Abstract
One specimen of the non-native European hake, Merluccius merluccius (Linnaeus, 1758), was caught in July 2023 in the offshore waters of the Bulgarian Black Sea. The species was identified based on its morphological characteristics (15 morphometric and 5 meristic) and DNA barcoding. The male specimen had a total length of 375 mm and a weight of 417.5 g. Its age was determined as 4 years. A fragment of the cytochrome b gene from its mitochondrial DNA was sequenced to support species identification. A phylogenetic analysis revealed a 100% genetic identity between the Bulgarian specimen and a M. merluccius sequence available in GenBank. This is the first documented report of this boreal-subtropic, benthopelagic fish species along the Bulgarian Black Sea coast. The record of the European hake confirms its presence along the western Black Sea coast.
1. Introduction
The European hake (Merluccius merluccius) is one of the most exploited demersal fish species in the Western and Eastern Mediterranean Sea [1,2]. Its distribution extends along the Atlantic coast of Europe and western North Africa, ranging from Norway and Iceland in the north to Mauritania in the south [1]). Longitudinally, the European hake can be found as far west as Iceland (around 27° W) and as far east as the Black Sea (40° E), having first colonized the entire Mediterranean Sea [3]. The species is non-native to the Black Sea [4], with its first recorded occurrence in the region mentioned by [5] in the marine fish checklist for Turkey. Various studies [1,6,7,8,9,10] have reported the presence of the European hake along the southern, eastern, and Crimean coasts of the Black Sea. Based on its occurrence at different times and across a wide depth range, ref. [9] concluded that the species has adapted to the eastern Black Sea coast of Turkey, suggesting a potential increase in its biomass. The presence of the European hake in the western Black Sea was noted by [11]. The European hake has been described as a benthopelagic predator caught in nearly all Mediterranean basins and is of high economic value [12], which can also feed in the water columns during migration. They may interact with nearly the entire food web in marine ecosystems [13]. Successful invaders are often characterized by their ability to utilize resources efficiently compared to native species [14], resulting in prey resource populations vulnerable to severe declines and possible extinctions [15]. The co-existence of an invasive species with other species might be explained by the availability of sufficient resources for all species and resource partitioning. The abundance of an invasive fish species and the degree of niche overlap with the native populations is a good indicator of the possible impacts on a fish community [16]. The hake could compete successfully for food with local whiting (Merlangius merlangus), which is also a benthopelagic predator in the Black Sea. The possible future expansion of the hake in the Black Sea and the local species diet overlap effects should be regarded with special attention.
The aim of this study is to report the first record of M. merluccius in Bulgarian Black Sea waters, based on morphological and molecular evidence.
2. Material and Methods
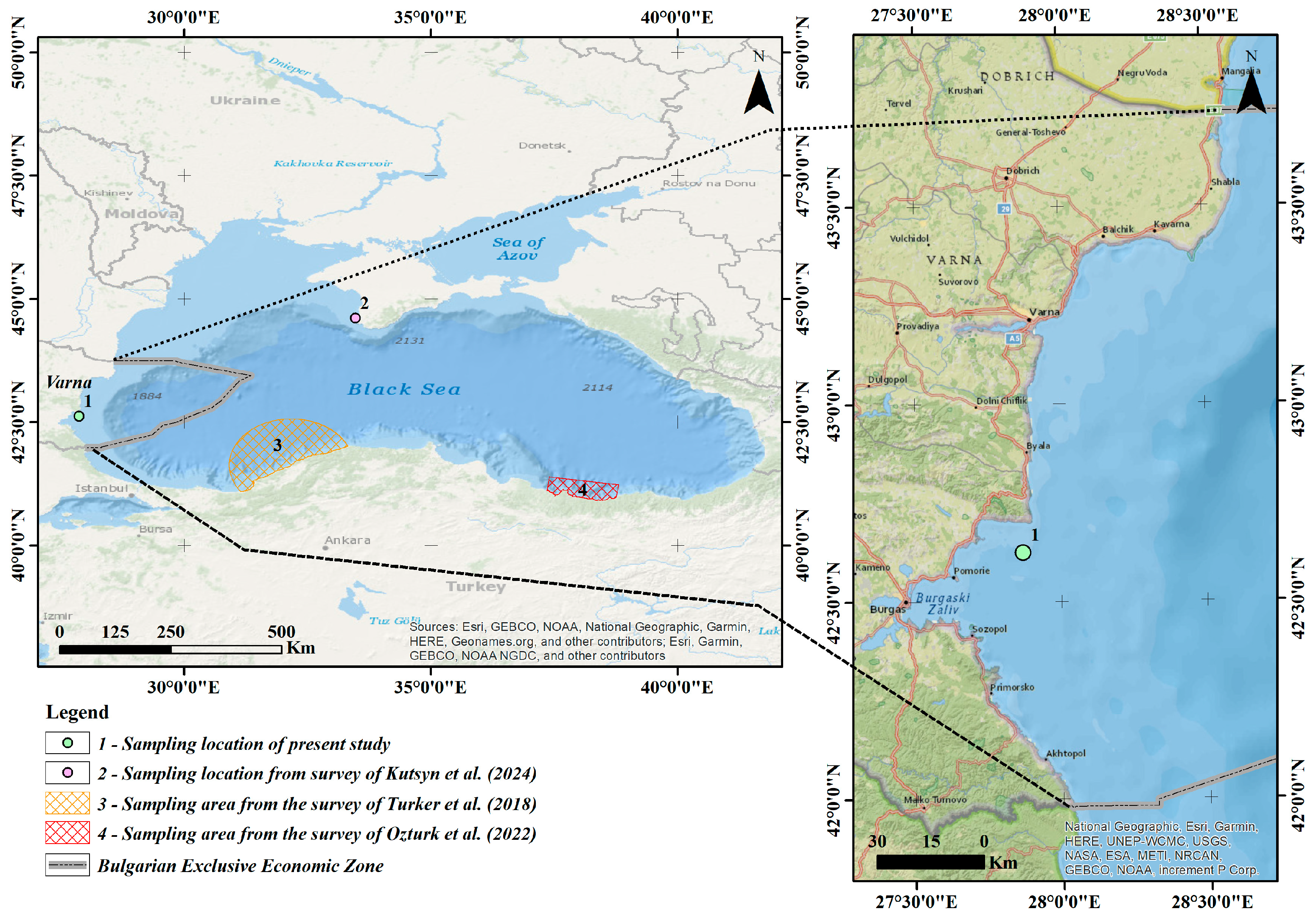
On 27 July 2023, during a scientific monitoring survey at the OTM trawl haul station (codend mesh 13 mm) (Figure 1), at a depth of 22 m, a sexually mature male specimen of M. merluccius (Linnaeus, 1758) was captured. The sea surface temperature at the capture location was 26.8 °C, and the water salinity was 17.5‰. The specimen was freshly processed onboard: measured, weighted, photographed and frozen until further analysis in the freezer at −20 °C. Later, the specimen was delivered to the laboratory using cooler bag.
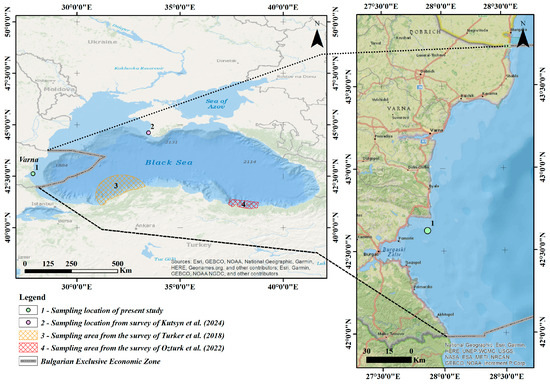
Figure 1.
Map showing the location of the M. merluccius specimen records in the Black Sea [9,10,11].
2.1. Morphometric and Meristic Measurements
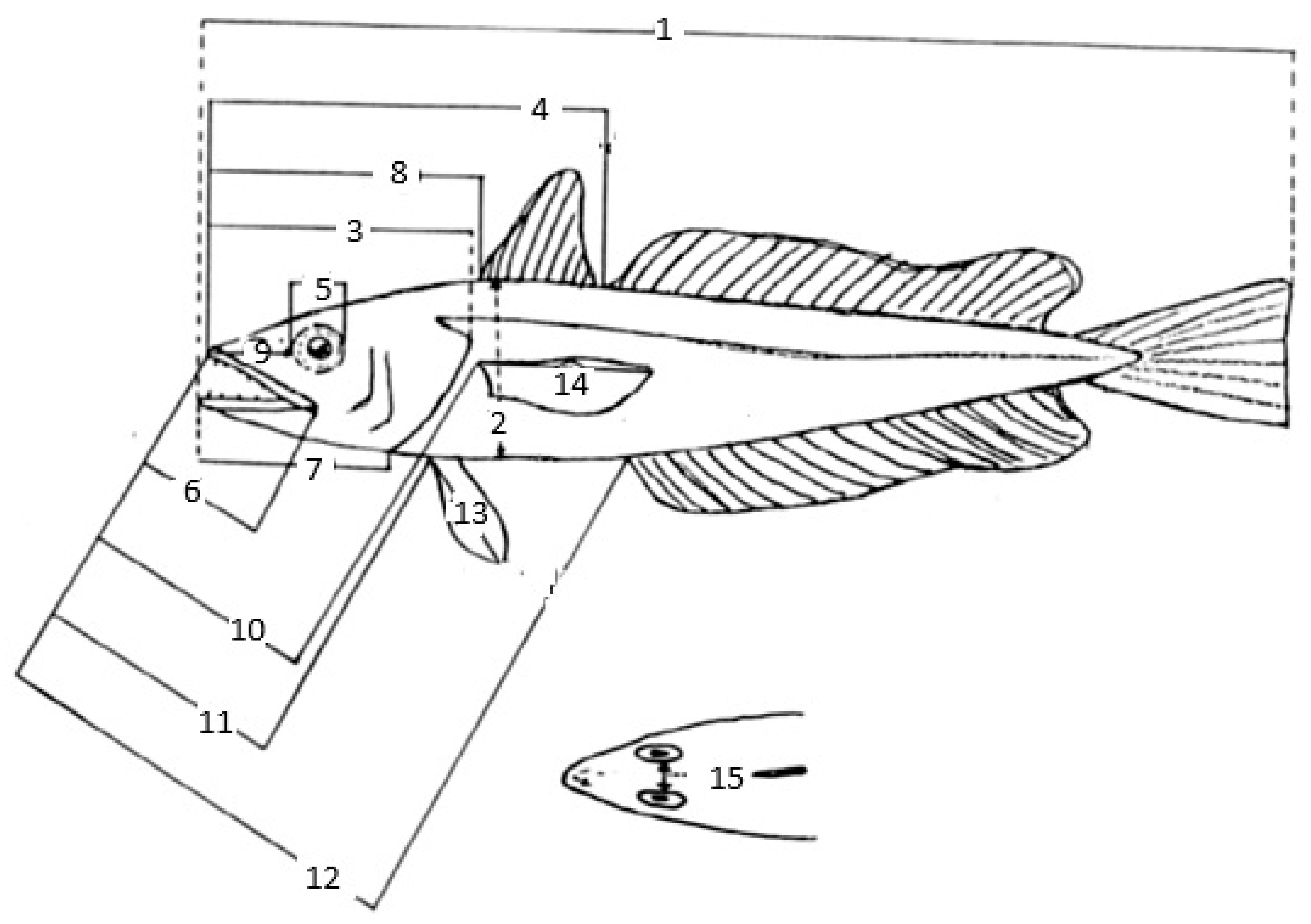
A total of 15 morphometric and 5 meristic characters were measured (Figure 2). The body measurements were recorded to the nearest 1 mm on linear axes for fish; and the body weight with an accuracy of 0.01 g.
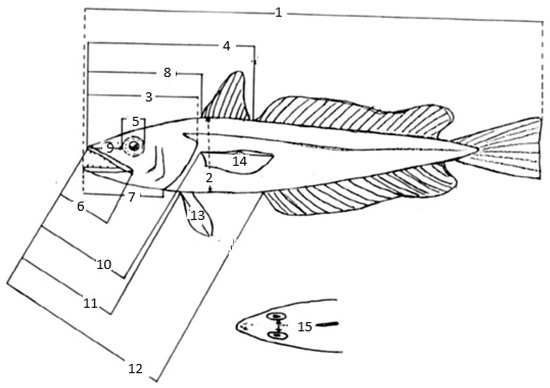
Figure 2.
Morphometric and meristic characters of M. merluccius (after [17]): total length 1, body depth 2, head length 3, snout length 4, diameter of orbit 5, upper jaw length 6, lower jaw length 7, length from tip of snout to first dorsal fin origin 8, length from tip of snout to second dorsal fin origin 9, length from tip of snout to pectoral fin insertion 10, length from tip of snout to pelvic fin insertion 11, length from tip of snout to anal fin origin 12, pelvic fin length 13, pectoral fin length 14, interorbital length 15.
The length, height, and width of the otoliths were measured using a caliper with an accuracy of 0.05 mm. The mass of the otoliths was determined using laboratory scales with an accuracy of 0.001 g.
To determine the age of the fish, otolith sections embedded in polymer (epoxy resin) and polished were used [18]. The cross-sections necessary for studying the increment sequences in the otoliths were placed in a 50% glycerol solution and observed under an incident light against a dark background at 10× magnification. The sectioned otoliths were also examined under a transmitted light using a binocular digital microscope set (Kern Digital Microscope Set OZL 464T241, KERN & SOHN GmbH., Balingen, Germany) linked to a Tablet camera (Kern ODC-2 Version 1.1 KERN & SOHN GmbH., Balingen, Germany). Oil was used to enhance the visibility of growth structures. The direct counting of opaque and hyaline zones was conducted, with false rings omitted to prevent the misinterpretation of the actual age. The first visible translucent zone from the center was interpreted as an additional demersal ring. Back-calculations were performed to describe individual growth, following the original descriptions by [19,20], who were the first to formally derive the back-calculation model based on the concept that “the growth increment of the scale is, on average, a constant proportion of the growth increment of the fish” [21].
In practice, the Fraser–Lee model modified the Dahl–Lea model by adjusting for the fish’s length at the time of structure formation (i.e., when R = 0, L = c), that is,
where “”comes from the length of the fish at the time of structure formation, the intercept of the length–structure relationship regression (e.g., from Equation (1)).
The gonadosomatic index (GSI) is the calculation of the gonad mass as a proportion of the total body mass [22]. It is represented by the formula:
GSI = [gonad weight/total tissue weight] × 100
2.2. Molecular Identification
The sequence analysis of the mitochondrial cytochrome b (cyt b) gene region was performed to genetically identify the specimen. A pectoral fin sample was preserved in 96% ethanol at 4 °C. Genomic DNA was extracted using the NucleoSpin Tissue kit (Machery-Nagel, Düren, Germany). A fragment of the cyt b (336 bp) gene was amplified via conventional polymerase chain reaction (PCR) using the primers L14841 5′-AAAAAGCTTCCATCCAACATCTCAGCATGATGAAA-3′ and H15149 5′-AAACTGCAGCCCCTCAGAATGATATTTGTCCTCA-3′ [23]. PCR was performed in a 50 μL reaction volume containing 1 μL of each primer, 25 μL of MyTaqTM HS Mix, and 2 μL of the target DNA. The PCR conditions were as follows: 95 °C for 1 min, followed by 35 cycles of 95 °C for 30 s, 55 °C for 30 s, 72 °C for 30 s, and a final extension step at 72 °C for 10 min. The PCR product quality was assessed using electrophoresis on a 1% agarose gel. The PCR product was purified and sequenced in both directions at Macrogen Europe B.V, Amsterdam, the Netherlands. The sequence was edited manually by using MEGA 12 [24] and is available in the GenBank database [24] under the accession number PV105570.
The obtained sequence was compared with reference sequences in the GenBank database [25] using the BLAST (Basic Local Alignment Search Tool, https://blast.ncbi.nlm.nih.gov, accessed on 3 February 2025), and species identification was performed by comparing sequence similarity. Preferably, sequences from published articles or from FishTrace [26] were selected to ensure greater taxonomic reliability. The sequence of the studied Black Sea specimen was aligned with the cyt b sequences of M. merluccius from other locations, Merluccius capensis, and Merluccius bilinearis, with Merlangus merlangus and Gadhus morhua used as an outgroup taxa, using MUSCLE [27] with the default settings in MEGA 12 [24]. The total number of nucleotide sequences was 20, and 333 positions were included in the final dataset used for the phylogenetic analyses. All the sites were used for the analyses. The phylogenetic relationships were determined using the maximum likelihood (ML) method in MEGA 12 [24] and the Bayesian inference (BI) method in MrBayes v.3.2 [28]. For the BI method, four Markov chain Monte Carlo (MCMC) chains were run for 1,000,000 generations, sampling every 100 generations. The first 25% of burn-in trees were discarded. The phylogenetic tree was represented using the ML results, with bootstrap values from the ML method (1000 replicates) and posterior probabilities from the BI method. The model used for both the ML and BI analyses was the general time-reversible (GTR) model with a discrete gamma distribution (+G), as selected by MEGA 12 [24] for ML and MrModeltest2 [29] for BI, based on the lowest Akaike information criterion (AIC) scores.
3. Results and Discussion
3.1. Morphological Analysis
The total length (TL) and total weight (TW) of the sampled specimen were 375 mm and 417.1 g, respectively (Figure 3). These results are comparable to the findings of [11] of the western Black Sea coast of Turkey (TL range: 12.5–37.8 cm; TW range: 13.53–494.95 g). The sampling and morphological details of the European hake specimen are provided in Table 1.

Figure 3.
(a,b) The sampled specimen, M. merluccius (♂), TL = 375 mm; W = 417.5 g, captured from the Bulgarian Black Sea offshore waters on 27 July 2023.

Table 1.
Morphometric and meristic characters of the M. merluccius specimen.
Additionally, the haul contained other fish such as sprat (Sprattus sprattus, Linnaeus, 1758), whiting (Merlangius merlangus, Linnaeus, 1758), turbot (Scophthalmus maximus, Linnaeus, 1758), horse mackerel (Trachurus mediterraneus, Steindachner, 1868), European flounder (Platichthys flesus, Linnaeus, 1758), and round goby (Neogobius melanostomus, Pallas, 1809).
The testes were at stage 2c—maturing, with a GSI of 0.82%, which is comparable with the estimate by [10] (1.01%). The otoliths were massive and elongated; the ventral edge was smooth and rounded, while the dorsal edge had multiple flukes and notches. The dimensions of the right otolith were as follows: length 1.510, height 0.714, and width 0.177; and its mass was 0.142 g. The age of the male specimen was determined to be 4 years. According to the reverse calculations based on the otolith cross-section, the total length of the specimen was estimated to be 17.6 cm, 26.1 cm, 31.2 cm, and 37.5 cm by the end of the first, second, third and fourth year of life, respectively (Figure 4, Table 2). The age and individual growth observed in the present study and are similar to those reported by [10] for the specimens found in the Black Sea but differ from those of other studied individuals in the Mediterranean waters.
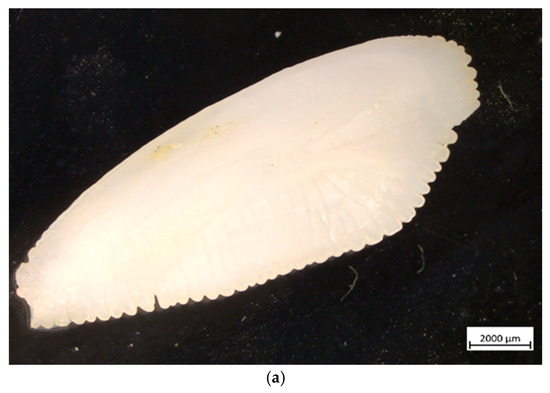
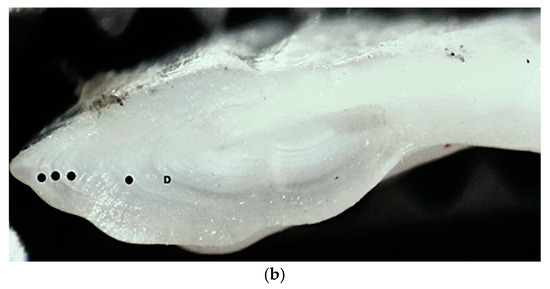
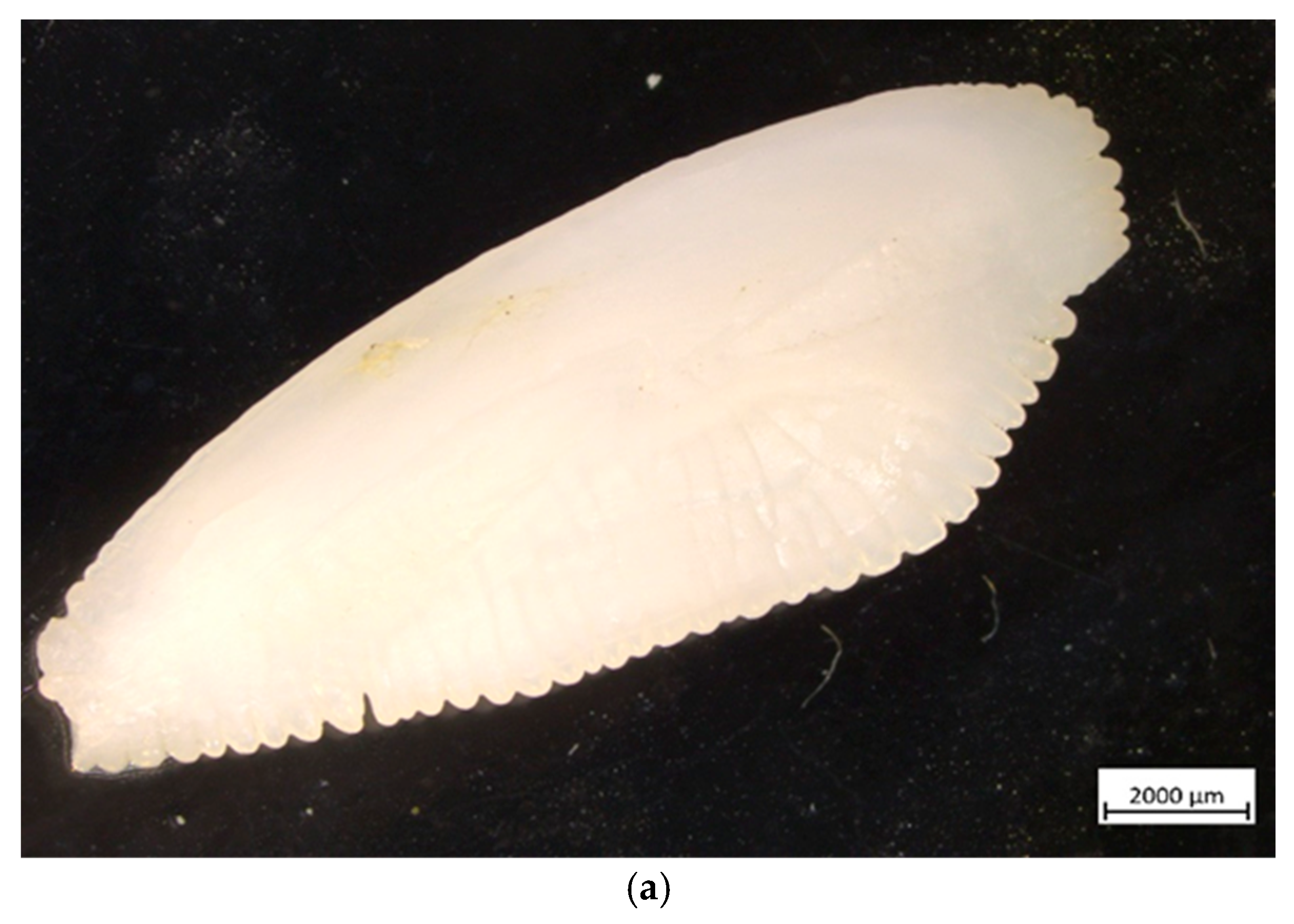
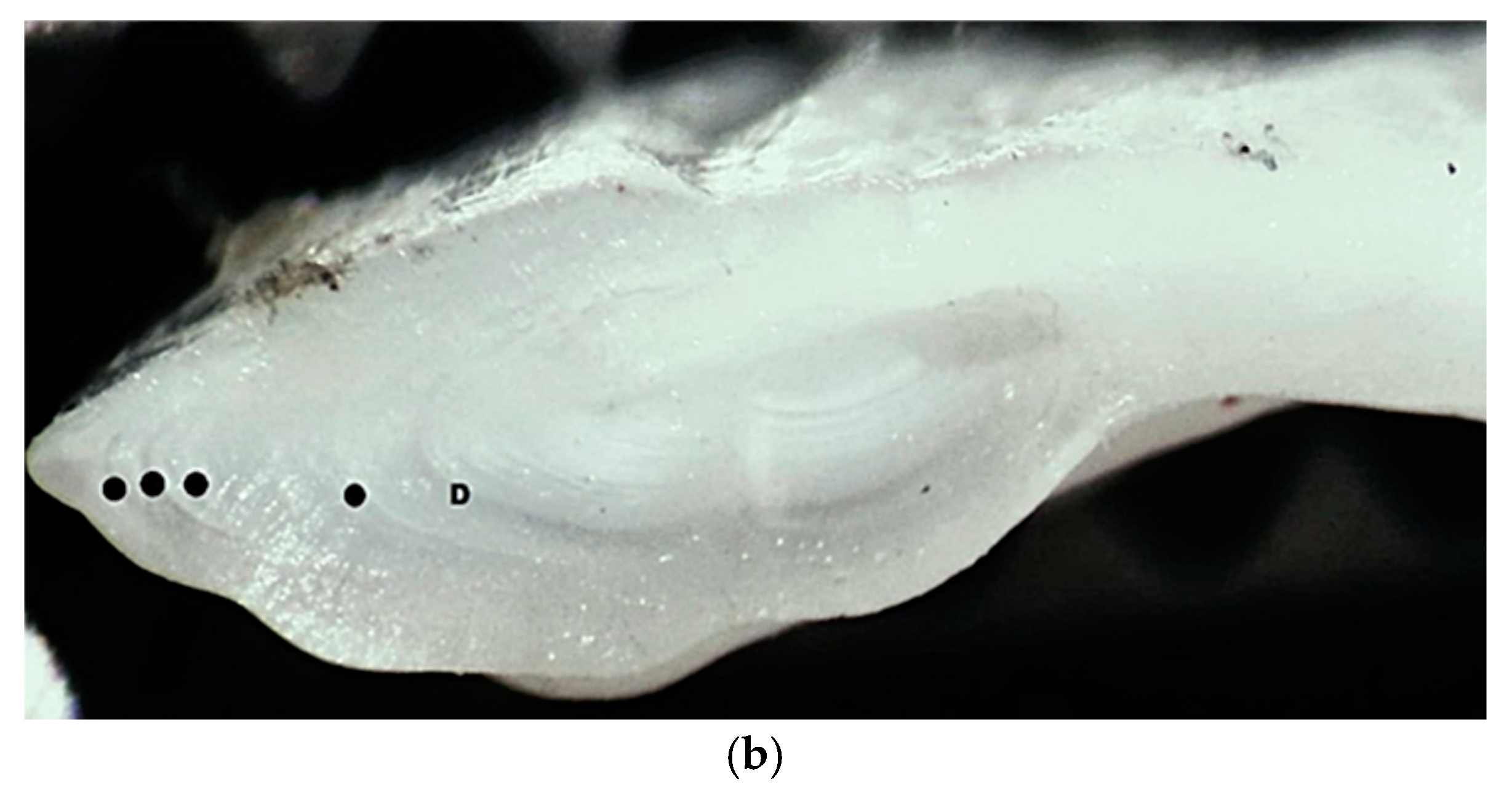
Figure 4.
European hake (M. merluccius) otolith (sagitta) from the studied specimen: (a) external appearance (magnification ×10); (b) cross-section—● annual rings, D demersal ring.

Table 2.
Total length at age for the M. merluccius obtained by several authors in different regions.
The age estimation of M. merluccius remains a major challenge due to the difficulty of otolith interpretation [30,34,35,36,37,38,39,40,41] and a lack of common interpretation criteria applicable to otoliths or scales [42]. Two main factors contribute to this challenge: (1) the continuous recruitment of juveniles due to multiple spawning events throughout the year [35,43] and the difficulty in identifying the first winter annulus due to the variable ring patterns influenced by the hatching season [37]. The age estimation of hake remains uncertain, and experimental studies suggest that the ageing of the hake in the Atlantic and Mediterranean Seas is often overestimated [44]. The age determination of M. merluccius is more complex than that of many other species due to the presence of checks or false rings, as well as the fact that each growth annulus is characterized by bands of several thinner translucent rings [45]. Additionally, the difficulties in identifying the first annulus have introduced biases in age interpretation [1].
3.2. DNA Barcoding
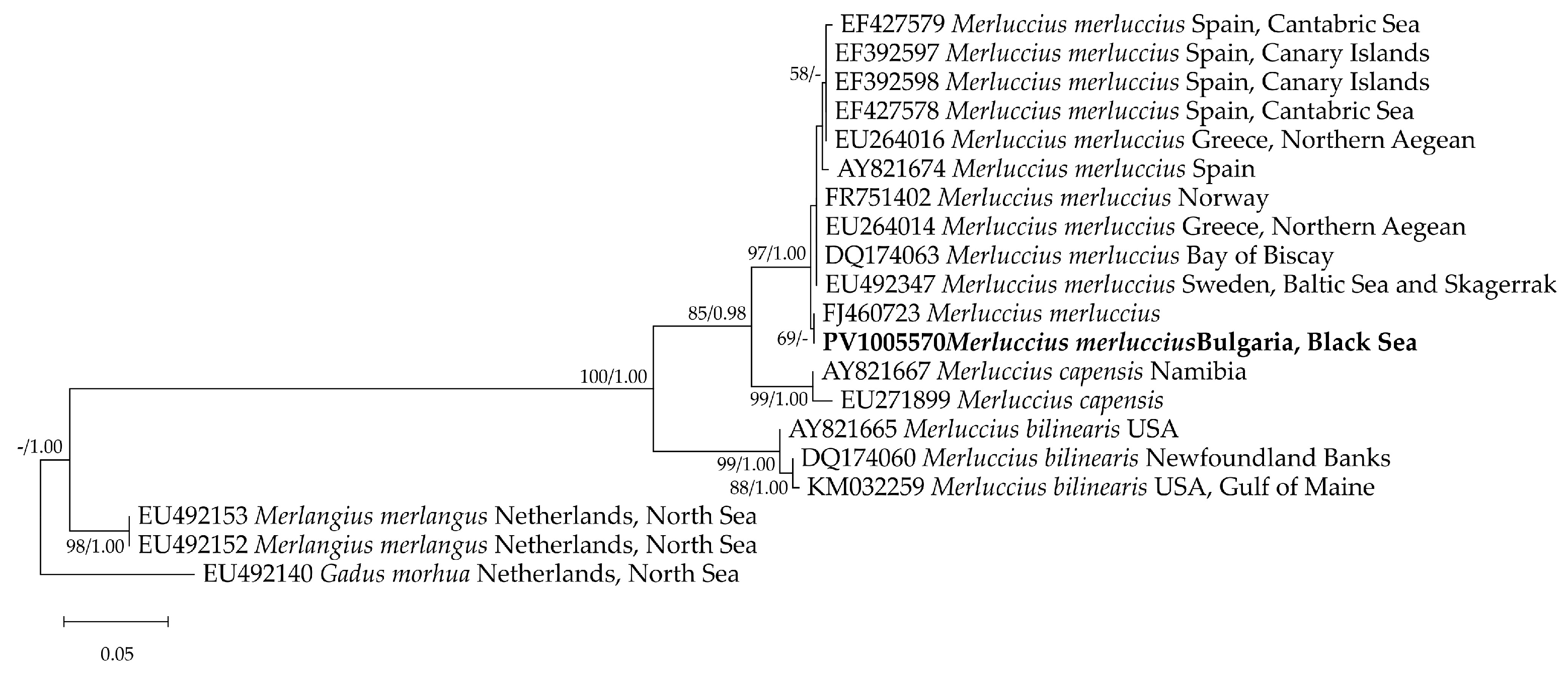
The cyt b sequence from the Black Sea specimen analyzed in the present study was identical to a sequence deposited in the GenBank database under the accession number FJ460723. In the phylogenetic tree, the representatives of the genus Merluccius clustered into a robust clade (ML 100%/BI 1.00). The Black Sea isolate was positioned within the well-supported M. merluccius clade (ML 97%/BI 1.00), alongside sequences from Spain, Greece, Norway, and Sweden (Figure 5). No clear separation based on the geographic origin was observed. This finding has been supported by the additional mitochondrial primers (16S rRNA and COI) that confirmed the species’ distribution range extending eastward into the Black Sea [9].
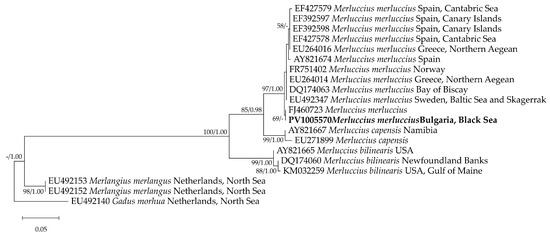
Figure 5.
A maximum likelihood tree derived from a cyt b alignment, including a total of 20 sequences–12 from M. merluccius, two from M. capensis, three from M. bilinearis, with Merlangus merlangus and Gadus morhua serving as outgroup taxa. The tree is drawn to scale, with branch lengths measured in the number of substitutions per site. The node labels correspond to the bootstrap values from the maximum likelihood method (1000 replicates) and the posterior probabilities from the Bayesian inference analyses (ML/BI); only bootstrap values > 50% and posterior probabilities > 0.9 are shown. The sequence from this study is indicated in bold.
4. Possible Vectors of Introduction and Effects
In recent years, the physical, chemical, and biological properties of the Black Sea have changed significantly with the impact of global climate change, and this is the reason for the constant development of Black Sea ichthyofauna [9]. A whole range of various factors is responsible for the penetration of Mediterranean species with subsequent naturalization in the Black Sea. These include not only a dramatic change in climate, but also a change in the natural coast landscape associated with economic activities [46]. Global climate change is driving shifts in the geographical distribution of species, leading to both local extinctions and range expansions. The Black Sea, characterized by low species diversity (indicating low competition) and high habitat diversity (providing a wide range of potential niches), creates favorable conditions for the introduction of alien species [47]. Natural and artificial transport pathways, such as ballast water transport, facilitate the redistribution of species, effectively eliminating the geographical barriers that once separated them [47]. The Turkish Straits System (TSS), consisting of the Istanbul Strait (Bosphorus), the Sea of Marmara, and the Çanakkale Strait, acts as a corridor for the two-way translocation of species between the Black and Mediterranean Seas [48,49]. Consequently, the diversity of the Black Sea ichthyofauna has increased due to the influx of the Mediterranean fish species [50,51,52]. The environmental conditions, such as temperature and food availability (crustaceans and small pelagic fish species), might play a key role in the spatial distribution of the biomass of the European hake [53]. As temperatures rise, the Mediterraneanization of the Black Sea fauna is underway, driven by the immigration of new species [54]. According to [46], a total of 25 new species of fish have been discovered in the Black Sea since 2014. The potential of crustaceans and small pelagic fish (anchovies and sardines) in the Black Sea [55] might support the existence of M. merluccius in the long term. Lower fishing pressure and the reduction of overfishing in the Black Sea could be another reason for the spatial distribution and further adaptation of the species in the Black Sea [9]. Humans and society are fundamentally involved with biological invasions in multiple ways, from the initial introduction, to recognition and management [56]. Many invasive alien species also change the ecosystem functioning and the delivery of ecosystem services by altering nutrient and contaminant cycling, hydrology, habitat structures, and disturbance regimes.
One additional and under-appreciated way in which humans have altered disturbance regimes is through the introduction of invasive non-native species, who themselves are capable of modifying existing disturbance regimes or introducing entirely new disturbances [57]. There is now evidence that the alteration of a disturbance regime may produce the most profound effects that a species or functional group can have on an ecosystem’s structure and function [58].
Consequently, many thousands of species may be transported around the world as stowaways in ballast water [59], and as contaminants of transported goods to regions that are becoming increasingly susceptible to new invasions owing to climate warming. Marine invasions are also being exacerbated by the dramatic increase in use of non-biodegradable plastics since the second half of the 20th century, depositing billions of tons of plastics globally at the land–sea interface. A new mechanism for ocean rafting is created when these plastics are swept into the ocean by tsunamis or by the increasing (owing to climate change) number and size of cyclonic storms (hurricanes, monsoons, typhoons) [60].
Recent studies [9,10] and the present study indicate that the European hake (Merluccius merluccius) continues to spread in the Black Sea, despite significantly different environmental conditions compared to its native habitats in the Mediterranean Sea and Northeast Atlantic. Ref. [61] reported that the reproduction of M. merluccius in the Black Sea has not been confirmed, as no mature individuals, eggs, or larvae have been documented. However, ref. [62] recorded a single instance of larvae found off the Crimean coast, though no adult specimens were observed. Ref. [9] suggest that the species has adapted to the Eastern Black Sea region, and further research is needed to determine whether it can successfully reproduce in this environment. If the species establishes a self-sustaining population, it could become a viable fishery resource in the long term. The potential expansion of the hake population in the Black Sea may lead to competition for food with native species, such as whiting (Merlangius merlangus). As both species may occupy similar ecological niches, competition for resources and environmental factors could arise. Therefore, the overlap of their niches and its ecological implications should be closely monitored in the near future.
5. Conclusions
This is the first confirmed report of M. merluccius along the Bulgarian Black Sea coast (West Black Sea region), based on both morphological and molecular identification. Consequently, further research is needed to enhance our understanding of the species’ biology, ecology, and reproduction, as well as to assess its ecological impact. This discovery underscores the need for long-term monitoring of the species’ effects on the Black Sea ecosystems.
Author Contributions
Conceptualization, V.S.R., P.I. and N.D.; methodology, P.I., N.D., V.S.R., M.Y. and D.P.D.; software, N.D. and B.P.; validation, P.I., N.D., V.S.R. and M.Y.; formal analysis, P.I., N.D., V.S.R. and M.Y.; investigation, P.I., N.D. and V.S.R.; resources, D.P.D. and V.S.R.; data curation, P.I., N.D. and V.S.R.; writing—original draft preparation, P.I., N.D., V.S.R. and M.Y.; writing—review and editing, P.I., N.D., V.S.R. and M.Y.; visualization, N.D. and B.P.; supervision, V.S.R. and P.I.; project administration, P.I. and V.S.R.; funding acquisition, P.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the grant BG14MFOP001-1.002-0001 “Collection, management and use of data for the purposes of scientific analysis and implementation of the Common Fisheries Policy for the period 2023–2024”, financed by the Maritime, Fisheries and Aquaculture Programme, co-financed by the European Union through the European Maritime, Fisheries and Aquaculture Fund.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The data are contained within the article.
Acknowledgments
This research received financial support from MASRI (project of the National Roadmap for Scientific Infrastructure (2020–2027) of the Republic of Bulgaria).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Casey, J.; Pereiro, J. European hake (M. merluccius) in the North-East Atlantic. In Hake: Fisheries, Ecology and Markets; Alheit, J., Pitcher, T.J., Eds.; Fish and Fisheries Series; Chapman and Hall: London, UK; New York, NY, USA, 1995; Volume 15, pp. 125–147. ISBN 0-412-57350-4. [Google Scholar]
- Gücü, C.A.; Bingel, F. Hake, Merluccius merluccius L., in the northeastern Mediterranean Sea: A case of disappearance. J. Appl. Ichthyol. 2011, 27, 1001–1012. [Google Scholar]
- Follesa, M.C.; Carbonara, P. Atlas of the Maturity Stages of Mediterranean Fishery Resources; Studies and Reviews; FAO: Rome, Italy, 2019; pp. 1–268. ISBN 978-92-5-131172-1. [Google Scholar]
- Parin, N.V.; Evseenko, S.À.; Vasileva, Å.D. Fishes of Russian Seas: Annotated Catalogue; Sysoev, A.V., Ed.; KMK Scientific Press: Moscow, Russia, 2014; pp. 2–733. ISBN 978-5-87317-967-1. [Google Scholar]
- Bilecenoğlu, M.; Kaya, M.; Cihangir, B.; Çiçek, E. An updated checklist of the marine fishes of Turkey. Turk. J. Zool. 2014, 38, 901–929. [Google Scholar] [CrossRef]
- Svetovidov, A.M. Gadiformes. Zoological Institute of the Academy of Sciences of the U.S.S.R.; National Science Foundation and Smithsonian Institution: Washington, DC, USA, 1948; Volume 9, pp. 5–222. [Google Scholar]
- Geldiay, R. Important Fishes Found in the Bay of Izmir and Their Possible Invasions. Ph.D. Thesis, Ege University of Turkkey, Izmir, Turkey, 1969. [Google Scholar]
- Svetovidov, A.N. Gadidae. In Fishes of the North-Eastern Atlantic and the Mediterranean; Whitehead, P.J., Bauchot, M.L., Hureau, J.C., Nielsen, J., Tortonese, E., Eds.; UNESCO: Paris, France, 1986; Volume 2, pp. 680–710. [Google Scholar]
- Öztürk, R.Ç.; Karadurmuş, U.; Aydın, M. Range extension of European hake from the Eastern Black Sea coasts of Turkey. J. Agric. Fac. Gaziosmanpaşa Univ. (JAFAG) 2022, 39, 19–24. [Google Scholar]
- Kutsyn, D.N.; Tamoykina, I.Y.; Vdodovicha, I.V.; Klimovaa, T.N.; Donchik, P.I. Finding of the European Hake Merluccius (Merlucciidae), off the Black Sea Shore of Crimea. J. Ichthyol. 2024, 64, 80–89. [Google Scholar] [CrossRef]
- Türker, D.; Bal, H. Length–weight relationships of 13 fish species from the western Black Sea (Zonguldak-Amasra), Turkey. J. Black Sea/Medit. Environ. 2018, 24, 115–127. [Google Scholar]
- Morales-Nin, B.; Pérez-Mayol, S.; MacKenzie, K.; Catalán, I.A.; Palmer, M.; Kersaudy, T.; Mahé, K. European hake (Merluccius merluccius) stock structure in the Mediterranean as assessed by otolith shape and microchemistry. Fish. Res. 2022, 254, 106419. [Google Scholar] [CrossRef]
- Gül, G. Feeding ecology of European hake: Insights from stomach content and stable isotope analyses. Reg. Stud. Mar. Sci. 2024, 69, 103314. [Google Scholar] [CrossRef]
- Correia, A.M. Niche breadth and trophic diversity: Feeding behavior of the red swamp cray fish (Procambarus clarkii) towards environmental availability of aquatic macroinvertebrates in a rice eld (Portugal). Acta Oecologica 2002, 23, 421–429. [Google Scholar] [CrossRef]
- Alexander, M.E.; Dick, J.T.A.; Weyl, O.L.F.; Robinson, T.B.; Richardson, D.M. Existing and emerging high impact invasive species are characterized by higher functional responses than natives. Biol. Lett. 2014, 10, 20130946. [Google Scholar] [CrossRef]
- Yalçın Özdilek, Ş.; Partal, N.; Jones, R.I. An invasive species, Carassius gibelio, alters the native fish community through trophic niche competition. Aquat. Sci. 2019, 81, 29. [Google Scholar] [CrossRef]
- Abaunza, P.; Mattiucci, S.; Nascetti, G.; Magoulas, A.; Cimmaruta, R.; Bullini, L. Morphometric and meristic variation in European Hake, Merluccius Merluccius, from the Northeast Atlantic and Mediterranean Sea. In Proceedings of the 2001 ICES Annual Science Conference, Oslo, Norway, 26–29 September 2001; pp. 1–20. [Google Scholar]
- Morales-Nin, B.; Torres, G.J.; Lombarte, A.; Recasens, L. Otolith growth and age estimation in the European hake. J. Fish Biol. 1998, 53, 1155–1168. [Google Scholar] [CrossRef]
- Fraser, C.M. Growth of the spring salmon. Trans. Pac. Fish. Soc. 1916, 1915, 29–39. [Google Scholar]
- Lee, R.M. A Review of the Methods of Age and Growth Determination in Fishes by Means of Scales; London Series United Kingdom; Fisheries Investigations: London, UK, 1920; pp. 1–32. [Google Scholar]
- Francis, R.I. Back-calculation of fish length: A critical review. J. Fish Biol. 1990, 36, 883–902. [Google Scholar] [CrossRef]
- Anderson, R.O.; Gutreuter, S.J. Length, weight and associated structural indices. In Fisheries Techniques; Nielsen, L.A., Johnson, D.L., Eds.; American Fisheries Society: Bethesda, MD, USA, 1983; pp. 283–300. ISBN 978-0-9132-3500-3. [Google Scholar]
- Kocher, T.D.; Thomas, W.K.; Meyer, A.; Edwards, S.V.; Pääbo, S.; Villablanca, F.X.; Wilson, A.C. Dynamics of mitochondrial DNA evolution in animals: Amplification and sequencing with conserved primers. Proc. Natl. Acad. Sci. USA 1989, 86, 6196–6200. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Suleski, M.; Sanderford, M.; Sharma, S.; Tamura, K. MEGA12: Molecular Evolutionary Genetic Analysis version 12 for adaptive and green computing. Mol. Biol. Evol. 2024, 41, msae263. [Google Scholar] [CrossRef] [PubMed]
- Clark, K.; Karsch-Mizrachi, I.; Lipman, D.J.; Ostell, J.; Sayers, E.W. GenBank. Nucleic Acids Res. 2016, 44, 67–72. [Google Scholar] [CrossRef]
- Zanzi, A.; Martinsohn, J.T. Fish Trace: A genetic catalogue of European fishes. Database 2017, bax075. [Google Scholar]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; Van Der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Nylander, J.A.A. MrModeltest, Version 2; Program Distributed by the Author; Evolutionary Biology Centre, Uppsala University: Uppsala, Sweden, 2004. [Google Scholar]
- Aldebert, Y. Contribution a la Biologie du Merlu du Golfe du lion: Premieres donneessur la croissance. Rapp. Comm. Int. Mer Médit. 1981, 275, 47–48. [Google Scholar]
- Abdel-Aziz, E.H. Biological Studies on the Hake Merluccius merluccius from the Mediterranean Sea off Alexandria. Ph.D. Thesis, Alexandria University, Alexandria, Egypt, 1976. [Google Scholar]
- Mugahid, A.R.; Hashem, M.T. Some aspects of the fishery biology of hake Merluccius merluccius L. in Libyan waters. Bull. Inst. Oceanogr. Fish. 1982, 8, 145–168. [Google Scholar]
- Philips, A.E. Age composition of the European hake Merluccius merluccius, Linnaeus, 1758 from the Egyptian Mediterranean waters off Alexandria. Egypt. J. Aquat. Res. 2014, 40, 163–169. [Google Scholar] [CrossRef]
- Aldebert, Y.; Carries, C. Le merlu du golfe du Lion et sa pêche. Rapp. Comm. Int. Mer Médit. 1983, 28, 5–41. [Google Scholar]
- Orsi-Relini, L.; Cappan Era, M.; Fior Entino, F. Spatial temporal distribution and growth of Merluccius merluccius recruits in the Ligurian Sea, observations on the 0 group. Cybium 1989, 13, 263–270. [Google Scholar]
- Oliver, P.A. Dinámica de la Población de Merluza (Merluccius merluccius L.) de Mallorca (Reclutamento, Crecimientoy Mortalidad). Ph.D. Thesis, University Illes Balears, Balearic Islands, Spain, 1991. [Google Scholar]
- Aldebert, Y.; Morales-Nin, B. La croissance des juvéniles de merlu dans le golfe du Lion: Nouvelles méthodes d’approche. Rapp. Comm. Int. Mer Médit. 1992, 33, 1–281. [Google Scholar]
- Recasens, L. L’état d’exploitation du merlu (Merluccius merluccius) de la mer Catalane (Nord-Ouest Méditerranée). Comm. Int. Explor. Mer. Médit 1992, 33, 1–309. [Google Scholar]
- Morales-Nin, B.; Aldebert, Y. Growth of juvenile Merluccius merluccius in the Gulf of Lions (NW Mediterranean) based on otolith microstructure and length frequency analysis. Fish. Res. 1997, 30, 77–85. [Google Scholar] [CrossRef]
- Morales-Nin, B.; Moranta, J. Recruitment and post-settlement growth of juvenile Merluccius merluccius on the western Mediterranean shelf. Sci. Mar. 2004, 63, 399–409. [Google Scholar] [CrossRef][Green Version]
- Courbin, N.; Fablet, R.; Mellon, C.; De Pontual, H. Are hake otolith macrostructures randomly deposited? Insights from an unsupervised statistical and quantitative approach applied to Mediterranean hake otoliths. J. Mar. Sci. Eng. 2007, 64, 1191–1201. [Google Scholar]
- Oliver, P.; Morillas, A.; Gaza, M. Âge et croissance du merlu (Merluccius merluccius L.) des îles Baleares. Bol. Inst. Esp. Oceanogr. 1992, 11, 163–178. [Google Scholar]
- Sarano, F. Cycle ovarien du merlu, Merluccius merluccius, poisson à ponte fractionnée. Rev. Trav. Inst. Peches Marit. 1986, 48, 65–76. [Google Scholar]
- Khoufi, W.; Dufour, J.L.; Jaziri, H.; Elfehri, S.; Elleboode, R.; Bellamy, E.; Ben Meriem, S.; Romdhane, M.S.; Mahé, K. Growth estimation of Merluccius merluccius off the northern coast of Tunisia. Cybium 2014, 38, 53–59. [Google Scholar]
- Carbonara, P.; Follesa, M.C. Handbook on Fish Age Determination: A Mediterranean Experience; Studies and Reviews; FAO: Rome, Italy, 2019; pp. 1–179. ISBN 978-92-5-131176-9. [Google Scholar]
- Gus’ kov, G.E.; Zherdev, N.A.; Bukhmin, D.A. New and Rare Fish Species off the Northern Shore of the Black Sea and Anthropogenic Factors Affecting their Penetration and Naturalization (review). Ecol. Saf. Coast. Shelf Zones Sea 2022, 1, 66–81. (In Russian) [Google Scholar] [CrossRef]
- Shiganova, T.A.; Ozturk, B. Trend on increasing Mediterranean species arrival into the Black Sea. In CIESM Workshop Monographs, No 39; Climate forcing and its impact on the Black Sea Biota, Briand, F., Eds.; CIESM: Monte Carlo, Monaco, 2010; pp. 75–93. [Google Scholar]
- Öztürk, B.; Öztürk, A.A. On the biology of Turkish Straits system. Bull. Inst. Océanogr Monaco 1996, 205–221. [Google Scholar]
- Kovalev, A.V.; Mazzocchi, M.G.; Siokou-Frangou, I.; Kideys, A.E. Zooplankton of the Black Sea and the Eastern editerranean: Similarities and dissimilarities. Mediterr. Mar. Sci. 2001, 2, 69–77. [Google Scholar] [CrossRef]
- Pusanov, I.I. Mediterranization of the Black Sea fauna and prospects of its strengthening. Zool. J. 1967, 46, 1287–1289. [Google Scholar]
- Boltachev, A.R.; Yurakhno, V.M. New evidence of ongoing mediterranization of the Black Sea ichthyofauna. J. Ichthyol. 2002, 42, 713–719. [Google Scholar]
- Yankova, M.H.; Pavlov, D.V.; Ivanova, P.P.; Karpova, E.; Boltachev, A.; Bat, L.; Oral, M.; Mgeladze, M. Annotated check list of the non-native fish species (Pisces). J. Black Sea/Medit. Environ. 2013, 19, 247–255. [Google Scholar]
- Sion, L.; Zupa, W.; Calculli, C.; Garofalo, G.; Hidalgo, M.; Jadaud, A.; Lefkaditou, E.; Ligas, A.; Peristeraki, P.; Bitetto, I.; et al. Spatial distribution pattern of European hake, Merluccius merluccius (Pisces: Merlucciidae), in the Mediterranean Sea. Sci. Mar. 2019, 83, 21–32. [Google Scholar] [CrossRef]
- Sezgin, M.; Bat, L.; Katagan, A.; Ates, A.S. Likely effects of global climate change on the Black Sea benthic ecosystem. J. Environ. Prot. Ecol. 2010, 11, 238–246. [Google Scholar]
- Gücü, A.C.; Genç, Y.; Dağtekin, M.; Sakinan, S.; Ak, O.; Ok, M.; Aydın, İ. On Black Sea anchovy and its fishery. Rev. Fish. Sci. Aquac. 2017, 25, 230–244. [Google Scholar] [CrossRef]
- Shackleton, R.T.; Larson, B.M.; Novoa, A.; Richardson, D.M.; Kull, C.A. The human and social dimensions of invasion science and management. J. Environ. Manag. 2019, 229, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Pyšek, P.; Hulme, P.E.; Simberloff, D.; Bacher, S.; Blackburn, T.M.; Carlton, J.T.; Dawson, W.; Essl, F.; Foxcroft, L.C.; Genovesi, P.; et al. Scientists’ warning on invasive alien species. Biol. Rev. 2020, 95, 1511–1534. [Google Scholar] [CrossRef]
- Mack, M.C.; D’Antonio, C. Impacts of biological invasions on disturbance regimes. TREE 1998, 13, 195–198. [Google Scholar] [CrossRef]
- Geller, J.B.; Carlton, J.T.; Powers, D.A. Interspecific and intrapopulation variation in mitochondrial ribosomal DNA sequences of Mytilus spp. (Bivalvia: Mollusca). Mol. Mar. Biol. Biotechnol. 1993, 2, 44–50. [Google Scholar] [PubMed]
- Peduzzi, P.; Chatenoux, B.; Dao, H.; De Bono, A.; Herold, C.; Kossin, J.; Mouton, F.; Nordbeck, O. Global trends in tropical cyclone risk. Nat. Clim. Change 2012, 2, 289–294. [Google Scholar] [CrossRef]
- Dehnik, T.V. The Ichthyoplankton of the Black Sea; Nauk. Dumka: Kiev, Ukraine, 1973; pp. 1–234. [Google Scholar]
- Klimova, T.N.; Subbotin, A.A.; Vdodovich, I.V.; Zagorodnyaya, Y.A.; Podrezova, P.S.; Garbazei, O.A. Distribution of ichthyoplankton in relation to specifics of hydrological regime off the Crimean coast (the Black Sea) in the spring–summer season 2017. J. Ichthyol. 2021, 61, 259–269. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).