting given the fact that Taraxacum is known for the high level of apomixis [89], meaning most dandelions do not need pollen for their propagation. On the other hand, the members of tribe Cardueae (knapweeds and thistles) are pointed out as the pollen sources for Megachille pilicrus, M. flabellipes, M. melanogaster, and M. picicornis [75].
4.3. Propagation Potential of the Food Plant Species
Almost all known botanical species successfully reproduce through seeds. Sexual reproduction via seeds generates genetically diverse individuals, which supports the maintenance of genetically rich populations with high adaptive potential. However, a significant challenge in the seed propagation of wild plant species lies in the inherent nature of their seeds. Wild plant seeds are typically adapted to ensure both reproductive success and the long-term survival of the species. These adaptations, beneficial in their natural environments, often become obstacles to intentional propagation efforts. The most notable characteristic of seeds from wild species (as opposed to cultivated ones) is dormancy, which can result in delayed or sporadic germination over extended periods [90,91,92,93,94]. Additionally, certain species exhibit highly specific requirements for seed activation and germination, such as exposure to periodic high temperatures, smoke, light, or interactions with specific biological agents, which are often difficult to replicate artificially [90,91]. Breaking seed dormancy is often a challenge. It can be achieved through various techniques depending on the plant species specifics. These methods include stratification, scarification, chemical or physical treatments of seeds, the application of growth regulators, inoculation with symbiotic organisms, and more [90,91,92,94,95,96].
One of the main drawbacks of seed propagation, especially when performed directly in the field, is the low survival rate of young plants. Plants are most sensitive and vulnerable to various adverse factors and environmental impacts during the early stages of development. For this reason, species should be sown in seasons and locations that closely resemble their natural habitats. Alternatively, young plants can be cultivated until they are robust enough to be reintroduced into their natural environment. For example, Sideritis scardica is propagated industrially by producing seedlings under artificially created conditions and later on transplanted in the field [97,98,99,100]. Astragalus dasyanthus and A. alopecurus are problematic for cultivation even if seedlings are produced under artificially created conditions; therefore, their native populations need to be protected efficiently [101].
Vegetative propagation methods are not widely used for wild plants. The primary limitation of vegetative propagation is that the resulting plants are genetically identical to the parent plants, leading to populations with low genetic diversity. However, vegetative propagation offers several advantages. It shortens the juvenile period in some perennial species, which can span several years [102]. Another significant benefit is the ability to propagate species for which seed propagation is challenging, inefficient, or excessively time-consuming. Vegetative propagation methods are particularly valuable for species with infrequent or poor seed production or in cases where seeds are extensively damaged by diseases and pests. These methods provide an effective alternative for conserving and propagating such species [90,92,95,103,104,105]. To mitigate the negative aspects of vegetative propagation, it is essential to use as many parent plants as possible. Additionally, planting vegetatively propagated individuals near natural populations of the same species can promote increased genetic diversity in subsequent generations.
When establishing wildflower strips, the interactions among the selected plant species and the environmental conditions in which they are introduced are of crucial significance. Some species are more competitive in dry and nutrient-poor soils, while others thrive in mesophilic or moist environments. Similarly, certain species perform well in open habitats, whereas others prefer proximity to trees and shrubs or grow as understory plants in forests. Some species are diffusely distributed within plant communities, while others tend to form dense clusters or groups. It is also important to consider that many wild plants naturally reproduce in stable plant communities, whereas others (ruderal species) require periodic habitat disturbances for successful reproduction [93]. Furthermore, the flowering phenology of different plant species should be taken into account to ensure that the strips provide a continuous food source for pollinators over the longest possible period.
All these factors highlight the necessity of carefully designing the plant communities to be included in the strips. Such a design ensures that the strips are as stable and effective as possible for maintaining the natural biodiversity of pollinators [106,107,108,109].

Table 3.
Life forms of the food plant species and their propagation potential.
Table 3.
Life forms of the food plant species and their propagation potential.
| Plant Family | Plant Species | Life Form | Propagation | Source of Information |
|---|---|---|---|---|
| Apiaceae | Eryngium campestre L. | perennial | Sowing seeds in autumn or spring | Unpublished data from Sokolov |
| Scandix pecten-veneris L. | annual | Sowing seeds in spring | Unpublished data from Sokolov | |
| Asparagaceae | Ornithogalum montanum Cirillo | perennial | Sowing seeds in autumn, divide abundance of small bulbs in summer | [110,111,112] |
| Asteraceae | Achillea clypeolata Sm. | perennial | Sowing seeds in spring; divide in winter or spring | [110,111,112] |
| Calendula officinalis L. | annual or biennial | Sowing seeds in autumn or spring | [111,112] | |
| Centaurea diffusa Lam. | biennial | Sowing seeds in autumn or spring | [110,111,112] | |
| Centaurea salonitana Vis. | perennial | |||
| Cirsium ligulare Boiss. | annual or biennial | Sowing seeds in spring | Unpublished data from Sokolov | |
| Crepis biennis L. | biennial | Sowing seeds in spring to autumn. | Unpublished data from Sokolov | |
| Leontodon biscutellifolius DC. | perennial | Sowing seeds in autumn to spring | Unpublished data from Sokolov | |
| Onopordum tauricum Willd. | annual or biennial | Sowing seeds in spring | Unpublished data from Sokolov | |
| Xeranthemum annuum L. | annual | Sowing seeds in spring | [110,111,112] | |
| Boraginaceae | Echium vulgare L. | biennial | Sowing seeds in autumn | [111,112] |
| Brassicaceae | Mutarda nigra (L.) Bernh. | annual | Sowing seeds in spring | [111,112] |
| Sisymbrium orientale L. | annual | Sowing seeds in spring | [112] | |
| Campanulaceae | Campanula bononiensis L. | perennial | Sowing seeds in spring | [110,111,112] |
| Campanula rapunculoides L. | perennial | Sowing seeds in spring | ||
| Campanula trachelium L. | perennial | Sowing seeds in spring | ||
| Caprifoliaceae | Cephalaria transsilvanica (L.) Roem. & Schult. | annual | Sowing seeds in autumn | Unpublished data from Sokolov |
| Knautia arvensis (L.) Coult. | biennial or perennial | Sowing seeds in autumn | Unpublished data from Sokolov | |
| Lomelosia argentea (L.) Greuter & Burdet biennial or perennial | Sowing seeds in autumn | Unpublished data from Sokolov | ||
| Caryophyllaceae | Gypsophila glomerata Pall. ex Adams | perennial | Sowing seeds in autumn | Unpublished data from Sokolov |
| Gypsophila trichotoma Wend. | perennial | Sowing seeds in autumn Temperature alternation or treatment with gibberelic acid promote germination | Unpublished data from Sokolov [113] | |
| Cistaceae | Helianthemum nummularium (L.) Miller | perennial | Sowing seeds in spring, rooting cuttings | [110,111,112] |
| Convolvulaceae | Convolvulus cantabrica L. | perennial | Sowing seeds in spring | [110,112] |
| Fabaceae | Astragalus alopecurus Pall. | perennial | Sowing seeds in spring Scarification is needed, but survival of the seedlings is very low | [101,110] |
| Astragalus dasyanthus Pall. | perennial | Sowing seeds in spring Scarification is needed, but survival of the seedlings is very low | [101,110] | |
| Astragalus glycyphyllos L. | perennial | Sowing seeds in spring | [110] | |
| Coronilla varia L. | perennial | Sowing seeds in autumn | [110] | |
| Lathyrus pratensis L. | climbing annual or perennial | Sowing seeds in spring | [110,111] | |
| Lotus corniculatus L. | perennial or annual | Sowing seeds in spring or autumn | [110,111,114] | |
| Medicago falcata L. | perennial | Sowing seeds in early spring | Well-established technology of cultivation of its close relative M. sativa L. | |
| Onobrychis alba (Waldst. et Kit.) Desv. | perennial | Sowing seeds in autumn | Unpublished data from Sokolov | |
| Onobrychis arenaria W.D.J.Koch | perennial | Sowing seeds in autumn | Unpublished data from Sokolov | |
| Trifolium alpestre Crantz | perennial | Sowing seeds in spring or autumn | [110,111,114] | |
| Trifolium medium L. | perennial | Sowing seeds in spring or autumn | [110,111,114] | |
| Trifolium pratense L. | perennial | Sowing seeds in spring or autumn | [110,111,114] | |
| Vicia dumetorum L. | perennial | Sowing seeds in autumn | Unpublished data from Sokolov | |
| Vicia grandiflora Scop. | annual or biennial | Sowing seeds in autumn | Unpublished data from Sokolov | |
| Vicia tenuifolia Roth. | annual or perennial | Sowing seeds in autumn | Unpublished data from Sokolov | |
| Gentianaceae | Gentiana cruciata L. | perennial | Sowing seeds in spring | [110,111] |
| Lamiaceae | Ajuga chia Schreb. | annual or subshrub | Sowing seeds in autumn or spring | - |
| Ballota nigra L. | perennial | Sowing seeds in spring, rooting cuttings | Unpublished data from Sokolov | |
| Clinopodium vulgare L. | perennial | Sowing seeds in spring, Separation of rooted shoots | [110,114] | |
| Lamium garganicum L. | perennial | Sowing seeds in spring | Unpublished data from Sokolov | |
| Lavandula angustifolia L. | subshrub | Cultivated, vegetative propagation (cuttings), adventive (by seed) | [111] | |
| Mentha spicata L. | perennial | Sowing seeds in spring, rooting cuttings | [111] | |
| Salvia pratensis L. | perennial | Sowing seeds in spring, rooting cuttings | [111] | |
| Salvia virgata Jacq. | perennial | Sowing seeds in spring | Unpublished data from Sokolov | |
| Satureja coerulea Janka | perennial | Sowing seeds in spring, rooting cuttings | [115] | |
| Sideritis scardica Griseb. | perennial (short living) | Seedlings need to be prepared and transplanted. Sowing seeds in February for stratification, giberelic acid treatment promotes germination if the seeds are not stratified divide clump, rooting cuttings | [97,98,99,100,110,116] Unpublished data from Kozuharova Unpublished data from Sokolov | |
| Stachys germanica L. | perennial | Sowing seeds in spring, divide clump, rooting cuttings | Unpublished data from Sokolov | |
| Teucrium chamaedrys L. | perennial | Sowing seeds in spring, divide clump, rooting cuttings | [115] | |
| Teucrium capitatum L. | perennial | Sowing seeds in spring, rooting cuttings | Unpublished data from Sokolov | |
| Onagrceae | Epilobium angustifolium L. | perennial | Sowing seeds in spring, root cuttings | [110,111,114] |
| Orobanchaceae | Rhinanthus wagneri Degen | annual | Sowing seeds in autumn | Unpublished data from Sokolov |
| Paeoniaceae | Paeonia peregrina Mill. | perennial | Seed sowing—germination occurs after 16 months of outdoor stratification, division of the rhizome at the end of summer | Unpublished data from Sokolov and Stoycheva; [111,117,118] |
| Plantaginaceae | Digitalis lanata Ehrh. | biennial or perennial | Sowing seeds in spring or early autumn | [110,111,112] |
| Digitalis viridiflora Lindl. | perennial | Sowing seeds in spring | ||
| Rhamnaceae | Paliurus spina-christi Mill. | shrub or tree | Sowing seeds extracted from the fruits in autumn | Unpublished data from Sokolov [112] |
| Rosaceae | Rosa pendulina L. | shrub | Sowing partially mature seeds in autumn | Vastly practiced method for all cultivars of Rosa spp. [111] |
| Rubiaceae | Galium verum L. | perennial | Sowing seeds in early autumn; divide clump in winter | [112] |
4.4. Principle Recommendations for the Composition of Pollinator-Friendly Mixes of Plants Seeds
Although this study lists certain plants and bees, it has some limitations. The seasonal variations, sample size, and time of observations on chosen plant species (some plants were observed longer than others) affect the records of wild bee diversity. Nevertheless, we documented certain food choices of wild bees. This study demonstrates that pollinator-friendly wildflower strips should be tailored considering a number of factors and principles. It is important to include suitable plant taxa in the pollinator-friendly mixes. The most important principle is to select locally specific entomophilous plants. Since we documented the bee visitors of only 64 plan taxa, while the flora of Bulgaria comprises of at least 2000 entomophilous plants, other local members of the same genera could be conditionally recommended for the restoration activities and pollinator-friendly wildflower strips. The first step to consider is to design pollinator-friendly wildflower strips, using plant species with a broader spectrum of visitors. These are the plants favored by most polylectic bees. The families with the largest number of observed associations are Fabaceae, Lamiaceae, and Asteraceae. They should be presented with locally sourced species. It is also essential to include plant species that support specialized oligolectic bees, such as most members of Melittidae and certain species from the families Apidae, Andrenidae, Colletidae, Halictidae, and Megachilidae. For example, Eryngium campestre and Coronilla varia are of utmost importance in potential plant mixes. Such key plant species should be prioritized in pollinator habitat restoration alongside other local species to support a large and diverse bee community.
The flowering period and the ability of plants to withstand drought conditions are increasingly important factors, especially as climate change continues to alter the timing and intensity of seasonal patterns. A well-balanced seed mix for wildflower strips should include species that bloom at different times of the year, ensuring a continuous food supply for wild bees throughout the season. Furthermore, the inclusion of drought-resistant plants is critical for ensuring habitat resilience under changing climatic conditions. These plants can maintain their floral resources even during periods of water scarcity, thereby supporting bee populations during stressful conditions.
Given the complex interactions between wild bees and their plant hosts, the best strategy for supporting these pollinators in agricultural and disturbed landscapes may be to sample plant communities from fragmented habitats, such as roadsides, field margins, and urban green spaces. These habitats often contain a diverse mix of generalist plants that can support a potentially wide range of bee species, as observed during our field studies. The restoration of natural habitats, however, would require specific sampling of local communities. The systematic, long-term monitoring of pollinators is needed. It is highly beneficial for ecological research, decision-making, and conservation action [119]. The EuPPollNet (European Plant- Pollinator Networks) database contains harmonized taxonomic data on plant–pollinator interactions. The implications for further research should address data gaps in plant–pollinator interactions and guide future efforts in conservation planning [120].
5. Conclusions
The necessity of pollinator-friendly habitat restoration is increasing as is the need for wildflower strips along large agricultural fields and roadsides. Furthermore, the efforts for natural habitats restoration and designed wildflower strips should focus on local native plant communities as models. The target should be specific plant species that are native to the region and are essential for maintaining the ecological integrity of these systems. Seed mixes for pollinator-friendly habitats’ restoration or flower strips should be composed to provide a diverse array of floral resources that are available throughout the whole growing season.
We provide a basic list of plants that offer food resources to certain wild bee taxa native to Bulgaria. This study also highlights the necessity of further and detailed research on the plants and their wild bee pollinators in Bulgaria. This will ensure comprehensive coverage in conservation and habitat restoration efforts. Future research should aim also to test the effectiveness of wildflower strips in different regions and habitats. The goal should be to determine the best combinations of plants for supporting both generalist and specialist wild bee species that also have the benefit of supporting other pollinator groups, such as flies, beetles, butterflies, etc.
The wild bees’ conservation may be supported by wildflower restoration activities, but the process depends on many factors including seed germination specifics. There are difficulties in the seed germination of many entomophilous plants. In order to overcome this barrier, more studies are needed for practical solutions in each specific case. The pollinator-friendly habitats’ restoration and wildflower strips creation activities should be aligned with initiatives and measures for the protection and preservation of natural grassland habitats.
Author Contributions
Conceptualization, E.K. and T.T.; methodology, E.K.; data collection, E.K., T.T. and R.S.S.; writing—original draft preparation, E.K.; writing—review and editing, E.K., T.T., R.S.S., N.Z. and. C.S.; visualization, E.K.; supervision, E.K. All authors have read and agreed to the published version of the manuscript.
Funding
Ekaterina Kozuharova is grateful for the financial support to the European Union—NextGenerationEU, through the National Recovery and Resilience Plan of the Republic of Bulgaria, Project No. BG-RRP-2.004-0004-C01.
Institutional Review Board Statement
Not applicable.
Acknowledgments
Special thank you to Nicolaos Vereeken, Justyna Kierat, and Mohamed Shebl for the help with the identification of some bees.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kevan, P.G.; Viana, B.F. The Global Decline of Pollination Services. Biodiversity 2003, 4, 3–8. [Google Scholar] [CrossRef]
- Goulson, D.; Hanley, M.E.; Darvill, B.; Ellis, J.S.; Knight, M.E. Causes of Rarity in Bumblebees. Biol. Conserv. 2005, 122, 1–8. [Google Scholar] [CrossRef]
- Potts, S.G.; Biesmeijer, J.C.; Kremen, C.; Neumann, P.; Schweiger, O.; Kunin, W.E. Global Pollinator Declines: Trends, Impacts and Drivers. Trends Ecol. Evol. 2010, 25, 345–353. [Google Scholar] [CrossRef]
- Vanbergen, A.J. The Insect Pollinators Initiative Threats to an Ecosystem Service: Pressures on Pollinators. Front. Ecol. Environ. 2013, 11, 251–259. [Google Scholar] [CrossRef]
- Goulson, D.; Nicholls, E.; Botías, C.; Rotheray, E.L. Bee Declines Driven by Combined Stress from Parasites, Pesticides, and Lack of Flowers. Science 2015, 347, 1255957. [Google Scholar] [CrossRef] [PubMed]
- Kremen, C.; Williams, N.M.; Thorp, R.W. Crop Pollination from Native Bees at Risk from Agricultural Intensification. Proc. Natl. Acad. Sci. USA 2002, 99, 16812–16816. [Google Scholar] [CrossRef]
- Kozuharova, E.; Vereecken, N.J. Lavender Production in SE Dobrudja—Intensive Agriculture Impacts Pollinators’ Density and Diversity. Euro-Mediterr. J. Environ. Integr. 2024, 9, 937–943. [Google Scholar] [CrossRef]
- Blaydes, H.; Gardner, E.; Whyatt, J.D.; Potts, S.G.; Armstrong, A. Solar Park Management and Design to Boost Bumble Bee Populations. Environ. Res. Lett. 2022, 17, 044002. [Google Scholar] [CrossRef]
- Yarkov, D.; Stankov, K.; Stankov, I. Historical Review of the Development of Bulgarian Livestock Production. Bulg. J. Agric. Sci. 2022, 28, 564–578. [Google Scholar]
- Vassilev, K.; Pedashenko, H.; Nikolov, S.C.; Apostolova, I.; Dengler, J. Effect of Land Abandonment on the Vegetation of Upland Semi-Natural Grasslands in the Western Balkan Mts., Bulgaria. Plant Biosyst. Int. J. Deal. Asp. Plant Biol. 2011, 145, 654–665. [Google Scholar] [CrossRef]
- Slavkova, S.; Shindarska, Z. Condition of Meadows and Pastures in Bulgaria and Tendencies for Their Development. Bulg. J. Anim. Husb. 2017, 54, 93–102. [Google Scholar]
- Kevan, P.G.; Phillips, T.P. The Economic Impacts of Pollinator Declines: An Approach to Assessing the Consequences. Conserv. Ecol. 2001, 5, 8. [Google Scholar] [CrossRef]
- Ferreira, P.A.; Boscolo, D.; Viana, B.F. What do we know about the effects of landscape changes on plant–pollinator interaction networks? Ecol. Indic. 2013, 31, 35–40. [Google Scholar] [CrossRef]
- Lippert, C.; Feuerbacher, A.; Narjes, M. Revisiting the economic valuation of agricultural losses due to large-scale changes in pollinator populations. Ecol. Econ. 2021, 180, 106860. [Google Scholar] [CrossRef]
- Scheper, J.; Badenhausser, I.; Kantelhardt, J.; Kirchweger, S.; Bartomeus, I.; Bretagnolle, V.; Clough, Y.; Gross, N.; Raemakers, I.; Vilà, M.; et al. Biodiversity and pollination benefits trade off against profit in an intensive farming system. Proc. Natl. Acad. Sci. USA 2023, 120, e2212124120. [Google Scholar] [CrossRef] [PubMed]
- Garratt, M.P.D.; O’Connor, R.S.; Carvell, C.; Fountain, M.T.; Breeze, T.D.; Pywell, R.; Redhead, J.W.; Kinneen, L.; Mitschunas, N.; Truslove, L.; et al. Addressing pollination deficits in orchard crops through habitat management for wild pollinators. Ecol. Appl. 2023, 33, e2743. [Google Scholar] [CrossRef]
- Cole, L.J.; Brocklehurst, S.; Robertson, D.; Harrison, W.; McCracken, D.I. Exploring the interactions between resource availability and the utilisation of semi-natural habitats by insect pollinators in an intensive agricultural landscape. Agric. Ecosyst. Environ. 2017, 246, 157–167. [Google Scholar] [CrossRef]
- Tassin De Montaigu, C.; Goulson, D. Factors influencing butterfly and bumblebee richness and abundance in gardens. Sci. Total Environ. 2024, 908, 167995. [Google Scholar] [CrossRef]
- Haaland, C.; Naisbit, R.E.; Bersier, L.-F. Sown wildflower strips for insect conservation: A review: Wildflower strips for insect conservation. Insect Conserv. Divers. 2011, 4, 60–80. [Google Scholar] [CrossRef]
- Balzan, M.V.; Bocci, G.; Moonen, A.-C. Augmenting flower trait diversity in wildflower strips to optimise the conservation of arthropod functional groups for multiple agroecosystem services. J. Insect Conserv. 2014, 18, 713–728. [Google Scholar] [CrossRef]
- Scheper, J.; Bommarco, R.; Holzschuh, A.; Potts, S.G.; Riedinger, V.; Roberts, S.P.M.; Rundlöf, M.; Smith, H.G.; Steffan-Dewenter, I.; Wickens, J.B.; et al. Local and landscape-level floral resources explain effects of wildflower strips on wild bees across four European countries. J. Appl. Ecol. 2015, 52, 1165–1175. [Google Scholar] [CrossRef]
- Ouvrard, P.; Transon, J.; Jacquemart, A.-L. Flower-strip agri-environment schemes provide diverse and valuable summer flower resources for pollinating insects. Biodivers. Conserv. 2018, 27, 2193–2216. [Google Scholar] [CrossRef]
- Buhk, C.; Oppermann, R.; Schanowski, A.; Bleil, R.; Lüdemann, J.; Maus, C. Flower strip networks offer promising long term effects on pollinator species richness in intensively cultivated agricultural areas. BMC Ecol. 2018, 18, 55. [Google Scholar] [CrossRef]
- Albrecht, M.; Kleijn, D.; Williams, N.M.; Tschumi, M.; Blaauw, B.R.; Bommarco, R.; Campbell, A.J.; Dainese, M.; Drummond, F.A.; Entling, M.H.; et al. The effectiveness of flower strips and hedgerows on pest control, pollination services and crop yield: A quantitative synthesis. Ecol. Lett. 2020, 23, 1488–1498. [Google Scholar] [CrossRef]
- Griffiths-Lee, J.; Davenport, B.; Foster, B.; Nicholls, E.; Goulson, D. Sown wildflowers between vines increase beneficial insect abundance and richness in a British vineyard. Agric. For. Entomol. 2023, 25, 139–151. [Google Scholar] [CrossRef]
- Willcox, B.K.; Garratt, M.P.D.; Breeze, T.D.; Mathimaran, N.; Potts, S.G.; Prasad, G.; Raj, R.; Senapathi, D. The benefits of floral border crops in smallholder rice production depends on agronomic inputs and landscape context. Agric. For. Entomol. 2024, 26, 327–338. [Google Scholar] [CrossRef]
- Scheper, J.; Bukovinszky, T.; Huigens, M.E.; Kleijn, D. Attractiveness of sown wildflower strips to flower-visiting insects depends on seed mixture and establishment success. Basic Appl. Ecol. 2021, 56, 401–415. [Google Scholar] [CrossRef]
- Nichols, R.N.; Holland, J.M.; Goulson, D. A novel farmland wildflower seed mix attracts a greater abundance and richness of pollinating insects than standard mixes. Insect Conserv. Divers. 2023, 16, 190–204. [Google Scholar] [CrossRef]
- Kuppler, J.; Neumüller, U.; Mayr, A.V.; Hopfenmüller, S.; Weiss, K.; Prosi, R.; Schanowski, A.; Schwenninger, H.-R.; Ayasse, M.; Burger, H. Favourite plants of wild bees. Agric. Ecosyst. Environ. 2023, 342, 108266. [Google Scholar] [CrossRef]
- Benvenuti, S. Wildflower strips in the agroecosystem for pollinator biodiversity restoration: Which plant species are capable of self-seeding? Ecol. Eng. 2025, 212, 107486. [Google Scholar] [CrossRef]
- Comba, L. Flowers, Nectar and Insect Visits: Evaluating British Plant Species for Pollinator-friendly Gardens. Ann. Bot. 1999, 83, 369–383. [Google Scholar] [CrossRef]
- Campbell, A.J.; Wilby, A.; Sutton, P.; Wäckers, F.L. Do sown flower strips boost wild pollinator abundance and pollination services in a spring-flowering crop? A case study from UK cider apple orchards. Agric. Ecosyst. Environ. 2017, 239, 20–29. [Google Scholar] [CrossRef]
- Herbertsson, L.; Jönsson, A.M.; Andersson, G.K.S.; Seibel, K.; Rundlöf, M.; Ekroos, J.; Stjernman, M.; Olsson, O.; Smith, H.G. The impact of sown flower strips on plant reproductive success in Southern Sweden varies with landscape context. Agric. Ecosyst. Environ. 2018, 259, 127–134. [Google Scholar] [CrossRef]
- Azpiazu, C.; Medina, P.; Adán, Á.; Sánchez-Ramos, I.; Del Estal, P.; Fereres, A.; Viñuela, E. The Role of Annual Flowering Plant Strips on a Melon Crop in Central Spain. Influence on Pollinators and Crop. Insects 2020, 11, 66. [Google Scholar] [CrossRef]
- Stout, J.C.; Dicks, L.V. From science to society: Implementing effective strategies to improve wild pollinator health. Philos. Trans. R. Soc. B Biol. Sci. 2022, 377, 20210165. [Google Scholar] [CrossRef]
- Schmied, H.; Getrost, L.; Diestelhorst, O.; Maaßen, G.; Gerhard, L. Between perfect habitat and ecological trap: Even wildflower strips mulched annually increase pollinating insect numbers in intensively used agricultural landscapes. J. Insect Conserv. 2022, 26, 425–434. [Google Scholar] [CrossRef]
- Ouvrard, P.; Jacquemart, A.-L. Agri-environment schemes targeting farmland bird populations also provide food for pollinating insects. Agric. For. Entomol. 2018, 20, 558–574. [Google Scholar] [CrossRef]
- Giovanetti, M.; Malabusini, S.; Zugno, M.; Lupi, D. Influence of Flowering Characteristics, Local Environment, and Daily Temperature on the Visits Paid by Apis mellifera to the Exotic Crop Phacelia tanacetifolia. Sustainability 2022, 14, 10186. [Google Scholar] [CrossRef]
- Maslo, S.; Šarić, Š. Two new neophytes in the flora of Bosnia and Herzegovina: Oenothera fruticosa and Phacelia campanularia. Glas. Future 2022, 5, 31–38. [Google Scholar] [CrossRef]
- Maurer, C.; Martínez-Núñez, C.; Dominik, C.; Heuschele, J.; Liu, Y.; Neumann, P.; Paxton, R.J.; Pellissier, L.; Proesmans, W.; Schweiger, O.; et al. Landscape simplification leads to loss of plant–pollinator interaction diversity and flower visitation frequency despite buffering by abundant generalist pollinators. Divers. Distrib. 2024, 30, e13853. [Google Scholar] [CrossRef]
- Bee fauna of Slovenia. Available online: https://www2.pms-lj.si/andrej/apoidea.htm (accessed on 16 February 2025).
- Olesen, J.M.; Jordano, P. Geographic Patterns in Plant–Pollinator Mutualistic Networks. Ecology 2002, 83, 2416–2424. [Google Scholar] [CrossRef]
- Dicks, L.V.; Corbet, S.A.; Pywell, R.F. Compartmentalization in plant–insect flower visitor webs. J. Anim. Ecol. 2002, 71, 32–43. [Google Scholar] [CrossRef]
- Basilio, A.M.; Medan, D.; Torretta, J.P.; Bartoloni, N.J. A year-long plant-pollinator network. Austral Ecol. 2006, 31, 975–983. [Google Scholar] [CrossRef]
- Bosch, J.; Martín González, A.M.; Rodrigo, A.; Navarro, D. Plant–pollinator networks: Adding the pollinator’s perspective. Ecol. Lett. 2009, 12, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.; Ștefan, V.; Knight, T.M. Oilseed Rape Shares Abundant and Generalized Pollinators with Its Co-Flowering Plant Species. Insects 2021, 12, 1096. [Google Scholar] [CrossRef]
- Marshall, L.; Leclercq, N.; Carvalheiro, L.G.; Dathe, H.H.; Jacobi, B.; Kuhlmann, M.; Potts, S.G.; Rasmont, P.; Roberts, S.P.M.; Vereecken, N.J. Understanding and addressing shortfalls in European wild bee data. Biol. Conserv. 2024, 290, 110455. [Google Scholar] [CrossRef]
- Kozuharova, E.K. Entomophilous plant species inhabiting the southern limestone slopes of Mt. VitoS’a (SW Bulgaria) and their pollinators. Flora Mediterr. 2000, 10, 227–234. [Google Scholar]
- Kozuharova, E.K.; Anchev, M.E.; Popov, P. The pollination ecology of Gentiana cruciata (Gentianaceae)-specifics of a Bulgarian population in comparison to Dutch populations. Nord. J. Bot. 2003, 23, 365–372. [Google Scholar] [CrossRef]
- Kozuharova, E.; Stoyanov, I. Honeybees, wild bees, and entomophilous plants on a meadow in Mt Konyavska, SW Bulgaria. Phytol. Balc. 2004, 9, 3. [Google Scholar]
- Kozuharova, E.; Firmage, D.H. On the pollination ecology of Astragalus alopecurus pallas (Fabaceae) in Bulgaria. Comptes Rendus L’académie Bulg. Des Sci. 2007, 60, 863–870. [Google Scholar]
- Kozuharova, E.; Firmage, D.H. Notes on the reproductive biology of Astragalus dasyanthus pall. (Fabaceae) a rare plant for Bulgaria. Comptes Rendus L’académie Bulg. Des Sci. 2009, 62, 1079–1088. [Google Scholar]
- Banaszak, J.; Dochkova, B. Bees (Hymenoptera, Apoidea, Apiformes) in the Agricultural Landscape of Bulgaria: Species Diversity. J. Apic. Sci. 2014, 58, 29–49. [Google Scholar] [CrossRef]
- Banaszak, J.; Szefer, P.; Dochkova, B. Relationships between bees (Hymenoptera: Apoidea: Apiformes) and flowers in the Bulgarian agricultural landscape. Pol. J. Entomol. 2015, 84, 101–126. [Google Scholar] [CrossRef]
- Kozuharova, E. Bumblebees and pollination of endemic Onobrychis pindicola (Fabaceae) in the subalpine habitats of Pirin Mts. Biol. Nyssana 2019, 9, 89–101. [Google Scholar] [CrossRef]
- Valchev, H.; Kolev, Z.; Stoykova, B.; Kozuharova, E. Pollinators of Lavandula angustifolia Mill., an important factor for optimal production of lavender essential oil. BioRisk 2022, 17, 297–307. [Google Scholar] [CrossRef]
- Valchev, H.; Kozuharova, E. In situ and Ex situ Investigations on Breeding Systems and Pollination of Sideritis scardica Griseb. (Lamiaceae) in Bulgaria. Proc. Bulg. Acad. Sci. 2022, 75, 527–535. [Google Scholar] [CrossRef]
- Plants of the World Online|Kew Science. Available online: https://powo.science.kew.org/ (accessed on 16 February 2025).
- Euro+Med PlantBase—Preview of the New Data Portal|Euro+Med-Plantbase. Available online: https://europlusmed.org/ (accessed on 16 February 2025).
- Bogusch, P.; Bláhová, E.; Horák, J. Pollen specialists are more endangered than non-specialised bees even though they collect pollen on flowers of non-endangered plants. Arthropod-Plant Interact. 2020, 14, 759–769. [Google Scholar] [CrossRef]
- Michez, D.; Patiny, S.; Rasmont, P.; Timmermann, K.; Vereecken, N.J. Phylogeny and host-plant evolution in Melittidae s.l. (Hymenoptera: Apoidea). Apidologie 2008, 39, 146–162. [Google Scholar] [CrossRef]
- Wood, T.J.; Holland, J.M.; Goulson, D. Diet characterisation of solitary bees on farmland: Dietary specialisation predicts rarity. Biodivers. Conserv. 2016, 25, 2655–2671. [Google Scholar] [CrossRef]
- Goulson, D. Bumblebees: Their Behaviour and Ecology; Oxford University Press: Oxford, UK; New York, NY, USA, 2003; ISBN 978-0-19-852606-3. [Google Scholar]
- Skyrpan, I.P.; Pytel, S.R. List of bee species (Hymenoptera, Apoidea) of Lviv city (Ukraine). Part I. Families Andrenidae Latreille, 1802 and Apidae Latreille, 1802. Stud. Biol. 2020, 14, 111–120. [Google Scholar] [CrossRef]
- Muller, A. Host-Plant Specialization in Western Palearctic Anthidine Bees (Hymenoptera: Apoidea: Megachilidae). Ecol. Monogr. 1996, 66, 235–257. [Google Scholar] [CrossRef]
- Kleijn, D.; Raemakers, I. A retrospective analysis of pollen host plant use by stable and declining bumble bee species. Ecology 2008, 89, 1811–1823. [Google Scholar] [CrossRef] [PubMed]
- Westrich, P. Die Wildbienen Baden-Württembergs; E. Ulmer: Stuttgart, Germany, 1990. [Google Scholar]
- Müller, A.; Kuhlmann, M. Pollen hosts of western palaearctic bees of the genus Colletes (Hymenoptera: Colletidae): The Asteraceae paradox: Pollen hosts of colletes bees. Biol. J. Linn. Soc. 2008, 95, 719–733. [Google Scholar] [CrossRef]
- Ducke, A. Die Bienengattung Osmia Panz. als Ergänzung zu Schmiedeknecht’s “Apidae europaeae”. In Ihren Palaearctischen Arten Monographisch Bearbeitet.-Berichte des Naturwissenschaftlich; Medizinischen Vereins in Innsbruck: Innsbruck, Austria, 1900; Volume 2, pp. 1–323. [Google Scholar]
- Müller, A. The hidden diet—Examination of crop content reveals distinct patterns of pollen host use by Central European bees of the genus Hylaeus (Hymenoptera, Colletidae). Alp. Entomol. 2023, 7, 21–35. [Google Scholar] [CrossRef]
- Baldock, D.W.; Wood, T.J.; Cross, I.; Smith, J. The bees of Portugal (Hymenoptera: Apoidea: Anthophila). In Entomofauna; Maximilian Schwarz: Ansfelden, Austria, 2018; pp. 1–164. [Google Scholar]
- Knerer, G. The biology and social behaviour of Evylaeus malachurus (K.) (Hymenoptera; Halictidae) in different climatic regions of Europe. Zool. Jahrbücher Abt. Syst. Okol. Geogr. Tiere 1992, 119, 261–290. [Google Scholar]
- Rust, R.W.; Cambon, G.; Grossa, J.-P.T.; Vaissière, B.E. Nesting Biology and Foraging Ecology of the Wood-boring Bee Lithurgus chrysurus (Hymenoptera: Megachilidae). J. Kans. Entomol. Soc. 2004, 77, 269–279. [Google Scholar] [CrossRef]
- Praz, C.J. Subgeneric classification and biology of the leafcutter and dauber bees (genus Megachile Latreille) of the western Palearctic (Hymenoptera, Apoidea, Megachilidae). J. Hymenopt. Res. 2017, 55, 1–54. [Google Scholar] [CrossRef]
- Müller, A.; Bansac, N. A specialized pollen-harvesting device in western palaearctic bees of the genus Megachile (Hymenoptera, Apoidea, Megachilidae). Apidologie 2004, 35, 329–337. [Google Scholar] [CrossRef]
- Müller, A. Palaearctic Osmia bees of the subgenera Hemiosmia, Tergosmia and Erythrosmia (Megachilidae, Osmiini): Biology, taxonomy and key to species. Zootaxa 2020, 4778, 201–236. [Google Scholar] [CrossRef]
- Müller, A. Palaearctic Osmia bees of the subgenera Allosmia and Neosmia (Megachilidae, Osmiini): Biology, taxonomy and key to species. Zootaxa 2022, 5188, 201–232. [Google Scholar] [CrossRef]
- Müller, A. Palaearctic Osmia bees of the subgenus Hoplosmia (Megachilidae, Osmiini): Biology, taxonomy and key to species. Zootaxa 2018, 4415, 297–329. [Google Scholar] [CrossRef] [PubMed]
- Hostinská, L.; Kuneš, P.; Hadrava, J.; Bosch, J.; Scaramozzino, P.L.; Bogusch, P. Comparative biology of four Rhodanthidium species (Hymenoptera, Megachilidae) that nest in snail shells. J. Hymenopt. Res. 2021, 85, 11–28. [Google Scholar] [CrossRef]
- Kasparek, M. The Cuckoo Bees of the Genus Stelis Panzer, 1806 in Europe, North Africa and the Middle East: A Review and Identification Guide; Maximilian Schwartz: Ansfelden, Austria, 2015; 144p, Available online: https://siris-libraries.si.edu/ipac20/ipac.jsp?uri=full=3100001~!1080769~!5&ri=8&aspect=basic&menu=search&source=~!silibraries&profile=liball (accessed on 13 March 2025).
- Kasparek, M. Revision of the Palaearctic Trachusa interrupta species complex (Apoidea: Anthidiini) with description of four new species. Zootaxa 2020, 4728, zootaxa.4728.1.1. [Google Scholar] [CrossRef]
- Corbet, S.A. Bee visits and the nectar of Echium vulgare L. and Sinapis alba L. Ecol. Entomol. 1978, 3, 25–37. [Google Scholar] [CrossRef]
- Hayot Carbonero, C.; Mueller-Harvey, I.; Brown, T.A.; Smith, L. Sainfoin (Onobrychis viciifolia): A beneficial forage legume. Plant Genet. Resour. 2011, 9, 70–85. [Google Scholar] [CrossRef]
- Pacini, E.; Nepi, M. Nectar production and presentation. In Nectaries and Nectar; Nicolson, S.W., Nepi, M., Pacini, E., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 167–214. ISBN 978-1-4020-5937-7. [Google Scholar]
- Efimov, S.V. Morphology of the staminodial disc of flowers of representatives of the paeoniaceae family and its possible connection with smell (aroma). Reg. Geosyst. 2011, 14, 254–258, (In Russian language). [Google Scholar]
- Weryszko-Chmielewska, E.; Sulborska, A. Staminodial Nectary Structure in Two Pulsatilla (L.) Species. Acta Biol. Cracoviensia Ser. Bot. 2011, 53, 94–103. [Google Scholar] [CrossRef]
- Müller, A.; Diener, S.; Schnyder, S.; Stutz, K.; Sedivy, C.; Dorn, S. Quantitative pollen requirements of solitary bees: Implications for bee conservation and the evolution of bee–flower relationships. Biol. Conserv. 2006, 130, 604–615. [Google Scholar] [CrossRef]
- Vanderplanck, M.; Gilles, H.; Nonclercq, D.; Duez, P.; Gerbaux, P. Asteraceae Paradox: Chemical and Mechanical Protection of Taraxacum Pollen. Insects 2020, 11, 304. [Google Scholar] [CrossRef]
- Van Dijk, P.J. Ecological and evolutionary opportunities of apomixis: Insights from Taraxacum and Chondrilla. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2003, 358, 1113–1121. [Google Scholar] [CrossRef]
- Vrijmoed, P. Collection, Propagation and Use of Native Plants. In Southern Forest Nursery Association Conference; Southern Research Station: Asheville, NC, USA, 1999; p. 156. [Google Scholar]
- Ruchala, S.L. Propagation of Several Native Ornamental Plants. Master’s Thesis, University of Maine, Orono, ME, USA, 2002. [Google Scholar]
- Thetford, M.; Heather, A.E.; Pérez, H.E.; Wilson, S.B. Propagation of Wildflowers from Wild-Collected Seeds or Cuttings. Int. Plant Propagators Soc. Comb. Proc. 2008, 58, 555–560. [Google Scholar]
- Krigas, N.; Mouflis, G.; Grigoriadou, K.; Maloupa, E. Conservation of important plants from the Ionian Islands at the Balkan Botanic Garden of Kroussia, N Greece: Using GIS to link the in situ collection data with plant propagation and ex situ cultivation. Biodivers. Conserv. 2010, 19, 3583–3603. [Google Scholar] [CrossRef]
- Alina, Z.; Lucia, D.; Liliana, C.E. Aspects regarding the “ex situ” propagation of some wild plants in order to introduce them into the culture. Șt. Hortic 2012, 55, 275–280. [Google Scholar]
- Heywood, V.H.; Dullo, M.E. In Situ Conservation of Wild Plant Species: A Critical Global Review of Good Practices; IPGRI Technical Bulletin 11: Rome, Italy, 2005. [Google Scholar]
- Hossain, M.A.; Sen, M.; Jewell, M.I.U.; Kabir, M.A. Propagation of Flacourtia jangomas: An approach towards the domestication of wild fruit species in Bangladesh. Dendrobiology 2011, 65, 63–71. [Google Scholar]
- Evstatieva, L.; Popova, I. Factors, Affecting Germination and Seedling Development of Sideritis scardica Griseb. and S. syriaca L.; CABI Digital Library: Oxford, UK, 1998; Volume 1, pp. 371–376. [Google Scholar]
- Evstatieva, L.; Alipieva, K.; Aneva, I. Introduction and Sustainable Use of Rare Medicinal Plants in Bulgaria. A Model Approach for Sideritis scardica Griseb. Medicinal Plants: Fundamental and Applied Problems. In Proceedings of the 1st International Scientific Conference, Novosibirsk, Russia, 21–22 May 2013; pp. 257–259. [Google Scholar]
- Aneva, I. Biological and Phytochemical In Situ and Ex Situ Study of Species of the Genus Sideritis with Conservation Importance in Bulgaria. Ph.D. Thesis, Institute of Biodiversity and Ecosystem Research, Bulgarian Academy of Sciences, Sofia, Bulgaria, 2016. [Google Scholar]
- Aneva, I.; Zhelev, P. Morphometric studies of Sideritis scardica Grsb. and S. syriaca L. in their natural populations in Bulgaria. Boletín Latinoam. Y Caribe Plantas Med. Y Aromáticas 2019, 18, 71–80. [Google Scholar] [CrossRef]
- Kožuharova, E.; Tzvetanova, V.; Firmage, D. Seed germination and seedling development of two rare Astragalus species (Fabaceae). Phytol. Balc. 2010, 16, 51–56. [Google Scholar]
- Singh, P. Propagation of 14 native prairie forbs by sexual and asexual methods. Nativ. Plants J. 2021, 22, 345–354. [Google Scholar] [CrossRef]
- Pence, V.C. Evaluating costs for the in vitro propagation and preservation of endangered plants. In Vitro Cell. Dev. Biol. Plant 2011, 47, 176–187. [Google Scholar] [CrossRef]
- Oseni, O.M.; Pande, V.; Nailwal, T.K. A Review on Plant Tissue Culture, A Technique for Propagation and Conservation of Endangered Plant Species. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 3778–3786. [Google Scholar] [CrossRef]
- Mikell, L.; Wilson, S.B.; Marble, S.C.; Vendrame, W.; Van Santen, E. Sexual and Asexual Propagation of Wild Lime (Zanthoxylum fagara L. Sarg.), a Native Florida Plant with Ornamental and Ecological Value. J. Environ. Hortic. 2024, 42, 131–139. [Google Scholar] [CrossRef]
- Jiang, X.L.; Zhang, W.G.; Wang, G. Effects of different components of diversity on productivity in artificial plant communities. Ecol. Res. 2007, 22, 629–634. [Google Scholar] [CrossRef]
- Fløjgaard, C.; Valdez, J.W.; Dalby, L.; Moeslund, J.E.; Clausen, K.K.; Ejrnæs, R.; Pärtel, M.; Brunbjerg, A.K. Dark diversity reveals importance of biotic resources and competition for plant diversity across habitats. Ecol. Evol. 2020, 10, 6078–6088. [Google Scholar] [CrossRef]
- Larionov, M.V.; Dogadina, M.A.; Tarakin, A.V.; Minakova, I.V.; Sentishcheva, E.A.; Bukreeva, T.N. Creation of artificial phytocenoses with controlled properties as a tool for managing cultural ecosystems and landscapes. IOP Conf. Ser. Earth Environ. Sci. 2021, 848, 012127. [Google Scholar] [CrossRef]
- Salomé-Castañeda, E.; Olivares-Esquivel, E. Artificial communities of plants as guides for design of urban areas of central Mexico. Acta Hortic. 2023, 1374, 125–132. [Google Scholar] [CrossRef]
- Yanev, A. Ornamental Plants form the Bulgarian Flora; Nauka i Izkustvo: Sofia, Bulgaria, 1959; 512p, (In Bulgarian language). [Google Scholar]
- Toogood, A. American Horticultural Society Plant Propagation: The Fully Illustrated Plant-by-Plant Manual of Practical Techniques; DC Publishing Inc.: New York, NY, USA, 1999. [Google Scholar]
- The Royal Horticultural Society. A–Z Encyclopedia of Garden Plants; Brickell, C., Royal Horticultural Society, Eds.; Dorling Kindersley: London, UK, 2000; ISBN 978-0-7513-0436-7. [Google Scholar]
- Balabanova, V.; Zdraveva, P.; Kozuharova, E.; Krasteva, I.; Nikolov, S. A possibility for cultivation and phytochemical study of endangered Gypsophila trichotoma Wend. Comptes Rendus L’académie Bulg. Sci. 2009, 62, 1247–1252. [Google Scholar]
- Naydenova, G.; Vasileva, V.; Mitev, D. Productivity of Bulgarian grazing ecotypes of perennial legumes. J. Mt. Agric. Balk. 2015, 18, 972–982. [Google Scholar]
- Sokolov, R.S. Preliminary test results of 15 species of Bulgarian flora cultivated as ornamental plants. Phytol. Balc. 2016, 22, 187–192. [Google Scholar]
- Sarrou, E.; Tsivelika, N.; Martens, S.; Irakli, M.; Bletsaki, F.; Broufa, S.; Panajiotidis, S.; Chatzopoulou, P.S.; Abraham, E.M. First steps towards pre-breeding of Sideritis scardica: A phenotypic, agronomic, and phytochemical profiling approach. Agronomy 2024, 14, 1448. [Google Scholar] [CrossRef]
- Prijić, Ž.; Mikić, S.; Peškanov, J.; Zhang, X.; Guo, L.; Dragumilo, A.; Filipović, V.; Anačkov, G.; Marković, T. Diversity of Treatments in Overcoming Morphophysiological Dormancy of Paeonia peregrina Mill. Seeds. Plants 2024, 13, 2178. [Google Scholar] [CrossRef]
- Prijić, Z.; Mikić, S.; Filipović, V.; Dragumilo, A.; Gordanić, S.; Batinić, P.; Čutović, N.; Marković, T. Seed Weight and Optimal Imbibition Period for some Herbaceous Peony (Paeonia spp.) Species Native to Serbia. In Proceedings of the XII International Symposium on Agricultural Sciences “AgroReS 2023”, Trebinje, Bosnia and Herzegovina, 24–26 May 2023. [Google Scholar]
- Breeze, T.D.; Bailey, A.P.; Balcombe, K.G.; Brereton, T.; Comont, R.; Edwards, M.; Garratt, M.P.; Harvey, M.; Hawes, C.; Isaac, N.; et al. Pollinator monitoring more than pays for itself. J. Appl. Ecol. 2021, 58, 44–57. [Google Scholar] [CrossRef]
- Lanuza, J.B.; Knight, T.M.; Montes-Perez, N.; Glenny, W.; Acuña, P.; Albrecht, M.; Artamendi, M.; Badenhausser, I.; Bennett, J.M.; Biella, P.; et al. EuPPollNet: A European Database of Plant-Pollinator Networks. Glob. Ecol. Biogeogr. 2025, 34, e70000. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).