Taxonomic Diversity and Abundance of Soil Macrofauna in Temperate Forests Under Different Types of Forest Management: A Case Study in European Russia
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Samples Collection
2.2. Animal Identification
2.3. Environmental Parameters Analysis
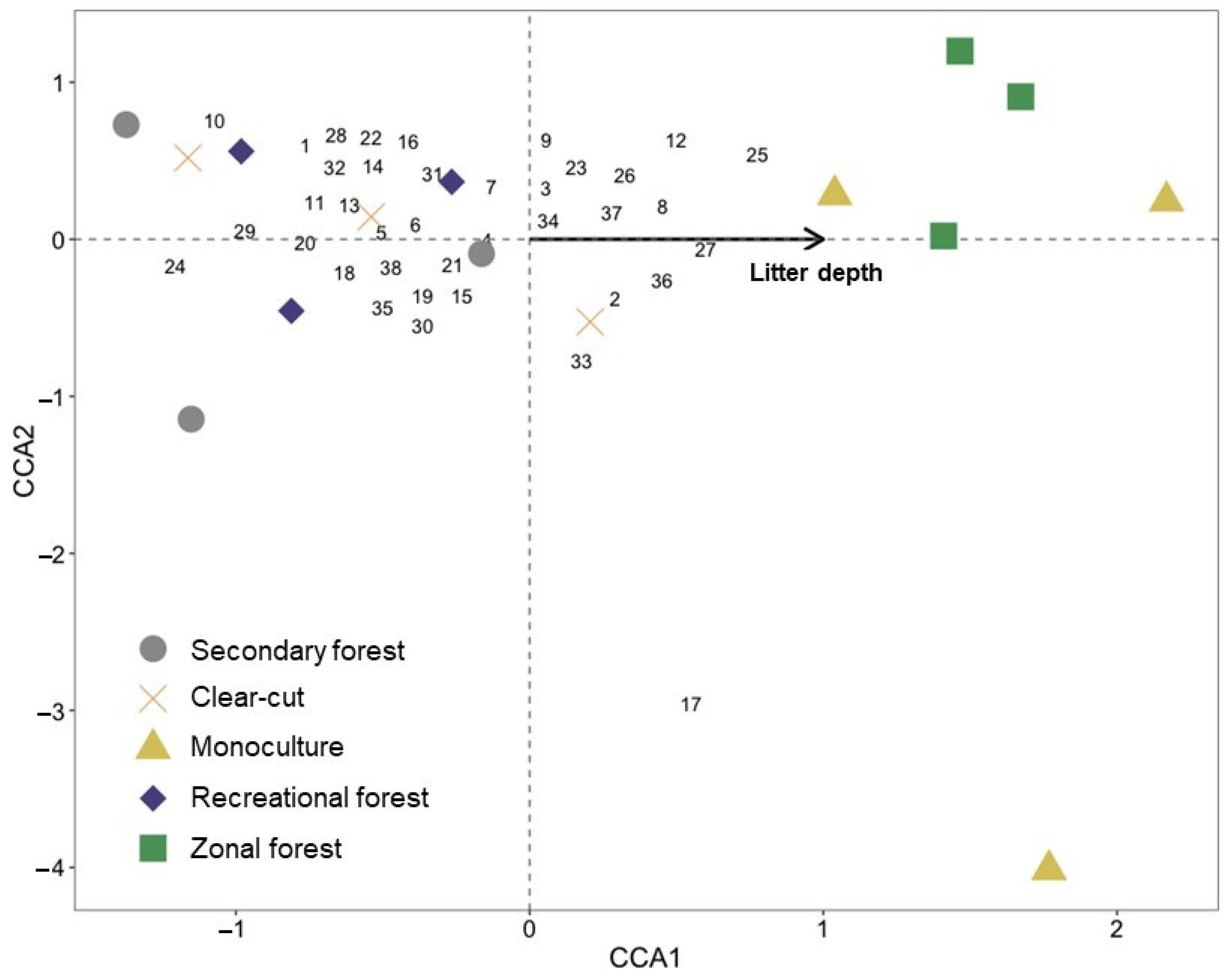
2.4. Statistical Analysis
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eisenhauer, N.; Powell, J.R. Plant trait effects on soil organisms and functions. Pedobiologia 2017, 65, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Lindenmayer, D.B.; Westgate, M.J.; Scheele, B.C.; Foster, C.N.; Blair, D.P. Key perspectives on early successional forests subject to stand-replacing disturbances. For. Ecol. Manag. 2019, 454, 117656. [Google Scholar] [CrossRef]
- Ogureeva, G.N.; Leonova, N.B.; Buldakova, E.V.; Kadetov, N.G.; Arkhipova, M.V.; Miklyaeva, I.M.; Bocharnikov, M.V.; Dudov, S.V.; Ignatova, E.A.; Ignatov, M.S.; et al. Map “The Biomes of Russia” (Scale 1: 7,500,000); WWF Russia: Moscow, Russia, 2018. [Google Scholar]
- Olson, D.M.; Dinerstein, E.; Wikramanayake, E.D.; Burgess, N.D.; Powell, G.V.N.; Underwood, E.C.; D’amico, J.A.; Itoua, I.; Strand, H.E.; Morrison, J.C.; et al. Terrestrial Ecoregions of the World: A New Map of Life on Earth: A new global map of terrestrial ecoregions provides an innovative tool for conserving biodiversity. BioScience 2001, 51, 933–938. [Google Scholar] [CrossRef]
- FAO-2020. Global Forest Resources Assessment 2020; FAO: Rome, Italy, 2018. [Google Scholar]
- Lukina, N.V.; Geraskina, A.P.; Gornov, A.V.; Shevchenko, N.E.; Kuprin, A.V.; Chernov, T.I.; Chumachenko, S.I.; Shanin, V.N.; Kuznetsova, A.I.; Tebenkova, D.N.; et al. Biodiversity and climate-regulating functions of forests: Current issues and research prospects. For. Sci. Issues 2021, 4, 1–90. [Google Scholar] [CrossRef]
- TEEB. The Economics of Ecosystems and Biodiversity: Mainstreaming the Economics of Nature: A Synthesis of the Approach, Conclusions and Recommendations of TEEB; Progress Press: Valetta, Malta, 2010. [Google Scholar]
- Anthony, M.A.; Bender, S.F.; van der Heijden, M.G. Enumerating soil biodiversity. Proc. Natl. Acad. Sci. USA 2023, 120, e2304663120. [Google Scholar] [CrossRef]
- De Vries, F.T.; Thébault, E.; Liiri, M.; Birkhofer, K.; Tsiafouli, M.A.; Bjørnlund, L.; Jørgensen, H.B.; Brady, M.V.; Christensen, S.; de Ruiter, P.C.; et al. Soil food web properties explain ecosystem services across European land use systems. Proc. Natl. Acad. Sci. USA 2013, 110, 14296–14301. [Google Scholar] [CrossRef]
- Coleman, D.C.; Crossley, D.A.; Hendrix, P.F., Jr. Fundamentals of Soil Ecology; Elsevier Academic Press: Amsterdam, The Netherlands, 2004; p. 386. [Google Scholar]
- Bardgett, R. The Biology of Soil: A Community and Ecosystem Approach; Oxford University Press: New York, NY, USA, 2005; p. 256. [Google Scholar]
- Chernenkova, T.; Kotlov, I.; Belyaeva, N.; Suslova, E. Spatiotemporal modeling of coniferous forests dynamics along the southern edge of their range in the Central Russian Plain. Remote Sens. 2021, 13, 1886. [Google Scholar] [CrossRef]
- Chernenkova, T.V.; Kotlov, I.P.; Belyaeva, N.G.; Suslova, E.G. Mapping and assessment of the cenotic diversity of the forests of the Moscow Region. Russ. J. Ecosyst. Ecol. 2023, 54, 682–692. [Google Scholar] [CrossRef]
- Gongalsky, K.B. Perfugia as a mechanism of recovery of soil fauna after ecosystem disturbances. Russ. J. Ecosyst. Ecol. 2017, 2, 3. [Google Scholar] [CrossRef]
- Viljur, M.; Abella, S.R.; Adámek, M.; Alencar, J.B.R.; Barber, N.A.; Beudert, B.; Burkle, L.A.; Cagnolo, L.; Campos, B.R.; Chao, A.; et al. The Effect of Natural Disturbances on Forest Biodiversity: An Ecological Synthesis. Biol. Rev. 2022, 97, 1930–1947. [Google Scholar] [CrossRef]
- Phillips, H.R.P.; Cameron, E.K.; Eisenhauer, N.; Burton, V.J.; Ferlian, O.; Jin, Y.; Kanabar, S.; Malladi, S.; Murphy, R.E.; Peter, A.; et al. Global changes and their environmental stressors have a significant impact on soil biodiversity—A meta-analysis. iScience 2024, 27, 110540. [Google Scholar] [CrossRef]
- Vorobeichik, E.L.; Ermakov, A.I.; Zolotarev, M.P.; Tuneva, T.K. Changes in diversity of soil macrofauna in industrial pollution gradient. Russ. Entomol. J. 2012, 21, 203–218. [Google Scholar] [CrossRef]
- Geisen, S.; Wall, D.H.; van der Putten, W.H. Challenges and Opportunities for Soil Biodiversity in the Anthropocene. Curr. Biol. 2019, 29, 1036–1044. [Google Scholar] [CrossRef]
- Filimonova, Z.V.; Gongalsky, K.B. Communities of soil macrofauna at a boundary between plots of high and low pollution from Kosogorsky metallurgical plant (Tula Region, Russia). Izv. Penz. Gos. Pedagog. Univ. V.G Belinsky 2011, 25, 472–477. [Google Scholar]
- García-Segura, D.; Castillo-Murrieta, I.M.; Martínez-Rabelo, F.; Gomez-Anaya, A.; Rodríguez-Campos, J.; Hernández-Castellanos, B.; Contreras-Ramos, S.M.; Barois, I. Macrofauna and mesofauna from soil contaminated by oil extraction. Geoderma 2018, 332, 180–189. [Google Scholar] [CrossRef]
- Vershinina, S.D. Structure of soil mesofauna in the urbanizasticon gradien. Bull. Udmurt Univ. Ser. Biol. Earth Sci. 2011, 2, 84–89. (In Russian) [Google Scholar]
- Ermakov, A.I.; Vorobeichik, E.L. Soil macrofauna of forest ecosystems in a large industrial city. Eurasian Entomol. J. 2013, 12, 519–528. (In Russian) [Google Scholar]
- Tuck, S.L.; Winqvist, C.; Mota, F.; Ahnström, J.; Turnbull, L.A.; Bengtsson, J. Land-use intensity and the effects of organic farming on biodiversity: A hierarchical meta-analysis. J. Appl. Ecol. 2014, 51, 746–755. [Google Scholar] [CrossRef]
- Stein-Bachinger, K.; Gottwald, F.; Haub, A.; Schmidt, E. To what extent does organic farming promote species richness and abundance in temperate climates? A review. Org. Agric. 2020, 11, 1–12. [Google Scholar] [CrossRef]
- Quine, C.P.; Humphrey, J.W. Plantations of exotic tree species in Britain: Irrelevant for biodiversity or novel habitat for native species? Biodivers. Conserv. 2010, 19, 503–1512. [Google Scholar] [CrossRef]
- Irwin, S.; Pedley, S.M.; Coote, L.; Dietzsch, A.C.; Wilson, M.W.; Oxbrough, A.; Sweeney, O.; Moore, K.M.; Martin, R.; Kelly, D.L.; et al. The value of plantation forests for plant, invertebrate and bird diversity and the potential for cross-taxon surrogacy. Biodivers. Conserv. 2014, 23, 697–714. [Google Scholar] [CrossRef]
- Malmström, A.; Persson, T.; Ahlström, K.; Gongalsky, K.B.; Bengtsson, J. Dynamics of soil meso-and macrofauna during a 5-year period after clear-cut burning in a boreal forest. Appl. Soil Ecol. 2009, 43, 61–74. [Google Scholar] [CrossRef]
- Calviño-Cancela, M.; Rubido-Bará, M. Invasive potential of Eucalyptus globulus: Seed dispersal, seedling recruitment and survival in habitats surrounding plantations. For. Ecol. Manag. 2013, 305, 129–137. [Google Scholar] [CrossRef]
- da Silva, L.P.; Heleno, R.H.; Costa, J.M.; Valente, M.; Mata, V.A.; Gonçalves, S.C.; da Silva, A.A.; Alves, J.; Jaime, A. Natural woodlands hold more diverse, abundant, and unique biota than novel anthropogenic forests: A multi-group assessment. Eur. J. For. Res. 2019, 138, 461–472. [Google Scholar] [CrossRef]
- Crotty, F.V.; Demirer, U.A.; Norris, S.L.; Liu, W.; Murray, P.J. Evaluating the Impact of Long-Term Land Use Change and Age since Disturbance on Soil Faunal Diversity. Forests 2023, 14, 1882. [Google Scholar] [CrossRef]
- Ogureeva, G.N.; Leonova, N.B.; Miklyaeva, I.M.; Bocharnikov, M.V.; Fedosov, V.E.; Muchnik, E.E.; Urbanavicius, G.P.; Emelyanova, L.G.; Khlyap, L.A.; Rumyantsev, V.Y.; et al. The Biodiversity of Russian Biomes. The Biomes of Plains; Institute of Global Climate and Ecology: Moscow, Russia, 2020. [Google Scholar]
- Dylis, N.V. Program and Methodology of Biogeocenological Research; Nauka: Moscow, Russia, 1974. (In Russian) [Google Scholar]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2014, Update 2015. International Soil Classification System for Naming Soils and Creating legends for Soil Maps; World Soil Resource Reports; Food and Agriculture Organization of the United Nations: Rome, Italy, 2015. [Google Scholar]
- Rabotnov, T.A. Phytocenology; MSU Press: Moscow, Russia, 1983. (In Russian) [Google Scholar]
- Zaleskaya, N.T. Opredelitel’ Mnogonozhek-Kostyanok SSSR (Chilopoda lithobiomorpha) [Identification Book of the Lithobiomorph Centipedes of the USSR (Chilopoda lithobiomorpha)]; Nauka: Moscow, Russia, 1978. (In Russian) [Google Scholar]
- Roberts, M.J. Spinnengids; Tirion: Baarn, The Netherlands, 1998. [Google Scholar]
- Bonato, L.; Minelli, A. Chilopoda Geophilomorpha of Europe: A revised list of species, with taxonomic and nomenclatorial notes. Zootaxa 2014, 3770, 1–136. [Google Scholar] [CrossRef]
- Krivosheina, M. Keys to the Palaearctic Families and Genera of Nematocerous Larvae (Diptera, Nematocera); KMK: Moscow, Russia, 2012. [Google Scholar]
- Bey-Bienko, G.J. (Ed.) Keys to the Insects of the European USSR. In Five Volumes; Nauka: Moscow, Russia, 1964. [Google Scholar]
- Korobushkin, D.I.; Gorbunova, A.Y.; Zaitsev, A.S.; Gongalsky, K.B. Trait-specific response of soil macrofauna to forest burning along a macrogeographic gradient. Appl. Soil Ecol. 2017, 112, 97–100. [Google Scholar] [CrossRef]
- Ellers, J.; Berg, M.P.; Dias, A.T.C.; Fontana, S.; Ooms, A.; Moretti, M. Diversity in form and function: Vertical distribution of soil fauna mediates multidimensional trait variation. J. Anim. Ecol. 2018, 87, 933–944. [Google Scholar] [CrossRef]
- Arinushkina, E.V. Manual on the Chemical Analysis of Soils; Publishing House of Moscow State University: Moscow, Russia, 1961. (In Russian) [Google Scholar]
- Anderson, J.P.; Domsch, K.H. A physiological method for the quantitative measurement of microbial biomass in soils. Soil Biol. Biochem. 1978, 10, 215–221. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://www.R-project.org/ (accessed on 11 December 2024).
- Posit Team. RStudio: Integrated Development Environment for R. Posit Software; PBC: Boston, MA, USA, 2024; Available online: http://www.posit.co/ (accessed on 11 December 2024).
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous Inference in General Parametric Models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef]
- Oksanen, J.; Simpson, G.; Blanchet, F.; Kindt, R.; Legendre, P.; Minchin, P.; O’Hara, R.; Solymos, P.; Stevens, M.; Szoecs, E.; et al. Vegan: Community Ecology Package. R Package Version 2.6-4. 2022. Available online: https://CRAN.R-project.org/package=vegan (accessed on 11 December 2024).
- Revelle, W. psych: Procedures for Psychological, Psychometric, and Personalityresearch [R Package Psych Version 2.4.6.26]. CRAN Contrib Packag. 2024. Available online: https://CRAN.R-project.org/package=psych (accessed on 11 December 2024).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Mordkovich, V.G. Invertebrate animals and diagnostics of elementary soil processes. Soil Sci. 1991, 10, 92–99. [Google Scholar]
- Gorbunova, A.Y.; Korobushkin, D.I.; Zaitsev, A.S.; Gongalsky, K.B. Forest fires increase variability of soil macrofauna communities along a macrogeographic gradient. Eur. J. Soil Biol. 2017, 80, 49–52. [Google Scholar] [CrossRef]
- Gongalsky, K.B.; Iurmanov, A.A.; Ukhova, N.L.; Korobushkin, D.I. The size of burnt areas has little effect on the recovery of soil macrofauna in the boreal forests of Middle Ural, Russia. Pedosphere 2020, 30, 714–718. [Google Scholar] [CrossRef]
- Wermelinger, B.; Moretti, M.; Duelli, P.; Lachat, T.; Pezzatti, G.B.; Obrist, M.K. Impact of windthrow and salvage-logging on taxonomic and functional diversity of forest arthropods. For. Ecol. Manag. 2017, 391, 9–18. [Google Scholar] [CrossRef]
- Moretti, M.; Duelli, P.; Obrist, M. Biodiversity and resilience of arthropod communities after fire disturbance in temperate forests. Oecologia 2006, 149, 312–327. [Google Scholar] [CrossRef]
- Ivonin, V.M.; Voskoboynikova, I.V. Theoretical concept of soil erosion for recreational forests. Sci. J. Russ. Sci. Res. Inst. Land Improv. Probl. 2015, 1, 61–71. [Google Scholar]
- Yang, X.; Li, T.; Gan, M.; Chen, M. Community characteristics and distribution patterns of soil fauna after vegetation restoration in the northern Loess Plateau. Ecol. Indic. 2021, 122, 107236. [Google Scholar] [CrossRef]
- Pyatina, E.V.; Bulgakova, M.A. Influence of megacity on the ecological structure of communities of soil-dwelling invertebrates. Ecol. Urban Areas 2018, 3, 33–39. [Google Scholar] [CrossRef]
- Stein, A.; Gerstner, K.; Kreft, H. Environmental heterogeneity as a universal driver of species richness across taxa, biomes and spatial scales. Ecol. Lett. 2014, 17, 866–880. [Google Scholar] [CrossRef]
- Birkhofer, K.; Diehl, E.; Wolters, V.; Smith, H.G. Global metawebs of spider predation highlight consequences of land-use change for terrestrial predator-prey networks. In Adaptive Food Webs: Stability and Transitions of Real and Model Ecosystems; Moore, J.C., Mccann, K.S., De Ruiter, P.C., Wolters, V., Eds.; Cambridge University Press: Cambridge, UK, 2017; pp. 193–213. [Google Scholar]
- Lapteva, E.M.; Vtyurin, G.M.; Bobkova, K.S.; Kaverin, D.A.; Dymov, A.A.; Simonov, G.A. Soil and soil cover changes in spruce forests after final logging. Siberian J. For. Sci. 2015, 5, 64–76. [Google Scholar] [CrossRef]
- Korboulewsky, N.; Perez, G.; Chauvat, M. How tree diversity affects soil fauna diversity: A review. Soil Biol. Biochem. 2016, 94, 94–106. [Google Scholar] [CrossRef]
- Gongalsky, K.B.; Zaitsev, A.S.; Korobushkin, D.I.; Saifutdinov, R.A.; Butenko, K.O.; Vries, F.T.; Ekschmitt, K.; Degtyarev, M.I.; Gorbunova, A.Y.; Kostina, N.V.; et al. Forest fire induces short-term shifts in soil food webs with consequences for carbon cycling. Ecol. Lett. 2021, 24, 438–450. [Google Scholar] [CrossRef]
- Feitosa de Souza, T.A.; Kormann, S.; Laurindo, L.K.; da Silva, L.J.R. Land use and soil properties shifts soil fauna community complexity in subtropical ecosystems. Arch. Agron. Soil Sci. 2023, 69, 2081–2091. [Google Scholar] [CrossRef]
- Karavanova, E.I.; Belyanina, L.A.; Stepanov, A.A. Water-soluble organic matter and soil solution acidity in the main soil types of the central forest state biosphere reserve. Eurasian Soil Sci. 2007, 40, 493–504. [Google Scholar] [CrossRef]
- Enríquez, S.; Duarte, C.M.; Sand-Jensen, K. Patterns in decomposition rates among photosynthetic organisms: The importance of detritus C:N:P content. Oecologia 1993, 94, 457–471. [Google Scholar] [CrossRef] [PubMed]
- Isidorov, V.A.; Zaitsev, A.A. Reviews and syntheses: VOC emissions from soil cover in boreal and temperate natural ecosystems of the Northern Hemisphere. Biogeoscie 2022, 19, 4715–4746. [Google Scholar] [CrossRef]
- Guggenberger, G. Humification and Mineralization in Soils. In Microorganisms in Soils: Roles in Genesis and Functions. Soil Biology; Varma, A., Buscot, F., Eds.; Springer: Berlin/Heidelberg, Germany, 2005; Volume 3, pp. 85–106. [Google Scholar] [CrossRef]
- Karhu, K.; Auffret, M.D.; Dungait, J.A.; Hopkins, D.W.; Prosser, J.I.; Singh, B.K.; Subke, J.; Wookey, P.A.; Ågren, G.I.; Sebastià, M.; et al. Temperature sensitivity of soil respiration rates enhanced by microbial community response. Nature 2014, 513, 81–84. [Google Scholar] [CrossRef]
- Ananyeva, N.D.; Susyan, E.A.; Gavrilenko, E.G. Determination of the soil microbial biomass carbon using the method of substrate-induced respiration. Eurasian Soil Sci. 2011, 11, 1327–1333. [Google Scholar] [CrossRef]
- Spohn, M.; Chodak, M. Microbial respiration per unit biomass increases with carbon-to-nutrient ratios in forest soils. Soil Biol. Biochem. 2015, 81, 128–133. [Google Scholar] [CrossRef]
- Eckert, M.; Gaigher, R.; Pryke, J.S.; Janion-Scheepers, C.; Samways, M.J. Timber plantations do not homogenize soil arthropod diversity but do alter species composition. Geoderma 2022, 428, 116190. [Google Scholar] [CrossRef]
- De Smedt, P.; Wuyts, K.; Baeten, L.; De Schrijver, A.; Proesmans, W.; De Frenne, P.; Ampoorter, E.; Remy, E.; Gigbels, M.; Hermy, M.; et al. Complementary distribution patterns of arthropod detritivores (woodlice and millipedes) along forest edge-to-interior gradients. Insect Conserv. Divers. 2016, 9, 456–469. [Google Scholar] [CrossRef]
- Pey, B.; Trân, C.; Cruz, P.; Hedde, M.; Jouany, C.; Laplanche, C.; Nahmani, J.; Chauvet, E.; Lecerf, A. Nutritive value and physical and chemical deterrents of forage grass litter explain feeding performances of two soil macrodetritivores. Appl. Soil Ecol. 2018, 133, 81–88. [Google Scholar] [CrossRef]
- Zheltukhina, V.I.; Korobov, E.D. Pauki i Zhestkokrylye Central’no-Lesnogo Zapovednika (Annotirovannyj Spisok Vidov). [Spiders and Coleopterance of the Central Forest Reserve: Annotated Species List]; Commission of Russian Academy of Science for the conservation of biological diversity: Moscow, Russia, 2009; 54p. (In Russian) [Google Scholar]
- Gongalsky, K.B. Soil macrofauna: Study problems and perspectives. Soil Biol. Biochem. 2021, 159, 108281. [Google Scholar] [CrossRef]
- Anderson, J.M.; Ingram, J.S.I. Tropical Soil Biology and Fertility: A Handbook of Methods, 2nd ed.; CAB International: Wallingford, UK, 1993; 221p. [Google Scholar]
- Sabatté, M.L.; Massobrio, M.J.; Cassani, M.T.; Momo, F.R. Macro and mesofauna soil food webs in two temperate grasslands: Responses to forestation with Eucalyptus. Heliyon 2021, 7, e05869. [Google Scholar] [CrossRef] [PubMed]

| Parameter | Zonal Forest | Secondary Forest | Monoculture | Recreational Forest | Clear-Cut |
|---|---|---|---|---|---|
| Total abundance, ind. m−2 | 503.7 ± 233.2 a | 792.6 ± 216.3 a | 592.6 ± 301 a | 1844.4 ± 607.1 a | 800.0 ± 66.7 a |
| Predators, ind. m−2 | 140.7 ± 39.2 b | 437.0 ± 137.2 ab | 185.2 ± 141.3 b | 1163.0 ± 543.2 a | 533.3 ± 67.9 ab |
| Saprophages, ind. m−2 | 81.5 ± 26.7 a | 103.7 ± 19.6 a | 140.7 ± 39.2 a | 259.3 ± 83.5 a | 111.1 ± 12.8 a |
| Phytophages, ind. m−2 | 281.5 ± 172.3 a | 229.6 ± 85.4 a | 266.7 ± 135.2 a | 303.7 ± 99.7 a | 118.5 ± 37 a |
| Unknown trophic position, ind. m−2 * | 0 ± 0 | 22.2 ± 12.8 | 0 ± 0 | 118.5 ± 51.9 | 37.0 ± 26.7 |
| Mobile, ind. m−2 | 459.3 ± 211.7 b | 688.9 ± 167.8 ab | 577.8 ± 299.0 b | 1666.7 ± 560.9 a | 688.9 ± 33.9 ab |
| Resident, ind. m−2 | 44.4 ± 22.2 ab | 103.7 ± 48.6 ab | 14.8 ± 7.4 b | 177.8 ± 46.3 a | 118.5 ± 26.6 ab |
| Aboveground, ind. m−2 | 355.6 ± 215.8 a | 311.1 ± 115.5 a | 200.0 ± 109.6 a | 474.1 ± 94.6 a | 318.5 ± 26.7 a |
| Belowground, ind. m−2 | 148.1 ± 19.6 a | 481.5 ± 232.8 a | 392.6 ± 194.3 a | 1370.4 ± 531.5 a | 488.9 ± 58.8 a |
| Total number of taxa ** | 13 | 17 | 12 | 26 | 25 |
| Average taxonomic richness *** | 4.5 ± 1.3 b | 7.7 ± 1.6 ab | 4.8 ± 1.9 b | 11.2 ± 1.3 a | 9.5 ± 0.6 ab |
| Litter weight, g | 11.69 ± 2 a | 8.93 ± 19.2 ab | 4.76 ± 0.25 bc | 5.27 ± 0.77 bc | 1.88 ± 0.24 c |
| Litter depth, cm | 4.42 ± 0.17 a | 0.95 ± 0.15 b | 3.67 ± 0.3 a | 1.58 ± 0.22 b | 1.13 ± 0.07 b |
| pH | 5.51 ± 0.27 a | 5.07 ± 0.15 a | 5.4 ± 0.13 a | 5.66 ± 0.2 a | 5.19 ± 0.15 a |
| C, % | 7.2 ± 1.23 a | 3.93 ± 0.49 a | 3.75 ± 0.48 a | 3.93 ± 0.9a | 3.95 ± 0.5 a |
| N, % | 0.58 ± 0.06 a | 0.3 ± 0.04 ab | 0.27 ± 0.03 b | 0.3 ± 0.08 ab | 0.28 ± 0.04 b |
| C/N | 11.9 ± 0.67 a | 13.1 ± 0.21 a | 13.8 ± 0.85 a | 13.04 ± 0.25 a | 14.2 ± 1.41 a |
| Cmic, mg C/g dry soil | 1.23 ± 0.21 a | 1.01 ± 0.05 a | 1.05 ± 0.27 a | 0.93 ± 0.14 a | 0.93 ± 0.02 a |
| Variable | Sum of Squares | df | Mean Square | F | p-Value |
|---|---|---|---|---|---|
| Litter weight | 176.2 | 4 | 44.1 | 8.8 | 0.003 |
| Litter depth | 5.9 | 4 | 1.5 | 45.1 | 0.001 |
| pH | 0.7 | 4 | 0.2 | 1.6 | 0.2 |
| Cmic | 0.2 | 4 | 0.05 | 0.5 | 0.7 |
| N, % | 1.3 | 4 | 0.3 | 4.6 | 0.03 |
| C, % | 0.9 | 4 | 0.2 | 3.1 | 0.07 |
| C/N | 0.05 | 4 | 0.01 | 1.3 | 0.3 |
| Total macrofauna abundance | 3.9 | 4 | 0.97 | 2.3 | 0.1 |
| Average taxonomic richness | 301.7 | 4 | 75.4 | 6.8 | 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korobushkin, D.I.; Pronina, N.A.; Saifutdinov, R.A.; Guseva, P.A.; Tsurikov, S.M.; Dudova, K.V. Taxonomic Diversity and Abundance of Soil Macrofauna in Temperate Forests Under Different Types of Forest Management: A Case Study in European Russia. Diversity 2025, 17, 216. https://doi.org/10.3390/d17030216
Korobushkin DI, Pronina NA, Saifutdinov RA, Guseva PA, Tsurikov SM, Dudova KV. Taxonomic Diversity and Abundance of Soil Macrofauna in Temperate Forests Under Different Types of Forest Management: A Case Study in European Russia. Diversity. 2025; 17(3):216. https://doi.org/10.3390/d17030216
Chicago/Turabian StyleKorobushkin, Daniil I., Nina A. Pronina, Ruslan A. Saifutdinov, Polina A. Guseva, Sergey M. Tsurikov, and Ksenia V. Dudova. 2025. "Taxonomic Diversity and Abundance of Soil Macrofauna in Temperate Forests Under Different Types of Forest Management: A Case Study in European Russia" Diversity 17, no. 3: 216. https://doi.org/10.3390/d17030216
APA StyleKorobushkin, D. I., Pronina, N. A., Saifutdinov, R. A., Guseva, P. A., Tsurikov, S. M., & Dudova, K. V. (2025). Taxonomic Diversity and Abundance of Soil Macrofauna in Temperate Forests Under Different Types of Forest Management: A Case Study in European Russia. Diversity, 17(3), 216. https://doi.org/10.3390/d17030216






